Back to Journals » Cancer Management and Research » Volume 17
Potential of Using New Indole- and Benzimidazo[1,2-C]quinazolines in Anticancer Therapy Based on Mesenchymal Stem Cells
Authors Radzikowska-Bűchner E, Radej S, Niezabitowska E, Sitarz R, Szewc M
Received 9 January 2025
Accepted for publication 25 April 2025
Published 11 June 2025 Volume 2025:17 Pages 1087—1097
DOI https://doi.org/10.2147/CMAR.S516593
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Elżbieta Radzikowska-Bűchner,1,2 Sebastian Radej,1 Ewa Niezabitowska,3 Robert Sitarz,4,5,* Monika Szewc5,*
1Institute of Medical Sciences, The John Paul II Catholic University of Lublin, Lublin, Poland; 2Radzikowska Clinic, Warszawa, Poland; 3Department of Urology and Urological Oncology, Multidisciplinary Hospital in Lublin, Lublin, Poland; 4l Department of Surgical Oncology, St. John’s Cancer Center, Lublin, Poland; 5Department of Normal, Clinical and Imaging Anatomy, Medical University of Lublin, Lublin, Poland
*These authors contributed equally to this work
Correspondence: Monika Szewc, Department of Normal, Clinical and Imaging Anatomy, Medical University of Lublin, Lublin, 20-950, Poland, Email [email protected]
Purpose: The study of the cytotoxic effect of variously substituted indole- and benzimidazo[1,2-c]quinazolines may prove particularly valuable in the context of developing new, effective anticancer therapies based on MSCs. The unique ability of MSCs to migrate and inhabit the tumor microenvironment makes them an ideal tool for transferring chemotherapeutic agents. The aim of this study was to evaluate the cytotoxic activity of 4-(6-indolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound A) and 4-(6-benzimidazolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound B) relative to the adipose-derived mesenchymal stem cells line (ASC52-telo) and the fibroblast line (HDFa).
Materials and Methods: The test was performed on commercial cell lines: ASC52-telo and HDFa which were incubated with compounds A and B at four concentrations: 1 μg/mL, 2 μg/mL, 4 μg/mL, 8 μg/mL for 48 and 72 hours. The MTT test was performed to assess the cytotoxicity and determine the IC50 value of compounds A and B against both tested cell lines.
Results: The results of the research indicate that both tested compounds showed stronger cytotoxic activity towards ASC52-telo than HDFa cells. In addition, compound A is characterized by greater cytotoxicity towards both tested cell lines compared to compound B.
Conclusion: The indole- and benzimidazo[1,2-c]quinazolines used in the study could potentially be used in MSCs-based therapy. There is a need to further investigate the safety of using MSCs as drug carriers, and to examine the anticancer activity of the tested compounds, as well as to perform additional and valuable assays, such as enzymatic and toxicity tests.
Keywords: substituted quinazoline derivatives, stem cells therapy, MSC-based cell therapy, anticancerstrategy
Introduction
The biological and pharmacological properties of quinazoline analogues determine the increasing role of these substances in cancer treatment.1,2 Over the past two decades, The Food and Drug Administration (FDA) has approved more than 20 anticancer agents containing a quinazoline ring or quinazolinone system.3 This group of drugs includes several quinazolines, such as: afatinib, erlotinib, gefitinib, lapatinib and vandetanib which have significant therapeutic effectiveness in the treatment of solid tumors.1 However, therapeutic success is limited due to the method of drug administration, which makes it impossible to achieve effective drug concentration directly at the tumor site and poses a risk of systemic side effects. Therefore, new therapeutic solutions are being sought to overcome these barriers. Mesenchymal stem cells (MSCs) seem to be a promising tool to address these challenges.
