Back to Journals » Infection and Drug Resistance » Volume 18
Predictive Value of Serum β2-Microglobulin for 28-Day Mortality in Sepsis Patients in the Emergency Department
Authors Zhang X , Yang L, Gu Y , Wu J, Mei X, Guo S
Received 29 January 2025
Accepted for publication 3 May 2025
Published 7 May 2025 Volume 2025:18 Pages 2365—2376
DOI https://doi.org/10.2147/IDR.S519987
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi Ruan
Xiangqun Zhang,1,2 Long Yang,1 Yu Gu,1 Junyuan Wu,1,2 Xue Mei,1,2,* Shubin Guo1,2,*
1Emergency Medicine Clinical Research Center, Beijing Chao-Yang Hospital, Capital Medical University, Beijing, People’s Republic of China; 2Clinical Center for Medicine in Acute Infection, Capital Medical University, Beijing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xue Mei, Email [email protected] Shubin Guo, Email [email protected]
Background: Sepsis is an infection-induced systemic inflammatory response syndrome with high morbidity and mortality. β 2-microglobulin (β 2-MG), a low-molecular-weight protein involved in immune processes, shows potential for predicting the prognosis of various diseases. However, its role in sepsis prognosis remains unclear, necessitating further exploration.
Objective: This study aimed to evaluate the predictive value of serum β 2-MG for 28-day mortality in sepsis patients and compare it with traditional indicators such as sequential organ failure assessment (SOFA) scores and lactate (Lac) levels.
Methods: A total of 346 sepsis patients were included in this single-center retrospective study conducted at the emergency department of Beijing Chao-Yang Hospital. Clinical and biochemical indicators, including β 2-MG, SOFA scores, and Lac levels, were collected. Predictive ability was assessed using receiver operating characteristic (ROC) curve analysis and binary logistic regression models, and β 2-MG was compared to SOFA scores and Lac levels.
Results: β 2-MG was significantly correlated with 28-day mortality and identified as an independent risk factor (P< 0.001, OR=1.142, 95% CI: 1.083– 1.204). The sensitivity of β 2-MG was 94%, and its specificity was 77% for predicting 28-day mortality. Combining β 2-MG with SOFA scores increased sensitivity to 94%, while combining it with Lac improved specificity to 88.9%. ROC analysis showed that β 2-MG’s predictive accuracy improved significantly when combined with these indicators.
Conclusion: The serum level of β 2-MG is an independent predictor of 28-day mortality in sepsis patients. While less sensitive and specific than SOFA scores and lactate, combining β 2-MG with these markers improves predictive accuracy, offering complementary prognostic value.
Keywords: 28-day mortality, β 2-microglobulin, lactate level, sepsis, sequential organ failure assessment score
Background
Sepsis is a life-threatening organ dysfunction syndrome caused by the dysregulated host response to infections,1 and this disease has high morbidity and mortality rates worldwide.2 The early and accurate identification of sepsis can assist clinicians in selecting appropriate therapies in time, thus significantly reducing the mortality of patients. However, as a group of heterogeneous syndromes, sepsis has a wide range of multidimensional clinical and biological characteristics. Additionally, it is difficult to make an early diagnosis and identification of patients with severe sepsis due to the rapid progression of this disease.3–6 Among many available predictors and scores, the sequential organ failure assessment (SOFA) score and lactate (Lac) level are relatively mature clinical evaluation indicators of sepsis.7 However, the complexity of the SOFA score and the fact that hyperlactacidemia may not always specifically reflect tissue hypoperfusion limit the accuracy of these indicators in reflecting the true condition of septic patients, hence their predictive ability is insufficient.8 Therefore, there is an urgent demand for identifying the predictors of sepsis with higher sensitivity and specificity.
