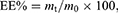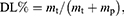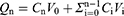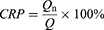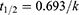Back to Journals » International Journal of Nanomedicine » Volume 20
Pluronic P123/L64 Mixed Micelles as Immediate Release Systems to Enhance the Bioavailability of Praziquantel in Rats
Authors Yuan J, Su W, Gao J, Ma X, Yin R, Jia T
Received 5 February 2025
Accepted for publication 25 June 2025
Published 7 July 2025 Volume 2025:20 Pages 8861—8871
DOI https://doi.org/10.2147/IJN.S520910
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Sachin Mali
Jing Yuan,1 Wenhao Su,1 Jingyang Gao,1 Xiaoqing Ma,1 Ronghuan Yin,1 Tong Jia2
1Key Laboratory of Livestock Infectious Diseases, Ministry of Education, and Key Laboratory of Ruminant Infectious Disease Prevention and Control (East), Ministry of Agriculture and Rural Affairs, College of Animal Science and Veterinary Medicine, Shenyang Agricultural University, Shenyang, 110866, People’s Republic of China; 2College of Information Science and Engineering, Northeastern University, Shenyang, 110819, People’s Republic of China
Correspondence: Ronghuan Yin, Key Laboratory of Livestock Infectious Diseases, Ministry of Education, and Key Laboratory of Ruminant Infectious Disease Prevention and Control (East), Ministry of Agriculture and Rural Affairs, College of Animal Science and Veterinary Medicine, Shenyang Agricultural University, 120 Dongling Road, Shenyang, 110866, People’s Republic of China, Email [email protected] Tong Jia, College of Information Science and Engineering, Northeastern University, No. 11, Lane 3, Wenhua Road, Shenyang, 110819, People’s Republic of China, Email [email protected]
Purpose: Praziquantel (PZQ) is currently the preferred medication for treating various parasites. However, its use is hindered by several issues, such as poor solubility, first-pass effect, and individual variability. A novel immediate release system was developed by loading PZQ onto Pluronic P123/L64 mixed micelles (PMMs). This approach aims to improve its bioavailability following oral administration.
Methods: PZQ-PMMs were prepared by thin film dispersion method. The encapsulation efficiency (EE), particle size, and polydispersity index (PDI) were utilized to identify the optimal formulation. Characterization techniques, including electron microscopy, infrared spectroscopy, thermal analysis, and X-ray diffraction were utilized to get an understanding of the molecular interactions between PZQ and micelles. This system was compared with commercially available preparations both in vitro and in vivo.
Results: The particle size of the prepared PZQ-PMMs (P123:L64 1:1, w/w) was 19.33 ± 0.22 nm, with a PDI value of 0.106 ± 0.044, an EE of 86.88 ± 4.60%, and a drug loading of 4.16 ± 0.21%. Structural characterization results indicated that the spherical micelles were uniformly dispersed, with the drug existing in an amorphous form within PMMs. In vitro, PZQ-PMMs exhibited a faster immediate release in both pH 1.2 and pH 6.8 buffers. In vivo, at the same dosage, the micelles rapidly produced higher blood drug concentrations. The relative bioavailability of PZQ-PMMs was comparable to that of the PZQ 30% ethanol solution and was 1.7 times greater than that of commercially available preparations, with the increase in bioavailability being highly significant (P < 0.01).
Conclusion: The findings of this study confirm that Pluronic P123/L64 PMMs represent a novel and promising approach for enhancing solubility and bioavailability of PZQ, both in vitro and in vivo. The development of immediate release formulations is anticipated to be an effective option for drugs exhibiting a notable first-pass effect.
Keywords: praziquantel, Pluronic mixed micelles, P123, L64, immediate release, pharmacokinetic study
Introduction
In 1980, Merck launched Praziquantel (PZQ) under the trade name Cesol. Because of its high efficiency, low toxicity and wide anti-parasitic spectrum, its market share expanded rapidly, replacing many other drugs, and it soon became the main drug for the treatment of schistosomiasis, Taenia, food-borne Trematodiasis, and a variety of other parasitic diseases in the world.1
According to the Biological Pharmaceutical Classification System (BSC), PZQ is categorized as a Class II compound, exhibiting a water solubility as low as 0.04%,2 and a high permeability through the intestinal membrane. As a result, the release of PZQ from the tablets occurs gradually, and once it is released, it is quickly absorbed. Additionally, it exhibits a notable first-pass effect, meaning that drugs that are absorbed gradually are swiftly metabolized by enzymes and quickly removed from the bloodstream. The plasma half-life typically ranges from 1 to 3 hours.3 Therefore, a significant challenge in the application of PZQ is the necessity for a high therapeutic dose of 40 mg/kg to achieve effective plasma concentrations with low bioavailability.4,5 It also leads to significant intra-individual variability in the drug’s effects and a broad range of peak blood concentrations.6 In this case, enhancing the release rate of PZQ by developing an immediate release formulation to maximize the saturation of liver drug enzymes may effectively improve its bioavailability.
