Back to Journals » International Journal of General Medicine » Volume 17
Prevalence and Risk Factors of Cardiovascular Disease in Rheumatoid Arthritis Patients: A Comparative Analysis of Real-World Data
Authors Tekeoglu S
Received 24 September 2024
Accepted for publication 27 November 2024
Published 6 December 2024 Volume 2024:17 Pages 5859—5868
DOI https://doi.org/10.2147/IJGM.S490916
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yuriy Sirenko
Senem Tekeoglu1,2
1Internal Medicine, Rheumatology, Halic University Medical Faculty, Istanbul, Turkey; 2Rheumatology, Private Bahcelievler Memorial Hospital, Istanbul, Turkey
Correspondence: Senem Tekeoglu, Internal Medicine, Rheumatology, Halic University Medical Faculty, 5. Levent Mahallesi, Istanbul, 34060, Eyupsultan, Turkey, Email [email protected]
Purpose: Rheumatoid arthritis (RA) is linked to cardiovascular disease (CVD), due to chronic inflammation and traditional CVD risk factors. This study evaluates CVD and related risk factors in RA patients compared to age and gender-matched controls without inflammatory diseases, and differences within RA patients with and without CVD.
Patients and Methods: This retrospective case-control study reviewed medical records of 405 RA patients (cases) and 950 control patients who attended rheumatology clinics in two branches of a private hospital between January 2021 and January 2024 to assess cardiovascular disease prevalence and associated risk factors.
Results: RA patients, with a mean age of 59 (± 23) years, disease duration of 89.5 months, and a female-to-male ratio of 4:1, exhibited a higher prevalence of CVD compared to controls (p = 0.01), despite similar classical risk factors. Logistic regression identified RA as an independent risk factor for CVD (p = 0.02, odds ratio = 1.9). RA patients with CVD were typically older males (p < 0.001), presenting with higher rates of hypertension (p < 0.001), hyperlipidemia (p < 0.001), diabetes (p = 0.002), and chronic kidney disease (p < 0.001). Arrhythmias (p < 0.001) and heart failure (p < 0.001) were prevalent among this subgroup, along with elevated creatinine levels and reduced glomerular filtration rates (p < 0.001 each). Treatment patterns indicated lower use of methotrexate (p = 0.003) and higher use of leflunomide (p = 0.02) among RA patients with CVD.
Conclusion: CVD in RA patients is multifactorial, involving both chronic systemic inflammation and classical CVD risk factors. Further research is necessary to advance our understanding of CVD in RA patients and to optimize treatment strategies for improved outcomes.
Keywords: Rheumatoid arthritis, cardiovascular diseases, comorbidity, mortality
Introduction
Rheumatoid arthritis (RA) is an inflammatory disease related to serious cardiovascular disease (CVD) risk.1 Studies indicate that RA patients have a 2- to 3-times elevated risk of cardiovascular morbidity and a 1.5-times elevated risk of mortality,2 with a high prevalence of heart failure.3 This elevated risk originates from a combination of classical CVD risk factors such as diabetes, hypertension, and hyperlipidemia, as well as the chronic systemic inflammation characteristic of RA.4,5 The Framingham and Systematic Coronary Risk Evaluation (SCORE) models tend to underestimate CVD risk in RA patients.6 Hence, the European League Against Rheumatism (EULAR) recommends applying a multiplier of 1.5 to calculated CVD risk in all RA patients.7
In recent decades, there has been a significant shift toward initiating and intensifying the treatment of RA at an earlier stage.8 Multiple studies have demonstrated that treatment with both traditional9,10 and biological disease-modifying anti-rheumatic drugs (DMARDs) is associated with a decreased risk of developing CVD.11 While EULAR recommendations have increased awareness of CVD in RA patients, they are not consistently followed in routine clinical practice.12
The main goal of this study was to evaluate the prevalence of CVD and associated risk factors among RA patients over a three-year period at rheumatology clinics in two branches of a private hospital. This investigation included a comparative analysis with patients without a history of inflammatory diseases, aiming to provide a picture of the burden of CVD in RA patients and the efficacy of current management strategies. Additionally, a comparative analysis was conducted to explore potential distinctions between RA patients with and without CVD.
