Back to Journals » Cancer Management and Research » Volume 17
Predicting the Efficacy of Immune Checkpoint Inhibitors in Esophageal Cancer: Changes in Peripheral Blood Lymphocyte Subsets Before and After Immunotherapy
Authors Cao R , Jiao P, Zhang S, Li J, Liu Q
Received 12 November 2024
Accepted for publication 4 April 2025
Published 16 April 2025 Volume 2025:17 Pages 815—825
DOI https://doi.org/10.2147/CMAR.S503171
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Ruijie Cao,1 Pengqing Jiao,1 Shasha Zhang,1 Jiasong Li,1 Qingyi Liu2
1Department of Immunology and Rheumatology, The Fourth Hospital of Hebei Medical University, Shijiazhuang, 050011, People’s Republic of China; 2Department of Cardiothoracic Surgery, The Fourth Hospital of Hebei Medical University, Shijiazhuang, 050011, People’s Republic of China
Correspondence: Qingyi Liu, Department of Cardiothoracic Surgery, The Fourth Hospital of Hebei Medical University, 12 Jiankang Road, Shijiazhuang, 050011, People’s Republic of China, Tel +86 311 86095734, Email [email protected]
Background: Immunotherapy has demonstrated potential in the treatment of esophageal cancer (EC); however, the overall response rate (ORR) remains below 30% among EC patients. Herein, the use of peripheral blood lymphocyte subsets as biomarkers was explored to evaluate the efficacy of immunotherapy in this patient population.
Methods: Sixty-three patients were enrolled. The patients were diagnosed with EC and treated with immune checkpoint inhibitors (ICIs) at The Fourth Hospital of Hebei Medical University from December 2019 to June 2023. Kaplan–Meier (KM) survival curves were used to reflect differences in survival benefit. The prognostic factors of survival were investigated using the Cox proportional hazards regression model for both univariate and multivariate analyses. Two-tailed P values were reported and statistical significance was defined as P < 0.05.
Results: The results of univariate and multifactorial Cox regression analysis for progression-free survival (PFS) revealed that only CD8+ T lymphocytes demonstrated a significant association with PFS (P = 0.034, P = 0.020). Additionally, the multifactorial Cox regression analysis results for overall survival (OS) revealed a significant association between natural killer (NK) cells and OS (P=0.049). Further, a systematic analysis was conducted on the CD8+ T cell biomarker. The KM survival curves indicated that the group with low CD8+ T cell levels experienced a significantly greater PFS benefit compared to the high CD8+ T cell group (P = 0.030).
Conclusion: The present study reveals that the reduction of both CD8+ T lymphocytes and NK cells in peripheral blood lymphocyte subsets after immunotherapy can serve as superior predictors for the effectiveness of ICIs in patients diagnosed with EC.
Keywords: esophageal cancer, lymphocyte subsets, immune checkpoint inhibitors, predictor, biomarker
Background
Esophageal cancer (EC) ranks as the seventh most prevalent malignant tumor in terms of incidence, with 604,000 new cases reported globally.1 It is also the sixth leading cause of cancer-related mortality, responsible for 544,000 deaths in 2020. This statistic underscores that EC accounts for approximately one in every eighteen cancer-related deaths.2 Despite advancements in multidisciplinary treatments, encompassing surgical interventions, chemotherapy, and radiotherapy, the prognosis of individuals diagnosed with EC remains unfavorable.3 Immune checkpoint inhibitors (ICIs) induce durable objective remission in patients with various cancers, including EC, by activating autologous lymphocytes.4 Following the release of the Keynote-590 study, immunotherapy has emerged as a first-line treatment option for EC.5 However, the overall response rate (ORR) for immunotherapy in EC patients remains below 30%,6 indicating a substantial proportion of patients do not derive benefits from ICI therapy. Consequently, identifying patients who are likely to respond to these agents is crucial. Several immune-related biomarkers, including programmed cell death protein ligand 1 (PD-L1) expression, tumor mutational burden (TMB), and microsatellite instability (MSI), have been identified as potentially valuable predictors for ICI-based treatment in EC.7 Nonetheless, it is important to acknowledge that the current accuracy of these biomarkers remains limited.8 It has been reported that patients with PD-L1 positive tumors commonly exhibit resistance to ICI therapy in the absence of a pre-existing immune response.9 Conversely, some investigations have reported that a substantial number of patients with PD-L1-negative tumors may still benefit from ICIs, particularly when receiving combination therapies that enhance T cell infiltration.10 The prognostic impact of TMB on solid tumors remains largely unexplored,11 and previous studies have reported that high TMB does not serve as a predictive factor for the response to ICIs in patients with EC.12–14 Further, deficient mismatch repair (dMMR) occurs in only 4.35% of EC cases, making MSI a rarely reported factor in this cancer type.15,16 Therefore, there is an urgent need to explore new biomarkers that can predict both the efficacy and safety of ICIs.
