Back to Journals » International Journal of Nanomedicine » Volume 19
Plant-Derived Exosome-Like Nanovesicles in Chronic Wound Healing
Authors Wu W, Zhang B, Wang W, Bu Q, Li Y, Zhang P, Zeng L
Received 3 July 2024
Accepted for publication 23 October 2024
Published 5 November 2024 Volume 2024:19 Pages 11293—11303
DOI https://doi.org/10.2147/IJN.S485441
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Prof. Dr. RDK Misra
Weiquan Wu,1,* Bing Zhang,1,* Weiqi Wang,2 Qiujin Bu,3 Yuange Li,3 Peihua Zhang,2 Li Zeng1
1Faculty of Chinese Medicine, Macau University of Science and Technology, Macau, People’s Republic of China; 2Institute of Plastic Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China; 3Department of Radiology, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Peihua Zhang, Institute of Plastic Surgery, Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China, Email [email protected] Li Zeng, Faculty of Chinese Medicine, Macau University of Science and Technology, Macau, People’s Republic of China, Email [email protected]
Abstract: The incidence of chronic wounds is steadily increasing each year, yet conventional treatments for chronic wounds yield unsatisfactory results. The delayed healing of chronic wounds significantly affects patient quality of life, placing a heavy burden on patients, their families, and the healthcare system. Therefore, there is an urgent need to find new treatment methods for chronic wounds. Plant-derived exosome-like nanovesicles (PELNs) may be able to accelerate chronic wound healing. PELNs possess advantages such as good accessibility (due in part to high isolation yields), low immunogenicity, and good stability. Currently, there are limited reports regarding the role of PELNs in chronic wound healing and their associated mechanisms, highlighting their novelty and the necessity for further research. This review aims to provide an overview of PELNs, discussing isolation methods, composition, and their mechanisms of action in chronic wound healing. Finally, we summarize future opportunities and challenges related to the use of PELNs for the treatment of chronic wounds, and offer some new insights and solutions.
Keywords: exosomes, exosome-like nanovesicles, plants, chronic wound healing
Introduction
Chronic wounds are wounds that persist with incomplete restoration of skin anatomy and function and are typically difficult to heal.1,2 With population ageing and lifestyle changes, the incidence of chronic wounds, such as diabetic ulcers,3 venous ulcers4 and pressure ulcers,5 is increasing annually.6 The clinical treatment efficacy for chronic wounds is still unsatisfactory, and the delayed healing of chronic wounds significantly affects patient quality of life,7 imposing substantial burdens on patients, their families, and the healthcare system.8,9 Therefore, there is an urgent need to find new, more effective treatment methods for chronic wounds. Conventional treatments for chronic wounds include physical therapy, surgical intervention, and pharmacological therapy. Physical therapy methods include heat therapy, cold therapy, and ultrasound therapy.10,11 Surgical treatments include wound debridement, tissue repair, and skin grafting.12,13 Pharmacological therapy entails the use of medication to promote healing, control infections, or alleviate symptoms.14 However, these conventional treatments still have drawbacks, such as unsatisfactory therapeutic effects and high costs. Research indicates that certain plant-derived compounds are effective for treating chronic wounds,15–17 but the complexity of their composition presents a challenge.
In 1987, researchers studying erythrocyte maturation discovered nanoscale vesicles and proposed the concept of exosomes.18 Exosomes are small vesicles that are formed within cells and then secreted into the extracellular space and are capable of transferring information between cells and regulating various biological processes.19,20 Exosomes exist not only in animal cells but also in plant cells.21,22 Animal-derived exosomes play important roles in intercellular communication, immune regulation, and disease development. However, animal-derived exosomes are inherently immunogenic, difficult to obtain, and prone to degradation.23–25 Therefore, plant-derived exosome-like nanovesicles (PELNs) isolated from natural plants have become a hot research topic. PELNs may play a certain role in chronic wound healing. Compared with animal-derived exosomes, PELNs have the advantages of being readily obtainable, having low immunogenicity, and having good stability.26,27 Compared to animal-derived exosomes, there are limited reports regarding the role of PELNs in chronic wound healing and their associated mechanisms, highlighting their novelty and the necessity for further research. In this review, methods for the isolation of PELNs and their components are summarized, and the mechanisms of their effects on chronic wound healing are analysed through recent studies. Finally, we summarize the future opportunities and challenges of PELNs in the treatment of chronic wounds and present several new insights and solutions.