Material and Methods
The Study Population
The electronic medical files of 405 consecutive RA patients and 950 controls with non-inflammatory complaints were reviewed retrospectively. The participants attended the rheumatology outpatient clinics at Bahcelievler Memorial Hospital and Hizmet Hospital in Istanbul between January 2021 and January 2024. These private hospitals serve patients from both Turkey and abroad. The clinics offer care to individuals aged 16 years and older with rheumatic diseases, as well as those with inflammatory and non-inflammatory musculoskeletal complaints.
RA diagnosis was based on either the 1987 American College of Rheumatology (ACR) criteria13 or the 2010 ACR/EULAR classification criteria for RA.14 Due to the low number of patients with peripheral vascular disease, CVD was considered as ischemic heart disease (IHD), and stroke for this study.
Data were extracted from electronic records, including details such as age, gender, and smoking status for both RA patients and controls. For controls the primary complaint was documented, while additional information for RA patients included disease duration, geriatric onset status, antibody profile (rheumatoid factor, anti-citrullinated protein antibody), disease activity score 28-C-reactive protein (DAS28-CRP) at the last visit, and details of secondary diseases and medications.
Self-reports were utilized to identify co-existing risk factors and secondary diseases in both groups. The Charlson comorbidity index (CCI), a well-established tool for predicting 10-year mortality based on 16 medical conditions,15 was calculated for each patient. This study focused on assessing the presence of IHD, diabetes, hyperlipidemia, hypertension, arrhythmias, heart failure, chronic kidney disease (CKD), and stroke.
In addition to DAS28-CRP, CRP and erythrocyte sedimentation rate (ESR) levels from the last visit were documented, due to fact that CRP is an independent predictor of CVD risk.16 Creatinine and glomerular filtration rate (GFR) were also recorded due to the known contraindications of certain medications in renal failure.17 GFR was calculated using the Modification of Diet in Renal Disease (MDRD) formula, and CKD was defined as a GFR <60 mL/min/1.73 m² persisting for 3 months or more.
The study was approved by the Bahcelievler Memorial Hospital ethics committee (Date: 11.06.2024, No. 125). Due to the retrospective nature of the study, patient consent to review medical records was not required by the ethics committee. All patient data were handled confidentially in accordance with the Declaration of Helsinki.
Statistical Analysis
Data analysis was conducted using Statistical Package for Social Sciences (SPSS) version 26. Differences in continuous variables such as age, creatinine, GFR, CRP, ESR, and CCI between groups (RA patients vs controls and RA patients with or without CVD) were assessed using the Mann–Whitney U-test. Descriptive statistics were expressed as median ± interquartile range, when these variables were not normally distributed.
Qualitative variables were presented as absolute and relative frequencies. Gender, smoking status, risk factors, and secondary diseases were compared between groups, and treatment history among RA patients with and without CVD was analyzed using the Chi-square test. Fisher’s exact test was used when expected frequencies were less than 5. Binary logistic regression analysis was employed to determine the effect of variables on the presence of CVD.
Statistical significance was considered when the probability (p) value was less than 0.05.
Results
RA Patients and Controls
The study included 405 patients with RA and 950 control patients. Among RA patients, the average duration of disease was 89.5 months. Of these patients, 137 (33.8%) had disease onset at or after 65 years, and 50.7% had seropositive disease. The median DAS28-CRP score at the last visit was 3.1. The most common secondary conditions among RA patients were osteoarthritis (17%) and fibromyalgia (11.4%) (Table 1).
