Back to Journals » International Journal of Nanomedicine » Volume 20
Potential Applications of Natural Components of Traditional Chinese Medicine Delivery via Nanoparticle Drug Delivery Systems in the Treatment of Alzheimer’s Disease
Authors Lai G , Wu H , Yang K, Hu K , Zhao W, Chen X , Li J , Wang H , Lv Z, Xie G , Wu X
Received 3 March 2025
Accepted for publication 31 May 2025
Published 17 June 2025 Volume 2025:20 Pages 7781—7810
DOI https://doi.org/10.2147/IJN.S525960
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Guogang Lai,1,* Hao Wu,2,* Kaixia Yang,1 Kaikai Hu,1 Wen Zhao,1 Xiao Chen,1 Jiayi Li,1 Haifeng Wang,1 Zhongyue Lv,1 Guomin Xie,1 Xiping Wu1
1Department of Neurology, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China; 2Ningbo Institute of Innovation for Combined Medicine and Engineering, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiping Wu; Guomin Xie, Department of Neurology, The Affiliated Lihuili Hospital of Ningbo University, Ningbo, Zhejiang, People’s Republic of China, Tel +86 13989315307 ; +86 13957881339, Email [email protected]; [email protected]
Abstract: Alzheimer’s disease (AD), a primary neurodegenerative disorder, is characterized by amyloid-β plaques and tau hyperphosphorylation-induced neurofibrillary tangles. Current treatments only alleviate symptoms, and Aβ monoclonal antibodies raise safety concerns in clinical use. Natural components (NCs) of Traditional Chinese Medicine (TCM) (eg, curcumin, quercetin, berberine, resveratrol) exhibit multi-target neuroprotective effects in AD, but poor blood-brain barrier (BBB) penetration and low bioavailability limit clinical use. Recent strategies to enhance TCM delivery include NP-based nanoparticle (NP) drug delivery systems (NDDS), structural modifications, and combination therapies. NDDS demonstrate superior performance in enabling brain-targeting delivery via passive (paracellular/transcellular) and active (adsorption-/receptor-/carrier-mediated transcytosis) approaches, improving NCs’ stability, controlled release, and bioavailability. With NCs of TCM delivery via NDDS, it is possible to develop intelligent therapeutic systems that combine multi-target regulation with precise drug delivery. This review summarizes the diverse neuroprotective effects of NCs of TCM in AD treatment and discusses the commonly used types of NPs for AD therapy. It particularly focuses on these NCs of TCM delivery via NDDS, covering aspects such as NPs types, fabrication techniques, characteristics, administration routes, and advantages. Finally, the challenges and potential solutions for NCs of TCM were examined, along with comparative advantages and limitations among different NPs and future research directions. Collectively, NCs of TCM delivery via NDDS demonstrate promising therapeutic potential for AD treatment.
Keywords: Alzheimer’s disease, Traditional Chinese Medicine, natural components, nanoparticle drug delivery systems
Introduction
Alzheimer’s disease (AD), the primary cause of dementia, accounts for 60–80% of global dementia cases, affecting nearly 50 million people.1 This number is expected to nearly double to 115 million by 2050, primarily due to aging populations, particularly in low- and middle-income countries.2,3 AD is characterized by progressive cognitive decline, memory loss, and, in advanced stages, personality changes and language impairments.4 While the precise mechanisms remain elusive, key pathological features include the accumulation of β-amyloid (Aβ) plaques, hyperphosphorylation of tau proteins leading to neurofibrillary tangles, and substantial neuronal and synaptic loss.2 Current treatments, such as cholinesterase inhibitors (eg, donepezil), N-Methyl-D-Aspartate receptor antagonists (eg, memantine), and medications targeting neuropsychiatric symptoms, provide only limited relief and often present significant side effects. Though newer drugs, including aducanumab and lecanemab, have been approved, their high costs and safety concerns restrict their accessibility.5,6 Therefore, the development of safe, affordable, and effective therapies remains crucial for improving the outcomes of patients with AD.
Traditional Chinese medicine (TCM) has been employed in the treatment of central nervous system (CNS) disorders for over two millennia.7 In AD, various natural components (NCs) of TCM, such as curcumin (Cur), quercetin (QT), berberine (BBR), and resveratrol (RES), have demonstrated considerable neuroprotective effects. Their mechanisms primarily involve inhibiting Aβ production and aggregation, modulating tau protein hyperphosphorylation, suppressing acetylcholinesterase (AChE) activity, and exerting antioxidant and anti-inflammatory actions. However, the clinical applicability of these NCs is hindered by their poor oral bioavailability and the restrictive nature of the blood-brain barrier (BBB), which prevents sufficient CNS concentrations and limits their therapeutic effectiveness. Consequently, enhancing the bioavailability and brain delivery efficiency of TCM has become a pivotal research focus.
Recent studies have explored various strategies to address these challenges, including nanoparticle (NP) drug delivery systems (NDDS), structural modifications of NCs, and combination therapies. Among these, NP-based NDDS have gained widespread use in disease treatment, offering innovative solutions to overcome the limitations of conventional drugs.8–10 NPs, as drug carriers, offer numerous advantages, including improved bioavailability, targeted delivery, enhanced drug concentration at the site of action, and reduced side effects.11–13 Additionally, NPs can traverse the BBB via active or passive targeting mechanisms, facilitating efficient CNS delivery.14–16 The feasibility of NDDS in AD treatment has been validated in numerous studies.16–18 Thus, employing NPs to deliver NCs of TCM not only enhances the multi-target therapeutic potential of these compounds but also significantly improves their efficacy, making this approach a highly promising strategy.
This review examined the neuroprotective effects of TCM in AD, focusing on the inhibition of Aβ generation and deposition, alleviation of tau protein hyperphosphorylation, suppression of AChE activity, and reduction of oxidative stress and neuroinflammation. The review further explored the types of NPs commonly used in AD treatment and highlights advancements in the delivery of TCM-derived NCs through NDDS. Key topics include the types of nanomedicines, fabrication processes, properties, routes of administration, and their respective advantages. Finally, the challenges and potential solutions for improving the delivery of NCs of TCM were discussed, alongside a comparison of the advantages and limitations of different NP types and future research directions.
The Neuroprotective Effect of Natural Components of Traditional Chinese Medicine on Alzheimer’s Disease
AD is a neurodegenerative disorder driven by multiple pathological mechanisms,19 including: 1) Aβ accumulation and deposition: Under normal physiological conditions, amyloid precursor protein (APP) is cleaved via the α-secretase/γ-secretase pathway. However, aberrant cleavage through the β-secretase (BACE1)/γ-secretase pathway results in the production of Aβ, which is secreted from cells and eventually aggregates into amyloid plaques.20 2) Tau hyperphosphorylation: In pathological conditions, excessive or abnormal phosphorylation of tau protein impedes its ability to assemble into microtubules, destabilizing the microtubule network, leading to depolymerization and axonal dysfunction, which in turn causes neuronal degeneration.21 3) Oxidative stress: This arises from an imbalance between pro-oxidants and antioxidants in the body. The brain, with its high oxygen consumption and relatively low antioxidant defense, is particularly vulnerable to oxidative damage. Persistent redox imbalance accelerates disease progression, contributing to the core neuropathological features of AD, such as neuronal degeneration, protein aggregates (including Aβ plaques and tau tangles), and neuroinflammation.22 Inflammation exacerbates neuronal damage, leading to cellular dysfunction and eventual neuronal death.23 4) Cholinergic neuronal degeneration: Cholinergic neurons, which secrete acetylcholine (ACh) vital for learning and memory, undergo degeneration in AD, reducing ACh levels.24 Additionally, elevated AChE activity further shortens ACh’s half-life.25,26
Emerging research indicates that these pathological mechanisms are interrelated. For instance, Aβ can trigger tauopathy, oxidative stress, and inflammation,27,28 while oxidative stress and inflammation exacerbate the production and aggregation of Aβ and tau.29 Consequently, therapeutic approaches should adopt a multi-target strategy rather than focusing on a single mechanism. Nanocarriers (NCs) derived from TCM, such as Cur, QT, BBR, and RES, exhibit various neuroprotective effects, including reducing Aβ accumulation, mitigating tau hyperphosphorylation, inhibiting AChE activity, and alleviating oxidative stress and neuroinflammation (Figure 1).26,30–33 However, the exact mechanisms underlying these neuroprotective effects remain unclear, necessitating further investigation into the pharmacological potential of TCM-based NCs. This section explores several TCM-derived NCs and their neuroprotective effects in AD (Table 1).
 |
Table 1 The Neuroprotective Effects of Different Traditional Chinese Medicines |
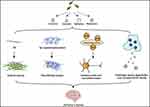 |
Figure 1 Neuroprotective effect of Traditional Chinese Medicine on Alzheimer’s disease. |
The Neuroprotective Effect of Curcumin on Alzheimer’s Disease
Numerous studies suggest that Cur offers neuroprotective benefits by reducing the formation and aggregation of Aβ. Zhang et al examined the effects of Cur on Aβ levels and APP processing in primary cortical neurons from mice and various cell lines.34 Their findings demonstrated that Cur effectively lowered Aβ levels by impairing APP maturation in the secretory pathway. Increasing evidence highlights the potential of activating the Wnt/β-catenin signaling pathway for neuroprotection in AD.80 Moreover, Aβ induces the downregulation of this pathway, contributing to disease progression. Cur has been shown to reduce Aβ generation and accumulation in a concentration- and time-dependent manner by activating the Wnt/β-catenin pathway, with this activation occurring via inhibition of glycogen synthase kinase-3 beta (GSK-3β) activity.35 Notably, presenilin 1 (PS1), a substrate of GSK-3β and an essential component of γ-secretase, is involved in this process, ultimately reducing Aβ production. In AD, impaired autophagy hampers Aβ clearance, exacerbating disease progression. Enhancing autophagy to promote Aβ clearance is a promising therapeutic strategy. Furthermore, the inhibition of mammalian target of rapamycin (mTOR) has been shown to positively regulate autophagy, with mTOR being primarily controlled by the phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/mTOR signaling pathway.81 While Cur’s anti-cancer effects, achieved through inhibition of the PI3K/Akt/mTOR pathway, are well-documented, its potential for treating AD via this mechanism remains unclear. Wang et al investigated Cur’s effects on autophagy in APP/PS1 double transgenic mice, finding that Cur downregulated the PI3K/Akt/mTOR signaling pathway, thereby enhancing autophagy and facilitating Aβ clearance.36
Further studies suggest that Akt activation can inhibit GSK-3β activity, thereby reducing tau hyperphosphorylation. SoukhakLari et al observed that Cur, particularly at doses of 50 and 100 mg/kg, prevented scopolamine-induced memory impairment in mice.37 This protective effect was attributed to Akt activation and GSK-3β inactivation. These findings were corroborated by Wang et al, who demonstrated that combining Cur with exosomes enhances its bioavailability and improves cognitive deficits in mice induced by OA.38 Additionally, Caveolin-1 has been implicated in GSK-3β-related signaling. Sun et al investigated the expression levels of Caveolin-1, GSK-3β, and tau following Cur treatment to explore potential mechanisms.39 The results suggest that Cur may reduce tau hyperphosphorylation in the brains of AD mice by inhibiting the Caveolin-1/GSK-3β pathway.
The effects of Cur on oxidative stress and inflammation have been extensively investigated. Over 20 years ago, Lim et al explored the neuroprotective effects of two different Cur doses (160 ppm and 5000 ppm) in mice with AD.40 Their findings indicated that both doses reduced oxidative damage and inhibited the inflammatory cytokine interleukin-1 (IL-1) without significant side effects. Notably, the lower dose of Cur further decreased Aβ levels by approximately 43–50%. Aβ is known to activate microglia, leading to the production of inflammatory cytokines. While Cur has been shown to inhibit lipopolysaccharide (LPS)-induced microglial activation, its effects on Aβ-induced microglial activation remain unclear. To address this, Shi et al examined the impact of Cur on Aβ-induced microglial activation.41 Their results demonstrated that Cur inhibited microglial activation and downregulated IL-1, interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) levels, with this effect being positively correlated with Cur dose. Further studies suggested that this inhibition might be mediated through the phosphorylation of ERK1/2 and P38. Cur also activates peroxisome proliferator-activated receptor gamma (PPARγ), which has been shown to suppress neuroinflammation induced by cerebral ischemia. However, its role in AD remains less understood. Liu et al demonstrated that Cur enhances PPARγ activity, inhibits the nuclear factor kappa-B (NF-κB) pathway, and alleviates neuroinflammatory responses.42 Additionally, Cur activates the adenosine 5′-monophosphate-activated protein kinase (AMPK) pathway, thereby inhibiting inflammation and oxidative stress. Specifically, Cur promotes the phosphorylation of AMPK in the brains of AD mice, resulting in a reduction in inflammatory factors (TNF-α, IL-6, interleukin-1β [IL-1β]) and an increase in superoxide dismutase (SOD) levels.43
In addition to its effects on oxidative stress and inflammation, Cur also inhibits AChE. Ahmed et al evaluated Cur’s inhibitory effects on AChE in scopolamine-induced AD rats.44 The inhibition of AChE increased with Cur doses ranging from 3 to 10 mg/kg. Another study found a similar dose-dependent inhibition of AChE in cadmium-induced AD rats, with Cur doses ranging from 12.5 to 25 mg/kg.45 However, experiments from the same group revealed no significant difference in AChE inhibition with Cur doses between 25 and 50 mg/kg.46
The Neuroprotective Effect of Quercetin on Alzheimer’s Disease
QT has demonstrated significant neuroprotective effects against Aβ aggregation. Treatment with QT in triple-transgenic AD (3xTg-AD) mice has been shown to reduce levels of Aβ1-40 and Aβ1-42, thereby improving cognitive function.47 Moreover, preventive oral administration of QT (100 mg/kg) also decreased Aβ levels in 3xTg-AD mice, mitigating cognitive decline.48 Elfiky et al further discovered that QT may reduce Aβ aggregation in aluminum chloride (AlCl3)-induced AD rats by upregulating the expression of ADAM10 and ADAM17 genes.49
Okadaic acid (OA) induces tau hyperphosphorylation in neuronal cells. Jiang et al created an in vitro AD model using HT22 cells and treated it with QT, demonstrating that QT significantly reduced OA-induced tau hyperphosphorylation.50 This neuroprotective effect appears to be mediated through the PI3K/Akt/GSK3β signaling pathway.
