Back to Journals » Infection and Drug Resistance » Volume 18
Predictors of Serological Cure in Syphilis Patients: A Retrospective Study at a Tertiary Hospital in Bandung, Indonesia
Authors Achdiat PA , Rowawi R, Pujiastuti NR , Amalia F , Suwarsa O , Anandita R , Maharani RH
Received 28 February 2025
Accepted for publication 6 June 2025
Published 17 June 2025 Volume 2025:18 Pages 3023—3035
DOI https://doi.org/10.2147/IDR.S518129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Pati Aji Achdiat, Rasmia Rowawi, Nindayu Rizki Pujiastuti, Fatimah Amalia, Oki Suwarsa, Rafithia Anandita, Retno Hesty Maharani
Department of Dermatology and Venereology, Faculty of Medicine Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Bandung, West Java, Indonesia
Correspondence: Pati Aji Achdiat, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +6281225955478 ext. 3449, Fax +62222032426, Email [email protected] Retno Hesty Maharani, Department of Dermatology and Venereology, Faculty of Medicine, Universitas Padjadjaran - Dr. Hasan Sadikin Hospital, Jl. Pasteur 38, Bandung, West Java, 40161, Indonesia, Tel +6281225955478 ext. 3449, Fax +62222032426, Email [email protected]
Introduction: Syphilis is an infection caused by the spirochete Treponema pallidum and transmitted through sexual intercourse. Regular monitoring is necessary to ensure affected patients have successfully attained a serological cure following medication. Despite receiving suitable medication, specific individuals in clinical settings do not succeed in achieving serological cure.
Purpose: This study aims to determine the factors associated with the serological cure of syphilis at Dr Hasan Sadikin General Hospital Bandung, Indonesia.
Material and Methods: The study procedures were carried out using a retrospective descriptive method. Secondary data were obtained from medical records at the Dermatology and Venereology Outpatient Clinic of Hasan Sadikin Hospital, Bandung, Indonesia, from 1 January 2018 to 31 December 2022.
Results: The sample population comprised 266 cases of syphilis, consisting of 185 male and 81 female patients. The bivariate analysis results showed that patients aged less than 35 years had a serological cure rate of 75.4%, with a 3.89 times higher chance of achieving serological cure compared to those ≥ 35 years (p < 0.0001, OR = 3.89, 95% CI 1.85– 8.13). In addition, the proportion of serological cure was higher in patients with Venereal disease research laboratory test (VDRL) titers of ≤ 1:8 before therapy (OR = 0.61, 95% CI 0.28– 1.35, P = 0.226). Similar results were also obtained in those with HIV-negative (OR = 0.83, 9% CI 0.38– 1.81, P=0.648), and benzathine penicillin G therapy (OR = Reff, P = 0.226).
Conclusion: An age of < 35 years was a highly influential factor in the success of syphilis therapy. This indicates that intervention and monitoring strategies should focus on this age group, especially in resource-limited settings. The most common time for serological cure in early syphilis was the 9th and 12th months, while the 3rd and 12th months were common in late syphilis.
Keywords: serological cure, serofast, syphilis
Introduction
Syphilis is a systemic infection caused by the spirochete Treponema pallidum and is usually transmitted through sexual intercourse.1–3 The disease consists of 4 stages, namely primary, secondary, early latent, and late latent.4–7 According to data from the Integrated Biological and Behavioral Survey published by the Indonesian Ministry of Health in 2015, which was conducted in 23 provinces throughout Indonesia, the proportion of syphilis incidence was 17.39% in waria. In addition, a proportion of 15.7% was found in men who have sex with men (MSM), 2.69% in high-risk men, 2.1% in women within correctional facilities, and 1.46% in injecting drug users.8 Ghanem et al9 reported a 60.5% serological cure, with a value 4 times higher than the initial titer after treatment, with 2.4 million international units (IU) of benzathine penicillin intramuscularly (IM). Febiyanto et al10 also found a serological cure rate of 62.5% at titer of >1:32 and 37.5% at titer of <1:32 before therapy among syphilis patients at the Central General Hospital Dr. Sardjito Yogyakarta. Syphilis is usually transmitted through sexual contact,2,3 but can also be acquired non-sexually during pregnancy, blood transfusions, work accidents, tattooing, close contact with lesions, and food chewed by mothers for their children.3 The Indonesian Guideline for Syphilis recommends that monitoring in affected patients must be carried out in the 1st, 3rd, 6th, 9th, 12th, 18th, and 24th months after therapy.3–6
Patients are often considered to have an acceptable serological response when their non-treponemal titers fall by fourfold or more. Meanwhile, a fourfold increase is an indicator of treatment failure or reinfection. Several studies have shown that a significant fraction (15–20%) of individuals with early syphilis have non-treponemal titers, which are known as serofast, as the titers neither rise nor fall by fourfold following therapy.4–8 The best way to manage serofast patients is currently unknown, and it is unclear what factors influence the serological response following treatment.9,10 Despite the use of appropriate therapy, some patients in clinical practice still experience a gradual decline in their serological titers and seldom obtain a negative value. Therefore, this study aims to assess the frequency of post-treatment serological cure and its predictive variables.