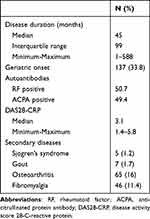 |
Table 1 Characteristics of Rheumatoid Arthritis Patients (N=405) |
In the control group, the most common primary complaint was non-specific musculoskeletal pain, followed by osteoarthritis and fibromyalgia. The primary complaints of the control group are presented in Figure 1.
 |
Figure 1 Primary complaints in the control group (%). Note: *Non-specific musculoskeletal pain besides osteoarthritis and fibromyalgia. |
The average age of RA patients was 59 ± 23 years, while the control group had an average age of 55 ± 17 years, showing no significant difference (p = 0.09). Both groups had a female to male ratio of 4:1 (p = 0.52). The proportion of ever-smokers was similar between the groups (p = 0.98) (Table 2).
 |
Table 2 Comparison of Rheumatoid Arthritis (RA) Patients with Controls |
RA patients exhibited a significantly higher rate of CVD (p = 0.01). In particular, IHD was more frequent in RA patients (p = 0.006), whereas stroke rates were comparable (p = 0.99; some patients had both IHD and stroke). Other cardiovascular risk factors, such as diabetes, hyperlipidemia, and hypertension, did not differ significantly between the groups (p = 0.28, p = 0.82, p = 0.08) (Figure 2). There was no significant difference in the prevalence of arrhythmia (p = 0.75). The prevalence of CKD, along with creatinine and GFR levels, were comparable (p = 0.45, p = 0.20, p = 0.23).
 |
Figure 2 Comparison of rheumatoid arthritis patients with controls (%). Abbreviations: RA, rheumatoid arthritis; CVD, cardiovascular disease; IHD, ischemic heart disease; CKD, chronic kidney disease. |
Despite the similar prevalence of individual diseases between the groups, RA patients had significantly higher CCI scores (p < 0.001, even after age adjustment). Heart failure was notably more prevalent among RA patients (p = 0.01) (Table 2).
Factors Influencing CVD Risk
To assess CVD risk in both RA patients and controls, logistic regression analysis was conducted using multiple parameters. Hypertension (p = 0.001), male gender (p < 0.001), hyperlipidemia (p < 0.001), RA (p = 0.02), diabetes (p = 0.04), creatinine level (p = 0.03), and age (p < 0.001) were found to be significant factors. Specifically, patients with RA were found to have a 1.9-fold higher risk of developing CVD (Table 3).
 |
Table 3 Logistic Regression Analysis for Ischemic Heart Disease and Significant Factors |
Comparison of RA Patients with or Without CVD
Among RA patients, those with CVD were predominantly older males (p < 0.001), with higher creatinine levels (p < 0.001) and lower GFRs (p < 0.001). While levels of disease activity markers (DAS28-CRP, CRP, ESR) and smoking status were similar between groups, those with CVD had higher CCI scores (p < 0.001), indicating a greater burden of comorbidities. Additionally, they exhibited higher prevalence of diabetes (p = 0.002), hyperlipidemia (p < 0.001), hypertension (p < 0.001), and CKD (p < 0.001)—known CVD risk factors (Figure 3). Arrhythmias (p < 0.001), particularly atrial fibrillation, and heart failure (p < 0.001), which are often consequences of CVD, were more common in this group. Treatment patterns showed that patients with CVD were less likely to receive methotrexate (MTX) (p = 0.003) but more likely to receive leflunomide (LEF) (p = 0.02) compared to those without CVD. Otherwise, the use of traditional and biological DMARDs was not different between the two groups (Table 4).
 |
Table 4 Comparisons of Demographics, Laboratory Tests, Comorbid Conditions, and Treatments Received by Rheumatoid Arthritis Patients Based on Their History of Cardiovascular Disease (CVD) |
 |
Figure 3 Comparison of rheumatoid arthritis patients according to cardiovascular disease (%). Abbreviations: CVD, cardiovascular disease; CKD, chronic kidney disease. |
Discussion
In addition to joint-related complications, RA is linked to an elevated risk of mortality, primarily attributed to CVD.4,7,18 The increased CVD risk in RA patients is linked to chronic systemic inflammation and classical risk factors such as hypertension, diabetes, and hyperlipidemia.2,3 This study compared RA patients with age and gender-matched controls without a history of inflammatory diseases, allowing for a focused examination of the impact of RA on CVD risk, independent of age and gender. Furthermore, within the RA patient group, further analysis compared those with CVD to those without CVD, revealing distinct differences between these subgroups.