In addition to its effects on Aβ and tau, QT exerts neuroprotective benefits through its antioxidant and anti-inflammatory properties. An in vitro study showed that QT protected neurons from Aβ-induced toxicity.51 At higher concentrations (20 and 40 μM), QT increased levels of 4-hydroxynonenal and 3-nitrotyrosine, indicating antioxidant activity, although this was not observed at lower concentrations (5 and 10 μM). Interestingly, QT mitigated Aβ neurotoxicity at both lower (5 and 10 μM) and higher (20 and 40 μM) concentrations, with no significant difference observed between the concentration groups. Cheng et al assessed the impact of QT on cognitive function in APP/PS1 mice, finding that QT improved cognitive abilities by increasing levels of glutathione (GSH), malondialdehyde (MDA), and SOD while decreasing ROS levels.52 This neuroprotective effect appears to be linked to QT’s activation of the Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor erythroid 2-related factor (Nrf2)/Heme Oxygenase-1 (HO-1) pathway. Lasure et al observed elevated levels of the astrocyte marker GFAP in the brains of Aβ1-42-induced AD mice, suggesting the activation of inflammatory pathways within astrocytes, along with increased levels of pro-inflammatory cytokines.33 QT treatment significantly reduced GFAP and pro-inflammatory cytokine levels in a dose-dependent manner within the 30–100 mg/kg range.
Additionally, QT has been shown to inhibit AChE activity. In cadmium-exposed rats, QT treatment at doses of 5, 25, and 50 mg/kg reduced AChE activity by 23%, 49%, and 54%, respectively.53 Similarly, a 50 mg/kg dose of QT prevented the increase in AChE activity in the cortex and hippocampus induced by diabetes, leading to restored memory in diabetic rats.54
The Neuroprotective Effect of Berberine on Alzheimer’s Disease
BBR effectively reduces the production and accumulation of Aβ, demonstrating significant neuroprotective effects. Previous studies have shown that BBR inhibits BACE1 activity, thereby reducing Aβ production and accumulation.55 Additionally, BBR enhances α-secretase activity, further decreasing Aβ levels in the hippocampus of AD mice. BBR has also been reported to activate autophagy through the class III PI3K/Beclin1 and AMPK/mTOR signaling pathways, promoting Aβ clearance.57,58 Notably, BBR improves cerebral vascular structure, function, and blood flow, facilitating the removal of Aβ and other toxic substances, which ultimately enhances cognitive function in AD mice.82 These findings confirm that BBR effectively reduces Aβ formation and accumulation.
BBR has also been shown to alleviate tau hyperphosphorylation, although the precise mechanism remains unclear.26,83 It has been observed that BBR reduces tau hyperphosphorylation by regulating the AKT/GSK-3β signaling pathway.59 Other studies suggest that BBR mitigates tau hyperphosphorylation by enhancing PP2A activity and reducing GSK-3β activity.60,61 Additionally, BBR promotes autophagy via the class III PI3K/Beclin1 pathway, facilitating tau clearance.59 Collectively, BBR plays a pivotal role in mitigating tau hyperphosphorylation.
Due to its antioxidant and anti-inflammatory properties, BBR is a promising candidate for AD treatment. BBR has been shown to alleviate cellular oxidative stress by increasing brain levels of GSH and GSH peroxidase (GPX).62–64 Further studies suggest that BBR exerts antioxidant effects by activating Nrf2, which regulates GSH, GPX4, and HO-1. Moreover, BBR inhibits the NF-κB signaling pathway in AD mice, reducing inflammatory cytokines such as IL-1β, TNF-α, and IL-6, thereby suppressing the inflammatory response and improving cognitive function.65 Microglia, which play a role in the neuroinflammatory process associated with AD,84 are also targeted by BBR, which inhibits their inflammatory response via the AMPK pathway.66 While the exact mechanisms require further exploration, the antioxidant and anti-inflammatory effects of BBR are well established.
BBR has been shown to inhibit AChE activity, contributing to improved cognitive function and memory in AD.67 In neuroblastoma cells (SH-SY5Y), BBR inhibited AChE activity.85 In a sporadic AD mouse model induced by intracerebroventricular injection of streptozotocin, BBR inhibited AChE activity and improved spatial learning and memory.68 Similarly, BBR reversed memory impairment in scopolamine-induced dementia mice by inhibiting AChE activity.69 Overall, BBR is a valuable therapeutic agent for improving AD symptoms through the inhibition of AChE activity.
The Neuroprotective Effect of Resveratrol on Alzheimer’s Disease
Previous studies suggested that RES promotes Aβ degradation not by directly affecting enzymes involved in Aβ production and secretion, but rather through proteasome-mediated mechanisms that inhibit Aβ generation.70 However, subsequent research has indicated that RES can reduce Aβ production by inhibiting BACE1.71 Additionally, some studies propose that RES decreases Aβ generation by inhibiting γ-secretase.72 These conflicting findings may arise from variations in the cell types utilized.
He et al were the first to demonstrate that RES mitigates formaldehyde-induced neuronal damage, potentially through tau protein dephosphorylation via reduced GSK-3β activity.73 Shati et al explored whether RES modulates the PI3K/Akt/GSK-3β signaling pathway to alleviate tau hyperphosphorylation.74 In vivo studies revealed that RES activates the PI3K/Akt/GSK-3β pathway and improves tau phosphorylation levels in AD rats. Given that protein phosphatase 2A (PP2A), a major tau phosphatase, shows reduced expression and activity in the brains of patients with AD, modulating PP2A activity may help alleviate tau hyperphosphorylation. Notably, RES has been shown to regulate PP2A, improving tau hyperphosphorylation and exerting neuroprotective effects.75
AMPK plays a pivotal role in the inflammatory and oxidative stress response. RES has been identified as an AMPK activator, suggesting its potential in mitigating neuroinflammation and oxidative damage. Chiang et al leveraged this property by treating Aβ-induced oxidative stress and neuroinflammation in human neural stem cells (hNSCs) with RES.76 Compared to controls, RES-treated hNSCs exhibited restored GSH, GPX1, and HO-1 levels, alongside a significant reduction in inflammatory cytokines (IL-1β and TNF-α). Similar results were observed in an AD mouse model induced by intracerebroventricular injection of Aβ and treated with RES.77
Furthermore, RES has been reported to inhibit AChE activity. Notably, multiple studies consistently show that RES treatment suppresses hippocampal AChE activity in rats with scopolamine-induced memory impairment.78,79
Limitations and solutions of natural components of Traditional Chinese Medicine in treating Alzheimer’s disease
Despite the promise of NDDS in enhancing the neuroprotective effects of TCM NCs, several challenges remain (Figure 2). First, the NCs of TCM are complex, and the primary active substances and mechanisms of action are not fully understood. Second, most NC formulations lack large-scale randomized controlled trials (RCTs) to validate their efficacy in AD treatment, necessitating improvements in clinical evidence. Lastly, improper combinations or dosages may lead to adverse drug reactions, such as the increased risk of hypoglycemic episodes when combining BBR with gliclazide.86 To address these challenges, future research should systematically integrate multi-omics technologies, including transcriptomics, proteomics, and metabolomics, to thoroughly analyze the therapeutic targets and molecular mechanisms of TCM NCs. Network pharmacology approaches should be utilized to predict key active components. Building on these insights, rigorously designed RCTs must be conducted in strict adherence to drug development standards. Moreover, a multidisciplinary evaluation system encompassing pharmacodynamics, safety, and clinical efficacy should be established, along with comprehensive risk assessment and management protocols. These measures will ensure the effectiveness and safety of TCM NCs in AD treatment, ultimately facilitating their clinical translation and application (Figure 2).
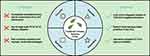 |
Figure 2 Limitations and solutions of natural components of Traditional Chinese Medicine in treating Alzheimer’s disease. |
Nanoparticle Drug Delivery Systems for Alzheimer’s Disease
NDDS have garnered considerable attention for their potential to overcome the challenges associated with AD treatment, particularly in circumventing the BBB, which limits the penetration of most drugs into the brain. Efflux transporters, such as P-glycoprotein (P-gp), further restrict drug concentrations,87 while traditional administration methods often result in suboptimal therapeutic effects and significant side effects.11,13 NDDS present a promising solution by enhancing drug bioavailability, enabling targeted delivery, and minimizing toxicity. The brain-targeting delivery mechanisms of NPs primarily involve passive and active targeting strategies.88 Passive targeting leverages the intrinsic physicochemical properties of NPs (eg, size, lipophilicity) and the physiological/pathological conditions of the BBB, allowing penetration through paracellular pathways (eg, tight junction disruption) or transcellular pathways (eg, passive diffusion). However, this approach often suffers from limited efficiency and specificity. In contrast, active targeting aims to deliver drugs specifically to target cells or tissues through mechanisms such as receptor-mediated transcytosis (RMT), adsorption-mediated transcytosis (AMT), and carrier-mediated transcytosis (CMT). RMT utilizes surface-modified ligands (eg, transferrin [TF]) that selectively bind to BBB endothelial cell receptors (eg, TF receptors), triggering endocytosis and transcytosis. AMT relies on electrostatic interactions between cationic carriers and negatively charged cellular membranes, promoting nonspecific endocytosis. CMT involves active internalization and intracellular delivery facilitated by functional carriers (eg, albumin), either naturally or through modification. In the realm of brain drug delivery, intranasal administration (nose-to-brain delivery) is a non-invasive method that bypasses the BBB via specialized pathways, including the olfactory and trigeminal nerve routes.89 This approach minimizes systemic drug accumulation in non-target tissues while achieving precise delivery to cerebral tissues. The olfactory pathway involves passive diffusion of small molecules through the olfactory epithelium to the olfactory bulb, while macromolecules are absorbed via receptor-mediated endocytosis followed by axonal transport along olfactory nerve fibers to the CNS through olfactory cortical projections.90 The trigeminal pathway involves drug absorption in the nasal respiratory region, with small molecules diffusing passively, and macromolecules entering trigeminal nerve terminals via receptor-mediated endocytosis, followed by bidirectional axoplasmic transport along trigeminal nerve axons to the CNS.91 Additionally, NDDS enhance pharmacokinetics and pharmacodynamics by encapsulating drugs within nanoscale particles, offering benefits such as improved solubility, controlled release, and reduced off-target effects.16,92–94 Among various NPs, polymeric, lipid-based, and protein-based NPs are particularly notable for their advantages, making them key subjects of research in AD therapy (Table 2). Overall, NDDS hold the potential to revolutionize the treatment of neurological disorders like AD by improving drug efficacy, safety, and targeted delivery.
 |
Table 2 Application of Nanoparticle Drug Delivery System in Alzheimer’s Disease |
Polymeric Nanoparticles
Poly(lactic-co-glycolic acid) (PLGA), a widely studied copolymer composed of lactic acid (LA) and glycolic acid (GA), exhibits excellent drug release properties in the NP form.112 By adjusting the LA/GA ratio, surface functionalization, and increasing the relative molecular weight of PLGA, improved drug release profiles can be achieved.112 Additionally, PLGA boasts favorable biocompatibility, biodegradability, and non-immunogenicity, minimizing adverse drug reactions. Donepezil, an FDA-approved drug for treating AD, is typically administered orally for long-term therapy. However, oral administration often leads to gastrointestinal side effects, such as diarrhea and nausea.93 To address this, researchers have developed donepezil-loaded PLGA NPs to reduce side effects while prolonging drug release and enhancing brain drug concentration. In vitro studies demonstrated that these NPs significantly improved BBB permeability and effectively disrupted Aβ fibril formation.95 In vivo experiments also showed notable improvements in learning and memory in rats treated with these NPs.96 Encapsulation of donepezil in PLGA has been reported to reduce Aβ accumulation and neuronal damage in mice, improving cognitive function.97 Furthermore, PLGA NPs loaded with donepezil significantly reduced AChE activity in the brain compared to donepezil alone, reinforcing the potential of PLGA as an effective delivery system.98
Chitosan (CS), a naturally occurring polymer, is known for its low immunogenicity, biodegradability, and excellent biocompatibility.113 Its unique adhesive properties and cationic nature enable strong interactions with negatively charged cell membranes, enhancing drug-targeting efficiency.114 These properties also extend its retention time on the nasal mucosa, making it ideal for intranasal delivery to the brain.114 Intranasal administration bypasses the BBB, avoids hepatic metabolism, minimizes systemic side effects, and provides rapid drug onset, making it an effective alternative drug delivery approach.115 Galantamine, an FDA-approved reversible AChE inhibitor, modulates nicotinic receptors and inhibits Aβ aggregation.116 Despite its high bioavailability (90%–100%), oral and injectable formulations often cause gastrointestinal disturbances and muscle tremors. To mitigate these effects, Hanafy et al developed intranasal galantamine/CS composite NPs (CX-NP2).94 Experimental results confirmed that CX-NP2 significantly reduced AChE protein levels and activity in rat brains without inducing toxicity, demonstrating both safety and efficacy. ElMosbah et al explored the therapeutic efficacy of oral versus intranasal administration of rivastigmine-loaded CS NPs.99 Notably, intranasal delivery led to superior therapeutic outcomes, as evidenced by histopathological findings and modulation of inflammatory pathway expression.
