Back to Journals » Drug Design, Development and Therapy » Volume 19
Procyanidin Improves Erectile Function in Rats by Inhibiting PDE5A Activity
Authors Su Y, Shui J, Qi D, Bai J, Xu X, Huang Y, Li R, Wu Q, Wang H, Cao C, Su Z, Zhang S
Received 31 December 2024
Accepted for publication 22 April 2025
Published 30 April 2025 Volume 2025:19 Pages 3351—3361
DOI https://doi.org/10.2147/DDDT.S514209
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Muzammal Hussain
Yudong Su,1,2,* Jinman Shui,1,* Danshi Qi,1 Jie Bai,1 Xiaoxia Xu,2 Yongchun Huang,1 Ruilian Li,1 Qiong Wu,2 Haiyan Wang,2 Chengzhu Cao,2 Zhanhai Su,2 Shoude Zhang1,3
1School of Pharmacy, Qinghai University, Xining, Qinghai, People’s Republic of China; 2Department of Basic Medical Sciences, Qinghai University Medical College, Xining, Qinghai, People’s Republic of China; 3State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, Qinghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhanhai Su, School of Pharmacy, Qinghai University, Xining, Qinghai, People’s Republic of China, Email [email protected] Shoude Zhang, School of Pharmacy, Qinghai University, Xining, Qinghai, People’s Republic of China, Email [email protected]
Purpose: Erectile dysfunction (ED), a prevalent form of male sexual dysfunction, is predominantly treated with Phosphodiesterase type 5 inhibitors (PDE5Is). Our previous research highlighted procyanidin, a natural compound, as a notably effective PDE5I. In the current study, we intend to further validate the inhibitory activity of procyanidin on PDE5A through in vitro and in vivo assessments. This study aims to validate the efficacy of procyanidin as a treatment for ED.
Methods: The binding affinity of procyanidin for PDE5A was assessed by molecular docking, molecular dynamics (MD) simulations, and microscale thermophoresis (MST) assay. The toxicity of procyanidin on penile corpus cavernosum smooth muscle (CCSM) cells (n=5) was evaluated. Additionally, its effects on intracellular cyclic guanosine monophosphate (cGMP) levels in CCSM cells (n=5) were evaluated. The absorption of procyanidin was evaluated by measuring plasma levels at various times after oral gavage to Sprague-Dawley (SD) rats (n=6). Subsequently, the effects of procyanidin on intracavernous pressure (ICP) and cGMP levels in penile cavernous tissue were evaluated in SD rats (n=6).
Results: Procyanidin forms three hydrogen bonds with PDE5A and stabilizes the complex structure, exhibiting equilibrium dissociation constants (KD) value of 7.77 ± 2.39 μmol/L. Additionally, procyanidin exhibits minimal cytotoxicity toward CCSM cells and significantly elevates intracellular cGMP levels compared to the control group. In vivo studies demonstrate that procyanidin is rapidly absorbed, achieving peak blood concentrations within one hour. Simultaneously, procyanidin significantly increases ICP and cGMP levels in rats compared to the control group.
Conclusion: These findings indicate that procyanidin sustains elevated cGMP levels within cells by targeting PDE5A, thereby exhibiting therapeutic efficacy in improving ICP. Procyanidin emerges as a promising PDE5I for treating ED and potentially other related conditions.