β2-microglobulin (β2-MG) is a low-molecular-weight protein, β2-microglobulin (β2-MG) is primarily secreted by karyocytes an is widely detected in all body fluids of humans. Under normal conditions, β2-MG is filtered through the glomeruli and reabsorbed and metabolized by the renal tubules. Elevated β2-MG levels are commonly considered a marker of renal dysfunction,9 as impaired renal function leads to reduced clearance and subsequent accumulation of β2-MG. However, beyond its role as a renal function indicator, β2-MG also plays a critical role in immune regulation. It serves as a crucial component of major histocompatibility complex class I molecules (MHC-I) and plays a pivotal role in orchestrating immune surveillance and regulation.10–12 During physiological stress or infection, the secretion of β2-MG substantially increases, reflecting the body’s immune response to pathogenic insults. Studies have indicated that in patients with infectious diseases, β2-MG not only serves as a predictor of renal function but also influences cognitive faculties.13 Furthermore, β2-MG has been closely linked to the prognosis of blood cancers and other tumor,14–17 and it is also associated with physiological processes related to aging and functional decline.18–20 While these associations are well-documented in cancer and other chronic conditions, research on the role of β2-MG in sepsis remains limited. Its involvement in the inflammatory and immune responses characteristic of sepsis is not yet fully understood. Therefore, further investigation into the prognostic potential of β2-MG in sepsis, particularly for early assessment, may provide valuable insights for clinical management and risk stratification.
This study was conducted to assess the potential value of β2-MG as a routine item in biochemical examinations during emergency admission for predicting the 28 day mortality of patients with sepsis.
Method
Study Design and Participants
We conducted a retrospective analysis of 489 adult patients (aged >18 years) diagnosed with sepsis within two weeks of admission to the emergency department at Beijing Chaoyang Hospital between January 1, 2022, and June 30, 2024. Patients were identified based on established diagnostic criteria for sepsis. A total of 74 patients (15%) were excluded due to advanced disease stages, including advanced or metastatic tumors, end-stage liver disease, and end-stage kidney disease. Additionally, 21 patients (4%) with documented immunosuppression and 48 patients (10%) with incomplete data were excluded from the analysis. As a result, the final study cohort consisted of 346 patients. The primary objective of this retrospective cohort study was to assess the prognostic value of β2-microglobulin (β2-MG) as a standard component of biochemical assessments during emergency admission, specifically for predicting 28-day mortality among sepsis patients. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Chaoyang Hospital, affiliated with Capital Medical University (Approval No.: 2021-KE-407). A waiver for informed consent was granted by the ethics committee, as all patient data were fully anonymized prior to access, in compliance with institutional and ethical guidelines.
Inclusion and Exclusion Criteria
The study’s inclusion criteria were based on the following parameters: (1) patients diagnosed with sepsis in accordance with the Third International Consensus Definitions for Sepsis (Sepsis-3); (2) patients undergoing admission assessments involving biochemical and clinical indicators such as β2-MG, SOFA score, and Lac; The exclusion criteria included: (1) patients with advanced stages of disease, including advanced or metastatic tumors, end-stage liver disease, or end-stage kidney disease; (2) patients who had been hospitalized within 14 days prior to the onset of symptoms; (3) patients with conditions such as cystic fibrosis, active pulmonary tuberculosis, and severe immunosuppression.
Data Collection
Patient demographic information, including age and gender, as well as vital signs, medical history, and admission concentrations of β2-MG, Lac, and other blood routine and biochemical examination indicators, were retrieved from the electronic information system of the hospital. The SOFA score of these patients was calculated based on their vital signs and laboratory findings. The β2-MG concentration was determined by the standard Latex Immunoturbidimetric Assay (Beckman AU5800 Analyzer, USA) with the normal range being 1.2–3.0mg/mL. The blood gas analyzer (GEM Premier 3000, Instrumentation Laboratory, Lexington, MA, USA) was used to measure the Lac level by the point of care testing (POCT) method, with the normal range being 0.7–2.5 mmol/L. The automated hematology detection analyzer (Sysmex XS-500i, Sysmex Corporation Kobe, Japan) was used to perform the white blood cell (WBC) counting analysis. The serum procalcitonin (PCT) concentration was measured using the Mini-VIDAS immunoanalyzer of BioMerieux (Block Scientific, Bohemia), with the limit of detection (LOD) being 0.05ng/L. The serum C-reactive protein (CRP) concentration was determined by immunoturbidimetry (BNII, Siemens Healthcare Diagnostic, Germany), with the normal range being 0–10ng/mL. All tests were completed within 6 hours of admission, and the results were meticulously recorded to ensure accurate representation of the early condition of the patients.
Study Endpoints and Grouping
In this study, 28-day mortality was selected as the endpoint. Among the 346 patients with sepsis, there were 85 patients in the death group and 261 patients in the survival group.
