Back to Journals » Drug Design, Development and Therapy » Volume 19
Population Pharmacokinetics of Prolonged Infusion for Meropenem: Tailoring Dosing Recommendations for Chinese Critically Ill Patients on Continuous Renal Replacement Therapy with Consideration for Renal Function
Authors Peng Y, Liu Y, Cheng Z, Zhang Q, Xie F , Zhu S, Li S
Received 1 August 2024
Accepted for publication 29 January 2025
Published 17 February 2025 Volume 2025:19 Pages 1105—1117
DOI https://doi.org/10.2147/DDDT.S489603
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Yaru Peng,1,2,* Yalan Liu,1,* Zeneng Cheng,1 Qiang Zhang,3 Feifan Xie,1 Sucui Zhu,3,4 Sanwang Li1,5,6
1Division of Biopharmaceutics and Pharmacokinetics, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, Hunan, People’s Republic of China; 2Office of Clinical Trial Institution, Department of Pharmacy, Peking University Shenzhen Hospital, Shenzhen, Guangdong, People’s Republic of China; 3Department of Respiratory and Critical Care Medicine, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 4Department of Nursing, The Third Xiangya Hospital, Central South University, Changsha, Hunan, People’s Republic of China; 5Department of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha, People’s Republic of China; 6Institute of Clinical Pharmacy, Central South University, Changsha, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sucui Zhu, Department of Respiratory and Critical Care Medicine, The Third Xiangya Hospital, Central South University, Tongzipo Road 138, Changsha, 410013, People’s Republic of China, Tel +86 0731 8861 8080, Email [email protected] Sanwang Li, Department of Pharmacy, The Second Xiangya Hospital, Central South University, Middle Renmin Road 139, Changsha, 410011, People’s Republic of China, Tel/Fax +86 0731 8529 2099, Email [email protected]
Objective: Extended meropenem infusion is increasingly employed to enhance clinical outcomes in critically ill patients. Nonetheless, investigations into such dosing regimens in renal-impaired patients undergoing continuous renal replacement therapy (CRRT) are scarce. This study aims to perform a population pharmacokinetic (PK) analysis of prolonged meropenem infusion in critically ill CRRT patients to inform optimal dosing regimens.
Methods: Ninety-four concentrations from 21 Chinese critically ill CRRT patients receiving 1 g meropenem every 8– 12 hours infused over 2– 3 hours were utilized to construct the population PK model. Monte Carlo simulations were employed to assess the efficacy based on PK/PD targets (100% fT>MIC or 100% fT> 4×MIC) and the risk of nephrotoxicity (trough concentration ≥ 45 mg/L) for extended meropenem dosing regimens (0.5– 2 g with a 3-hour infusion administered every 6– 12 hours).
Results: Meropenem concentration data was adequately described by a one-compartment model with linear elimination, and creatinine clearance (CLCR) significantly influenced meropenem’s endogenous clearance. 0.5 g q6h and 1 g q8h could achieve desirable attainment of 100% fT>MIC target against an MIC≤ 4 mg/L, with negligible risk of toxicity for CRRT patients across a CLCR range of 10– 50 mL/min. 2 g q6h and 2 g q8h is required for targeting 100% fT> 4×MIC for the patients, but the associated risk of toxicity is very high (> 20%).
Conclusion: A population PK model was developed for prolonged meropenem infusion in Chinese CRRT patients, and 0.5 g q6h and 1 g q8h may be the optimal regimen for prolonged infusion.
Keywords: continuous renal replacement therapy, meropenem, prolonged infusion, population pharmacokinetics, critically ill
Introduction
Nosocomial infections and sepsis pose significant life-threatening risks to critically ill patients, necessitating prompt and optimal antibiotic therapy. To effectively target a wide spectrum of prevalent pathogens, meropenem, a prototypical beta-lactam antibiotic, is the primary clinical choice for initial administration.1
Meropenem is a hydrophilic compound characterized by a low distribution volume (~0.3 L/kg) and minimal protein binding (~2%). Consequently, 98% of meropenem circulates freely as microbiologically active fraction within the body. The compound is eliminated through the kidneys without any alteration and has a half-life of 1 hour under normal renal function.2 Pharmacokinetic (PK) studies have demonstrated a direct correlation between plasma clearance of meropenem and creatinine clearance (CLCR). Guidelines from the US Food and Drug Administration as well as the European Medicines Agency recommend dosage adjustments for individuals with renal impairment (CLCR≤50 mL/min).3,4 Meropenem shows time-dependent bactericidal effects, with the most reliable pharmacodynamic (PD) indicator being the duration that unbound plasma meropenem levels surpass the minimal inhibitory concentration (fT>MIC). In critically ill patients, achieving PK/PD targets, such as maintaining 100% fT>MIC or 100% fT>4×MIC, is advocated to enhance clinical efficacy and avoid antimicrobial resistance.5,6
Acute kidney injury (AKI) frequently occurs as a complication among critically ill patients, particularly those who are afflicted by severe infections or sepsis.7 Consequently, continuous renal replacement therapy (CRRT), which encompasses continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF), has been employed to address this challenge.8 The extracorporeal clearance (CLCRRT) for solutes in various CRRT modalities is determined by the effluent flow rate and either the sieving coefficient (Sc) or the saturation coefficient (Sd).