Overview of PELNs
PELNs were first isolated by researchers in 2009.28 PELNs are exosome-like nanosized vesicles, encapsulated by a lipid bilayer membrane, that are released by plant cells. Their diameter typically ranges from 30 to 150 nanometres, although larger PELNs more similar in size to animal exosomes have also been observed.29 PELNs have been isolated from various plant parts, including roots,30–32 leaves,33,34 fruits35–38 and seeds.39 PELNs contain bioactive molecules that participate in biological processes and regulate various cell functions, such as those of stem cells,40 tumour cells,41,42 fibroblasts43 and osteoblasts.44,45 Research indicates that PELN activities can modulate various diseases, such as cancers,41,42 autoimmune diseases,46 liver diseases,47 and inflammatory diseases.48,49 PELNs are also considered to have significant potential applications in the field of chronic wound healing.50
Isolation of PELNs
In 2023, the International Society for Extracellular Vesicles (ISEV) provided some guidance on methods for the isolation of extracellular vesicles based on yield and specificity.51 Currently, there is no single method for PELNs isolation that simultaneously achieves the highest yield and specificity. Therefore, the isolation method should be chosen based on the specific requirements of the intended research or application. The commonly used methods for isolating PELNs, as reported in the literature, are summarized in Table 1.
 |
Table 1 The Isolation Methods for PELNs |
Differential Ultracentrifugation
Differential ultracentrifugation (dUC) is a technique that is commonly employed for PELNs isolation. This method utilizes the centrifugal force generated by a centrifuge to separate and purify PELNs based on their density and size. Differential ultracentrifugation effectively removes large particles, dead cells, sticky proteins, fibers, and cell debris by sequentially applying low-speed, medium-speed, and high-speed centrifugation, followed by ultracentrifugation to obtain PELNs. PELNs can be obtained from Pueraria lobata,52 apples,53 yams,44 and Phellinus linteus54 by dUC. In general, differential ultracentrifugation enables the effective separation of PELNs based on their density and size, offering advantages such as simplicity, ease of operation, and high separation efficiency. However, this approach also requires specialized equipment and meticulous handling to prevent sample degradation.
Density Gradient Centrifugation
Density gradient centrifugation is another method commonly used for separating PELNs. This method utilizes centrifugation in density gradient media, such as sucrose or iodixanol, to separate plant samples. PELNs precipitate in layers of different densities, thereby achieving separation. Density gradient centrifugation effectively removes large particles, dead cells, sticky proteins, fibers, and cell debris by sequentially applying low-speed, medium-speed, and high-speed centrifugation, subsequent sucrose gradient ultracentrifugation to collect vesicles between the sucrose layers to obtain PELNs. PELNs can be obtained from Momordica charantia,55 Edible,56 Ginseng,31 and Artemisia annua57 by density gradient centrifugation. Overall, density gradient centrifugation enables effective separation of PELNs based on differences in density, yielding relatively pure and intact nanovesicles. This method is highly valuable for studying the composition and functions of PELNs. However, this process is complex and involves considerable loss of nanovesicles during multiple washing and transfer steps.
Polyethylene Glycol (PEG) Precipitation
Polyethylene glycol (PEG) precipitation is a commonly used method for isolating PELNs from plants. This method exploits the precipitating properties of PEG to concentrate PELNs in solution. For example, blueberry juice was subjected to differential centrifugation, and the supernatant was incubated with PEG overnight and then centrifuged at 10,000 × g for 30 minutes to obtain blueberry-derived exosome-like nanovesicles.58 Differential centrifugation of lantern fruit juice followed by overnight PEG incubation of the supernatant and subsequent low-speed centrifugation allowed the isolation of plant-derived exosome-like nanoparticles from Physalis peruviana fruit.59 This method is relatively simple and cost-effective; hence, it is widely utilized in laboratory settings. However, importantly, this method may leave behind residual PEG, which could impact certain downstream experiments or therapeutic applications and must be carefully considered.
Ultrafiltration
Ultrafiltration is another commonly used technique for isolating PELNs from plants. This method utilizes membrane filters (with pore sizes typically ranging from 100 to 300 nanometres) to perform molecular sieving, removing cellular debris and larger particulate matter, thereby yielding relatively pure extracellular vesicles. Research in Pueraria lobata has shown that after the supernatant is filtered through a 0.22 μm membrane to remove large debris, followed by centrifugation, edible exosome-like nanovesicles can be obtained.60 The ultrafiltration method is relatively simple to perform, requires no complex instrumentation, and does not introduce exogenous substances, as is the case for PEG precipitation. However, this method has certain limitations in terms of large-scale application and achieving high purity.
Size Exclusion Chromatography
Size exclusion chromatography (SEC) is a commonly used chromatographic technique that can also be employed to separate PELNs. Size exclusion chromatography separates molecules in a sample the basis of their size as they pass through a matrix, typically a porous gel or gel beads. Larger molecules are eluted more rapidly from the matrix, while smaller molecules experience greater hindrance and elute more slowly, establishing the basis of the molecular separation. Studies have shown that size exclusion chromatography can be used to isolate exosome-like nanovesicles from cabbage.61 Size exclusion chromatography offers a method for the separation of high-purity PELNs under mild conditions. However, it has drawbacks such as operational complexity, the need for specialized equipment, and sample loss/low recovery rates.
Capillary Channel Polymer (C‒CP) Separation
Capillary channel polymer (C-CP) technology is an emerging method used for the separation and capture of PELNs. This approach utilizes microfluidics technology and polymer materials to create microscale capillary channels, enabling the efficient separation of PELNs through these channels. The C-CP method has been used to isolate exosome-like nanovesicles from common fruits and vegetables.62 C-CP technology features high throughput, high efficiency, and miniaturization, allowing the separation and capture of PELNs at microscale. This method has potential applications in PELNs research, aiding researchers in gaining a better understanding of the composition and functions of exosomes; however, it exhibits low recovery rates. Importantly, as C-CP technology is still in the developmental stage, it lacks standardization, and further experimental validation and optimization are required to ensure its stability and reliability in the isolation of PELNs.
