Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 19
Processing of Sedentary Time and Its Reference Equation in Patients with COPD
Authors Minakata Y , Sasaki S, Murakami Y, Kawabe K, Ono H
Received 17 April 2024
Accepted for publication 22 August 2024
Published 27 August 2024 Volume 2024:19 Pages 1931—1942
DOI https://doi.org/10.2147/COPD.S474273
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Yoshiaki Minakata, Seigo Sasaki, Yusuke Murakami, Kazumi Kawabe, Hideya Ono
Department of Respiratory Medicine, NHO Wakayama Hospital, Hidaka-gun, Wakayama, Japan
Correspondence: Yoshiaki Minakata, Department of Respiratory Medicine, NHO Wakayama Hospital, 1138 Wada Mihama-cho, Hidaka-gun, Wakayama, 644-0044, Japan, Tel +81 738 22 3256, Email [email protected]
Purpose: Sedentary time (ST) is associated with mortality independent of moderate-to-vigorous physical activity in patients with COPD. The proper processing methods for the measurement data and factors related to ST are still unknown. We investigated several conditions for determining the proper processing of ST accelerometric data and created a reference equation for ST using ST-related factors.
Patients and Methods: In Study 1, we evaluated the minimum required number of days to obtain repeatability at different measurement times and assessed the effects of rainy days or weekend days on ST in patients with COPD. In Study 2, we detected the ST-related factors among 28 parameters and created a reference equation for ST using the detected factors.
Results: In Study 1, 38 patients with stable COPD were analyzed. The minimum number of days required for repeatability was 3 for 8-h wearing and 2 for 10-h wearing. The ST was significantly prolonged on rainy days, but not on weekends. In Study 2, 216 patients with stable COPD were analyzed. BMI, FEV1%pred, 6MWD, and mMRC were detected as ST-related factors, and a reference equation could be created using these four factors. The equation was validated for patients whose ST was ≥ 6 h.
Conclusion: By using properly processed measurement data of ST, we created a reference equation for assessing ST that is expected to be useful for providing individual guidance on the shortening of ST to patients with COPD.
Plain Language Summary: When measuring sedentary time (ST) objectively, we should recognize that the minimum required number of days is three and that ST is prolonged on rainy days. The reference equation for ST could be created using four ST-related factors. It might serve as a guide for shortening ST in COPD.
Keywords: sedentary behavior, physical activity, accelerometer, chronic obstructive pulmonary disease
Introduction
COPD is a leading cause of morbidity and mortality worldwide.1,2 Physical activity (PA) may be an important predictor of the prognosis of patients.3,4 Recently, sedentary behavior has received increasing attention because sedentary time (ST) is associated with COPD-related mortality, independent of PA.5
Sedentary behavior is typically defined by both low energy expenditure (eg, resting metabolic rate, typically ≤1.5 metabolic equivalents [METs]) and a sitting or reclining posture.6–9 Accelerometers are more accurate than questionnaires in evaluating ST. However, when PA or ST is evaluated with an accelerometer, proper processing of measurement data is important for generalizing conclusions from different studies.10,11 Among 110 interventional trials reporting objective PA outcomes, only one-third fulfilled the certain processing of measurement data, including assessment days, minimum required number of days, and total measurement hours per day.12 In our previous report, to assess the duration of PA at ≥3.0 METs or ≥2.0 METs using an accelerometer Active Style Pro HJA-750C (HJA; Omron Healthcare, Kyoto, Japan), at least three days of measurements, excluding rainy days, were required for repeatability.13
Unlike PA, ST accounts for more than half of the measurement time,14 so ST could be more affected by accelerometer wearing than PA. However, the proper processing of ST measurement data to obtain reproducibility is still unknown. Furthermore, factors associated with PA have been studied; however, those associated with ST remain unclear. By detecting associated factors and creating a prediction equation for ST using these factors, it is possible to understand the current status of each patient’s sedentary behavior, which is expected to be useful for individual patient management.
Therefore, in Study 1, we evaluated the processing of the ST measurement data to improve data reproducibility. First, we evaluated the minimum measurement time required per day and the minimum number of days required to obtain repeatability. We then evaluated the effects of rainy days or weekends on ST in patients with COPD. In Study 2, we detected ST-related factors among the 28 parameters. Finally, we created a reference equation for ST using the detected factors and verified its validity.