Recent studies had shown that the advancement of drug nanocarriers for the oral administration is an interesting approach to overwhelm the redundant PZQ shortcomings.7–16 Luciana Nalone Andrade et al9 developed solid lipid nanoparticles (SLN) to encapsulate PZQ, resulting in an average particle size of around 300 nm and an encapsulation efficiency (EE) of 92.31%. In vitro studies showed that PZQ-SLN released about 18% of the drug over a 3-hour period, which is roughly four times greater than the release rate of free PZQ. Furthermore, PZQ-SLN demonstrated enhanced insecticidal effectiveness in these in vitro tests. Andressa Daniele Artico Silva et al17 utilized the wet milling technique to create micro and nanocrystals with an average particle size of 346.2 nm, which greatly improved the dissolution of PZQ. Remarkably, the MC5 formulation boosted the dissolution rate by 13 times. In vivo experiments demonstrated that the developed formulation could enhance PZQ’s efficacy in treating parasitic eggs in tissues and may potentially promote early improvement of granulomatous lesions. Waleed M. Arafa and colleagues8 reported that newly developed micelles made from F127 and Gelucire 44 were created to encapsulate PZQ. The optimized formulation showed a controlled release over 24 hours, with a release rate of 78.22%. Additionally, a single dose of the drug-loaded micelles significantly improved the anthelmintic effectiveness of PZQ in rats infected with Hymenolepis nana. While the Lutrol F127/Gelucire 44/14 micelle demonstrated sustained release in vitro, it exhibited a similar release rate and achieved 1.31 times the peak blood concentration of PZQ in in vivo studies. E. S. Meteleva et al18 indicated that the formation of micelles with PZQ in a glycyrrhizic acid disodium salt solution also enhanced the drug release rate. The bioavailability of this formulation was roughly three times greater than that of the PZQ suspension.
Among these nanocarriers, polymer micelles (PMs) had emerged as one of the most effective and straightforward methods for enhancing the physicochemical properties of insoluble drugs. These amphiphilic polymers spontaneously form core-shell micelles at concentrations exceeding the critical micelle concentration (CMC) in water. In this structure, hydrophobic segments constitute the core, while hydrophilic segments form the outer corona.7,19,20 The hydrophobic core of these micelles can accommodate poorly soluble, hydrophobic drugs, while the hydrophilic shell, which is not present in many nanosystems, allows the micelles to be well dispersed in digestive fluids. Additionally, the particle size of the micelles is generally less than 100 nm; smaller particle sizes facilitate the rapid release of drugs.
The representatives of these polymers are Pluronic block copolymers, which are structurally denoted as PEO-PPO-PEO, where PEO and PPO represent poly(ethylene oxide) and poly(propylene oxide), respectively. These copolymers are non-ionic and are offered in a diverse array of molecular weights and PEO/PPO ratios, which render them flexible and promising pharmaceutical excipients.21–25 Pluronic is widely considered to be safe for various routes of administration, including oral, topical, ophthalmic, periodontal, intratympanic, and parenteral applications. Elif İspir et al26 devised Pluronic F127 micelles with controlled release effects, which successfully improved the solubility of quercetin and yielded promising results in in vivo experiments. Huan Gao et al27 encapsulated Doxorubicin (DOX) within the hydrophobic core of Pluronic P123-based polymeric micelles, achieving a high drug loading capacity of 3.44%. They were efficiently taken up by breast cancer cells and significantly inhibited tumor growth in tumor-bearing nude mice.
In this study, PZQ was incorporated into Pluronic mixed micelles (PMMs) to develop a novel immediate release system. The performance of this system was compared with commercial preparations both in vitro and in vivo. The effects of different Pluronic species and their concentrations on PMMs were evaluated through EE and particle size distribution analysis. To gain a thorough comprehension of the formation of the PMMs, they were examined using Transmission Electron Microscopy (TEM), Fourier Transform Infrared Spectroscopy (FTIR), Differential Scanning Calorimetry (DSC), and X-Ray Diffraction (XRD) to elucidate the molecular interactions between PZQ and the micelles. Finally, in vitro release and in vivo absorption studies of the PMM formulations, powder, and commercially available preparations of PZQ were conducted to illustrate the advantages of polymeric micelles for oral administration.
Materials and Methods
Materials
PZQ (≥98%, Aladdin Industrial Corporation, Shanghai, China), Pluronic F127 (99.8%, Beyotime Biotechnology Co., Ltd., Shanghai, China), Pluronic P123 (99.8%, Sigma-Aldrich, Germany) and Pluronic L31, L61, L64 (99.8%, Aladdin Industrial Corporation, Shanghai, China) were utilized as received, without any additional purification; acetonitrile, methanol were obtained from Xilong Scientific Co., Ltd. (HPLC grade, Guangdong, China); dichloromethane, methyl tert-butyl ether were obtained from Jiangsu Aikang Biopharmaceutical Co., Ltd. (HPLC grade, Jiangsu, China).