Each of these methods has its own advantages and disadvantages, and the choice of method depends on the nature of the PELNs of interest, the research objectives, and the laboratory’s equipment and technical capabilities. When studying PELNs, it is usually necessary to consider each of these methods and select the most suitable separation method based on specific circumstances.
PELNs Composition
PELNs may contain a variety of biomolecules, and their composition varies depending on the plant species, tissue type, growth conditions, and physiological status. However, these components typically include the following constituents (Figure 1): i. Proteins. PELNs contain a variety of proteins, including structural proteins, signalling proteins, and regulatory proteins. These proteins play crucial roles in intercellular communication and signal transduction.63,64 ii. Nucleic acids. PELNs may contain nucleic acid molecules such as DNA, mRNA, and miRNA. These nucleic acids might play a role in regulating gene expression, thereby influencing cellular functions and biological processes.49,65,66 iii. Lipids. The lipid components of PELNs include membrane lipids and free phospholipids, which may play crucial roles in the organization and regulation of cell membrane structure and function.33,63,67 iv. Other small molecules. PELNs may also contain other small molecules, such as vitamin C, ions, polysaccharides, oligosaccharides, and metabolites.40,53,63,67,68
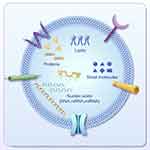 |
Figure 1 The composition of PELNs. Abbreviation: PELNs, plant-derived exosome-like nanovesicles. |
The components of PELNs, such as proteins and lipids, exhibit a degree of similarity to those found in mammalian cells, which reduces the likelihood of their being recognized as foreign substances by the host immune system.63 The bilayer lipid membrane of PELNs offers excellent physical stability, maintaining the integrity of their contents and allowing them to effectively withstand environmental changes.27
The specific composition of PELNs varies among different types of plant cells and under different environmental conditions and greatly influences their biological functions and effects. Therefore, research on the composition of PELNs is of great importance.
Role of PELNs in Chronic Wound Healing
The current most common types of chronic wounds are as follows: i. Diabetic foot ulcers. These ulcers, which occur on the feet of diabetic patients, can easily develop into chronic wounds due to neuropathy, poor blood circulation, and infection associated with diabetes.69,70 ii. Venous leg ulcers. Poor venous circulation in the lower extremities causes tissue ischaemia and hypoxia, leading to ulcers that are difficult to heal.71 iii. Arterial leg ulcers. Inadequate blood supply to the lower limbs results in tissue ischaemia, necrosis, and the formation of ulcers that are difficult to heal.72 iv. Radiation ulcers. Skin damage and chronic wounds caused by radiation therapy are common in cancer patients who have undergone radiation treatment.73 v. Pressure ulcers. Prolonged pressure on the skin and tissues in patients who are bedridden or confined to a wheelchair causes localized damage that does not heal, resulting in chronic wounds.74 vi. Allergic eczema ulcers. Skin damage and ulceration caused by chronic skin inflammation or allergic reactions can have difficulty in healing.75
Chronic wound healing is a relatively complex biological process that requires a closely coordinated cascade of responses to restore and repair the damaged site. These processes include cell proliferation and migration, inflammatory response, angiogenesis, granulation tissue formation, extracellular matrix deposition, and remodelling, ultimately completing the healing process.76–78 The mechanisms of action of PELNs in chronic wound healing are still under investigation, but some studies have suggested that they may involve the following mechanisms (Figure 2 and Table 2).
 |
Table 2 The Role of PELNs in Chronic Wound Healing |
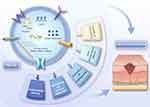 |
Figure 2 The role of PELNs in chronic wound healing. Abbreviation: PELNs, plant-derived exosome-like nanovesicles. |
Promotion of Healing
PELNs components such as proteins, lipids, and nucleic acids can promote cell proliferation, migration, and differentiation, aiding in the formation and repair of new tissue and thus accelerating the chronic wound healing process. One study showed that plant-derived exosome-like nanovesicles from Physalis peruviana fruit promote the proliferation and migration of human skin fibroblasts, upregulate the expression of type I collagen, and have the potential to promote the healing of chronic wounds.59 Furthermore, exosome-like nanovesicles from aloe vera peels enhanced the proliferation and migration of human keratinocytes and human fibroblasts and reduced the levels of reactive oxygen species (ROS) in human keratinocytes, indicating antioxidant effects and the ability to promote the healing of chronic wounds.79 Solanum lycopersicum (tomato)-derived exosome-like nanovesicles induced the migration of human keratinocytes and mouse fibroblasts, promoting wound healing, suggesting their potential therapeutic effects in chronic wound healing.80 Finally, Grapefruit-derived exosome-like nanovesicles have been shown to increase cell viability and migration in human epidermal keratinocytes, indicating their potential to promote chronic wound healing.81
Anti-Inflammatory Effects
Certain components of PELNs may possess anti-inflammatory properties, helping to reduce the inflammatory response in wounds and create a more favourable microenvironment for chronic wound healing. Currently, researches have shown that exosome-like nanovesicles derived from Lemon,89 Cabbage,61 and Turmeric,90 have anti-inflammatory effects. In addition, Aloe vera peel-derived exosome-like nanovesicles reduced the expression of the proinflammatory cytokines TNFα, IL-1β, and IL-6, demonstrating anti-inflammatory properties that are beneficial for chronic wound healing,43 and pomegranate-derived exosome-like nanovesicles have exhibited anti-inflammatory effects that are beneficial for the healing of chronic wounds.82 Further, Dendrobium-derived exosome-like nanovesicles can inhibit IL-1β expression in mouse wounds, exhibiting anti-inflammatory effects and promoting wound healing.83
Promotion of Angiogenesis
Certain components in PELNs may promote angiogenesis, which helps improve the blood supply to the wound and facilitates healing. Researches have shown that PELNs from Grapefruit,81 Aloe saponaria,50Wheat84 and Ginseng85 enhance the tube formation capability of human umbilical vein endothelial cells (HUVECs), promote angiogenesis and exhibit the potential to facilitate the healing of chronic wounds.