The activation of lymphocytes is a pivotal factor in immunotherapy; thus, the quantity and subset of lymphocytes are intricately linked to the efficacy of ICIs. T cells serve as the primary effector cells involved in the immune response, which can be broadly classified into cytotoxic T cells (CD8+) and regulatory T cells (CD4+).17 The cytotoxicity and migratory capacity of CD8+ T cells make them crucial to the anti-tumor immune response, as they can infiltrate tumor tissues from the peripheral blood.18 In contrast to the direct tumor-killing effect exerted by CD8+ T cells, CD4+ T cells primarily function in an immune-modulatory and paracrine capacity. CD4+ Th1 cells facilitate the differentiation of initial CD8+ T cells into cytotoxic T lymphocytes (CTL) via the CD70-CD27 pathway, and enhance the infiltration of tumor-reactive CD8+ T cells into the tumor microenvironment.19
Herein, a retrospective study was conducted to analyze the correlation between changes in peripheral blood lymphocyte subsets before and after immunotherapy and prognosis of EC, with the aim of exploring the potential applicability of peripheral blood lymphocyte subsets as a biomarker.
Methods
Study Participants
After excluding 8 patients who lacked data on peripheral blood lymphocyte subsets, 63 patients were ultimately included. The included patients were diagnosed with EC and treated with ICIs at The Fourth Hospital of Hebei Medical University between December 2019 and June 2023. The inclusion criteria were: (i) pathological confirmation of EC; (ii) receipt of at least 3 cycles of ICIs during the treatment period; and (iii) assessment of peripheral blood lymphocyte subsets both before and after immunotherapy. The exclusion criteria were: (i) a follow-up period of less than 3 months after ICI therapy; and (ii) simultaneous receipt of two or more types of ICI therapy.
Data Collection
For each patient meeting the inclusion criteria, data were collected on several aspects: basic patient information, including gender, age, disease stage according to the Tumor, Node, and Metastasis (TNM) classification system, and Eastern Cooperative Oncology Group Performance Status (ECOG PS) score; treatment specifics, detailing the lines of ICIs administered and whether the patient had received radiation therapy or surgical interventions; lymphocyte subset data, including the total counts of T lymphocytes, B lymphocytes, and natural killer (NK) cells, as well as the counts and percentages of CD4+ and CD8+ T cells both before and after immunotherapy; and efficacy parameters, such as overall survival (OS) and progression-free survival (PFS).
Specimen Collection and Lymphocyte Subset Detection
Sample Preparation: Peripheral blood samples were collected in EDTA-coated tubes and processed within 6 hours.