Administering meropenem rationally in critically ill patients is challenging due to its dynamic PK, which is affected by pathophysiological changes like an expanded volume of distribution during sepsis and modified renal function.9 Extracorporeal clearance by the implementation of CRRT further complicated its PK behavior in vivo.10 A recent multicenter study revealed that approximately 25% of critically ill patients fail to attain optimal therapeutic concentrations of the given antibiotics.11 This trend is especially pronounced in patients suffering from infections caused by pathogens with high minimum inhibitory concentration (MIC), like Pseudomonas aeruginosa and Acinetobacter baumannii. Within ICUs, inadequate beta-lactam antibiotic exposure (less than 100% fT>MIC) has been associated with elevated mortality rates.11 Conversely, elevated beta-lactam levels (eg, ≥45 mg/L for meropenem) are tied to increased neurological decline in septic ICU patients.12 This highlights the delicate dosing balance needed in this special patient population.
Short-term infusion, typically lasting 30 minutes, is a conventional dosing method for beta-lactams in hospitalized patients. Nonetheless, this approach may fall short in meeting the stringent PK/PD targets of maintaining drug levels at 100% fT>MIC or 100% fT>4×MIC, particularly among critically ill patients. Studies across various beta-lactams have highlighted the superiority of prolonged infusion (eg 3 hours) dosing regimens in meeting PK/PD targets and enhancing patient outcomes in critically ill compared to short-term infusion.13,14 The Surviving Sepsis Campaign advocates for prolonged beta-lactam infusion (following an initial bolus) over traditional bolus infusion for adults with sepsis or septic shock.15 The PK of meropenem in critically ill patients without CRRT (short-term and prolonged infusions) or with CRRT (short-term infusion) was extensively explored.16–19 Unfortunately, the pharmacokinetics and effectiveness of prolonged meropenem infusion in critically ill CRRT patients were scarcely investigated, particularly in the context of Chinese patients.
This study aimed to describe the population PK and assess the attainment of PK/PD targets for prolonged meropenem infusion in Chinese critically ill patients with renal impairment undergoing CRRT. Furthermore, the study aimed to explore the optimal prolonged infusion regimen for meropenem tailored to different levels of renal function using a simulation approach.
Methods
Study Design and Subjects
We conducted a prospective clinical PK study on meropenem within the Respiratory Intensive Care Unit (RICU) at the Third Xiangya Hospital affiliated with Central South University (Changsha, China) between May 2021 and April 2023. Eligible participants were adult patients requiring CRRT and receiving meropenem as their standard antimicrobial therapy. Patients who had undergone major surgical procedures within the preceding 4 weeks or were pregnant at enrollment were excluded. The study protocol complied with the Declaration of Helsinki and was approved by the Ethics Committee at the Third Xiangya Hospital (Approval number: I21115) before the research commenced. Prior to initiating any study-related procedures, explicit consent was obtained from each participating subject.
Detailed demographic and clinical data for each patient were thoroughly documented, such as age, gender, body weight, clinical diagnosis or sepsis, and Acute Physiology and Chronic Health Evaluation (APACHE) II scores. Microbiological factors, including pathogen types and MIC against meropenem, were documented. Laboratory examinations, such as serum creatinine (SCR), 24-hour urine volume, C-reactive protein (CRP), procalcitonin (PCT), and albumin, were also collected. Simultaneously, CRRT parameters, medication administration details, and sampling times were recorded. Furthermore, microbiological response (negative/positive) and clinical outcomes, indicating cure, improvement, or deterioration, along with the overall patient status regarding survival or mortality, were documented as PD endpoints.