Modulating the Immune Response
Some PELNs components may have immunomodulatory effects, helping to balance immune responses, reduce inflammatory reactions, and lower the risk of complications during the wound healing process. Research has shown that PELNs from Catharanthus roseus can promote macrophage polarization and lymphocyte proliferation, alleviate leukopenia and bone marrow cell cycle arrest in immunosuppressed mice, and have an immunomodulatory effect.86 Other studies showed that PELNs from edible Pueraria lobata60 and Turmeric87 can promote M2 polarization of macrophages, exerting immunomodulatory and anti-inflammatory effects, and subsequently facilitate wound healing in diabetes.
Antibacterial Activity
Certain PELNs components may possess antibacterial activity, helping to reduce bacterial infections in chronic wounds and thereby improve the success rate of wound healing. Research has shown that dandelion-derived exosome-like nanovesicles possess antibacterial activity and can neutralize staphylococcal exotoxins, reduce bacterial infections, and expedite the healing of chronic wounds.88
These mechanisms of action may interact through multiple pathways, contributing to the improvement of chronic wound healing, accelerating the healing process, reducing healing time, and minimizing the occurrence of complications. Notably, there are still unknowns regarding the possible role of PELNs in chronic wound healing. Further research is also needed to elucidate the underlying molecular mechanisms involved.
Conclusions and Future Perspective
PELNs can promote the healing of chronic wounds through mechanisms such as enhancing cell proliferation and migration, exerting anti-inflammatory effects, promoting angiogenesis, modulating immune responses, and providing antibacterial activity. Additionally, factors such as the isolation and purification methods used and the variations in PELNs components can influence their ability to promote chronic wound healing. PELNs hold great promise for research and applications in chronic wound healing; however, there remain challenges such as standardization, purity, yield of isolation and storage, as well as a lack of clarity regarding their functional mechanisms and biosafety.86,91,92. Collaborating with the International Organization for Standardization to promote the standardization of PELNs, thereby establishing globally recognized standards, is considered a viable approach for the standardization of PELNs.93 Optimizing extraction techniques, improving separation methods, and automating production for scale-up are strategies to enhance the purity and yield of PELNs.94 PELNs can be refrigerated at 4°C, which is suitable for temporary storage.51,95 PELNs are stored in a −80°C to maintain their activity and stability.51,96 Lyophilization of PELNs is considered beneficial for long-term preservation and reducing the risk of degradation during transport and storage.51,97 Clarifying the functional mechanisms and biosafety of PELNs requires long-term scientific research and assessment.98 In the future, with continuous research efforts and technological advancements, we anticipate that these challenges will be gradually overcome, harnessing the immense potential of PELNs in the field of chronic wound healing and enabling their widespread application.
Acknowledgments
We would like to thank all the participants who participated in this study.
Funding
This work was supported by the Natural Science Foundation of Guangdong Province, China (No. 2024A1515012994) and the Medical Science and Technology Research Fund of Guangdong, China (Nos. A2023249 and A2023247).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Falanga V, Isseroff RR, Soulika AM, et al. Chronic wounds. Nat Rev Dis Primers. 2022;8(1):50. doi:10.1038/s41572-022-00377-3
2. Kathawala MH, Ng WL, Liu D, et al. Healing of chronic wounds: an update of recent developments and future possibilities. Tissue Eng Part B Rev. 2019;25(5):429–444. doi:10.1089/ten.TEB.2019.0019
3. Mieczkowski M, Mrozikiewicz-Rakowska B, Kowara M, Kleibert M, Czupryniak L. The problem of wound healing in diabetes-from molecular pathways to the design of an animal model. Int J Mol Sci. 2022;23(14):7930. doi:10.3390/ijms23147930