Keywords: sexual dysfunctions, erectile dysfunction, cyclic guanosine monophosphate, microscale thermophoresis, cavernosum smooth muscle cells
Graphical Abstract:

Introduction
Erectile dysfunction (ED) was defined by the 4th International Consultation on Sexual Medicine as the persistent or recurrent inability to obtain and/or maintain a penile erection sufficient for satisfactory sexual activity.1 ED is a common condition affecting up to 30 million men in the United States.2 As of 1995 estimates, over 150 million men globally were afflicted by ED, with projections indicating that this number could soar to 322 million by 2025.3 Erectile function is governed by coordinated vascular and neural processes. The internal pudendal artery primarily supplies blood to the penis through its cavernous branches, while venous outflow is facilitated by a network of easily compressible veins to occur.4 When sexual arousal occurs, parasympathetic activity in the sacral segments of the spinal cord triggers a cascade of events that releases nitric oxide and increases intracellular cyclic guanosine monophosphate (cGMP). This increase in cGMP leads to the relaxation of vascular smooth muscle and a surge in blood flow into the cavernous body.5 This rapid influx of blood compresses the micro-vein network, reducing venous outflow and thereby increasing the pressure within the cavernous body of the penis, resulting in an erection.4–6 Thus, ED may arise from any pathological process that impairs the integrity of the neural or vascular mechanisms essential for penile erection. Chronic diseases such as diabetes and hypertension are recognized as risk factors for the development of ED,7 which itself is a risk factor for cardiovascular and metabolic diseases.8 Studies have shown that the severity of a patient’s ED may be linked to an increased risk of cardiovascular disease, with ED occurring up to five years prior to a cardiovascular event.9 Although ED is not life-threatening, it can significantly diminish the quality of life for both the affected individual and their partner. Treatment for ED, according to the American Urological Association guidelines, includes both non-invasive and invasive options such as oral phosphodiesterase type 5 inhibitors (PDE5I), vacuum-loaded erection devices (VEDs), intracorporeal penile injections (ICIs), intraurethral suppositories, and penile prostheses.10 Oral PDE5Is, including sildenafil, tadalafil, vardenafil, and avanafil, are often preferred by clinicians as first-line treatment due to their clinical efficacy and safety.11,12 These medications function by preventing the degradation of cGMP by inhibiting PDE5A activity, thereby maintaining high levels of cGMP.13,14 Up to 65% of men taking PDE5I report a favorable response after initial treatment.15 However, due to the high degree of homology among members of the PDEs family, these drugs can exhibit various side effects, including headaches, flushing, indigestion, and visual disturbances.16,17
Traditional Chinese medicines are characterized by fewer adverse effects and higher safety, providing a rich resource for exploring promising PDE5I. Therefore, traditional Chinese medicines would be another important strategy for screening PDE5Is.18 Owing to these considerations, recent investigations have increasingly concentrated on traditional Chinese medicines as a means of identifying PDE5Is from their phytochemical constituents to ameliorate ED.
Procyanidins are polyphenolic compounds that are widely found in fruits, barks, leaves, and seeds of many plants and derived foods such as apples, cocoa, grapes, fruit juices, green tea, chocolate, and red wine.19,20 Procyanidins are recognized as natural compounds endowed with a spectrum of biological activities, including antioxidant, anti-inflammatory, immune-enhancing, anti-tumor, and neuroprotective effects.21 Our previous research established a novel method for assessing PDE5A enzyme activity using LC-MS/MS and identified procyanidin as a new PDE5I.22 However, the in vivo efficacy of procyanidin in treating diseases associated with PDE5A remains to be elucidated. In this paper, we further affirmed the binding affinity of procyanidin for PDE5A through both in vitro and in vivo studies, providing a theoretical basis for procyanidin as a new PDE5I and a potential treatment for ED.
Materials and Methods
Compounds and Reagents
Most of the reagents were obtained from BBI Life Sciences Corporation (Shanghai, China). Procyanidin was purchased from Herbest Biotechnology (Baoji, China). Sildenafil citrate powder was purchased from Aladdin (Shanghai, China) for cellular experiments, and sildenafil citrate tablets were purchased from Pfizer Pharmaceuticals Inc (New York, USA) for animal experiments. Cyclic guanosine monophosphate (cGMP) enzyme immunoassay kit and heparin sodium were purchased from BBI Life Sciences Corporation (Shanghai, China). HPLC-grade acetonitrile, HPLC-grade formic acid, and HPLC-grade methanol were purchased from Thermo Fisher Scientific (Boston, USA). Dulbecco’s modified eagle medium/Nutrient Mixture F-12 (DMEM/F12) and fetal bovine serum (FBS) were purchased from Procell Life Science (Wuhan, China). The purchase of dimethyl sulfoxide (DMSO ≥ 99%) was from Solarbio (Beijing, China). Other commonly used consumable materials, such as CCK-8 kits, penicillin-streptomycin, and phosphate-buffered saline (PBS), were purchased from Beyotime Biotechnology (Shanghai, China).
Molecular Docking
The X-ray crystal structure of the PDE5A (PDB: 8W4S) was prepared by MGL Tools, and the procyanidin structure was retrieved from PubChem. The docking site, defined as residues within 40 Å of the ligand sildenafil, was specified by the coordinates X [38.749, 55.709], Y [−24.131, 8.675], and Z [163.739, 180.582]. Docking was performed using Ledock, generating 10 conformational isomers of procyanidin.23 To validate the method, ligands in 8W4S were redocked, achieving a low RMSD value to confirm accuracy. Docking results were ranked by affinity to identify the optimal procyanidin conformation for binding to PDE5A.