Preparation of Praziquantel-Loaded Pluronic Mixed Micelles (PZQ-PMMs)
PZQ-PMMs was prepared using the most commonly employed the thin film dispersion method.28–31 The operational method was outlined as follows: 5 mg PZQ and 100 mg Pluronic were precisely weighed, placed in a round-bottom flask, dissolved by adding 20 mL absolute ethanol and placed on a rotary evaporator to remove the solvent to form a film at 40°C, 15 r/min, dried in vacuum at 25°C overnight. Then, added 5 mL water, hydrated on a rotary evaporator for approximately 30 min at a temperature of 50°C and a speed of 100 r/min. The solution was allowed to sit for 30 min, vortexed for 5 min, and subsequently filtered through a 0.22 μm filter membrane. Mannitol (1%, w/v) was then added as a protective agent and freeze-dried. Blank PMMs were obtained without the addition of PZQ during the preparation process.
The effects of various types and proportions of Pluronic polymer on particle size distribution and EE were examined during the formulation screening process.
Characterization
After proper dilution, PZQ-PMMs was gently dripped onto carbon film copper net, negative dyeing was carried out, and placed in a dust-free environment to dry naturally. The morphology of the particles was observed under TEM (HT7700, Hitachi, Japan). The thermal behaviors of samples were conducted on a DSC (DSC25, TA, USA). All measurements were conducted from 25°C to 250°C. The heating rate was set at 10°C/min, and the protective gas used was nitrogen. FTIR spectra were obtained between 400 and 4000 wavenumber using an FTIR Spectrophotometer (IRTracer 100, Shimadzu, Japan). XRD patterns for samples were acquired using an X-ray diffractometer (D8 Advance, Bruker, Germany). The scanning range spanned from 5° to 90°, 2θ angle, with a step size of 0.04° and a step time of 0.3 s. The analysis of particle size distribution and zeta potential was conducted utilizing Dynamic Light Scattering techniques (DLS, Zeta sizer 3000 HSA, Malvern, UK).32 Ultrapure water was produced by NANOpure Diamond Water Purification System (Thermo Fisher Scientific, USA).
Encapsulation Efficiency and Drug Loading
The EE and drug loading (DL) of PZQ-PMMs were assessed by a direct method. The micelles were filtered with microporous filter (0.22 μm), and the continued filtrate was collected for injection into high performance liquid chromatography (HPLC, Dionex Ultimate 3000, Thermo Fisher Scientific, USA). Data recording and processing were conducted using Chromeleon 7.0 software.33 A Venusil XBP C18 column (4.6 × 150 mm, 5 μm, Dikma Technologies, China) with acetonitrile-water 60:40 (v/v) as the mobile phase was used, and the detection wavelength was 264 nm.
EE and DL was calculated by Eq. (1) and (2):
where m0 or mp represents the total amount of the drug or polymer incorporated into the system, while mt denotes the amount of the drug present in PZQ-PMMs.
In vitro Release Studies
The in vitro release of PZQ powder, PZQ commercial preparation powder and PZQ-PMMs lyophilized powder were studied by dialysis in pH 1.2 and 6.8 buffer (containing 0.2% of SDS).34,35 Briefly, a precise amount of 4 mL of PZQ-PMMs was collected and packed into dialysis bags (3.5 kDa, Viskase, USA). The bags were then immersed in 96 mL of release medium (sink) and shaken in a constant temperature incubator at 100 rpm and 37°C. Samples were taken at regular intervals and supplemented with an equal volume of fresh buffer. The refill filtrate was then injected into HPLC to analyze PZQ concentration. Time-dependent release curves were calculated using Eq. (3) and (4):
where Qn represents the calculated cumulative release, CRP represents the cumulative release percentage, Cn denotes the concentration of the drug in the release medium at time t, V0 refers to the total volume of the release medium, Vi indicates the sample volume, and Q signifies the initial total amount of the drug.
Ethical Approval
The experimental protocol was agreed upon by the Institutional Animal Care and Use Committee (IACUC) of Shenyang Agricultural University with approval No. 2023050701. The date of approval is 7/5/2023. The guidelines followed for the welfare of the laboratory animals is the National standards of the People’s Republic of China “Laboratory animal - Guideline for ethical review of animal welfare” (GB/T 35892–2018).
In vivo Absorption Studies
Twelve male SD rats (Liaoning Changsheng Biotechnology Co., Ltd., Liaoning, China), each weighing approximately 200 g, were randomly divided into three groups. The rats were kept under controlled environmental conditions (25 ± 2°C; humidity 50–60%) and were provided with a standard diet and water ad libitum. The animals were administered 20 mg/kg of the test substance orally after fasting. 0.5 mL of blood was collected at regular intervals, centrifuged at 3500 rpm for 10 min, and plasma was separated and stored at −20°C.