Due to their unique properties (ability to repair tissues, immunomodulation,4,5 low immunogenicity),6 MSCs are used in the treatment of various diseases.7 The immunomodulatory effects of MSCs may regulate the immune response at sites of inflammation, injury, or cancer.8–10 Research conducted by Mian et al highlights the ability of these cells to escape the control of innate immune mechanisms. Therefore, these cells are able to migrate and inhabit tumor microenvironment. This effect is also related to the activity of chemokines - proteins released by cancer cells.11 Apart from that, MSCs are able to actively influence the fate of cancer cells by paracrine communication with other cells, as well as by modulating the immune response.12 Studies have shown the ability of MSCs to migrate to the tumor sites in ovarian cancer,13 colon cancer,14 breast cancer,15 lung cancer,16 and gliomas.17
Numerous reports about the unique ability of MSCs to migrate and inhabit the tumor microenvironment have aroused interest in the possibility of using MSCs in anticancer therapy.13,14,18 Literature data show that MSCs can load nanoparticles containing anticancer drugs and then release them at the tumor site, causing a therapeutic effect.13,18 MSCs can release anticancer substances in a time-dependent manner, so that the drug can be distributed slowly and locally, which guarantees the achievement of a therapeutically effective concentration of the drug directly in the tumor site.19 It is worth noting, that the ability of MSCs to accept drugs is limited, and chemotherapeutics absorbed into the cytoplasm reduce the viability and migratory capacity of these cells.18 Due to the ambiguous nature of MSCs: the ability to inhibit tumor growth and progression, as well as the ability to support tumorigenesis,20 it seems important to eliminate MSCs from the patient’s body after achieving a therapeutic effect. Clinical studies related to MSCs infusions have proven that their use in treatment is generally safe and does not impair the function of healthy tissues.21 However, in order to maintain safety, genetic engineering techniques can be used to program MSCs so that after releasing the drug at the tumor site, they undergo inactivation or apoptosis.22
Quinazolines are one of the most active classes of nitrogen-containing heterocyclic compounds, whose inhibitory effect at various stages of carcinogenesis is widely described in the literature. Quinazolines may act as receptor tyrosine kinases (RTKs) inhibitors,1 phosphatidylinositol-3-kinase (PI3K) inhibitors,23 cassette transporter inhibitors adenosine-5′-triphosphate-binding cassette (ABC) inhibitors,24 heat shock protein 90 (Hsp90) inhibitors,25 and tubulin blockers.26 Data on these compounds also indicate their anticancer activity at the molecular level, which includes the interaction of quinazoline derivatives with DNA, their intercalation properties, and their activity against topoisomerases I and II.27,28
The synthesis and study of the properties of compounds containing the quinazoline system and differently fused indole and benzimidazole rings seems to be particularly interesting due to the fact that the structure of the compounds is similar to the natural alkaloid hickdentine A, which has a strong anticancer effect.29,30 The synthesis of analogs of this alkaloid is a great challenge, because there are few possibilities to obtain indolo[1,2-c]quinazoline compounds and the related ring system. Moreover, there are no data in the literature on such compounds with a β-resorcyl ring in the structure.30 Therefore, the study of the cytotoxic effect of variously substituted indole- and benzimidazo[1,2-c]quinazolines and their analogs condensed in polycyclic systems, which are characterized by, among others, the ability to interact with cellular DNA, may prove particularly valuable in the context of developing new, effective anticancer therapies based on MSCs.31,32 There is little data on the full mechanism of anticancer action of compounds from the group of indole- and benzimidazo[1,2-c]quinazolines. Mainly, the intercalation effect and the influence on the activity of topoisomerase I and II as well as the influence on DNA replication and transcription are described.27,28,33,34
The aim of this research was to evaluate the cytotoxic activity of chemical compounds from the quinazoline group: 4-(6-indolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound A) and 4-(6-benzimidazolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound B) against MSC line (ASC52-telo) and fibroblast cell line (HDFa). The study was conducted to determine the potential use of the tested compounds in MSC-based therapy. The cytotoxic activity of both substances against MSCs is intended to provide a preliminary assessment of the possibility of transferring these substances by MSCs. The analysis of the cytotoxic activity of both tested compounds against HDFa is intended to demonstrate the potential safety of using these compounds against healthy cells of the body.
Materials and Methods
Materials
Two commercial cell lines were used to conduct the research:
- ASC52-telo cell line (ATCC, SCRC-4000, hTERT immortalized adipose derived mesenchymal stem cells) - a normal, adherent line with a morphology similar to fibroblasts. This line was isolated in 2006. from the adipose tissue of a white woman.35
- Human skin fibroblast cell line (HDFa) (ATCC, PCS-201-012, Primary Dermal Fibroblast; Normal, Human, Adult) - a normal, adherent, skin cell line with research applications in responding to pathogens, skin aging, wound healing, gene delivery and skin diseases including scleroderma. It was a control line in the study.36
In order to carry out the research, two isomole compounds from two constitutional groups were used. The compounds are described in Table 1. and their general formula is presented in Figure 1.
 |
Table 1 Chemical Compounds Used in the Research |
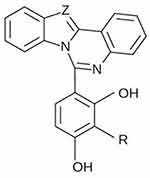 |
Figure 1 The general formula of the tested compounds (R: Me; Z: – CH = or – N =). |
The tested compounds were obtained by reacting appropriately substituted aryl-amines with an original thioaryloylating reagent developed by the research team of the University of Life Sciences in Lublin. The structure of the compounds was confirmed by elemental semi-microanalysis, electron ionization mass spectrometry (EI-MS) and 1H-NMR and IR spectroscopy. The purity of the connections was determined using chromatographic methods. Research on the design, synthesis and assessment of the physicochemical and biological properties of the tested compounds (Ludwik Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences in Wrocław) was financed by Druga Sowiniec Group Sp. z o. o. S. K. A. (formerly: Druga Świtalski&Synowie Sp. z o. o. S. K. A. (Poznań)).