4. Bonkemeyer Millan S, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physician. 2019;100(5):298–305.
5. Mervis JS, Phillips TJ. Pressure ulcers: prevention and management. J Am Acad Dermatol. 2019;81(4):893–902. doi:10.1016/j.jaad.2018.12.068
6. Bowers S, Franco E. Chronic wounds: evaluation and management. Am Fam Physician. 2020;101(3):159–166.
7. Reinboldt-Jockenhofer F, Babadagi Z, Hoppe HD, et al. Association of wound genesis on varying aspects of health-related quality of life in patients with different types of chronic wounds: results of a cross-sectional multicentre study. Int Wound J. 2021;18(4):432–439. doi:10.1111/iwj.13543
8. Sen CK. Human wound and its burden: updated 2022 Compendium of estimates. Adv Wound Care. 2023;12(12):657–670. doi:10.1089/wound.2023.0150
9. desJardins-Park HE, Gurtner GC, Wan DC, Longaker MT. From chronic wounds to scarring: the growing health care burden of under- and over-healing wounds. Adv Wound Care. 2022;11(9):496–510. doi:10.1089/wound.2021.0039
10. Fernandez-Guarino M, Bacci S, Perez Gonzalez LA, Bermejo-Martinez M, Cecilia-Matilla A, Hernandez-Bule ML. The role of physical therapies in wound healing and assisted scarring. Int J Mol Sci. 2023;24(8):7487. doi:10.3390/ijms24087487
11. Chen H, Yu Z, Liu N, et al. The efficacy of low-frequency ultrasound as an added treatment for chronic wounds: a meta-analysis. Int Wound J. 2023;20(2):448–457. doi:10.1111/iwj.13893
12. Thomas DC, Tsu CL, Nain RA, Arsat N, Fun SS, Sahid Nik Lah NA. The role of debridement in wound bed preparation in chronic wound: a narrative review. Ann Med Surg Lond. 2021;71:102876. doi:10.1016/j.amsu.2021.102876
13. Zhang X, Wang G, Sun Y, Ding P, Yang X, Zhao Z. The Z-plasty contributes to the coalescence of a chronic non-healing wound. Int Wound J. 2021;18(6):796–804. doi:10.1111/iwj.13583
14. Ohnstedt E, Lofton Tomenius H, Vagesjo E, Phillipson M. The discovery and development of topical medicines for wound healing. Expert Opin Drug Discov. 2019;14(5):485–497. doi:10.1080/17460441.2019.1588879
15. El-Sherbeni SA, Negm WA. The wound healing effect of botanicals and pure natural substances used in in vivo models. Inflammopharmacology. 2023;31(2):755–772. doi:10.1007/s10787-023-01157-5
16. Moses RL, Prescott TAK, Mas-Claret E, Steadman R, Moseley R, Sloan AJ. Evidence for natural products as alternative wound-healing therapies. Biomolecules. 2023;13(3):444. doi:10.3390/biom13030444
17. Albahri G, Badran A, Hijazi A, et al. The therapeutic wound healing bioactivities of various medicinal plants. Life. 2023;13(2):317. doi:10.3390/life13020317
18. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7
19. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi:10.1146/annurev-biochem-013118-111902
20. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
21. Krylova SV, Feng D. The machinery of exosomes: biogenesis, release, and uptake. Int J Mol Sci. 2023;24(2):1337. doi:10.3390/ijms24021337
22. Kim J, Li S, Zhang S, Wang J. Plant-derived exosome-like nanoparticles and their therapeutic activities. Asian J Pharm Sci. 2022;17(1):53–69. doi:10.1016/j.ajps.2021.05.006
23. Asghar S, Litherland GJ, Lockhart JC, Goodyear CS, Crilly A. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology. 2020;59(1):57–68. doi:10.1093/rheumatology/kez462
24. Castillo-Pena A, Molina-Pinelo S. Landscape of tumor and immune system cells-derived exosomes in lung cancer: mediators of antitumor immunity regulation. Front Immunol. 2023;14:1279495. doi:10.3389/fimmu.2023.1279495
25. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochim Biophys Acta Rev Cancer. 2019;1871(2):455–468. doi:10.1016/j.bbcan.2019.04.004
26. Narauskaite D, Vydmantaite G, Rusteikaite J, et al. Extracellular vesicles in skin wound healing. Pharmaceuticals. 2021;14(8):811. doi:10.3390/ph14080811
27. Li A, Li D, Gu Y, et al. Plant-derived nanovesicles: further exploration of biomedical function and application potential. Acta Pharm Sin B. 2023;13(8):3300–3320. doi:10.1016/j.apsb.2022.12.022
28. Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L. Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. FEBS Lett. 2009;583(20):3363–3366. doi:10.1016/j.febslet.2009.09.041
29. Bai C, Liu J, Zhang X, et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543. doi:10.1016/j.biopha.2024.116543
30. Kim DK, Rhee WJ. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics. 2021;13(8):1203. doi:10.3390/pharmaceutics13081203
31. Seo K, Yoo JH, Kim J, et al. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale. 2023;15(12):5798–5808. doi:10.1039/d2nr07018a
32. Li Z, Wang H, Yin H, Bennett C, Zhang HG, Guo P. Arrowtail RNA for ligand display on ginger exosome-like nanovesicles to systemic deliver siRNA for cancer suppression. Sci Rep. 2018;8(1):14644. doi:10.1038/s41598-018-32953-7
33. Jiang D, Li Z, Liu H, Liu H, Xia X, Xiang X. Plant exosome-like nanovesicles derived from sesame leaves as carriers for luteolin delivery: molecular docking, stability and bioactivity. Food Chem. 2024;438:137963. doi:10.1016/j.foodchem.2023.137963
