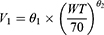Back to Journals » Drug Design, Development and Therapy » Volume 19
Population Pharmacokinetics of Tranexamic Acid in Chinese Population Undergoing Cardiac Surgery with Cardiopulmonary Bypass
Authors Liu Y , Zhou C, Lv H , Tian L , Jiang J, Shi J
Received 28 August 2024
Accepted for publication 6 May 2025
Published 26 May 2025 Volume 2025:19 Pages 4343—4353
DOI https://doi.org/10.2147/DDDT.S493485
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Georgios Panos
Yue Liu,1 Chenghui Zhou,2 Hong Lv,1 Lei Tian,3 Juanjuan Jiang,3 Jia Shi1
1Department of Anesthesiology, State Key Laboratory of Cardiovascular Disease, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; 2Center for Anesthesiology, Beijing Anzhen Hospital, Capital Medical University, Beijing, People’s Republic of China; 3NHC Key Laboratory of Clinical Research for Cardiovascular Medications, National Clinical Research Center of Cardiovascular Diseases, Fuwai Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Jia Shi, Email [email protected]
Background: Population pharmacokinetics (PK) models could provide specific references for the formulation of personal drug delivery protocols, however, there is no population PK study of tranexamic acid (TXA) have been conducted in the Chinese population. The aim of this study was to establish a population PK model based on the data of perioperative plasma concentrations in Chinese participants, and to provide a reference for individualized administration of TXA.
Methods: Participants undergoing cardiac surgery were randomly assigned to high-dose of TXA group (a 30-mg/kg bolus, a 16-mg/kg/h maintenance dose, and a 2-mg/kg prime, n = 7) and low-dose group of TXA (a 10-mg/kg bolus, a 2-mg/kg/h maintenance dose, and a 1-mg/kg prime, n = 9). Blood samples were collected at 14 time points and the concentration of TXA was determined by liquid chromatography-tandem mass spectrometry. Modelling was performed using Phoenix NLME 8.3 software.
Results: The primary covariate identified was body weight, while no significant influence of cardiopulmonary bypass (CPB) on the PK was detected. The population estimates for clearance (CL1), volume of the central compartment (V1), diffusional clearance (CL2), and volume of peripheral compartment (V2) were 4.7 L/h, 4.9 L, 17.0 L/h, and 11.1 L, respectively, assuming a bodyweight of 70 kg.
Conclusion: This study provides the first population PK model of TXA in the Chinese population undergoing cardiac surgery with CPB. The model could serve as a reference for the future development of individualized TXA administration strategies, with target-controlled infusion (TCI) emerging as a viable option.
Plain Language Summary: What is already known on this topic?
Tranexamic acid is an antifibrinolytic drug that has been demonstrated to reduce the requirement for allogeneic blood transfusions in cardiac surgery with CPB. However, prolonged or high-dose sustained tranexamic acid transfusions have been associated with an increased risk of seizures.
What this study adds?
This study presents the population pharmacokinetics of tranexamic acid in a Chinese population undergoing cardiac surgery with cardiopulmonary bypass, and develops the first population pharmacokinetic model for the Chinese population.
How this study might affect research, practice or policy?
The model will facilitate the future promotion of individualized dosing of tranexamic acid, ensuring adequate antifibrinolytic effects while reducing adverse effects. Target-controlled infusion based on population modelling is a potential avenue for further consideration.
Keywords: tranexamic acid, population pharmacokinetics, blood conservation, cardiac surgery, cardiopulmonary bypass
Introduction
Massive bleeding and resulting allogeneic transfusion are significant clinical issues in cardiac surgery. In particular, hyperfibrinolysis in cardiac surgery with cardiopulmonary bypass (CPB) could cause premature and excessive fibrin degradation, leading to a significant increase in bleeding. Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine that exerts its antifibrinolytic effect by competitively binding to the lysine-binding sites on plasminogen.1 Numerous clinical guidelines have recommended the TXA usage in cardiac surgery.2–5 To ensure its full antifibrinolytic effect, adequate blood levels should be maintained. However, high doses or prolonged continuous infusions, while beneficial in maintaining effective blood levels, may also increase the risk of adverse events, such as thrombotic adverse events and seizures.6–10 Currently, more than 150,000 cardiac bypass surgeries are performed in China annually, with a significant number of patients requiring TXA during the procedure. This association between high TXA expose and seizures has been well-documented in large international multi-center studies, which have included Chinese participants,11 as well as in case reports from China.12
The search for an optimal dosing regimen that balances effectiveness and safety has been ongoing. Many researches have reported on the pharmacokinetics (PK) of TXA13–15 resulting in the basis for several commonly used dosing regimens. However, the optimal dose of TXA in cardiac surgery has not yet been standardized, with different dosing regimens used in different institutions. As the exploration of TXA dosing regimens continues and the concept of individualized dosing becomes increasingly prevalent, accumulating studies have begun to use population PK analysis to guide clinical use of the TXA.16–20 However, the pharmacokinetic characteristics of drugs are generally influenced by factors such as genetic background, dietary habits, and environmental conditions. Consequently, population PK models developed based on data from foreign populations may not be directly applicable to the Chinese population. More importantly, the lack of studies on TXA pharmacokinetics in Chinese population underscores the need for dedicated research to characterize the PK profile of TXA in this population, thereby ensuring its safe and effective use in Chinese clinical practice.
