Back to Journals » Infection and Drug Resistance » Volume 18
Prevalence of Site-Specific Mycoplasma genitalium Infection and Macrolide and Fluoroquinolone-Associated Mutations in Men Who Have Sex with Men in Shenzhen, China
Authors Leng X, Zhu R, Ao X, Zhou Y, Zhang K, Hu T, Wu J, Chen Z, Huang L, Huang N, Li X, Ahmed Alnour R , Xue Z , Zhang X , Liu H , Axirejiang T, Ke W, Zou H
Received 14 September 2024
Accepted for publication 12 November 2024
Published 13 January 2025 Volume 2025:18 Pages 239—252
DOI https://doi.org/10.2147/IDR.S489403
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Xinying Leng,1,* Rui Zhu,2,* Xian Ao,1 Ying Zhou,3 Kechun Zhang,4 Tian Hu,4 Jiaxin Wu,1 Zhaoqi Chen,1 Lixia Huang,1 Nanxuan Huang,1 Xinyuan Li,1 Ruaa Ahmed Alnour,1 Zhantu Xue,1 Xiangcai Zhang,1 Han Liu,1 Tuerhongjiang Axirejiang,5 Wujian Ke,1,2 Huachun Zou6
1Dermatology Hospital, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 2The First School of Clinical Medicine, Southern Medical University, Guangzhou, Guangdong, People’s Republic of China; 3Affiliated Hospital of Guangdong Medical University, Zhanjiang, Guangdong, People’s Republic of China; 4Longhua District Center for Disease Control and Prevention, Shenzhen, Guangdong, People’s Republic of China; 5The First People’s Hospital of Kashi Prefecture, Kashi, Xinjiang, People’s Republic of China; 6School of Public Health, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huachun Zou, School of Public Health, Fudan University, Shanghai, People’s Republic of China, Email [email protected] Wujian Ke, The First School of Clinical Medicine, Southern Medical University, Email [email protected]
Background: Mycoplasma genitalium (MG) poses a growing public health concern due to the escalating antimicrobial resistance. We aimed to assess site-specific MG infection and its correlates and macrolide and fluoroquinolones mutations among men who have sex with men (MSM) in Shenzhen, China.
Methods: Samples were obtained from different anatomic sites of MSM based on their sexual behavior. MG infection was detected using nested polymerase chain reaction (nested PCR). Identifying macrolide and fluoroquinolone resistance involved targeting the V region of the 23S rRNA, topoisomerase IV and DNA gyrase genes. Logistic regression was used to evaluate correlates of MG infection.
Results: We collected 124 pharynx swabs, 132 urethral swabs, and 89 rectal swabs from 162 MSM participants based on their sexual behavior. MG was detected in 13.0% (21/162) of MSM. The prevalence of MG in the pharynx, urethra, and rectum was 9.7% (12/124), 6.1% (8/132), and 7.9% (7/89), respectively. Among the 21 MG-positive participants, 4.8% (1/21) were infected at all three sites, and 19.0% (4/21) were infected at two sites. Of the 27 MG-positive specimens, 22.2% (2/9) exhibited mutations at position A2071G, with A2071T being the predominant mutation in the 23S rRNA gene, accounting for 77.8% (7/9) of cases. Mutations in the parC and gyrA genes were detected in 33.3% (1/3) and 33.3% (2/6) of specimens, respectively.
Conclusion: We observed a high prevalence of MG infections at different anatomic sites among the MSM population in Shenzhen, China. The high prevalence of macrolide and fluoroquinolone-resistant MG underscores the importance of implementing resistance-guided therapy, establishing surveillance networks, and exploring new antibiotics against MG.
Keywords: antimicrobial resistance, Mycoplasma genitalium, MSM, macrolide, fluoroquinolone
Background
Mycoplasma genitalium (MG) is recognized as a causative agent of non-gonococcal urethritis (NGU) and has the potential to impair male fertility by affecting sperm DNA integrity.1–3 Risk factors associated with MG infection among MSM encompass elements such as younger age, inconsistent condom use during receptive anal sex, higher numbers of sexual partners, engagement in chemsex, stable partnerships with increased condomless sex, and unprotected anal intercourse within the past six months, especially in individuals living with HIV.4–7 While the prevalence of MG in the general population typically ranges from 1% to 2%,8–10 in MSM populations, rates have been reported as high as 9.5% to 10.5%.11,12 The available data on MG prevalence at various anatomical sites within the MSM population in China can vary significantly. Reported figures indicate a prevalence of 5.4% in the rectal site in Shenzhen at 20106, 11.8% in Jiangsu during 2008 to 200913 and 15.3% in Shenyang at 2018;14 For the urethral site, the data shows a prevalence of 3.4% in Shenzhen at 2010,6 17.2% in non-HIV individuals in Jiangsu during 2008 to 2009,13 and 25.5% in HIV-1 infected MSM in Jiangsu during 2009 to 2010.15 Pharyngeal prevalence of MG has been reported as high as 13.5% in Jiangsu during.2008−200913 However, it is important to note that despite the potential for pharyngeal sites to serve as reservoirs for MG transmission, most published data have focused on estimating urethral and rectal MG infections, with comparatively limited data available for pharyngeal sites.