Materials and Methods
Study 1: Assessment of Processing of Measurement Data for ST
Subjects
We used the data obtained from the patients of the NHO Wakayama Hospital among the patients who were included in our prospective observational study to create a reference equation for physical activity (UMIN000025459). Stable outpatients with COPD diagnosed based on a post-bronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) <0.7 and aged ≥40 years were eligible for inclusion. The following exclusion criteria were applied: 1) clinically relevant bronchial asthma, 2) receiving oxygen therapy, 3) a history of lung resection, 4) a history of an exacerbation within three months, 5) PA extremely suppressed by other diseases (including neuromuscular disease, bone and joint disease, active malignant disease, myocardial infarction, etc)., and 6) participation in this study deemed inappropriate by the attending physician. This study was conducted in accordance with the provisions of the Declaration of Helsinki, was approved by the ethics committee of the NHO Wakayama Hospital (approval number: 04–3; approval date: February 21, 2023), and registered with the University Hospital Medical Information Network (UMIN 000050745, April 1, 2023). We explained the research details on the website of the NHO Wakayama Hospital and provided an opportunity to refuse participation in the study.
ST Measurement and Diary
ST was assessed with a triaxial accelerometer, HJA, which is a waist-worn type and can evaluate the intensity of physical activity every 10 seconds.15,16 Patients were instructed to wear the HJA for 24 h, except when bathing or engaged in water-based activities from day 0 to day 14 (up to day 28). They were also instructed to keep a diary during that time. Among the data obtained, the data of days with less than 8 hours of wearing time were excluded as invalid. Then, the data of days with 8 h or more of wearing time (8-h wearing) or those with 10 h or more of wearing time (10-h wearing) were extracted. The non-wearing time was defined according to Choi’s algorithm, the elements of which are: 1) zero-count threshold during a non-wearing time interval, 2) 90-min time window for consecutive zero/nonzero counts, and 3) allowance of a 2-min interval of nonzero counts with the up/downstream 30-min consecutive zero count window for the detection of artifactual movements.17 The mean values of the duration at 1.0–1.5 METs among the valid days’ data were used as ST. In the diary, participants were asked to write down the weather and any special activities that occurred.
Evaluation
Wearing Time and Minimum Required Number of Days
ST could be more influenced by the accelerometer wearing time because ST occupies more than half (64%) of the total wearing time.14 Therefore, the influence of wearing time on the repeatability of the obtained data should be evaluated. Among the enrolled subjects, only those who had five or more days of 10-h wearing data were enrolled. The minimum required number of measurement days to obtain repeatability for both 8-h wearing and 10-h wearing was assessed using the intraclass correlation coefficient (ICC) between 2 and 5 days.
Effects of Weather and Weekend Days
Based on the results of the minimum required number of days, subsequent studies adopted the data of 10-h wearing. Based on the recorded diaries, valid days were divided into two groups according to weather (rainy days vs non-rainy days) or days of the week (weekdays vs weekend days or holidays). To assess the effect of weekends, only non-rainy days were analyzed. The differences in ST on weather and weekend days were then compared between the two groups.
Study 2: Creation of ST Reference Equation
Subjects
Patients were recruited from institutes belonging to the NHO group in Japan between January 2017 and February 2020. The entry and exclusion criteria were described in Study 1. This study was conducted as part of a prospective observational study to create a reference equation for PA. Written informed consent was obtained from all participants. This study was approved by the NHO Central Review Board (approval number H28-0411001, April 11, 2016) and registered with the UMIN-CTR (UMIN000025459, January 10, 2017).
ST Measurement
Patients were instructed to wear the HJA for 24 h, except when bathing or engaged in water-based activities from day 0 to day 14 (up to day 28), and to keep a diary during that time, as in Study 1. Days with less than 10 hours of wearing time, days with special activity, and rainy days were excluded as invalid data. Patients with three or more valid days were included in the analysis.
Parameters
Demographic information (ie, age, sex, height, weight, body mass index (BMI), smoking history, presence or absence of medication for COPD, whether pulmonary rehabilitation was conducted including inpatient and outpatient setting, and presence or absence of comorbidities obtained from medical record), post-bronchodilator spirometry, grip strength, upper arm circumference, triceps branch subcutaneous fat thickness, modified Medical Research Council (mMRC) dyspnea score, Hospital Anxiety and Depression Scale (HADS), and blood tests were evaluated on day 0. Blood tests included red blood cell, hemoglobin, fasting blood sugar, hemoglobin A1c, albumin, and brain natriuretic peptide (BNP) levels. A 6-minute walk test was performed on day 14 (or up to day 28).