Statistical Analysis
Statistical analysis was performed using SPSS 16.0 software (SPSS Inc., Chicago, IL, USA). Data distribution was assessed using the Shapiro–Wilk test. Normally distributed data were presented as mean ± standard deviation (SD) and analyzed using independent t-tests for two-group comparisons and one-way analysis of variance (ANOVA) for multiple-group comparisons. Non-normally distributed data were presented as median (P25, P75) and analyzed using the Mann–Whitney U-test for two-group comparisons and the Kruskal–Wallis test for multiple-group comparisons. White blood cell count (WBC), lactate (Lac), procalcitonin (PCT), C-reactive protein (CRP), β2-microglobulin (β2-MG), and SOFA scores were analyzed, and receiver operating characteristic (ROC) curves were plotted to calculate the area under the curve (AUC). Sensitivity and specificity were calculated, and differences in AUCs were assessed using the formula Z = (A1 - A2) / √(SE1² + SE2²) (Z0.05 = 1.96, Z0.01 = 2.58). Binary logistic regression analysis was performed to identify independent predictors of 28-day mortality in patients with sepsis. All statistical analyses were conducted using two-tailed tests, and a P-value of <0.05 was considered statistically significant.
Results
Baseline Data of Patients with Sepsis
A total of 346 patients with sepsis were included in this study based on the inclusion criteria. Based on 28-day mortality, these patients were divided into the death group (N=85) and the survival group (N=261). The analysis revealed notable statistical variances in age (p<0.001), WBC, CRP, PCT, Lac, β2-MG (p<0.001), and SOFA scores (p<0.001) among these patients (Table 1).
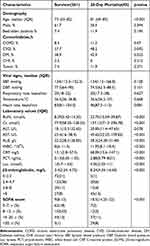 |
Table 1 Baseline Characteristics of the 346 Included Patients |
With the findings demonstrating a marked increase in both β2-MG levels and SOFA scores, there is a significant rise in 28-day mortality rates among sepsis patients. As shown in Figure A, these patients were divided into four groups (0–2.3 mg/L, 2.4–4.7 mg/L, 4.8–8 mg/L, and >8 mg/L) according to the β2-MG level. This stratification revealed a discernible escalation in 28-day mortality rates corresponding to higher β2-MG levels. As shown in Figure B, these patients were also divided into four groups (2–7 points, 8–13 points, 14–20 points, and >20 points) according to the SOFA score. The results indicated that the higher the SOFA score, the higher the 28-day mortality (Table 1 and Figure 1).
 |
Figure 1 Distribution ofβ2-MG and SOFA Scores in Relation to 28-day Mortality. |
Correlation between β2-MG and Other Indicators
The correlation of β2-MG with WBC (r=0.067, P=0.213), CRP (r=0.09, p=0.093), PCT (r=0.296, P<0.001), Lac (r=0.343, P<0.001), and SOFA score (r=0.067, p=0.213) was explored using the Spearman correlation analysis (Table 2). The results revealed that β2-MG was correlated with PCT and Lac (Table 2 and Figure 2).
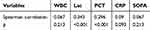 |
Table 2 The Correlation Between Serum β2-MG and Other Indicator |
 |
Figure 2 Spearman Correlation Between β2-MG and PCT (A), and β2-MG and Lactate (B). |
Logistic Regression Analysis of Various Variables Regarding the 28-Day Mortality of Patients with Sepsis
In this study, the independent predictors for sepsis were analyzed using multivariate logistic regression, including β2-MG (p<0.001, OR=1.142, 95% CI: 1.083–1.204), Lac (p<0.001, OR=1.224, 95% CI: 1.111–1.348), and SOFA score (p<0.001, OR=1.388, 95% CI: 1.249–1.542), as listed in Table 3. After excluding influencing factors such as age and renal functions, β2-MG was identified as an independent risk factor for the 28-day mortality of patients with sepsis (Table 3).