The 200 μL plasma sample was vortexed and mixed with 1.2 mL methyl tert-butyl ether and dichloromethane (2:1, v/v) for 5 min. After centrifugation at 3500 rpm for 30 min, the supernatant was removed, dried at 55°C under nitrogen, redissolved in 100 μL methanol, filtered and injected for HPLC analysis. A Diamonsil C18 column (4.6 × 250 mm, 5 μm, Dikma Technologies, China) was used, the mobile phase was methanol-water 70:30 (v/v), the detection wavelength was 223 nm, and the external standard method was used for quantification.
Pharmacokinetic Evaluation
The peak concentration (Cmax) and time to peak (tmax) were read from the plasma drug concentration–time curve. The area under the curve (AUC0-24) was calculated by the trapezoidal method to evaluate bioavailability. The elimination half-life (t1/2) was calculated using Eq. (5):
where k is the first-order elimination rate constant of the drug, determined through linear regression of the terminal phase of the concentration–time plot. The mean residence time (MRT) of PZQ-PMMs was calculated using Eq. (6):
where AUMC refers to the area under the moment curve from 0 to ∞, while AUC denotes the area under the plasma drug concentration–time curve from 0 to ∞.36
Statistical Analysis
The data is presented as mean ± standard deviation (SD). One-way ANOVA was used to perform the statistical analyses. Each experiment was conducted in triplicate at least.
Results and Discussion
Preparation of PZQ-PMMs
Considering the strong hydrophobicity of PZQ, Pluronic P123, which has a high ratio of hydrophobic to hydrophilic chain lengths, was selected as the micelle carrier material for the experiments. The structure of Pluronic P123 is PEO20PPO70PEO20, with a molecular weight of 5800 and an HLB value ranging from 7 to 12. However, due to its short hydrophilic chain, the prepared micelles tended to agglomerate and settle after standing. Meanwhile, the micelles prepared solely with P123 did not re-dissolve effectively after freeze-drying, resulting in an increase in particle size from approximately 20 nm to 987.6 ± 375.5 nm. To enhance the stability of the micelles, the more hydrophilic Pluronic L64 was compounded with P123. L64 has a structural formula of PEO13PPO30PEO13, a molecular weight of 2900, and an HLB value of 12 to 18. Other Pluronic polymers, such as F127 (molecular weight 12600, HLB 20–29), L61 (molecular weight 2000, HLB 3), and L31 (molecular weight 1100, HLB 3.5), were also used either separately or in mixtures for the preparation of PMMs. However, these alternatives exhibited issues with low EE or poor stability (see Table S1).
The influence of the P123 to L64 ratio on micelles properties was examined in more detail. The results (see Table 1 and Figure S1) indicated that the addition of L64 had little impact on the EE of the PZQ-PMMs. When P123, P123: L64 3:1, and P123: L64 1:1 were used as carriers, the EE of the micelles was comparable, and all formulations met the requirements for particle size uniformity. However, as previously mentioned, the re-dissolution effect of freeze-dried PZQ-PMMs (P123) was suboptimal. PZQ-PMMs (P123:L64 3:1) exhibited similar issues. In contrast, PZQ-PMMs (P123:L64 1:1) demonstrated good stability after standing for 24 hours post-preparation, showed no collapse in appearance after freeze-drying, and exhibited effective re-dissolution (see Table S2). Conversely, when the amount of L64 exceeded P123, both the particle size and PDI of the micelles increased, leading to a decrease in uniformity and stability. Therefore, PZQ-PMMs (P123:L64 1:1) were selected as the optimal formulation in this study. Since Pluronic is a non-ionic polymer and praziquantel (PZQ) lacks acidic and basic groups, the drug-loaded micelles are nearly electrically neutral and slightly negatively charged. The electrostatic repulsion between the particles is small; therefore, they should be lyophilized after preparation.
 |
Table 1 Effects of Different P123/L64 Mass Ratios on PZQ-PMMs Properties (Mean ± SD) |
Characterization of PZQ-PMMs
A representative TEM images (Figure 1) of the optimal PZQ-PMMs formulation revealed that the micelle particles were consistently shaped and uniformly distributed. Additionally, the morphological analysis aligned with the average micellar size measured by DLS.
