Back to Journals » Infection and Drug Resistance » Volume 18
Prevalence, Risk Factors, and Clinical Outcomes with Advanced HIV Disease Among People with Newly Diagnosed HIV During the “Treat-All” Era: A Retrospective Cohort Study From Xi’an City, China
Authors Jin J, Pan S, Chen J, Yin J, Ba H, Hou H, Zhang Y, Ma K
Received 22 January 2025
Accepted for publication 5 May 2025
Published 9 May 2025 Volume 2025:18 Pages 2427—2438
DOI https://doi.org/10.2147/IDR.S518809
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Juan Jin,1,* Songnan Pan,2,* Jie Chen,1 Jinling Yin,1 Huanhuan Ba,1 Haohua Hou,1 Yuan Zhang,1 Kangxiao Ma1
1Department of Infectious Diseases, Xi’an Eighth’s Hospital, Xi’an, Shaanxi, People’s Republic of China; 2Department of Infectious Diseases, Infectious Disease Hospital of Heilongjiang Province, Harbin, Heilongjiang, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kangxiao Ma, Department of Infectious Diseases, Xi’an Eighth’s Hospital, 2 Zhangba East Road, Yanta District, Xi’an, Shaanxi, 710061, People’s Republic of China, Tel/Fax +86 029 85393973, Email [email protected]
Purpose: China launched “treat-all” in 2016 to make all HIV-positive people eligible for ART regardless of disease stage. Widespread treatment may not eliminate advanced HIV. This study investigated the prevalence, characteristics, risk factors, and clinical outcomes of advanced HIV disease (AHD) in newly diagnosed HIV-positive individuals in China during the “treat-all” period.
Patients and methods: We performed a retrospective cohort study on newly diagnosed adult ART-naive people with HIV (PWH) in Xi’an from 2016 to 2022. The prevalence of AHD and six-month mortality/loss to follow-up (LTFU) were investigated. Risk variables for AHD and predictors of mortality or LTFU in the cohort were investigated using multivariate logistic and Cox regression, respectively.
Results: Of the PWH, 47.5% (2999/6318) had AHD at HIV diagnosis. At enrollment, being ≥ 50 years (aOR: 1.75, 95% CI: 1.44– 2.12, P < 0.001; ≥ 50 vs 18– 29), 30– 49 years (aOR: 1.43, 95% CI: 1.24– 1.65, P < 0.001; 30– 49 vs 18– 29), opportunity infections (aOR: 7.43, 95% CI: 5.96– 9.35, P < 0.001), severe anemia (aOR: 3.56, 95% CI: 1.81– 7.70, P = 0.001) and liver disease (aOR: 3.09, 95% CI: 1.48– 7.05, P = 0.004) were independently associated with AHD. Within 6 months of enrollment, 95.6% and 58.3% of those who died or were LTFU had AHD. AHD (aHR: 14.30, 95% CI: 4.42– 46.30, P < 0.001), ≥ 50 years (aHR: 5.39, 95% CI: 2.10– 13.82, P < 0.001; ≥ 50 years vs 18– 29 years), those with opportunistic infections (aHR: 2.59, 95% CI: 1.54– 4.34, P < 0.001), and severe anemia (aHR: 9.89, 95% CI: 5.19– 18.87, P < 0.001) were independent predictors of six-month mortality.
Conclusion: Under the “treat-all” policy, Xi’an had a high prevalence of AHD upon HIV diagnosis. AHD predicted 6-month mortality. Urgent implementation of targeted strategies is necessary to minimize AHD.