Inflammation plays a pivotal role in the development and progression of atherosclerosis, with involvement in plaque formation, destabilization, and rupture. Atherosclerosis shares common inflammatory pathways with RA, suggesting a link between joint inflammation and plaque instability. Pro-inflammatory molecules such as CRP, fibrinogen, interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF) contribute to this process.19 In current study, findings indicate a higher prevalence of CVD in RA patients, despite similar major CVD risk factors between RA patients and control groups. This underscores the significant role of chronic inflammation in CVD pathogenesis. Logistic regression analysis identified RA as an independent risk factor for CVD (Odds ratio [OR] = 1.9), with a risk level similar to that of diabetes (OR = 1.8), consistent with previous studies.20 Other relevant risk factors included hypertension, male gender, older age, hyperlipidemia, diabetes, and higher creatinine levels.
Previous studies have indicated that CRP levels can independently predict cardiovascular risk in the general population.21 While many observational studies have linked higher disease activity in RA with worse cardiovascular outcomes,22 current study did not find evidence supporting this association. This discrepancy could be attributed to the majority of patients being in remission, and DAS28-CRP scores and CRP levels noted only at the last visit, potentially masking their true impact.
A previous study noted that hypertension, dyslipidemia, and insulin resistance showed stronger associations with CVD in RA compared to inflammatory markers.23 Classical CVD risk factors such as obesity, diabetes, hypertension, and smoking appear to be more prevalent in patients with RA. Smoking, in particular, has been recognized as a significant and common risk factor for both CVD and RA development.24 In the current study, RA patients with CVD tended to be older males with a history of smoking and had higher CCI scores, indicating a greater number of coexisting conditions such as hypertension, diabetes, and hyperlipidemia.
Patients with CKD have a higher risk of CVD, IHD, congestive heart failure, arrhythmias, and sudden cardiac death. CKD leads to a persistent, systemic pro-inflammatory state, contributing to changes in blood vessels and the heart, including the development of atherosclerosis and vascular calcification, resembling an accelerated aging process.25 In this study, RA patients with CVD exhibited higher creatinine levels, lower GFRs, and higher rates of CKD. Both RA and CKD may have contributed to CVD in these patients. While the higher prevalence of old age, diabetes, and hypertension likely played a role in the increased risk of CVD and CKD in this subgroup, logistic regression analysis identified higher creatinine levels as an independent risk factor (OR = 1.6), along with diabetes, hypertension, and hyperlipidemia.
Cardiac dysfunction in RA patients was initially thought to be secondary to IHD. Nevertheless, atherosclerotic disease alone cannot account for the prevalence of congestive heart failure.12 A previous study showed that patients diagnosed with RA faced a rapidly escalating risk of developing non-ischemic congestive heart failure.3 RA disease activity strongly correlates with cardiac function, with greater left ventricular strain observed in RA patients with high disease activity compared to controls, as shown by speckle-tracking echocardiography.26 Rheumatoid arthritis patients often exhibit a higher prevalence of diastolic dysfunction, which is linked to elevated levels of circulating IL-6.27 These studies indicate that cardiac dysfunction cannot be solely explained by atherosclerosis. Consistent with prior research, RA patients in the current study exhibited a higher frequency of heart failure, with an even higher prevalence observed among RA patients with CVD.