The aim of this study was to collect perioperative blood concentration data from Chinese subjects and construct a population PK model to obtain key PK parameters, in order to reveal the distribution and excretion patterns of TXA in the Chinese population, and then to provide a reference for the individualized administration of TXA in the future.
Methods
Study Subjects and Dosing Regimen
The study was approved by the Ethics Committee of Fuwai Hospital, Chinese Academy of Medical Sciences, in accordance with the Declaration of Helsinki (Ethics No. 2022–1866), and written informed consent was obtained from all participants. Eligible participants were screened for inclusion in the study, and the inclusion criteria included: (1) aged 18–70 years; (2) undergoing cardiac surgery with CPB; and (3) intraoperative infusion of TXA. Exclusion criteria were: (1) allergy or contraindication to TXA; (2) women who were breastfeeding or pregnant; (3) in end-stage disease with an expected survival of less than 3 months; and (4) concurrent participation in other clinical trials.
In this study, TXA was administered as a “loading dose + maintenance dose + pump prime dose” regimen: the loading dose was given by intravenous infusion within 20 minutes after induction of anesthesia, followed by continuous intravenous infusion at a maintenance dose until the end of the operation, and the pump prime dose was added to the priming solution of the CPB machine before the start of the CPB. Participants who met the inclusion criteria were randomly assigned to either the high-dose or low-dose group. Simple randomization, which had been approved by the ethics committee, was performed using a computer-generated random number sequence. A dedicated research coordinator, who was blinded to other clinical data, assigned participants to the respective dose groups according to the randomization list. In the high-dose group, the loading dose was 30 mg/kg, the maintenance dose was 16 mg/kg/h, and the pump prime dose was 2 mg/kg, in the low-dose group, the loading dose was 10 mg/kg, the maintenance dose was 2 mg/kg/h, and the pump prime dose was 1 mg/kg.
Sample Collection and Measurement
Arterial blood samples were collected at the following 14 time points: before loading dose administration (T1), 10 minutes after the start of the loading dose (T2), immediately after the end of the loading dose (T3), 30 minutes (T4), 1 hour (T5), 2 hours (T6) after the start of the maintenance dose, and immediately after the end of the maintenance dose (T7), 15 minutes (T8), 30 minutes (T9), 1 hour (T10), 2 hours (T11), 3 hours (T12), 4 hours (T13), 6 hours (T14) after the end of the maintenance dose. The plasma was separated by centrifugation and stored in a −20°C fridge, and finally the concentration of TXA in plasma was determined by liquid chromatography-tandem mass spectrometry, with a lower limit of quantification of 1μg/mL.
Exploratory Data Analyses
After identifying and processing missing data, values below the lower limit of quantification, and abnormal data, exploratory analyses were conducted to describe the baseline characteristics of the participants, including number of participants, group assignment, age, weight, sex, routine laboratory findings, and perioperative data. SPSS 26.0 software was used for analysis. Prior to model development, normality tests (Shapiro–Wilk test) were performed for continuous variables. Variables with a normal distribution were expressed as mean ± standard deviation (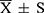 ), whereas those with a skewed distribution were expressed as median (interquartile range) [M (IQR)]. Categorical variables were summarized as counts and percentages.
), whereas those with a skewed distribution were expressed as median (interquartile range) [M (IQR)]. Categorical variables were summarized as counts and percentages.