The emergence of drug-resistant MG strains has become a frequent concern globally,16 with heightened apprehension regarding macrolide and fluoroquinolones (FQs) resistance, particularly in the Western Pacific Region.16 While guidelines frequently recommend azithromycin as a first-line therapy for MG, macrolide resistance is progressively widespread and escalating in many countries. Even a single nucleotide polymorphism (SNP) at positions 2071 (E. coli numbering 2058) or 2072 (E. coli numbering 2059) within the 23S rRNA gene can lead to macrolide resistance.17,18 Notably, macrolide resistance appears to be more prevalent in specimens from MSM16,19, with reported rates as high as 95.4% in France,20 79.9% in Berlin,21 88.4% in Belgium,22 84.2–89.7% in Australia4,23 and 70.6–80.0% in America.24 Moreover, treatment failure with FQs can be attributed to mutations in the quinolone resistance-determining regions (QRDR) of topoisomerase IV and DNA gyrase genes, predominantly in the parC and gyrA genes. Concurrent gyrA mutations further elevate the risk of treatment failure.25 Resistance to FQs in the MSM population has been less studied and has been estimated at rates ranging from 13% in Berlin,21 24.2% in Belgium,22 to 33.3% in Dublin.26 The efficacy of macrolides and FQs has declined worldwide due to the dissemination of resistant MG strains, leaving limited alternatives for treatment.
Data on macrolide and FQs resistance genes of MG in China are predominantly derived from sexually transmitted disease (STD) clinics, with reported rates of 88.9% and 89.5% in Nanjing, 66.4% and 77.7% in Guangzhou, and 23.1% and 65.0% in Hong Kong among patients in STD clinics,27–29 it is important to note that the antimicrobial resistance (AMR) statistics in China are strikingly high. However, there is limited data available to assess AMR of MG at various anatomical sites among MSM in China. The objectives of this study are threefold: (1) to investigate the prevalence of MG infection at the pharynx, urethra, and rectum; (2) to identify potential risk factors for MG infection, including demographics and sexual behavior; and (3) to screen for antimicrobial resistance associated with macrolides and FQs among MSM in Shenzhen, China.
Methods
Sample Collection
Between June 2020 and December 2020, participants were recruited at the Shenzhen Longhua District Center for Disease Control and Prevention. To be eligible for participation in this study, patients had to be: a. at least 18 years old; b. agree to complete an online questionnaire; c. receive at least one site detection for MG detection (pharyngeal, urethral and/or rectal swab). Exclusion criteria included: a. under the age of 18; b. those with severe visual, hearing or cognitive dysfunction who cannot complete the questionnaire; c. refuse the sample collection. The recruitment methods of our study are as follows: 1) Recruitment by CDC of Shenzhen Longhua District: Posters were displayed at the Longhua CDC health check-up center to promote the project, facilitating eligible MSM to join the study; promotional activities were conducted at the VCT site at Longhua CDC and among MSM populations already included online, encouraging participation;
2) Recruitment through MSM social media and dating apps: Popular dating apps widely used by the domestic MSM community, such as Blued (with over 27 million registered users) and Zank, were utilized to create dedicated accounts for this study. The apps were used to search for target populations in Shenzhen with set conditions. If individuals met the inclusion and exclusion criteria for the project, they were invited to participate; 3) Recruitment at MSM gathering spots: Posters were displayed at places frequently visited by MSM, such as barbecue bars and nightclubs, to promote the study. Personnel were also arranged to visit these locations on weekends for on-site promotion and recruitment; 4) Snowball recruitment: Participants in the study were able to refer other eligible MSM individuals to join the study.
