Back to Journals » Infection and Drug Resistance » Volume 18
Point Prevalence Survey of Antibiotic Use in Level 1 hospitals in Zambia: Future Prospects for Antimicrobial Stewardship Programs
Authors Mudenda S , Lubanga AF , Jamshed S, Biemba B, Sakala R, Chiyabi M, Kavubya L, Milambo LT, Bumbangi FN , Chizimu JY, Yamba K, Wesangula E, Chigome A, Kalungia AC , Sefah IA, Mustafa ZU , Massele AY, Saleem Z , Mutemwa R , Kazonga E, Sartelli M, Meyer JC, Muma JB, Chilengi R, Godman B
Received 30 November 2024
Accepted for publication 7 February 2025
Published 15 February 2025 Volume 2025:18 Pages 887—902
DOI https://doi.org/10.2147/IDR.S509522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Steward Mudenda,1– 3 Adriano Focus Lubanga,4,5 Shazia Jamshed,6 Bibian Biemba,1 Racheal Sakala,1 Mervis Chiyabi,1 Lorraine Kavubya,1 Linda Twaambo Milambo,1 Flavien Nsoni Bumbangi,7 Joseph Yamweka Chizimu,3 Kaunda Yamba,8 Evelyn Wesangula,9 Audrey Chigome,10 Aubrey Chichonyi Kalungia,1 Israel Abebrese Sefah,11 Zia UI Mustafa,12,13 Amos Yared Massele,14 Zikria Saleem,15 Richard Mutemwa,16 Eustarckio Kazonga,16 Massimo Sartelli,17 Johanna Catharina Meyer,10,18 John Bwalya Muma,19 Roma Chilengi,3 Brian Godman10,20
1Department of Pharmacy, School of Health Sciences, University of Zambia, Lusaka, Zambia; 2Education and Continuous Professional Development Committee, Pharmaceutical Society of Zambia, Lusaka, Zambia; 3Antimicrobial Resistance Coordinating Committee, Zambia National Public Health Institute, Lusaka, Zambia; 4Education and Research, Clinical Research Education and Management Services (CREAMS), Lilongwe, Malawi; 5Department of Clinical Services, Kamuzu Central Hospital (KCH), Lilongwe, Malawi; 6Department of Pharmacy Practice, School of Pharmacy, International Medical University, Kuala Lumpur, Malaysia; 7Department of Medicine and Clinical Sciences, School of Medicine, Eden University, Lusaka, Zambia; 8Action on Antibiotic Resistance (React) Africa, Lusaka, Zambia; 9Strengthening Pandemic Preparedness, Eastern, Central, and Southern Africa Health Community, Arusha, Tanzania; 10Department of Public Health Pharmacy and Management, School of Pharmacy, Sefako Makgatho Health Sciences University, Garankuwa, Pretoria, South Africa; 11Pharmacy Practice Department, School of Pharmacy, University of Health and Allied Sciences, Volta Region, Ghana; 12Discipline of Clinical Pharmacy, School of Pharmaceutical Sciences, Universiti Sains Malaysia, Gelugor, Penang, Malaysia; 13Department of Pharmacy Services, District Headquarter (DHQ) Hospital, Pakpattan, 57400, Pakistan; 14Department of Clinical Pharmacology and Therapeutics, Kairuki University, Dar Es Salaam, Tanzania; 15Department of Pharmacy Practice, Faculty of Pharmacy, Bahauddin Zakariya University, Multan, Pakistan; 16Department of Public Health, School of Medicine and Health Sciences, University of Lusaka, Lusaka, Zambia; 17Department of Surgery, Macerata Hospital, Macerata, Italy; 18South African Vaccination and Immunisation Centre, Sefako Makgatho Health Sciences University, Garankuwa, Pretoria, South Africa; 19Department of Disease Control, School of Veterinary Medicine, University of Zambia, Lusaka, Zambia; 20Department of Pharmacoepidemiology, Strathclyde Institute of Pharmacy and Biomedical Sciences, University of Strathclyde, Glasgow, UK
Correspondence: Adriano Focus Lubanga, Clinical Research Education and Management Services (CREAMS), Anderson House, Area 43, P. O Box 31045, Lilongwe, Malawi, Tel +265992744497, Email [email protected]
Introduction: The inappropriate prescribing and use of antibiotics have contributed to the emergence and spread of antimicrobial resistance (AMR). In Zambia, there is a paucity of information on the prescribing patterns and use of antibiotics among hospitalized patients in level 1 hospitals. This study investigated antibiotic use in five level 1 hospitals in Lusaka, Zambia.
Methods: This cross-sectional study utilized the World Health Organization (WHO) Point Prevalence Survey (PPS) methodology among in-patients admitted in level 1 hospitals before 08:00 a.m. on the survey day in August 2024. Data were analysed using IBM SPSS version 23.0.
Results: The prevalence of antibiotic use among inpatients was 59.0%, with ceftriaxone being the most prescribed. Antibiotics were prescribed mainly for paediatrics and male inpatients. This study found that 53.0% of prescribed antibiotics were from the Access group while 38.2% were from the Watch group of the World Health Organization Access, Watch, and Reserve (AWaRe) classification. Adherence to national treatment guidelines was 36.0%, with most antibiotics prescribed empirically without evidence of culture and sensitivity tests.
Conclusion: This study found a high use of antibiotics and low adherence to treatment guidelines in level 1 hospitals in Lusaka, Zambia. The findings of this study demonstrate the need to establish and strengthen antimicrobial stewardship programs and strengthen laboratory capacity to aid clinicians in diagnosing, treating, and managing patients across level 1 hospitals in Zambia.
Keywords: antibiotic use, antimicrobial resistance, antimicrobial stewardship, Point Prevalence Survey, Zambia
Introduction
Antimicrobial resistance (AMR) is a global public health problem appreciably impacting morbidity, mortality and costs, with the highest burden in sub-Saharan Africa (SSA).1–6 Despite AMR occurring as a natural phenomenon, it is increased by inappropriate consumption and use of antimicrobials.7–12 Consequently, measures to improve antibiotic utilization must be developed and implemented to prevent the escalation of AMR as the next global pandemic.13–15 There have been several global and regional initiatives in recent years to reduce AMR in humans.13,16–20 These incorporate initiatives by the World Health Organization (WHO) including the launch of the Global Action Plan (GAP) in 2015, which translated into National Action Plans (NAPs),21–24 as well as initiatives to improve surveillance of AMR.25,26 It is against this background that this study investigated antibiotic use among in-patients admitted in five level 1 hospitals in Lusaka, Zambia.