Drug Administration and Sampling
Patients were empirically administered 1 g of meropenem every 8–12 hours through a 2–3 hour infusion. Blood samples (2 mL each) were opportunistically collected at 0–3 hours, 3–5 hours, and 5 hours following the initial meropenem dose or subsequent doses. Simultaneously, effluent samples (2 mL each) were collected from the CRRT post-filter line. Following collection, blood samples underwent immediate centrifugation to obtain plasma samples, which were subsequently kept at −80°C. Effluent samples, on the other hand, were directly frozen at −80°C.
Quantification of Meropenem and Its CRRT Clearance
Meropenem concentrations in both plasma and effluent samples were quantified employing a validated ultra-performance liquid chromatography coupled with photodiode array (UPLC-PDA) method.20 Sc was determined by dividing the meropenem concentration in effluent samples by the corresponding plasma concentration at the same time point. Given the utilization of CVVH in our RICU, the calculation of CLCRRT for each patient involved the multiplication of the ultrafiltrate flow rate (Quf, mL/h) by Sc.21 In practical clinical settings, the effluent flow rate is typically adjusted based on body weight (measured in mL/h/kg). This weight-based adjustment is known as the CRRT dose.
Population Pharmacokinetic Modeling
The analysis of meropenem concentration data was performed using the first-order conditional estimation with interaction (FOCE-I) algorithm in NONMEM® (Version 7.5). This process was facilitated by Perl-speaks-NONMEM (PsN) (version 5.3) and the Pirana workbench (version 3.0). Data processing and visualization were carried out using R® software (Version 4.0.5).
Structural Model Development
We assessed one- and two-compartment models to determine the optimal structural pharmacokinetic model. Total meropenem clearance (CLtotal) was characterized as the sum of endogenous clearance (CLbody) and CLCRRT. CLbody was estimated from the concentration data, while CLCRRT was calculated individually as detailed in the preceding section. Inter-individual variability (IIV) in typical population parameter estimates was modeled using a log-normal distribution. Residual unexplained variability was assessed using additive error, proportional error, and combined proportional and additive error models.
Covariate Analysis
Following the establishment of structural model, demographic data, clinical characteristics and laboratory examinations of the study population were assessed for potential inclusion as covariates in the population PK model. Continuous covariates evaluated included age, body weight, Cockcroft-Gault-based creatinine clearance (CLCR), APACHE II score, CRP and PCT. Categorical covariates tested included sex, sepsis or other infections, AKI or chronic renal failure, and urine output (anuric or non-anuric).
Initially, we analyzed plots showing empirical Bayes estimates of PK parameters plotted against subjects’ covariates to detect any observable patterns. Following this, covariates demonstrating potential associations based on graphical analysis were further explored using a stepwise approach: forward addition (p<0.05) and backward elimination (p<0.001).
Model Evaluation and Validation
The model selection process considered various criteria such as the objective function value (OFV), relative standard error (RSE) of parameter estimates, condition number, and goodness-of-fit (GOF) plots.22 A decrease of 3.84 in the OFV was deemed statistically significant (p<0.05) for adding a single parameter. To validate the final PK model internally, prediction-corrected visual predictive check (pcVPC) was performed, evaluating its predictive accuracy across 1000 virtual datasets. Parameter uncertainty was evaluated through bootstrap analysis, which involved resampling the dataset 1000 times.
Probability of Target Attainment
The final PK model was reparametrized, and Monte Carlo simulations were conducted using the mrgsolve package (version 1.4.1) in R. These simulations aimed to assess the probability of target attainment (PTA) and the associated risk of toxicity for various extended infusion (3-hour infusion) meropenem dosing regimens (0.5 g q6h, 0.5 g q8h, 1.0 g q6h, 1.0 g q8h, 1.0 g q12h, 2.0 g q6h, 2.0 g q8h, and 2.0 g q12h), stratified by retained covariates. Simulations were conducted for each scenario using 10,000 virtual subjects, all standardized to a fixed weight of 65 kg, representing the median weight of the study population. The CRRT dose was set at 25 mL/h/kg. PK/PD targets, indicative of efficacy, included achieving 100% fT>MIC and 100% fT>4×MIC. Toxicity was defined by a trough concentration ≥45 mg/L. Pathogen MIC values ranging from 0.25 to 16 mg/L were considered, based on distributions for Pseudomonas spp. and Acinetobacter spp. sourced from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) database.23 The simulations evaluated the PTA and potential toxicity risk based on predicted steady-state meropenem concentrations, aiming for ≥90% PTA as the desired PK/PD target achievement.