Detection of peripheral blood lymphocyte subsets: Whole blood samples (100 μL) were incubated with a pre-mixed antibody cocktail (BECKMAN COULTER, Cyto-STAT tetra CHROME CD45-FITC /CD4-RD1/CD8-ECD /CD3-PC5 and Cyto-STAT tetra CHROME CD45- FITC/CD56-RD1/CD19-ECD/CD3-PC5) for 15 minutes at room temperature in the dark. Following incubation, OptiLyse C Lysing Solution (BECKMAN COULTER) was added to lyse red blood cells, and the mixture was incubated for an additional 10 minutes. The lysed sample was centrifuged at 300 × g for 5 minutes, after which the supernatant was carefully discarded. The resulting cell pellet was washed twice with phosphate-buffered saline (PBS) and subsequently resuspended in 500 μL of PBS. Samples were analyzed using a BECKMAN COULTER CytoFLEX flow cytometer equipped with 488 nm and 638 nm lasers. Absolute lymphocyte counts were precisely determined by the addition of Flow-Count Fluorospheres (BECKMAN COULTER) prior to data acquisition. Lymphocyte subsets, namely B cells, NK cells, CD3+T cells, CD4+ helper T cells, and CD8+ cytotoxic T cells, were quantitatively analyzed using Kaluza Analysis 2.0 Software with gating strategies based on forward/side scatter and fluorescence parameters.
Treatment and Assessment
Patients received standard programmed cell death 1 (PD-1) inhibitors every 3 weeks until disease progression, unacceptable toxicity, clinical deterioration, or patient refusal. The PD-1 inhibitors included toripalimab at a dose of 240 mg every 2 weeks, as well as sintilimab, camrelizumab, and pembrolizumab, each administered at a dose of 200 mg every 3 weeks. The objective tumor response was evaluated using the New Response Evaluation Criteria in Solid Tumours: revised RECIST guideline (version 1.1),20 based on repeated assessments with computed tomography (CT), magnetic resonance imaging (MRI), upper gastrointestinal contrast, or endoscopy conducted every 2 or 3 cycles.
Data Analysis
IBM SPSS version 21.0 was used for statistical analysis, and visualization was accomplished using the “ggplot2” package running in R software version 4.2.2 (www.R-project.org, 2022). Categorical variables were described using frequency and percentage, and group comparisons were conducted using the Chi-square test. Continuous variables were reported as Mean ± SD if they followed a normal distribution, or as median and interquartile range (IQR) if they did not follow a normal distribution. Between-group comparisons were conducted using either the Student’s t-test or Mann–Whitney test, depending on the appropriateness of each method. Patients who achieved disease control and had a PFS of > 6 months were categorized as the benefit group, while those with PFS ≤6 months were classified as the non-benefit group. The difference in CD8+ T cell counts and NK cell counts before and after immunotherapy was calculated, with a positive result indicating the high-level group and a negative result indicating the low-level group. Kaplan–Meier (KM) survival curves were used to reflect differences in survival benefit. The area under the curve (AUC) of the receiver operating characteristic (ROC) curve was used to assess the value of efficacy prediction. The prognostic factors for survival were analyzed using the Cox proportional hazards regression model for both univariate and multivariate analyses. Two-tailed P values were calculated, with statistical significance defined as P < 0.05.
Results
Basic Characteristics of the Patients
A total of 63 patients diagnosed with EC met the inclusion criteria. The cohort comprised 46 males and 17 females, with a mean age of 66.4 years. All patients received immunotherapy in combination with chemotherapy; 14 patients (22.2%) also underwent radiation therapy, and 15 patients (23.8%) received surgical intervention. The majority of patients (55) received ICIs as their first-line treatment, while 8 patients (12.7%) received ICIs as second-line or later therapy. Most patients were in good physical condition, with 39 patients (61.9%) having an ECOG performance status score of 0–1 (see Table 1). The mean or median number of each lymphocyte subpopulation is also detailed in Table 1. The overall objective response rate (ORR) was 23.8%, and the disease control rate (DCR) was 84.1%. The median PFS for the overall population was 436 days.