Polymeric micelles (PMs) are self-assembled NPs composed of amphiphilic copolymers designed for optimal hydrophobicity/lipophilicity balance, NP size, drug loading efficiency, and stability in systemic circulation.117 However, they are prone to premature drug release. To address this, researchers have employed stimuli-responsive strategies (eg, temperature, pH, and magnetic) to achieve controlled drug release.118 Certain polymers, such as Pluronic and D-α-tocopheryl polyethylene glycol succinate (TPGS), can inhibit P-gp, enhancing intracellular drug accumulation by blocking efflux pumps and thereby improving therapeutic efficacy.119 Guareschi et al developed cyclosporine A-loaded TPGS micelles for AD treatment, demonstrating that intranasal administration increased cyclosporine A concentrations in the brain and enhanced its anti-inflammatory effects, likely through P-gp efflux inhibition.100
Lipid-Based Nanoparticles
Liposomes (Lips) are spherical vesicles composed of a phospholipid bilayer, typically ranging from 50 to 100 nm in size. They can encapsulate both hydrophilic and hydrophobic drugs, enhancing drug stability and extending circulation time.120 Surface functionalization of Lips significantly improves drug solubility and bioavailability in brain regions, making them an effective tool for brain-targeted drug delivery. Lee et al utilized Lips to deliver donepezil, memantine, and BACE1 siRNA, evaluating their in vitro BBB penetration and therapeutic effects on AD.101 The results demonstrated that Lip-encapsulated drugs exhibited significantly higher BBB permeability compared to free drugs, with a more pronounced reduction in Aβ deposition and suppression of pro-inflammatory cytokine expression. These findings were further confirmed through in vivo experiments.102 Notably, the surface charge of Lips can be easily modified, and both cationic and anionic Lips offer distinct advantages for AD therapy.121 Cationic Lips enhance cellular uptake through electrostatic interactions with the anionic surfaces of endothelial cell membranes. Yang et al engineered cationic cell-penetrating peptide (CPP)-modified rivastigmine Lips (CPP-Lp) for intranasal delivery.103 In vitro BBB model evaluations demonstrated that CPP-Lp exhibited significantly greater BBB penetration efficiency compared to unmodified Lips. In vivo studies revealed that intranasal administration of these formulations substantially improved drug accumulation and retention in CNS regions, particularly the hippocampus and cerebral cortex, when compared to intravenous injection. Histopathological assessments confirmed minimal nasal mucosal irritation following CPP-Lp treatment. Remarkably, CPP-Lp administration resulted in sustained and potent inhibition of AChE activity, attributed to its prolonged release profile and enhanced tissue retention. In contrast, anionic Lips, such as phosphatidylserine (PS)-incorporated Lips, modulate microglial polarization by suppressing pro-inflammatory cytokines (eg, TNF-α, IL-6) and enhancing anti-inflammatory factors (eg, IL-10, TGF-β), thereby alleviating Aβ-induced neuroinflammation.122 Saffari et al successfully developed PS-based Lip NPs for metformin (MET) delivery (MET-PSL) in AD treatment.104 Experimental results showed that, compared to free MET, the MET-PSL treatment group exhibited significantly reduced levels of hippocampal inflammatory cytokines (IL-1β, TNF-α, and TGF-β), confirming the pronounced anti-neuroinflammatory efficacy of this NDDS.
Solid lipid NPs (SLNs) are nano-carriers that utilize solid lipids as their core matrix. These NPs are considered safer than metal and polymer-based carriers due to the absence of organic solvents during the manufacturing process.123 SLNs offer various advantages, including the ability to cross the BBB, high stability, high drug loading capacity, low toxicity, and controlled drug release.8 In comparison to traditional Lips, which suffer from low drug loading capacity and short drug release times, SLNs offer significant advantages. Erythropoietin (EPO), known for its neuroprotective effects in AD, has shown promise in improving the pathological manifestations of the disease.124 However, its poor ability to cross the BBB remains a major limitation. Dara et al addressed this by loading EPO into SLNs (EPO-SLN) for AD treatment.105 The results demonstrated that EPO-SLN was more effective than free EPO in reducing Aβ deposition and alleviating oxidative stress, providing enhanced neuroprotection. Another study utilized SLNs as carriers to co-load tramiprosate (TMPS, which inhibits Aβ monomer oligomerization) and memantine hydrochloride (MeHCl), creating an M+T SLN drug delivery system to enhance neuroprotective effects.106 Both in vitro and in vivo studies showed that M+T SLNs outperformed individual drugs in inhibiting Aβ deposition. In addition to intranasal administration, the facial trigeminal nerve pathway has emerged as an effective route for brain drug delivery. Permana et al developed a thermosensitive gel loaded with rivastigmine SLNs, which was delivered to the brain via facial intradermal injection.107 Compared to intravenous administration, facial intradermal injection significantly enhanced drug accumulation in the brain.
Nanostructured lipid carriers (NLCs), considered a new generation of SLNs, are composed of a blend of solid and liquid lipids.125 NLCs aim to address some limitations of SLNs, such as enhancing drug loading capacity and preventing drug release during storage.125 Cunha et al developed a thermosensitive in situ nasal gel based on NLCs loaded with rivastigmine (G-RVG-NLC).108 Compared to free rivastigmine, G-RVG-NLC demonstrated significantly enhanced delivery efficiency. Studies showed that G-RVG-NLC not only prolonged drug retention time on the nasal mucosa but also facilitated controlled drug release via its thermosensitive properties, significantly improving brain-targeting efficiency through intranasal administration. This approach offers a highly effective nasal delivery strategy for AD treatment. Another study evaluated the brain delivery efficiency of donepezil-loaded NLCs (DNZ NLCs).109 The results revealed that DNZ NLCs significantly enhanced drug utilization compared to free DNZ, with intranasal administration achieving brain drug concentrations nine times higher than intravenous administration, further underscoring the superiority of intranasal delivery for brain-targeted drug delivery.
Protein-Based Nanoparticles
Serum albumin, the most abundant functional protein in human plasma, has emerged as one of the most clinically translatable NCs for neurological disorders. Its unique molecular structure enables efficient binding of both lipophilic and hydrophilic drugs, while its low immunogenicity, biodegradability, and excellent biocompatibility make it an attractive option.126 Among various albumin variants, bovine serum albumin (BSA) and human serum albumin (HSA) are the most widely utilized protein-based drug delivery platforms. In a pioneering study, Luppi et al systematically characterized tacrine-loaded BSA NPs, demonstrating favorable physicochemical properties for drug delivery: the NCs exhibited a monodisperse size distribution (mean diameter <300 nm) and a negative surface charge, which collectively enhanced drug permeation across biological barriers and provided remarkable mucoadhesive properties.110 These findings strongly support the potential application of BSA-based NPs in intranasal delivery systems for targeted AD therapy. In another study, Avachat et al developed HSA NPs loaded with RT to enhance sustained drug release.111 In vitro evaluations revealed significantly modulated release kinetics: free RT exhibited rapid release (86% within 5 hours), whereas the HSA-based formulation achieved a prolonged release (53.93% over 12 hours), highlighting its potential for controlled drug delivery. From a clinical translation and safety perspective, HSA demonstrates superior potential as a drug carrier compared to other alternatives, due to its low immunogenicity and established regulatory approval history, as evidenced by FDA-approved HSA-based therapeutics such as Abraxane (paclitaxel-bound albumin NPs) for cancer treatment.127 These findings underscore the significant potential of HSA as a nanocarrier platform for AD therapy.
Advantages and Limitations of Different Delivery Systems
Numerous NPs have been successfully developed, with polymeric NPs, lipid-based NPs, and protein-based NPs emerging as the three primary delivery systems (Figure 3). Polymeric NPs offer distinct advantages, including controlled release and a broad drug-loading capacity for various therapeutic agents. However, unmodified polymeric NPs exhibit limited BBB permeability, requiring surface functionalization to enhance penetration efficiency. Additionally, degradation byproducts, such as LA from PLGA, may disrupt the delicate brain microenvironment. Lipid-based NPs exhibit excellent biocompatibility due to their biomimetic phospholipid bilayer structure, making them especially effective for delivering lipophilic compounds and nucleic acids. Despite their advantages, these systems face challenges such as rapid clearance by serum proteins, which can be mitigated by PEGylation to extend circulation time,128 suboptimal targeting specificity relying mainly on passive accumulation,103 and limited loading efficiency for hydrophilic drugs. Protein-based NPs possess unique endogenous BBB transport capabilities, mediated through gp60 receptors and SPARC (secreted protein acidic and rich in cysteine)-facilitated pathways.129 Their inherent antioxidative and anti-inflammatory properties offer additional therapeutic benefits for neurodegenerative disorders. However, the high production costs of materials like HSA limit their applicability, and current formulations are mainly restricted to hydrophobic small molecules, presenting difficulties in delivering nucleic acids or large protein-based therapeutics.
 |
Figure 3 Advantages and limitations of different delivery systems. |
As research progresses, single-component nanomaterials are proving insufficient to meet the complex therapeutic needs of various diseases. The synergistic integration of multiple nanomaterials into composite NPs is expected to offer a more efficient drug delivery solution. Additionally, the safety profile of nanomedicines remains a critical concern. Encapsulating drugs within biomimetic nanomaterials may significantly enhance their biocompatibility and safety, potentially mitigating some of the current challenges. These innovative approaches open new frontiers for future research, with the potential to drive significant advancements in the field of nanomedicine.
The potential application of Natural components of Traditional Chinese Medicine delivery via nanoparticle drug delivery systems in the treatment of Alzheimer’s disease
As discussed earlier, NCs of TCM exhibit various neuroprotective effects, making them promising therapeutic agents for AD.130 However, their low bioavailability and limited BBB penetration have hindered their therapeutic potential and clinical application.131 NDDS can overcome these limitations by encapsulating NCs of TCM in NPs, improving their bioavailability and targeting them to the brain. NDDS also allow for prolonged release, reducing dosing frequency and enhancing patient compliance. Thus, the delivery of NCs of TCM via NDDS represents a promising and feasible approach for AD treatment. The following section discusses several NCs of TCM that have been studied in delivery via NDDS for AD treatment and research. This section summarizes their therapeutic effects, mechanisms of action, the structure of NPs used for drug loading, and their delivery mechanisms (Supplementary Tables 1–4).
Curcumin
Curcumin Delivery via Polymeric Nanoparticles for the Treatment of Alzheimer’s Disease
Over a decade ago, Mathew et al successfully using a single emulsion solvent evaporation method and synthesized Cur-PLGA NPs by encapsulating Cur in PLGA.132 These NPs maintained the physicochemical properties of Cur while demonstrating good water solubility. In vitro studies indicated that this formulation could inhibit Aβ aggregation, exhibiting potential anti-AD effects. In another study, the team conjugated an anti-Aβ protein aptamer (NN2) to the Cur-PLGA NPs to further reduce Aβ aggregation.133 Additionally, the Tet-1 peptide, which specifically targets neurons and facilitates retrograde delivery within neuronal cells, was combined with Cur-PLGA NPs (Tet-Cur-PLGA). This formulation demonstrated anti-Aβ and antioxidant properties in vitro.134 Interestingly, Tet-Cur-PLGA was more effective than Cur-PLGA in inhibiting Aβ aggregation, while showing comparable antioxidant activity to Cur-PLGA. Although these NP formulations hold promise for AD treatment, in vivo validation is still pending. Fan et al conjugated the B6 peptide, a brain-targeting peptide, to Cur-loaded NPs (PLGA-PEG-B6/Cur) for testing in transgenic mice.135 They found that PLGA-PEG-B6/Cur significantly enhanced the spatial learning abilities and memory function of the mice. Furthermore, it reduced Aβ formation and deposition, and decreased tau protein phosphorylation levels. In another study, researchers enhanced the targeting capability of Cur-PLGA NPs by modifying their surface with a glycopeptide (g7) capable of crossing the BBB. In vitro studies demonstrated that Cur-PLGA-g7 was more effective than Cur-PLGA in reducing Aβ aggregation.136 Huang et al developed a novel NP formulation, CRT-PLGA-S1-Cur, to improve AD treatment.137 This formulation combines PLGA loaded with Cur and the S1 peptide, an Aβ generation inhibitor, and is further modified with the brain-targeting peptide CRT, which targets the TF receptor to enhance BBB permeability. The results indicated that CRT-PLGA-S1-Cur exhibited the most significant improvement in transgenic AD mice compared to other treatment groups, including Cur alone, PLGA-Cur, and PLGA-S1-Cur. Additionally, CRT-PLGA-S1-Cur significantly reduced levels of Aβ, oxidative stress, and inflammatory factors in the brains of AD mice. Similar findings were reported in another study using this NP formulation.138 Huo et al reported that Cur-loaded selenium-PLGA (Cur/Se-PLGA) nanospheres enhanced BBB penetration, attributed to the presence of selenium NPs.139 These nanospheres significantly reduced Aβ aggregation and decreased inflammation in AD mice compared to Cur and Cur-PLGA. Doggui found that PLGA synthesized with a GA to LA ratio of 65:35 (Cur-PLGA 65:35) exhibited small size and high encapsulation efficiency.140 In vitro studies demonstrated that Cur-PLGA 65:35 protected SK-N-SH cells from H2O2-induced damage by inducing Nrf2 expression. Djiokeng Paka et al emphasized the importance of controlling drug release capacity as a critical parameter for enhancing nano-delivery efficiency. By adjusting the ratio of GA to LA to 50:50, they improved the overall efficiency of the drug delivery system.141 Research showed that Cur-PLGA 50:50 released Cur at a faster rate compared to Cur-PLGA 65:35. Moreover, Cur-PLGA 50:50 demonstrated superior anti-inflammatory and antioxidant activities compared to free Cur. The Wnt/β-catenin pathway plays a critical role not only in regulating Aβ levels but also in neuronal development, differentiation, and self-renewal. Targeting this pathway has thus emerged as a promising strategy for AD treatment. Tiwari et al observed that Cur-PLGA NPs mitigated the toxic effects of Aβ on neurogenesis in AD mice and enhanced the brain’s self-repair mechanisms, likely due to Cur’s activation of the Wnt/β-catenin pathway.142 This approach proved to be more effective than Cur alone. Sublingual administration is an effective strategy for enhancing patient compliance and reducing dosing frequency compared to oral administration. Yekeler et al pioneered the use of 3D printing to embed Cur-loaded PLGA NPs into a sodium alginate/gelatin matrix.143 This matrix dissolves rapidly in artificial saliva (dissolution time of 2.3 seconds), enabling sublingual drug delivery. In vitro studies suggest that Cur may exert neuroprotective effects in AD treatment by modulating the Wnt/β-catenin and PI3K/Akt/GSK-3β pathways. Therefore, further validation of the feasibility and safety of this NP-based drug is required through in vivo animal studies and clinical trials. Lin et al proposed a thermosensitive hydrogel delivery system using PLGA-PEG-PLGA loaded with Cur (PGC) to prevent the progression of AD.144 Their study indicated that PGC reduced cell toxicity and oxidative stress in N2a cells induced by AlCl3, while also suppressing inflammation in BV2 cells. Further research revealed that weekly intramuscular injections of PGC in an AD rat model effectively improved cognitive function and prevented disease progression. Additionally, PGC can transition from a solution to a gel at 37°C, allowing for controlled, sustained release of Cur.