Evaluation
Factors associated with ST were extracted using simple and multiple regression analyses of the 28 parameters. The reference equation for ST was created using a multiple linear regression equation with ST-related factors. Then, the reproducibility between the measured ST values and the ST values calculated with the equation was evaluated.
Sample Size Determination
In our previous study, the correlation coefficients between the duration of physical activity at ≥3.0 METs and FEV1% of predicted value (FEV1%pred) or mMRC score were 0.500 or −0.313, respectively.18 Assuming a 2-sided α-value of 0.05 and β-value of 0.10, the required number of patients was 38 for r = 0.5 and 113 for r = 0.3; assuming a 20% dropout rate, the numbers should be 48 and 142, respectively. Furthermore, to perform a multiple regression analysis, the number of samples per independent variable should be ≥10. Assuming that 20 of the 28 parameters were detected as related factors by a simple regression analysis and were employed in the multiple regression analysis, the required number of patients was 200. Assuming a 20% dropout rate, the number should be 250. Therefore, the sample size was set to 250.
Statistical Analysis
The repeatability of the data among measured days of both 8-h wearing and 10-h wearing was assessed by ICC. In accelerometer studies, multiple ICCs with a value of ICC >0.8 are generally accepted.19–22 The influence of weather and weekend days was assessed using paired t-tests. Simple and multiple linear regression analyses were used to assess the relationship between the ST and several parameters. The correlation between the measured and calculated ST values was assessed using Pearson’s correlation coefficient, and reproducibility was assessed using Bland–Altman plots. The normality of distribution was assessed using the D’Agostino and Pearson test. The statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, San Diego, CA, USA) and IBM SPSS Statistics (IBM Japan, Tokyo, Japan).
Results
Study 1: Assessment of Processing of Measurement Data for ST
Thirty-eight patients were recruited and two were excluded. Among the 36 enrolled patients, two were excluded because the number of days with 10-h wearing was less than five, so 34 were registered for analysis (Figure S1 in the Supporting Information). The average age was 72.0 years, and the study population included 32 male patients (94.1%) and an average FEV1%pred of 62.3%. The step count was 3796.3 ± 2193.5 steps and the duration at 1.0–1.5 METs was 330.1 ± 93.6 min (Table S1 in the Supporting Information).
Wearing Time and Minimum Required Number of Days
On 8-h wearing, the ICC values for 2 days were <0.8, but those for 3, 4, or 5 days were >0.8. On the other hand, on 10-h wearing, the ICC values for 2, 3, 4, or 5 days were >0.8 and were all higher than on 8-h wearing (Figure 1).
Effects of Weather and Weekend Days
Six patients had no rainy weather among 10-h wearing days and one patient had no weekend days or holidays among 10-h wearing and non-rainy days, so the effects of weather and weekend days on ST were assessed in 28 and 33 patients, respectively. The numbers of non-rainy days and rainy days were 10.4 ± 3.6 and 2.4 ± 1.3, respectively, and those of weekdays and weekend days were 6.9 ± 3.0 and 3.3 ± 1.0, respectively. The ST duration on rainy days was significantly longer than that on non-rainy days (p = 0.001). The ST duration on weekends was not significantly different from that on weekdays (p = 0.102) (Figure 2).
 |
Figure 2 Effects of weather and weekend days. (A) Patients who experienced at least one rainy day (n = 28). (B) patients who had at least one valid weekend day (n = 33). |
Study 2: Creation of ST Reference Equation
Two hundred fifty-three patients were recruited and 14 were excluded. Among the 239 enrolled patients, 23 were excluded because the number of valid days (days with 10-h wearing, days without special activity, and non-rainy days) was <3; therefore, 216 patients were included in the analysis (Figure S2 in Supporting Information). The average age was 73.1 years, and the study population included 202 male patients (93.5%) and mild to very severe COPD patients with an average FEV1%pred of 62.6%. The step count was 3893.0 ± 2493.8 steps and the duration at 1.0–1.5 METs was 370.7 ± 101.6 min (Table 1). The duration at 1.0–1.5 METs was normally distributed (Figure S3 in the Supporting Information). In the simple regression analysis, age, weight, BMI, inspiratory capacity (IC), FVC, FVC %pred, FEV1/ FVC, FEV1%pred, 6-minute walk distance (6MWD), upper arm circumference, grip strength, mMRC, and presence of rehabilitation were found to be associated with ST (Table 2). In the multiple regression analysis after adjusting for multicollinearity, BMI, FEV1%pred, 6MWD, and mMRC were associated with ST (Table 3). Although rehabilitation tended to be associated with ST, only 14 (6.5%) patients received rehabilitation, suggesting that this trend was due to chance. Then, the reference equation for ST could be created using a multiple linear regression formula with ST-related factors (Figure 3).