Another initiative was the introduction of the Access, Watch and Reserve (AWaRe) classification of antibiotics with an emphasis on reducing the overuse of antibiotics from the “Watch” and “Reserve” lists due to their greater resistance potential.9,27,28 This is particularly important in low- and middle-income countries (LMICs), including countries in sub-Saharan Africa, with rapid increases in the utilization of “Watch” antibiotics in recent years.29 The WHO AWaRe classification classifies antibiotics into three categories based on their spectrum of activity and potential to promote resistance.30–32 The Access group includes antibiotics recommended for first- and second-line treatments of common infections.30,33,34 The Watch group comprises broad-spectrum antibiotics that carry a higher risk of resistance development.30,34,35 Finally, the Reserve group consists of last-resort antibiotics, reserved for treating multidrug-resistant infections.30,34 According to the WHO AWaRe classification, it was recommended that hospitals must be prescribing at least 60% of antibiotics from the Access group.31,36 Recently, the United Nations General Assembly (UNGA) of 2024 guided that hospitals must be prescribing at least 70% of antibiotics from the Access group.37 More recently, the WHO AWaRe book was launched providing treatment guidance on a range of infectious diseases seen in ambulatory and hospital care, including alternatives to antibiotics where pertinent to enhance their appropriate use.30,31,38 Therefore, monitoring the consumption of antibiotics is a necessary first step in developing pertinent strategies to enhance the rational use of antibiotics within healthcare settings.39–44 However, there have been concerns with implementing NAPs on AMR, especially in sub-Saharan Africa as a result of a shortage of personnel and limited resources.45–47
Point prevalence surveys (PPS) have been used to monitor antibiotic use and prescribing patterns, especially in hospitals across countries including many sub-Saharan African countries.17,39,41,48–50 The WHO PPS and Global PPS have been used extensively globally to monitor the use of antibiotics in many countries building on European initiatives.39,41,51–56 The basic version of the PPS is used for surveillance of antimicrobial use, hospital-acquired infections (HAI) and AMR. This is essential to help hospitals compare their ongoing antimicrobial utilization patterns with national and regional benchmark figures to improve future antimicrobial prescribing.39,41,48 Additionally, through a PPS, hospitals can monitor adherence to antibiotic prescribing guidelines including the WHO AWaRe classification, which is used to visualize antibiotic prescribing patterns in healthcare facilities with an initial target of at least 60% of total antibiotic utilization being antibiotics from the ‘Access’ category.29,41,51 Adherence to guidelines is increasingly being used across countries to assess the quality of prescribing across sectors including among hospitals.39,41,49,57,58
Published studies have shown extensive concerns with the prescribing of antimicrobials in hospitals across sub-Saharan Africa.41,49,59–61 In Zambia, a PPS conducted among adult in-patients found that antibiotics were prescribed for 59.0% of in-patients, with low adherence to current treatment guidelines.62 In addition, in the study by D’Arcy et al (2020) involving one hospital from Zambia, antibiotics were prescribed in 57.0% of in-patients.53 This rate was higher than seen among participating hospitals in Tanzania, Uganda and Ghana at between 30.0%–55.0% of in-patients.53 Additionally, 58.0% of prescribed antibiotics were in the ‘Access’ category, similar to the other countries.53 Other observational studies conducted in Zambia, including during the recent COVID-19 pandemic, have also shown high rates of antibiotic prescribing alongside concerns with adherence to current guidelines among different hospital types, including primary healthcare hospitals, as well as among outpatients.63–66
However, there is still a paucity of information on antibiotic use in hospitals in Zambia, especially those predominantly treating the general population including faith-based hospitals, which typically serve more rural areas,67 as well as level 1 hospitals, which are usually situated in areas of ease of reach and typically the first point of contact with the healthcare system.62 Level 1 hospitals serve the majority of community members, with patients only typically referred to secondary or tertiary care hospitals for diseases or procedures that cannot be addressed in Level 1 hospitals. However, there are concerns generally with diagnostic capabilities in these hospitals similar to other LMICs.68–70 Overall, there are concerns with antibiotic prescribing patterns, including in ambulatory care, in Zambia exacerbating AMR rates.62,64,65,71–74
Addressing inappropriate antibiotic prescribing practices in Zambia is crucial to attain its NAP goals given concerns generally with attaining NAPs in Africa.45,47,75 To address the gaps, this study was conducted to investigate current antibiotic use among in-patients admitted in five level 1 hospitals in Lusaka, Zambia, building on earlier studies among all hospital types in Zambia including during the recent COVID-19 pandemic.62–65,76,77 The study also assessed the commonly prescribed antibiotics, prescribing of antibiotics by WHO AWaRe classification, commonly diagnosed diseases, adherence to national treatment guidelines, and the use of culture and sensitivity in initiating antibiotic therapy. The combined findings can be used to guide future activities among hospitals in Zambia to improve future antibiotic prescribing, which potentially includes Antimicrobial Stewardship (AMS) Programs despite previous concerns in Zambia, which is similar to other LMICs.78–80 However, this is changing with the increasing instigation of AMS Programs across sub-Saharan Africa guiding hospital groups across Zambia and beyond.41,81–84
Materials and Methods
Study Design, Period, and Setting
This PPS was conducted in five level 1 hospitals in Lusaka, Zambia in August 2024. Lusaka was chosen as the setting for this initial study since it is the capital city of Zambia housing the five level 1 hospitals. Consequently, if there are problems in the level 1 hospitals in Lusaka, these are likely to be worse in more rural areas and lower levels of practice in Zambia. The five chosen sites were purposively selected to provide a range of bed capacities and catchment areas. They included Kanyama Level 1 hospital, with a bed capacity of 163 and a catchment population of 276, 000, Chilenje Level 1 hospital, with a bed capacity of 87 serving a catchment population of over 500,000, Chawama Level 1 hospital with a bed capacity of 106 and a catchment population of 97,958, Matero Level 1 hospital with a bed capacity of 152 and serving 131,592 people, and Chipata Level 1 hospital with a bed capacity of 76 and a catchment population of over 489,000.
The selected hospitals offer Outpatient Department (OPD) services (medical, dental, physiotherapy, and pharmacy), Inpatient Department services (general medical, maternity, and surgical), and diagnostic services (laboratory and radiology). In-patients who were admitted before or at 08:00 a.m. on the day of the survey were included in the study in line with recommended PPS methods.51,52,70 The PPS included in-patients who were admitted to Obstetrics and Gynaecology wards, Adults’ Medical and Surgical wards, and Children’s Medical and Surgical wards.
Sample Size, Inclusion and Exclusion Criteria
The bed spaces for the surveyed hospitals ranged from 76 to 163. Consequently, based on the WHO PPS methodology, all in-patients that meet the inclusion criteria must be included in the survey provided there are 500 or fewer bed spaces in a particular hospital.52 As a result, sample size calculation did not apply, because we had prior information on the number of bed spaces per hospital before conducting the PPS. All patients who were admitted after 08:00 a.m. on the day of the PPS were excluded from the study. Additionally, we also excluded any documentation and pertinent patients prescribed topical antibiotics, antivirals, anti-tuberculosis, antifungals, or anti-parasitic antimicrobials to concentrate on parenterally and orally administered antibiotics. All antibiotic therapy that were started after 08:00 a.m. or stopped before 08:00 a.m. on survey day were excluded. Hence, such patients were not counted in the numerator as being on antibiotic therapy.
Data Collection
Data was collected using Research Electronic Data Capture (REDCap) software version 9.1.15.85,86 The WHO PPS methodology questionnaire was utilized to collect data on antibiotic use and prescribing patterns among in-patients in the study hospitals.52 This included information about (i) the hospital, (ii) ward, (iii) patient (iv) indication and (v) antibiotic use and microbiology data. Microbiology data for culture and sensitivity included blood, urine, would, stool, sputum/ respiratory samples, sterile fluids including cerebrospinal fluid, peritoneal fluid, and synovial fluid. Data collection was executed by six data collectors who were specifically trained for this purpose and the team visited each hospital for a period of two days translating into a 10-day data collection period. The trainers were part of the Antimicrobial Resistance Coordinating Committee (AMRCC) who are experts in conducting PPS in Zambia. The training was done for a period of three days to ensure that the data collectors understood the need to collect complete and good quality data. REDCap accounts were opened for all the data collectors and testing of data entry was done on day two and three of training. After conducting the PPS, a meeting was held with the hospital management and staff where the findings were disseminated and recommendations were provided.
Data Analysis
The collected data were extracted from REDCap and exported to Microsoft Excel 2013. Data analysis was performed using IBM SPSS version 23.0. Descriptive statistics were performed for hospital and ward demographic characteristics, patient data, indication, and antibiotic prescribing patterns and the results were presented in tables and charts as frequencies and percentages. To determine the prevalence of antibiotic use, the denominator was the number of patients who met the inclusion criteria and were included in the survey. The numerator was the number of recruited patients who were currently on antibiotic treatment on the day of the survey. Prescribing compliance was assessed using the Zambia Standard Treatment Guidelines (STGs).87 Antibiotics were also classified by WHO AWaRe classification.27,28,88
Ethical Approval
Ethical approval was obtained from the University of Zambia Health Sciences Research Ethics Committee (UNZAHSREC) with approval number 20231270137. Further approval was obtained from the Zambia National Health Authority (NHRA) with approval number NHRA5949/13/08/2024. Official permission to collect data from the selected hospitals was obtained from the district authority at the Lusaka District Office (DHO). There was no need for informed patient consent as this was a review of the patient’s medical records, with all data anonymized, which is similar to other PPS studies.89–91 The study was conducted according to the guidelines of the Declaration of Helsinki.
Results
In this study, a total of 580 patients were enrolled with 112 being from Chawama, 134 from Chilenje, 104 from Chipata, 108 from Kanyama, and 122 from Matero Level 1 hospitals. The overall prevalence of antibiotic use among hospitalized patients was 59% (342/580), p=0.190. The highest prescribing of antibiotics was noted for Kanyama (69%), Chilenje (59%), and Matero (59%) Level 1 hospitals (Table 1). Additionally, the prevalence of antibiotic use of highest among patients admitted to the female surgical ward (79%), followed by the male medical ward (76%), and children’s ward (75%), p=0.001. The lowest use of antibiotics was reported in the Obstetrics and Gynaecology wards (35%) (Table 1).
 |
Table 1 Prevalence of Antibiotic Use by Hospital and Ward Among Hospitalized Patients |
Most antibiotics were prescribed for children aged below the age of two years (87.5%) followed by those aged between two and five years (68.2%). Additionally, 66.3% of antibiotics were prescribed for adults above the age of 35 years old. A lower prescribing rate of antibiotics (47.1%) was recorded for children aged between six and 12 years (Table 2). Our study also found that antibiotics were prescribed mostly for male patients (76.1%) (Table 2).
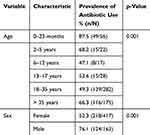 |
Table 2 Antibiotic Prescribing Patterns by Age and Sex of Hospitalized Patients |
A total of 631 antibiotics were prescribed for the in-patients across the five level 1 hospitals in Lusaka, Zambia. Most of the in-patients received ceftriaxone (36.6%) of which Chilenje, Chawama, and Kanyama Level 1 hospitals had the highest rates of prescribing of ceftriaxone (Table 3). Of the 34 antibiotics prescribed, 18 (53.0%) were from the “Access” group, 13 (38.2%) were from the “Watch” group, and 3 (8.8%) were from the “Reserve” group (Table 3).
 |
Table 3 Antibiotic Prescribing Patterns by WHO AWaRe Classification of Antibiotics |
The commonly prescribed antibiotic classes were cephalosporins, penicillins, and quinolones (Figure 1). On the other hand, the least prescribed classes of antibiotics were tetracyclines, imidazoles, sulfonamide-trimethoprim-combinations, amphenicols, and carbapenems (Figure 1).
 |
Figure 1 Antibiotic prescribing patterns by class of antibiotics in level 1 hospitals in Lusaka, Zambia. |
Most patients received antibiotics for empirical treatment, pneumonia, OBGY infections, cellulitis, wounds, soft skin infections, and gastrointestinal tract infections (Figure 2). Of the 61 patients who underwent surgery, 61 (100%) received antibiotics. Of the 81 patients who were at high risk of developing an infection, 80 (99%) received antibiotics for medical prophylaxis. Of the 150 patients with community-acquired infections, 148 (97%) received antibiotics. Further, of the 19 patients with HAIs, 18 (95%) received antibiotics. Therefore, most antibiotics were used for surgical and medical prophylaxis and to treat community-acquired infections.
 |
Figure 2 Distribution of diagnoses found in the surveyed level 1 hospitals in Lusaka District, Zambia. |
Compliance with the Zambia STGs was 36.0% with Matero level 1 hospital being the most compliant at 47% while Chilenje (29%) and Kanyama (32%) level 1 hospitals, respectively, were the least compliant (Table 4). Of the 342 in-patients who received antibiotics, only 3 (0.9%) received directed antibiotic treatment (Table 4).