Results
Patient Characteristics
The population PK analysis included 21 critically ill patients undergoing CRRT, for whom 94 serum concentrations were collected to construct the model. The patients’ demographic information, clinical profiles, and laboratory test results are summarized in Table 1. The cohort predominantly comprised male patients (76.2%). Patients exhibited an average age of 58 years, a body weight of 65 kg, an APACHE II score of 19, and a CLCR of 13.6 mL/min. Most patients were diagnosed with AKI (n=7) or chronic renal failure (n=13), except for one young male patient exhibiting normal renal function. Sepsis or pneumonia constituted the primary infections, but only 5 patients exhibited a negative microbiological response following meropenem therapy. The median dose of CRRT administered was 25.76 mL/h/kg.
 |
Table 1 Summary of Patient Characteristics Presented as Median and Interquartile Range (IQR) or Number of Subjects (%) |
Population Pharmacokinetic Analysis
A one-compartment model with linear elimination effectively captured the concentration profile of meropenem, incorporating log-normal inter-individual variability in endogenous clearance. Residual variability was effectively captured by a combined proportional and additive error model. Due to limitations in our dataset, we were unable to precisely estimate IIV on the distribution volume (V). Therefore, to maintain the numerical stability of the model, the variability of this population parameter was fixed at zero, resulting in an increase of 9.94 points in the OFV.
In the univariable analysis, CLCR, age and sex were found to be significant covariates affecting CLbody (p<0.05). Through rigorous scrutiny employing forward addition and backward deletion, it was determined that age and sex did not remain significant covariates for CLbody after accounting for creatinine clearance. Consequently, CLCR emerged as the sole significant covariate retained for CLbody using an exponential model. Equation 1 in the final PK model characterized the CLtotal.
Where 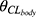 is the typical value of endogenous clearance, and
is the typical value of endogenous clearance, and 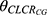 represents exponential factor of CLCR on CLbody.
represents exponential factor of CLCR on CLbody. 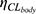 is the IIV of
is the IIV of 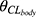 .
.
Table 2 presents the population PK parameter estimates obtained from the final model. The typical values for CLbody and V were determined to be 2.89 L/h and 26.0 L, respectively. These population parameters were accurately estimated, and their precision was confirmed by the non-parametric bootstrap analysis, detailed in Table 2.
 |
Table 2 Population Pharmacokinetic Estimates for the Final Model and Bootstrap Results |
The GOF plots of the final model are illustrated in Figure 1. Clearly, the scatterplots depicting observed concentrations against both population and individual predicted concentrations showed a consistent alignment with the line of identity. Additionally, conditional weighted residuals (CWRES) exhibited symmetric distribution around zero across the entire population predictions and time after dose range. Figure 2 displays the pcVPC plot assessing the model’s predictive performance, illustrating close alignment between the 5th, 50th, and 95th percentiles of observed and simulated concentrations. These diagnostic plots affirm the robustness and predictive accuracy of our final model. The solid black line signifies the correlation between observations and predictions, with dashed lines indicating the unity reference line or CWRES boundary.
Simulations and Dosing Recommendations
Influence of Creatinine Clearance on Target Attainment
Since CLCR stood as the sole covariate included in the final population PK model, simulations were performed for virtual patients with stratified CLCR values of 10, 25, and 50 mL/min. Among patients with CLCR of approximately 10 mL/min, all investigated prolonged infusion regimens demonstrated the ability in achieving >90% PTA against MICs≤4 mg/L for the 100% fT>MIC target (Figure S1). Similar observations were found for patients whose CLCR is 25 mL/min, except for the 1 g q12h regimen (Figure 3). However, for patients with CLCR of 50 mL/min, the regimens 0.5 g q8h, 1 g q12h, and 2 g q12h resulted in unacceptable attainment (PTA<70%) at an MIC of 4 mg/L (Figure S2).
When considering 100% fT>4×MIC as the efficacy marker, only dosage schedules of 1 g q6h, 2 g q6h, and 2 g q8h attained the desired PTAs near or above 90% against an MIC at 4 mg/L for patients whose CLCR ranges from 10 mL/min to 25 mL/min (Figures S1 and 3). However, only the 2 g q6h regimen achieved the anticipated PTA for an MIC of 4 mg/L for patients with CLCR of 50 mL/min (Figure S2). Overall, the regimens of 1 g q6h, 2 g q6h, and 2 g q8h demonstrated desirable target achievement for patients across the studied range of creatinine clearance (10–50 mL/min) for an MIC of 2 mg/L.