 |
Figure 1 TEM image of PZQ-PMMs prepared by P123: L64=1:1. |
FTIR spectra were utilized to confirm the intermolecular interactions between PZQ and PMMs (Figure 2A). In the PZQ spectrum (a), the peaks at 2930 and 2853 cm−1 were linked to the C-H stretching vibrations of CH2, and the band at 1651 cm−1 was the C=O stretching vibration. The peak at 1422 cm−1 indicated the -CH in-plane bending vibration of CH2. Additionally, between 1350 and 1000 cm−1, axial deformation was noted, characterized by overlapping bands resulting from the symmetrical angular stretching of C-N and C-H in CH2.37 In the blank micelles spectrum (b), the peak observed at 3269 cm−1 was linked to O-H stretching vibrations, and the peak at 1090 cm−1 signified C-O-C stretching vibrations. Additionally, C-H stretching vibrations of CH2 were also detected in this spectrum. In contrast, the spectrum of the physical mixture (d) exhibited distinct peaks of both PZQ and PMMs. Lastly, in the spectrum of PZQ-PMMs (c), the characteristic peak at 1651 cm−1 for PZQ disappeared, indicating that PZQ was dispersed in the micelles in an amorphous state.38
 |
Figure 2 FTIR spectra (A), DSC curves (B), and XRD curves (C) of (a) PZQ (b) PMMs prepared by P123: L64=1:1(w/w) (c) PZQ-PMMs prepared by P123: L64=1:1(w/w) (d) PZQ and PMMs physical mixture. |
The formation of PZQ-PMMs was confirmed by DSC, as shown in Figure 2B. The DSC curves for PZQ (a) clearly indicated melting temperatures (Tm) at 139°C, which corresponds to the drug’s melting point of 136–142°C.17 In the curve representing the physical mixture of PZQ and PMMs (d), the melting peak of PZQ appeared as a broad peak, starting at 120°C. The curves for PZQ-PMMs (c) and the blank micelles (b) were nearly identical, exhibiting endothermic peaks for the micelles near 158°C, while the endothermic peak for PZQ was absent. This observation suggested that the drug formed a binding material with the micelles that differed from the physical mixture.
The resulting XRD patterns were depicted in Figure 2C, providing comprehensive insights into the crystalline nature of the drugs and formulations. Sharp crystalline peaks were evident in the PZQ diffractogram (a). Due to the incorporation of mannitol during the freeze-drying process, sharp crystal peaks were observed in the pattern of PMMs (b). The pattern of PZQ-PMMs (c) closely resembled that of PMMs, with the characteristic peak of PZQ being indistinguishable. In contrast, the pattern of the physical mixture of PZQ and PMMs (d) exhibited a straightforward superposition of the crystal peaks from both PZQ and PMMs. Consequently, it could be concluded that PZQ in the drug-loaded micelles may exist in either a molecularly distributed or an amorphous condition.39 These results are consistent with the findings from the FTIR spectra and DSC curves.
In vitro Release Studies
When compared to PZQ powder and commercially available preparations, PZQ-Q and PZQ-B, the three formulations of PZQ-PMMs exhibited similar rapid and complete release profiles (see Figure 3), with highly significant differences in both the maximum CRP and the CRP at 2 hours (p < 0.001). In a release medium with a pH of 1.2, the maximum CRP of PZQ-PMMs was 83.01 ± 2.00%, which increased with the dosage of L64, demonstrating a significant difference (p < 0.05). This might be attributed to the higher HLB value of L64 compared to that of P123, along with its shorter hydrophobic chain, which weakened the interaction forces within the hydrophobic core and facilitates drug release. In the release medium at pH 6.8, the maximum CRP of PZQ-PMMs did not show significant differences across the three ratios, with the highest CRP being approximately 93%. All six samples exhibited more complete release in the pH 6.8 medium than in the pH 1.2 medium. Ana C. Mengarda et al40 observed that the release behavior of PZQ powder using the dialysis method was also faster in a pH 6.8 buffer medium. Although PZQ was expected to exist in molecular form in both release media, literature41 indicated that its solubility in hydrochloric acid solution was lower than in pH 6.8 phosphate buffer solution, which may explain the observed results.
 |
Figure 3 Cumulative release profiles of PZQ from different preparations. (Left: pH1.2, Right: pH6.8). |
In vivo Absorption Studies
A HPLC technique was established for the measure PZQ concentration in vivo. The retention time for PZQ in chromatography was 9.785 minutes, with no interference from endogenous substances in plasma, demonstrating good specificity (see Figure 4). A standard curve for PZQ was established, with the regression equation given by y = 0.3164x + 0.0228 and an R² value of 0.9996. A strong linear correlation was found between drug concentration of plasma and peak area within the 0.1 to 10 μg/mL range. The limit of detection (LOD) was established at 0.05 μg/mL, and the limit of quantification (LOQ) was set at 0.10 μg/mL. Recovery rates varied from 98% to 110%, with a relative standard deviation (RSD) between 4.21% and 5.76%. The RSD values for both intra-day and inter-day precision were below 6%.
 |
Figure 4 HPLC chromatogram of (a) PZQ (b) blank plasma (c) blank plasma with PZQ for specificity. |
Figure 5 displayed the plasma drug concentration–time curves of PZQ after the oral intake of a 30% ethanol solution, commercially available PZQ-Q suspension, and PZQ-PMMs (P123: L64 = 1:1) solution. The associated pharmacokinetic parameters could be found in Table 2.