Materials and Methods
Participants
This retrospective study used secondary data from medical records at the Division of Venereology, Dermatology, and Venereology Outpatient Clinic, Hasan Sadikin Central General Hospital (RSHS) Bandung, Indonesia, from January 2018 to December 2022. The inclusion criteria comprised syphilis patients with available socio-demographic and clinical data, including gender, marital status, highest education level, occupation, venereal disease research laboratory test (VDRL) titer before and after treatment, type of therapy, HIV coinfection status, serological cure, and characteristics of patients who achieved serological cure. The VDRL titers serial follow-ups at 1, 3, 6, 9, 12, and 18 months were also recorded. Patients with incomplete medical records were excluded from the study.
The serological cure is a situation characterized by a reduction of more than fourfold in the non-treponemal titer. Meanwhile, patients who experience an increase of ≥ fourfold have the potential for treatment failure or reinfection. Non-treponemal titers that remain unchanged following therapy are classified as being in a serofast state.
Although the timeframe used to assess serological response was considered appropriate, it is important to acknowledge the potential for bias due to loss of follow-up. In this study, a total of 7 participants did not complete the follow-up assessment. While the proportion of dropouts was relatively low, their exclusion from the final analysis may have influenced the overall serological response data, particularly when clinical outcomes differed systematically from those who remained in the study. However, baseline characteristics of those lost to follow-up were comparable to others who completed the study, suggesting that attrition was unlikely to introduce significant bias. Despite this outcome, the dropouts remain a limitation and must be considered when interpreting the results.
Statistical Analysis
Statistical analysis was carried out using a Chi‐square test to compare differences in the categorical data. In addition, a p-value of <0.05 was considered statistically significant. The odds ratio with a 95% confidence interval was calculated for each relevant variable. All analyses were performed using IBM SPSS Statistics for Windows, version 22.0.
Result
The flowchart in Figure 1 showed the selection process. This study included 273 patients diagnosed with syphilis between January 2018 and December 2022. However, incomplete data or unreadable records led to the exclusion of 7 patients, leaving a total of 266 cases. The results showed that 232 cases achieved serological cure, while 34 remained in a serofast state. Among these 34 cases, 28 were reinfections that achieved serological cure after retreatment, 1 case of neurosyphilis showed a twofold decrease in VDRL titer 6 months after receiving a 14-day course of procaine penicillin, and 5 cases were lost to follow-up.
 |
Figure 1 Flow of Participants Used in the Study. |
Socio-Demographic Characteristics of Syphilis Patients
In this study, 273 medical records of syphilis patients were obtained but 7 were incomplete and excluded. Among the 266 syphilis patient records that could be analyzed, 185 were male patients, while 81 were female patients. The socio-demographic characteristics presented were age, gender, highest education level, occupation, and marital status, which can be seen in detail in Table 1.
 |
Table 1 Socio-Demographic Characteristics of Syphilis Patient |
Overall, there were more male syphilis patients, specifically 185 patients (69.5%), compared to female patients, who numbered 81 patients (30.5%). The largest age group was between 26 and 35 (40.5%). The youngest patient was 14 years old, while the oldest patient was 63 years old, and both were male. A total of 42.7% of males and 60.5% of females had completed their high school education. The most common occupation among male patients was private employees (65.4%), while among female patients, it was housewives (64.2%). A significant portion of male patients (81.7%) were unmarried, while most female patients (65.5%) were married.
Clinical, Serological, Therapy Type, and Serological Cure Characteristics of Syphilis Patients
The clinical and serological characteristics studied in this study included age, gender, stage of syphilis, VDRL titer before therapy, and HIV coinfection, which was presented in Table 2. This pattern of serological cure based on the month of the visit was observed in Table 3. However, Table 4 showed a bivariate analysis of characteristics associated with serological cure in syphilis patients.
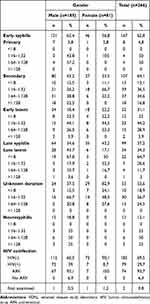 |
Table 2 Clinical and Serologic Characteristics of Syphilis Patients Before Therapy |
 |
Table 3 Serological Cure Pattern of Syphilis Patient Based on Follow-up |
 |
Table 4 Bivariate Analysis of Characteristics Associated with Serological Cure in Syphilis Patients |
Based on the above data, the majority of patients achieved serological cure in early syphilis (62.9%) and 37.1% in late syphilis. Among the total of 34 patients who experienced serofast, 29 patients did not achieve a serological cure, and 1 was diagnosed with neurosyphilis and was treated with procaine penicillin (PP) 2.4 million IU IM for 14 days. However, this patient only experienced a twofold decrease in VDRL titer from the previous titer in the sixth-month post-therapy observation and was subsequently lost to follow-up. The other 28 patients who did not achieve serological cure were diagnosed with reinfection and received re-treatment. After re-treatment, their titer decreased four-fold.