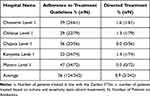 |
Table 4 Adherence Rates to Current National Treatment Guidelines and Treatment Type in Level 1 hospitals in Lusaka District, Zambia |
Discussion
We believe this is the first study in Zambia to investigate antibiotic use among in-patients in level 1 hospitals, building on previous limited studies. Our study found that antibiotics were prescribed in 59.0% of the in-patients seen across all the surveyed facilities. Further, ceftriaxone, an antibiotic from the “Watch” group was widely used in the surveyed hospitals, potentially exacerbated by the highly empirical use of antibiotics in the surveyed hospitals with low use of culture and sensitivity results and low adherence to the national treatment guidelines.
At each hospital surveyed, a high prevalence of antibiotic use was evident with 54% at Chawama, 59% at Chilenje, 54% at Chipata, 69% at Kanyama, and 59% at Matero level 1 hospital, respectively. A similar average prevalence of antibiotic use among hospitalized patients was reported in an earlier PPS that was conducted in Zambia.62 The high use of antibiotics in Zambia has been reported to be due to a high burden of disease, a lack of diagnostic capacity, non-adherence to treatment guidelines, lack of awareness and knowledge regarding antibiotic use and AMR among prescribers.92,93 Our findings agree with the relatively high antibiotic use patterns among hospitalized patients in the sub-Saharan African region.62,94,95 The high prevalence of antibiotic use in hospitals has been reported in other PPS ranging from 62.3% up to 94.6% globally.49,60,68,96–100 The prevalence of antibiotic use in our study is higher than reported in other PPS globally.101–110 The differences in the prevalence of antibiotic use could be attributed to different disease burdens, diagnostic capabilities, and availability of a full range of antibiotics in various hospitals and countries.
In this study, ceftriaxone, a Watch group antibiotic was widely prescribed and used across the five hospitals. The high use of ceftriaxone indicates low adherence to the treatment guidelines and a deviation from the WHO AWaRe classification of antibiotics. Ceftriaxone, like most cephalosporins, is frequently prescribed and used inappropriately.111–116 Arguably, Ceftriaxone is overused due to its high clinical usage across many diseases covering a variety of bacteria and its ease of administration.59,111,117–119 Further evidence has shown that the overuse of ceftriaxone could be due to its availability in the supply chain compared to other antibiotics.120 The prescriber preference to prescribe broad-spectrum antibiotics such as ceftriaxone over other antibiotics could also be a leading cause of overuse of this antibiotic in hospitals. A Zambian survey revealed that 36.1% of hospitalised patients were inappropriately prescribed ceftriaxone, lacking culture and sensitivity testing.62 Studies conducted globally have revealed an overuse of ceftriaxone in hospitals including 54% reported in Malawi,121 78.6% in Ethiopia,122 53.3% in Uganda,112 62.4% in Sanandaj, 20.5% in Southeast Asia, and 4.5% in Europe,98 51.1% in Tanzania,123 21.6% in Eswatini,124 and 12.1% in Ghana,20 respectively. The overuse and misuse of broad-spectrum antibiotics such as ceftriaxone are contributing factors to the observed resistance against cephalosporins.55,112,125,126
Our study found that 53% of antibiotics prescribed in level 1 hospitals belonged to the Access group while 38.2% and 8.8% belonged to the Watch and Reserve groups, respectively. The WHO recommends that 60% of antibiotics prescribed for in-patients must belong to the Access group.36,127 The present study found that the prescribing practices in investigated hospitals did not meet the WHO recommendations, indicating more concerted efforts to address this. Even though this is the case, our findings are in keeping with the recent Mapping Antimicrobial Resistance and Antimicrobial Use (MAAP) report released by the African Society of Laboratory Medicine (ASLM) in 2022.93 The report indicated a high use of Access antibiotics compared to the Watch and Reserve categories. Even though our study indicated that the use of Access category drugs was only at 53%, which is lower as compared to the findings in 14 countries as reported in MAAP. This may be attributed to the small number of patients evaluated during a PPS.43 This requires monitoring and improvement. Our study findings are in line with those reported in other countries where the prescribing of Access group antibiotics was less than the recommended 60%. A PPS in Bangladesh found that 64% of Watch group antibiotics were prescribed for in-patients.128 The overuse of Watch group antibiotics has also been reported in other studies62,121,129,130 This practice requires monitoring and probably strengthening of AMS programs to improve prescribing patterns of antibiotics.
In this study, most of the in the inpatients were diagnosed with community-acquired infections (CAIs). The present study found a high use of antibiotics for surgical and medical prophylaxis and for the treatment of CAIs. Additionally, the most diagnoses for antibiotic use were pneumonia, OBGY infections, gastrointestinal tract infections, and respiratory and urinary tract infections. A systematic review reported that community-acquired infections tend to be the major reason for initiating antibiotics among inpatients.94 Our findings are in line with existing evidence that has demonstrated high overuse of antibiotics for the treatment of community-acquired infections, surgical prophylaxis, urinary tract infections and obstetric-associated infections.131,132 For both adults and paediatrics, respiratory illnesses constitute the most common community-acquired infections. The overuse of antibiotics for the treatment of respiratory tract infections including pneumonia was also reported in China.133 Due to the diagnostic challenges to separate viral and bacterial infections, empirical use of antibiotics grossly remains high leading to overuse and misuse of antibiotics.134 The use of antibiotics in surgical care as prophylaxis is routine in most countries.135,136 It is part of the WHO and CDC recommendations for the infection prevention care package.137–139 Furthermore, in obstetrics, most obstetric complications such as puerperal sepsis demand the use of antibiotics. Due to diagnostic challenges, these antibiotics are usually prescribed empirically leading to irrational use and antimicrobial resistance.140
Our results showed that most of the antibiotic use was empirical with a low use of culture and sensitivity results. Additionally, only 0.9% of in-patients received directed treatment in which culture and sensitivity tests were performed indicating a high empirical prescribing of antibiotics in level 1 hospitals of Lusaka, Zambia. In Zambia, the high prescribing of antibiotics empirically could be due to the low diagnostic capacity of laboratories to conduct microbiological to guide treatment as reported previously.67,141 A study in Tanzania also found that less than 1% of prescriptions were informed by culture and sensitivity test results also demonstrating high empirical prescribing of antibiotics.127 Our study findings corroborate with those reported in Central India where most antibiotics were prescribed and used empirically.99 Similarly, the high prevalence of antibiotic prescribing empirically was reported in Iran and was highly due to low diagnostic laboratory capacity.98 A PPS across 10 hospitals in Peru found that 74% of antibiotics were prescribed empirically, with only 4.4% as a targeted treatment.109
In this study, compliance with the Zambia STGs was low at 36% (Chawama 39%, Chilenje 29%, Chipata 36%, Kanyama 32%, and Matero 47%). Non-adherence to treatment guidelines has been found to contribute to the increase in antibiotic-resistant pathogens.74 Our study findings are in line with those reported in Uganda in which adherence to treatment guidelines was 30%.59 Our study findings also corroborate those found in Ghana where a 25% adherence to treatment guidelines was reported.20 A study in Germany found a 33% adherence to treatment guidelines for patients who were being treated for bloodstream and urinary tract infections, indicating a low adherence to the guidelines.142 Once more, the low adherence to treatment guidelines may be attributed to several factors such as patient preference, availability of alternative antibiotics, weak regulations, and limited diagnostic capacity which usually lead to empirical use of antibiotics. These findings have been reported in various studies done across the globe.17,143–146 Non-adherence to standard treatment guidelines can lead to the irrational use of antibiotics and eventually the development of drug resistance.