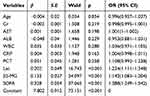 |
Table 3 Binary Logistic Regression Analysis of Factors Associated with 28-Day Mortality in Sepsis |
Prediction of β2-MG and Other Indicators and Their Combinations on the 28-Day Mortality of Patients with Sepsis
The AUC of β2-MG, Lac, and SOFA scores was 0.786 (95% CI: 0.727–0.845, p<0.001), 0.843 (95% CI: 0.79–0.896, p<0.001), and 0.846 (95% CI: 0.79–0.901, p<0.001), respectively. The AUC of β2-MG+SOFA and β2-MG+Lac was 0.92 (95% CI: 0.89–0.95, p<0.001) and 0.889 (95% CI: 0.84–0.929, p<0.001), respectively, (Table 4 and Figure 3).
 |
Table 4 Characteristics of Predictors for 28-Day Mortality in Sepsis |
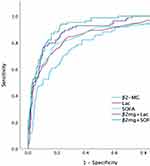 |
Figure 3 The ROC curves of variables for 28-day mortality in patients with sepsis. |
Comparison of β2-MG and Other Indicators Regarding Their Ability to Predict the 28-Day Mortality of Patients with Sepsis
The study compared the predictive capabilities of β2-MG and other indicators for 28-day mortality among sepsis patients, utilizing their respective AUC values. The findings indicated that there was no statistically significant difference between β2-MG and SOFA scores in predicting 28-day mortality in sepsis patients (Z=1.46; p=0.143). Similarly, there was no discernible variance between the predictive abilities of β2-MG and Lac for 28-day mortality in these patients (Z=1.41; p=0.158). Moreover, β2-MG+SOFA was superior to β2-MG (Z=4; p<0.001) and SOFA (Z=2.33; p=0.02) in predicting the 28-day mortality of patients with sepsis. β2-MG+Lac was also superior to β2-MG in predicting the 28-day mortality of these patients (Z=2.81; p=0.005), but there was no significant difference between β2-MG+Lac and Lac (Z=1.34; p=0.179) (Table 5).
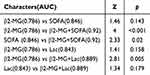 |
Table 5 Comparison of AUC for Predicting 28-Day Mortality in Sepsis Patients |
Discussion
This study represents the first scientific attempt to systematically evaluate the predictive ability of β2-microglobulin (β2-MG) as an independent predictor for the mortality of patients with sepsis. The findings evinced a strong correlation between β2-MG levels and 28-day mortality among these patients. Besides, β2-MG was also validated to be an independent prognosis indicator after adjusting for other key variables, such as the sequential organ failure assessment (SOFA) score, lactate (Lac), age, blood urea nitrogen (BUN), and creatinine. The multivariate Logistic regression analysis results indicated that β2-MG (OR=1.105, p<0.001), SOFA scores (OR=1.35, p<0.001), and Lac (OR=1.261, p<0.001) were independent predictors for the mortality of patients with sepsis. Additionally, statistical comparisons revealed no significant difference between the predictive power of β2-MG and that of SOFA scores (Z=1.46, p=0.143) or Lac (Z=1.41, p=0.158) in predicting the 28-day mortality of patients with sepsis. Although SOFA scores and Lac have been widely used to assess the prognosis of patients with sepsis, β2-MG can still provide additional and important prognostic information. The different correlation patterns observed between β2-MG and these markers may be attributed to their underlying pathophysiological mechanisms. The SOFA score is a comprehensive indicator of multi-organ dysfunction, whereas β2-MG primarily reflects renal function and immune responses, particularly inflammation and immune activation. Lactate and procalcitonin are more directly associated with systemic inflammatory responses and metabolic disturbances, which are more closely related to the role of β2-MG in sepsis. Therefore, the significant correlations between β2-MG and lactate or procalcitonin, but not with the SOFA score, suggest that β2-MG is more reflective of inflammatory and immune processes rather than the broader multi-organ dysfunction captured by the SOFA score.
Of considerable significance is the observation that the combination of β2-MG with SOFA scores or Lac markedly enhances their predictive capability concerning mortality in these patients. ROC curve analysis demonstrated that the AUC for β2-MG combined with SOFA scores reached 0.92, which was significantly higher than that of β2-MG or SOFA scores alone. Similarly, the AUC of β2-MG combined with Lac also reached 0.889, exhibiting a strong predictive ability. The combination of β2-MG and these conventional indicators can better reflect the inflammatory status and organ failure severity of patients with sepsis, thus significantly improving the accuracy of mortality prediction. These findings signify that β2-MG serves as a potent independent predictor for assessing the condition of sepsis patients and that its amalgamation with other markers may refine prognostic assessment. These insights are poised to aid clinicians in carrying out more precise risk stratification and treatment decisions.