RA patients also have a higher risk of sudden cardiac death after acute coronary syndromes compared to those without RA, possibly due to an increased incidence of malignant ventricular arrhythmias.28 Population-based studies suggest a significantly higher incidence of atrial fibrillation in RA patients, indicating widespread abnormal electrical activity in the heart.29 Additionally, inflammation-related non-structural heart abnormalities may contribute to arrhythmias in RA, potentially amplifying the arrhythmic risk caused by structural damage associated with IHD and heart failure.30 Similar to previous research, RA patients with CVD in this study had a higher prevalence of arrhythmias compared to those without CVD.
Various drugs have differing effects on CVD risk in RA. Non-steroidal anti-inflammatory drugs (NSAIDs) generally increase the risk of CVD.31 Glucocorticoids can lead to several cardiovascular adverse effects, such as hypertension, dyslipidemia, insulin resistance, and diabetes, with the risk increasing with the dose and duration of treatment.32 MTX has a beneficial effect in slowing the progression of atherosclerosis in RA patients, reducing CVD mortality, and delaying CVD risk.33 Hydroxychloroquine is recognized for its vascular protective effects, inhibiting platelet aggregation, preventing thrombosis, improving insulin sensitivity, and reducing glucose, total cholesterol, and low-density lipoprotein levels, all contributing to a reduced risk of CVD.34 LEF has been shown to reduce myocardial hypertrophy and inhibit cardiac fibrosis.35
TNF inhibitors in RA patients markedly decrease CVD risk and offer various myocardial protective effects, such as improving glucose and cholesterol metabolism, and mitigating inflammation’s impact on blood clotting.36 However, TNF’s role can vary; while high levels can lead to ventricular dysfunction, low levels protect the myocardium. TNF inhibitors are not advisable for patients with congestive heart failure due to safety concerns.37 Abatacept might be a safer option compared to TNF inhibitors, particularly for older RA patients with CVD.38 IL-6 inhibitors have been shown to inhibit atherosclerosis.35 A high dose of tofacitinib is linked to a higher incidence of pulmonary embolism compared to TNF inhibitors in patients aged 50 years and older with at least one CVD risk factor.39
When comparing treatment patterns in RA patients with or without CVD, NSAID use was low in both groups, and around 80% of patients in both groups using prednisone 5 mg or less. Patients with CVD were less likely to use MTX compared to those without CVD, with LEF being the preferred alternative due to its efficacy, ease of use, and safety profile, particularly in mild renal impairment, since patients with CVD had significantly higher creatinine levels and higher CKD rates. The use of biological treatments was similar between patients with and without CVD, with TNF inhibitor etanercept being the most commonly used drug due to its safety profile. Notably, patients with lower ejection fraction did not receive TNF inhibitor treatment, possibly due to concerns about worsening cardiac function. Tofacitinib and baricitinib were not used in patients with CVD risk factors due to safety concerns.
The main limitation is retrospective nature of the study. Real-world data, despite its retrospective nature in this study, holds significant value in understanding the practical implications of disease management and outcomes in a diverse patient population. Unlike controlled clinical trials, real-world data captures the complexities of patient care in routine clinical practice, offering insights into the effectiveness and challenges of treatment strategies in a broader patient population. While prospective, multi-center registries are crucial for examining real-world data comprehensively, experience from everyday clinical practice at a single center over an extended period is also valuable. This study contributes to the body of evidence by providing insights into the real-world management and outcomes of RA patients with CVD risk factors.
Another limitation is the lack of calculated RA disease activity scores or CRP levels at each visit due to missing data. Only the latest CRP level is reported, which may explain the similar CRP levels in RA patients and control groups, as most RA patients were in remission at their last visit.
Additionally, data on confounding lifestyle factors, such as dietary habits40 and physical activity levels,41 were not consistently available in patient records due to the retrospective design. Consequently, their effects could not be measured or analyzed in this study. Future prospective studies could more comprehensively examine these lifestyle factors alongside clinical data.