Both compounds contain a quinazoline system and fused indole and benzimidazole rings, respectively. The confirmed mechanisms and directions of action of this group of connections allow to assume that both compounds used in the research should have intercalation properties and activity towards topoisomerase I and II. Due to the substitution - in both representative compounds - of tetracyclic systems with a β-resorcyl ring, the possibility of local interactions is also assumed, including: with Hsp90 and ATP tyrosine kinase proteins.37
Reagents and tests which were used: buffered saline solution (PBS) without the addition of Ca(II) and Mg(II) ions (ATCC, USA), Mesenchymal Stem Cell Basal Medium (ATCC, USA), Mesenchymal Stem Cell Growth Kit – Low Serum (ATCC, USA), trypsin/EDTA solution (0.05% trypsin, 0.02% EDTA in PBS without the addition of Ca(II) and Mg(II) ions) (ATCC, USA), 2% dimethyl sulfoxide (DMSO) (ATCC, USA), fetal bovine serum (FBS) (ATCC, USA), RPMI-1640 Medium (ATCC, USA), 1% penicillin – streptomycin – amphotericin B solution (ATCC, USA), 0.1% trypan blue solution (Invitrogen Thermo Fisher Scientific, USA), CyQUANT MTT Cell Viability Assay (Invitrogen Thermo Fisher Scientific, USA).
Laboratory equipment which was used: laminar chamber HS 9 (Kendro-Heraeus, USA), Zeiss Axiolab inverted microscope (Carl Zeiss Microscopy GmbH, Germany), Victor IV spectrophotometer camera (PerkinElmer, USA), HeraCell 150 CO2 incubator for cell culture (Kendro Heraeus, USA), Sigma 2–26KL centrifuge (Sigma Laborzentrifugen GmbH, Germany), DUB 15 water bath (Thermo Fisher Scientific, USA), and small laboratory equipment: bottles with a growth surface of 25 cm2, pipettes, filters, etc.; (Eppendorf, Millipore, Greiner, Falcon).
Methods
The study has been carried out on freshly thawed commercial cell lines with microbiological purity guaranteed and confirmed by the manufacturer. Cryogenic ampoules with cells, after being removed from liquid nitrogen vapor (−196°C) and after waiting for about 1 minute at room temperature (21°C), were placed in a water bath (37°C) and brought to a liquid state. HDFa and ASC52-telo cell lines were cultured according to the manufacturer’s protocol. After thawing, the cells were suspended in a medium dedicated for each line: RPMI-1640 with the addition of fetal bovine serum and 1% penicillin-streptomycin-amphotericin B solution for the HDFa and Mesenchymal Stem Cell Basal Medium lines combined with Mesenchymal Stem Cell Growth Kit - Low Serum for ASC52-telo cell line. Cultures were incubated in culture bottles with a growth area of 25 cm2, incubating them at a temperature of 37°C and an atmosphere of 5% CO2 until monolayers were obtained.35,36
In order to assess the cytotoxicity and determine the IC50 value of compounds A and B against the tested cell lines: ASC52-telo and HDFa, the MTT test was performed in accordance with the manufacturer’s protocol.38 2% DMSO solution was used to dissolve the tested compounds. Before the actual test, a control test was carried out, which showed no influence of the solvent on the viability of cells of both lines. A 0.1% trypan blue solution was used to assess the viability of cells prepared for the experiment. Cell viability <95% was considered insufficient to conduct the study. The cell culture density used in the test was 104 cells/mL and was within the limits recommended by the manufacturer (103–106 cells/mL of suspension). HDFa and ASC52-telo cell lines treated with compound A and compound B were incubated at four concentrations: 1 μg/mL, 2 μg/mL, 4 μg/mL, 8 μg/mL for 48 and 72 hours.