 |
Table 1 Basic Information |
Correlation of Lymphocyte Subsets with the Efficacy of ICIs
To investigate the correlation between changes in peripheral blood lymphocyte subsets before and after immunotherapy and the efficacy of ICIs, patients were categorized into two groups based on their survival outcomes. The benefit group comprised patients who achieved disease control and PFS for a duration of > 6 months, while the non-benefit group included those who experienced PFS ≤ 6 months. The univariate Cox regression analysis for PFS and OS incorporated patient basic information, as well as the difference between total lymphocytes, B cells, NK cells, CD4+ T cell count and percentage, and CD8+ T cell count and percentage before and after immunotherapy (Table 2). The results of the univariate Cox regression analysis for PFS are as follows: age (P = 0.423), ECOG performance status score (P = 0.232), lines of ICIs usage (P = 0.411), TNM staging (P = 0.693), receipt of radiotherapy (P = 0.937), and surgical intervention (P = 0.021). The only lymphocyte subset significantly associated with PFS was the reduction in CD8+ T lymphocytes following immunotherapy (P = 0.034). Consequently, both patient basic information and the reduction in CD8+ T cell counts were included in a multivariate analysis, which consistently demonstrated a strong correlation between CD8+ T cell reduction and PFS (P = 0.008). For OS, the univariate Cox regression analysis identified only the ECOG performance status score (P = 0.020) as significantly associated with OS. However, a subsequent multivariate analysis incorporating ECOG PS score, lines of ICIs usage, TNM staging, receipt of radiotherapy, surgical intervention, and all lymphocyte subsets revealed a significant association between NK cell depletion after immunotherapy and OS (P = 0.049).
 |
Table 2 Univariate and Multivariate Analyses of PFS and OS with Cox Regression Models |
Further, a systematic analysis was performed on the CD8+ T cell biomarker. The results showed that the high-level and low-level groups of CD8+ T cells were well matched in terms of all basic information, including sex, age, line of therapy, ECOG PS, TNM staging, and whether patients had undergone radiation therapy or surgical intervention (Table 3). The decline of CD8+T cells after immunotherapy was more pronounced in patients belonging to the benefit group compared to non-benefit group, but there was no statistical difference between the benefit and non-benefit groups (P = 0.52) (Figure 1). The KM survival curves showed that the low CD8+ T-cell group (median PFS: 480 days) had a significantly better PFS benefit than the high CD8+ T-cell group (median PFS: 300 days) (P = 0.030) (Figure 2). However, CD8+ T cells were not an ideal prognostic biomarker for EC, with an AUC of only 0.553 (see Figure 3). Additionally, the KM curves were used to evaluate the association between changes in NK cell levels before and after immunotherapy and OS, but no statistical difference was found between the two groups (P = 0.920) (see Figure S1).
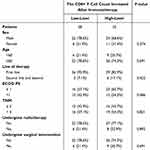 |
Table 3 Analysis of Basic Information Matching Between High and Low CD8+ T Cell Groups |
 |
Figure 1 Differential analysis of CD8+ T cells before and after immunotherapy in the benefit group and non-benefit group. |
 |
Figure 2 Kaplan-Meier survival curve illustrating the PFS across varying levels of CD8+ T cell differences before and after immunotherapy. |
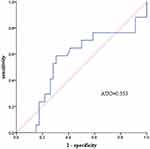 |
Figure 3 ROC curve of the difference in CD8+ T cell levels before and after immunotherapy. |
Discussion
Prior to 2020, the standard first-line treatment for advanced EC was chemotherapy. However, with the publication of the Keynote-590 study, immunotherapy has now become the first-line treatment for EC.5 At present, the biomarkers primarily utilized for predicting the efficacy of ICIs encompass PD-L1 expression, TMB, and MSI.7 However, the credibility of these biomarkers remains a subject of controversy, each with its own set of advantages and disadvantages. Moreover, mainstream biomarkers rely on tumor tissue acquisition, which is limited in terms of accessibility. Notably, no peripheral blood biomarkers have received approval from regulatory agencies such as the Food and Drug Administration (FDA), European Medicines Agency (EMA), and Pharmaceuticals and Medical Devices Agency (PMDA).21,22
CD4+ helper T cells and CD8+ cytotoxic T cells are generated in the thymus from common progenitor cells that express both CD4 and CD8.23 Extensive evidence supports the cytotoxic anti-tumor functionality of CD4+ and CD8+ T cells, highlighting their potential therapeutic significance in cancer immunotherapy.24 Single-cell Ribonucleic acid (RNA) transcriptomic analysis identified granzyme B+ tumor-specific CD4+ T cells in human melanoma patients, both within the tumor microenvironment and in peripheral blood.25 Similar to T cells, the anti-tumor effects of NK cells are well-established and have been extensively studied and reviewed in numerous publications.26 Numerous studies have demonstrated that peripheral blood biomarkers, such as baseline CD8+ T lymphocytes,27,28 and CD4+CD45RA- T cells,17 can serve as predictive biomarkers for the efficacy of ICIs and immune-related adverse events (irAEs) in non–small cell lung cancer (NSCC) and metastatic melanoma.29 Henceforth, peripheral blood lymphocytes exhibit potential as a non-invasive biomarker for ICI treatment.