Keywords: advanced HIV disease, HIV, mortality, loss to follow-up, treat all, China
Introduction
Thirty-nine million people globally were infected with the human immunodeficiency virus (HIV) by the end of 2022, and despite advances in the scaling up of antiretroviral medication (ART), 630,000 people died away from illnesses related to the acquired immunodeficiency syndrome (AIDS).1 The percentage of people with advanced HIV disease (AHD)—which is defined by a CD4 count of <200 cells/μL or World Health Organization (WHO) stage 3 or 4 events—remains high at over 4 million,2 increasing the risk of severe opportunistic infections and mortality. AIDS and AHD are similar but vary in definition, clinical usage, and therapy. AIDS is the most severe stage of HIV infection, characterized by CD4 count <200 or specific opportunistic infections or cancers. Even if CD4 levels recover, a patient’s AIDS history remains (for legal/statistical reasons), whereas AHD is a wider clinical designation for people at high risk of severe outcomes. AHD requires prompt attention (eg, rapid ART, fungal infection screening), whereas AIDS requires long-term monitoring and disability benefits in certain countries. All AIDS cases are AHD, but not all AHD meets the strict AIDS definition. For example: a patient with tuberculosis (WHO Stage 3) and CD4 = 250 has “AHD” but “not AIDS”. Modern HIV treatment has made “AIDS” less common, but advanced disease remains a critical focus to prevent deaths from late diagnosis or treatment failure.
Despite a stabilization of HIV prevalence and a decline in incidence from 1990 to 2017, AIDS-related mortality in China has increased dramatically in recent years.3 At the end of 2020, China had 1.05 million people with HIV (PWH) and 351,000 recorded deaths.4 AHD remains a significant concern in China, as it substantially contributes to AIDS-related mortality. According to data from a nationwide cohort in China between 2006 and 2014, AHD accounted for 34% of newly diagnosed individuals.5 Health systems were burdened more while AHD was more prevalent.6 Data from different regions in China up to 2019 reported varying proportions of AHD in PWH newly diagnosed ranging from 29.7% to 47.2%.5,7–9 The prevalence of HIV infection and AHD exhibits significantly regional variations across China.4 For instance, provinces of Yunnan, Guangxi, Henan, and Xinjiang are considered high-risk based on statistics on new HIV infections, AIDS cases, and AIDS-related mortality.10
Previous studies identified risk factors associated with AHD, including older age, lower levels of education, poor risk perception, and ignorance of HIV-related symptoms.7,8 The preceding study focusses only on demographic and socioeconomic variables, leaving out clinical issues. There are presently no studies that investigate clinical outcomes in the Chinese AHD population. Since China implemented “treat-all” policy in June 2016, PWH are being started on ART earlier instead of based on CD4 criteria, which may reduce AHD prevalence. It remains crucial to investigate the local AHD epidemiological characteristics and clinical outcomes under the “treat-all” policy.
Xi’an, Shaanxi’s provincial capital, is the most populous city in central China, with a population of more than 13 million. Xi’an’s first HIV case was recorded in 1992, and the number of people with HIV/AIDS increased rapidly from 1,200 in 2010 to more than 9,000 in 2022.11 The prevalence and determinants of AHD in Xi’an are unclear. As a result, the current study investigated the prevalence of AHD, risk factors, and clinical outcomes in people newly diagnosed with HIV/AIDS under the “treat-all” era in Xi’an, China.
Methods
Study Participants
The study included all persons newly diagnosed with HIV who enrolled in the HIV/AIDS information management system of the Xi’an Centers for Disease Control (CDC) between June 2016 and December 2022. The inclusion criteria were: (1) HIV-positive, (2) residing in Xi’an city, (3) at least 18 years at diagnosis, (4) ART-naïve, (5) with a CD4 count record within three months of diagnosis. Exclusion criteria included individuals who: (1) had no record of CD4 count, (2) had previous ART experience; (3) had less than 6 months between enrollment and last visit; (4) transferred out; (5) missing data. Newly diagnosed people with HIV were defined as those who received a serologically verified diagnosis of HIV infection that either caused their present hospital visit or was recorded during their current hospital stay. We were unable to discriminate between newly infected and chronically infected individuals since most newly diagnosed HIV patients in the study were not consistently tested prior to becoming serologically positive. The study was approved by the Human Medical Ethics Committee of Xi’an Eighth’s Hospital (No. 2024–12) and followed the Helsinki Declaration. At the time of diagnosis, every PWH provided informed consent for the use of their clinical records.
Data Collection
Upon recruitment, all participants received a medical history and physical evaluation, as well as a demographic and behavioral survey. Participants’ routine following up visits were scheduled at 2, 4, and 12 weeks after ART started and every three months thereafter. Blood routine, serum creatinine, and aspartate aminotransferase (AST) levels were tested for each participant at the previously stated follow-up visits. Free CD4 count testing was done at the start of therapy and every 6 months, whereas free viral load assessment was done yearly following ART.