The studies were performed in triplicate for each cell line. The test was performed in a 24-well plate where 100 μL of MTT was added per 1 mL of cell suspension. A volume of MTT solution equal to 10% of the well volume was added to each well of a multi-well plate. Then everything was incubated for 4 hours in an incubator at 37°C, 5% CO2. After incubation, the formed formazan crystals were dissolved with acidic isopropanol. To accomplish this, a volume of isopropanol equal to the volume of the cell suspension was added to each multi-well plate. Gently pipetting dissolved the formazan crystals. Using a spectrophotometric reader, the absorbance of the solutions was measured at a wavelength of 540 nm. The obtained results were processed as follows: average absorbance values were calculated for each concentration of the tested compound and for the control, it was calculated what percentage of the value obtained for control cells (control cells are 100%) is the calculated average absorbance values (A) for individual concentrations of tested compounds A and B, and a graph was made of the relationship between cell viability (% of control) and the concentration of the tested compound. From the graph prepared in this way, the value of the IC50 parameter for compound A and IC50 for compound B was read.
Statistical Analysis
The values of the analyzed data were presented as: median, arithmetic mean ± standard deviation (SD), the lowest value of the statistical series (Min)., the highest value of the statistical series (Max). and the value of the lower and upper quartiles. The compliance of the distribution of individual variables with the normal distribution was checked using the Shapiro–Wilk test. Since the studied variables were not normally distributed, non-parametric tests were used in further analysis. For this purpose, the Wilcoxon test and the Mann–Whitney U-test were used. All p values lower than 0.05 were considered significant. Statistical analysis was performed using Statistica 7.1 PL software (StatSoft Inc, USA, RRID:SCR_014213).
Results
IC50 values of compound A and compound B were determined for 48- and 72-hour culture of ASC52-telo and HDFa cell lines. The study was carried out in three repetitions: for both time intervals, three cell cultures of both cell lines were established, and the IC50 value of both tested compounds was determined for each sample. The obtained IC50 values of compounds A and B are presented in Table 2.
 |
Table 2 IC50 Values of Compound A and Compound B Obtained for ASC52-Telo and HDFa Cells in 48- and 72-Hour Cell Cultures |
It was shown that the IC50 values of both tested compounds determined for ASC52-telo line cells are statistically significantly lower than the IC50 values of these compounds obtained for HDFa line cells (p≤0.05). In addition, a statistically significant difference was demonstrated by comparing the IC50 values of compound B determined for ASC52-telo and HDFa line cells in 72 hours cell culture (p≤0.05). For ASC52-telo and HDFa cell lines, statistically significant higher IC50 values of compound B were obtained in a 48-hour culture compared to a 72-hour culture (p≤0.05).
The research revealed statistically significant higher IC50 values of compound B compared to IC50 values of compound A in 48-hour cell cultures of both tested lines (p≤0.05). Assessment of the IC50 values of compound A and compound B for HDFa cell culture conducted for 72 hours showed similar statistically significant differences (p≤0.05), where the IC50 values of compound B were higher compared to the IC50 values of compound A. The assessment of the IC50 value of both tested compounds with respect to the culture time and the type of cell line tested is presented in Figures 2–4.
 |
Figure 2 Evaluation of the IC50 values of compound A and compound B determined for ASC52-telo line cells in 48-hour cell culture. |
 |
Figure 3 Assessment of the IC50 values of compound A and compound B determined for HDFa line cells in 48-hour cell culture. |
 |
Figure 4 Assessment of the IC50 values of compound A and compound B determined for HDFa line cells in 72-hour cell culture. |
When assessing the IC50 value of compound A, a statistically significantly higher result was obtained for the HDFa cell line compared to the ASC52-telo cell line (p≤0.05). The difference between the IC50 values of compound A obtained for HDFa line cells in 48- and 72-hours cell culture turned out to be statistically significant. In this case, the IC50 values of compound A were higher in the 48-hour culture compared to the IC50 values of compound A in the 72-hour culture (p≤0.05). Analysis of the mean ± SD of the IC50 parameter of compound A obtained for cells of both tested cell lines in 48- and 72-hour cultures is shown in Figure 5.
 |
Figure 5 Evaluation of the IC50 values of compound A determined for ASC52-telo cells and HDFa cells in 48- and 72-hour cell cultures. |
In 48- and 72-hour cultures, higher statistically significant IC50 values of compound B were obtained for HDFa line cells compared to ASC52-telo line cells (p≤0.05) (Figure 6).