34. Alnusaire TS, Sayed AM, Elmaidomy AH, et al. An in vitro and in silico study of the enhanced antiproliferative and pro-oxidant potential of Olea europaea L. cv. arbosana leaf extract via elastic nanovesicles (spanlastics). Antioxidants. 2021;10(12). doi:10.3390/antiox10121860
35. Rabienezhad Ganji N, Urzi O, Tinnirello V, et al. Proof-of-concept study on the use of tangerine-derived nanovesicles as siRNA delivery vehicles toward colorectal cancer cell line SW480. Int J Mol Sci. 2023;25(1):546. doi:10.3390/ijms25010546
36. Castelli G, Logozzi M, Mizzoni D, et al. Ex vivo anti-leukemic effect of exosome-like grapefruit-derived nanovesicles from organic farming-the potential role of ascorbic acid. Int J Mol Sci. 2023;24(21):15663. doi:10.3390/ijms242115663
37. Fang Z, Song M, Lai K, Cui M, Yin M, Liu K. Kiwi-derived extracellular vesicles for oral delivery of sorafenib. Eur J Pharm Sci. 2023;191:106604. doi:10.1016/j.ejps.2023.106604
38. Pocsfalvi G, Turiak L, Ambrosone A, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. J Plant Physiol. 2018;229:111–121. doi:10.1016/j.jplph.2018.07.006
39. Eom JY, Choi SH, Kim HJ, et al. Hemp-derived nanovesicles protect leaky gut and liver injury in dextran sodium sulfate-induced colitis. Int J Mol Sci. 2022;23(17):9955. doi:10.3390/ijms23179955
40. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi:10.3390/biom11010087
41. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi:10.18632/oncotarget.4004
42. Sharma V, Sinha ES, Singh J. Investigation of in vitro anti-cancer and apoptotic potential of onion-derived nanovesicles against prostate and cervical cancer cell lines. Appl Biochem Biotechnol. 2024. doi:10.1007/s12010-024-04872-z
43. Ramirez O, Pomareda F, Olivares B, et al. Aloe vera peel-derived nanovesicles display anti-inflammatory properties and prevent myofibroblast differentiation. Phytomedicine. 2024;122:155108. doi:10.1016/j.phymed.2023.155108
44. Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184–198. doi:10.1016/j.jconrel.2023.01.071
45. Sim Y, Seo HJ, Kim DH, et al. The effect of apple-derived nanovesicles on the osteoblastogenesis of osteoblastic MC3T3-E1 cells. J Med Food. 2023;26(1):49–58. doi:10.1089/jmf.2022.K.0094
46. Huang R, Jia B, Su D, et al. Plant exosomes fused with engineered mesenchymal stem cell-derived nanovesicles for synergistic therapy of autoimmune skin disorders. J Extracell Vesicles. 2023;12(10):e12361. doi:10.1002/jev2.12361
47. Kim JS, Kim DH, Gil MC, et al. Pomegranate-derived exosome-like nanovesicles alleviate binge alcohol-induced leaky gut and liver injury. J Med Food. 2023;26(10):739–748. doi:10.1089/jmf.2023.K.0060
48. Choi SH, Eom JY, Kim HJ, et al. Aloe-derived nanovesicles attenuate inflammation and enhance tight junction proteins for acute colitis treatment. Biomater Sci. 2023;11(16):5490–5501. doi:10.1039/d3bm00591g
49. Zhu Z, Liao L, Gao M, Liu Q. Garlic-derived exosome-like nanovesicles alleviate dextran sulphate sodium-induced mouse colitis via the TLR4/MyD88/NF-kappaB pathway and gut microbiota modulation. Food Funct. 2023;14(16):7520–7534. doi:10.1039/d3fo01094e
50. Kim M, Park JH. Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;14(9):1905. doi:10.3390/pharmaceutics14091905
51. Welsh JA, Goberdhan DCI, O’Driscoll L, et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J Extracell Vesicles. 2024;13(2):e12404. doi:10.1002/jev2.12404
52. Zhan W, Deng M, Huang X, et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J Control Release. 2023;364:644–653. doi:10.1016/j.jconrel.2023.11.020
53. Trentini M, Zanolla I, Tiengo E, et al. Link between organic nanovescicles from vegetable kingdom and human cell physiology: intracellular calcium signalling. J Nanobiotechnol. 2024;22(1):68. doi:10.1186/s12951-024-02340-8
54. Han J, Wu T, Jin J, et al. Exosome-like nanovesicles derived from Phellinus linteus inhibit Mical2 expression through cross-kingdom regulation and inhibit ultraviolet-induced skin aging. J Nanobiotechnol. 2024;20(1):455. doi:10.1186/s12951-022-01657-6
55. Cai H, Huang LY, Hong R, et al. Momordica charantia exosome-like nanoparticles exert neuroprotective effects against ischemic brain injury via inhibiting matrix metalloproteinase 9 and activating the AKT/GSK3beta signaling pathway. Front Pharmacol. 2022;13:908830. doi:10.3389/fphar.2022.908830
56. Zhu MZ, Xu HM, Liang YJ, et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4(+)CD8(+)T cells expansion. J Nanobiotechnol. 2023;21(1):309. doi:10.1186/s12951-023-02065-0
57. Liu J, Xiang J, Jin C, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21(1):78. doi:10.1186/s12951-023-01835-0
58. Zhao WJ, Bian YP, Wang QH, et al. Blueberry-derived exosomes-like nanoparticles ameliorate nonalcoholic fatty liver disease by attenuating mitochondrial oxidative stress. Acta Pharmacol Sin. 2022;43(3):645–658. doi:10.1038/s41401-021-00681-w
59. Natania F, Iriawati I, Ayuningtyas FD, Barlian A. Potential of plant-derived exosome-like nanoparticles from physalis peruviana fruit for human dermal fibroblast regeneration and remodeling. Pharm Nanotechnol. 2024;12. doi:10.2174/0122117385281838240105110106.