Data was extracted from the hospital’s computerized medical records and the HIV/AIDS information management system of the China CDC. Variables that were obtained included age, sex, routes of transmission, marital status, CD4 count at enrollment, WHO stage, hemoglobin, platelet, creatinine, AST, dates of HIV diagnosis, historical ART initiation date, ART regimen, date of transfer out, and death. CD4 count at enrollment was determined using test results from three months prior to the initiation of ART. Severe anemia is defined as hemoglobin <8 g/dL. Chronic kidney disease (CKD) stage 3–5 was defined as having an estimated glomerular filtration rate (eGFR) of less than 60 mL/min/1.73 m2 for over 3 months,12 as calculated using the Modification of Diet in Renal Disease equation and blood creatinine obtained at enrollment. Liver disease was defined as AST-to-platelet ratio index >1.5.13,14
Outcome Measures
The primary treatment outcome was 6-month mortality following ART initiation, while the secondary outcome was loss to follow-up (LTFU), which was defined as the person’s (who had not died or been transferred) lack to contact the health center 60 days after the last drug pick-up in the six months following enrollment.
Statistical Analysis
The baseline characteristics were shown as frequencies and proportions for categorical variables, and as medians and interquartile ranges (IQR) for continuous variables. Continuous and categorical data were compared using the Kruskal–Wallis and χ2 tests, respectively. The predictors of mortality and LTFU within six months after enrollment were assessed using multivariate Cox regression models. The hazard ratio (HR) and adjusted hazard ratio (aHR) were reported with 95% confidence intervals (CI). Odd ratio (OR), adjusted odds ratios (aOR) and 95% CI were also obtained from the investigation of variables associated with AHD using multivariate logistic regression models. The variables that had a P-value of less than 0.05 in the univariate analysis were included in the multivariate analysis. A P value below 0.05 was considered statistically significant. All analyses were performed using the SPSS 26.0 software (SPSS, Chicago, IL).
Results
Study Population
Table 1 shows participant characteristics at enrollment. Between June 2016 and December 2022, 6529 PWH aged ≥18 years were recruited in Xi’an city. Of them, 6318 (96.8%) had accessible data and were analyzed in the study (Supplementary Figure 1). Of the participants, 2999 (47.5%) had an AHD at diagnosed (67.3% had a CD4 count of less than 200 and 91.5% had WHO stage 3 or 4).
 |
Table 1 Characteristics of People Presenting with and without Advanced HIV Disease |
Although most people with HIV were male (92.9%), a higher proportion of men than women presented with advanced HIV disease (92.9% vs 7.1%). The median age was 34 (IQR 27–46) years, with AHD individuals significantly older than non-AHD individuals (37 vs 31). The median time between diagnosis and ART was 28 (IQR 14–62) days, with comparable rates of rapid ART initiation (≤7 days following diagnosis) in both groups (10.8% and 10.0%). (Table 1)
At enrollment, the median CD4 count was 296 cells/μL (IQR 153–437), with 144 cells/μL in AHD individuals and 418 cells/μL in non-AHD individuals (Table 2). In total, 39.2%, 28.8%, 13.8%, and 18.2% of participants had CD4 counts of ≥350, 200–349, 100–199, and <100 cells/μL, respectively. About 43.4% of participants had a diagnosed of WHO stage 3 or 4 irrespective of CD4 level, which accounted for 91.5% of individuals with AHD.
 |
Table 2 Laboratory Characteristics of People Presenting with and without Advanced HIV Disease |
At the time of enrollment, 39 (0.6%) had liver disease, 35 (0.6%) had CKD (stages 3–5), and 63 (1.0%) had severe anemia. When comparing those with AHD to those without AHD, severe anemia and liver disease were much more common (P < 0.001).