It took approximately 10 minutes for participants to complete an online questionnaire on demographics, sexual behavior preference, and symptoms onset in the previous week. Fixed partner in the questionnaire refers to a committed or steady sexual partner, often in contrast to casual or non-monogamous sexual relationships. “Recreational drugs” refers to illegal drugs used in the context of sexual activity, such as nitrite inhalants (commonly known as rush), methamphetamine (crystal meth), and ecstasy (MDMA). After completing the questionnaire, a trained volunteer collected swabs from at least one of the sites (pharyngeal, urethral, and rectal swabs) from participants according to their sexual behavior. For instance, the volunteer will collect the rectal swab if the participants perform exclusively receptive anal sex. If the participants perform versatile sexual behavior (eg receive and give both oral and anal sex), the volunteer will collect all three sites. The participants could get free consultations from a doctor specializing in sexually transmitted diseases (STDs).
MG Detection and Sequence Analysis of Samples
MG was detected via nested polymerase chain reaction (PCR) as described by Jiang J et al13 (Supplementary Table 1). MG-positive samples were sent to Sangon Biotech Co. for detection of macrolide and FQs resistance mediating mutations on the same day or stored at − 20 °C until molecular analysis of resistance (within 4 weeks after sampling). DNA is extracted using a DNA extraction kit (Sangon Biotech Co). From these extracted DNA, mutations associated with macrolide resistance were detected using primers targeting region V of the 23S rRNA gene. FQs resistance mutations in the gyrA and parC genes were screened using primers as reported previously.28 PCR amplification of the V region of 23S rRNA, parC and gyrA sequence was performed as in a previous study28 (Supplementary Table 2).
Data Analyses
Descriptive analyses (demographics and sexual behavior) were performed on IBM SPSS Statistics for OS Version 26.0 (IBM Corp, New York, USA). Univariate logistic regressions were conducted to assess whether participants’ demographics and sexual behavior could be associated with the detection of MG. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were estimated. Only significant variables with a P value <0.20 in univariate analysis were included in the multivariate logistic regression model. The adjusted odds ratio (aOR) and its corresponding 95% confidence interval (CI) were calculated, and P < 0.05 was considered statistically significant. The mutation sequencing data in 23S rRNA, gyrA and parC genes from MG-positive DNA specimens were analyzed with the software program BioEdit (http://www.mbio.ncsu.edu/bioedit/bioedit.html). The obtained sequences were aligned against corresponding parts of the genome sequence of MG strain G37 (GenBank accession no. NC_000908.2). Amino acid changes in the QRDRs of the gyrA and parC genes in this study were compared with previous studies.28
Ethics Approval
The study adheres to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Shenzhen Longhua District Center for Disease Control and Prevention (No. 2020005). Written information and consent were obtained from all participants, and we used a private phone specially for this study to inform the participants of the test result. The test result would be uncovered only when participants themselves receive the call.
Results
Socio-Demographic Characteristics
A total of 169 MSM were initially recruited for the study. However, 7 participants were excluded due to questionnaire loss, resulting in the analysis based on the remaining 162 participants. The median age of the participants was 29 years (range 20–57), with a majority (89.5%) identifying as Han ethnicity. Educational attainment varied, with 77.8% of participants having completed senior high school or lower, while 19.1% held a Bachelor’s degree, and 3.1% possessed a Master’s degree. Most participants (83.3%) reported their marital status as single. In terms of sexual orientation, 59.3% identified as homosexual, 2.5% as heterosexual, and 32.1% as bisexual. A small proportion (6.2%) chose not to disclose their sexual orientation. The median age for first oral or anal sexual encounters with men was 21 years (range 8–45) and 22 years (range 13–43), respectively. The minimum reported ages for first oral and anal sexual encounters were 8 and 13, respectively. About 11.7% of participants reported engaging in their first oral sex experience before the age of 18, while 8.6% reported the same for anal sex. A significant portion of participants reported recent symptoms in the pharynx (17.9%), urethra (18.5%), and anorectum (22.2%), including symptoms like itch, discomfort, discharge, dysuria, or bleeding (Table 1).
 |
Table 1 Demographic and Behavioral Characteristics of MSM in Shenzhen, China (n = 162) |
Sexual Behaviors and Potential Risk Factors Associated with MG Infection
Among the 162 MSM participants, 38.3% reported having a fixed sex partner. In our study, an estimated 26.5% of participants reported consistent condom use when engaging in sexual activity with a fixed partner. Specifically, 29.6% of MSM reported consistent condom use during oral sex and 42.6% reported consistent condom for anal sex. A noteworthy 8.6% of MSM had previously engaged in commercial sex, and 22.2% reported having multiple sexual partners in the past 12 months. Additional details regarding participants’ sexual behaviors are available in Table 1. No significant risk factors, such as demographic or sexual behavior, were found to be associated with MG infection (Table 2).