 |
Table 1 Patient Characteristics in Study 2 |
 |
Table 2 Simple Regression Analysis |
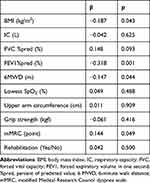 |
Table 3 Multiple Regression Analysis |
The correlation between measured and calculated ST values was statistically significant (r = 0.474, p <0.001). The 95% confidence interval of the mean difference between the measured and calculated values of ST was −1.725 to 1.722 min, which indicated that there was no fixed bias. The correlation coefficient between the difference and mean values on Bland-Altman Plots was 0.651 (p<0.001). This suggests that there was a proportional bias. The calculated values were higher than the measured values in patients with low ST values (Figure S4 in the Supporting Information). However, when patients with an ST value of 360 minutes or more were selected, neither fixed nor proportional biases were observed (Figure 4).
Discussion
For the accelerometer evaluation of ST, the minimum required number of days for repeatability was 3 for 8-h wearing and 2 for 10-h wearing. The ST was significantly prolonged on rainy days, but not on weekends. When the 10-h wearing data, excluding rainy days and days with special activities, were analyzed, BMI, FEV1%pred, 6MWD, and mMRC were detected as ST-related factors. A reference equation could be created with these four factors. The measured and calculated ST values were well correlated, but there was a proportional bias between them when all cases were included. When patients whose ST was 6 h or more were selected, there was no systematic bias.
At least three days were needed to obtain repeatability when ST was evaluated using 8-h wearing data whereas two days was sufficient when using 10-h wearing data. The ICC values obtained using the 10-h wearing data were consistently higher than those obtained using the 8-h wearing data. In COPD, the ST, which includes time spent sitting and lying, accounts for 64% of the total measurement time, which is much longer than the time spent standing or walking (33%).14 This suggests that the ST might be more influenced by the measurement time than by PA. Unexpectedly, the minimum required number of days to obtain repeatability of ST with 8-h wearing data using the HJA was 3, which is the same as that of the duration of PA at 3.0 METs.13 These results suggest that that both ST and PA could be evaluated using 8-h wearing data in patients with three or more days of data. However, the use of 10-h wearing data may achieve better repeatability.
Bertoche et al23 reported that the minimum required number of days for evaluating sedentary behavior in COPD patients was 4, which is different from the current result. They assessed repeatability with an ICC of >0.9, which is more stringent than our assessment. Although a higher ICC value indicates better repeatability, an ICC of ≥0.8 is considered to be sufficiently reliable.19–22 Therefore, we believe that at least three days are sufficient for evaluating ST.
ST was prolonged on rainy days, and this result could be inferred from previous reports that the duration of PA was shortened on rainy days.13,24–27 In contrast, ST did not change between weekends and weekdays. Some reports showed that the duration of PA was shortened on weekends in healthy subjects,20,21,28 but other reports showed that there was no difference between weekends and weekdays in patients with COPD.13,29 This difference may have been due to employment status because the PA in patients with a job is higher than that in patients without a job.30 Similar to the duration of PA, there may be no difference in ST in patients with COPD who are mostly retired.
BMI, FEV1%pred, 6MWD, and mMRC were found to be associated with ST in the multiple regression analysis. Among these factors, the association of FEV1%pred (beta = −0.318) was stronger than other factors (BMI: beta = −0.187, 6MWD: beta = −0.147, mMRC: beta = 0.144). FEV1 was associated with PA14,22,29,31,32 but its association was not so strong.14 However, in the case of ST, the association of FEV1 may be higher than expected.
Exercise capacity evaluated using the 6MWD was associated with ST. It is expected that patients with low exercise capacity tend to have longer sitting times. However, the degree of relationship between ST and exercise capacity was lower than that of FEV1. This result was different from reports of PA, in which the degree of relationship between exercise capacity and PA was higher than that of FEV1.14,18,22,29,33 Although exercise capacity could influence ST, the degree of association may be lower for ST than for PA. This might be one of the differences between ST and PA. The mMRC was also associated with ST, but the degree of relationship with ST was lower than that of FEV1. Although exercise capacity and dyspnea were strongly associated with PA,34 the degree of association of mMRC might be lower for ST than for PA, similar to exercise capacity.