 |
Figure 6 Evaluation of the IC50 values of compound B determined for ASC52-telo cells and HDFa cells in 48- and 72-hour cell cultures. |
Discussion
Reports of preclinical studies and ongoing clinical trials evaluating the efficacy of therapies based on MSCs loaded with therapeutic anticancer agents emphasize the importance of the topic discussed in this article.39 Studies indicate that the use of MSCs as a carrier of anticancer drugs results in a better therapeutic effect compared to the traditional method of administering chemotherapy, due to the possibility of achieving the therapeutic concentration of the drug (with prolongation of its release time and reduction of the administered amount) directly at the site of the tumor, and at the same time limiting its toxic effect on healthy tissues. This is confirmed by studies that examined the possibility of MSCs carrying and then releasing into the tumor microenvironment drugs known and used in anticancer therapy, such as doxorubicin (DOX), paclitaxel (PTX), gemcitabine, sorafenib and others.22,40 Bonomi et al showed in an in vitro experiment on a human multiple myeloma cell line that PTX-loaded MSCs cause strong suppression of cancer cells compared to MSCs without the drug.41 Nicolay et al noticed in an in vivo study that MSCs used as a PTX carrier caused a strong inhibition of murine melanoma lung metastases.42 In a study on the effect of DOX-loaded MSCs on colorectal cancer cells, a significantly inhibitory effect of the drug was demonstrated compared to the use of free DOX.43 MSCs can be a vehicle for delivering therapeutic chemicals as well as genes to target tumor sites. Furthermore, extracellular vesicles (EVs) released from MSCs, can be used as a potent cell-free tool for cancer therapy.40,44 For example Wu et al showed that human umbilical cord MSC-derived small extracellular vesicles (hucMSC-sEVs) can play a significant role as inhibitors of tumor progression in gastric cancer (GC). The researchers found that hucMSC-sEVs are captured by GC cells, and then inhibited their proliferation and induced apoptosis.45 In another study, Zhou et al demonstrated a negative effect of human placenta MSCs-derived EVs (hPMSC-EVs) on breast cancer growth by inhibiting tumor cell proliferation and migration via an indirect antiangiogenic mechanism.46
In order to use MSCs as a carrier of newly synthesized chemotherapeutics, it is important to assess the impact of these substances on the viability, proliferation, differentiation and self-renewal capacity of MSCs cells. The obtained results show that both tested compounds are characterized by statistically significantly higher cytotoxic activity towards ASC52-telo line cells compared to cytotoxic activity towards HDFa line cells, which represent the patient’s healthy tissue cells in this study. It allows to assume that the tested substances will not be the cause of severe side effects during therapy. Moreover, in the case of both tested cell lines, it was found that the cytotoxic effect of both compounds was statistically significantly stronger in a 72-hour culture compared to a 48-hour culture. The effect of compound A on ASC52-telo line cells in a 72-hour culture turned out to be so strong that the determination of the IC50 coefficient went beyond the test reference values.
As the literature indicates, a decisive factor in the development of MSC-based therapy, in addition to the type of chemotherapeutic agent, is the origin of the MSC.22 In the study we human adipose-derived MSCs (AD-MSCs) were used. There have been reports of assessing the sensitivity of AD-MSCs to known chemotherapeutic agents. Rimoldi et al showed that AD-MSCs are moderately resistant to cisplatin and the cationic platinum (II) complex. The authors also noted the ability of MSCs to uptake similar amounts of both drugs.47 Similar results regarding cisplatin were presented by the authors of another study.48 Liang et al demonstrated the resistance of AD-MSCs to vincristine and camptothecin, and also found that the tested MSCs retained their characteristics after in vitro exposure to all medicinal substances used by the authors.49 The authors of another study proved that the viability of AD-MSCs after a 3-day exposure to cisplatin and camptothecin was 70%, and PTX (at a concentration of up to 10,000 ng/mL) caused the death of only 20% of the cells.50 Studies also suggest that AD-MSCs are more resistant to genotoxic damage caused by anticancer drugs compared to bone marrow-derived MSCs.22 This information confirms the correctness of the selection of AD-MSCs for the study.
Compounds containing a quinazoline ring or quinazoline system are among the therapeutic agents currently used in the treatment of cancer.3,40 Due to the significant anticancer effectiveness of these chemotherapeutics, numerous attempts are being made to synthesize new analogues of quinazoline compounds, whose properties and activity against various cancer cell lines are being checked.27–30,33,34,51,52 The confirmed mechanisms and directions of action of this group of connections indicate that both tested compounds may exhibit, among others: intercalation properties, activity towards topoisomerases I and II, as well as the possibility of interaction with Hsp90 proteins and ATP tyrosine kinases.37 However, this requires confirmation in further studies.