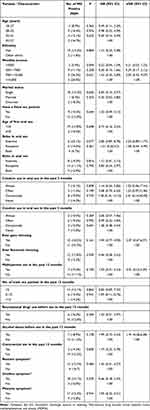 |
Table 2 Correlates of Mycoplasma Genitalium Positivity in MSM in Shenzhen, China (n = 162) |
The Prevalence of MG Among MSM
In our study, each of the 162 MSM participants underwent testing at least one anatomical site according to their self-reported sexual behavior. A total of 124 pharyngeal swabs, 132 urethral swabs, and 89 rectal swabs were collected from these participants, ensuring comprehensive testing. MG was detected in 13.0% (21/162) of the participants, and the prevalence of MG at different anatomical sites was as follows: a) MG was detected in 9.7% (12/124) of pharyngeal swabs. b) MG was found in 6.1% (8/132) of urethral swabs. c) The prevalence of MG in rectal swabs was 7.9% (7/89). Among the 21 MG-positive participants, 1 individual was infected at all three anatomical sites, and 4 participants were found to be infected at 2 of the tested sites (Figure 1).
 |
Figure 1 Summary of Mutations Detected in 23S rRNA, parC, and gyrA for Mycoplasma genitalium Isolates from Various Anatomical Sites of 169 MSM in Shenzhen, China. |
Antimicrobial Resistance Mutations in MG
27 MG-positive specimens (out of 21 MG-positive participants) were collected and available for the detection of mutations associated with resistance to macrolides and FQs (Table 3). In terms of amplicon production for detecting resistance mutations, 33.3% (9/27) of specimens had sufficient amplicons for the 23S rRNA gene, 11.1% (3/27) for the parC gene, and 22.2% (6/27) for the gyrA gene. Among the specimens for which sequencing data were available, 77.8% (7/9) displayed an A2071T single nucleotide polymorphism (SNP), while 22.2% (2/9) exhibited an A2071G SNP. For the three successfully amplified parC genes, 66.7% (2/3) were identified as having a wild-type parC sequence, while 33.3% (1/3) displayed a G241T SNP, leading to alterations in the amino acid at position 81 (Gly81Cys). This specimen that carried the G241T mutation in parC concurrently exhibited macrolide resistance (A2071T). Regarding the six successfully amplified gyrA genes, 66.7% (4/6) of specimens had a wild-type gyrA sequence, while 33.3% (2/6) harbored mutations in the gyrA genes. Specifically, 16.7% (1/6) displayed a base missing at position 428 (C428-), and another 16.7% (1/6) contained two SNPs (C353G and A256G), resulting in alterations in the amino acids at positions 118 (Ala118Gly) and 86 (Met86Val), respectively. Notably, all two specimens with gyrA SNPs exhibited concurrent macrolide resistance (A2071T).
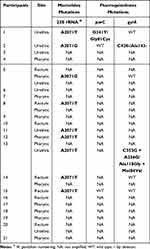 |
Table 3 Resistance-Associated Mutations to Macrolides and Fluoroquinolones in Mycoplasma Genitalium Isolates from Various Anatomical Sites of 21 MSM in Shenzhen, China |
Discussion
In recent years, increased scholarly attention has been directed towards the prevalence of MG infections among MSM. A 2021 meta-analysis conducted in China revealed a notable MG infection rate of 9.7% among the Chinese MSM population,12 a figure consistent with findings from an Australian study involving 1001 MSM, wherein 9.5% tested positive for MG.4 Additionally, a 2021 French study investigating MG infection and drug resistance among MSM reported a baseline MG infection rate of approximately 10.5% within the MSM community.11 However, our study conducted in Shenzhen, involving 162 MSM, identified an even higher MG infection rate of 13.0% (21/162). This rate surpasses those observed in China, Australia, and France, underscoring a pronounced MG prevalence among MSM in Shenzhen.4,11,12 These findings emphasize the imperative for intensified interventions aimed at curbing MG infection rates within this specific demographic.