This study revealed that most antibiotics prescribed were for the paediatric age group. The high use of antibiotics in paediatrics has been reported to be a leading cause of adverse effects and AMR.147 The use of antibiotics in pediatric patients is a common practice due to the pattern of their illnesses. Respiratory tract infections form most of the common illnesses in younger populations and are usually difficult to differentiate between viral and bacterial.134,148,149 Hence, antibiotics are mostly prescribed empirically. This may have a catastrophic impact on the future of antimicrobial medicines and their efficacy. The high use of antibiotics in hospitals requires careful monitoring and innovative instigation of AMS programs to optimize the prescribing practices by adhering to recommended guidelines, especially in the sub-Saharan African region.94 Establishing and implementing effective AMS programs while providing on-site orientation and mentorship to multidisciplinary teams of healthcare workers in hospitals is critical in promoting the rational use of antibiotics.150,151 Implementing AMS programs has been found to improve the prescribing of antibiotics for hospitalized patients in hospitals.84,152–156
Our study highlights the prescribing patterns and use of antibiotics in level 1 hospitals in Lusaka which can provide information for conducting internal quality improvements. Our findings can also be used to provide interventions to improve antibiotic use in the surveyed hospitals. Therefore, going forward, we recommend the establishment and implementation of AMS programs in level 1 hospitals, similar to previous recommendations and guidance.79,150,157,158 This should start with establishing multidisciplinary AMS committees and focal point persons with clear terms of references. AMS programs are critical in optimizing antibiotic use through the education of healthcare workers, patients, communities, and students.159–161 Additionally, we propose the strengthening of laboratory capacity to conduct microbiological tests to promote diagnostic stewardship.162–169 This is built on previous studies that we conducted in Zambia that found a low capacity of laboratories to conduct AMR surveillance.67,93,141
We are aware that our study had limitations. This study only covered level 1 hospitals in the Lusaka District of Zambia and provided some useful insights into antibiotic prescription and use at that level. Hence, the findings cannot be generalized to hospital hospitals offering other levels of care like secondary and tertiary levels. Thus, future studies are recommended for secondary and tertiary healthcare facilities. Future studies should also consider the inclusion of private and faith-based hospitals. Finally, this study was not designed to investigate any effect that the factor “province” may have on antibiotic prescription and use behaviour; it is arguable that provincial-level latent determinants such as provincial health leadership, staff adherence to relevant clinical protocols, and other health system factors, may be at play.
Conclusions
The prevalence of antibiotic use in level 1 hospitals in Zambia was higher than recommended by the WHO for hospitalized patients. Additionally, this study found a high use of ceftriaxone, a Watch group antibiotic with low adherence to the treatment guidelines across all level 1 hospitals. Additionally, this study found a high prevalence of antibiotic prescribing for hospitalized patients usually without laboratory culture and sensitivity results. Therefore, these findings demonstrate the need for establishing and implementing antimicrobial stewardship programs to promote the rational use of antibiotics and reduce AMR. The study also indicates a need to promote and strengthen laboratory capacity to conduct clinical microbiological tests to guide antibiotic use and improve patient outcomes.
Acknowledgments
We are grateful to the hospital management and staff for granting us permission and support to collect data from their hospitals. We acknowledge the University of Zambia for the logical support provided to the data collectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Salam MA, Al-Amin MY, Salam MT. et al. Antimicrobial Resistance: a Growing Serious Threat for Global Public Health. Healthcare. 2023;11(13):1946. doi:10.3390/healthcare11131946
2. Aljeldah MM. Antimicrobial Resistance and Its Spread Is a Global Threat. Antibiotics. 2022;11(8):1082. doi:10.3390/antibiotics11081082
3. Murray CJ, Ikuta KS, Sharara F, et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629–655. doi:10.1016/S0140-6736(21)02724-0
4. Sartorius B, Gray AP, Davis Weaver N, et al. The burden of bacterial antimicrobial resistance in the WHO African region in 2019: a cross-country systematic analysis. Lancet Glob Heal. 2024;12(2):e201–e216. doi:10.1016/s2214-109x(23)00539-9
5. Jain A, Mahajan M, Upaganlawar A, Upasani C. Impact of Antimicrobial Resistance in Health and Economic Outcomes: a Review. Adv Pharmacol Clin Trials. 2024;9(2):000234. doi:10.23880/apct-16000234
6. Dadgostar P. Antimicrobial resistance: implications and costs. Infect Drug Resist. 2019;12:3903–3910. doi:10.2147/IDR.S234610
7. Baran A, Kwiatkowska A, Potocki L. Antibiotics and Bacterial Resistance—A Short Story of an Endless Arms Race. Int J mol Sci. 2023;24(6):5777. doi:10.3390/ijms24065777
8. Belay WY, Getachew M, Tegegne BA, et al. Mechanism of antibacterial resistance, strategies and next-generation antimicrobials to contain antimicrobial resistance: a review. Front Pharmacol. 2024;15:1444781. doi:10.3389/fphar.2024.1444781
9. Sulis G, Sayood S, Katukoori S, et al. Exposure to World Health Organization’s AWaRe antibiotics and isolation of multi-drug resistant bacteria: a systematic review and meta-analysis. Clin Microbiol Infect. 2022:
10. Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health. 2015;109(7):309–318. doi:10.1179/2047773215Y.0000000030
11. Byrne MK, Miellet S, McGlinn A, et al. The drivers of antibiotic use and misuse: the development and investigation of a theory driven community measure. BMC Public Health. 2019;19(1):1425. doi:10.1186/s12889-019-7796-8
12. Irfan M, Almotiri A, AlZeyadi ZA. Antimicrobial Resistance and Its Drivers—A Review. Antibiotics. 2022;11(10):1362. doi:10.3390/antibiotics11101362
13. Mudenda S, Chabalenge B, Daka V, et al. Global Strategies to Combat Antimicrobial Resistance: a One Health Perspective. Pharmacol Pharm. 2023;14(8):271–328. doi:10.4236/PP.2023.148020
14. Godman B, Egwuenu A, Haque M, et al. Strategies to improve antimicrobial utilization with a special focus on developing countries. Life. 2021;11(6):528. doi:10.3390/life11060528
15. Gautam A. Antimicrobial Resistance: the Next Probable Pandemic. J Nepal Med Assoc. 2022;60(246):225–228. doi:10.31729/jnma.7174
16. Sartelli M, Barie PS, Coccolini F, et al. Ten golden rules for optimal antibiotic use in hospital settings: the WARNING call to action. World J Emerg Surg. 2023;18(1):50. doi:10.1186/S13017-023-00518-3
17. Global Alliance for Infections in Surgery. Global Infection Prevention and Management in Healthcare: antimicrobial Resistance and One Health.; 2024.
18. Oliveira M, Antunes W, Mota S, Madureira-Carvalho Á, Dinis-Oliveira RJ, Dias da Silva D. An Overview of the Recent Advances in Antimicrobial Resistance. Microorganisms. 2024;12(9):1920. doi:10.3390/microorganisms12091920
19. Gunasekara Y, Kottawatta S, Nisansala T, Silva-Fletcher A, Kalupahana R. Tackling Antimicrobial Resistance Needs One Health Approach. In: One Health: Human, Animal, and Environment Triad. John Wiley & Sons, Ltd; 2023:309–323. doi:10.1002/9781119867333.ch22
20. Agyare E, Acolatse JEE, Dakorah MP, et al. Antimicrobial stewardship capacity and antibiotic utilisation practices in the Cape Coast Teaching Hospital, Ghana: a point prevalence survey study. PLoS One. 2024;19(1):e0297626. doi:10.1371/JOURNAL.PONE.0297626
21. World Health Organization. Monitoring and Evaluation of the Global Action Plan on Antimicrobial Resistance.; 2019.
22. World Health Organization. Global action plan on antimicrobial resistance. 2015.
23. Willemsen A, Reid S, Assefa Y. A review of national action plans on antimicrobial resistance: strengths and weaknesses. Antimicrob Resist Infect Control. 2022;11(1):90. doi:10.1186/s13756-022-01130-x
24. Charani E, Mendelson M, Pallett SJC, et al. An analysis of existing national action plans for antimicrobial resistance—gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob Heal. 2023;11(3):e466–e474. doi:10.1016/S2214-109X(23)00019-0
25. Tornimbene B, Eremin S, Abednego R, et al. Global Antimicrobial Resistance and Use Surveillance System on the African continent: early implementation 2017–2019. Afr J Lab Med. 2022;11(1):1594. doi:10.4102/ajlm.v11i1.1594
26. Nabadda S, Kakooza F, Kiggundu R, et al. Implementation of the World Health Organization Global Antimicrobial Resistance Surveillance System in Uganda, 2015–2020: mixed-Methods Study Using National Surveillance Data. JMIR Public Heal Surveill. 2021;7(10):e29954. doi:10.2196/29954
27. Sharland M, Gandra S, Huttner B, et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—the new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect Dis. 2019;19(12):1278–1280. doi:10.1016/S1473-3099(19)30532-8
28. Sharland M, Pulcini C, Harbarth S, et al. Classifying antibiotics in the WHO Essential Medicines List for optimal use—be AWaRe. Lancet Infect Dis. 2018;18(1):18–20. doi:10.1016/S1473-3099(17)30724-7
29. Klein EY, Milkowska-Shibata M, Tseng KK, et al. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–15: an analysis of pharmaceutical sales data. Lancet Infect Dis. 2021;21(1):107–115. doi:10.1016/S1473-3099(20)30332-7
30. Zanichelli V, Sharland M, Cappello B, et al. The WHO AWaRe (Access, Watch, Reserve) antibiotic book and prevention of antimicrobial resistance. Bull World Health Organ. 2023;101(4):290–296. doi:10.2471/BLT.22.288614
31. Sharland M, Zanichelli V, Ombajo LA, et al. The WHO essential medicines list AWaRe book: from a list to a quality improvement system. Clin Microbiol Infect. 2022;28(12):1533–1535. doi:10.1016/j.cmi.2022.08.009
32. Hsia Y, Lee BR, Versporten A, et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): an analysis of paediatric survey data from 56 countries. Lancet Glob Heal. 2019;7(7):e861–e871. doi:10.1016/S2214-109X(19)30071-3
33. Mudenda S, Wataya MD, Mufwambi W, Chizimu JY. The World Health Organization Access, Watch, and Reserve classification of antibiotics: an awareness survey among pharmacy professionals in a sub-Saharan country, Zambia. Antimicrob Steward Healthc Epidemiol. 2024;4(1):e212. doi:10.1017/ASH.2024.403
34. Bou-Antoun S, Oettle RC, Leanord A, et al. Adaptation of the WHO AWaRe (Access, Watch, Reserve) antibiotic classification to support national antimicrobial stewardship priorities in the UK: findings from a modified Delphi approach to achieve expert consensus. JAC-Antimicrobial Resist. 2025;7(1):dlae218. doi:10.1093/JACAMR/DLAE218
35. Abdelsalam Elshenawy R, Umaru N, Aslanpour Z. WHO AWaRe classification for antibiotic stewardship: tackling antimicrobial resistance – a descriptive study from an English NHS Foundation Trust prior to and during the COVID-19 pandemic. Front Microbiol. 2023;14:1298858. doi:10.3389/fmicb.2023.1298858
36. Mudenda S, Daka V, Matafwali SK. World Health Organization AWaRe framework for antibiotic stewardship: where are we now and where do we need to go? An expert viewpoint. Antimicrob Steward Healthc Epidemiol. 2023;3(1):e84. doi:10.1017/ASH.2023.164