 |
Table 2 Pharmacokinetic Parameters Measured Following the Oral Intake of a 30% Ethanol Solution of PZQ, PZQ-Q Suspension and PZQ-PMMs in Rats (Mean ± SD) |
 |
Figure 5 Plasma drug concentration–time curves in vivo after the oral intake of a 30% ethanol solution of PZQ, PZQ-Q suspension and PZQ-PMMs in rats (Mean±SD). |
After gavage administration, the in vivo release behavior of PZQ-PMMs and PZQ-Q was similar, with both exhibiting a rapid peak in the blood drug concentration curve followed by a gradual decline. The tmax of PZQ-Q was 1.3 ± 0.6 hours, while PZQ-PMMs achieved their peak more quickly, with a tmax of 0.5 ± 0.4 hours. In contrast, the blood drug concentration curve of the PZQ 30% ethanol solution displayed double peaks, with tmax values of 0.7 ± 0.3 hours and 6.0 ± 0.0 hours, respectively. This phenomenon may be attributed to the high concentration of ethanol used to dissolve the drug, which could affect its absorption in rats.42 The Cmax of PZQ-PMMs was 1659 ± 441 ng/mL, which is 1.9 times greater than that of PZQ (P < 0.05) and 1.6 times greater than that of PZQ-Q. This suggests that, at the same dosage, micelles can rapidly produce higher blood drug concentrations. The Cmax of the two PZQ micelle formulations mentioned in the introduction was 380.75 ± 47.67 ng/mL (for a single oral dose of 12.5 mg/kg) and 20.5 μg/mL (for a single oral dose of 400 mg/kg), respectively.8,18 The relative bioavailability of PZQ-PMMs was comparable to that of the PZQ 30% ethanol solution and was 1.7 times that of PZQ-Q, with the increase in bioavailability being highly significant (P < 0.01). As envisioned, PZQ-PMMs did exhibit faster drug release, higher peak blood concentrations, and improved bioavailability compared to the commercially available formulation. The first-pass metabolism of PZQ is dose-dependent with regard to capacity, with saturation of the metabolic routes.1 It is speculated that the enhanced bioavailability of PZQ-PMMs results from the transiently elevated blood concentrations, which help to saturate liver drug-metabolizing enzymes, thereby reducing the first-pass effect.
Conclusions
PZQ is a very slightly soluble drug, with a notable first-pass effect after oral administration, along with considerable individual variability. Developing an immediate release formulation is an effective strategy to enhance the instantaneous plasma concentration, thereby maximizing the saturation of liver drug-metabolizing enzymes and improving its bioavailability. In this study, the newly discovered Pluronic P123/L64 micelles were utilized to encapsulate PZQ. The optimized PZQ-PMMs measured with 20 nm in size and demonstrated high DL and EE, leading to enhanced solubility of drugs that are not easily soluble. Furthermore, PZQ-PMMs exhibited superior immediate release behavior compared to the commercial preparations in both in vitro release experiments and in vivo absorption studies. Following a single oral dose of PZQ-PMMs given to rats, the Cmax was increased, and the bioavailability was significantly improved. Additional studies on taste masking effects, safety, long-term stability, and clinical trials are warranted. Moreover, it may be beneficial to combine ionic amphiphilic polymers with Pluronic to address the current low zeta potential of the micelles, which could further improve their stability.
Acknowledgments
This research was funded by the Introducing Talent Scientific Research Foundation of Shenyang Agricultural University (Grant No. 880415024).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Olliaro P, Delgado-Romero P, Keiser J. The little we know about the pharmacokinetics and pharmacodynamics of praziquantel (racemate and R-enantiomer). J Antimicrob Chemoth. 2014;69(4):863–870. doi:10.1093/jac/dkt491