Modelling
Structural Model
Based on the PK characteristics of TXA and population PK models reported in previous literatures,16,17 a two-compartment model with first-order elimination processes was chosen as the structural model. The PK parameters included in this structural model were clearance (CL1), volume of the central compartment (V1), diffusional clearance (CL2) and volume of peripheral compartment (V2).
Random Effect Model
The between-subject variability (BSV) and within-subject variability (WSV) in random effects were represented by exponential and proportional models, respectively. Based on the non-linear mixed-effects modelling approach, the population parameters were calculated using first order conditional estimation (FOCE) supplemented by extended least square (ELS).
Covariate Models
Introduction of covariates to optimize the base model and explain BSV in PK parameters. The study considered several factors, including body weight, sex, age, state of CPB, CPB duration, minimum rectal temperature during CPB, total bilirubin, direct bilirubin, alanine aminotransferase, aspartate aminotransferase, serum creatinine, and blood urea nitrogen. To ensure accurate parameter estimates, correlations between covariates were examined to avoid collinearity and instability. Covariate models were then constructed using stepwise methods. The relationships between continuous variables and PK parameters were described by power exponential models, and the relationships between dichotomous variables and PK parameters were described by proportional models. To improve the precision of parameter estimates in the covariate model and avoid estimates outside the observed range, the covariate effects were centered. As an example of the effect of body weight (WT) on V1, the reparametrized power exponent model, centered on 70 kg, is shown below:
This value was chosen because the mean body weight of participants enrolled in this study was close to 70 kg, and it has also been adopted in previous study.17 Furthermore, an allometric scaling model was introduced in the parameterization between the PK parameters and body weight. Specifically, exponent θ2 was set as a fixed value, while the exponent for the clearance parameter was typically 0.75 and the exponent for the volume of distribution parameter was typically 1.21
Evaluation of the Model
Model selection and evaluation were performed in accordance with the guidelines provided by the National Medical Products Administration (NMPA) for population pharmacokinetic studies. Initially, models were screened using the likelihood ratio test, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC), as these are standard methods for assessing model fit and balancing complexity. Model diagnostics, including prediction and residual plots, were used to evaluate the model’s fit to the observed data and to detect any prediction bias. To assess model stability, the bootstrap method with 1000 resamples was applied for the final model. Additionally, Monte Carlo simulations (1000 iterations) were conducted to perform the visual predictive check (VPC), which is commonly used to assess the predictive performance of the model across the observed data range.
Results
Exploratory Data Analysis
A total of 16 participants were included in this study, 7 in the high dose group and 9 in the low dose group. All participants had blood samples collected and drug concentrations measured at the specified 14 time points, with no data below the lower limit of quantification. No missing values or outliers were found during data verification. The perioperative TXA concentration-time profiles for the 16 participants are shown in Figure 1. Group-level drug exposure trends and individual concentration-time profiles are provided in Figures S1 and S2, respectively. The demographic and perioperative data for all participants are shown in Tables 1 and 2 respectively. The differences between the two groups of participants in each of the indicators of the demographic profiles were not statistically significant (all P > 0.05). There were no statistically significant differences in preoperative laboratory tests, preoperative medication between the two groups (all P > 0.05). Furthermore, there were no statistically significant differences in surgery duration, CPB duration, and Length of intensive care unit (ICU) stay between the two groups (all P > 0.05).
 |
Table 1 Demographic Data for All Participants |
 |
Table 2 Perioperative Data for All Participants |
 |
Figure 1 The TXA concentration-time profiles for all participants. Abbreviation: TXA, tranexamic acid. Note: The horizontal axis indicates the time after administration of the loading dose. |
Model Construction and Optimization
The results showed that the two-compartment model could better describe the PK characteristics of TXA, and the PK parameters included in this structural model were CL1, CL2, V1, and V2. The BSV was suitable for the exponential model and the WSV was suitable for the proportional model. A total of 73 covariate models were successively constructed using the stepwise method. Among these, the 23rd model was identified as the best-performing model. This model retained gender and age as covariates, which were found to influence the PK parameters CL2 and CL1, respectively. However, compared to the base model, this model was more complex but did not explain additional variability. Therefore, drawing on previous literature, we also examined the inclusion of body weight as a main covariate. A comparison of the population PK models that were included as candidates is shown in Table 3. The model with body weight as the main covariate had the smallest values of −2LL (minus two times the log likelihood), AIC and BIC. This means that the model was able to account for more sources of BSV in a relatively concise model structure. Therefore, it was considered the final choice, and Table 4 displays the estimated typical values for each PK parameter.