37. United Nations Environment Programme. World leaders commit to decisive action on antimicrobial resistance. 2024.
38. Moja L, Zanichelli V, Mertz D, et al. WHO’s essential medicines and AWaRe: recommendations on first- and second-choice antibiotics for empiric treatment of clinical infections. Clin Microbiol Infect. 2024;30(Suppl 2):S1–S51. doi:10.1016/j.cmi.2024.02.003
39. Versporten A, Zarb P, Caniaux I, et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: results of an internet-based global point prevalence survey. Lancet Glob Heal. 2018;6(6):e619–e629. doi:10.1016/S2214-109X(18)30186-4
40. da Silva RMR, de Mendonça SCB, Leão IN, et al. Use of monitoring indicators in hospital management of antimicrobials. BMC Infect Dis. 2021;21(1):827. doi:10.1186/s12879-021-06542-5
41. Saleem Z, Godman B, Cook A, et al. Ongoing Efforts to Improve Antimicrobial Utilization in Hospitals among African Countries and Implications for the Future. Antibiotics. 2022;11(12):1824. doi:10.3390/antibiotics11121824
42. Chigome A, Ramdas N, Skosana P, et al. A Narrative Review of Antibiotic Prescribing Practices in Primary Care Settings in South Africa and Potential Ways Forward to Reduce Antimicrobial Resistance. Antibiotics. 2023;12(10):1540. doi:10.3390/antibiotics12101540
43. Lubanga AF, Bwanali AN, Kambiri F, et al. Tackling antimicrobial resistance in Sub-Saharan Africa: challenges and opportunities for implementing the new people-centered WHO guidelines. Expert Rev Anti Infect Ther. 2024;22(6):379–386. doi:10.1080/14787210.2024.2362270
44. Waswa JP, Kiggundu R, Konduri N, Kasujja H, Lawry LL, Joshi MP. What is the appropriate antimicrobial use surveillance tool at the health facility level for Uganda and other low- and middle-income countries? J Glob Antimicrob Resist. 2023;34:145–149. doi:10.1016/j.jgar.2023.07.003
45. Godman B, Egwuenu A, Wesangula E, et al. Tackling antimicrobial resistance across sub-Saharan Africa: current challenges and implications for the future. Expert Opin Drug Saf. 2022;21(8):1089–1111. doi:10.1080/14740338.2022.2106368
46. Iwu CD, Patrick SM. An insight into the implementation of the global action plan on antimicrobial resistance in the WHO African region: a roadmap for action. Int J Antimicrob Agents. 2021;58(4):106411. doi:10.1016/j.ijantimicag.2021.106411
47. Ohemu GP. Starved of ACTION: a Critical Look at the Antimicrobial Resistance Action Plans of African Countries. ACS Infect Dis. 2022;8(8):1377–1380. doi:10.1021/acsinfecdis.2c00303
48. Saleem Z, Godman B, Hassali MA, Hashmi FK, Azhar F, Rehman IU. Point prevalence surveys of health-care-associated infections: a systematic review. Pathog Glob Health. 2019;113(4):191–205. doi:10.1080/20477724.2019.1632070
49. Anand Paramadhas BD, Tiroyakgosi C, Mpinda-Joseph P, et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev Anti Infect Ther. 2019;17(7):535–546. doi:10.1080/14787210.2019.1629288
50. Haseeb A, Saleem Z, Maqadmi AF, et al. Ongoing Strategies to Improve Antimicrobial Utilization in Hospitals across the Middle East and North Africa (MENA): findings and Implications. Antibiotics. 2023;12(5):827. doi:10.3390/antibiotics12050827
51. Pauwels I, Versporten A, Drapier N, Vlieghe E, Goossens H. Hospital antibiotic prescribing patterns in adult patients according to the WHO Access, Watch and Reserve classification (AWaRe): results from a worldwide point prevalence survey in 69 countries. J Antimicrob Chemother. 2021;76(6):1614–1624. doi:10.1093/jac/dkab050
52. World Health Organization. WHO Methodology for Point Prevalence Survey on Antibiotic Use in Hospitals. 2018.
53. D’Arcy N, Ashiru-Oredope D, Olaoye O, et al. Antibiotic prescribing patterns in Ghana, Uganda, Zambia and Tanzania hospitals: results from the global point prevalence survey (G-PPS) on antimicrobial use and stewardship interventions implemented. Antibiotics. 2021;10(9):1122. doi:10.3390/antibiotics10091122
54. Al-Taani GM, Scott M, Farren D, et al. Longitudinal point prevalence survey of antibacterial use in Northern Ireland using the European Surveillance of Antimicrobial Consumption (ESAC) PPS and Global-PPS tool. Epidemiol Infect. 2018;146(8):985–990. doi:10.1017/S095026881800095X
55. Yehouenou CL, Kpangon AA, Affolabi D, et al. Antimicrobial resistance in hospitalized surgical patients: a silently emerging public health concern in Benin. Ann Clin Microbiol Antimicrob. 2020;19(1):54. doi:10.1155/2024/6698387
56. Mustafa ZU, Saleem MS, Ikram MN, et al. Co-infections and antimicrobial use among hospitalized COVID-19 patients in Punjab, Pakistan: findings from a multicenter, point prevalence survey. Pathog Glob Health. 2022;116(7):421–427. doi:10.1080/20477724.2021.1999716
57. Campbell SM, Meyer JC, Godman B. Why compliance to national prescribing guidelines is important especially across sub-Saharan Africa and suggestions for the future. J Biomed Pharm Sci. 2021;4(6):316.