Molecular Dynamics (MD) Simulation
In this study, Gromacs (v2023.3) was employed for comprehensive molecular dynamics (MD) simulation.24 Initially, proteins were separated from small-molecule ligands based on docking results. Ligand force field parameters were generated using the antechamber tool in AmberTools and converted to Gromacs-compatible format with ACPYPE. During simulations, ligands were modeled with the Generalized Amber Force Field (GAFF), while proteins were described using the AMBER14SB force field paired with the TIP3P water model. The processed protein and ligand files were then combined to create the simulation system. Simulations were conducted at constant temperature (298 K, V-rescale thermostat) and pressure (1 bar, Berendsen barostat), with periodic boundary conditions applied for system stability. The LINCS algorithm constrained hydrogen bonds, with a 2 fs time step. Electrostatic interactions were computed via the Particle Mesh Ewald (PME) method (1.2 nm cutoff), and non-bonded interactions used a 10 Å cutoff, updated every 10 steps. The system was equilibrated for 100 ps under NVT and NPT ensembles before a 100 ns production MD run, with snapshots recorded every 10 ps. Trajectories were analyzed using VMD and PyMOL.
MicroScale Thermophoresis (MST)
MST measurements of procyanidin binding to PDE5A were performed using a Monolith system (NanoTemper). PDE5A was expressed by constructing a pET21b recombinant plasmid encoding its catalytic domain, followed by transfection into Escherichia coli BL21(DE3). The protein was purified via Ni-NTA affinity chromatography and HiPrep 26/60 Sephacryl S-200 hR columns (GE Healthcare), achieving >95% purity as confirmed by SDS-PAGE. For the experiment, PDE5A was diluted to 1 µM using standard buffer [PBS containing 0.05% Tween-20, 150 mm NaCl, 10 mm MgCl2, pH 7.5] and labeled with RED-NHS. Procyanidin was then diluted to different concentrations and incubated with labeled PDE5A away from light for 30 min at room temperature. Each sample was loaded into a NanoTemper glass capillary tube and assayed with 30% excitation light. Fluorescence signals were normalized to correct for intensity variations, and a binding curve (fluorescence signal vs ligand concentration) was plotted, yielding an inverted S-shaped curve. A single-site binding model was fitted using nonlinear least-squares regression to determine the KD. The experiment was conducted in triplicate.
Cell Culture
CCSM cells, obtained from Procell Life Science (Cat no.: CP-R203), were cultured in DMEM/F12 supplemented with 20% FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere with 5% CO2. For experiments, cells passaged fewer than five times were used. Prior to experimentation, cell health was assessed by evaluating growth density and morphology, selecting cells in the exponential growth phase at 90% confluence.
Cell Counting Kit-8 (CCK-8) Assay
CCSM cells in the logarithmic growth phase were seeded into 96-well plates at 5000 cells per well and incubated overnight at 37°C in a 5% CO2 incubator. The following day, the medium was replaced with serum-free DMEM/F12 containing varying concentrations of test compounds. After 24 hours of treatment, 10 µL of CCK-8 solution was added to each well, followed by a 2-hour incubation period. Cell viability was determined by measuring absorbance at 450 nm using a Varioskan LUX multimode microplate reader (Thermo Fisher Scientific).
Quantification of cGMP of Corpus Cavernosum Smooth Muscle (CCSM) Cells
CCSM cells in the logarithmic growth phase were seeded into 6-well plates at 2×104 cells per well and cultured at 37°C in a 5% CO2 incubator until 70–80% confluence. The medium was then removed, cells were washed three times with prewarmed PBS, and treated with drug-containing medium for 24 hours. Subsequently, cells were detached using 0.25% trypsin, lysed by ultrasonic disruption, and centrifuged at 12,500 rpm for 10 minutes at 4°C. The supernatant was collected, and cGMP content was measured using an ELISA kit.