 |
Table 3 Comparison of Alternative Models |
 |
Table 4 Parameter Estimates of the Final Model |
Model Evaluation
Diagnostic Plots
The diagnostic plots of the final model are shown in Figure 2. In the prediction-based Figure 2A and B, the trend lines closely overlap with the reference lines, indicating that both individual predictions (IPRED) and population predictions (PRED) accurately capture the central tendency and dispersion of individual and overall data, respectively. In the residuals-based Figure 2C and D, the majority of conditional weighted residuals (CWRES) are symmetrically distributed on both sides of the reference line, concentrated within ± 2, and do not show a clear trend with the independent variable. This distribution suggests that the model is robust and well-fitting.
Bootstrap Method
Fit the 1000 bootstrap datasets generated after resampling 1000 times resulted in 95.8% of the datasets converging successfully, ie, they were able to estimate the PK parameters successfully. A comparison of the results of the final model and the bootstrap method is shown in Table 5. The population typical values of PK parameters in the final model were similar to the median of typical values in the models constructed based on 1000 bootstrap data sets, and were within the 95% CI of the bootstrap results, indicating that the final model has good stability.
 |
Table 5 Comparison of the Results of the Final Model and the Bootstrap Method |
Visual Predictive Check
Based on the parameter estimates of the final model and the structure of the real measurement data based on the original data (including dosing regimen, sampling time points, covariate information, etc)., a total of 1000 Monte Carlo simulations were performed, and 1000 simulated datasets were generated. The distribution characteristics of the 25th and 75th percentiles of the measured and simulated datasets at each time point are shown in Figure 3. The high degree of agreement between the measured and predicted values around the 75th and 25th percentiles and the fact that the lines of the measured values were within the 95% CI of the predicted values suggested that the final model was able to accurately characterize the drug concentration of the target population.
Discussion
This study presents the first population PK model of TXA in the Chinese population. It characterizes the distribution and excretion patterns of TXA and provides key PK parameters. With an intensive sampling design and rigorous data management, this study offers a more precise description of the individual PK processes. The final model demonstrated higher estimation accuracy and more reliable predictive performance, as evidenced by lower %RSE of PK parameter estimates and smaller shrinkage values of BSV. Additionally, various evaluation methods with stringent criteria further validated the reliability of the model.
The analysis of drug concentration data indicated that the two-compartment model was the most suitable for describing the PK characteristics of TXA, and the PK parameters included in this structural model were CL1, V1, CL2 and V2. In this study, the typical population estimates for these parameters were as follows: CL1 = 4.7 L/h, V1 = 4.9 L, CL2 = 17.0 L/h and V2 = 11.1 L. However, variability in these estimates has been observed across different studies. For instance, in other population PK studies involving adult participants undergoing cardiac surgery with CPB,16–20 typical estimates for CL1 ranged from 4.8 L/h to 0.17 L/min, for V1 from 5.0 L to 17.3 L, for CL2 from 0.011 L/h to 32.2 L/h and for V2 from 8.5 L to 10.8 L. These variations in PK parameter estimates could be attributed to differences in study design, population characteristics, dosing regimens, and analytical techniques. In terms of covariate inclusion, previous models typically incorporated one or two covariates, with weight being the most commonly included, while CPB was less frequently included as a covariate in the models. Dowd et al reported that CPB could affect PK parameters: V1 and V2 increase during and after CPB, while CL1 slows down during CPB and CL2 speeds up during and after CPB.16 In examining the covariate effects of CPB on PK parameters, this study attempted to introduce the time of CPB and the minimum rectal temperature during CPB, which are continuous variables, as covariates for fitting, in addition to referring to the method of Dowd et al, which used the state of CPB as a dichotomous variable. However, no significant effect of CPB on the PK parameters was detected, which is consistent with the findings of Grassin-Delyle et al.17 As both this study and the study by Grassin-Delyle et al used dosing regimens that included the pump prime dose, which was added to the priming solution of the CPB machine before the start of the CPB. The dilution of the plasma drug concentration by the priming solution in the line would be counteracted to some extent at the immediate start of CPB. This would mask the effects of CPB on PK parameters.