58. Sefah IA, Essah DO, Kurdi A, et al. Assessment of adherence to pneumonia guidelines and its determinants in an ambulatory care clinic in Ghana: findings and implications for the future. JAC-Antimicrobial Resist. 2021;3(2):dlab080. doi:10.1093/jacamr/dlab080
59. Kiggundu R, Wittenauer R, Waswa JP, et al. Point Prevalence Survey of Antibiotic Use across 13 hospitals in Uganda. Antibiotics. 2022;11(2):199. doi:10.3390/antibiotics11020199
60. Seni J, Mapunjo SG, Wittenauer R, et al. Antimicrobial use across six referral hospitals in Tanzania: a point prevalence survey. BMJ Open. 2020;10(12):e042819. doi:10.1136/bmjopen-2020-042819
61. Akintan P, Oshun P, Osuagwu C, et al. Point Prevalence Surveys of Antibiotic Prescribing in Children at a Tertiary Hospital in a resource constraint, low-income sub-Saharan African country. BMC Pediatr. 2024;24(1):383. doi:10.1186/S12887-024-04847-3
62. Kalungia AC, Mukosha M, Mwila C, et al. Antibiotic Use and Stewardship Indicators in the First- and Second-Level Hospitals in Zambia: findings and Implications for the Future. Antibiotics. 2022;11(11):1626. doi:10.3390/ANTIBIOTICS11111626
63. Masich AM, Vega AD, Callahan P, et al. Antimicrobial usage at a large teaching hospital in Lusaka, Zambia. PLoS One. 2020;15(2):e0228555. doi:10.1371/journal.pone.0228555
64. Mudenda S, Nsofu E, Chisha P, et al. Prescribing Patterns of Antibiotics According to the WHO AWaRe Classification during the COVID-19 Pandemic at a Teaching Hospital in Lusaka, Zambia: implications for Strengthening of Antimicrobial Stewardship Programmes. Pharmacoepidemiology. 2023;2(1):42–53. doi:10.3390/PHARMA2010005
65. Mudenda S, Chomba M, Chabalenge B, et al. Antibiotic Prescribing Patterns in Adult Patients According to the WHO AWaRe Classification: a Multi-Facility Cross-Sectional Study in Primary Healthcare Hospitals in Lusaka, Zambia. Pharmacol Pharm. 2022;13(10):379–392. doi:10.4236/PP.2022.1310029
66. Kalonga J, Hangoma J, Banda M, Munkombwe D, Mudenda S. Antibiotic Prescribing Patterns in Paediatric Patients at Levy Mwanawasa University Teaching Hospital in Lusaka, Zambia. Int J Pharm Pharmacol. 2020;4(1):1–9. doi:10.31531/2581-3080.1000138
67. Shempela DM, Mudenda S, Kasanga M, et al. A Situation Analysis of the Capacity of Laboratories in Faith-Based Hospitals in Zambia to Conduct Surveillance of Antimicrobial Resistance: opportunities to Improve Diagnostic Stewardship. Microorganisms. 2024;12(8):1697. doi:10.3390/MICROORGANISMS12081697
68. Afriyie DK, Sefah IA, Sneddon J, et al. Antimicrobial point prevalence surveys in two Ghanaian hospitals: opportunities for antimicrobial stewardship. JAC-Antimicrobial Resist. 2020;2(1):dlaa001. doi:10.1093/jacamr/dlaa001
69. Sefah IA, Nyamadi D, Kurdi A, et al. Assessment of the quality of antimicrobial prescribing among hospitalized patients in a teaching hospital in Ghana: findings and implications. Hosp Pract. 2023;51(4):223–232. doi:10.1080/21548331.2023.2241344
70. Mustafa ZU, Salman M, Yasir M, et al. Antibiotic consumption among hospitalized neonates and children in Punjab province, Pakistan. Expert Rev Anti Infect Ther. 2022;20(6):931–939. doi:10.1080/14787210.2021.1986388
71. Nowbuth AA, Asombang AW, Tazinkeng NN, Makinde OY, Sheets LR. Antimicrobial resistance from a One Health perspective in Zambia: a systematic review. Antimicrob Resist Infect Control. 2023;12(1):15. doi:10.1186/s13756-023-01224-0
72. Kasanga M, Mukosha R, Kasanga M, et al. Antimicrobial resistance patterns of bacterial pathogens their distribution in university teaching hospitals in Zambia. Future Microbiol. 2020;16(11):811–824. doi:10.2217/fmb-2021-0104
73. Kasanga M, Kwenda G, Wu J, et al. Antimicrobial Resistance Patterns and Risk Factors Associated with ESBL-Producing and MDR Escherichia coli in Hospital and Environmental Settings in Lusaka, Zambia: implications for One Health, Antimicrobial Stewardship and Surveillance Systems. Microorganisms. 2023;11(8):1951. doi:10.3390/MICROORGANISMS11081951
74. Yamba K, Mudenda S, Mpabalwani E, et al. Antibiotic prescribing patterns and carriage of antibiotic-resistant Escherichia coli and Enterococcus species in healthy individuals from selected communities in Lusaka and Ndola districts, Zambia. JAC-Antimicrobial Resist. 2024;6(2):dlae027. doi:10.1093/JACAMR/DLAE027
75. ZNPHI. Multi-Sectoral National Action Plan on Antimicrobial Resistance.; 2017.
76. Mudenda W, Chikatula E, Chambula E, et al. Prescribing Patterns and Medicine Use at the University Teaching Hospital, Lusaka, Zambia. Med J Zambia. 2016;43(2):94–102.
77. Mudenda S, Chilimboyi R, Matafwali SK, et al. Hospital prescribing patterns of antibiotics in Zambia using the WHO prescribing indicators post-COVID-19 pandemic: findings and implications. JAC-Antimicrobial Resist. 2024;6(1):dlae023. doi:10.1093/JACAMR/DLAE023
78. Cox JA, Vlieghe E, Mendelson M, et al. Antibiotic stewardship in low- and middle-income countries: the same but different? Clin Microbiol Infect. 2017;23(11):812–818. doi:10.1016/j.cmi.2017.07.010
79. Kalungia AC, Mwambula H, Munkombwe D, et al. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: implications for future policy and practice. J Chemother. 2019;31(7–8):378–387. doi:10.1080/1120009X.2019.1622293
80. Nathwani D, Varghese D, Stephens J, Ansari W, Martin S, Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: a systematic review. Antimicrob Resist Infect Control. 2019;8(1):35. doi:10.1186/s13756-019-0471-0
81. Ashiru-Oredope D, Nabiryo M, Zengeni L, et al. Tackling antimicrobial resistance: developing and implementing antimicrobial stewardship interventions in four African commonwealth countries through a health partnership model. J Public Health Africa. 2023;14(6):2335. doi:10.4081/jphia.2023.2335
82. Otieno PA, Campbell S, Maley S, Obinju Arunga T, Otieno Okumu M. A Systematic Review of Pharmacist-Led Antimicrobial Stewardship Programs in Sub-Saharan Africa. Int J Clin Pract. 2022;2022:3639943. doi:10.1155/2022/3639943
83. Akpan MR, Isemin NU, Udoh AE, Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: a systematic literature review. J Glob Antimicrob Resist. 2020;22:317–324. doi:10.1016/j.jgar.2020.03.009
84. Siachalinga L, Mufwambi W, Lee IH. Impact of antimicrobial stewardship interventions to improve antibiotic prescribing for hospital inpatients in Africa: a systematic review and meta-analysis. J Hosp Infect. 2022;129:124–143. doi:10.1016/j.jhin.2022.07.031
85. REDCap. Vanderbilt. Software – rEDCap - Res Electron Data Capture. 2017.
86. Levy Hara G, Rojas-Cortes R, Molina León HF, et al. Point prevalence survey of antibiotic use in hospitals in Latin American countries. J Antimicrob Chemother. 2022;77(3):807–815. doi:10.1093/jac/dkab459
87. Republic of Zambia Ministry of Health. Zambia Standard Treatment Guidelines. 2020.
88. World Health Organization. Anatomical Therapeutic Chemical (ATC) Classification. 2021.
89. Dlamini NN, Meyer JC, Kruger D, Kurdi A, Godman B, Schellack N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: a pilot study and implications. Hosp Pract. 2019;47(2):88–95. doi:10.1080/21548331.2019.1592880
90. Skosana PP, Schellack N, Godman B, et al. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev Anti Infect Ther. 2021;19(10):1353–1366. doi:10.1080/14787210.2021.1898946
91. Saleem Z, Hassali MA, Versporten A, et al. A multicenter point prevalence survey of antibiotic use in Punjab, Pakistan: findings and implications. Expert Rev Anti Infect Ther. 2019;17(4):285–293. doi:10.1080/14787210.2019.1581063
92. Mudenda S, Mufwambi W, Mohamed S. The Burden of Antimicrobial Resistance in Zambia, a Sub-Saharan African Country: a One Health Review of the Current Situation, Risk Factors, and Solutions. Pharmacol Pharm. 2024;15(12):403–465. doi:10.4236/PP.2024.1512024