The highest serological cure for early-stage syphilis patients was achieved sequentially in the twelfth month. As for late-stage syphilis, the highest serological cure was achieved in the sixth month, with a total of 23 patients.
Overall, the highest serological cure was found in the age group of <35 years (75.4%, P:0.0001, OR: 3.89 (95% CI 1.85–8.13), among male patients (68.1%, P: 0.181 OR: 0.55 (0.23–1.32), and in cases of early syphilis (62.89%, P:0.648, OR: 0.83 (0.38–1.81). In addition, the proportion of serological cure was also higher in patients with VDRL titers before therapy ≤1:8 (P; 0.226, OR: 0.61 (0.28–1.35), those with HIV-negative status (P:0.648, OR: 0.83 (0.38–1.81), and those who received BPG therapy.
Discussion
Socio-Demographic Characteristics of Study Participants
Gender Characteristics
Based on this study, it was found that there were 266 syphilis cases included in the data, consisting of 185 male patients and 81 female patients. These findings were consistent with what was reported by the Australian Government from 2009 to 2019, which showed that out of 281 cases, 82% were male patients, and the remaining 18% were female.11 Similar results were obtained from a study on the characteristics of HIV/AIDS patients with syphilis coinfection at the Teratai Clinic of RSHS Bandung from 2018 to 2020, where the majority were male patients, accounting for 126 patients (96.2%).12
Factors contributing to the higher incidence of syphilis in males included their greater engagement in unprotected sexual activities, having multiple sexual partners, engaging in unprotected sexual behaviors, and using drugs during sexual intercourse to enhance satisfaction. However, societal norms often limited female sexual activity, leading to less frequent engagement in risky sexual behaviors.13,14 Females were less likely to notice painless early syphilis lesions, which could delay seeking medical attention.14
Age Characteristics
In this study, the most common age group for male and female patients was 20 to 29 years, followed by the 30 to 39 age group. Another study from the Dermatology and Venereology Clinic at Dr Soetomo Hospital in Surabaya, East Java, Indonesia, for the period from 2009 to 2017, found 37 new cases of latent syphilis. The majority of patients in the 26 to 35 years age group were male, with 26 patients (73.1%) out of a total of 37 patients diagnosed with syphilis.15,16
Syphilis was more commonly found in the second and third decades of life because these age groups were typically sexually active.17 The age group most frequently infected was the late adolescent age group, which is 17 to 25. Adolescents had an intense curiosity and a tendency to explore new things, including sexual activities. Some could seek information related to sexuality and engage in various activities to satisfy their curiosity, often without considering the potential consequences in the future. Therefore, adolescents were more prone to engage in behavior that deviated from societal norms.18,19 Other studies, such as one conducted by Gao et al20 had reported that syphilis in very young individuals was associated with early coitarche. Those under the age of 20 were less likely to be infected with syphilis because adolescents rarely visit clinics for check-ups.
Educational Characteristics
The education level of most male and female syphilis patients in this study was at the high school level. A study conducted at the Kota Raja Jayapura Reproductive Health Center in August–September 2022 with 89 patients who visited the Reproductive Health Center revealed that 55 patients (61.9%) had a high school education, 21 patients (23.6%) had a junior high school education, 8 patients (9%) had a college education, and 5 patients (5.6%) had a primary school education.18 This was similar to RSHS, a tertiary referral hospital in West Java, where most hospital visitors had completed high school. A study of 345 patients seeking treatment at an STD clinic in Brazil found that 175 patients had 3 years of schooling, 115 patients had 4 to 11 years of education, and 55 patients had more than 12 years of schooling. The higher the level of education, the easier it was for individuals to receive information, leading to more significant knowledge acquisition.19
Occupational Characteristics