Unfavorable Clinical Outcomes Among People with Advanced HIV Disease
Within 6 months of enrollment, 69 (1.1%) of these individuals died, and 108 (1.7%) became LTFU. Among those who died or were LTFU, 66 (95.6%) and 63 (58.3%) had advanced HIV disease, respectively (Figure 1). The median time from enrollment to death was 52 (IQR 15–91) days.
 |
Figure 1 Advanced human immunodeficiency virus disease contribution to death and loss to follow-up. Abbreviations: AHD, Advanced human immunodeficiency virus disease; LTFU, Loss to follow-up. |
Factors Associated with Advanced HIV Disease at Diagnosis
The results of logistic regression analyses that were performed to determine the risk factors correlated with AHD are shown in Table 3. AHD was more common in PWH ≥50 years (aOR: 1.75, 95% CI: 1.44–2.12, P < 0.001) and 30–49 years (aOR: 1.43, 95% CI: 1.24–1.65, P < 0.001) compared to those 18–29. AHD was less common in single PWH (aOR: 0.72, 95% CI: 0.62–0.84, P < 0.001) than in married/cohabiting individuals, and in those who took integrase strand transfer inhibitor-based regimen (aOR: 0.80, 95% CI: 0.70–0.91, P = 0.001) than those who chose nonnucleoside reverse transcriptase inhibitor-based regimen. As well, those with opportunity infections (aOR: 7.44, 95% CI: 5.97–9.37, P < 0.001), severe anemia (aOR: 3.56, 95% CI: 1.81–7.70, P = 0.001) and liver disease (aOR: 3.09, 95% CI: 1.48–7.05, P = 0.004) were more likely to suffer from AHD.
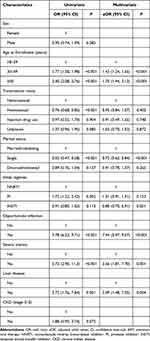 |
Table 3 Risk Factors Associated with Advanced HIV Disease Among 6318 Newly Diagnosed People with HIV |
Predictors of Six-Month Mortality Among People with HIV
In the Cox regression analysis, AHD (aHR: 14.30, 95% CI: 4.42–46.30, P < 0.001), ≥50 years (aHR: 5.39, 95% CI: 2.10–13.82, P < 0.001; ≥50 years vs 18–29 years), and those with opportunistic infection (aHR: 2.59, 95% CI: 1.54–4.34, P < 0.001), and severe anemia (aHR: 9.89, 95% CI: 5.19–18.87, P < 0.001) were independent predictors of six-month mortality (Table 4).
 |
Table 4 Factors Associated with 6-month Mortality Among People with HIV |
Predictors of LTFU Among People with HIV
Predictors of LTFU in multivariate Cox regression analysis included ≥50 years (aHR: 2.81, 95% CI: 1.70–4.62, P < 0.001; ≥50 vs 18–29), and those with unknown transmission route (aHR: 2.75, 95% CI: 1.31–5.77, P = 0.007; unknown vs heterosexual) (Table 5).
 |
Table 5 Factors Associated with Loss to Follow-up Among People with HIV |
Discussion
We evaluated the prevalence, risk factors, and unfavorable clinical outcomes of AHD in a large HIV cohort in Xi’an during the “treat-all” era. We observed that the high burden of AHD resulted in increased mortality. We also found AHD risk variables and predictors of six-month mortality or LTFU in PWH.
Upon diagnosis, 47.5% of subjects had AHD. About 87.5% of PWH with CD4 counts <200 was in WHO clinical stages 3/4, highlighting the distinction between WHO stage and CD4 count level,15,16 which objectively reflects immune and clinical status and the risk of opportunistic infections.17 At baseline, 32% of participants had CD4 levels of below 200 cells/μL, which is comparable to findings from previous Chinese studies,5,18 suggesting that late diagnosis is a significant issue in China. An increased risk of AIDS and mortality has been related to delayed presentation, especially in the first year after diagnosis.19 Reducing advanced HIV disease and late HIV diagnoses is still a major public health concern and challenge.
Our study showed that a higher risk of AHD was related to age ≥30, especially ≥50. A study carried out in South Africa observed that people aged 30–50 were 2.6 times more probable to present with AHD than those aged 12–20.14 AHD may be more prevalent among PWH aged 30 years or older due to delays in HIV diagnosis, which may result in delayed ART initiation or interruptions in ART.20 These findings indicate an urgent need for strategies that involve young adults and elderly PWH earlier in the care process and assure the continuation of treatment to reduce the risk of AHD.