Based on these findings, the initial investigation focused on the association between baseline lymphocyte subsets and the prognosis of immunotherapy in EC patients. Nevertheless, due to the limited sample size, no statistically significant indicators were identified. As such, the focus of the study shifted to exploring changes in lymphocyte subsets before and after immunotherapy as potential predictors of therapeutic efficacy with ICIs. The present study is the first to examine the correlation between alterations in lymphocyte subsets before and after immunotherapy and their prognostic implications for patients with EC. A strong association was observed between the difference in CD8+ lymphocytes before and after immunotherapy and PFS in both univariate and multivariate analyses. The greater the reduction of CD8+ lymphocytes in peripheral blood following immunotherapy, the more favorable the prognosis. Moreover, although univariate analyses did not yield any statistically significant findings regarding lymphocyte subsets and OS in EC patients treated with ICIs, multivariate analyses revealed a significant association between the difference in NK cells before and after immunotherapy and OS. Correspondingly, a lower count of peripheral blood NK cells following immunotherapy is indicative of a more favorable prognosis.
The presence of peritumoral tumor-infiltrating lymphocytes (TILs), particularly CD8+ and FOXP3+ TILs, is widely recognized as being associated with a favorable prognosis in EC.30,31 However, the invasive nature and limited accessibility of tissue samples hinder the widespread use of TILs as predictive biomarkers for immunotherapy efficacy. Consequently, in recent years, researchers have increasingly explored the utility of peripheral blood lymphocyte subsets as prognostic biomarkers for immunotherapy effectiveness, owing to their simplicity and accessibility.32 The present study suggests that the reduction of CD8+ lymphocytes and NK cells in peripheral blood lymphocyte subsets after immunotherapy may be superior predictors of immunotherapy efficacy compared to baseline lymphocyte subsets. This phenomenon could be attributed to the hypothesis that a decrease in peripheral blood lymphocytes following immunotherapy is linked to increased lymphocyte infiltration within tumor tissue. Notably, a recent study demonstrated clonotypic expansion of effector-like T cells in both tumor and blood, proposing that circulating counterparts might replenish existing TILs with non-exhausted cells.33 Several scholars have confirmed that regulatory T (Treg) II cells in peripheral blood enter the breast cancer tumor tissue through a series of complex immune reactions mediated by cytokines, and inhibit the tumor immunity.34 However, whether CD8+ T lymphocytes and NK cells in the peripheral blood of EC patients can be recruited by cytokine signals and migrate into tumor tissues remains unexplored in the existing literature. To further investigate this relationship, the present authors intend to expand the sample size while concurrently assessing peripheral blood lymphocyte subsets and lymphocyte infiltration in tumor tissue among patients diagnosed with EC, both before and after immunotherapy. The aim is to explore the correlation between peripheral blood lymphocytes and tumor tissue infiltrating lymphocytes. Additionally, the optimal cut-off value for changes in CD8+ T cells after immunotherapy as a predictor of prognosis in EC patients remains unclear, with the current study achieving an AUC of only 0.553. Consequently, further investigations with larger sample sizes are needed to explore this aspect in greater depth.