60. Wu J, Ma X, Lu Y, et al. Edible PUERARIA LOBATA-DERIVED EXOSOMES promote M2 macrophage polarization. Molecules. 2022;27(23):8184. doi:10.3390/molecules27238184
61. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
62. Jackson KK, Mata C, Marcus RK. A rapid capillary-channeled polymer (C-CP) fiber spin-down tip approach for the isolation of plant-derived extracellular vesicles (PDEVs) from 20 common fruit and vegetable sources. Talanta. 2023;252:123779. doi:10.1016/j.talanta.2022.123779
63. Woith E, Guerriero G, Hausman JF, et al. Plant extracellular vesicles and nanovesicles: focus on secondary metabolites, proteins and lipids with perspectives on their potential and sources. Int J Mol Sci. 2021;22(7):3719. doi:10.3390/ijms22073719
64. Stanly C, Moubarak M, Fiume I, Turiak L, Pocsfalvi G. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int J Mol Sci. 2019;20(24):6205. doi:10.3390/ijms20246205
65. Wang S, He B, Wu H, et al. Plant mRNAs move into a fungal pathogen via extracellular vesicles to reduce infection. Cell Host Microbe. 2024;32(1):93–105e6. doi:10.1016/j.chom.2023.11.020
66. Yan G, Xiao Q, Zhao J, et al. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J Control Release. 2024;367:425–440. doi:10.1016/j.jconrel.2024.01.060
67. Li S, Ye Z, Zhao L, Yao Y, Zhou Z. Evaluation of antioxidant activity and drug delivery potential of cell-derived extracellular vesicles from citrus reticulata blanco cv. ‘Dahongpao’. Antioxidants. 2023;12(9). doi:10.3390/antiox12091706
68. Kim WS, Ha JH, Jeong SH, et al. Immunological effects of aster yomena callus-derived extracellular vesicles as potential therapeutic agents against allergic asthma. Cells. 2022;11(18):2805. doi:10.3390/cells11182805
69. Burgess JL, Wyant WA, Abdo Abujamra B, Kirsner RS, Jozic I. Diabetic wound-healing science. Medicina. 2021;57(10). doi:10.3390/medicina57101072
70. Chen P, Vilorio NC, Dhatariya K, et al. Guidelines on interventions to enhance healing of foot ulcers in people with diabetes (IWGDF 2023 update). Diabetes Metab Res Rev. 2024;40(3):e3644. doi:10.1002/dmrr.3644
71. Raffetto JD, Ligi D, Maniscalco R, Khalil RA, Mannello F. Why venous leg ulcers have difficulty healing: overview on pathophysiology, clinical consequences, and treatment. J Clin Med. 2020;10(1):29. doi:10.3390/jcm10010029
72. Hafner J, Schaad I, Schneider E, Seifert B, Burg G, Cassina PC. Leg ulcers in peripheral arterial disease (arterial leg ulcers): impaired wound healing above the threshold of chronic critical limb ischemia. J Am Acad Dermatol. 2000;43(6):1001–1008. doi:10.1067/mjd.2000.108375
73. Punchera J, Vuagnat H, Laubach HJ. Radiation-induced chronic ulcerations and fistulae successfully treated with photobiomodulation. J Eur Acad Dermatol Venereol. 2024. doi:10.1111/jdv.20009
74. Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81(4):881–890. doi:10.1016/j.jaad.2018.12.069
75. Monari P, Fusano M, Moro R, et al. Allergic contact versus irritant contact dermatitis in patients with hard-to-heal leg ulcer: clinical and diagnostic approach. J Wound Care. 2021;30(5):394–398. doi:10.12968/jowc.2021.30.5.394
76. Pastar I, Balukoff NC, Marjanovic J, Chen VY, Stone RC, Tomic-Canic M. Molecular pathophysiology of chronic wounds: current state and future directions. Cold Spring Harb Perspect Biol. 2023;15(4):a041243. doi:10.1101/cshperspect.a041243
77. Wilkinson HN, Hardman MJ. Wound healing: cellular mechanisms and pathological outcomes. Open Biol. 2020;10(9):200223. doi:10.1098/rsob.200223
78. Veith AP, Henderson K, Spencer A, Sligar AD, Baker AB. Therapeutic strategies for enhancing angiogenesis in wound healing. Adv Drug Deliv Rev. 2019;146:97–125. doi:10.1016/j.addr.2018.09.010