The prevalence of non-AIDS-related diseases in our study is lower than a previous Tanzania study,16 where we found that liver disease and severe anemia were linked to AHD. Anemia has previously been reported to be associated with AHD, although it is not specific to persons with AHD.16,21,22 The prevalence of anemia in HIV is comparatively high, as PWH are at greater risk of developing anemia because of inadequate iron ingestion, opportunistic infections, such as tuberculosis, and inflammation.23 Our results emphasize the necessity of incorporating non-AIDS-related diseases care within a differentiated service delivery approach for PWH, including those who have AHD.
In our study, individuals with AHD experienced a high rate of six-month mortality, with many deaths occurring within the first two months of enrollment. AHD, older age (≥50 years), severe anemia, and opportunistic infection were independent predictors of mortality. Mortality was associated with advanced age, which is well recognized as a risk factor for AIDS-related diseases.24 Severe anemia, which usually results from severe opportunistic infections, has previously been related to increased mortality.16,25 There is an urgent need for an extensive package of care that includes screening and prophylaxis for major opportunistic infections.
Our study has several limitations: The study’s retrospective design may limit its power to identify variables related to mortality or LTFU. In addition, our study does not examine the influence of the coronavirus disease epidemic on the prevalence of AHD. Finally, co-infections with other viruses, such as hepatitis B and C viruses, may disrupt the natural history of HIV infection and are associated with AHD. The large number of missing co-infection data points may have an impact on the overall findings.
Conclusion
Nearly half of the PWH included in our cohort following Treat-All adoption still had AHD at diagnosis in Xi’an, China. Age, opportunistic infections, severe anemia, and liver disease at enrollment were risk factors related to AHD. AHD was a predictor of 6-month mortality, but it was not a predictor of LTFU. Crucial measures to lower mortality include expanding HIV testing and adopting the AHD recommendations for opportunistic infection early detection, diagnosis, and therapy into practice.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Statement
The study was approved by the Human Medical Ethics Committee of Xi’an Eighth’s Hospital and followed the Helsinki Declaration.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. UNAIDS. Global HIV & AIDS Statistics — 2022 Fact Sheet. 2023.
2. Ford N, Ehrenkranz P, Jarvis J. Advanced HIV as a Neglected Disease. N Engl J Med. 2024;390(6):487–489. doi:10.1056/NEJMp2313777
3. Liu XJ, McGoogan JM, Wu ZY. Human immunodeficiency virus/acquired immunodeficiency syndrome prevalence, incidence, and mortality in China, 1990 to 2017: a secondary analysis of the Global Burden of Disease Study 2017 data. Chin Med J. 2021;134(10):1175–1180. doi:10.1097/CM9.0000000000001447
4. He N. Research Progress in the Epidemiology of HIV/AIDS in China. China CDC Wkly. 2021;3(48):1022–1030. doi:10.46234/ccdcw2021.249
5. Tang H, Mao Y, Tang W, Han J, Xu J, Li J. Late for testing, early for antiretroviral therapy, less likely to die: results from a large HIV cohort study in China. BMC Infect Dis. 2018;18(1):272. doi:10.1186/s12879-018-3158-x
6. Krentz HB, Auld MC, Gill MJ. The high cost of medical care for patients who present late (CD4 <200 cells/microL) with HIV infection. HIV Med. 2004;5(2):93–98. doi:10.1111/j.1468-1293.2004.00193.x
7. Hu X, Liang B, Zhou C, et al. HIV late presentation and advanced HIV disease among patients with newly diagnosed HIV/AIDS in Southwestern China: a large-scale cross-sectional study. AIDS Res Ther. 2019;16(1):6. doi:10.1186/s12981-019-0221-7