Diverging from conventional blood testing practices, the collection of MG samples in clinical settings hinges on patients’ sexual behavior histories, notably involving activities like active oral/anal sex affecting the oropharynx/rectal area. Consequently, detecting MG infection entails sampling from diverse anatomical sites. Studies indicate variability in MG infection rates across these sites. An Australian study observed infection rates of 2.7% in urine samples and 7.0% in rectal samples among MSM.4 Similarly, a French study reported rates of 6.3% in urine, 4.3% in rectal swabs, and 0.5% in pharynx swabs.11 In a 2014 cross-sectional study in Shenzhen, China, the prevalence of MG among MSM stood at 8.1%, with rectal positivity at 5.4% and urine positivity at 3.4%.6 However, a 2018 cross-sectional study in Shenyang, China, found a notably higher rectal MG infection rate among MSM at 15.3%,14 approximately threefold to Shenzhen (5.4%),6 France (4.3%),11 and Italy (4.8%).30 Our investigation revealed MG infection rates of 6.1% (8/132) in urethral swabs, 7.9% (7/89) in rectal swabs, and 9.7% (12/124) in pharyngeal swabs. Notably, the prevalence of MG at urethral swabs was lower than in France in 2021 but higher than in Australia in 2017 and Shenzhen in 2010; at rectal swabs was lower than in Shenyang in 2018 but higher than in Shenzhen in 2010, France in 2021 and Italy in 2017; and pharyngeal swabs significantly exceeded that of France in 2021. These findings underscore a high prevalence of pharyngeal MG infection in Shenzhen, emphasizing the need for focused preventive measures to curb MG infections in this specific anatomical region.
Findings from a 2018 national cross-sectional study in Germany underscored several key risk factors for STDs among MSM.31 These factors included having more than 5 male sex partners within 6 months, engaging in unprotected sex, using recreational drugs, being HIV positive, and utilizing Pre-Exposure Prophylaxis (PrEP). Notably, younger age emerged as a significant independent risk factor for infection.31 Similarly, a 2020 clinical study conducted in Sydney revealed that MSM individuals on PrEP were twice as likely to be infected by MG compared to those not on this preventive regimen.32 While previous studies have suggested that sexual behaviors such as early sexual age, a high number of sexual partners, infrequent condom use during sexual activity, and the consumption of alcohol or drugs before sex could be potential risk factors for MG infection, our study did not identify significant associations, possibly due to the relatively small sample size.
Tetracyclines (such as doxycycline), macrolides (such as azithromycin), and FQs (such as moxifloxacin) constitute the primary treatment options for MG. However, a concerning trend has emerged in recent years regarding increased resistance among MSM-infected patients to macrolides and FQs. The escalating rates of macrolide-resistant MG and the diminishing efficacy of azithromycin over time, coupled with mounting reports of resistance to the second-line antibiotic moxifloxacin, signal significant challenges in MG treatment. A systematic review detailing global MG drug resistance highlighted an alarming surge in macrolide resistance, soaring from 10% before 2010 to 51% in.2016−201716 Regional disparities were evident, with the Americas (0% to 67%) and Australia (18.8% to 66.0%) experiencing the most drastic increases.16 FQs resistance stood at 7.7%, notably higher in the Western Pacific region compared to the European region.16 Moreover, MSM cohorts exhibit higher macrolide resistance rates than heterosexual men. A study in southern Spain demonstrated macrolide and FQs resistance rates of 62.5% and 16.7% among MSM, whereas non-MSM populations showed rates of 26.6% and 10.0%, respectively.33 Notably, macrolide consumption among MSM was significantly higher than in non-MSM groups. In a Chinese study on MG in gay sex workers, macrolide resistance reached 83.0%, with 79.8% and 21.1% of the parC and gyrA genes showing potential FQs resistance mutations, respectively.34 Our findings reveal concerning resistance rates among MSM populations, with 100.0% (9/9) and 33.3% (3/9) exhibiting resistance to macrolides and FQs, respectively. Of these, 33.3% (1/3) displayed parC mutations, and 33.3% (2/6) exhibited gyrA mutations. This highlights an alarming scenario of MG drug resistance in Shenzhen. Despite limited research, this concerning trend demands immediate attention and calls for targeted strategies to mitigate the spread of drug-resistant strains.