Curcumin Delivery via Lipid-Based NPs for the Treatment of Alzheimer’s Disease
Kakkar et al developed Cur-loaded SLNs (Cur-SLNs) and validated their oral bioavailability in rats.145 Compared to free Cur (50 mg/kg), the oral bioavailability of Cur-SLNs at varying dosages was found to be 155 times higher (1 mg/kg), 59 times higher (12.5 mg/kg), 32 times higher (25 mg/kg), and 39 times higher (50 mg/kg). Notably, Cur-SLNs can be stored for up to 12 months at 2–8°C without any loss of drug potency. Aluminum deposition in the brain is a contributing factor to AD pathology, as it induces neurotoxicity that leads to Aβ deposition, tau phosphorylation, and neuronal loss.17 In previous studies, the same research team evaluated the efficacy of Cur-SLNs in orally administered AlCl3-induced AD mice.146 The results indicated that Cur-SLNs were more effective in improving the learning and cognitive abilities of the mice. AChE activity in the mouse brains showed that the 50 mg/kg dose of Cur-SLNs achieved significantly better results, with a 73% inhibition rate of AChE activity, compared to only 22% inhibition with Cur alone. Furthermore, histological examination of the mouse brain tissue revealed that the cellular composition and structure were more intact after treatment with Cur-SLNs than with Cur, even at lower doses. Tissue transglutaminase (TG2) is a multifunctional protein that promotes the formation of amyloid aggregates in AD pathology.147 Recent studies have shown that Cur can exert neuroprotective effects in AD mice by modulating TG2 expression. However, the low bioavailability of Cur limits its in vivo application. Campisi et al utilized SLNs as a carrier to deliver Cur (Cur-SLN) for the treatment of AD mice.148 This study provided the first evidence that Cur-SLNs can modulate TG2 expression in vivo, significantly enhancing the concentration of Cur and improving cognitive abilities and memory function in AD mice. In summary, SLN-based Cur delivery represents an effective pharmaceutical formulation for AD treatment.
NLCs address the limitations of SLNs, enhancing the bioavailability of Cur in vivo. Malvajerd et al developed two types of nanomedicines: Cur-SLNs and Cur-NLCs, aimed at optimizing lipid NP preparations and selecting a more suitable drug carrier.149 Experimental results demonstrated that both Cur-SLNs and Cur-NLCs increased Cur absorption in the brain compared to free Cur. Notably, Cur-NLCs facilitated over four times greater brain absorption than Cur-SLNs. In terms of drug encapsulation efficiency, Cur-SLNs achieved 82 ± 0.49%, while Cur-NLCs reached 94 ± 0.74%. These findings suggest that Cur-NLCs may be a superior option for AD treatment. Building on these findings, the same authors applied Cur-loaded NLCs (Cur-NLCs) in an Aβ-induced rat model of AD.92 In vivo results confirmed that Cur-NLCs significantly enhanced Cur accumulation in the brain and serum levels in the rats. Interestingly, both pre-treatment and treatment groups were evaluated for neuroprotective effects. The pre-treatment group showed superior neuroprotection against AD, likely due to the creation of a more favorable environment for maintaining neuronal function, thus reducing damage from Aβ and reactive oxygen species (ROS). These findings highlight the potential of Cur-NLCs in AD treatment and provide a basis for further research into their prevention and management. To optimize Cur-NLCs for higher drug encapsulation, prolonged release, and enhanced stability, Agrawal et al employed an enhanced melt-emulsification-ultrasonication method to prepare drug-loaded NLCs.150 They used the Box-Behnken design for experimental design and optimization. The optimized formulation yielded a material ratio of 3.092, a surfactant concentration of 2.131%, and an ultrasonication time of 4.757 minutes. The resulting NPs had a size of 121.8 ± 55.81 nm and exhibited a spherical and uniform shape, with an encapsulation efficiency of 93.62 ± 0.68% and a drug release rate of 92.73 ± 0.06%. Notably, Cur-NLCs demonstrated an initial burst release followed by sustained release over 48 hours. These results meet the criteria for brain delivery and support further development of Cur-NLCs as a therapeutic option for AD.
Curcumin Delivery via Protein-Based NPs for the Treatment of Alzheimer’s Disease
To enhance Aβ clearance efficiency and improve Cur’s ability to cross the BBB, Yang et al utilized electrostatic interactions to conjugate positively charged CS with negatively charged BSA, forming NPs loaded with Cur (CS-BSA@Cur).151 In an in vitro BBB model, the permeability of CS-BSA@Cur at 1, 2, and 3 hours was 37.7%, 45.6%, and 60.2%, respectively, compared to free Cur, which exhibited only 12.3% permeability at 1 hour, 20.3% at 2 hours, and 29.8% at 3 hours. These results demonstrate that CS-BSA@Cur significantly improves the bioavailability of Cur. The enhanced permeability is facilitated through vesicular and micellar transport mechanisms. Notably, CS-BSA@Cur also better activates microglia, accelerating Aβ clearance and providing neuroprotective effects compared to Cur alone. In summary, CS-BSA@Cur shows considerable potential in enhancing AD treatment.
Quercetin
Quercetin Delivery via Polymeric Nanoparticles for the Treatment of Alzheimer’s Disease
PLGA, known for its excellent biocompatibility, is widely used as a drug delivery vehicle. In this regard, Sun et al developed PLGA-encapsulated QT (PLGA@QT) to inhibit and degrade Aβ42 fibrils, assessing its therapeutic effects on AD.152 In vitro studies demonstrated that PLGA@QT could suppress Aβ42 aggregation induced by excess metal ions, thereby reducing neuronal cell damage. Additionally, in vitro toxicity tests revealed low cytotoxicity, and further in vivo studies showed no pathological abnormalities in various organs, including the heart, liver, spleen, lungs, and kidneys, confirming the safety of PLGA@QT. Behavioral experiments indicated that PLGA@QT significantly improved cognitive and memory functions in AD mice, outperforming the QT group. Collectively, these findings provide strong evidence for the potential of PLGA@QT in AD treatment.
Quercetin Delivery via Lipid-Based Nanoparticles for the Treatment of Alzheimer’s Disease
A study prepared QT-loaded Lips (QT Lips) as a delivery system and enhanced cognitive function in AD animals through nasal administration.153 The results demonstrated that QT Lips significantly improved cognitive performance in AD rats compared to free QT. Histological analysis showed that QT Lip treatment alleviated the reduction in hippocampal and cholinergic neuron densities in AD rats.
In a related study, QT-loaded SLNs (SLN-QT) improved cognitive impairment and spatial learning abilities in AlCl3-induced AD mice.154 The study also found that SLN-QT maintained levels of GSH and nitrite in the brain, suggesting that SLN-QT can effectively target the CNS for AD treatment. Similarly, Rishitha et al investigated the therapeutic effects of SLN-QT in a pentylenetetrazol (PTZ)-induced AD zebrafish model.155 The results indicated that SLN-QT reduced learning and memory impairments caused by PTZ in the hippocampus. The primary mechanisms included an increase in reduced GSH levels and a decrease in thiobarbituric acid-reactive substances and AChE levels. Furthermore, no significant difference in therapeutic effects was observed between SLN-QT and donepezil at the same dosage, highlighting its effective neuroprotective properties. NLCs represent a new generation of nano-lipid delivery systems that overcome the limitations of SLNs. Patil et al utilized NLCs to deliver QT (NLC-QT) via intranasal administration, enhancing the drug’s targeting capability.156 Research indicated that NLC-QT did not damage the nasal mucosa during delivery and achieved a higher brain concentration compared to free QT. Additionally, NLC-QT demonstrated sustained release properties, maintaining drug release over 24 hours. To further optimize NLC-QT, Sonawane et al employed methods such as melt emulsification-high-pressure homogenization and a Box-Behnken design to prepare an in situ gel of NLC-QT.157 Results showed that the nasal mucosal permeability of the NLC-QT in situ gel was three times greater than that of free QT. Notably, pharmacokinetic data confirmed that the targeting efficiency of intranasal administration of NLC-QT in situ gel was 117.47% compared to free QT. Further studies revealed that NLC-QT in situ gel exhibited superior therapeutic effects compared to donepezil, underscoring the significant potential of NLCs as a delivery carrier for QT in AD treatment. To evaluate and improve lipid NCs, Aditya et al conducted a comparative study between NLCs and SLNs.158 The results showed that NLCs loaded with QT (NLC-QT) exhibited smaller particle size, higher drug loading capacity, and enhanced bioavailability compared to SLN-QT. Kumar et al also compared NLCs and SLNs to assess their performance as drug delivery systems for QT.159 In vitro studies demonstrated that both NLC-QT and SLN-QT effectively penetrated Caco-2 cells. Further in vivo research indicated that both NLC-QT and SLN-QT had better bioavailability compared to free QT, with enhancements of 5.4-fold and 3.5-fold, respectively. Additionally, the drug clearance time was significantly prolonged, by 5.8-fold for NLC and 3.5-fold for SLN. These findings highlight that both NLCs and SLNs can significantly enhance the therapeutic efficacy of QT. In an AlCl3-induced rat model, administration of NLC-QT and SLN-QT significantly reduced the decline in GSH levels, with NLC-QT showing a more pronounced effect. Pinheiro et al further enhanced the ability of NPs to cross the BBB by modifying NLCs loaded with QT (RVG29-NLC-QT) and SLNs loaded with QT (RVG29-SLN-QT) using RVG29, a peptide that targets nicotinic ACh receptors (nAChR) and facilitates binding to neuronal cells and the BBB.160 The results indicated that the modified NPs (RVG29-NLC-QT and RVG29-SLN-QT) exhibited 1.5 times greater BBB penetration compared to their unmodified counterparts (NLC-QT and SLN-QT). Furthermore, both RVG29-modified NPs demonstrated enhanced inhibition of Aβ aggregation. In another study, TF was used to modify NLC-QT (TF-NLC-QT) and SLN-QT (TF-SLN-QT) to improve their BBB-crossing ability and Aβ aggregation inhibition.161 However, the permeability of TF-modified NPs (TF-NLC-QT and TF-SLN-QT) did not show a significant increase, likely due to the saturation of endogenous TF transport, which hindered receptor-mediated active transport of the TF-modified NPs.
Quercetin Delivery via Protein-Based Nanoparticles for the Treatment of Alzheimer’s Disease
Dou et al developed novel albumin-based NPs by encapsulating QT within HSA to form HSA@QT NPs (HQ NPs) for potential therapeutic use in advanced AD.162 The HQ NPs exhibited significant antioxidant activity, effectively protecting PC12 cells from H2O2-induced oxidative damage. When administered intranasally to 11-month-old APP/PS1 mice—an established model for advanced AD—these NPs demonstrated multiple therapeutic benefits: prevention of body weight loss, improved survival rates, and substantial reduction in key pathological features, including oxidative stress, neuronal apoptosis, Aβ aggregation, and synaptic dysfunction in brain tissues. Notably, administration of HQ NPs led to a significant recovery of severely impaired cognitive function. In addition to their potent anti-AD effects, HQ NPs exhibited excellent biosafety and biocompatibility due to their natural composition. While this study confirmed the neuroprotective effects of HQ NPs, a comprehensive comparative analysis of their therapeutic efficacy against QT is still required and merits further exploration.
Berberine
Berberine Delivery via Polymeric Nanoparticles for the Treatment of Alzheimer’s Disease
Saleh et al investigated whether Tet-modified PLGA NPs loaded with BBR (BBR/PLGA-Tet) could enhance the bioavailability and therapeutic efficacy of BBR.163 The results demonstrated that, compared to BBR alone, the BBR/PLGA-Tet treatment group showed significantly reduced levels of Aβ42 and phosphorylated tau protein, alongside notable improvements in neuroplasticity and cognitive function in rats. These findings indicate that BBR/PLGA-Tet enhances both the bioavailability and therapeutic effects of BBR. Another study reported that CS BBR NPs (BBR-NPs) effectively alleviated scopolamine-induced cognitive impairment.164 Specifically, BBR-NPs exhibited superior inhibition of AChE, reduced Aβ42 and tau protein levels, and demonstrated stronger anti-inflammatory and antioxidant properties than BBR or donepezil. Notably, BBR-NPs (7 mg/kg) significantly improved learning and memory in AD rats, requiring only one-sixth of the recommended BBR dose (50 mg/kg).
Berberine Delivery via Lipid-Based Nanoparticles for the Treatment of Alzheimer’s Disease
Recent research has shown that lactoferrin (Lf)-modified polyethylene glycol (PEG)ylated BBR Lips (BR-Lf) can enhance the anti-AD effects of BBR.16 Both modified and unmodified Lf NP groups exhibited superior neuroprotective effects compared to free BBR. Notably, the BR-Lf group demonstrated the most significant improvement in behavioral performance relative to the unmodified Lf groups, likely due to the presence of Lf receptors (LfRs) on the BBB endothelial cells, which facilitated increased brain targeting of the NPs.165 Additionally, PEGylation extended the in vivo circulation time of BBR-loaded nanoLips, significantly reducing the clearance rate of BBR in plasma and tissues. These findings suggest that BR-Lf may hold promise as a potential therapeutic agent for AD. However, further investigation is required to assess the dose-dependent effects of BR-Lf’s neuroprotective role and to determine the optimal administration method. The use of imidacloprid has been linked to potential irreversible harm, particularly neurotoxicity. In a study by El Gazzar et al, the neuroprotective effects of BBR-loaded PEGylated nanoLips (Ber-Lip) against imidacloprid-induced neuronal damage were explored.166 Experimental results revealed that Ber-Lip significantly alleviated neuronal injury caused by imidacloprid exposure, primarily by inhibiting NOD-like receptor protein 3 (NLRP3)/Caspase-1/gasdermin D (GSDMD)-mediated pyroptosis.