79. Kim MK, Choi YC, Cho SH, Choi JS, Cho YW. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng Regen Med. 2021;18(4):561–571. doi:10.1007/s13770-021-00367-8
80. Daniello V, De Leo V, Lasalvia M, et al. Solanum lycopersicum (Tomato)-derived nanovesicles accelerate wound healing by eliciting the migration of keratinocytes and fibroblasts. Int J Mol Sci. 2024;25(5):2452. doi:10.3390/ijms25052452
81. Savci Y, Kirbas OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12(11):5144–5156. doi:10.1039/d0fo02953j
82. Sanchez-Lopez CM, Manzaneque-Lopez MC, Perez-Bermudez P, Soler C, Marcilla A. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022;13(24):12870–12882. doi:10.1039/d2fo01806c
83. Tu J, Jiang F, Fang J, et al. Anticipation and verification of dendrobium-derived nanovesicles for skin wound healing targets, predicated upon immune infiltration and senescence. Int J Nanomed. 2024;19:1629–1644. doi:10.2147/IJN.S438398
84. Sahin F, Kocak P, Gunes MY, Ozkan I, Yildirim E, Kala EY. In vitro wound healing activity of wheat-derived nanovesicles. Appl Biochem Biotechnol. 2019;188(2):381–394. doi:10.1007/s12010-018-2913-1
85. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47(1):133–143. doi:10.1016/j.jgr.2022.07.005
86. Ou X, Wang H, Tie H, et al. Novel plant-derived exosome-like nanovesicles from Catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-alpha/NF-kappaB/PU.1 axis. J Nanobiotechnol. 2023;21(1):160. doi:10.1186/s12951-023-01919-x
87. Wu B, Pan W, Luo S, et al. Turmeric-derived nanoparticles functionalized aerogel regulates multicellular networks to promote diabetic wound healing. Adv Sci. 2024;11:e2307630. doi:10.1002/advs.202307630
88. Tan S, Liu Z, Cong M, et al. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing Staphylococcus aureus exotoxins for the care of invasive wounds. J Control Release. 2024;368:355–371. doi:10.1016/j.jconrel.2024.02.045
89. Raimondo S, Urzi O, Meraviglia S, et al. Anti-inflammatory properties of lemon-derived extracellular vesicles are achieved through the inhibition of ERK/NF-κB signalling pathways. J Cell Mol Med. 2022;26(15):4195–4209. doi:10.1111/jcmm.17404
90. Gao C, Zhou Y, Chen Z, et al. Turmeric-derived nanovesicles as novel nanobiologics for targeted therapy of ulcerative colitis. Theranostics. 2022;12(12):5596–5614. doi:10.7150/thno.73650
91. Bokka R, Ramos AP, Fiume I, et al. Biomanufacturing of tomato-derived nanovesicles. Foods. 2020;9(12):1852. doi:10.3390/foods9121852
92. Lee BH, Wu SC, Shen TL, Hsu YY, Chen CH, Hsu WH. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem. 2021;340:128104. doi:10.1016/j.foodchem.2020.128104
93. Yadav A, Xuan Y, Sen CK, Ghatak S. Standardized reporting of research on exosomes to ensure rigor and reproducibility. Adv Wound Care. 2024. doi:10.1089/wound.2024.0093
94. Martinez-Santillan A, Gonzalez-Valdez J. Novel technologies for exosome and exosome-like nanovesicle procurement and enhancement. Biomedicines. 2023;11(5):1487. doi:10.3390/biomedicines11051487
95. Pratiwi FW, Thomas RT, Karzarjeddi M, et al. Scalable Purification, storage, and release of plant-derived nanovesicles for local therapy using nanostructured all-cellulose composite membranes. Biomacromolecules. 2024;25(9):5847–5859. doi:10.1021/acs.biomac.4c00535
96. Chen X, He L, Chen Y, et al. Evaluating stability and bioactivity of rehmannia-derived nanovesicles during storage. Sci Rep. 2024;14(1):19966. doi:10.1038/s41598-024-70334-5
97. Trenkenschuh E, Richter M, Heinrich E, Koch M, Fuhrmann G, Friess W. Enhancing the stabilization potential of lyophilization for extracellular vesicles. Adv Healthc Mater. 2022;11(5):e2100538. doi:10.1002/adhm.202100538
98. Dad HA, Gu TW, Zhu AQ, Huang LQ, Peng LH. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