Consistency in MG treatment guidelines across different countries remains elusive. European guidelines advocate azithromycin (500 mg on the first day, followed by 250 mg daily for the next 4 days) as first-line treatments for uncomplicated MG infection, with moxifloxacin (400 mg daily for 7–10 days) as the second line. Doxycycline (100 mg twice daily for 14 days) or pristinamycin (1g four times daily for 10 days) are recommended if initial and secondary treatments fail, while complicated cases suggest moxifloxacin (400 mg daily for 14 days).3 The US CDC recommends doxycycline (100 mg twice daily for 7 days) followed by azithromycin (1g for the first day, then 250 mg daily for the next 4 days). Moxifloxacin (400 mg once daily for 7 days) as an alternative for macrolide-resistant cases.35 British guidelines propose a sequential regimen involving doxycycline (100 mg twice daily for a week) followed by azithromycin (1g, 500 mg, and 500 mg orally on the first, second and third days, respectively).36 For resistant or complicated cases, the regimen aligns with European guidelines. A recent Melbourne study on MG urethritis highlighted its prevalence among MSM (39%).37 Notably, MSM displayed nearly double the macrolide resistance compared to women or heterosexual men (76% in MSM versus 39% in women and heterosexual men combined).37 This discrepancy may arise from concurrent treatment of other STIs in MSM, inadvertently exposing MG to azithromycin, escalating macrolide resistance, and hindering subsequent treatments. Despite doxycycline’s limited efficacy and low microbial cure rates, sequential therapy with it reduces bacterial load, potentially enhancing success rates with moxifloxacin.35 A domestic study assessing doxycycline-moxifloxacin sequential therapy revealed an overall efficacy of 83.3%.38 The 16.7% failure rate is potentially linked to antibiotic misuse. Therefore, clinicians should prioritize sensitive antibacterial drugs to curb MG’s drug resistance development and enhance treatment effectiveness. All of the MG-positive participants (21/21) received a 14-day doxycycline regimen. Only 19.0% (4/21) of participants returned for the test of cure (TOC) 2 weeks after treatment and all 4 participants were cured. No significant correlates of MG infection were identified. This absence of MG in the reexamined patients prompts a crucial need for further validation, particularly regarding the suitability of doxycycline in treating MG infection among MSM. Studies have found that the microbiological cure rate of minocycline, a tetracycline antibiotic, in patients with macrolide resistance is approximately 68%–71%.39,40 Microbial cure was defined as a negative TOC 14–90 days after therapy in an Australian study.41 However, it is possible that TOC was performed too soon to generate false-negative results. Few data exist to inform the optimal time to conduct TOC3. Current limited evidence suggests that performing a TOC sooner than three weeks after treatment may generate false-negative results because of the initial fall but then rise in organism load following the development of macrolide resistance mutations.3 Our study has several limitations that should be noted. First, the inclusion and exclusion criteria were not fully comprehensive. For instance, HIV infection and co-infections with other sexually transmitted pathogens, which may increase susceptibility to MG among MSM, were not accounted for, potentially influencing our results. Additionally, a history of STD treatment within the 3–6 months before sample collection could have been included as an exclusion criterion, as recent treatment may affect the study’s findings. Our study did not identify significant associations of MG infection, possibly due to the relatively small sample size.
Conclusion
Our study of MSM in Shenzhen revealed a high MG infection rate and resistance rates to macrolides and fluoroquinolones, highlighting the need for intensified interventions. We also observed anatomical variations in infection, with a notably high prevalence of pharyngeal infection, pointing to the need for targeted prevention efforts. Remarkably, doxycycline treatment in macrolide-resistant cases led to undetectable MG upon follow-up. This suggests doxycycline’s potential effectiveness against MG in MSM, underscoring the need for larger studies to confirm these results.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Qing L, Song QX, Feng JL, et al. Prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium and Ureaplasma infections using a novel isothermal simultaneous RNA amplification testing method in infertile males. Ann Clin Microbiol Antimicrob. 2017;16(1):45. doi:10.1186/s12941-017-0220-2