To address the challenges of poor oral bioavailability associated with BBR, SLNs have emerged as a promising delivery strategy. In a recent study, Nguyen et al developed BBR-SLNs using stearic acid (SA), glyceryl monostearate (GMS), and Poloxamer 407 (P407).167 Through systematic optimization, a formulation with a weight ratio of 1:9:2 (SA: GMS: P407) exhibited optimal physicochemical properties. Additionally, BBR-SLNs produced via spray drying showed enhanced stability and sustained release characteristics compared to their suspended counterparts, likely due to the solid-state form of SLNs improving their physicochemical attributes. These results suggest that spray-dried SLNs could be an effective delivery method for expanding BBR’s therapeutic potential in AD. BBR-loaded NLCs (Berb-NLC) have been developed for their therapeutic potential in AlCl3-induced AD rat models.17 Experimental data demonstrated notable improvements in cognitive function and spatial learning in AD rats following Berb-NLC treatment. A comparative therapeutic evaluation showed superior efficacy of oral Berb-NLCs over conventional treatments, including free BBR and donepezil, indicating successful overcoming of BBR’s bioavailability limitations. Controlled release analysis revealed that Berb-NLCs achieved approximately 80% drug release within 24 hours, confirming the system’s sustained-release properties. While these findings position Berb-NLCs as a promising alternative for AD treatment, the study did not assess cerebral distribution patterns, a critical area for future research. Intranasal delivery has gained attention as a promising approach for CNS disorders, due to its ability to bypass the BBB and provide direct drug delivery to the brain. Abo El-Enin et al pioneered the design of CS-coated BBR-loaded NLCs (BER-CTS-NLCs) and evaluated their efficacy through intranasal administration.168 Pharmacokinetic profiling and brain-targeting assessments revealed significantly higher cerebral drug concentrations and improved brain-to-blood area under the curve (AUC) ratios in AD rats treated with BER-CTS-NLCs compared to conventional BBR administration. Additionally, BER-CTS-NLCs exhibited prolonged drug release and enhanced drug penetration into the nasal mucosa. Histological evaluations confirmed the excellent biocompatibility of BER-CTS-NLCs, with no mucosal damage, establishing their safety for intranasal applications. Collectively, these findings suggest that intranasal BER-CTS-NLCs could enhance therapeutic outcomes in neurodegenerative diseases, particularly AD. However, clinical data is essential to evaluate the efficacy and risk/benefit ratio of BER-CTS-NLCs in human subjects.
Berberine Delivery via Protein-Based Nanoparticles for the Treatment of Alzheimer’s Disease
Zhang et al successfully developed BBR NPs based on HSA (BBR-HSA) through spontaneous electrostatic interactions.169 Comprehensive characterization revealed that the NPs exhibited excellent physicochemical properties: uniform spherical morphology with an average diameter of approximately 100 nm, a drug loading capacity of 19.37%, encapsulation efficiency of 70.34%, and a production yield of 88.91%. In vitro release studies showed that free BBR released rapidly within 24 hours, while BBR-HSA exhibited significantly sustained release over the same period. In an AD cellular model, both BBR and BBR-HSA provided significant protection against H2O2-induced oxidative damage, as indicated by enhanced catalase (CAT) activity. Notably, BBR-HSA demonstrated superior antioxidative protection compared to free BBR. These findings support the potential application of natural product-based nanodelivery systems (NDDS) in AD treatment, positioning BBR-HSA as a promising therapeutic strategy for oxidative stress-related neurodegenerative disorders.
Resveratrol
Resveratrol Delivery via Polymeric Nanoparticles for the Treatment of Alzheimer’s Disease
Emerging evidence underscores the substantial impact of glucolipid metabolic disorders on cognitive function, particularly through insulin resistance mechanisms that exacerbate Aβ aggregation and tau protein hyperphosphorylation.170 Additionally, growing research identifies the gut microbiota as a critical regulator of glucolipid homeostasis.171 To address both microbial dysbiosis and metabolic dysfunction in AD pathology, Yang’s team engineered a novel flower-like nanoplatform: RES-loaded selenium NPs/CS NPs (Res@SeNPs@Res-CS-NPs).172 Experimental validation demonstrated that this nanoconstruct alleviated cognitive deficits in AD models through several mechanisms, including gut microbiota restoration, oxidative stress reduction, neuroinflammation suppression, and regulation of glucolipid metabolism. However, a direct comparison of therapeutic efficacy between Res@SeNPs@Res-CS-NPs and RES has not been explored in current research. In a subsequent study, the team developed an advanced nanodelivery system by using ionic crosslinking of CS with sodium tripolyphosphate (TPP) for RES encapsulation (Res-CS/TPP-NPs).173 To enhance BBB penetration, they created a targeted delivery system by conjugating a brain-targeting peptide (TG: TGNYKALHPHNG) to the NP surface, resulting in TG-Res-CS/TPP-NPs. Studies revealed that this targeted formulation effectively mitigated lipid accumulation-induced insulin resistance and modulated AD-related pathological markers (Aβ plaque formation and tau protein hyperphosphorylation) via the JNK/AKT/GSK3β signaling pathway. Moreover, TG-Res-CS/TPP-NPs demonstrated superior therapeutic outcomes compared to RES and Res-CS/TPP-NPs.
Resveratrol Delivery via Lipid-Based Nanoparticles for the Treatment of Alzheimer’s Disease
NPs, such as SLNs and NLCs, offer an effective strategy for encapsulating RES, thereby enhancing the oral bioavailability of this poorly soluble lipophilic compound.174 Neves et al developed RES-loaded SLNs and NLCs to improve the physicochemical properties of RES and enhance its oral bioavailability for potential neurological disorder treatments.174 Their findings demonstrated that both formulations significantly reduced RES instability in vivo while facilitating its sustained release, positioning these nanomedicines as promising candidates for oral drug delivery. Vascular dementia (VaD), a leading cognitive disorder caused by cerebrovascular abnormalities, has shown increasing associations with mitochondrial dysfunction and oxidative stress. Recent studies have investigated the therapeutic efficacy of RES-loaded SLNs (R-SLNs) in a VaD animal model induced by permanent bilateral common carotid artery occlusion.175 The formulation exhibited superior pharmacokinetic properties, achieving a 4.5-fold increase in cerebral RES bioavailability compared to the non-encapsulated form. Behavioral assessments revealed that R-SLN treatment resulted in significantly greater cognitive improvements in VaD rats than conventional RES treatment. Additionally, oral delivery of R-SLNs effectively suppressed mitochondrial-derived reactive oxygen species (ROS) production and inhibited lipid peroxidation cascades within the VaD model. These results suggest that R-SLNs are a promising therapeutic platform for both VaD management and neurodegenerative diseases, such as AD, by enhancing BBB penetration and providing targeted antioxidant delivery. Khishvand et al developed RES-loaded SLNs (RSV-SLNs) and optimized their physicochemical properties.176 Behavioral assessments demonstrated that RSV-SLNs significantly reduced escape latency and improved target quadrant retention time, outperforming free RES. Histopathological analysis showed that RSV-SLNs exhibited neuroprotective effects, reducing neurodegeneration and preserving the morphological integrity of CA1 pyramidal cells. Additionally, the nanoformulation demonstrated superior therapeutic potential in modulating oxidative stress, significantly lowering brain lipid peroxidase levels and elevating GSH concentrations. These findings indicate that RSV-SLNs offer enhanced therapeutic benefits over conventional RES in alleviating AD-related pathological features. To further enhance the brain-targeting ability of RSV-SLNs, a novel formulation of apolipoprotein E (ApoE)-functionalized RSV-SLNs (SLN-DSPE-ApoE RSV and SLN-Palmitate-ApoE RSV) was developed. By exploiting the recognition of ApoE by low-density lipoprotein (LDL) receptors on the BBB, this formulation aimed to improve NP targeting and delivery efficiency.177 In vitro permeability assays using hCMEC/D3-derived BBB models demonstrated a 1.8-fold increase in transendothelial penetration for the surface-functionalized NPs compared to their non-functionalized counterparts. Loureiro et al designed a RES-loaded SLN functionalized with OX26 antibodies to inhibit Aβ aggregation.178 The results indicated that this nanomedicine exhibited a stronger inhibitory effect compared to free RES. Furthermore, in vitro studies using a BBB model demonstrated that the nanomedicine’s BBB penetration was four times greater than that of free RES. These findings suggest potential therapeutic benefits for the prevention or treatment of AD. However, further in vivo experiments are necessary to confirm these results. As an advanced generation of lipid-based NCs, NLCs have been effectively utilized for RES encapsulation. Rajput et al developed an in situ gel formulation incorporating RES-loaded NLCs (RES-NLCs) for intranasal administration in scopolamine-induced AD rats.179 Comparative analysis revealed that the RES-NLCs in situ gel exhibited superior therapeutic efficacy in enhancing cognitive functions compared to its orally administered RES suspension-based in situ gel. Notably, the RES-NLCs in situ gel enhanced nasal mucosal permeability five-fold. In an innovative approach to targeted drug delivery, researchers developed a biomimetic nanoplatform by encapsulating RES within erythrocyte membrane-coated NLCs.180 This system, designated as RVG/TPP NPs@RBCm, features dual surface modifications with RVG29 and TPP to improve both BBB penetration and mitochondrial targeting. Comparative analysis demonstrated that RVG/TPP NPs@RBCm administration significantly reduced Aβ accumulation and oxidative stress markers in both in vitro and in vivo AD models, outperforming control and other treatment groups. Following intravenous administration, the nanoplatform showed sustained drug release, improved biocompatibility, and extended systemic circulation. These findings suggest that this biomimetic delivery system could offer a promising therapeutic strategy for neurodegenerative disorders.
Resveratrol Delivery via Protein-Based Nanoparticles for the Treatment of Alzheimer’s Disease
Malaiya et al developed and evaluated CS-coated BSA NPs for enhancing the therapeutic efficacy of RES (CS-RES-BSANPs) in the targeted treatment of AD in elderly females.181 Characterization results demonstrated that the CS-RES-BSANPs exhibited spherical morphology with smooth surfaces and maintained excellent stability for 90 days under ambient refrigeration. In vitro release studies showed sustained drug release from CS-RES-BSANPs over 48 hours (cumulative release: 60.74%), compared to near-complete release of free RES (94.28%). Additionally, in vitro studies confirmed the biosafety of CS-RES-BSANPs, showing no significant alteration in the histological integrity of nasal mucosa. Behavioral assessments in AD rat models indicated that intranasally administered CS-RES-BSANPs significantly improved cognitive deficits compared to native RES, highlighting their potential as effective nose-to-brain drug delivery carriers.
Conclusions
Currently, there is still a lack of therapeutic drugs that can effectively prevent or reverse the progression of AD. Notably, NCs of TCM delivery via NDDS for AD treatment bring hope to patients. Although both NCs of TCM and NDDS have limitations and there are relatively few clinical studies, these issues may be effectively resolved through the solutions described earlier. In conclusion, NDDS opens up new prospects for the application of NCs of TCM in AD treatment. In the future, with the rapid development of nanotechnology and continuous breakthroughs in research, the NCs of TCM delivery via NDDS will fully exert their pharmacological effects, enabling the exploration of more efficient and innovative therapeutic strategies for AD treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 32272250), Zhejiang Provincial Natural Science Foundation of China (Grant No. LTGY24B050001), Key Project of Ningbo Science and Technology (Grant No. 2024Z184), Ningbo Key Research and Development Program (Grant No. 2023Z196), Zhejiang Health Science and Technology Project (Grant No. 2022PY020), Project of Ningbo Leading Medical & Health Discipline (Grant No.2022-F05), and Ningbo Natural Science Foundation (Grant No. 2022J252). We thank Bullet Edits Limited for linguistic editing and proofreading of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gopalan D, Pandey A, Udupa N, Mutalik S. Receptor specific, stimuli responsive and subcellular targeted approaches for effective therapy of Alzheimer: role of surface engineered nanocarriers. J Controlled Rel. 2020;319:183–200. doi:10.1016/j.jconrel.2019.12.034
2. Jain U, Johari S, Srivastava P. Current Insights of Nanocarrier-Mediated Gene Therapeutics to Treat Potential Impairment of Amyloid Beta Protein and Tau Protein in Alzheimer’s Disease. Mol Neurobiol. 2023;61(4):1969–1989. doi:10.1007/s12035-023-03671-7
3. Lane CA, Hardy J, Schott JM. Alzheimer’s disease. Eur J Neurol. 2018;25(1):59–70. doi:10.1111/ene.13439
4. Knopman DS, Amieva H, Petersen RC, et al. Alzheimer disease. Nat Rev Dis Primers. 2021;7(1):33. doi:10.1038/s41572-021-00269-y
5. Wojtunik-Kulesza K, Rudkowska M, Orzel-Sajdlowska A. Aducanumab-Hope or Disappointment for Alzheimer’s Disease. Int J Mol Sci. 2023;24(5):4367. doi:10.3390/ijms24054367
6. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. New Engl J Med. 2023;388(1):9–21. doi:10.1056/NEJMoa2212948
7. Sun K, Fan J, Han J. Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta pharmaceutica Sinica B. 2015;5(1):8–24. doi:10.1016/j.apsb.2014.11.002
8. Taliyan R, Kakoty V, Sarathlal KC, et al. Nanocarrier mediated drug delivery as an impeccable therapeutic approach against Alzheimer’s disease. J Controlled Rel. 2022;343:528–550.