In this study, the most common occupation among male syphilis patients, in descending order, was private employees (private companies, banks, stores) with 121 patients (65.4%), followed by self-employed individuals with 16 patients (8.6%), students with 19 patients (10.2%), service sector workers (cashiers, security guards, salon workers, cleaning staff) with 12 patients (6.4%), 1 police officer (0.5%), 4 healthcare workers (nurses and laboratory technicians, 2.1%), and 12 patients (6.4%) unemployed. Among female participants, the most common occupations, in descending order, were housewives with 52 patients (64.1%), 9 private employees (11.1%), 4 self-employed individuals (4.9%), 2 service sector workers (2.4%), 7 students (8.6%), 4 unemployed (4.9%), 2 commercial sex workers (2.4%), and 1 healthcare worker (1.2%). Similar results were obtained from a descriptive study conducted at the Melati Clinic of Dr. Soedarso General Hospital in Pontianak from November 2015 to May 2016 on syphilis-HIV/AIDS coinfection patients, with 39.8% being private employees, 30.6% housewives, 19.3% without data, 3.06% unemployed, 2.04% civil servants, 1.02% laborers, 1.02% Indonesian National Army personnel, 1.02% students, 1.02% prisoners, and 1.02% security guards.21 The participants who were employed and had sufficient income were more likely to engage in sexual relationships with multiple partners. However, the situation was different for female sex workers who provide sexual services to earn money, goods, or other benefits.13
Marital Status Characteristics
The majority of male participants, 151 patients (81.6%), were unmarried, and 27 females (33.3%) were unmarried. A study by Niode et al22 reported that among MSM, 75% were unmarried, 23.3% were married, and 1.7% were divorced. Another study conducted by Kusumawaty et al23 in the IMS Clinic of Dr. Wahidin Sudirohusodo Hospital in Makassar from 2017 to 2018 found that 44 patients (55.7%) were unmarried, and 35 patients (44.3%) were married. Unmarried participants had the opportunity to have more than 1 sexual partner because marital bonds could limit individuals from having multiple sexual partners. Men engaged in extramarital sexual relationships to express their masculinity and improve their social status by having multiple sexual partners, which increased the risk of STIs.21,24
Clinical and Serological Characteristics of Syphilis in Participants
Syphilis Stage Characteristics
The results of this study indicated that the stages of syphilis in both male and female participants were 107 patients (40.2%) had secondary syphilis, 53 patients (19.9%) had latent syphilis of unknown duration, 52 patients (19.5%) had early latent syphilis, 34 patients (12.8%) had late latent syphilis, and 8 patients (3%) had primary syphilis. Atsawawaranunt et al25 conducted a study in 2016 on the time to serological cure in syphilis patients with or without coinfection in Bang Rak, Thailand. This found 255 patients (51.3%) with secondary syphilis, 154 patients (31%) with late latent syphilis, 67 patients (13.5%) with early latent syphilis, and 21 patients (4.2%) with primary syphilis. Another study by Arando et al26 in 2015 on symptoms and risk factors in syphilis patients with HIV coinfection in Barcelona, Spain, found 51.3% with secondary syphilis, 27.5% with primary syphilis, and 21.2% with early latent syphilis. Kusumawaty et al23 2018 conducted a study on the seroepidemiology and risk factors of syphilis patients in Makassar and observed 63.3% with latent syphilis, 24.1% with secondary syphilis, and 12.6% with primary syphilis.
Syphilis consisted of several stages classified according to symptoms and the time since the initial infection.27 Accurate staging was essential as it helped assess the extent of the T. pallidum infection and determine the duration of therapy.28 Syphilis was divided into early and late stages in adults and congenital syphilis in infants.29 Early syphilis was further divided into primary, secondary, and early latent syphilis, while late-stage syphilis included late latent syphilis and tertiary syphilis (gumma, cardiovascular syphilis, or neurosyphilis).30 Approximately 50 to 75% of participants who came into contact with syphilis lesions, whether primary or secondary, become infected with syphilis.31 The diagnosis of early-stage syphilis showed that the T. pallidum infection occurred in the preceding year. Late-stage syphilis indicated manifestations of infection occurring more than one year, or even several decades, after the initial infection. Late-stage syphilis was non-infectious and included late latent syphilis and tertiary syphilis.28,31
Treatment for STIs was conducted alongside screening for HIV, syphilis, and hepatitis B as a form of integrated care, both for patients and their sexual partners. This approach helped detect asymptomatic diseases like latent syphilis. Therefore, some studies found latent syphilis to be the most prevalent stage.32
Characteristics of VDRL Titers Before Treatment
Most patients had VDRL titers before treatment in the range of 1:16 to 1:32 (51.5%), followed by titers <1:8 (24.4%), 1:64 to 1:128 (17.6%), and >1:128 (6.4%). In Kusumawaty’s23 study, the most common rapid plasma reagin (RPR) titer before treatment was 1:16 (55.7%), followed by 1:4 (32.9%), 1:8 (6.3%), and 1:32 (5.1%). The most frequently encountered VDRL titer in this study was 1:16 to 1:32. This was because the most common stage found in this study was secondary syphilis. These findings were consistent with a study by Talwar et al33 on VDRL titers in syphilis patients before and after treatment in India between 1976 and 1981, where the most common VDRL titer in secondary syphilis was 1:16 to 1:32.