2. Idahl A, Jurstrand M, Olofsson JI, et al. Mycoplasma genitalium serum antibodies in infertile couples and fertile women: table 1. Sex Transm Infect. 2015;91(8):589–591. doi:10.1136/sextrans-2015-052011
3. Jensen JS, Cusini M, Gomberg M, et al. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36(5):641–650. doi:10.1111/jdv.17972
4. Read TRH, Murray GL, Danielewski JA, et al. Symptoms, sites, and significance of Mycoplasma genitalium in men who have sex with men. Emerg Infect Dis. 2019;25(4):719–727. doi:10.3201/eid2504.181258
5. Ring A, Balakrishna S, Imkamp F, et al. High rates of asymptomatic Mycoplasma genitalium infections with high proportion of genotypic resistance to first-line macrolide treatment among men who have sex with men enrolled in the Zurich Primary HIV Infection Study. Open Forum Infect Dis. 2022;9(6):ofac217. doi:10.1093/ofid/ofac217
6. Zheng BJ, Yin YP, Han Y, et al. The prevalence of urethral and rectal Mycoplasma genitalium among men who have sex with men in China, a cross-sectional study. BMC Public Health. 2014;14:195. doi:10.1186/1471-2458-14-195
7. Latimer RL, Shilling HS, Vodstrcil LA, et al. Prevalence of Mycoplasma genitalium by anatomical site in men who have sex with men: a systematic review and meta-analysis. Sex Transm Infect. 2020;96(8):563–570. doi:10.1136/sextrans-2019-054310
8. Andersen B, Sokolowski I, Ostergaard L, et al. Mycoplasma genitalium: prevalence and behavioural risk factors in the general population. Sex Transm Infect. 2007;83(3):237–241. doi:10.1136/sti.2006.022970
9. Sonnenberg P, Ison CA, Clifton S, et al. Epidemiology of Mycoplasma genitalium in British men and women aged 16-44 years: evidence from the third national survey of sexual attitudes and lifestyles (Natsal-3). Int J Epidemiol. 2015;44(6):1982–1994. doi:10.1093/ije/dyv194
10. Baumann L, Cina M, Egli-Gany D, et al. Prevalence of Mycoplasma genitalium in different population groups: systematic review and meta-analysis. Sex Transm Infect. 2018;94(4):255–262. doi:10.1136/sextrans-2017-053384
11. Bercot B, Charreau I, Rousseau C, et al. High prevalence and high rate of antibiotic resistance of Mycoplasma genitalium infections in men who have sex with men: a substudy of the ANRS IPERGAY pre-exposure prophylaxis trial. Clin Infect Dis. 2021;73(7):e2127–e2133. doi:10.1093/cid/ciaa1832
12. Xuan Y, Wei LX, Hong X, et al. A meta-analysis on the infection rates on Mycoplasma genitalium in the genitourinary tract of different populations in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2021;42(2):335–342. doi:10.3760/cma.j.cn112338-20200530-00791
13. Jiang J, Liu P, Cao N, et al. The Prevalence of Mycoplasma genitalium and Chlamydia Trachomatis at Various Anatomical Sites of Men Who Have Sex with Men in Five Cities of China. Sex Transm Infect. 2015;91(Suppl 2): A30.3–A31. doi:10.1136/sextrans-2015-052270.93
14. Zhao N, Li KT, Gao YY, et al. Mycoplasma Genitalium and Mycoplasma Hominis are prevalent and correlated with HIV risk in MSM: a cross-sectional study in Shenyang, China. BMC Infect Dis. 2019;19(1):494. doi:10.1186/s12879-019-4138-5
15. Wu JR, Wang B, Chen LS, et al. Alarming incidence of genital mycoplasmas among HIV-1-infected MSM in Jiangsu, China. Eur J Clin Microbiol Infect Dis. 2014;33(2):189–195. doi:10.1007/s10096-013-1942-5
16. Machalek DA, Tao Y, Shilling H, et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20(11):1302–1314. doi:10.1016/S1473-3099(20)30154-7
17. Unemo M, Jensen JS. Antimicrobial-resistant sexually transmitted infections: gonorrhoea and Mycoplasma genitalium. Nat Rev Urol. 2017;14(3):139–152. doi:10.1038/nrurol.2016.268
18. Jensen JS, Bradshaw CS, Tabrizi SN, et al. Azithromycin treatment failure in Mycoplasma genitalium –positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008;47(12):1546–1553. doi:10.1086/593188
19. Fifer H, Merrick R, Pitt R, et al. Frequency and correlates of Mycoplasma genitalium antimicrobial resistance mutations and their association with treatment outcomes: findings from a national sentinel surveillance pilot in England. Sex Transm Dis. 2021;48(12):951–954. doi:10.1097/OLQ.0000000000001493
20. Guiraud J, Helary M, Le Roy C, et al. Molecular typing reveals distinct Mycoplasma genitalium transmission networks among a cohort of men who have sex with men and a cohort of women in France. Microorganisms. 2022;10(8):1587. doi:10.3390/microorganisms10081587
21. Dumke R, Ziegler T, Abbasi-Boroudjeni N, et al. Prevalence of macrolide- and fluoroquinolone-resistant Mycoplasma genitalium strains in clinical specimens from men who have sex with men of two sexually transmitted infection practices in Berlin, Germany. J Glob Antimicrob Resist. 2019;18:118–121. doi:10.1016/j.jgar.2019.06.015