Among the population PK studies conducted in adult patients undergoing cardiac surgery with CPB, only a few studies retained CPB as a covariate.16,20 Both this study and others did not find a significant effect of CPB on TXA pharmacokinetics. However, this does not rule out the possibility of an effect, as current studies often focus on sampling points based on drug administration events and may not adequately capture critical time points during CPB. Consequently, the limited number of sampling time points during CPB makes it challenging to accurately observe PK changes during this period. During CPB, the distribution of TXA can be influenced by various factors, including hemodilution, heparin administration, reduced tissue perfusion and hypothermia.22,23 The clearance may also be impacted by reduced renal perfusion due to hypotension, nonpulsatile perfusion, and hypothermia.24,25 After the end of CPB, the above changes may be gradually reversed, accompanied by the restoration of body temperature, pulsatile perfusion, blood pressure and cardiac output.26,27 There is a limited number of studies examining the impact of CPB on PK, and a consensus conclusion has not been reached. The discrepancy in the results is mainly due to the differences in management of anesthesia or CPB in cardiac surgery. These differences include the materials of extracorporeal circulatory lines, volumes and compositions of priming solution, degrees of hypothermia, management of hemodynamics, and numbers and distributions of time points for sampling. And these differences can all contribute to varying results, finally. Furthermore, it is important to note that drugs require several half-lives to reach equilibrium within the human body, and the relatively short duration of CPB is not sufficient to support a complete observation of the PK process, thus limiting the estimation of PK parameters.
The most common clinical application of population PK models is to simulate relevant dose and recommend optimized drug dosing strategies. Previous studies have made significant advancements in this area, providing robust models for TXA in various clinical scenarios.17,19,28,29 However, despite these advances, the practical implementation of these dosing strategies in clinical settings is often hindered by the complexity of the regimens. Many dosing recommendations based on population models involve dose adjustments for multiple covariates, which can be difficult for clinicians to remember and apply in real time. As a result, clinicians tend to favor simpler dosing regimens, which limits the widespread adoption of well-designed, evidence-based dosing strategies that could improve patient outcomes. To address this challenge, we propose the use of target-controlled infusion (TCI) based on the population PK parameters derived from this study. TCI is a technique that allows for the real-time adjustment of drug delivery by maintaining a constant target plasma concentration, using algorithms that consider patient-specific covariates. It has been demonstrated to be safe and effective in numerous studies, including in the context of anesthetic agents.30 TCI simplifies the process by only requiring the operator to input basic patient information and a target drug concentration, significantly enhancing convenience and reducing the risk of dosing errors. In our previous large-scale, randomized controlled trial,31 we demonstrated that a high dose of TXA significantly reduced the need for allogeneic red blood cell transfusions while meeting the noninferiority criterion for a composite safety endpoint, including 30-day mortality, seizures, renal dysfunction, and thrombotic events. In this study, TXA concentrations in the high-dose group during CPB were generally maintained within the range of 150–180 mg/L, with some participants briefly exceeding 200 mg/L. Based on these findings, we recommend targeting a plasma drug concentration of 150–180 mg/L for TCI during cardiac surgery with CPB. Furthermore, to mitigate dilution of TXA due to the priming solution during CPB, we recommend adding TXA to the priming solution. The concentration of TXA in the priming solution should also fall within the target range of 150–180 mg/L. By using TCI, clinicians can achieve optimal plasma concentrations with minimal effort, ensuring individualized dosing that matches each patient’s pharmacokinetic profile, thereby improving the safety and efficacy of antifibrinolytic therapy during cardiac surgery with CPB.
The primary limitation of this study is the relatively small sample size. However, the dense sampling schedule and appropriate distribution of time points partially mitigate this limitation. Nonetheless, it is important to acknowledge that due to the baseline characteristics of the included participants, the model’s applicability and the recommended target concentrations are limited to adult patients undergoing cardiac surgery with CPB. The findings may not be generalizable to pediatric patients or those undergoing other types of surgeries or clinical scenarios. Another limitation of this study is the lack of use of the population pharmacokinetics-pharmacodynamics (PK-PD) analysis methods. As a result, TCI based on this model only supports plasma concentration targeting, rather than effector compartment targeting. Previous studies also rarely incorporated pharmacodynamics (PD) endpoints into population PK modeling, this is mainly due to the lack of reliable, real-time, and specific pharmacodynamic markers for TXA effects. However, recent studies have explored using maximum lysis (ML) from thromboelastography (TEG) as a pharmacodynamic marker of antifibrinolytic activity, showing promising results.32 This provides a potential reference for future PK-PD analyses of TXA, helping to identify appropriate pharmacodynamic endpoints. Although drug exposure levels play an important role in drug development and clinical application, clinicians should focus more on the body’s response to drugs, which can have either positive effects, such as the benefits of antifibrinolytic effects in reducing bleeding and blood transfusion, or negative effects, such as an increased risk of thrombotic adverse events and seizures. Clarification of the exposure-effect relationship of a TXA can be decisive for the development of an individualized dosing regimen.