Sena et al34 conducted a study on serological cure and serofast stage in syphilis patients without HIV coinfection before and after treatment in the USA and Madagascar between 2000 and 2009. 92.1% of serological cures were associated with an RPR titer before treatment of >1:32, which increased the likelihood of serological cure. Non-treponemal tests could be used to monitor disease activity. A fourfold decrease in the initial titer tested, from 1:8 to 1:2, indicated successful treatment. Serofast (persistently low positive nontreponemal antibodies) could occur with appropriate treatment, and non-treponemal tests did not rely on subjective interpretation.9,35 Biological false positives occurred in autoimmune diseases, ulcerative colitis, vasculitis, chickenpox, hepatitis, infectious mononucleosis, measles, mumps, pneumonia, bacterial endocarditis, malignancies, and pregnancy. False negatives appeared as a prozone phenomenon, where high antibody titers interfered with antigen-antibody complex formation. This phenomenon was overcome by diluting the sample to at least 1:16.36
Characteristics of HIV Coinfection
Coinfection of syphilis with HIV was observed to be more prevalent in male patients compared to females. Among HIV-positive male patients, 73 patients (39%) received HIV therapy, while 67 patients (93.1%) did not. However, among female patients, the majority, 73 (90.1%), did not have HIV. Salman et al12 conducted a study in 2020, showing that 28.2% of male patients had a positive HIV coinfection, while 1.5% of female patients did. Another study conducted by David et al on the profile of syphilis with HIV/AIDS coinfection at the Melati Clinic of Dr Soedarso Regional Public Hospital in Pontianak in 2019 found that 5.1% of syphilis patients had coinfection with HIV, and all of these patients were male.21
This study showed that syphilis increased the spread of HIV through sexual transmission. Coinfection with syphilis and HIV could lead to atypical clinical manifestations, accelerate disease progression, make diagnosis more challenging, increase the risk of neurological complications, and raise the risk of treatment failure with standard regimens. Syphilis reduced the number of clusters of differentiation (CD4) cells or increased plasma viral load in HIV-infected patients. Horberg et al37 on the epidemiology and clinical manifestations of syphilis patients with HIV coinfection in California from 1995 to 2005, the incidence of syphilis was 62.3 per 1000 individuals per year in the HIV-infected group and 8 per 1000 persons per year in the HIV-uninfected group. Individuals infected with HIV were more vulnerable to coinfection with syphilis due to both lifestyle factors and immunosuppression. A study by Pattanasin et al38 in 2022 in Bangkok found that in the transgender group with HIV infection, there was a twofold increased risk of syphilis compared to HIV-negative individuals. Clinical manifestations of syphilis were more commonly seen in HIV-infected patients, particularly with multiple chances in the primary stage. Ulcers in primary syphilis compromised mucosal and epithelial integrity, making HIV transmission and acquisition easier. Inflammation and CD4 cells migrating to the ulcer area could also increase the risk of HIV transmission. The risk of eye infections in syphilis-infected HIV individuals included uveitis, keratitis, and conjunctivitis. Gumma and neurosyphilis occurred more rapidly in syphilis-infected individuals with HIV.39–41
Data on Serological Cure in Study Participants
In this study, 232 patients (87.2%) experienced serological cures, while 34 (12.8%) experienced serofast. The factors associated with serological cure were compared, including the patient’s age, gender, HIV status, syphilis stage, baseline VDRL, and treatment regimen given (Table 4). Atsawawaranunt et al25 in 2016 found that 309 patients (62.1%) achieved serological cure after treatment, 105 patients (21.2%) were lost to follow-up, 49 patients (9.9%) experienced serofast, 23 patients (4.6%) had treatment failure, and 11 patients (2.2%) did not respond to treatment. In general, the time required to achieve a serological cure in syphilis patients was 110 days (range: 100 to 120 days). For early latent syphilis, the time needed for serological cure was 99 days (range: 94 to 104 days), and for late latent syphilis, it was 144 days (range: 69 to 219 days). Based on VDRL titers before treatment, patients with titers >1:32 required serological cure for 131 days, while those >1:64 required 192 days. Factors influencing the success of a serological cure included a history of previous syphilis, stage of infection, baseline serological titers, immune status, and the treatment received.42
This study found an association between the younger age group and the likelihood of achieving a serological cure. Statistically significant differences in patients <35 years old were observed, having more than 3 3-fold probability of achieving serological cure compared to patients ≥35 years old. A study by Sena et al34 reported similar findings, stating that patients in an age group younger than 24 were more likely to achieve a serological cure. Li-tong et al43 also reported that age <23 years was associated with a 2-fold greater probability of a serological cure than an age >40. This older group typically exhibited immunosuppression and immune system senescence, which could influence the serological response to syphilis treatment.4,44
Treatment success was analyzed by assessing whether clinical symptoms persisted or serological responses occurred. Non-treponemal antibody titers decreased in most syphilis patients after treatment (in this study, the non-treponemal test used was VDRL). VDRL retesting must be minimal in the first, third, sixth, and twelfth months. For patients with HIV infection, stricter monitoring was recommended at the first, third, sixth, ninth, twelfth, and twenty-fourth months, especially when the CD4 cell count was ≤350/mL and when the patient had not received antiretroviral therapy. Since 1993, it was agreed that VDRL response after treatment, reflecting cure, occurred when seroconversion to negative or at least a fourfold decrease in titer from before treatment (equivalent to a twofold dilution decrease).45
Several specific conditions could be found in the post-treatment follow-up of syphilis. When, during the follow-up period, clinical signs and symptoms of syphilis persisted or recurred, the patient was diagnosed with reinfection or treatment failure. Supposed clinical signs and symptoms of syphilis were not found in the patient, but VDRL titers remained positive after treatment. This condition was categorized into reinfection, treatment failure, and VDRL titer persistence (serofast and serologic nonresponse).44,45 The non-treponemal titer before treatment was associated with the likelihood of achieving a serological cure. Tong et al43 reported that a low VDRL titer before treatment increased the chances of attaining a serological cure. This was consistent with this study that patients with baseline VDRL titer ≤1:8 were more likely to achieve serological cure. In a study by Sena et al34 and Leeyaphan et al,42 patients with baseline VDRL titer ≤1:32 were more likely to achieve a serological cure.