The results of the conducted research indicate that 4-(6-indolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound A) is characterized by high cytotoxicity at relatively low concentrations towards tested cell lines compared to 4-(6-benzimidazolo[1,2-c]quinazoline)2-methyl-benzene-1,3-diol (compound B). Moreover, both tested compounds showed stronger cytotoxic activity towards ASC52-telo cells than towards HDFa cells. This may be related to the nature of MSCs, which, like cancer cells, have the ability to unlimited number of cell divisions and are distinguished by their metabolic activity among other healthy cells in the human body.4,5,8–10,12 A deeper understanding of the effects of both tested compounds on healthy and cancer cells, as well as the discovery of the possibility of using these compounds in MSC-based therapy requires extensive research analysis, both in vitro and in vivo.
Conclusion
The indole- and benzimidazo[1,2-c]quinazolines used in the study could potentially be used in MSCs-based therapy. However, the study has its limitations and should be continued and expanded, including by performing additional tests, eg enzymatic analyses and toxicity studies. There is also a need to explore the anticancer activity of both tested compounds and, above all, to determine in which cancers they may be most effective in treatment. It is also necessary to conduct further research on the possibility of transporting the tested compounds by MSCs to the tumor microenvironment and then the ability to release them in order to obtain the therapeutic concentration of the compounds directly in the area of tumor tissue. There is also a need to explore the nature of MSCs and the safety of using these cells as carriers of chemotherapy drugs.
Abbreviations
MSCs, mesenchymal stem cells; ASC52-telo, adipose-derived mesenchymal stem cells line; HDFa, fibroblast line; FDA, The Food and Drug Administration; RTKs, receptor tyrosine kinases; PI3K, phosphatidylinositol-3-kinase; ABC, adenosine-5′-triphosphate-binding cassette; Hsp90, heat shock protein 90; DOX, doxorubicin; PTX, paclitaxel; AD-MSCs, adipose-derived MSCs; EI-MS, electron ionization mass spectrometry; EVs, extracellular vesicles; hPMSC-EVs, human placenta MSCs-derived EVs; GC, gastric cancer; SD, standard deviation; Min, the lowest value of the statistical series; Max, the highest value of the statistical series.
Acknowledgments
Appreciating the involvement in the implementation of the research project resulting in this work, we would like to thank Professor Ryszard Maciejewski and Professor Andrzej Niewiadomy for their help and support.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bathula R, Monda P, Raparla R, et al. Evaluation of antitumor potential of synthesized novel 2-substituted 4- anilinoquinazolines as quinazoline-pyrrole hybrids in MCF-7 human breast cancer cell line and A-549 human lung adenocarcinoma cell lines. Future J Pharm Sci. 2020;6:44. doi:10.1186/s43094-020-00059-5
2. Ghorab MM, Alsaid MS, Al-Dosari MS, et al. Design, Synthesis and Anticancer Evaluation of Novel Quinazoline-Sulfonamide Hybrids. Molecules. 2016;21(2):189. doi:10.3390/molecules21020189
3. Haider K, Das S, Joseph A, et al. An appraisal of anticancer activity with structure-activity relationship of quinazoline and quinazolinone analogues through EGFR and VEGFR inhibition: a review. Drug Dev Res. 2022;83(4):859–890. doi:10.1002/ddr.21925
4. Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27(8):1954–1962. doi:10.1002/stem.118
5. Su J, Chen X, Huang Y, et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014;21(3):388–396. doi:10.1038/cdd.2013.149
6. Lan T, Luo M, Wei X. Mesenchymal stem/stromal cells in cancer therapy. J Hematol Oncol. 2021;14(1):195. doi:10.1186/s13045-021-01208-w
7. Naji A, Eitoku M, Favier B, et al. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol Life Sci. 2019;76(17):3323–3348. doi:10.1007/s00018-019-03125-1
8. Ahn SY, Sung DK, Chang YS, et al. BDNF-overexpressing engineered mesenchymal stem cells enhances their therapeutic efficacy against severe neonatal hypoxic ischemic brain injury. Int J Mol Sci. 2021;22:11395. doi:10.3390/ijms222111395
9. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. doi:10.3322/caac.21763
10. Sharma RR, Pollock K, Hubel A, et al. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418–1437. doi:10.1111/trf.12421
11. Miana VV, Gonzalez EAP. Adipose tissue stem cells in regenerative medicine. Ecancermedicalscience. 2018;12:822. doi:10.3332/ecancer.2018.822
12. Rastegar F, Shenaq D, Huang J, et al. Mesenchymal stem cells: molecular characteristics and clinical applications. World J Stem Cells. 2010;2:67–80. doi:10.4252/wjsc.v2.i4.67
13. Takayama Y, Kusamori K, Tsukimori C, et al. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J Control Release. 2021;329:1090–1101. doi:10.1016/j.jconrel.2020.10.037
14. Komarova S, Roth J, Alvarez R, et al. Targeting of mesenchymal stem cells to ovarian tumors via an artificial receptor. J Ovarian Res. 2010;3:12. doi:10.1186/1757-2215-3-12
15. Hung SC, Deng WP, Yang WK, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11(21):7749–7756. doi:10.1158/1078-0432.CCR-05-0876
16. Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–63.