Toxicity Risk Evaluation Across Dosing Regimens
Table 3 displays the probability of reaching the toxicity threshold for various meropenem dosing regimens in CRRT patients across varying levels of renal function. Among the dosing regimens considered, the following led to a risk exceeding 15% of reaching the toxicity threshold: 1 g q6h, 2 g q6h, and 2 g q8h for patients with a CLCR of 10 mL/min; 2 g q6h and 2 g q8h for patients with a CLCR of 25 mL/min; and 2 g q6h for patients with a CLCR of 50 mL/min. However, for all other cases, the risk of toxicity is negligible.
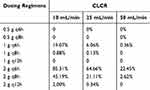 |
Table 3 Risk of Toxicity for Different Prolonged Infusion (3 h) Dosing Regimens of Meropenem in CRRT Patients with Different Creatinine Clearance (CLCR) |
Overall, the regimens of 0.5 g q6h and 1 g q8h could achieve desirable attainment of the 100% fT>MIC target against an MIC≤4 mg/L with negligible risk of toxicity for CRRT patients with a CLCR of 10–50 mL/min.
Discussion
In this study, we developed a population PK model for extended meropenem infusion in 21 critically ill patients undergoing CRRT. We systematically evaluated optimal dosing strategies for patients with varying degrees of renal impairment, assessing the likelihood of achieving PK/PD targets and potential toxicity risks. To our knowledge, this is the first population PK model of meropenem specifically tailored for extended infusion in CRRT patients.
Our final meropenem PK model embraced a one-compartment structure, deviating from the commonly reported two-compartment model. Similar to other one-compartment models derived from prolonged or continuous infusion data, the extended infusion method in our patient cohort potentially obscured the distribution phase and hindered the capture of a peripheral compartment.24,25 Although the early profile of the concentration–time curve varied between the one- and two-compartment models, simulation-based PTA predictions may show comparable results between the two models, considering meropenem’s time-dependent killing mechanism.
In our population PK analysis, we found that the typical CLbody of meropenem in CRRT patients is 2.89 L/h, with a typical distribution volume of 26.0 L. These values closely align with previously reported parameters from a two-compartment PK model in CRRT patient population given short-term infusion of meropenem, which indicated a 3.03 L/h for endogenous clearance and 28.8 L for total distribution volume.26 Notably, in our study, the endogenous clearance is significantly lower than healthy subjects (15.5 L/h) and critically ill patients not undergoing CRRT (9.25 to 9.89 L/h).19,27,28 The observed decrease in endogenous clearance in our study can be attributed to a substantial decline in renal function (a median CLCR of 13.6 mL/min) among critically ill CRRT patients, highlighting the impact of renal impairment on meropenem pharmacokinetics.
Our covariate analysis revealed CLCR as the sole covariate influencing meropenem clearance, consistent with the findings of the majority of previously published meropenem population PK models in critically ill patients.24,29–32 However, incorporating the covariate effect of CLCR on CLbody only resulted in a reduction of IIV of CLbody from 62.8% to 42.1%, indicating persistent variability. This variability, documented in other meropenem population PK reports,33,34 is likely attributed to dynamic and complex pathophysiological changes whose impact on meropenem PK is challenging to capture.28,35
Our model-based simulation analysis demonstrated that 0.5 g q6h and 1 g q8h could achieve a balanced attainment of 100% fT>MIC target against an MIC≤4 mg/L, while minimizing the risk of toxicity for critically ill CRRT patients with CLCR ranging from 10 mL/min to 50 mL/min. Furthermore, 1 g q6h is also a feasible treatment option for CRRT patients having a CLCR of 50 mL/min. This finding is consistent with the study by Lan et al, where they showed that 1 g q6h with a 2-hour infusion duration was adequate to achieve 100% fT>MIC target in their critically ill population. In their cohort, which included 16 out of 48 patients receiving CRRT, the mean CLCR was 45.9 mL/min.36 It is important to highlight that critically ill CRRT patients with varying degrees of renal functions may yield different dosing recommendations across reported studies. For instance, Seyler et al demonstrated 1 g q12h with a 0.5 h infusion is adequate for targeting 100% fT>MIC