Most patients did not need retreatment, especially when some were asymptomatic and had shown an adequate decline in titers. Clinicians must assess the full clinical picture history, serologic trends, and risk factors before deciding on additional therapy or workup.
Retreatment could be considered in certain cases, particularly when there was evidence suggesting reinfection, treatment failure, or ongoing clinical activity. A fourfold increase in non-treponemal titers after initial treatment was indicative of possible reinfection or relapse and typically warrants retreatment. Similarly, patients who failed to show an adequate serological response, defined as a fourfold decline in titers within 6 to 12 months for early syphilis or within 12 to 24 months for late latent syphilis, must be carefully evaluated, especially in high-risk groups such as those with HIV. The presence of new or persistent symptoms, or findings suggestive of neurosyphilis, also justified further investigation, including cerebrospinal fluid analysis, and possible retreatment. However, in asymptomatic patients who had shown an appropriate decline in titers and remained serofast without clinical evidence of relapse, retreatment was generally not necessary, and ongoing monitoring could suffice.
Follow-up strategies for the patients could be improved by implementing standardized post-treatment monitoring at defined intervals, such as 3, 6, 12, and 24 months. Strengthening patient communication through reminder systems and digital health tools reduced the loss of follow-up. In addition, creating clear clinical protocols for interpreting titer trends and deciding when further evaluation was necessary, such as cerebrospinal fluid examination or retreatment, could aid in consistent patient management. Ensuring accurate documentation and better coordination across healthcare providers also contributed to improved long-term follow-up, particularly in high-risk or mobile populations.
Limitations of the Study
In this retrospective study, the data analyzed relied solely on findings that were recorded by doctors in medical records, and not all the required data was available. In this study, the number of patients who achieved serological cure was limited, as only a portion of the patients followed up regularly. Therefore, further prospective cohort study was needed to determine the number of serological cures and the factors associated with them. Medical records were obtained for only 266 out of 273 syphilis patients who were included, because some medical records were incomplete data, and there were unreadable writings. Follow-up loss could affect outcome assessment, as a result, further prospective studies were needed.
Conclusion
In conclusion, serological cure was more common in early syphilis, with the most common time being the 12th and 9th months. However, in late syphilis, serological cure was most common in the 12th and 3rd months. Characteristics of patients who achieved serological cure were most common in the younger age (p:0.002), male patients, secondary syphilis stage, VDRL titer before treatment of 1:16–1:32, HIV negative status, and treatment with BPG.
The conclusions were generally aligned with the findings, particularly regarding the prevalence and characteristics of serofast status following syphilis treatment. However, further clarification and expansion on future study directions were warranted. Prospective, longitudinal studies were needed to validate these results and to better understand the long-term immunological profiles of serofast patients. In addition, the identification and validation of novel biomarkers that could predict serological cure was of great clinical value, potentially guiding individualized follow-up and retreatment strategies. Such a study could refine the current management of the patients and reduce unnecessary interventions.
Ethics Statement
All participants provided full permission in the form of written consent for the publication of photographs and textual material (case histories) in the publication, which was documented in their medical records. Patients aged 18 years or older gave consent independently, while those under 18 received authorization from a parent or guardian through a signed written consent form to include the case details in the medical record. Institutional approval for the dissemination of case details was obtained from the Ethics Committee of Dr. Hasan Sadikin Hospital, under approval number DP.04.03/D.XIV.6.5/362/2024. This study was conducted strictly following the Declaration of Helsinki.
Consent Statement
The authors attested to be in the position of all necessary patient permission paperwork. A consent form allowing the release of the case data was signed.