β2-MG is a naturally occurring polypeptide consisting of 100 amino acids and encoded by chromosome 15, with a total molecular weight of 11,800 Dalton.21 It can be detected in all karyocytes that bind to major histocompatibility complex class II (MHC II)/human leukocyte antigen I (HLA I).22 The interaction of β2-MG with the α-chain of HLA-I is critical for antigen presentation. β2-MG can trigger immune responses by presenting antigenic peptides from pathogen-infected or transformed cells to CD8+ T cells. Therefore, β2-MG plays a key role in adaptive immunity.23 The serum level of β2-MG is dynamically associated with a variety of diseases. Further, the elevated serum level of β2-MG is associated with the increased tumor burden and poor prognosis of multiple diseases.24,25 However, in certain cancer studies, the deletion or variation of β2-MG implies that the tumor is insensitive to anti-tumor drugs, and the loss of β2-MG may make tumor cells evade immune control.26 In healthy individuals, the majority of β2-MG is excreted via renal filtration, and the serum concentration of β2-MG is maintained at a normal range from 1 to 3 μg/mL.27 In patients with impaired renal functions, the concentration of β2-MG is significantly elevated. In those with renal failure and on dialysis, the β2-MG level may reach 20–30 times the upper limit of the normal range.28
β2-MG is intricately linked to immune system activation during the course of infection. When a viral infection occurs, lymphocytes are activated, leading to a notable increase in the β2-MG level. Elevated levels of β2-MG have also been observed in patients infected with the human immunodeficiency virus (HIV) and cytomegalovirus.29,30 It has been reported that the concentration of β2-MG may change in the treatment process in patients with hepatitis B virus infection.31 Conca W corroborated that a higher β2-MG level may be an early indicator for predicting the severity of COVID-19, and it can also be employed to predict the prognosis of this disease.32 In terms of bacterial infections, Collazos et al proposed that the detection of β2-MG combined with other clinical and laboratory indicators may contribute to assessing the treatment response of patients with tuberculosis.33 In addition, the elevated serum level of β2-MG can be considered an independent risk factor for the 28-day mortality of patients with acute respiratory distress syndrome (ARDS) caused by bacterial infections.34 Bacteria, viruses, and other pathogens usually cause extensive inflammatory responses and immune activation in patients with sepsis. Therefore, β2-MG may be closely related to the severity of sepsis and can be used to predict the prognosis of this disease. However, limited research has been conducted on the correlation between β2-MG and the prognosis of sepsis. The findings of this study indicate that, after controlling for influencing factors such as age and renal function, β2-MG emerged as an independent risk factor for 28-day mortality in sepsis patients, and was also correlated with Lac and procalcitonin (PCT) levels. With an increase in the β2-MG level, the 28-day mortality of patients with sepsis increased significantly. The AUC of β2-MG was lower than that of the SOFA score and Lac. Nevertheless, β2-MG did not exhibit statistical difference compared to the SOFA score (Z=1.46, p=0.143) and Lac (Z=1.41, p=0.158) in predicting the 28-day mortality of patients with sepsis. This outcome suggests that β2-MG is comparable to the SOFA score and Lac in predicting the 28-day mortality of these patients.
The SOFA score and Lac are widely used biomarkers and assessment tools in the prognosis evaluation of patients with sepsis. The SOFA score is a comprehensive tool for assessing the severity of multiple organ dysfunction, including respiratory, cardiovascular, renal, hepatic, coagulation, and nervous system functions. Its multidimensional assessments provide a thorough reflection of the organ function status of patients. Consequently, the SOFA score has been extensively applied in the clinical diagnosis, severity assessment, and prognosis prediction of sepsis.35–39 However, it requires the assessment of multiple laboratory and clinical parameters, making it a complex tool, especially in resource-limited settings. Moreover, the SOFA score often reflects organ failure resulting from advanced diseases, posing challenges in the early identification of high-risk patients. Lactate, as a metabolic marker, can indicate tissue perfusion and oxidative stress status, particularly in hypoperfusion and hypoxia caused by sepsis. Elevated lactate is considered a direct indicator of tissue damage and has high clinical value in identifying potential septic shock in the early stages.7 Nevertheless, studies have suggested that an increase in blood lactate may not necessarily be related to poor tissue perfusion in patients with severe sepsis.40 Furthermore, treatment strategies targeting normal lactate levels for septic shock did not significantly reduce the 28-day all-cause mortality.41 This is attributed to lactate metabolism being influenced by various factors, such as liver functions and the imbalance between oxygen supply and demand, making it easily influenced by non-septic factors. Different from the SOFA score and Lac, β2-MG cannot directly reflect organ functions or tissue hypoxia. However, it can reflect the body’s immune response to infections, which is closely related to the early and progressive stages of inflammation. The results of this study suggested that β2-MG was not statistically different from the SOFA score and Lac in predicting the 28-day mortality of patients with sepsis. Moreover, the combination of β2-MG and SOFA scores or Lac significantly improved the sensitivity or specificity for predicting the mortality of these patients compared with a single indicator alone. The sensitivity of β2-MG combined with SOFA scores was higher (94%) in predicting the 28-day mortality of patients with sepsis; while the specificity of β2-MG combined with Lac was also higher (88.9%) in predicting the 28-day mortality of these patients. These two combinations provide a comprehensive assessment of the pathogenesis and organ function status of patients with sepsis, contributing to the earlier detection of changes in these patients.