93. ASLM. MAAP Report Zambia 2016–2018. 2022.
94. Boltena MT, Wolde M, Hailu B, et al. Point prevalence of evidence-based antimicrobial use among hospitalized patients in sub-Saharan Africa: a systematic review and meta-analysis. Sci Rep. 2024;14(1):12652. doi:10.1038/s41598-024-62651-6
95. Siachalinga L, Godman B, Mwita JC, et al. Current Antibiotic Use Among Hospitals in the sub-Saharan Africa Region; Findings and Implications. Infect Drug Resist. 2023;16:2179–2190. doi:10.2147/IDR.S398223
96. Kamara IF, Kanu J, Maruta A, et al. Antibiotic use among hospitalised patients in Sierra Leone: a national point prevalence survey using the WHO survey methodology. BMJ Open. 2023;13(12):e078367. doi:10.1136/bmjopen-2023-078367
97. Ambreen S, Safdar N, Ikram A, et al. Point Prevalence Survey of Antimicrobial Use in Selected Tertiary Care Hospitals of Pakistan Using WHO Methodology: results and Inferences. Medicina (B Aires). 2023;59(6):1102. doi:10.3390/medicina59061102
98. Soltani J, Behzadi S, Pauwels I, Goossens H, Versporten A. Global-PPS targets for antimicrobial stewardship in paediatric patients at hospitals in Sanandaj, Western Iran, compared with Southeast Asian and European hospitals. J Glob Antimicrob Resist. 2024;36:473–481. doi:10.1016/j.jgar.2024.01.011
99. Kumar S, Shukla P, Goel P, et al. Point Prevalence Study (PPS) of Antibiotic Usage and Bacterial Culture Rate (BCR) among Secondary Care Hospitals of Small Cities in Central India: consolidating Indian Evidence. J Lab Physicians. 2023;15(2):259–263. doi:10.1055/s-0042-1757585
100. Alosaimi HM, Alshammari MK, Fetyani MM, et al. Point prevalence survey of antibiotics use among hospitalised neonates and children in Saudi Arabia: findings and implications. J Pharm Policy Pract. 2024;17(1):2371411. doi:10.1080/20523211.2024.2371411
101. Jing F-H, Wang Q, He T-J, et al. Three-Year Point Prevalence Survey of Antimicrobial Use in a Chinese University Hospital. Can J Infect Dis Med Microbiol. 2024;2024:6698387. doi:10.1155/2024/6698387
102. Gkentzi D, Kortsalioudaki C, Cailes BC, et al. Epidemiology of infections and antimicrobial use in Greek Neonatal Units. Arch Dis Child Fetal Neonatal Ed. 2019;104(3):F293–F297. doi:10.1136/archdischild-2018-315024
103. Osowicki J, Gwee A, Noronha J, et al. Australia-wide point prevalence survey of antimicrobial prescribing in neonatal units: how much and how good? Pediatr Infect Dis J. 2015;34(8):e185–e190. doi:10.1097/INF.0000000000000719
104. Omulo S, Oluka M, Achieng L, et al. Point-prevalence survey of antibiotic use at three public referral hospitals in Kenya. PLoS One. 2022;17(6):e0270048. doi:10.1371/journal.pone.0270048
105. Skosana PP, Schellack N, Godman B, et al. A national, multicentre, web-based point prevalence survey of antimicrobial use and quality indices among hospitalised paediatric patients across South Africa. J Glob Antimicrob Resist. 2022;29:542–550. doi:10.1016/j.jgar.2021.12.003
106. Nwafia I, Nwachukwu P, Orakwe O, et al. Point Prevalence Survey of Antimicrobial Prescription and Consumption in a Nigerian Tertiary Hospital: a Gateway to the Antimicrobial Stewardship Program. Niger J Clin Pract. 2024;27(6):702–707. doi:10.4103/NJCP.NJCP_449_23
107. Anugulruengkitt S, Charoenpong L, Kulthanmanusorn A, et al. Point prevalence survey of antibiotic use among hospitalized patients across 41 hospitals in Thailand. JAC-Antimicrobial Resist. 2022;5(1):dlac140. doi:10.1093/jacamr/dlac140
108. Tapha O, Degbey CC, Yacouba A, et al. Antimicrobial use in hospitalized patients: a point prevalence survey across four tertiary hospitals in Niger. JAC-Antimicrobial Resist. 2024;6(5):dlae175. doi:10.1093/JACAMR/DLAE175
109. Rondon C, Garcia C, Krapp F, et al. Antibiotic point prevalence survey and antimicrobial resistance in hospitalized patients across Peruvian reference hospitals. J Infect Public Health. 2023;16:52–60. doi:10.1016/j.jiph.2023.10.030
110. German GJ, Frenette C, Caissy J-A, et al. The 2018 Global Point Prevalence Survey of antimicrobial consumption and resistance in 47 Canadian hospitals: a cross-sectional survey. CMAJ Open. 2021;9(4):E1242–E1251. doi:10.9778/cmajo.20200274
111. Tafere C, Endeshaw D, Demsie DG, et al. Inappropriate ceftriaxone utilization and predictor factors in Ethiopia: a systematic review and meta-analysis. Sci Rep. 2024;14(1):25035. doi:10.1038/s41598-024-75728-z
112. Kutyabami P, Munanura EI, Kalidi R, et al. Evaluation of the clinical use of ceftriaxone among in-patients in selected health facilities in Uganda. Antibiotics. 2021;10(7):779. doi:10.3390/antibiotics10070779
113. Ayele AA, Gebresillassie BM, Erku DA, et al. Prospective evaluation of Ceftriaxone use in medical and emergency wards of Gondar university referral hospital, Ethiopia. Pharmacol Res Perspect. 2018;6(1):e00383. doi:10.1002/prp2.383
114. Afriyie DK, Amponsah SK, Dogbey J, et al. A pilot study evaluating the prescribing of ceftriaxone in hospitals in Ghana: findings and implications. Hosp Pract. 2017;45(4):143–149. doi:10.1080/21548331.2017.1348139
115. Amare F, Gashaw T, Sisay M, Baye Y, Tesfa T. The appropriateness of ceftriaxone utilization in government hospitals of Eastern Ethiopia: a retrospective evaluation of clinical practice. SAGE Open Medicine. 2021;9:20503121211051524. doi:10.1177/20503121211051525
116. Sileshi A, Tenna A, Feyissa M, Shibeshi W. Evaluation of ceftriaxone utilization in medical and emergency wards of Tikur Anbessa specialized hospital: a prospective cross-sectional study. BMC Pharmacol Toxicol. 2016;17(1):7. doi:10.1186/s40360-016-0057-x
117. Kizito M, Lalitha R, Kajumbula H, Ssenyonga R, Muyanja D, Byakika-Kibwika P. Antibiotic prevalence study and factors influencing prescription of who watch category antibiotic ceftriaxone in a tertiary care private not for profit hospital in Uganda. Antibiotics. 2021;10(10):1167. doi:10.3390/antibiotics10101167
118. Hurst AL, Olson D, Somme S, et al. Once-Daily Ceftriaxone Plus Metronidazole Versus Ertapenem and/or Cefoxitin for Pediatric Appendicitis. J Pediatric Infect Dis Soc. 2017;6(1):57–64. doi:10.1093/jpids/piv082
119. Lin H-A, Yang Y-S, Wang J-X, et al. Comparison of the effectiveness and antibiotic cost among ceftriaxone, ertapenem, and levofloxacin in treatment of community-acquired complicated urinary tract infections. J Microbiol Immunol Infect. 2016;49(2):237–242. doi:10.1016/j.jmii.2014.12.010
120. Tadesse TY, Molla M, Yimer YS, Tarekegn BS, Kefale B. Evaluation of antibiotic prescribing patterns among inpatients using World Health Organization indicators: a cross-sectional study. SAGE Open Medicine. 2022;10:205031212210966. doi:10.1177/20503121221096608
121. Mithi B, Luhanga M, Kaminyoghe F, Chiumia F, Banda DL, Nyama L. Antibiotic use and resistance patterns at Rumphi District Hospital in Malawi: a cross-sectional study. BMC Infect Dis. 2024;24(1):445. doi:10.1186/s12879-024-09333-w
122. Muhammed OS, Nasir BB. Drug Use Evaluation of Ceftriaxone in Ras-Desta Memorial General Hospital, Ethiopia. Drug Healthc Patient Saf. 2020;12:161–168. doi:10.2147/DHPS.S260364
123. Sonda TB, Horumpende PG, Kumburu HH, et al. Ceftriaxone use in a tertiary care hospital in Kilimanjaro, Tanzania: a need for a hospital antibiotic stewardship programme. PLoS One. 2019;14(8):e0220261. doi:10.1371/journal.pone.0220261
124. Gwebu PC, Meyer JC, Schellack N, Matsebula-Myeni ZC, Godman B. A web-based point prevalence survey of antimicrobial use and quality indicators at Raleigh Fitkin Memorial Hospital in the Kingdom of Eswatini and the implications. Hosp Pract. 2022;50(3):214–221. doi:10.1080/21548331.2022.2069247
125. Gelaw LY, Bitew AA, Gashey EM, Ademe MN. Ceftriaxone resistance among patients at GAMBY teaching general hospital. Sci Rep. 2022;12(1):12000. doi:10.1038/s41598-022-16132-3
126. Van Besien RF, Hampton N, Micek ST, Kollef MH, Saranathan M. Ceftriaxone resistance and adequacy of initial antibiotic therapy in community onset bacterial pneumonia. Med. 2022;101(20):e29159. doi:10.1097/MD.0000000000029159
127. Nsojo A, George L, Mwasomola D, et al. Prescribing patterns of antimicrobials according to the WHO AWaRe classification at a tertiary referral hospital in the southern highlands of Tanzania. Infect Prev Pract. 2024:100347. doi:10.1016/J.INFPIP.2024.100347.
128. Rashid MM, Akhtar Z, Chowdhury S, et al. Pattern of Antibiotic Use among Hospitalized Patients according to WHO Access, Watch, Reserve (AWaRe) Classification: findings from a Point Prevalence Survey in Bangladesh. Antibiotics. 2022;11(6):810. doi:10.3390/antibiotics11060810
129. Chizimu JY, Mudenda S, Yamba K, et al. Antibiotic use and adherence to the WHO AWaRe guidelines across 16 hospitals in Zambia: a point prevalence survey. JAC-Antimicrobial Resist. 2024;6(5):dlae170. doi:10.1093/JACAMR/DLAE170
130. Negi G, Arjun K, Panda PK. Ground level utility of Access, Watch, Reserve classification: insights from a tertiary care center in North India. World J Exp Med. 2023;13(5):123–133. doi:10.5493/wjem.v13.i5.123
131. Kim YC, Park JY, Kim B, et al. Prescriptions patterns and appropriateness of usage of antibiotics in non-teaching community hospitals in South Korea: a multicentre retrospective study. Antimicrob Resist Infect Control. 2022;11(1):40. doi:10.1186/s13756-022-01082-2
132. Kotwani A, Kumar S, Swain PK, Suri JC, Gaur SN. Antimicrobial drug prescribing patterns for community-acquired pneumonia in hospitalized patients: a retrospective pilot study from New Delhi, India. Indian J Pharmacol. 2015;47(4):375–382. doi:10.4103/0253-7613.161258
133. Zhang J, Lin L, Lu G, et al. Patterns of antibiotic administration in Chinese neonates: results from a multi-center, point prevalence survey. BMC Infect Dis. 2024;24(1):186. doi:10.1186/s12879-024-09077-7
134. Lubanga AF, Khuluza C, Muhyuddin J, et al. A retrospective review of the common childhood illnesses and the indications for antibiotic prescription at community hospital in Malawi. Front Antibiot. 2024;3:1447435. doi:10.3389/frabi.2024.1447435
135. Sartelli M, Coccolini F, Labricciosa FM, et al. Surgical Antibiotic Prophylaxis: a Proposal for a Global Evidence-Based Bundle. Antibiotics. 2024;13(1):100. doi:10.3390/ANTIBIOTICS13010100
136. Schrama TJ, Vliegenthart-Jongbloed KJ, Gemuwang M, Nuwass EQ. Surgical prophylaxis in Haydom Lutheran Hospital, Tanzania – learning from a point prevalence survey. Infect Prev Pract. 2025;100429. doi:10.1016/J.INFPIP.2024.100429
137. Allegranzi B, Zayed B, Bischoff P, et al. New WHO recommendations on intraoperative and postoperative measures for surgical site infection prevention: an evidence-based global perspective. Lancet Infect Dis. 2016;16(12):e288–e303. doi:10.1016/S1473-3099(16)30402-9