In conclusion, this study presents the first population model of TXA for the Chinese population. Following external validation, this model could serve as a powerful tool for promoting individualized TXA dosing, thereby reducing the risk of drug-related adverse events while ensuring an adequate antifibrinolytic effect. The exploration of the TCI of TXA, based on a population PK model, may provide a more feasible and practical approach than creating fine-grained individualized dosing regimens for each patient. This technique not only simplifies the dosing process but also enhances the accuracy and safety of TXA administration, ultimately contributing to improved patient outcomes in clinical settings, particularly in cardiac surgery with CPB.
Acknowledgment
We thank the participants, anesthesiologists and anesthesia assistants at Fuwai Hospital in this study.
Funding
This work was supported by the Noncommunicable Chronic Diseases-National Science and Technology Major Project (2023ZD0504705) and the CAMS Innovation Fund for Medical Sciences (CIFMS) (Grant Nos. 2024-I2M-C&T-B-044).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Dunn CJ, Goa KL. Tranexamic acid: a review of its use in surgery and other indications. Drugs. 1999;57(6):1005–1032. doi:10.2165/00003495-199957060-00017
2. Ferraris VA, Brown JR, Brown JR, et al. Society of Thoracic Surgeons Blood Conservation Guideline Task Force. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–982. doi:10.1016/j.athoracsur.2010.11.078.
3. Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30(6):270–382. doi:10.1097/EJA.0b013e32835f4d5b
4. Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol. 2017;34(6):332–395. doi:10.1097/EJA.0000000000000630
5. National Clinical Guideline Centre (UK). Blood Transfusion. London: National Institute for Health and Care Excellence (NICE); 2015 Nov. Available from: https://www.ncbi.nlm.nih.gov/books/NBK327570/.
6. Murkin JM, Falter F, Granton J, Young B, Burt C, Chu M. High-dose tranexamic acid is associated with nonischemic clinical seizures in cardiac surgical patients. Anesth Analg. 2010;110(2):350–353. doi:10.1213/ANE.0b013e3181c92b23
7. Shi J, Ji H, Ren F, et al. Protective effects of tranexamic acid on clopidogrel before coronary artery bypass grafting: a multicenter randomized trial. JAMA Surg. 2013;148(6):538–547. doi:10.1001/jamasurg.2013.1560
8. Bhat A, Bhowmik DM, Vibha D, Dogra M, Agarwal SK. Tranexamic acid overdosage-induced generalized seizure in renal failure. Saudi J Kidney Dis Transpl. 2014;25(1):130–132. doi:10.4103/1319-2442.124529
9. Lin Z, Xiaoyi Z. Tranexamic acid-associated seizures: a meta-analysis. Seizure. 2016;36:70–73. doi:10.1016/j.seizure.2016.02.011
10. Couture P, Lebon JS, Laliberté É, et al. Low-dose versus high-dose tranexamic acid reduces the risk of nonischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2017;31(5):1611–1617. doi:10.1053/j.jvca.2017.04.026
11. Myles PS, Smith JA, Forbes A, et al. tranexamic acid in patients undergoing coronary-artery surgery. N Engl J Med. 2017;376(2):136–148. doi:10.1056/NEJMoa1606424
12. Wu Y, Xiong X, Hu Q, Wang M, Wu Y. A seizure case induced by topical application of tranexamic acid in a local incision. Kor J Anesthesiol. doi:10.4097/kja.24931
13. Andersson L, Nilsoon IM, Colleen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 1968;146(2):642–658. doi:10.1111/j.1749-6632.1968.tb20322.x
14. Pilbrant A, Schannong M, Vessman J. Pharmacokinetics and bioavailability of tranexamic acid. Eur J Clin Pharmacol. 1981;20(1):65–72. doi:10.1007/BF00554669
15. Horrow JC, Van Riper DF, Strong MD, Grunewald KE, Parmet JL. The dose-response relationship of tranexamic acid. Anesthesiology. 1995;82(2):383–392. doi:10.1097/00000542-199502000-00009