17. Loebinger MR, Kyrtatos PG, Turmaine M, et al. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–8867. doi:10.1158/0008-5472.CAN-09-1912
18. Chartouni A, Mouawad A, Boutros M, et al. Mesenchymal stem cells: a trojan horse to treat glioblastoma. Invest New Drugs. 2023;41(2):240–250. doi:10.1007/s10637-023-01352-9
19. Foglietta F, Duchi S, Canaparo R, et al. Selective sensitiveness of mesenchymal stem cells to shock waves leads to anticancer effect in human cancer cell co-cultures. Life Sci. 2017;173:28–35. doi:10.1016/j.lfs.2017.01.009
20. Lin W, Huang L, Li Y, et al. Mesenchymal stem cells and cancer: clinical challenges and opportunities. Biomed Res Int. 2019;2019:2820853. doi:10.1155/2019/2820853
21. Dotoli GM, De Santis GC, Orellana MD, et al. Mesenchymal stromal cell infusion to treat steroid-refractory acute GvHD III/IV after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52(6):859–862. doi:10.1038/bmt.2017.35
22. Babajani A, Soltani P, Jamshidi E, et al. Recent advances on drug-loaded mesenchymal stem cells with anti-neoplastic agents for targeted treatment of cancer. Front BioengBiotechnol. 2020;8:748.
23. Raj A, Kumar A, Singh AK, et al. Synthetic methodologies and SAR of quinazoline derivatives as PI3K inhibitors. Anticancer Agents Med Chem. 2023;16.
24. Braconi L, Teodori E, Contino M, et al. Overcoming multidrug resistance (MDR): design, biological evaluation and molecular modelling studies of 2,4-substituted quinazoline derivatives. Chem Med Chem. 2022;17(12):e202200027. doi:10.1002/cmdc.202200027
25. El-Shafey HW, Gomaa RM, El-Messery SM, et al. Quinazoline based HSP90 inhibitors: synthesis, modeling study and ADME calculations towards breast cancer targeting. Bioorg Med Chem Lett. 2020;30(15):127281. doi:10.1016/j.bmcl.2020.127281
26. Li W, Yin Y, Shuai W, et al. Discovery of novel quinazolines as potential anti-tubulin agents occupying three zones of colchicine domain. Bioorg Chem. 2019;83:380–390. doi:10.1016/j.bioorg.2018.10.027
27. Via LD, Gia O, Magno SM, et al. Synthesis, in vitro antiproliferative activity and DNA-interaction of benzimidazoquinazoline derivatives as potential anti-tumor agents. Il Farmaco. 2001;56:159–167.
28. Guo S, Tao L, Zhang W, et al. Regioselective synthesis of Indolo[1,2- c]quinazolines and 11 h -Indolo[3,2- c]quinolines via copper-catalyzed cascade reactions of 2-(2-Bromoaryl)-1 h -indoles with aldehydes and aqueous ammonia. J Org Chem. 2015;80(21):10955–10964. doi:10.1021/acs.joc.5b02076
29. Rohini R, Shanker K, Reddy PM, et al. 6-substituted indolo[1,2-c]quinazolines as new Antimicrobial agents. Arch Pharm. 2009;342(9):533–540. doi:10.1002/ardp.200900068
30. McWhorter WW, Liu Y. Progress toward the synthesis of hinckdentine A. Curr Opin Drug Discov Devel. 2003;6(6):930–944.
31. Frère S, Thiéry V, Ch B, et al. Novel 6-substituted benzothiazol-2-yl indolo[1,2-c]quinazolines and benzimidazo[1,2-c]quinazolines. Tetrahedron. 2003;59:773–779.
32. Lamazzi C, Léonce S, Pfeiffer B, et al. Expeditious synthesis and cytotoxic activity of new cyanoindolo[3,2-c]quinolines and benzimidazo[1,2-c]quinazolines. Bioorg Med Chem Lett. 2000;10(19):2183–2185. doi:10.1016/S0960-894X(00)00427-3
33. Braña MF, Castellano JM, Keilhauer G, et al. Benzimidazo[1,2-c]quinazolines: a new class of antitumor compounds. Anti-Cancer Drug Design. 1994;9(6):527–538.