BMI showed a weak negative association with ST, indicating that patients with a lower BMI had a longer ST. BMI in patients with reduced PA was significantly lower than in patients with maintained PA,35 and it was positively associated with PA in Japanese COPD patients.36 The patients with a worse BMI or BODE index (consisting of BMI, FEV1%pred, mMRC, and 6MWD) had a shorter PA duration.18,37 From these reports, it is assumed that the lower the BMI, the longer the ST, which is consistent with the current results. A meta-analysis that included subjects other than COPD found that increased BMI was associated with prolonged ST,38 which was not consistent with the current results. In that report, most patients were obese (BMI ≥30). In contrast, COPD patients with a BMI of 22.4±3.3 were recruited for the current study. The difference may have been responsible for the different results regarding the association between BMI and ST. These results suggest that increased BMI in obese subjects and decreased BMI in normal-weight or thin COPD patients might be associated with prolonged ST.
We could create a reference equation for ST using BMI, FEV1%pred, 6MWD, and mMRC. The calculated values of ST using the equation were significantly correlated with the measured values of ST; however, a proportional error was found among them using Bland-Altman Plots. The calculated values were higher than the measured values in patients with lower values. We then re-evaluated patients with an ST of ≥6 h. The results showed that both ST values were significantly correlated, and there was no systematic error among them in the Bland-Altman Plots. As sitting time in Japanese subjects of 18 to 39 years of age is reported to be 7 hours,39 an ST of <6 hours is considered to be short enough for patients with COPD who are mostly elderly. Therefore, this reference equation could be useful for reducing the ST in patients with COPD.
The present study was associated with several limitations. First, the determination of the required number of patients was based on the relationships between PA and related factors but not between ST and the factors. Therefore, it is unclear whether this number of recruited patients was sufficient for evaluation. Accordingly, a larger number of patients may be required for evaluation. Second, we could not evaluate the effects of osteoporosis, the presence or absence of work or hobbies, or household environment (whether the participant lived alone or with others), although these factors have been reported to be associated with PA.30,40–43 Third, this study only targeted Japanese patients with COPD, and most of the subjects were men; therefore, further studies are required for patients from other countries and women.
Conclusion
We verified the proper processing of the ST measurement data, which was evaluated with an accelerometer. We then created a reference equation for assessing ST using ST-related factors, including BMI, FEV1%pred, 6MWD, and mMRC. This equation is expected to be useful for providing individual guidance to shorten ST in patients with COPD.
Acknowledgments
The subjects were recruited from following institutes belonging to the NHO group Japan: NHO Asahikawa Medical Center, NHO Takasaki General Medical Center, NHO Yokohama Medical Center, NHO Kanazawa Medical Center, NHO Tenryu Hospital, NHO Minami Kyoto Hospital, NHO Kinki-chuo Chest Medical Center, NHO Osaka Toneyama Medical Center, NHO Osaka Minami Medical Center, NHO Himeji Medical Center, NHO Nara Medical Center, NHO Minami Wakayama Medical Center, NHO Wakayama Hospital, NHO Minami-Okayama Medical Center, NHO Yamaguchi-Ube Medical Center, NHO Ehime Medical Center, NHO Kochi National Hospital, NHO Fukuoka Hospital. The authors thank Brian Quinn for reviewing the manuscript.
Disclosure
Y. Minakata received grants from Environmental Restoration and Conservation Agency, Japan and NHO-Evidence-based Medicine (EBM) study group, Japan, during the conduct of the study, and lecture fees from Nippon Boehringer Ingelheim, Japan, outside the submitted work. The authors declare no other conflicts of interest in association with the present study.
References
1. Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the global burden of disease study. Lancet. 2010;380(9859):2095–2128. doi:10.1016/S0140-6736(12)61728-0
2. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the global burden of disease study. Lancet. 2010;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
3. Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi:10.1378/chest.10-2521
4. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. 2024; Available from: www.goldcopd.org/2024-gold-report/.