16. Dowd NP, Karski JM, Cheng DC, et al. Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology. 2002;97(2):390–399. doi:10.1097/00000542-200208000-00016
17. Grassin-Delyle S, Tremey B, Abe E, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111(6):916–924. doi:10.1093/bja/aet255
18. Yang QJ, Jerath A, Bies RR, Wąsowicz M, Pang KS. Pharmacokinetic modeling of tranexamic acid for patients undergoing cardiac surgery with normal renal function and model simulations for patients with renal impairment. Biopharm Drug Dispos. 2015;36(5):294–307. doi:10.1002/bdd.1941
19. Jerath A, Yang QJ, Pang KS, et al. Tranexamic acid dosing for cardiac surgical patients with chronic renal dysfunction: a new dosing regimen. Anesth Analg. 2018;127(6):1323–1332. doi:10.1213/ANE.0000000000002724
20. Strauss ER, Li S, Henderson R, et al. A pharmacokinetic and plasmin-generation pharmacodynamic assessment of a tranexamic acid regimen designed for cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2022;36(8):2473–2482. doi:10.1053/j.jvca.2021.12.029
21. Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi:10.1146/annurev.pharmtox.48.113006.094708
22. Ingrande J, Gutierrez K, Lemmens HJ, et al. Pharmacokinetics of cefazolin and vancomycin in infants undergoing open-heart surgery with cardiopulmonary bypass. Anesth Analg. 2019;128(5):935–943. doi:10.1213/ANE.0000000000003876
23. Wilkinson GR. Pharmacokinetics of drug disposition: hemodynamic considerations. Ann Rev Pharmacol. 1975;15:11–27. doi:10.1146/annurev.pa.15.040175.000303
24. van Saet A, Tibboel D. The influence of cardiopulmonary bypass on pediatric pharmacokinetics. Expert Opin Drug Metab Toxicol. 2023;19(6):333–344. doi:10.1080/17425255.2023.2227556
25. Favié LMA, de Haan TR, Bijleveld YA, et al. Prediction of drug exposure in critically ill encephalopathic neonates treated with therapeutic hypothermia based on a pooled population pharmacokinetic analysis of seven drugs and five metabolites. Clin Pharmacol Ther. 2020;108(5):1098–1106. doi:10.1002/cpt.1917
26. van Saet A, de Wildt SN, Knibbe CA, et al. The effect of adult and pediatric cardiopulmonary bypass on pharmacokinetic and pharmacodynamic parameters. Curr Clin Pharmacol. 2013;8(4):297–318. doi:10.2174/15748847113089990067
27. Adiraju SKS, Shekar K, Fraser JF, Smith MT, Ghassabian S. Effect of cardiopulmonary bypass on cytochrome P450 enzyme activity: implications for pharmacotherapy. Drug Metab Rev. 2018;50(2):109–124. doi:10.1080/03602532.2017.1417423
28. Wesley MC, Pereira LM, Scharp LA, Emani SM, Fx M Jr, DiNardo JA. Pharmacokinetics of tranexamic acid in neonates, infants, and children undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2015;122(4):746–758. doi:10.1097/ALN.0000000000000570
29. Grassin-Delyle S, Theusinger OM, Albrecht R, et al. Optimisation of the dosage of tranexamic acid in trauma patients with population pharmacokinetic analysis. Anaesthesia. 2018;73(6):719–729. doi:10.1111/anae.14184
30. Schnider TW, Minto CF, Struys MM, Absalom AR. The safety of target-controlled infusions. Anesth Analg. 2016;122(1):79–85. doi:10.1213/ANE.0000000000001005
31. Shi J, Zhou C, Pan W, et al. Effect of high- vs low-dose tranexamic acid infusion on need for red blood cell transfusion and adverse events in patients undergoing cardiac surgery: the OPTIMAL randomized clinical trial. JAMA. 2022;328(4):336–347. doi:10.1001/jama.2022.10725
32. Li S, Ahmadzia HK, Guo D, et al. Population pharmacokinetics and pharmacodynamics of Tranexamic acid in women undergoing caesarean delivery. Br J Clin Pharmacol. 2021;87(9):3531–3541. doi:10.1111/bcp.14767
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.