Acknowledgments
The authors thanked the Department of Dermatology and Venereology staff, Faculty of Medicine, Universitas Padjadjaran, Bandung, West Java, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sparling P, Swartz M, Musher D, et al. Editor. Sexually Transmitted Disease.
2. Lukehart S, Holmes K, Sparling M, et al. Sexually transmitted disease. 37. 2008;647–659.
3. Tuddenham S, Dalam ZJS, Kang S, et al. Editor. Fitzpatrick Dermatology.
4. European Centre for Disease Prevention and Control. Syphilis. In: ECDC. Annual Epidemiological Report For 2019. Stockholm: ECDC; 2022.
5. Sadoghi B, Stary G, Wolf P. Syphilis. J Dtsch Dermatol Ges. 2023;21(5):504–519. doi:10.1111/ddg.14999
6. Kidd S, Grey J, Torrone E, Weinstock H. Increased methamphetamine, injection drug, and heroin use among women and heterosexual men with primary and secondary syphilis.United States, 2013–2017. Morb Mortal. 2019;68(6):144–148. doi:10.15585/mmwr.mm6806a4
7. Ministry of health of the republic of Indonesia. infodatin: executive report on HIV and AIDS and Sexually Transmitted Infectious Diseases (STIs) Progress for Quarter II; 2022. Available from: https://siha.kemkes.go.id/.
8. Ministry of Health of the Republic of Indonesia. [infodatin: Integrated Biological and Behavioural]; 2017. Available from: https://survey-terpadu-biologis-dan-perilaku.go.id/.
9. Ghanem KG, Hook EW. The terms “serofast”“serological nonresponse” in the modern syphilis era. Sex Transm Dis. 2021;48(6):4512. doi:10.1097/OLQ.0000000000001387
10. Febiyanto N, Budiyanto A, Pudjiati SR. serological responses to benzathine penicillin–g treatment in early syphilis patients with different HIV status. Indian J Dermatol. 2021;66(5):575. doi:10.4103/ijd.ijd_877_20
11. Kakar SR, Truman G, Thomas J, Jackson EY, Forssman BL. Epidemiology of syphilis in the Nepean and blue mountains local health district between 1. Commun Dis Intell. 2023;47.
12. Salman A, Tony S, Ratna D. Original research: insidensi dan karakteristik pasien hiv/aids dengan koinfeksi sifilis di klinik teratai bandung year 2018-2020. bandung conference series: medical science. JACS Au. 2022;2(1):1169–1175. doi:10.1021/jacsau.2c00150
13. Dela H, Attram N, Behene E, et al. Risk factors associated with gonorrhea and chlamydia transmission in selected health facilities in Ghana. BMC Infect Dis. 2019;19(1):425. doi:10.1186/s12879-019-4035-y
14. Maruti S, Hwang L, Ross M, Leonard L, Paffel J, Hollins L. 1997. The epidemiology of early syphilis in Houston, Texas, 1994–1995. Sexually transmitted. diseases. 24(8):475–480.
15. Ministry of Health of the Republic of Indonesia. [infodatin: Indonesia Health Profile 2020. Available from: https://kemkes.go.id/.
16. Repository U. Infodatin: latent Syphilis. Periodical Skin Genital Health Sci. 2019;31.
17. Yu W, You X, Luo W. Global, regional, and national burden of syphilis, 1990–2021 and predictions by Bayesian age-period-cohort analysis: a systematic analysis for the global burden of disease study. Front Med. 2021;11:1448–1841.
18. Gomes N, Meier D, Pieri F, et al. Prevalence and factors associated with syphilis in a reference center. Rev Soc Bras Med Trop. 2017;50(1):27–34. doi:10.1590/0037-8682-0102-2016
19. Matar I, Raudeliuniene J. The role of knowledge acquisition in enhancing knowledge management processes in higher education institutions. 2021. doi:10.3846/cibmee.2021.646
20. Faustina N, Dankwa K, Ampiah C, Boampong JN, Nuvor SV. Seroprevalence of syphilis infection in individuals at cape coast metropolis, Ghana. Br J Med Res. 2015;8:157–164. doi:10.9734/BJMMR/2015/16267
21. Mampan DAGKSPPH, Dr ADKMRSUD. Soedarso Pontianak. Jurnal Mahasiswa PPDS FK Universitas Tanjungpura. 2019;5(1):1–18.
22. Niode NJ, Minarto H, Mitaart AF, Kapantow GM, Kandou RT. Seroprevalence of syphilis and herpes simplex virus type 2 and its association with sexual behavior factors (a cross-sectional study among men who have sex with men in Manado, Indonesia).2017.