5. Furlanetto KC, Donaria L, Schneider LP, et al. Sedentary behavior is an independent predictor of mortality in subjects with COPD. Respir Care. 2017;62(5):579–587. doi:10.4187/respcare.05306
6. Pate RR, O’Neill JR, Lobelo F. The evolving definition of ”sedentary”. Exerc Sport Sci Rev. 2008;36(4):173–178. doi:10.1097/JES.0b013e3181877d1a
7. Owen N, Healy GN, Matthews CE, Dunstan DW. Too much sitting: the population health science of sedentary behavior. Exerc Sport Sci Rev. 2010;38(3):105–113. doi:10.1097/JES.0b013e3181e373a2
8. Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35(6):725–740. doi:10.1139/H10-079
9. Sedentary Behavior Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37(3):540–542. doi:10.1139/h2012-024
10. Minakata Y, Sasaki S. Data reproducibility and effectiveness of bronchodilators for improving physical activity in COPD patients. J Clin Med. 2020;9(11):3497. doi:10.3390/jcm9113497
11. Demeyer H, Mohan D, Burtin C, et al. Objectively measured physical activity in patients with COPD: recommendations from an international task force on physical activity. Chronic Obstr Pulm Dis. 2021;8(4):528–550. doi:10.15326/jcopdf.2021.0213
12. Burtin C, Mohan D, Troosters T, et al. Objectively measured physical activity as a COPD clinical trial outcome. Chest. 2021;160(6):2080–2100. doi:10.1016/j.chest.2021.06.044
13. Miyamoto S, Minakata Y, Azuma Y, et al. Verification of a motion sensor for evaluating physical activity in COPD patients. Can Respir J. 2018;2018:8343705. doi:10.1155/2018/8343705
14. Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi:10.1164/rccm.200407-855OC
15. Oshima Y, Kawaguchi K, Tanaka S, et al. Classifying household and locomotive activities using a triaxial accelerometer. Gait Posture. 2010;31(3):370–374. doi:10.1016/j.gaitpost.2010.01.005
16. Ohkawara K, Oshima Y, Hikihara Y, Ishikawa-Takata K, Tabata I, Tanaka S. Real-time estimation of daily physical activity intensity by a triaxial accelerometer and a gravity-removal classification algorithm. Br J Nutr. 2011;105(11):1681–1691. doi:10.1017/S0007114510005441
17. Choi L, Liu Z, Matthews CE, Buchowski MS. Validation of accelerometer wear and nonwear time classification algorithm. Med Sci Sports Exerc. 2011;43(2):357–364. doi:10.1249/MSS.0b013e3181ed61a3
18. Minakata Y, Sugino A, Kanda M, et al. Reduced level of physical activity in Japanese patients with chronic obstructive pulmonary disease. Respir Investig. 2014;52(1):41–48. doi:10.1016/j.resinv.2013.06.002
19. Baranowski T, de Moor C. How many days was that? Intra-individual variability and physical activity assessment. Res Q Exerc Sport. 2000;71(2 Suppl):S74–8. doi:10.1080/02701367.2000.11082789
20. Matthews CE, Ainsworth BE, Thompson RW, Bassett DR. Sources of variance in daily physical activity levels as measured by an accelerometer. Med Sci Sports Exerc. 2002;34(8):1376–1381. doi:10.1097/00005768-200208000-00021
21. Tudor-Locke C, Burkett L, Reis JP, Ainsworth BE, Macera CA, Wilson DK. How many days of pedometer monitoring predict weekly physical activity in adults? Prev Med. 2005;40(3):293–298. doi:10.1016/j.ypmed.2004.06.003
22. Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi:10.1183/09031936.00024608
23. Bertoche MP, Furlanetto KC, Hirata RP, et al. Assessment of sedentary behaviour in individuals with COPD: how many days are necessary? ERJ Open Res. 2023;9(4):732–2022. doi:10.1183/23120541.00732-2022
24. Sugino A, Minakata Y, Kanda M, et al. Validation of a compact motion sensor for the measurement of physical activity in patients with chronic obstructive pulmonary disease. Respiration. 2012;83(4):300–307. doi:10.1159/000330046
25. Alahmari AD, Mackay AJ, Patel AR, et al. Influence of weather and atmospheric pollution on physical activity in patients with COPD. Respir Res. 2015;16(1):71. doi:10.1186/s12931-015-0229-z
26. Balish SM, Dechman G, Hernandez P, et al. The relationship between weather and objectively measured physical activity among individuals with COPD. J Cardiopulm Rehabil Prev. 2017;37(6):445–449. doi:10.1097/HCR.0000000000000244