8. Jiang H, Liu J, Tan Z, et al. Prevalence of and factors associated with advanced HIV disease among newly diagnosed people living with HIV in Guangdong Province, China. J Int AIDS Soc. 2020;23(11):e25642. doi:10.1002/jia2.25642
9. Yue T, Zhang P, Hao Y, et al. Epidemiology and Clinical Outcomes of HIV Infection in South-Central China: a Retrospective Study From 2003 to 2018. Front Public Health. 2022;10:902537. doi:10.3389/fpubh.2022.902537
10. Qiao YC, Xu Y, Jiang DX, et al. Epidemiological analyses of regional and age differences of HIV/AIDS prevalence in China, 2004-2016. Int J Infect Dis. 2019;81:215–220. doi:10.1016/j.ijid.2019.02.016
11. Xia H, Jin J, Ba H, et al. Genetic Diversity and Characteristics of Drug Resistance Among Treatment-Naive People Living with HIV in Xi’an, China. Drug Des Devel Ther. 2023;17:1485–1494. doi:10.2147/DDDT.S406255
12. Kidney Disease: improving Global Outcomes CKDWG. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024;105(4S):S117–S314. doi:10.1016/j.kint.2023.10.018
13. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66(5):1486–1501. doi:10.1002/hep.29302
14. Mauss S, Pol S, Buti M, et al. Late presentation of chronic viral hepatitis for medical care: a consensus definition. BMC Med. 2017;15(1):92. doi:10.1186/s12916-017-0856-y
15. Hakim J, Musiime V, Szubert AJ, et al. Enhanced Prophylaxis plus Antiretroviral Therapy for Advanced HIV Infection in Africa. N Engl J Med. 2017;377(3):233–245. doi:10.1056/NEJMoa1615822
16. Stoger L, Katende A, Mapesi H, et al. Persistent High Burden and Mortality Associated With Advanced HIV Disease in Rural Tanzania Despite Uptake of World Health Organization “Test and Treat” Guidelines. Open Forum Infect Dis. 2022;9(12):ofac611. doi:10.1093/ofid/ofac611
17. Ford N, Meintjes G, Vitoria M, Greene G, Chiller T. The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS. 2017;12(2):123–128. doi:10.1097/COH.0000000000000348
18. Xia H, Li L, Wu Y, Gao L, Zhang D, Ma P. Rapid Initiation of Antiretroviral Therapy Under the Treat-All Policy Reduces Loss to Follow-Up and Virological Failure in Routine Human Immunodeficiency Virus Care Settings in China: a Retrospective Cohort Study (2016-2022). AIDS Patient Care STDS. 2024;38(4):168–176. doi:10.1089/apc.2024.0045
19. Mocroft A, Lundgren JD, Sabin ML, et al. Risk factors and outcomes for late presentation for HIV-positive persons in Europe: results from the Collaboration of Observational HIV Epidemiological Research Europe Study (COHERE). PLoS Med. 2013;10(9):e1001510. doi:10.1371/journal.pmed.1001510
20. Oboho IK, Esber AL, Dear N, et al. Advanced HIV disease in East Africa and Nigeria, in The African Cohort Study. J Acquir Immune Defic Syndr. 2024;96(1):51–60. doi:10.1097/QAI.0000000000003392
21. Nandlal V, Moodley D, Grobler A, Bagratee J, Maharaj NR, Richardson P. Anaemia in pregnancy is associated with advanced HIV disease. PLoS One. 2014;9(9):e106103. doi:10.1371/journal.pone.0106103
22. Abioye AI, Andersen CT, Sudfeld CR, Fawzi WW. Anemia, Iron Status, and HIV: a Systematic Review of the Evidence. Adv Nutr. 2020;11(5):1334–1363. doi:10.1093/advances/nmaa037
23. Cao G, Wang Y, Wu Y, Jing W, Liu J, Liu M. Prevalence of anemia among people living with HIV: a systematic review and meta-analysis. EClinicalMedicine. 2022;44:101283. doi:10.1016/j.eclinm.2022.101283
24. Ren N, Li Y, Wan Z, et al. Patterns of HIV or AIDS Mortality Among Older People From 1990 to 2019 in China: age-Period-Cohort Analysis. JMIR Public Health Surveill. 2022;8(11):e35785. doi:10.2196/35785
25. Haider BA, Spiegelman D, Hertzmark E, et al. Anemia, Iron Deficiency, and Iron Supplementation in Relation to Mortality among HIV-Infected Patients Receiving Highly Active Antiretroviral Therapy in Tanzania. Am J Trop Med Hyg. 2019;100(6):1512–1520. doi:10.4269/ajtmh.18-0096
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