Conclusion
This study highlights the significantly increased risk of CVD in patients with RA. The interplay between chronic systemic inflammation and traditional CVD risk factors necessitates comprehensive management strategies that address both RA and CVD to reduce the substantial burden on these patients. These findings underscore the importance of early and aggressive treatment of RA and regular cardiovascular monitoring. Further research is warranted to enhance our understanding of the mechanisms linking RA to CVD and to develop optimized treatment protocols to improve clinical outcomes for RA patients with heightened CVD risk.
Funding
No funding was received for this study.
Disclosure
The author declares no conflict of interest.
References
1. Logstrup BB, Ellingsen T, Pedersen AB, et al. Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology(Oxford). 2021;60(3):1400–1409. doi:10.1093/rheumatology/keaa374
2. Meune C, Touzé E, Trinquart L, Allanore Y. Trends in cardiovascular mortality in patients with rheumatoid arthritis over 50 years: a systematic review and meta-analysis of cohort studies. Rheumatology. 2009;48(10):1309–1313. doi:10.1093/rheumatology/kep252
3. Mantel A, Holmqvist M, Andersson DC, Lund LH. Askling J Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69(10):1275–1285. doi:10.1016/j.jacc.2016.12.033
4. Crowson CS, Rollefstad S, Ikdahl E, et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis. 2018;77(1):48–54. doi:10.1136/annrheumdis-2017-211735
5. Raadsen R, Agca R, Boers M, et al. In RA patients without prevalent CVD, incident CVD is mainly associated with traditional risk factors: a 20-year follow-up in the CARRÉ cohort study. Semin Arthritis Rheum. 2023;58:152132. doi:10.1016/j.semarthrit.2022.152132
6. Arts EE, Popa C, Den Broeder AA, et al. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann Rheum Dis. 2015;74(4):668–674. doi:10.1136/annrheumdis-2013-204024
7. Peters MJ, Symmons DP, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis. 2010;69(2):325–331. doi:10.1136/ard.2009.113696
8. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. 2023;82(1):3–18. doi:10.1136/ard-2022-223356
9. De Vecchis R, Baldi C, Palmisani L. Protective effects of methotrexate against ischemic cardiovascular disorders in patients treated for rheumatoid arthritis or psoriasis: novel therapeutic insights coming from a meta-analysis of the literature data. Anatol J Cardiol. 2016;16(1):2–9. doi:10.5152/akd.2015.6136
10. Rempenault C, Combe B, Barnetche T, et al. Metabolic and cardiovascular benefits of hydroxychloroquine in patients with rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2018;77(1):98–103. doi:10.1136/annrheumdis-2017-211836
11. Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74(3):480–489. doi:10.1136/annrheumdis-2014-206624
12. Hansildaar R, Vedder D, Baniaamam M, Tausche AK, Gerritsen M, Nurmohamed MT. Cardiovascular risk in inflammatory arthritis: rheumatoid arthritis and gout. Lancet Rheumatol. 2021;3(1):e58–e70. doi:10.1016/S2665-9913(20)30221-6
13. Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31(3):315–324. doi:10.1002/art.1780310302
14. Aletaha D, Neogi T, Silman AJ, et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
15. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
16. Ridker PM, Buring JE, Shih J, Matias M, Hennekens CH. Prospective study of C-reactive protein and the risk of future cardiovascular events among apparently healthy women. Circulation. 1998;98(8):731–733. doi:10.1161/01.CIR.98.8.731
17. Tyczyńska KM, Augustyniak-Bartosik H, Świerkot J. Rheumatoid arthritis - medication dosage in chronic kidney disease. Reumatologia. 2023;61(6):481–491. doi:10.5114/reum/177005
18. Choy E, Ganeshalingam K, Semb AG, Szekanecz Z, Nurmohamed M. Cardiovascular risk in rheumatoid arthritis: recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology. 2014;53(12):2143–2154. doi:10.1093/rheumatology/keu224