Despite the valuable insights gained from this study, certain limitations warrant consideration. Due to its retrospective, single-center design, the study is confined by a relatively modest sample size, potentially constraining the generalizability of the findings. Additionally, the absence of long-term follow-up constitutes a notable limitation, as the study focused only on 28-day mortality, thereby overlooking the comprehensive assessment of long-term prognostic implications for sepsis patients. Moreover, β2-MG levels can be influenced by renal function, and using a more sensitive renal function marker such as cystatin C could potentially provide a more accurate adjustment. However, as a retrospective study, cystatin C was not routinely measured as part of standard clinical practice during the study period, and serum creatinine remains the most commonly measured renal marker in our clinical setting. To mitigate the potential confounding impact of renal dysfunction on β2-MG levels, we adjusted for serum creatinine in the multivariate logistic regression analysis, allowing us to minimize the influence of renal function and more accurately evaluate the independent prognostic value of β2-MG. While creatinine adjustment was deemed appropriate in this study, we acknowledge that future prospective studies incorporating cystatin C could provide a more comprehensive assessment of renal function’s impact on β2-MG levels. Additionally, the impact of β2-MG on long-term survival and other pivotal outcomes, including readmission rate and quality of life, remains undefined. Finally, despite adjusting for certain confounders such as age and renal function, potential unmeasured confounding factors may still distort the association between β2-MG and sepsis prognosis.
Conclusion
The serum level of β2-MG upon admission serves as an independent predictor for 28-day mortality among sepsis patients. Although its sensitivity and specificity are lower than conventional biomarkers such as SOFA scores and lactate, β2-MG provides complementary prognostic value. Notably, combining β2-MG with SOFA scores or lactate significantly improves predictive accuracy. These findings suggest that while SOFA scores and lactate remain reliable predictors, incorporating β2-MG may enhance prognostic assessment in sepsis patients.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Chaoyang Hospital, affiliated with Capital Medical University (Approval No.: 2021-KE-407). The requirement for informed consent was waived by the ethics committee as all data were fully anonymized before access, in compliance with institutional and ethical guidelines.
Consent for Publication
All authors have read and approved the final manuscript and have given their consent for publication.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Supported by National Key R&D Program of China (grant number:2023YFC0872400).
Disclosure
All authors report no conflicts of interest in this work.
References
1. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–810. doi:10.1001/jama.2016.0287
2. Chiu C, Legrand M. Epidemiology of sepsis and septic shock. Curr Opin Anaesthesiol. 2021;34(2):71–76. doi:10.1097/ACO.0000000000000958
3. Wang W, Liu CF. Sepsis heterogeneity. World J Pediatr. 2023;19(10):919–927. doi:10.1007/s12519-023-00689-8
4. Caraballo C, Jaimes F. Organ dysfunction in sepsis: an ominous trajectory from infection to death. Yale J Biol Med. 2019;92(4):629–640.