for an MIC≤4 mg/L in CRRT patients with AKI.37 In contrast, our study revealed that a regimen of 1 g q12h with a 3-hour infusion failed to achieve a desirable attainment of 100% fT>MIC in CRRT patients having a CLCR of 50 mL/min. Furthermore, varying proposed infusion durations can introduce additional considerations when comparing recommended regimens across studies (eg, 2 hours versus 3 hours). For example, Padulles et al suggested a regimen of a 0.5 g q8h with a 2-hour infusion for CRRT patients with AKI,38 while our study focused on a 3-hour prolonged infusion. In recent years, a 24-hour continuous infusion of meropenem has also been reported for critically ill patients.14 While continuous infusion is expected to offer significant advantages in achieving PK/PD targets, the susceptibility of meropenem to degradation at room temperature should not be overlooked. Fawaz et al declaimed meropenem could be administered continuously for a minimum of 7 hours under conditions where the temperature does not exceed 22°C, and for 5 hours if the temperature remains below 33°C.39
Choosing different PK/PD targets, for example 100% fT>MIC versus 100% fT>4×MIC, could also yield distinct dosing recommendations, as demonstrated in our simulations. Despite in vitro studies demonstrating that 100% fT>4×MIC is associated with maximal bacterial killing,40 the microbiological response does not necessarily translate to clinical outcome benefits. In a sepsis rat model, our previous study showed that the treatment groups attaining 100% fT>MIC and 100% fT>4×MIC did not exhibit a significant difference in survival outcomes.41 Once again, our multistate survival model in critically ill patients administered meropenem revealed that attaining 100% fT>4×MIC target does not provide additional survival benefits compared to achieving 100% fT>MIC.42 Considering these facts, our dosing recommendation for CRRT patients was mainly based on the target of 100% fT>MIC.
Several limitations warrant note in this study. First, the limited sample size impedes our ability to correlate the computed fT>MIC with the microbiological and clinical outcomes. Second, this was a single-center study involving meropenem administration to CRRT patients with minimal residual renal function. As a result, the dosing recommendations derived from our model may not be generalizable to other CRRT patients with preserved renal function.
Conclusion
In summary, we developed a population PK model for extended meropenem infusion in critically ill Chinese patients undergoing CRRT. Creatinine clearance was identified as the only factor influencing endogenous clearance. According to our simulations, we recommend administering 0.5 g every 6 hours or 1 g every 8 hours as a 3-hour infusion to achieve adequate plasma exposure for patients infected with non-resistant pathogens.
Institutional Review Board Statement
The study protocol received approval from the Ethics Committee of the Third Xiangya Hospital before commencing the research (Approval number: I21115).
Informed Consent Statement
All patients signed an informed consent before inclusion in this clinical study. Informed consent form can be made available from the corresponding author on reasonable request.
Data Sharing Statement
The data used to support the findings of the study are available from the corresponding author upon request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (Project number: 82073940), the Science and Technology Innovation Program of Hunan Province (Grant number: 2022RC1229), the Foundation of Peking University Shenzhen Hospital (Grant number: LCYJ2021044), and the Hunan Provincial Innovation Foundation for Postgraduate (Grant number: CX20240321).
Disclosure
The authors declare no conflicts of interest.
References
1. Baldwin CM, Lyseng-Williamson KA, Keam SJ. Meropenem: a review of its use in the treatment of serious bacterial infections. Drugs. 2008;68:803–838. doi:10.2165/00003495-200868060-00006
2. Wiseman LR, Wagstaff AJ, Brogden RN, Bryson HMM. A review of its antibacterial activity, pharmacokinetic properties and clinical efficacy. Drugs. 1995;50:73–101. doi:10.2165/00003495-199550010-00007
3. FDA. Highlights of meropenem prescribing information. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/202106s007lbl.pdf.
4. EMA. Summary of meronem characteristics. Available from: https://www.ema.europa.eu/en/documents/referral/meronem-article-30-referral-annex-i-ii-iii_en.pdf.