9. Batty CJ, Bachelder EM, Ainslie KM. Historical Perspective of Clinical Nano and Microparticle Formulations for Delivery of Therapeutics. Trends Mol Med. 2021;27(6):516–519. doi:10.1016/j.molmed.2021.04.002
10. Wang X, Zhong X, Li J, Liu Z, Cheng L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem Soc Rev. 2021;50(15):8669–8742. doi:10.1039/d0cs00461h
11. Jain S, Dongare K, Nallamothu B, et al. Enhanced stability and oral bioavailability of erlotinib by solid self nano emulsifying drug delivery systems. Int J Pharm. 2022;622:121852. doi:10.1016/j.ijpharm.2022.121852
12. Kumbhar P, Kole K, Khadake V, et al. Nanoparticulate drugs and vaccines: breakthroughs and bottlenecks of repurposing in breast cancer. J Controlled Rel. 2022;349:812–830. doi:10.1016/j.jconrel.2022.07.039
13. Stater EP, Sonay AY, Hart C, Grimm J. The ancillary effects of nanoparticles and their implications for nanomedicine. Nat Nanotechnol. 2021;16(11):1180–1194. doi:10.1038/s41565-021-01017-9
14. Kamanzi A, Gu Y, Tahvildari R, et al. Simultaneous, Single-Particle Measurements of Size and Loading Give Insights into the Structure of Drug-Delivery Nanoparticles. ACS Nano. 2021;15(12):19244–19255. doi:10.1021/acsnano.1c04862
15. Escriche-Navarro B, Escudero A, Lucena-Sanchez E, Sancenon F, Garcia-Fernandez A, Martinez-Manez R. Mesoporous Silica Materials as an Emerging Tool for Cancer Immunotherapy. Adv Sci. 2022;9(26):e2200756. doi:10.1002/advs.202200756
16. Wang L, Zhou BQ, Li YH, et al. Lactoferrin modification of berberine nanoliposomes enhances the neuroprotective effects in a mouse model of Alzheimer’s disease. Neural Regen Res. 2023;18(1):226–232. doi:10.4103/1673-5374.344841
17. Raju M, Kunde SS, Auti ST, Kulkarni YA, Wairkar S. Berberine loaded nanostructured lipid carrier for Alzheimer’s disease: design, statistical optimization and enhanced in vivo performance. Life Sci. 2021;285:119990. doi:10.1016/j.lfs.2021.119990
18. Lohan S, Raza K, Mehta SK, Bhatti GK, Saini S, Singh B. Anti-Alzheimer’s potential of berberine using surface decorated multi-walled carbon nanotubes: a preclinical evidence. Int J Pharm. 2017;530(1–2):263–278. doi:10.1016/j.ijpharm.2017.07.080
19. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/S0140-6736(20)32205-4
20. Tautou M, Descamps F, Larchanche PE, et al. A Polyaminobiaryl-Based beta-secretase Modulator Alleviates Cognitive Impairments, Amyloid Load, Astrogliosis, and Neuroinflammation in APP(Swe)/PSEN1(DeltaE9) Mice Model of Amyloid Pathology. Int J Mol Sci. 2023;24(6):5285. doi:10.3390/ijms24065285
21. Trejo-Lopez JA, Yachnis AT, Prokop S. Neuropathology of Alzheimer’s Disease. Neurotherapeutics. 2022;19(1):173–185. doi:10.1007/s13311-021-01146-y
22. Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022;77:101619. doi:10.1016/j.arr.2022.101619
23. Twarowski B, Herbet M. Inflammatory Processes in Alzheimer’s Disease-Pathomechanism, Diagnosis and Treatment: a Review. Int J Mol Sci. 2023;24(7):6518. doi:10.3390/ijms24076518
24. Bowen DM, Smith CB, White P, Davison AN. Neurotransmitter-related enzymes and indices of hypoxia in senile dementia and other abiotrophies. Brain. 1976;99(3):459–496. doi:10.1093/brain/99.3.459
25. Wenk GL. Neuropathologic changes in Alzheimer’s disease. J Clin Psychiatry. 2003;64(9):7–10.
26. Akbar M, Shabbir A, Rehman K, Akash MSH, Shah MA. Neuroprotective potential of berberine in modulating Alzheimer’s disease via multiple signaling pathways. J Food Biochem. 2021;45(10):e13936. doi:10.1111/jfbc.13936
27. Carrano A, Hoozemans JJ, van der Vies SM, Rozemuller AJ, van Horssen J, de Vries HE. Amyloid Beta induces oxidative stress-mediated blood-brain barrier changes in capillary amyloid angiopathy. Antioxid Redox Signal. 2011;15(5):1167–1178. doi:10.1089/ars.2011.3895
28. Choi SH, Kim YH, Hebisch M, et al. A three-dimensional human neural cell culture model of Alzheimer’s disease. Nature. 2014;515(7526):274–278.
29. Cai Z, Xiao M, Chang L, Yan LJ. Role of insulin resistance in Alzheimer’s disease. Metab Brain Dis. 2015;30(4):839–851. doi:10.1007/s11011-014-9631-3
30. Yu H, Yamashita T, Hu X, et al. Protective and anti-oxidative effects of curcumin and resveratrol on Aβ-oligomer-induced damage in the SH-SY5Y cell line. J Neurol Sci. 2022;441:120356. doi:10.1016/j.jns.2022.120356
31. Qais FA, Parveen N, Afzal M, Furkan M, Khan RH. Preventing amyloid-beta oligomerization and aggregation with berberine: investigating the mechanism of action through computational methods. Int J Biol Macromol. 2024;258(Pt 2):128900. doi:10.1016/j.ijbiomac.2023.128900
32. Farihi A, Bouhrim M, Chigr F, et al. Exploring Medicinal Herbs’ Therapeutic Potential and Molecular Docking Analysis for Compounds as Potential Inhibitors of Human Acetylcholinesterase in Alzheimer’s Disease Treatment. Medicina (Kaunas). 2023;59(10). doi:10.3390/medicina59101812.
33. Lasure VU, Singh Gautam A, Singh RK. Quercetin ameliorates neuroinflammatory and neurodegenerative biomarkers in the brain and improves neurobehavioral parameters in a repeated intranasal amyloid-beta exposed model of Alzheimer’s disease. Food Funct. 2024;15(17):8712–8728. doi:10.1039/D4FO02602K
34. Zhang C, Browne A, Child D, Tanzi RE. Curcumin decreases amyloid-beta peptide levels by attenuating the maturation of amyloid-beta precursor protein. J Biol Chem. 2010;285(37):28472–28480. doi:10.1074/jbc.M110.133520
35. Zhang X, Yin WK, Shi XD, Li Y. Curcumin activates Wnt/β-catenin signaling pathway through inhibiting the activity of GSK-3β in APPswe transfected SY5Y cells. Eur JPharml Sci. 2011;42(5):540–546. doi:10.1016/j.ejps.2011.02.009
36. Wang C, Zhang X, Teng Z, Zhang T, Li Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur J Pharmacol. 2014;740:312–320. doi:10.1016/j.ejphar.2014.06.051
37. SoukhakLari R, Moezi L, Pirsalami F, Ashjazadeh N, Moosavi M. Curcumin ameliorates scopolamine-induced mice memory retrieval deficit and restores hippocampal p-Akt and p-GSK-3β. Eur J Pharmacol. 2018;841:28–32. doi:10.1016/j.ejphar.2018.10.012
38. Wang H, Sui H, Zheng Y, et al. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3β pathway. Nanoscale. 2019;11(15):7481–7496. doi:10.1039/C9NR01255A
39. Sun J, Zhang X, Wang C, Teng Z, Li Y. Curcumin Decreases Hyperphosphorylation of Tau by Down-Regulating Caveolin-1/GSK-3β in N2a/APP695swe Cells and APP/PS1 Double Transgenic Alzheimer’s Disease Mice. Am J Chin Med. 2017;45(8):1667–1682. doi:10.1142/S0192415X17500902
40. Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–8377. doi:10.1523/JNEUROSCI.21-21-08370.2001
41. Shi X, Zheng Z, Li J, et al. Curcumin inhibits Aβ-induced microglial inflammatory responses in vitro: involvement of ERK1/2 and p38 signaling pathways. Neurosci Lett. 2015;594:105–110. doi:10.1016/j.neulet.2015.03.045
42. Liu ZJ, ZH L, Liu L, et al. Curcumin Attenuates Beta-Amyloid-Induced Neuroinflammation via Activation of Peroxisome Proliferator-Activated Receptor-Gamma Function in a Rat Model of Alzheimer’s Disease. Front Pharmacol. 2016;7:261. doi:10.3389/fphar.2016.00261
43. Shao S, Ye X, Su W, Wang Y. Curcumin alleviates Alzheimer’s disease by inhibiting inflammatory response, oxidative stress and activating the AMPK pathway. J Chem Neuroanat. 2023;134:102363. doi:10.1016/j.jchemneu.2023.102363
44. Ahmed T, Gilani AH. Inhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s disease. Pharmacol Biochem Behav. 2009;91(4):554–559. doi:10.1016/j.pbb.2008.09.010
45. Akinyemi AJ, Okonkwo PK, Faboya OA, et al. Curcumin improves episodic memory in cadmium induced memory impairment through inhibition of acetylcholinesterase and adenosine deaminase activities in a rat model. Metab Brain Dis. 2017;32(1):87–95. doi:10.1007/s11011-016-9887-x
46. Akinyemi AJ, Oboh G, Fadaka AO, Olatunji BP, Akomolafe S. Curcumin administration suppress acetylcholinesterase gene expression in cadmium treated rats. Neurotoxicology. 2017;62:75–79. doi:10.1016/j.neuro.2017.05.004
47. Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea-Rodriguez M, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology. 2015;93:134–145. doi:10.1016/j.neuropharm.2015.01.027
48. Paula PC, Angelica Maria SG, Luis CH, Gloria Patricia CG. Preventive Effect of Quercetin in a Triple Transgenic Alzheimer’s Disease Mice Model. Molecules. 2019;24(12):2287. doi:10.3390/molecules24122287
49. Elfiky AM, Mahmoud AA, Elreedy HA, Ibrahim KS, Ghazy MA. Quercetin stimulates the non-amyloidogenic pathway via activation of ADAM10 and ADAM17 gene expression in aluminum chloride-induced Alzheimer’s disease rat model. Life Sci. 2021;285:119964. doi:10.1016/j.lfs.2021.119964
50. Jiang W, Luo T, Li S, et al. Quercetin Protects against Okadaic Acid-Induced Injury via MAPK and PI3K/Akt/GSK3β Signaling Pathways in HT22 Hippocampal Neurons. PLoS One. 2016;11(4):e0152371. doi:10.1371/journal.pone.0152371
51. Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Abeta(1-42): relevance to Alzheimer’s disease. J Nutr Biochem. 2009;20(4):269–275. doi:10.1016/j.jnutbio.2008.03.002
52. Cheng M, Yuan C, Ju Y, et al. Quercetin Attenuates Oxidative Stress and Apoptosis in Brain Tissue of APP/PS1 Double Transgenic AD Mice by Regulating Keap1/Nrf2/HO-1 Pathway to Improve Cognitive Impairment. Behav Neurol. 2024;2024(1):5698119. doi:10.1155/2024/5698119
53. Abdalla FH, Cardoso AM, Pereira LB, et al. Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem. 2013;381(1–2):1–8. doi:10.1007/s11010-013-1659-x
54. Maciel RM, Carvalho FB, Olabiyi AA, et al. Neuroprotective effects of quercetin on memory and anxiogenic-like behavior in diabetic rats: role of ectonucleotidases and acetylcholinesterase activities. Biomed Parmacothe. 2016;84:559–568. doi:10.1016/j.biopha.2016.09.069
55. Wu Y, Chen Q, Wen B, Wu N, He B, Chen J. Berberine Reduces Abeta(42) Deposition and Tau Hyperphosphorylation via Ameliorating Endoplasmic Reticulum Stress. Front Pharmacol. 2021;12:640758. doi:10.3389/fphar.2021.640758
56. Cai Z, Wang C, He W, Chen Y. Berberine Alleviates Amyloid-Beta Pathology in the Brain of APP/PS1 Transgenic Mice via Inhibiting beta/gamma-Secretases Activity and Enhancing alpha-Secretases. Curr Alzheimer Res. 2018;15(11):1045–1052. doi:10.2174/1567205015666180702105740
57. Huang M, Jiang X, Liang Y, Liu Q, Chen S, Guo Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp Gerontol. 2017;91:25–33. doi:10.1016/j.exger.2017.02.004
58. Wang YY, Yan Q, Huang ZT, et al. Ameliorating Ribosylation-Induced Amyloid-beta Pathology by Berberine via Inhibiting mTOR/p70S6K Signaling. J Alzheimer Dis. 2021;79(2):833–844. doi:10.3233/JAD-200995
59. Chen Y, Chen Y, Liang Y, Chen H, Ji X, Huang M. Berberine mitigates cognitive decline in an Alzheimer’s Disease Mouse Model by targeting both tau hyperphosphorylation and autophagic clearance. Biomed Parmacothe. 2020;121:109670. doi:10.1016/j.biopha.2019.109670
60. Yu G, Li Y, Tian Q, et al. Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J Alzheimer Dis. 2011;24(3):525–535. doi:10.3233/JAD-2011-101779
61. Liu X, Zhou J, Abid MD, et al. Berberine attenuates axonal transport impairment and axonopathy induced by Calyculin A in N2a cells. PLoS One. 2014;9(4):e93974. doi:10.1371/journal.pone.0093974
62. Li X, Chen J, Feng W, et al. Berberine ameliorates iron levels and ferroptosis in the brain of 3 x Tg-AD mice. Phytomedicine. 2023;118:154962. doi:10.1016/j.phymed.2023.154962
63. Hsu YY, Chen CS, Wu SN, Jong YJ, Lo YC. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mechanism in NSC34 motor neuron-like cells. Eur JPharml Sci. 2012;46(5):415–425. doi:10.1016/j.ejps.2012.03.004
64. Shou JW, Li XX, Tang YS, et al. Novel mechanistic insight on the neuroprotective effect of berberine: the role of PPARdelta for antioxidant action. Free Radic Biol Med. 2022;181:62–71. doi:10.1016/j.freeradbiomed.2022.01.022
65. Sadraie S, Kiasalari Z, Razavian M, et al. Berberine ameliorates lipopolysaccharide-induced learning and memory deficit in the rat: insights into underlying molecular mechanisms. Metab Brain Dis. 2019;34(1):245–255. doi:10.1007/s11011-018-0349-5
66. Lu DY, Tang CH, Chen YH, Wei IH. Berberine suppresses neuroinflammatory responses through AMP-activated protein kinase activation in BV-2 microglia. J Cell Biochem. 2010;110(3):697–705. doi:10.1002/jcb.22580
67. Cao TQ, Ngo QT, Seong SH, et al. Cholinesterase inhibitory alkaloids from the rhizomes of Coptis chinensis. Bioorg Chem. 2018;77:625–632. doi:10.1016/j.bioorg.2018.01.038
68. de Oliveira JS, Abdalla FH, Dornelles GL, et al. Berberine protects against memory impairment and anxiogenic-like behavior in rats submitted to sporadic Alzheimer’s-like dementia: involvement of acetylcholinesterase and cell death. Neurotoxicology. 2016;57:241–250. doi:10.1016/j.neuro.2016.10.008
69. Lee B, Sur B, Shim I, Lee H, Hahm DH. Phellodendron amurense and Its Major Alkaloid Compound, Berberine Ameliorates Scopolamine-Induced Neuronal Impairment and Memory Dysfunction in Rats. Korean J Physiol Pharmacol. 2012;16(2):79–89. doi:10.4196/kjpp.2012.16.2.79
70. Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J Biol Chem. 2005;280(45):37377–37382. doi:10.1074/jbc.M508246200
71. Choi C, Choi YH, Cha M-R, et al. In Vitro BACE-1 Inhibitory Activity of Resveratrol Oligomers from the Seed Extract of Paeonia lactiflora. Planta Med. 2011;77(4):374–376. doi:10.1055/s-0030-1250370
72. Choi B, Kim S, Jang B-G, Kim M-J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. Journal of Functional Foods. 2016;23:124–134.