22. De Baetselier I, Kenyon C, Vanden Berghe W, et al. An alarming high prevalence of resistance-associated mutations to macrolides and fluoroquinolones in Mycoplasma genitalium in Belgium: results from samples collected between 2015 and 2018. Sex Transm Infect. 2021;97(4):297–303. doi:10.1136/sextrans-2020-054511
23. McIver R, Jalocon D, McNulty A, et al. Men who have sex with men with Mycoplasma genitalium-positive nongonococcal urethritis are more likely to have macrolide-resistant strains than men with only female partners: a prospective study. Sex Transm Dis. 2019;46(8):513–517. doi:10.1097/OLQ.0000000000001009
24. Dionne-Odom J, Geisler WM, Aaron KJ, et al. High prevalence of multidrug-resistant Mycoplasma genitalium in human immunodeficiency virus-infected men who have sex with men in Alabama. Clin Infect Dis. 2018;66(5):796–798. doi:10.1093/cid/cix853
25. Murray GL, Bodiyabadu K, Danielewski J, et al. Moxifloxacin and sitafloxacin treatment failure in Mycoplasma genitalium infection: association with parC mutation G248T (S83I) and concurrent gyrA mutations. J Infect Dis. 2020;221(6):1017–1024. doi:10.1093/infdis/jiz550
26. Mulligan V, Lynagh Y, Clarke S, et al. Prevalence, macrolide resistance, and fluoroquinolone resistance in Mycoplasma genitalium in men who have sex with men attending an sexually transmitted disease clinic in Dublin, Ireland in 2017-2018. Sex Transm Dis. 2019;46(4):e35–e37. doi:10.1097/OLQ.0000000000000940
27. Li Y, Su X, Le W, et al. Mycoplasma genitalium in symptomatic male urethritis: macrolide use is associated with increased resistance. Clin Infect Dis. 2020;70(5):805–810. doi:10.1093/cid/ciz294
28. Ke W, Li D, Tso LS, et al. Macrolide and fluoroquinolone associated mutations in Mycoplasma genitalium in a retrospective study of male and female patients seeking care at a STI Clinic in Guangzhou, China, 2016-2018. BMC Infect Dis. 2020;20(1):950. doi:10.1186/s12879-020-05659-3
29. KKM Ng, Leung PKL, Cheung TKM. Molecular detection of Mycoplasma genitalium in endocervical swabs and associated rates of macrolide and fluoroquinolone resistance in Hong Kong. Hong Kong Med J. 2020;26(5):390–396. doi:10.12809/hkmj208507
30. Foschi C, Gaspari V, Sgubbi P, et al. Sexually transmitted rectal infections in a cohort of ‘men having sex with men’. J Med Microbiol. 2018;67(8):1050–1057. doi:10.1099/jmm.0.000781
31. Jansen K, Steffen G, Potthoff A, et al. STI in times of PrEP: high prevalence of chlamydia, gonorrhea, and mycoplasma at different anatomic sites in men who have sex with men in Germany. BMC Infect Dis. 2020;20(1):110. doi:10.1186/s12879-020-4831-4
32. Couldwell DL, Jalocon D, Power M, et al. Mycoplasma genitalium: high prevalence of resistance to macrolides and frequent anorectal infection in men who have sex with men in western Sydney. Sex Transm Infect. 2018;94(6):406–410. doi:10.1136/sextrans-2017-053480
33. de Salazar A, Barrientos-Duran A, Espadafor B, et al. Macrolide and fluoroquinolone resistance of Mycoplasma genitalium in southern Spain, 2018–2019. Sex Transm Infect. 2021;97(1):8–10. doi:10.1136/sextrans-2019-054386
34. Wang L, Li Z, Wan C, et al. Prevalence of Mycoplasma genitalium infection with antimicrobial resistance mutations among gay sex workers in China. Int J STD AIDS. 2023;34(8):518–524. doi:10.1177/09564624231160676
35. Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1–187. doi:10.15585/mmwr.rr7004a1
36. Soni S, Horner P, Rayment M, et al. British association for sexual health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018). Int J STD AIDS. 2019;30(10):938–950. doi:10.1177/0956462419825948
37. Read TR, Fairley CK, Tabrizi SN, et al. Azithromycin 1.5g over 5 days compared to 1g single dose in urethral Mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis. 2017;64(3):250–256. doi:10.1093/cid/ciw719
38. Li S, Xue H, Zhang S, et al. Doxycycline-moxifloxacin sequential therapy for Mycoplasma genitalium urethritis/cervicitis: a clinical observation. Chin Jl of Dermatology. 2022;55(12):1092–1095. doi:10.35541/cjd.20210631
39. Clarke EJ, Vodstrcil LA, Plummer EL, et al. Efficacy of minocycline for the treatment of Mycoplasma genitalium. Open Forum Infect Dis. 2023;10(8):ofad427. doi:10.1093/ofid/ofad427
40. Doyle M, Vodstrcil LA, Plummer EL, et al. Nonquinolone Options for the treatment of Mycoplasma genitalium in the era of increased resistance. Open Forum Infect Dis. 2020;7(8):ofaa291. doi:10.1093/ofid/ofaa291
41. Vodstrcil LA, Plummer EL, Doyle M, et al. Combination therapy for mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve cure. Clin Infect Dis. 2022;75(5):813–823. doi:10.1093/cid/ciab1058
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