34. Sang P, Xie Y, Zou J, et al. Copper-catalyzed sequential Ullmann N -arylation and aerobic oxidative C–H amination: a convenient route to Indolo[1,2- c]quinazoline derivatives. Org Let. 2012;14(15):3894–3897. doi:10.1021/ol3016435
35. “ASC52telo, hTERT immortalized adipose derived mesenchymal stem cells, SCRC-4000” (ATCC, USA). 2023.
36. “Primary dermal fibroblast; normal, human, adult (HDFa), PCS-201-012”. (ATCC, USA). 2023.
37. Stevens MFG, Wells G, Westwell AD, Poole TD. PCT Int. Appl. WO 0304479, 2003.
38. “CyQUANT MTT cell viability assay protocol” (Invitrogen thermo fisher scientific, USA). 2021.
39. Yu Y, Tao Y, Ma J, et al. Targeting the tumor microenvironment with mesenchymal stem cells based delivery approach for efficient delivery of anticancer agents: an updated review. BiochemPharmacol. 2025;232:116725.
40. Szewc M, Radzikowska-Bűchner E, Wdowiak P, et al. MSCs as tumor-specific vectors for the delivery of anticancer agents-A potential therapeutic strategy in cancer diseases: perspectives for quinazoline derivatives. Int J Mol Sci. 2022;23(5):2745. doi:10.3390/ijms23052745
41. Bonomi A, Steimberg N, Benetti A, et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. HematolOncol. 2017;35(4):693–702.
42. Nicolay NH, Rühle A, Perez RL, et al. Mesenchymal stem cells are sensitive to bleomycin treatment. Sci Rep. 2016;6:26645. doi:10.1038/srep26645
43. Bagheri E, Abnous K, Farzad SA, et al. Targeted doxorubicin-loaded mesenchymal stem cells-derived exosomes as a versatile platform for fighting against colorectal cancer. Life Sci. 2020;261:118369. doi:10.1016/j.lfs.2020.118369
44. Kangari P, Salahlou R, Vandghanooni S. Harnessing the therapeutic potential of mesenchymal stem cells in cancer treatment. Adv Pharm Bull. 2024;14(3):574–590. doi:10.34172/apb.2024.052
45. Wu P, Wang M, Jin C, et al. Highly efficient delivery of novel MiR-13896 by human umbilical cord mesenchymal stem cell-derived small extracellular vesicles inhibits gastric cancer progression by targeting ATG2A-mediated autophagy. Biomater Res. 2024;28:0119. doi:10.34133/bmr.0119
46. Zhou M, Li H, Zhao J, et al. Extracellular vesicles derived from mesenchymal stem cells suppress breast cancer progression by inhibiting angiogenesis. Mol Med Rep. 2024;30(5):192. doi:10.3892/mmr.2024.13316
47. Rimoldi I, Coccè V, Facchetti G, et al. Uptake-release by MSCs of a cationic platinum(II) complex active in vitro on human malignant cancer cell lines. Biomed Pharmacother. 2018;108:111–118. doi:10.1016/j.biopha.2018.09.040
48. Gilazieva Z, Tazetdinova L, Arkhipova S, et al. Effect of cisplatin on ultrastructure and viability of adipose-derived mesenchymal stem cells. BioNanoSci. 2016;6:534–539. doi:10.1007/s12668-016-0283-0
49. Liang W, Xia H, Li J, et al. Human adipose tissue derived mesenchymal stem cells are resistant to several chemotherapeutic agents. Cytotechnology. 2011;63(5):523–530. doi:10.1007/s10616-011-9374-5
50. Bonomi A, Coccè V, Cavicchini L, et al. Adipose tissue-derived stromal cells primed in vitro with paclitaxel acquire anti-tumor activity. Int J ImmunopatholPharmacol. 2013;26:33–41. doi:10.1177/03946320130260S105
51. Catanzaro E, Betari N, Arencibia JM, et al. Targeting topoisomerase II with trypthantrin derivatives: discovery of 7-((2-(dimethylamino)ethyl)amino)indolo[2,1-b]quinazoline-6,12-dione as an antiproliferative agent and to treat cancer. Eur J Med Chem. 2020;202:112504. doi:10.1016/j.ejmech.2020.112504
52. Zou Y, Zhang G, Li C, et al. Discovery of tryptanthrin and its derivatives and its activities against NSCLC in vitro via both apoptosis and autophagy pathways. Int J Mol Sci. 2023;24(2):1450. doi:10.3390/ijms24021450
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