73. He X, Li Z, Rizak JD, et al. Resveratrol Attenuates Formaldehyde Induced Hyperphosphorylation of Tau Protein and Cytotoxicity in N2a Cells. Front Neurosci. 2016;10:598. doi:10.3389/fnins.2016.00598
74. Shati AA, Alfaifi MY. Trans-resveratrol Inhibits Tau Phosphorylation in the Brains of Control and Cadmium Chloride-Treated Rats by Activating PP2A and PI3K/Akt Induced-Inhibition of GSK3β. Neurochem Res. 2019;44(2):357–373. doi:10.1007/s11064-018-2683-8
75. Schweiger S, Matthes F, Posey K, et al. Resveratrol induces dephosphorylation of Tau by interfering with the MID1-PP2A complex. Sci Rep. 2017;7(1):13753.
76. Chiang MC, Nicol CJ, Cheng YC. Resveratrol activation of AMPK-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced inflammation and oxidative stress. Neurochem Int. 2018;115:1–10. doi:10.1016/j.neuint.2017.10.002
77. Qi Y, Shang L, Liao Z, et al. Intracerebroventricular injection of resveratrol ameliorated Aβ-induced learning and cognitive decline in mice. Metab Brain Dis. 2019;34(1):257–266. doi:10.1007/s11011-018-0348-6
78. Lazarova M, Stefanova M, Tsvetanova E, et al. Resveratrol-Loaded Pluronic Micelles Ameliorate Scopolamine-Induced Cognitive Dysfunction Targeting Acetylcholinesterase Activity and Programmed Cell Death. Int J Mol Sci. 2024;25(23):1.
79. Foudah AI, Devi S, Alam A, Salkini MA, Ross SA. Anticholinergic effect of resveratrol with vitamin E on scopolamine-induced Alzheimer’s disease in rats: mechanistic approach to prevent inflammation. Front Pharmacol. 2023;14:1115721. doi:10.3389/fphar.2023.1115721
80. Ashrafizadeh M, Ahmadi Z, Mohamamdinejad R, et al. Curcumin Therapeutic Modulation of the Wnt Signaling Pathway. Current Pharm Biotechnol. 2020;21(11):1006–1015. doi:10.2174/1389201021666200305115101
81. Forouzanfar F, Read MI, Barreto GE, Sahebkar A. Neuroprotective effects of curcumin through autophagy modulation. IUBMB Life. 2020;72(4):652–664. doi:10.1002/iub.2209
82. Ye C, Liang Y, Chen Y, et al. Berberine Improves Cognitive Impairment by Simultaneously Impacting Cerebral Blood Flow and beta-Amyloid Accumulation in an APP/tau/PS1 Mouse Model of Alzheimer’s Disease. Cells. 2021;10(5):1161. doi:10.3390/cells10051161
83. He W, Wang C, Chen Y, He Y, Cai Z. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-kappaB signaling, oxidative stress and neuroinflammation. Pharmacol Rep. 2017;69(6):1341–1348. doi:10.1016/j.pharep.2017.06.006
84. Hemonnot AL, Hua J, Ulmann L, Hirbec H. Microglia in Alzheimer Disease: well-Known Targets and New Opportunities. Front Aging Neurosci. 2019;11:233. doi:10.3389/fnagi.2019.00233
85. Amat-Ur-Rasool H, Ahmed M, Hasnain S, Carter WG. Anti-Cholinesterase Combination Drug Therapy as a Potential Treatment for Alzheimer’s Disease. Brain Sci. 2021;11(2):184. doi:10.3390/brainsci11020184
86. Singh A, Zhao K, Bell C, Shah AJ. Effect of berberine on in vitro metabolism of sulfonylureas: a herb-drug interactions study. RCM. 2020;34(Suppl 4):e8651. doi:10.1002/rcm.8651
87. Elmeliegy M, Vourvahis M, Guo C, Wang DD. Effect of P-glycoprotein (P-gp) Inducers on Exposure of P-gp Substrates: review of Clinical Drug-Drug Interaction Studies. Clin Pharmacokinet. 2020;59(6):699–714.
88. Liu J, Wang T, Dong J, Lu Y. The blood-brain barriers: novel nanocarriers for central nervous system diseases. J Nanobiotechnol. 2025;23(1):146.
89. Xie J, Shen Z, Anraku Y, Kataoka K, Chen X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials. 2019;224:119491.
90. Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–170.
91. Costa CP, Moreira JN, Sousa Lobo JM, Silva AC. Intranasal delivery of nanostructured lipid carriers, solid lipid nanoparticles and nanoemulsions: a current overview of in vivo studies. Acta pharmaceutica Sinica B. 2021;11(4):925–940. doi:10.1016/j.apsb.2021.02.012
92. Sadegh Malvajerd S, Izadi Z, Azadi A, et al. Neuroprotective Potential of Curcumin-Loaded Nanostructured Lipid Carrier in an Animal Model of Alzheimer’s Disease: behavioral and Biochemical Evidence. J Alzheimer Dis. 2019;69(3):671–686. doi:10.3233/JAD-190083
93. Md S, Ali M, Baboota S, Sahni JK, Bhatnagar A, Ali J. Preparation, characterization, in vivo biodistribution and pharmacokinetic studies of donepezil-loaded PLGA nanoparticles for brain targeting. Drug Dev Ind Pharm. 2014;40(2):278–287. doi:10.3109/03639045.2012.758130
94. Hanafy AS, Farid RM, Helmy MW, ElGamal SS. Pharmacological, toxicological and neuronal localization assessment of galantamine/chitosan complex nanoparticles in rats: future potential contribution in Alzheimer’s disease management. Drug Deliv. 2016;23(8):3111–3122. doi:10.3109/10717544.2016.1153748
95. Baysal I, Ucar G, Gultekinoglu M, Ulubayram K, Yabanoglu-Ciftci S. Donepezil loaded PLGA-b-PEG nanoparticles: their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J Neural Transmission. 2017;124(1):33–45. doi:10.1007/s00702-016-1527-4
96. Çınar E, Mutluay SU, Baysal İ, et al. Donepezil-loaded PLGA-b-PEG Nanoparticles Enhance the Learning and Memory Function of Beta-Amyloid Rat Model of Alzheimer’s Disease. Noro psikiyatri arsivi. 2022;59(4):281–289. doi:10.29399/npa.28275
97. Rani V, Chawla R. Design, fabrication, optimization and characterization of memantine-loaded biodegradable PLGA nanoscaffolds for treatment of Alzheimer’s disease. Biomedl Mater. 2022;17(6):065024. doi:10.1088/1748-605X/ac9811
98. Imam F, Mukhopadhyay S, Kothiyal P, et al. Formulation and characterization of polymeric nanoparticle of Rivastigmine for effective management of Alzheimer’s disease. SPJ. 2024;32(5):102048. doi:10.1016/j.jsps.2024.102048
99. ElMosbah DE, Khattab MS, Ibrahim MA, El-Asssal MI, Miniawy H. Preclinical efficacy of oral and nasal rivastigmine-loaded chitosan nano-particles on AlCl(3)-induced Alzheimer’s-like disease in rats. Inflammopharmacology. 2024;32(6):3943–3952. doi:10.1007/s10787-024-01541-9
100. Guareschi F, Fonseca C, Silva S, et al. Therapeutic effect of cyclosporine A-loading TPGS micelles on a mouse model of LPS-induced neuroinflammation. Eur JPharml Sci. 2025;205:106994. doi:10.1016/j.ejps.2024.106994
101. Lee D, Shen AM, Garbuzenko OB, Minko T. Liposomal Formulations of Anti-Alzheimer Drugs and siRNA for Nose-to-Brain Delivery: design, Safety and Efficacy In Vitro. AAPS J. 2024;26(5):99. doi:10.1208/s12248-024-00967-x
102. Lee D, Shen AM, Shah M, Garbuzenko OB, Minko T. In Vivo Evaluation of Nose-to-Brain Delivery of Liposomal Donepezil, Memantine, and BACE-1 siRNA for Alzheimer’s Disease Therapy. Int J Mol Sci. 2024;25(19):2.
103. Yang ZZ, Zhang YQ, Wang ZZ, Wu K, Lou JN, Qi XR. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int J Pharm. 2013;452(1–2):344–354. doi:10.1016/j.ijpharm.2013.05.009
104. Saffari PM, Alijanpour S, Takzaree N, et al. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci. 2020;255:117861. doi:10.1016/j.lfs.2020.117861
105. Dara T, Vatanara A, Sharifzadeh M, et al. Improvement of memory deficits in the rat model of Alzheimer’s disease by erythropoietin-loaded solid lipid nanoparticles. Neurobiol Learn Mem. 2019;166:107082. doi:10.1016/j.nlm.2019.107082
106. Shivananjegowda MG, Hani U, Osmani RAM, et al. Development and Evaluation of Solid Lipid Nanoparticles for the Clearance of Aβ in Alzheimer’s Disease. Pharmaceutics. 2023;15(1):221. doi:10.3390/pharmaceutics15010221
107. Permana AD, Mahfud MAS, Munir M, et al. A Combinatorial Approach with Microneedle Pretreatment and Thermosensitive Gel Loaded with Rivastigmine Lipid Nanoparticle Formulation Enables Brain Delivery via the Trigeminal Nerve. ACS Appl. Mater. Interfaces. 2024;16(49):68388–68406. doi:10.1021/acsami.4c16024
108. Cunha S, Swedrowska M, Bellahnid Y, et al. Thermosensitive in situ hydrogels of rivastigmine-loaded lipid-based nanosystems for nose-to-brain delivery: characterisation, biocompatibility, and drug deposition studies. Int J Pharm. 2022;620:121720. doi:10.1016/j.ijpharm.2022.121720
109. Tekade A, Susar R, Kulkarni G, Surwade S, Gaikwad A. Nanostructured Lipid Carriers of Donepezil Hydrochloride for the Treatment of Alzheimer’s Disease. Curr Alzheimer Res. 2024;2024:10.
110. Luppi B, Bigucci F, Corace G, et al. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-Alzheimer drug tacrine. Eur JPharml Sci. 2011;44(4):559–565. doi:10.1016/j.ejps.2011.10.002
111. Avachat AM, Oswal YM, Gujar KN, Shah RD. Preparation and characterization of rivastigmine loaded human serum albumin (HSA) nanoparticles. Curr Drug Deliv. 2014;11(3):359–370. doi:10.2174/15672018113109990050
112. Narmani A, Jahedi R, Bakhshian-Dehkordi E, et al. Biomedical applications of PLGA nanoparticles in nanomedicine: advances in drug delivery systems and cancer therapy. Expert Opin Drug Delivery. 2023;20(7):937–954. doi:10.1080/17425247.2023.2223941
113. Almajidi YQ, Ponnusankar S, Chaitanya M, et al. Chitosan-based nanofibrous scaffolds for biomedical and pharmaceutical applications: a comprehensive review. Int J Biol Macromol. 2024;264(Pt 2):130683. doi:10.1016/j.ijbiomac.2024.130683
114. Yu S, Xu X, Feng J, Liu M, Hu K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int J Pharm. 2019;560:282–293. doi:10.1016/j.ijpharm.2019.02.012
115. P A, Agrawal M, Dethe MR, et al. Nose-to-brain drug delivery for the treatment of Alzheimer’s disease: current advancements and challenges. Expert Opin Drug Delivery. 2022;19(1):87–102. doi:10.1080/17425247.2022.2029845
116. Manek E, Darvas F, Petroianu GA. Use of Biodegradable, Chitosan-Based Nanoparticles in the Treatment of Alzheimer’s Disease. Molecules. 2020;25(20):4866. doi:10.3390/molecules25204866
117. Awad R, Avital A, Sosnik A. Polymeric nanocarriers for nose-to-brain drug delivery in neurodegenerative diseases and neurodevelopmental disorders. Acta pharmaceutica Sinica B. 2023;13(5):1866–1886. doi:10.1016/j.apsb.2022.07.003
118. Ghosh B, Biswas S. Polymeric micelles in cancer therapy: state of the art. J Controlled Rel. 2021;332:127–147. doi:10.1016/j.jconrel.2021.02.016
119. Nguyen TT, Duong VA, Maeng HJ. Pharmaceutical Formulations with P-Glycoprotein Inhibitory Effect as Promising Approaches for Enhancing Oral Drug Absorption and Bioavailability. Pharmaceutics. 2021;13(7):1103. doi:10.3390/pharmaceutics13071103
120. Jang YJ, Kang SJ, Park HS, et al. Drug delivery strategies with lipid-based nanoparticles for Alzheimer’s disease treatment. J Nanobiotechnol. 2025;23(1):99. doi:10.1186/s12951-025-03109-3
121. Hernandez C, Shukla S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen Res. 2022;17(6):1190–1198. doi:10.4103/1673-5374.327328
122. Ma X, Li X, Wang W, Zhang M, Yang B, Miao Z. Phosphatidylserine, inflammation, and central nervous system diseases. Front Aging Neurosci. 2022;14:975176. doi:10.3389/fnagi.2022.975176
123. Li L, Zhang J, Huang X, et al. Research Progress of Nanocarriers for the Treatment of Alzheimer’s Disease. Curr Pharm Des. 2023;29(2):95–115. doi:10.2174/1381612829666221216114912
124. Sun J, Martin JM, Vanderpoel V, Sumbria RK. The Promises and Challenges of Erythropoietin for Treatment of Alzheimer’s Disease. Neuromolecular Med. 2019;21(1):12–24. doi:10.1007/s12017-019-08524-y
125. Ashkar A, Sosnik A, Davidovich-Pinhas M. Structured edible lipid-based particle systems for oral drug-delivery. Biotechnol Adv. 2022;54. doi:10.1016/j.biotechadv.2021.107789
126. Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta pharmaceutica Sinica B. 2018;8(1):34–50. doi:10.1016/j.apsb.2017.11.005
127. Yardley DA. nab-Paclitaxel mechanisms of action and delivery. J Controlled Rel. 2013;170(3):365–372. doi:10.1016/j.jconrel.2013.05.041
128. Kim JK, Choi SH, Kim CO, Park JS, Ahn WS, Kim CK. Enhancement of polyethylene glycol (PEG)-modified cationic liposome-mediated gene deliveries: effects on serum stability and transfection efficiency. J Pharm Pharmacol. 2003;55(4):453–460. doi:10.1211/002235702928
129. Ji Q, Zhu H, Qin Y, et al. GP60 and SPARC as albumin receptors: key targeted sites for the delivery of antitumor drugs. Front Pharmacol. 2024;15:1329636. doi:10.3389/fphar.2024.1329636
130. Tian E, Sharma G, Dai C. Neuroprotective Properties of Berberine: molecular Mechanisms and Clinical Implications. Antioxidants. 2023;12(10). doi:10.3390/antiox12101883
131. Murakami T, Bodor E, Bodor N. Approaching strategy to increase the oral bioavailability of berberine, a quaternary ammonium isoquinoline alkaloid: part 1. Physicochemical and pharmacokinetic properties. Expert Opin Drug Metab Toxicol. 2023;19(3):129–137. doi:10.1080/17425255.2023.2203857
132. Mathew A, Aravind A, Fukuda T, et al. Curcumin nanoparticles-a gateway for multifaceted approach to tackle Alzheimer’s disease. IEEE. 2011;2011:833–836.