27. Vaidya T, Thomas-Ollivier V, Hug F, et al. Translation and cultural adaptation of proactive instruments for COPD in French and influence of weather and pollution on its difficulty score. Int J Chron Obstruct Pulmon Dis. 2020;15:471–478. doi:10.2147/COPD.S214410
28. Gretebeck RJ, Montoye HJ. Variability of some objective measures of physical activity. Med Sci Sports Exerc. 1992;24(10):1167–1172. doi:10.1249/00005768-199210000-00016
29. Steele BG, Holt L, Belza B, Ferris S, Lakshminaryan S, Buchner DM. Quantitating physical activity in COPD using a triaxial accelerometer. Chest. 2000;117(5):1359–1367. doi:10.1378/chest.117.5.1359
30. Ichinose M, Minakata Y, Motegi T, et al. A non-interventional, cross-sectional study to evaluate factors relating to daily step counts and physical activity in Japanese patients with chronic obstructive pulmonary disease: STEP COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:3385–3396. doi:10.2147/COPD.S277782
31. Waschki B, Spruit MA, Watz H, et al. Physical activity monitoring in COPD: compliance and associations with clinical characteristics in a multicenter study. Respir Med. 2012;106(4):522–530. doi:10.1016/j.rmed.2011.10.022
32. Saunders T, Campbell N, Jason T, et al. Objectively measured steps/day in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Phys Act Health. 2016;13(11):1275–1283. doi:10.1123/jpah.2016-0087
33. Kawagoshi A, Kiyokawa N, Sugawara K, et al. Quantitative assessment of walking time and postural change in patients with COPD using a new triaxial accelerometer system. Int J Chron Obstruct Pulmon Dis. 2013;8:397–404. doi:10.2147/COPD.S49491
34. Minakata Y, Sasaki S, Azuma Y, Kawabe K, Ono H. Reference equations for assessing the physical activity of Japanese patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:3041–3053. doi:10.2147/COPD.S336670
35. Kobayashi S, Chiba F, Ishida M, et al. Physical activity and outcomes in Japanese patients with chronic obstructive pulmonary disease: from the Ishinomaki COPD Network registry. Respir Investig. 2024;62(1):107–112. doi:10.1016/j.resinv.2023.10.007
36. Yoshida M, Hiramoto T, Moriwaki A, Osoreda H, Iwanaga T, Inoue H. Impact of extrapulmonary comorbidities on physical activity in chronic obstructive pulmonary disease in Japan: a cross-sectional study. PLoS One. 2022;17(7):e0270836. doi:10.1371/journal.pone.0270836
37. Ramon MA, Esquinas C, Barrecheguren M, et al. Self-reported daily walking time in COPD: relationship with relevant clinical and functional characteristics. Int J Chron Obstruct Pulmon Dis. 2017;12:1173–1181. doi:10.2147/COPD.S128234
38. Silveira EA, Mendonça CR, Delpino FM, et al. Sedentary behavior, physical inactivity, abdominal obesity and obesity in ad ults and older adults: a systematic review and meta-analysis. Clin Nutr ESPEN. 2022;50:63–73. doi:10.1016/j.clnesp.2022.06.001
39. Bauman A, Ainsworth BE, Sallis JF, et al. The descriptive epidemiology of sitting. A 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med. 2011;41(2):228–235. doi:10.1016/j.amepre.2011.05.003
40. Li Y, Gao H, Zhao L, Wang J. Osteoporosis in COPD patients: risk factors and pulmonary rehabilitation. Clin Respir J. 2022;16(7):487–496. doi:10.1111/crj.13514
41. Liu WT, Kuo HP, Liao TH, et al. Low bone mineral density in COPD patients with osteoporosis is related to low daily physical activity and high COPD assessment test scores. Int J Chron Obstruct Pulmon Dis. 2015;10:1737–1744. doi:10.2147/COPD.S87110
42. Donesky D, Janson SL, Nguyen HQ, Neuhaus J, Neilands TB, Carrieri-Kohlman V. Determinants of frequency, duration, and continuity of home walking in patients with COPD. Geriatr Nurs. 2011;32(3):178–187. doi:10.1016/j.gerinurse.2011.01.011
43. Mesquita R, Nakken N, Janssen DJA, et al. Activity levels and exercise motivation in patients with COPD and their resident loved ones. Chest. 2017;151(5):1028–1038. doi:10.1016/j.chest.2016.12.021
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