23. Kusumawaty M, Djawad K, Nasrum Massi M, Adam AM, Wahab S, Bahar B. Sero-epidemiology and syphilis risk factors in Makassar, Indonesia. Serbian J of Dermatology and Venereol. 2019;11:43–49. doi:10.2478/sjdv-2019-0006
24. Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021;35(3):57488. doi:10.1111/jdv.16946
25. Atsawawaranunt K, Kittiyaowamarn R, Phonrat B, Kamolratanakul S, Kangvalpornroj T, Dhitavat J. Time to serological cure and associated factors among syphilis patients with and without hiv in a sexually transmitted infections center, Thailand. Sex Transm Dis. 2020;47(5):283–289. doi:10.1097/OLQ.0000000000001154
26. Arando M, Fernandez-Naval C, Mota-Foix M, et al. Early syphilis: risk factors and clinical manifestations focusing on HIV-positive patients. BMC Infect Dis. 2019;19(1):727. doi:10.1186/s12879-019-4269-8
27. Schmidt R, Carson PJ, Jansen RJ. Resurgence of Syphilis in the United States: an assessment of contributing factors. Infect Dis. 2019;12:1178633719883282.
28. Norkin L. Virology Molecular Biology and Parthenogenesis. ASM Press. 2010;1.
29. Dfn DCR, da Cunha Rosa LR, de Almeida Silva C, et al. Epidemiology of HIV, syphilis, and hepatitis B and C among manual cane cutters in low-income regions of Brazil. BMC Infect Dis. 2018;18(1):546. doi:10.1186/s12879-018-3439-4
30. Adeyemi EO. Gender inequities in sexually transmitted infections: implications for HIV infection and control in Lagos State, Nigeria. Infect Dis Rep. 2011;3(1):e7. doi:10.4081/idr.2011.1049
31. Mwakagile D, Mmari E, Makwaya C, et al. Sexual behavior among youths at high risk for HIV-1 infection in Dar es Salaam, Tanzania. Sex Transm Infect. 2001;77(4):255–259. doi:10.1136/sti.77.4.255
32. Sawyer SM, Azzopardi PS, Wickremarathne D, Patton GC. The age of adolescence. Lancet Child Adolesc Health. 2018;2(3):223–228. doi:10.1016/S2352-4642(18)30022-1
33. Talwar S, Tutakne MA, Tiwari VD. VDRL titers in early syphilis before and after treatment. Genitourin Med. 1992;68(2):120–122. doi:10.1136/sti.68.2.120
34. Seña AC, Wolff M, Martin DH, et al. Predictors of serological cure and Serofast State after treatment in HIV-negative persons with early syphilis. Clin Infect Dis. 2011;53(11):1092–1099. doi:10.1093/cid/cir671
35. Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clin Vaccine Immunol. 2015;22(2):137–147. doi:10.1128/CVI.00681-14
36. Fan L, Yu A, Zhang D, Wang Z, Ma P. Consequences of HIV/Syphilis Coinfection on HIV Viral Load and Immune Response to Antiretroviral Therapy. Infect Drug Resist. 14:2851–2862. doi:10.2147/IDR.S320648
37. Horberg MA, Ranatunga DK, Quesenberry CP, Klein DB, Silverberg MJ. Syphilis epidemiology and clinical outcomes in HIV–infected and HIV-uninfected patients in Kaiser Permanente Northern California. Sex Transm Dis. 2010;37(1):53–58. doi:10.1097/OLQ.0b013e3181b6f0cc
38. Pattanasin S, Griensven FV, Mock PA, et al. HIV and syphilis prevalence among transgender women and men who have sex with men, Silom Community Clinic. AIDS Care. 2022;34(10):1305–1313. doi:10.1080/09540121.2021.1967854
39. Baetena JM, Overbaughb J. Measuring the infectiousness of persons with HIV–: opportunities for preventing sexual HIV-1 transmission. Current HIV Res. 2003;1:69–86. doi:10.2174/1570162033352110
40. Zetola N, Klausner J. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44(9):1222–1228. doi:10.1086/513427
41. Sato N. Serologic Response to Treatment in Syphilis.
42. Leeyaphan C, Punyaratabandhu P, Jiamton S, et al. Predictors of serological cure after treatment in patients with early syphilis: a retrospective observational study in Thailand. Indian J Dermatol Venereol Leprol. 2019;85(2):235. doi:10.4103/ijdvl.IJDVL_810_17
43. Tong ML, Lin LR, Liu GL, et al. Factors associated with serological cure and the serofast state of HIV-negative patients with primary, secondary, latent, and tertiary syphilis. PLoS One. 2013;8(7):e70102. doi:10.1371/journal.pone.0070102
44. Satyaputra F, Hendry S, Braddick M, Sivabalan P, Norton R. The laboratory diagnosis of syphilis. J Clin Microbiol. 2021;59(10):e0010021. doi:10.1128/JCM.00100-21
45. Turner AN, Feldblum P, Hoke TH. Condom use and sexually transmitted infections among Malagasy sex workers. Int J STDs and AIDS. 2011;22:552–557. doi:10.1258/ijsa.2011.010311
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