19. Risk Factors Collaboration, E Kaptoge S, Di Angelantonio E. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140.
20. Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis. 2011;70(6):929–934. doi:10.1136/ard.2010.143396
21. Rho YH, Chung CP, Oeser A, et al. Inflammatory mediators and premature coronary atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2009;61(11):1580–1585. doi:10.1002/art.25009
22. Solomon DH, Reed GW, Kremer JM, et al. Disease activity in rheumatoid arthritis and the risk of cardiovascular events. Arthritis Rheumatol. 2015;67(6):1449–1455. doi:10.1002/art.39098
23. Sandoo A, Chanchlani N, Hodson J, Smith JP, Douglas KM, Kitas GD. Classical cardiovascular disease risk factors associate with vascular function and morphology in rheumatoid arthritis: a six-year prospective study. Arthritis Res Ther. 2013;15(6):R203. doi:10.1186/ar4396
24. Sugiyama D, Nishimura K, Tamaki K, et al. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Ann Rheum Dis. 2010;69(1):70–81. doi:10.1136/ard.2008.096487
25. Jankowski J, Floege J, Fliser D, Bohm M, Marx N. Cardiovascular Disease in Chronic Kidney Disease: pathophysiological Insights and Therapeutic Options. Circulation. 2021;143(11):1157–1172. doi:10.1161/CIRCULATIONAHA.120.050686
26. Fine NM, Crowson CS, Lin G, Oh JK, Villarraga HR, Gabriel SE. Evaluation of myocardial function in patients with rheumatoid arthritis using strain imaging by speckle-tracking echocardiography. Ann Rheum Dis. 2014;73(10):1833–1839. doi:10.1136/annrheumdis-2013-203314
27. Liang KP, Myasoedova E, Crowson CS, et al. Increased prevalence of diastolic dysfunction in rheumatoid arthritis. Ann Rheum Dis. 2010;69(9):1665–1670. doi:10.1136/ard.2009.124362
28. Maradit-Kremers H, Crowson CS, Nicola PJ, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2005;52(2):402–411. doi:10.1002/art.20853
29. Ungprasert P, Srivali N, Kittanamongkolchai W. Risk of incident atrial fibrillation in patients with rheumatoid arthritis: a systematic review and meta-analysis. Int J Rheum Dis. 2017;20(4):434–441. doi:10.1111/1756-185X.12820
30. Lazzerini PE, Capecchi PL, Acampa M, Galeazzi M, Laghi-Pasini F. Arrhythmic risk in rheumatoid arthritis: the driving role of systemic inflammation. Autoimmun Rev. 2014;13(9):936–944. doi:10.1016/j.autrev.2014.05.007
31. Varga Z, Sabzwari SRA, Vargova V. Cardiovascular Risk of Nonsteroidal Anti-Inflammatory Drugs. An Under-Recog Public Health Issue Cureus. 2017;9(4):e1144.