2. The Merck Index*Online. [2025-02-05]. Available from: https://merckindex.rsc.org/monographs/m9107.
3. Cioli D, Pica-Mattoccia L. Praziquantel. Parasitol Res. 2003;90(Supp 1):S3–S9. doi:10.1007/s00436-002-0751-z
4. Meyer T, Sekljic H, Fuchs S, Bothe H, Schollmeyer D, Miculka C. Taste, a new incentive to switch to (R)-praziquantel in schistosomiasis treatment. PLoS Neglect Trop D. 2009;3(1):e357. doi:10.1371/journal.pntd.0000357
5. Mengarda AC, Iles B, Longo JPF, de Moraes J. Recent trends in praziquantel nanoformulations for helminthiasis treatment. Expert Opin Drug Deliv. 2022;19(4):383–393. doi:10.1080/17425247.2022.2051477
6. Kuevi DNO, Acquah FA, Amuquandoh A, et al. Challenges and proven recommendations of praziquantel formulation. J Clin Pharm Ther. 2023;2023(3976392). doi:10.1155/2023/3976392
7. Thanitwatthanasak S, Sagis LM, Chitprasert P. Pluronic F127/Pluronic P123/vitamin E TPGS mixed micelles for oral delivery of mangiferin and quercetin: mixture-design optimization, micellization, and solubilization behavior. J Mol Liq. 2019;274:223–238. doi:10.1016/j.molliq.2018.10.089
8. Arafa WM, Elkomy MH, Aboud HM, et al. Tunable polymeric mixed micellar nanoassemblies of lutrol F127/Gelucire 44/14 for oral delivery of praziquantel: a promising nanovector against hymenolepis nana in experimentally-infected rats. Pharmaceutics. 2022;14(10):2023. doi:10.3390/pharmaceutics14102023
9. Andrade LN, Marques C, Barbosa T, et al. Praziquantel-loaded solid lipid nanoparticles: production, physicochemical characterization, release profile, cytotoxicity and in vitro activity against Schistosoma mansoni. J Drug Deliv Sci Tec. 2020;58(1):101784. doi:10.1016/j.jddst.2020.101784
10. Santiago-Villarreal O, Rojas-González L, Bernad-Bernad MJ, Miranda-Calderón JE. Self-emulsifying drug delivery system for praziquantel with enhanced ex vivo permeation. J Pharm Innov. 2023;18(2):525–537. doi:10.1007/s12247-022-09649-7
11. El-Attar NA, El-Sawi MR, El-Shabasy EA. The synergistic effect of Ficus carica nanoparticles and praziquantel on mice infected by Schistosoma mansoni cercariae. Sci Rep-UK. 2024;14(1):18944. doi:10.1038/s41598-024-68957-9
12. Pereira ED, da Silva Dutra L, Paiva TF, et al. In vitro release and in vivo pharmacokinetics of praziquantel loaded in different polymer particles. Materials. 2023;16(9):3382. doi:10.3390/ma16093382
13. Salas-Zuniga R, Mondragon-Vasquez K, Alcala-Alcala S, et al. Nanoconfinement of a pharmaceutical cocrystal with praziquantel in mesoporous silica: the influence of the solid form on dissolution enhancement. Molecular Pharmaceutics. 2022;19(2):414–431. doi:10.1021/acs.molpharmaceut.1c00606
14. Borrego-Sánchez A, Sánchez-Espejo R, García-Villén F, Viseras C, Sainz-Díaz CI. Praziquantel–clays as accelerated release systems to enhance the low solubility of the drug. Pharmaceutics. 2020;12(10):914. doi:10.3390/pharmaceutics12100914
15. Mahmoud M, Allam AF, Essawy AE, Shalaby TI, El-Sherif SS. Therapeutic efficacy of praziquantel loaded-chitosan nanoparticles on juvenile Schistosoma mansoni worms in murine model. Exp Parasitol. 2024;266:108843. doi:10.1016/j.exppara.2024.108843
16. Mathews PD, Patta ACMF, Madrid RRM, Ramirez CAB, Pimenta BV, Mertins O. Efficient treatment of fish intestinal parasites applying a membrane-penetrating oral drug delivery nanoparticle. ACS Biomater Sci Eng. 2023;9(6):2911–2923. doi:10.1021/acsbiomaterials.1c00890
17. Silva ADA, Sarcinelli MA, de Carvalho Patricio BF, et al. Pharmaceutical development of micro and nanocrystals of a poorly water-soluble drug: dissolution rate enhancement of praziquantel. J Drug Deliv Sci Tec. 2023;81:104260. doi:10.1016/j.jddst.2023.104260
18. Meteleva E, Chistyachenko Y, Suntsova L, Tsyganov M, Lyakhov N. Physicochemical properties and anti-opisthorchosis effect of mechanochemically synthesized solid compositions of praziquantel with glycyrrhizic acid disodium salt. Dokl Biochem Biophys. 2018;481:228–231. doi:10.1134/S1607672918040142
19. Gaggero A, Dukovski BJ, Radić I, et al. Co-grinding with surfactants as a new approach to enhance in vitro dissolution of praziquantel. J Pharmaceut Biomed. 2020;189:113494. doi:10.1016/j.jpba.2020.113494
20. Ghezzi M, Pescina S, Padula C, Santi P, Nicoli S. Polymeric micelles in drug delivery: an insight of the techniques for their characterization and assessment in biorelevant conditions. J Control Release. 2021;332:312–336. doi:10.1016/j.jconrel.2021.02.031
21. Adekiya TA, Kumar P, Kondiah PP, Ubanako P, Choonara YE. In vivo evaluation of praziquantel-loaded solid lipid nanoparticles against S. mansoni infection in preclinical murine models. Int J Mol Sci. 2022;23(16):9485. doi:10.3390/ijms23169485
22. Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Control Release. 2021;332(6):127–147. doi:10.1016/j.jconrel.2021.02.016
23. Wang Z, Chen P, Guo M, Yang X, Song W, Huang F. Physicochemical characterization of Berberine-loaded Pluronic F127 polymeric micelles and in vivo evaluation of hypoglycemic effect. J Pharm Innov. 2022;18(2):538–547. doi:10.1007/s12247-022-09658-6
24. Akhlaghi N, Najafpour-Darzi G. Amino-Functionalized Pluronic F127 micelles as a dual drug delivery nanostructure for controlled therapeutics release. J Mol Liq. 2024;400:124489. doi:10.1016/j.molliq.2024.124489
25. Singh G, Kaur K, Bhalla V, et al. Pluronic L121-Chrysin conjugated polymeric micelles of exemestane: improved synergistic effect, in vitro and in vivo anticancer activity. Colloid Surf A. 2024;698:134458. doi:10.1016/j.colsurfa.2024.134458
26. Spir E, Nal M, Gk ZG, Yiitolu M. Synthesis, characterization and in vitro release analysis of pluronic F127 copolymer micelles containing quercetin as a hydrophobic drug. Polym Bull. 2024;81(8):6801–6822. doi:10.1007/s00289-023-05040-9
27. Gao H, Zhang J, Gkleijn T, et al. Dual ligand-targeted Pluronic P123 polymeric micelles enhance the therapeutic effect of breast cancer with bone metastases. Oncol Res. 2024;32(4):769–784. doi:10.32604/or.2023.044276
28. Patel P, Pardhi V, Jain K. Bedaquiline loaded Soluplus® micelles for improving solubility and intestinal permeability: formulation, optimization, and ex vivo evaluation. J Drug Deliv Sci Tec. 2025;105:106656. doi:10.1016/j.jddst.2025.106656
29. Dayani L, Haddadi F, Aliomrani M, Taheri A. Preparation and in vitro/in vivo evaluation of Fingolimod hydrochloride loaded polymeric mixed nano-micelles for treatment of multiple sclerosis. J Neuroimmune Pharm. 2025;20(1):41. doi:10.1007/s11481-025-10203-8
30. Gu J, Cai X, Raza F, et al. Preparation of a minocycline polymer micelle thermosensitive gel and its application in spinal cord injury. Nanoscale Adv. 2024;6(23):5874–5888. doi:10.1039/d4na00625a
31. Shaikh R, Bhattacharya S, Saoji SD. Development, optimization, and characterization of polymeric micelles to improve dasatinib oral bioavailability: Hep G2 cell cytotoxicity and in vivo pharmacokinetics for targeted liver cancer therapy. Heliyon. 2024;10(21):e39632. doi:10.1016/j.heliyon.2024.e39632
32. Yuan J, Gao Y, Tian X, et al. Computational and experimental comparison of molecularly imprinted polymers prepared by different functional monomers—quantitative parameters defined based on molecular dynamics simulation. Molecules. 2024;29(17):4236. doi:10.3390/molecules29174236
33. Yuan J, Wang C, Gao Y, et al. Probing the molecular basis for sulfonamides recognition in surface molecularly imprinted polymers using computational and experimental approaches. React Funct Polym. 2021;170:105105. doi:10.1016/j.reactfunctpolym.2021.105105
34. Yuan J, Guo L, Wang S, et al. Preparation of self-assembled nanoparticles of ε-polylysine-sodium alginate: a sustained-release carrier for antigen delivery. Colloid Surf B. 2018;171:406–412. doi:10.1016/j.colsurfb.2018.07.058
35. Raghunath I, Koland M, Sarathchandran SR, et al. Design and optimization of chitosan-coated solid lipid nanoparticles containing insulin for improved intestinal permeability using piperine. Intl J Biol Macromol. 2024;280:135849. doi:10.1016/j.ijbiomac.2024.135849
36. Yuan J, Liu T, Li H, et al. Oral sustained-release suspension based on a novel taste-masked and mucoadhesive carrier–ion-exchange fiber. Int J Pharmaceut. 2014;472(1):74–81. doi:10.1016/j.ijpharm.2014.05.048
37. Chaud M, Lima A, Vila M, et al. Development and evaluation of praziquantel solid dispersions in sodium starch glycolate. Trop J Pharm Res. 2013;12(2):163–168. doi:10.4314/tjpr.v12i2.5
38. Sun DH, Sun DX, Hao Y. Synthesis and characterization of superparamagnetic NiFe2O4 nanoparticles. Mater Sci Forum. 2010;663-665(2):1325–1328. doi:10.4028/www.scientific.net/MSF.663-665.1325
39. Usapkar P, Saoji S, Jagtap P, et al. QbD-guided phospholipid-tagged nanonized boswellic acid naturosomal delivery for effective rheumatoid arthritis treatment. Int J Pharmaceut: X. 2024;7:100257. doi:10.1016/j.ijpx.2024.100257
40. Mengarda AC, Iles B, Rodrigues VC, et al. Praziquantel nanoparticle formulation for the treatment of schistosomiasis. ACS Appl Nano Mater. 2025;8:3985–3997. doi:10.1021/acsanm.4c06757
41. Eason T, Ramirez G, Clulow AJ, Salim M, Boyd BJ. Revisiting the dissolution of praziquantel in biorelevant media and the impact of digestion of milk on drug dissolution. Pharmaceutics. 2022;14(2228). doi:10.3390/pharmaceutics14102228
42. Linnoila M, Mattila M, Kitchell B. Drug interactions with alcohol. Drugs. 1979;18:299–311. doi:10.2165/00003495-197918040-00003
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.