The present study has the following limitations. First, the study was conducted in a single institution, employing an observational and retrospective design, which may introduce potential biases. Second, the sample size of the patients was relatively limited. Third, the limited duration of follow-up in this study meant that some patients did not reach the specified endpoints for disease progression or mortality. Consequently, continuous monitoring will be conducted for these individuals. Fourth, patients of EC necessitate comprehensive treatment, with surgery and radiotherapy being widely recognized as influential factors in prognosis. Due to the limited sample size, cases involving combination therapy were not excluded; however, the inclusion of surgical and radiotherapeutic combinations was balanced across both low and high CD8+ lymphocytes groups. Nonetheless, the present study represents the first investigation to establish a correlation between changes in peripheral blood lymphocyte subsets before and after immunotherapy and the efficacy of immunotherapy in patients with EC.
Conclusions
This study identified that reductions in both CD8+ T lymphocytes and NK cells in peripheral blood lymphocyte subsets after immunotherapy can serve as effective predictors of ICI efficacy in EC patients. To validate these findings, further prospective randomized studies with larger sample sizes are necessary, and the optimal threshold for these biomarkers still needs to be determined.
Data Sharing Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study protocol was reviewed and approved by the ethics committee of the Fourth Hospital of Hebei Medical University (2023KS090). All participants provided written informed consent to participate voluntarily, in accordance with the principles of the Declaration of Helsinki. Additionally, respondents were assured that their participation would be treated with strict confidentiality and anonymity.
Funding
The present study was supported by the Key Science and Technology Research Program from Health Commission of Hebei Province (grant no. 20230149).
Disclosure
The authors declare that they have no competing interests.
References
1. Baba Y, Nomoto D, Okadome K, et al. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020;111(9):3132–3141. doi:10.1111/cas.14541
2. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
3. Baba Y, Yoshida N, Kinoshita K, et al. Clinical and prognostic features of patients with esophageal cancer and multiple primary cancers: a retrospective single-institution study. Ann Surg. 2018;267(3):478–483. doi:10.1097/SLA.0000000000002118
4. Zou LQ, Yang X, Li YD, et al. Immune checkpoint inhibitors: a new era for esophageal cancer. Expert Rev Anticancer Ther. 2019;19(8):731–738. doi:10.1080/14737140.2019.1654379
5. Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, Phase 3 study. Lancet. 2021;398(10302):759–771. doi:10.1016/S0140-6736(21)01234-4
6. Fang P, Zhou J, Liang Z, et al. Immunotherapy resistance in esophageal cancer: possible mechanisms and clinical implications. Front Immunol. 2022;13:975986. doi:10.3389/fimmu.2022.975986
7. Wu HX, Wang ZX, Zhao Q, et al. Tumor mutational and indel burden: a systematic pan-cancer evaluation as prognostic biomarkers. Ann Transl Med. 2019;7(22):640. doi:10.21037/atm.2019.10.116
8. Noori M, Yousefi AM, Zali MR, et al. Predictive value of PD-L1 expression in response to immune checkpoint inhibitors for esophageal cancer treatment: a systematic review and meta-analysis. Front Oncol. 2022;12:1021859.