32. Pujades-Rodriguez M, Morgan AW, Cubbon RM, Wu J, Rahimi K. Dose-dependent oral glucocorticoid cardiovascular risks in people with immune-mediated inflammatory diseases: a population-based cohort study. PLoS Med. 2020;17(12):e1003432. doi:10.1371/journal.pmed.1003432
33. Westlake SL, Colebatch AN, Baird J, et al. The effect of methotrexate on cardiovascular disease in patients with rheumatoid arthritis: a systematic literature review. Rheumatology. 2010;49(2):295–307. doi:10.1093/rheumatology/kep366
34. Hage MP, Al-Badri MR, Azar ST. A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Ther Adv Endocrinol Metab. 2014;5(4):77–85. doi:10.1177/2042018814547204
35. Baoqi Y, Dan M, Xingxing Z, et al. Effect of Anti-Rheumatic Drugs on Cardiovascular Disease Events in Rheumatoid Arthritis. Front Cardiovasc Med. 2022;8:812631. doi:10.3389/fcvm.2021.812631
36. Barnabe C, Martin BJ, Ghali WA. Systematic review and meta-analysis: anti-tumor necrosis factor α therapy and cardiovascular events in rheumatoid arthritis. Arthritis Care Res. 2011;63(4):522–529. doi:10.1002/acr.20371
37. Chung ES, Packer M, Lo KH, Fasanmade AA, Willerson JT. Anti-TNF Therapy Against Congestive Heart Failure Investigators. Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF Therapy Against Congestive Heart Failure (ATTACH) trial. Circulation. 2003;107(25):3133–3140. doi:10.1161/01.CIR.0000077913.60364.D2
38. Jin Y, Kang EH, Brill G, Desai RJ, Kim SC. Cardiovascular (CV) Risk after Initiation of Abatacept versus TNF Inhibitors in Rheumatoid Arthritis Patients with and without Baseline CV Disease. J Rheumatol. 2018;45(9):1240–1248. doi:10.3899/jrheum.170926
39. Mease P, Charles-Schoeman C, Cohen S, et al. Incidence of venous and arterial thromboembolic events reported in the tofacitinib rheumatoid arthritis, psoriasis and psoriatic arthritis development programmes and from real-world data. Ann Rheum Dis. 2020;79(11):1400–1413. doi:10.1136/annrheumdis-2019-216761
40. Hulander E, Bärebring L, Turesson Wadell A, et al. Diet intervention improves cardiovascular profile in patients with rheumatoid arthritis: results from the randomized controlled cross-over trial ADIRA. Nutr J. 2021;20(1):9. doi:10.1186/s12937-021-00663-y
41. Malani K, Pradhan S, Roberts M, et al. Joint effect of rheumatoid arthritis and diet quality on cardiovascular and mortality outcomes: insights from the Women’s Health Initiative. Clin Rheumatol. 2024;43(10):3089–3104. doi:10.1007/s10067-024-07092-2
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Association of Elevated Thyroid Stimulating Hormone with Atherosclerotic Cardiovascular Disease and Its Mortality in Elderly Community-Dwelling Chinese
Wang Y, Liu C, Liu L, Chen X, Wei L, Liu J, Peng S, Pi J, Zhang Q, Tomlinson B, Chan P, Zhang L, Fan H, Zheng L, Liu Z, Zhang Y
Clinical Interventions in Aging 2022, 17:1139-1150
Published Date: 2 August 2022
Comorbid Heart Disease in Patients with COPD is Associated with Increased Hospitalization and Mortality – A 15-Year Follow-Up
Giezeman M, Sundh J, Athlin Å, Lisspers K, Ställberg B, Janson C, Montgomery S, Kisiel MA, Nager A, Sandelowsky H, Hasselgren M
International Journal of Chronic Obstructive Pulmonary Disease 2023, 18:11-21
Published Date: 9 January 2023
The Relationship Between the Neutrophil Percentage-to-Albumin Ratio and Rates of 28-Day Mortality in Atrial Fibrillation Patients 80 Years of Age or Older
Cai J, Li M, Wang W, Luo R, Zhang Z, Liu H
Journal of Inflammation Research 2023, 16:1629-1638
Published Date: 17 April 2023
Current Trends in Comorbidity Prevalence and Associated Mortality in a Population-Based Cohort of Hip Fracture Patients in Denmark
Kristensen PK, Hjelholt TJ, Madsen M, Pedersen AB
Clinical Epidemiology 2023, 15:839-853
Published Date: 18 July 2023
Patient Characteristics and Clinical and Economic Outcomes Associated with Unplanned Medical and Surgical Intensive Care Unit Admissions: A Retrospective Analysis
Khanna AK, Moucharite MA, Benefield PJ, Kaw R
ClinicoEconomics and Outcomes Research 2023, 15:703-719
Published Date: 25 September 2023