5. Li C, Du X, Kuti JL, Nicolau DP. Clinical pharmacodynamics of meropenem in patients with lower respiratory tract infections. Antimicrob Agents Chemother. 2007;51:1725–1730. doi:10.1128/AAC.00294-06
6. Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current beta-lactam antibiotic doses sufficient for critically ill patients? Clin Infect Dis. 2014;58:1072–1083. doi:10.1093/cid/ciu027
7. Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96:1083–1099. doi:10.1016/j.kint.2019.05.026
8. Hoste EA, Lameire NH, Vanholder RC, Benoit DD, Decruyenaere JM, Colardyn FA. Acute renal failure in patients with sepsis in a surgical ICU: predictive factors, incidence, comorbidity, and outcome. J Am Soc Nephrol. 2003;14:1022–1030. doi:10.1097/01.asn.0000059863.48590.e9
9. Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014;14:498–509. doi:10.1016/s1473-3099(14)70036-2
10. Investigators RS. Renal replacement therapy for acute kidney injury in Australian and New Zealand intensive care units: a practice survey. Crit Care Resusc. 2008;10:225–230.
11. Roberts JA, Joynt GM, Lee A, et al. The effect of renal replacement therapy and antibiotic dose on antibiotic concentrations in critically ill patients: data from the multinational sampling antibiotics in renal replacement therapy study. Clin Infect Dis. 2021;72:1369–1378. doi:10.1093/cid/ciaa224
12. Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of beta-lactam concentration-toxicity relationships. J Antimicrob Chemother. 2017;72:2891–2897. doi:10.1093/jac/dkx209
13. Vardakas KZ, Voulgaris GL, Maliaros A, Samonis G, Falagas ME. Prolonged versus short-term intravenous infusion of antipseudomonal beta-lactams for patients with sepsis: a systematic review and meta-analysis of randomised trials. Lancet Infect Dis. 2018;18:108–120. doi:10.1016/S1473-3099(17)30615-1
14. Roberts JA, Abdul-Aziz MH, Davis JS, et al. Continuous versus intermittent beta-lactam infusion in severe sepsis. a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med. 2016;194:681–691. doi:10.1164/rccm.201601-0024OC
15. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181–1247. doi:10.1007/s00134-021-06506-y
16. Onichimowski D, Bedzkowska A, Ziolkowski H, et al. Population pharmacokinetics of standard-dose meropenem in critically ill patients on continuous renal replacement therapy: a prospective observational trial. Pharmacol Rep. 2020;72(3):719–729. doi:10.1007/s43440-020-00104-3
17. Grensemann J, Busse D, Konig C, et al. Acute-on-chronic liver failure alters meropenem pharmacokinetics in critically ill patients with continuous hemodialysis: an observational study. Ann Intensive Care. 2020;10:48. doi:10.1186/s13613-020-00666-8
18. Zhao YC, Zou Y, Xiao YW, et al. Does prolonged infusion time really improve the efficacy of meropenem therapy? A prospective study in critically ill patients. Infect Dis Ther. 2022;11:201–216. doi:10.1007/s40121-021-00551-2
19. Ehmann L, Zoller M, Minichmayr IK, et al. Development of a dosing algorithm for meropenem in critically ill patients based on a population pharmacokinetic/pharmacodynamic analysis. Int J Antimicrob Agents. 2019;54:309–317. doi:10.1016/j.ijantimicag.2019.06.016
20. Xie F, Liu L, Wang Y, Peng Y, Li S. An UPLC-PDA assay for simultaneous determination of seven antibiotics in human plasma. J Pharmaceut Biomed Anal. 2022;210:114558. doi:10.1016/j.jpba.2021.114558
21. Schetz M. Drug dosing in continuous renal replacement therapy: general rules. Curr Opin Crit Care. 2007;13:645–651. doi:10.1097/MCC.0b013e3282f0a3d3
22. Nguyen TH, Mouksassi MS, Holford N, et al. Model evaluation of continuous data pharmacometric models: metrics and graphics. CPT Pharmacometrics Syst Pharmacol. 2017;6:87–109. doi:10.1002/psp4.12161
23. EUCAST. Clinical breakpoints - breakpoints and guidance. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_14.0_Breakpoint_Tables.pdf.