5. Pruinelli L, Westra BL, Yadav P, et al. Delay within the 3-hour surviving sepsis campaign guideline on mortality for patients with severe sepsis and septic shock. Crit Care Med. 2018;46(4):500–505. doi:10.1097/CCM.0000000000002949
6. Weinberger J, Rhee C, Klompas M. A critical analysis of the literature on time-to-antibiotics in suspected sepsis. J Infect Dis. 2020;222(Suppl 2):S110–s8. doi:10.1093/infdis/jiaa146
7. Liu Z, Meng Z, Li Y, et al. Prognostic accuracy of the serum lactate level, the SOFA score and the qSOFA score for mortality among adults with Sepsis. Scand J Trauma Resusc Emerg Med. 2019;27(1):51. doi:10.1186/s13049-019-0609-3
8. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi:10.1016/S0140-6736(18)30696-2
9. Uemura T, Nishimoto M, Eriguchi M, et al. Utility of serum β2-microglobulin for prediction of kidney outcome among patients with biopsy-proven diabetic nephropathy. Diabetes Obes Metab. 2024;26(2):583–591. doi:10.1111/dom.15347
10. Wang H, Liu B, Wei J. Beta2-microglobulin(B2M) in cancer immunotherapies: biological function, resistance and remedy. Cancer Lett. 2021;517:96–104. doi:10.1016/j.canlet.2021.06.008
11. Bernal M, Ruiz-Cabello F, Concha A, Paschen A, Garrido F. Implication of the β2-microglobulin gene in the generation of tumor escape phenotypes. Cancer Immunol Immunother. 2012;61(9):1359–1371. doi:10.1007/s00262-012-1321-6
12. Doukas J, Rolland A. Mechanisms of action underlying the immunotherapeutic activity of allovectin in advanced melanoma. Cancer Gene Ther. 2012;19(12):811–817. doi:10.1038/cgt.2012.69
13. Gao R, Li G, Yang R, Yuan H, Zhang S. Hippocampal β2‑microglobulin mediates sepsis‑induced cognitive impairment. Mol Med Rep. 2018;17(6):7813–7820. doi:10.3892/mmr.2018.8858
14. Langerbeins P, Giza A, Robrecht S, et al. Reassessing the chronic lymphocytic leukemia international prognostic index in the era of targeted therapies. Blood. 2024;143(25):2588–2598. doi:10.1182/blood.2023022564
15. Kim HD, Cho H, Kim S, et al. Prognostic stratification of patients with Burkitt lymphoma using serum β2-microglobulin levels. Cancer Res Treat. 2021;53(3):847–856. doi:10.4143/crt.2020.1060
16. Sequeira J, Sengupta S, Mhatre B. Serum beta-2 microglobulin analysis in patients with oral squamous cell carcinoma. Natl J Maxillofac Surg. 2021;12(2):227–232. doi:10.4103/njms.NJMS_242_20
17. Kim HD, Cho H, Sohn BS, et al. Prognostic significance of serum β2-microglobulin levels in patients with peripheral T-cell lymphoma not otherwise specified. Leuk Lymphoma. 2022;63(1):124–130. doi:10.1080/10428194.2021.1971220
18. Dong XM, Cai R, Yang F, et al. Predictive value of plasma β2-microglobulin on human body function and senescence. Eur Rev Med Pharmacol Sci. 2016;20(11):2350–2356.
19. Załęska-Kocięcka M, Jezierski P, Grabowski M, et al. Role of β2-microglobulin in postoperative cognitive decline. Biomark Med. 2017;11(3):245–253. doi:10.2217/bmm-2016-0274
20. Chen F, Liu J, Li FQ, et al. β2-microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats. Neural Regen Res. 2023;18(3):603–608. doi:10.4103/1673-5374.350204
21. Ploegh HL, Orr HT, Strominger JL. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981;24(2):287–299. doi:10.1016/0092-8674(81)90318-4
22. Argyropoulos CP, Chen SS, Ng YH, et al. Rediscovering beta-2 microglobulin as a biomarker across the spectrum of kidney diseases. Front Med. 2017;4:73.