133. Mathew A, Aravind A, Brahatheeswaran D, et al. Amyloid-Binding Aptamer Conjugated Curcumin–PLGA Nanoparticle for Potential Use in Alzheimer’s Disease. BioNanoScience. 2012;2(2):83–93. doi:10.1007/s12668-012-0040-y
134. Mathew A, Fukuda T, Nagaoka Y, et al. Curcumin loaded-PLGA nanoparticles conjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One. 2012;7(3):e32616. doi:10.1371/journal.pone.0032616
135. Fan S, Zheng Y, Liu X, et al. Curcumin-loaded PLGA-PEG nanoparticles conjugated with B6 peptide for potential use in Alzheimer’s disease. Drug Deliv. 2018;25(1):1091–1102. doi:10.1080/10717544.2018.1461955
136. Barbara R, Belletti D, Pederzoli F, et al. Novel Curcumin loaded nanoparticles engineered for Blood-Brain Barrier crossing and able to disrupt Abeta aggregates. Int J Pharm. 2017;526(1–2):413–424. doi:10.1016/j.ijpharm.2017.05.015
137. Huang N, Lu S, Liu XG, Zhu J, Wang YJ, Liu RT. PLGA nanoparticles modified with a BBB-penetrating peptide co-delivering Aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in Alzheimer’s disease mice. Oncotarget. 2017;8(46):81001–81013. doi:10.18632/oncotarget.20944
138. Zeng H, Xu L, Zou Y, Wang S. The impacts of curcumin on learning memory function in Alzheimer’s disease under the poly lactic-co-glycolic acid nanoparticle drug carrier. Appl Nanosci. 2023;13(5):3483–3491. doi:10.1007/s13204-022-02638-9
139. Huo X, Zhang Y, Jin X, Li Y, Zhang L. A novel synthesis of selenium nanoparticles encapsulated PLGA nanospheres with curcumin molecules for the inhibition of amyloid β aggregation in Alzheimer’s disease. J Photochem Photobiol B. 2019;190:98–102. doi:10.1016/j.jphotobiol.2018.11.008
140. Doggui S, Sahni JK, Arseneault M, Dao L, Ramassamy C. Neuronal uptake and neuroprotective effect of curcumin-loaded PLGA nanoparticles on the human SK-N-SH cell line. J Alzheimer Dis. 2012;30(2):377–392. doi:10.3233/JAD-2012-112141
141. Djiokeng Paka G, Doggui S, Zaghmi A, et al. Neuronal Uptake and Neuroprotective Properties of Curcumin-Loaded Nanoparticles on SK-N-SH Cell Line: role of Poly(lactide-co-glycolide) Polymeric Matrix Composition. Mol Pharmaceut. 2016;13(2):391–403. doi:10.1021/acs.molpharmaceut.5b00611
142. Tiwari SK, Agarwal S, Seth B, et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/β-catenin pathway. ACS Nano. 2014;8(1):76–103. doi:10.1021/nn405077y
143. Yekeler HB, Guler E, Beato PS, et al. Design and in vitro evaluation of curcumin-loaded PLGA nanoparticle-embedded sodium alginate/gelatin 3D printed scaffolds for Alzheimer’s disease. Int J Biol Macromol. 2024;268(Pt 2):131841. doi:10.1016/j.ijbiomac.2024.131841
144. Lin YW, Fang CH, Yang CY, Liang YJ, Lin FH. Investigating a Curcumin-Loaded PLGA-PEG-PLGA Thermo-Sensitive Hydrogel for the Prevention of Alzheimer’s Disease. Antioxidants. 2022;11(4). doi:10.3390/antiox11040727
145. Kakkar V, Singh S, Singla D, Kaur IP. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol Nutr Food Res. 2011;55(3):495–503. doi:10.1002/mnfr.201000310
146. Kakkar V, Kaur IP. Evaluating potential of curcumin loaded solid lipid nanoparticles in aluminium induced behavioural, biochemical and histopathological alterations in mice brain. Food Chem Toxicol. 2011;49(11):2906–2913. doi:10.1016/j.fct.2011.08.006
147. Grosso H, Mouradian MM. Transglutaminase 2: biology, relevance to neurodegenerative diseases and therapeutic implications. Pharmacol Ther. 2012;133(3):392–410. doi:10.1016/j.pharmthera.2011.12.003
148. Campisi A, Sposito G, Pellitteri R, et al. Effect of Unloaded and Curcumin-Loaded Solid Lipid Nanoparticles on Tissue Transglutaminase Isoforms Expression Levels in an Experimental Model of Alzheimer’s Disease. Antioxidants. 2022;11(10). doi:10.3390/antiox11101863.
149. Sadegh Malvajerd S, Azadi A, Izadi Z, et al. Brain Delivery of Curcumin Using Solid Lipid Nanoparticles and Nanostructured Lipid Carriers: preparation, Optimization, and Pharmacokinetic Evaluation. ACS Chem Neurosci. 2019;10(1):728–739. doi:10.1021/acschemneuro.8b00510
150. Agrawal M, Saraf S, Pradhan M, et al. Design and optimization of curcumin loaded nano lipid carrier system using Box-Behnken design. Biomed Parmacothe. 2021;141:111919. doi:10.1016/j.biopha.2021.111919
151. Yang R, Zheng Y, Wang Q, Zhao L. Curcumin-loaded chitosan-bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res Lett. 2018;13(1):330. doi:10.1186/s11671-018-2759-z
152. Sun D, Li N, Zhang W, et al. Design of PLGA-functionalized quercetin nanoparticles for potential use in Alzheimer’s disease. Colloids Surf B. 2016;148:116–129. doi:10.1016/j.colsurfb.2016.08.052
153. Tong-un T, Muchimapura S, Wattanathorn J, Phachonpai W. Nasal Administration of Quercetin Liposomes Improves Memory Impairment and Neurodegeneration in Animal Model of Alzheimer’s Disease. Am J Agric Biol Sci. 2010;5(3):286–293. doi:10.3844/ajabssp.2010.286.293
154. Dhawan S, Kapil R, Singh B. Formulation development and systematic optimization of solid lipid nanoparticles of quercetin for improved brain delivery. J Pharm Pharmacol. 2011;63(3):342–351. doi:10.1111/j.2042-7158.2010.01225.x
155. Rishitha N, Muthuraman A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018;199:80–87. doi:10.1016/j.lfs.2018.03.010
156. Patil N, Mahajan H. Quercetin Loaded Nanostructured Lipid Carriers for Nose to Brain Delivery: in Vitro and In Vivo Studies. Am J Adv Drug Delivery. 2018;06(01). doi:10.21767/2321-547X.1000022
157. Sonawane D, Pokharkar V. Quercetin-Loaded Nanostructured Lipid Carrier In Situ Gel for Brain Targeting Through Intranasal Route: formulation, In Vivo Pharmacokinetic and Pharmacodynamic Studies. AAPS Pharm Sci Tech. 2024;25(2):30. doi:10.1208/s12249-024-02736-7
158. Aditya NP, Macedo AS, Doktorovova S, et al. Development and evaluation of lipid nanocarriers for quercetin delivery: a comparative study of solid lipid nanoparticles (SLN), nanostructured lipid carriers (NLC), and lipid nanoemulsions (LNE). LWT Food Sci Technol. 2014;59(1):115–121. doi:10.1016/j.lwt.2014.04.058
159. Kumar P, Sharma G, Kumar R, et al. Promises of a biocompatible nanocarrier in improved brain delivery of quercetin: biochemical, pharmacokinetic and biodistribution evidences. Int J Pharm. 2016;515(1–2):307–314. doi:10.1016/j.ijpharm.2016.10.024
160. Pinheiro RGR, Granja A, Loureiro JA, et al. RVG29-Functionalized Lipid Nanoparticles for Quercetin Brain Delivery and Alzheimer’s Disease. Pharm Res. 2020;37(7):139. doi:10.1007/s11095-020-02865-1
161. Pinheiro RGR, Granja A, Loureiro JA, et al. Quercetin lipid nanoparticles functionalized with transferrin for Alzheimer’s disease. Eur JPharml Sci. 2020;148:105314. doi:10.1016/j.ejps.2020.105314
162. Dou Y, Zhao D, Yang F, Tang Y, Chang J. Natural Phyto-Antioxidant Albumin Nanoagents to Treat Advanced Alzheimer’s Disease. ACS Appl. Mater. Interfaces. 2021;13(26):30373–30382. doi:10.1021/acsami.1c07281
163. Saleh SR, Abd-Elmegied A, Aly Madhy S, et al. Brain-targeted Tet-1 peptide-PLGA nanoparticles for berberine delivery against STZ-induced Alzheimer’s disease in a rat model: alleviation of hippocampal synaptic dysfunction, Tau pathology, and amyloidogenesis. Int J Pharm. 2024;658:124218. doi:10.1016/j.ijpharm.2024.124218
164. Saleh SR, Abady MM, Nofal M, et al. Berberine Nanoencapsulation Attenuates Hallmarks of Scoplomine Induced Alzheimer’s-Like Disease in Rats. Curr Rev Clin Exp Pharmacol. 2021;16(2):139–154. doi:10.2174/27724336MTA31NzQh0
165. Fillebeen C, Descamps L, Dehouck MP, et al. Receptor-mediated transcytosis of lactoferrin through the blood-brain barrier. J Biol Chem. 1999;274(11):7011–7017. doi:10.1074/jbc.274.11.7011
166. El Gazzar WB, Farag AA, Samir M, et al. Berberine chloride loaded nano-PEGylated liposomes attenuates imidacloprid-induced neurotoxicity by inhibiting NLRP3/Caspase-1/GSDMD-mediated pyroptosis. Biofactors. 2025;51(1):e2107. doi:10.1002/biof.2107
167. Nguyen VH, Le KNM, Nguyen MCN. Spray-dried Solid Lipid Nanoparticles for Enhancing Berberine Bioavailability via Oral Administration. Curr Pharm Des. 2023;29(38):3050–3059. doi:10.2174/0113816128263982231102062745
168. HA AE-E, Elkomy MH, Naguib IA, et al. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceuticals. 2022;15(3):3.
169. Zhang Y, Wang L, Li G, Gao J. Berberine-Albumin Nanoparticles: preparation, Thermodynamic Study and Evaluation Their Protective Effects Against Oxidative Stress in Primary Neuronal Cells as a Model of Alzheimer’s Disease. J Biomed Nanotechnol. 2021;17(6):1088–1097. doi:10.1166/jbn.2021.2995
170. Galizzi G, Palumbo L, Amato A, et al. Altered insulin pathway compromises mitochondrial function and quality control both in in vitro and in vivo model systems. Mitochondrion. 2021;60:178–188. doi:10.1016/j.mito.2021.08.014
171. Bonfili L, Cuccioloni M, Gong C, et al. Gut microbiota modulation in Alzheimer’s disease: focus on lipid metabolism. Clin Nutr. 2022;41(3):698–708. doi:10.1016/j.clnu.2022.01.025
172. Yang L, Wang Y, Zheng G, Li Z, Mei J. Resveratrol-loaded selenium/chitosan nano-flowers alleviate glucolipid metabolism disorder-associated cognitive impairment in Alzheimer’s disease. Int J Biol Macromol. 2023;239:124316. doi:10.1016/j.ijbiomac.2023.124316
173. Yang L, Wang Y, Li Z, Wu X, Mei J, Zheng G. Brain targeted peptide-functionalized chitosan nanoparticles for resveratrol delivery: impact on insulin resistance and gut microbiota in obesity-related Alzheimer’s disease. Carbohydr Polym. 2023;310:120714. doi:10.1016/j.carbpol.2023.120714
174. Neves AR, Lúcio M, Martins S, Lima JL, Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int J Nanomed. 2013;8:177–187. doi:10.2147/IJN.S37840
175. Yadav A, Sunkaria A, Singhal N, Sandhir R. Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway. Neurochem Int. 2018;112:239–254. doi:10.1016/j.neuint.2017.08.001
176. Khishvand MA, Yeganeh EM, Zarei M, Soleimani M, Mohammadi M, Mahjub R. Development, Statistical Optimization, and Characterization of Resveratrol-Containing Solid Lipid Nanoparticles (SLNs) and Determination of the Efficacy in Reducing Neurodegenerative Symptoms Related to Alzheimer’s Disease: in Vitro and In Vivo Study. Biomed Res Int. 2024;2024(1):7877265. doi:10.1155/2024/7877265
177. Neves AR, Queiroz JF, Reis S. Brain-targeted delivery of resveratrol using solid lipid nanoparticles functionalized with apolipoprotein E. J Nanobiotechnol. 2016;14(1):27. doi:10.1186/s12951-016-0177-x
178. Loureiro JA, Andrade S, Duarte A, et al. Resveratrol and Grape Extract-loaded Solid Lipid Nanoparticles for the Treatment of Alzheimer’s Disease. Molecules. 2017;22(2):277. doi:10.3390/molecules22020277
179. Rajput A, Bariya A, Allam A, Othman S, SB B. In situ nanostructured hydrogel of resveratrol for brain targeting: in vitro-in vivo characterization. Drug Deliv Transl Res. 2018;8(5):1460–1470. doi:10.1007/s13346-018-0540-6
180. Han Y, Chu X, Cui L, et al. Neuronal mitochondria-targeted therapy for Alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020;27(1):502–518. doi:10.1080/10717544.2020.1745328
181. Malaiya A, Kenwat R, Mamgain A, et al. Intranasal resveratrol delivery to the brain with chitosan-decorated bovine serum albumin nanoparticles: advancing Alzheimer’s management in old female rats through QbD-based optimization, in vitro evaluation, and in vivo exploration. Int J Biol Macromol. 2025;311:143300. doi:10.1016/j.ijbiomac.2025.143300
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