9. Yi M, Niu M, Xu L, et al. Regulation of PD-L1 expression in the tumor microenvironment. J Hematol Oncol. 2021;14(1):10. doi:10.1186/s13045-020-01027-5
10. Wang C, Wang HN, Wang L. Biomarkers for predicting the efficacy of immune checkpoint inhibitors. J Cancer. 2022;13(2):481–495. doi:10.7150/jca.65012
11. Saeed A, Salem ME. Prognostic value of tumor mutation burden (TMB) and INDEL burden (IDB) in cancer: current view and clinical applications. Ann Transl Med. 2020;8(9):575. doi:10.21037/atm-2020-75
12. Jardim DL, Goodman A, de Melo Gagliato D, et al. The challenges of tumor mutational burden as an immunotherapy biomarker. Cancer Cell. 2021;39(2):154–173. doi:10.1016/j.ccell.2020.10.001
13. McGrail DJ, Pilié PG, Rashid NU, et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann Oncol. 2021;32(5):661–672. doi:10.1016/j.annonc.2021.02.006
14. Huang J, Xu B, Mo H, et al. Safety, activity, and biomarkers of SHR-1210, an Anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24(6):1296–1304. doi:10.1158/1078-0432.CCR-17-2439
15. Zang YS, Dai C, Xu X, et al. Comprehensive analysis of potential immunotherapy genomic biomarkers in 1000 Chinese patients with cancer. Cancer Med. 2019;8(10):4699–4708. doi:10.1002/cam4.2381
16. Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127(5):1001–1010. doi:10.1002/ijc.25283
17. Muroyama Y, Wherry EJ. Memory T-Cell heterogeneity and terminology. Cold Spring Harb Perspect Biol. 2021;13(10):a037929. doi:10.1101/cshperspect.a037929
18. Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21(11):718–738. doi:10.1038/s41577-021-00537-8
19. Bos R, Sherman LA. CD4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res. 2010;70(21):8368–8377. doi:10.1158/0008-5472.CAN-10-1322
20. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
21. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi:10.1126/science.aaa1348
22. Cristescu R, Mogg R, Ayers M, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi:10.1126/science.aar3593
23. Naito T, Tanaka H, Naoe Y, et al. Transcriptional control of T-cell development. Int Immunol. 2011;23(11):661–668. doi:10.1093/intimm/dxr078
24. Preglej T, Ellmeier W. CD4(+) cytotoxic T cells - phenotype, function and transcriptional networks controlling their differentiation pathways. Immunol Lett. 2022;247:27–42. doi:10.1016/j.imlet.2022.05.001
25. Cachot A, Bilous M, Liu YC, et al. Tumor-specific cytolytic CD4 T cells mediate immunity against human cancer. Sci Adv. 2021;7(9):eabe3348. doi:10.1126/sciadv.abe3348
26. Yuan X, Rasul F, Nashan B, et al. Innate lymphoid cells and cancer: role in tumor progression and inhibition. Eur J Immunol. 2021;51(9):2188–2205. doi:10.1002/eji.202049033
27. Watson RA, Tong O, Cooper R, et al. Immune checkpoint blockade sensitivity and progression-free survival associates with baseline CD8+ T cell clone size and cytotoxicity. Sci Immunol. 2021;6(64):eabj8825. doi:10.1126/sciimmunol.abj8825
28. Wu K, Xia B, Zhang J, et al. Positive correlation of peripheral CD8(+) T lymphocytes with immune-related adverse events and combinational prognostic value in advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Cancers. 2022;14(15):3568. doi:10.3390/cancers14153568
29. Lozano AX, Chaudhuri AA, Nene A, et al. T cell characteristics associated with toxicity to immune checkpoint blockade in patients with melanoma. Nat Med. 2022;28(2):353–362. doi:10.1038/s41591-021-01623-z
30. Baba Y, Yagi T, Kosumi K, et al. Morphological lymphocytic reaction, patient prognosis and PD-1 expression after surgical resection for oesophageal cancer. Br J Surg. 2019;106(10):1352–1361. doi:10.1002/bjs.11301
31. Schumacher K, Haensch W, Röefzaad C, et al. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res. 2001;61(10):3932–3936.
32. Miao K, Zhang X, Wang H, et al. Peripheral blood lymphocyte subsets predict the efficacy of immune checkpoint inhibitors in non-small cell lung cancer. Front Immunol. 2022;13:912180. doi:10.3389/fimmu.2022.912180
33. Wu TD, Madireddi S, de Almeida PE, et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature. 2020;579(7798):274–278. doi:10.1038/s41586-020-2056-8
34. Wang L, Simons DL, Lu X, et al. Connecting blood and intratumoral T(reg) cell activity in predicting future relapse in breast cancer. Nat Immunol. 2019;20(9):1220–1230. doi:10.1038/s41590-019-0429-7
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