24. Westermann I, Gastine S, Muller C, et al. Population pharmacokinetics and probability of target attainment in patients with sepsis under renal replacement therapy receiving continuous infusion of meropenem: sustained low-efficiency dialysis and continuous veno-venous haemodialysis. Br J Clin Pharmacol. 2021;87:4293–4303. doi:10.1111/bcp.14846
25. An G, Creech CB, Wu N, et al. Evaluation of empirical dosing regimens for meropenem in intensive care unit patients using population pharmacokinetic modeling and target attainment analysis. Antimicrob Agents Chemother. 2023;67:e0131222. doi:10.1128/aac.01312-22
26. Peng Y, Cheng Z, Xie F. Population pharmacokinetic meta-analysis and dosing recommendation for meropenem in critically ill patients receiving continuous renal replacement therapy. Antimicrob Agents Chemother. 2022;66:e0082222. doi:10.1128/aac.00822-22
27. Mouton JW, van den Anker JN. Meropenem clinical pharmacokinetics. Clin Pharmacokinet. 1995;28:275–286. doi:10.2165/00003088-199528040-00002
28. Dhaese SAM, Farkas A, Colin P, et al. Population pharmacokinetics and evaluation of the predictive performance of pharmacokinetic models in critically ill patients receiving continuous infusion meropenem: a comparison of eight pharmacokinetic models. J Antimicrob Chemother. 2019;74:432–441. doi:10.1093/jac/dky434
29. Roberts JA, Kirkpatrick CM, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction: intermittent bolus versus continuous administration? Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother. 2009;64:142–150. doi:10.1093/jac/dkp139
30. Ulldemolins M, Soy D, Llaurado-Serra M, et al. Meropenem population pharmacokinetics in critically ill patients with septic shock and continuous renal replacement therapy: influence of residual diuresis on dose requirements. Antimicrob Agents Chemother. 2015;59:5520–5528. doi:10.1128/AAC.00712-15
31. Ehmann L, Zoller M, Minichmayr IK, et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care. 2017;21:263. doi:10.1186/s13054-017-1829-4
32. Eisert A, Lanckohr C, Frey J, et al. Comparison of two empirical prolonged infusion dosing regimens for meropenem in patients with septic shock: a two-center pilot study. Int J Antimicrob Agents. 2021;57:106289. doi:10.1016/j.ijantimicag.2021.106289
33. Burger R, Guidi M, Calpini V, et al. Effect of renal clearance and continuous renal replacement therapy on appropriateness of recommended meropenem dosing regimens in critically ill patients with susceptible life-threatening infections. J Antimicrob Chemother. 2018;73:3413–3422. doi:10.1093/jac/dky370
34. Gijsen M, Elkayal O, Annaert P, et al. Meropenem target attainment and population pharmacokinetics in critically ill septic patients with preserved or increased renal function. Infect Drug Resist. 2022;15:53–62. doi:10.2147/IDR.S343264
35. Dhaese SAM, Thooft ADJ, Farkas A, et al. Early target attainment of continuous infusion piperacillin/tazobactam and meropenem in critically ill patients: a prospective observational study. J Crit Care. 2019;52:75–79. doi:10.1016/j.jcrc.2019.04.013
36. Lan J, Wu Z, Wang X, et al. Population pharmacokinetics analysis and dosing simulations of meropenem in critically ill patients with pulmonary infection. J Pharm Sci. 2022;111(6):1833–42. doi:10.1016/j.xphs.2022.01.015
37. Seyler L, Cotton F, Taccone FS, et al. Recommended beta-lactam regimens are inadequate in septic patients treated with continuous renal replacement therapy. Crit Care. 2011;15:R137. doi:10.1186/cc10257
38. Padulles Zamora A, Juvany Roig R, Leiva Badosa E, et al. Optimized meropenem dosage regimens using a pharmacokinetic/pharmacodynamic population approach in patients undergoing continuous venovenous haemodiafiltration with high-adsorbent membrane. J Antimicrob Chemother. 2019;74:2979–2983. doi:10.1093/jac/dkz299
39. Fawaz S, Barton S, Whitney L, Swinden J, Nabhani-Gebara S. Stability of meropenem after reconstitution for administration by prolonged infusion. Hospital Pharm. 2018;54:190–196. doi:10.1177/0018578718779009
40. Mouton JW, Den hollander JG. Killing of Pseudomonas aeruginosa during continuous and intermittent infusion of ceftazidime in an in vitro pharmacokinetic model. Antimicrob Agents Chemother. 1994;38:931–936. doi:10.1128/AAC.38.5.931
41. Wang Y, Liu L, Wu Q, Yin Q, Xie F. Defining exposure predictors of meropenem that are associated with improved survival for severe bacterial infection: a preclinical PK/PD study in sepsis rat model. Antibiotics. 2022;11(11):1660. doi:10.3390/antibiotics11111660
42. Peng Y, Minichmayr IK, Liu H, Xie F, Friberg LE. Multistate modeling for survival analysis in critically ill patients treated with meropenem. CPT Pharmacometrics Syst Pharmacol. 2023;13:222–233. doi:10.1002/psp4.13072
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