138. Berriós-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017;152(8):784–791. doi:10.1001/JAMASURG.2017.0904
139. World Health Organization. Global Guidelines for the Prevention of Surgical Site Infection, 2nd Ed.; 2018.
140. Kvalvik SA, Zakariassen SB, Overrein S, Rasmussen S, Skrede S, Baghestan E. Obstetric infections and clinical characteristics of maternal sepsis: a hospital-based retrospective cohort study. Sci Rep. 2024;14(1):6067. doi:10.1038/s41598-024-56486-4
141. Yamba K, Chizimu JY, Mudenda S, et al. Assessment of Antimicrobial Resistance Laboratory-based Surveillance Capacity of Hospitals in Zambia: findings and Implications for System Strengthening. J Hosp Infect. 2024;148:129–137. doi:10.1016/j.jhin.2024.03.014
142. Biniek JP, Schwab F, Graf K, Vonberg RP. Adherence to Antibiotic Prescription Guidelines in Four Community Hospitals in Germany. Antibiotics. 2024;13(7):635. doi:10.3390/antibiotics13070635
143. Moyo P, Moyo E, Mangoya D, et al. Prevention of antimicrobial resistance in sub-Saharan Africa: what has worked? What still needs to be done? J Infect Public Health. 2023;16(4):632–639. doi:10.1016/j.jiph.2023.02.020
144. Chiumia FK, Muula AS, Chimimba F, Nyirongo HM, Kampira E, Khuluza F. Effect of antibiotic medicines availability on adherence to standard treatment guidelines among hospitalized adult patients in southern Malawi. PLoS One. 2023;18(10 October):e0293562. doi:10.1371/journal.pone.0293562
145. Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S Afr Med J. 2018;108(4):304–310. doi:10.7196/SAMJ.2018.v108i4.12564
146. Pant S, Corwin A, Adhikari P, et al. Evaluating Antibiotic Treatment Guideline Adherence to Ongoing Antibiotic Stewardship in a Tertiary Care Setting: a Retrospective Observational Study. Can J Infect Dis Med Microbiol. 2024;2024(1):6663119. doi:10.1155/2024/6663119
147. Meesters K, Buonsenso D. Antimicrobial Stewardship in Pediatric Emergency Medicine: a Narrative Exploration of Antibiotic Overprescribing, Stewardship Interventions, and Performance Metrics. Children. 2024;11(3):276. doi:10.3390/children11030276
148. Sirota SB, Doxey MC, Dominguez RMV, et al. Global, regional, and national burden of upper respiratory infections and otitis media, 1990–2021: a systematic analysis from the Global Burden of Disease Study 2021. Lancet Infect Dis. 2024. doi:10.1016/S1473-3099(24)00430-4/ATTACHMENT/B7DAB1CD-806A-4D46-B52B-77526E763257/MMC3.PDF
149. Marom T. Burden of upper respiratory infections and otitis media. Lancet Infect Dis. 2024. doi:10.1016/s1473-3099(24)00532-2
150. Kalungia AC, Kampamba M, Banda D, et al. Impact of a hub-and-spoke approach to hospital antimicrobial stewardship programmes on antibiotic use in Zambia. JAC-Antimicrobial Resist. 2024;6(6):dlae178. doi:10.1093/JACAMR/DLAE178
151. Avent ML, Cosgrove SE, Price-Haywood EG, Van Driel ML. Antimicrobial stewardship in the primary care setting: from dream to reality? BMC Fam Pract. 2020;21(1):134. doi:10.1186/s12875-020-01191-0
152. Brigadoi G, Gres E, Barbieri E, et al. Impact of a multifaceted antibiotic stewardship programme in a paediatric acute care unit over 8 years. JAC-Antimicrobial Resist. 2024;6(6):dlae181. doi:10.1093/JACAMR/DLAE181
153. Cassim J, Essack SY, Chetty S. Building an antimicrobial stewardship model for a public-sector hospital: a pre-implementation study. J Med Microbiol. 2024;73(7):001853. doi:10.1099/jmm.0.001853
154. Al-Omari A, Al Mutair A, Alhumaid S, et al. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: results of a five-years pre-post analysis. Antimicrob Resist Infect Control. 2020;9(1):95. doi:10.1186/s13756-020-00751-4
155. Amponsah OKO, Courtenay A, Kwame Ayisi-Boateng N, et al. Assessing the impact of antimicrobial stewardship implementation at a district hospital in Ghana using a health partnership model. JAC-Antimicrobial Resist. 2023;5(4):dlad084. doi:10.1093/JACAMR/DLAD084
156. Ture Z, Güner R, Alp E. Antimicrobial stewardship in the intensive care unit. J Intensive Med. 2023;3(3):244–253. doi:10.1016/j.jointm.2022.10.001
157. Chizimu JY, Mudenda S, Yamba K, et al. Antimicrobial stewardship situation analysis in selected hospitals in Zambia: findings and implications from a national survey. Front Public Health. 2024;12:1367703. doi:10.3389/FPUBH.2024.1367703
158. Mudenda S, Chabalenge B, Daka V, et al. Knowledge, awareness and practices of healthcare workers regarding antimicrobial use, resistance and stewardship in Zambia: a multi-facility cross-sectional study. JAC-Antimicrobial Resist. 2024;6(3):dlae076. doi:10.1093/JACAMR/DLAE076
159. McKenzie D, Rawlins M, Del Mar C. Antimicrobial stewardship: what’s it all about? Aust Prescr. 2013;36(4):116–120. doi:10.18773/austprescr.2013.045
160. Mendelson M, Morris AM, Thursky K, Pulcini C. How to start an antimicrobial stewardship programme in a hospital. Clin Microbiol Infect. 2020;26(4):447–453. doi:10.1016/j.cmi.2019.08.007
161. Nyoloka N, Richards C, Mpute W, et al. Pharmacist-Led Antimicrobial Stewardship Programme in Two Tertiary Hospitals in Malawi. Antibiotics. 2024;13(6):480. doi:10.3390/ANTIBIOTICS13060480
162. Morency-Potvin P, Schwartz DN, Weinstein RA. Antimicrobial stewardship: how the microbiology laboratory can right the ship. Clin Microbiol Rev. 2017;30(1):381–407. doi:10.1128/CMR.00066-16
163. Jacobs J, Hardy L, Semret M, et al. Diagnostic Bacteriology in District Hospitals in Sub-Saharan Africa: at the Forefront of the Containment of Antimicrobial Resistance. Front Med. 2019;6:20. doi:10.3389/fmed.2019.00205
164. Ombelet S, Ronat JB, Walsh T, et al. Clinical bacteriology in low-resource settings: today’s solutions. Lancet Infect Dis. 2018;18(8):e248–e258. doi:10.1016/S1473-3099(18)30093-8
165. Patel R, Fang FC. Diagnostic Stewardship: opportunity for a Laboratory-Infectious Diseases Partnership. Clin Infect Dis. 2018;67(5):799–801. doi:10.1093/cid/ciy077
166. Umutesi G, Velin L, Muwanguzi M, et al. Strengthening antimicrobial resistance diagnostic capacity in rural Rwanda: a feasibility assessment. Ann Global Health. 2021;87(1):1–13. doi:10.5334/aogh.3416
167. Singh HK, Claeys KC, Advani SD, et al. Diagnostic stewardship to improve patient outcomes and healthcare-associated infection (HAI) metrics. Infect Control Hosp Epidemiol. 2024;45(4):405–411. doi:10.1017/ice.2023.284
168. Saha SK, Thursky K, Kong DC, Mazza D. A Novel GPPAS Model: guiding the Implementation of Antimicrobial Stewardship in Primary Care Utilising Collaboration between General Practitioners and Community Pharmacists. Antibiotics. 2022;11(9):1158. doi:10.3390/antibiotics11091158
169. Jinks T, Subramaniam S, Bassetti M, et al. Opportunities to Enhance Diagnostic Testing and Antimicrobial Stewardship: a Qualitative Multinational Survey of Healthcare Professionals. Infect Dis Ther. 2024:1–17. doi:10.1007/S40121-024-00996-1.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