23. Cosma GL, Eisenlohr LC. Impact of epitope density on CD8(+) T cell development and function. Mol Immunol. 2019;113:120–125. doi:10.1016/j.molimm.2019.03.010
24. Nosratzehi F, Nosratzehi T, Alijani E, Rad SS. Salivary β2-microglobulin levels in patients with erosive oral lichen planus and squamous cell carcinoma. BMC Res Notes. 2020;13(1):294. doi:10.1186/s13104-020-05135-w
25. Lin Q, Jiang Z, Mo D, et al. Beta2-microglobulin as predictive biomarkers in the prognosis of hepatocellular carcinoma and development of a new nomogram. J Hepatocell Carcinoma. 2023;10:1813–1825. doi:10.2147/JHC.S425344
26. Germano G, Lu S, Rospo G, et al. CD4 T cell-dependent rejection of beta-2 microglobulin null mismatch repair-deficient tumors. Cancer Discov. 2021;11(7):1844–1859. doi:10.1158/2159-8290.CD-20-0987
27. Kuharić M, Zibar L. Screening for carpal tunnel syndrome in patients on chronic hemodialysis. Acta Med Acad. 2019;48(2):167–176. doi:10.5644/ama2006-124.255
28. Coppolino G, Bolignano D, Rivoli L, Mazza G, Presta P, Fuiano G. Tumour markers and kidney function: a systematic review. Biomed Res Int. 2014;2014:647541. doi:10.1155/2014/647541
29. Bardeguez AD, Connor E, Stephens R, Denny TN, Holland B, Oleske J. Effect of human immunodeficiency virus infection on serum beta2-microglobulin levels in pregnant women. Obstet Gynecol. 1999;94(4):537–542. doi:10.1016/s0029-7844(99)00343-9
30. Alarcon A, Garcia-Alix A, Cabañas F, et al. Beta2-microglobulin concentrations in cerebrospinal fluid correlate with neuroimaging findings in newborns with symptomatic congenital cytomegalovirus infection. Eur J Pediatr. 2006;165(9):636–645. doi:10.1007/s00431-006-0160-x
31. Elefsiniotis IS, Scarmeas N, Glynou I, Pantazis KD, Kada H, Mavrogiannis C. Serum beta2-microglobulin levels in hepatitis B e antigen-negative chronic hepatitis B patients under long term lamivudine monotherapy: relationship with virological breakthrough. Can J Gastroenterol. 2004;18(5):307–313. doi:10.1155/2004/864292
32. Conca W, Alabdely M, Albaiz F, et al. Serum β2-microglobulin levels in coronavirus disease 2019 (Covid-19): another prognosticator of disease severity? PLoS One. 2021;16(3):e0247758. doi:10.1371/journal.pone.0247758
33. Collazos J, Martínez E, Mayo J. Evolution of serum beta2-microglobulin concentrations during treatment of tuberculosis patients. Scand J Infect Dis. 1999;31(3):265–267. doi:10.1080/00365549950163554
34. Cui N, Feng X, Zhang Y, Zhang L, Wang J. Serum β2-microglobulin as an independent risk factor for mortality in patients with acute respiratory distress syndrome caused by bacterial infection. Sci Rep. 2024;14(1):22999. doi:10.1038/s41598-024-73922-7
35. Schertz AR, Lenoir KM, Bertoni AG, Levine BJ, Mongraw-Chaffin M, Thomas KW. Sepsis prediction model for determining sepsis vs SIRS, qSOFA, and SOFA. JAMA Netw Open. 2023;6(8):e2329729. doi:10.1001/jamanetworkopen.2023.29729
36. Raith EP, Udy AA, Bailey M, et al. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317(3):290–300. doi:10.1001/jama.2016.20328
37. Morkar DN, Dwivedi M, Patil P. Comparative study of sofa, apache ii, saps ii, as a predictor of mortality in patients of sepsis admitted in medical ICU. J Assoc Physicians India. 2022;70(4):11–12.
38. Majidazar M, Hamidi F, Masoudi N, Vand-Rajabpour Z, Paknezhad SP. Comparing the predictive value of SOFA and SIRS for mortality in the early hours of hospitalization of sepsis patients: a systematic review and meta-analysis. Arch Iran Med. 2024;27(8):439–446. doi:10.34172/aim.28567
39. Khan AM, Aslam SM. Comparison of qSOFA score, SIRS criteria, and SOFA score as predictors of mortality in patients with sepsis. Ghana Med J. 2022;56(3):191–197. doi:10.4314/gmj.v56i3.9
40. Marik PE. Lactate guided resuscitation-nothing is more dangerous than conscientious foolishness. J Thorac Dis. 2019;11(Suppl 15):S1969–s72. doi:10.21037/jtd.2019.07.67
41. Hernández G, Ospina-Tascón GA, Damiani LP, et al. Effect of a resuscitation strategy targeting peripheral perfusion status vs serum lactate levels on 28-day mortality among patients with septic shock: the ANDROMEDA-SHOCK randomized clinical trial. JAMA. 2019;321(7):654–664. doi:10.1001/jama.2019.0071
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