Animals and Experimental Design
Male Sprague-Dawley rats (8 weeks old, 200–240 g) were sourced from Xi’an Jiaotong University. They were housed in a controlled facility with a 12-hour light/dark cycle, ad libitum access to food and water, and bedding changed thrice weekly. Following a one-week acclimatization, rats with body weights within ± 20 g were selected, numbered, and randomly assigned to three groups (n=6 each) using computer-generated random numbers: control (saline, 1 mL/100 g), sildenafil (sildenafil citrate, 10 mg/kg), and procyanidin (procyanidin, 100 mg/kg). The experimental design yielded a statistical power of approximately 0.7. For the procyanidin absorption study, rats (n=6) received procyanidin via gavage at 100 mg/kg. Each experiment was conducted as an independent observation. During invasive procedures, rats were maintained under deep anesthesia (confirmed by loss of eyelid reflexes), and at the study’s end, they were euthanized with an overdose of pentobarbital.
Intracavernous Pressure (ICP) Measurement
Rats were deeply anesthetized with sodium pentobarbital and secured to a small-animal surgical table The abdomen was shaved and disinfected with povidone-iodine. A 5 cm midline abdominal incision was made, and the intestines and other organs were gently displaced to the upper abdominal cavity using a sterile cotton swab. Under a surgical microscope, the pelvic ganglion was identified at the dorsal aspect of the prostate’s posterior lobe, at the junction with the vas deferens. The largest downward branch from the pelvic ganglion was confirmed as the cavernous nerve. The cavernous nerve was isolated, and the abdominal cavity was kept moist. Subsequently, a modified crus of penis intubation method was employed,25 which better preserves penile structural integrity compared to cavernous cannulation. A 1–2 cm incision was made at the intersection of the pubic symphysis and parapenis, and muscle tissue was bluntly dissected with curved forceps to expose the crus of penis. An infusion needle was inserted into the penile pedicle, secured to prevent displacement. A small volume of heparinized saline was slowly injected, with successful cannulation confirmed by corpus cavernosum expansion. The cavernous nerve was then stimulated using a bipolar electrode (7 V, 25 HZ, 1 minute), avoiding short-circuiting. ICP was recorded with a BL420N Integrated Biosignal Acquisition and Analysis System (Techman Corporation).
Mean Arterial Pressure (MAP) Measurement
Rats were anesthetized via intraperitoneal injection of sodium pentobarbital, and the left carotid artery was isolated through a left cervical incision. An infusion needle, pre-connected to a pressure transducer and filled with heparinized saline, was carefully inserted into the artery and secured. MAP changes were detected using a BL420N Integrated Biosignal Acquisition and Analysis System (Techman Corporation). The MAP curve was continuously recorded for 10–15 minutes until stabilization, after which formal measurements were initiated.
cGMP Quantification of Penile Corpus Cavernosum Tissue
Rats from each group were deeply anesthetized with sodium pentobarbital, and the penis was exposed by carefully incising the skin. Tissue was excised from the penile root. The glans was removed, and the corpus cavernosum was isolated. The tissue was washed three times with pre-chilled PBS to remove residual blood, and the leucomembranous tissue was meticulously separated on ice. The cavernous tissue was then snap-frozen in liquid nitrogen for storage. A 100 mg tissue sample was placed in a small beaker. 0.86% ice-cold saline was added, and the beaker was kept in an ice-water bath while the tissue was minced with ophthalmic scissors. The tissue suspension was homogenized in a tissue grinder at 10,000–15,000 rpm to prepare a 10% homogenate. The homogenate was centrifuged at 3000 rpm for 10–15 minutes at 4°C. The supernatant was collected, and the pellet was discarded. The supernatant was stored at −80°C. An appropriate volume of supernatant was analyzed using an ELISA kit, following the manufacturer’s instructions, to measure absorbance and calculate the target concentration.
Determination of Procyanidin in Blood
The night before the experiment, Sprague-Dawley (SD) rats were fasted but had ad libitum access to water. Prior to the experiment, rats were deeply anesthetized via intraperitoneal injection of sodium pentobarbital. Procyanidin solution (100 mg/kg) was administered by gavage. Venous blood was collected from the retro-orbital plexus at 0, 0.5, 1, 2, 4, 6, 8, and 10 hours post-administration. Blood samples were incubated at room temperature for 1 hour, then centrifuged at 3500 rpm for 10 minutes to isolate serum, which was stored at 4°C. To 100 µL of serum, 400 µL of methanol was added, vortexed for 2 minutes to precipitate proteins, and centrifuged at 13,000 rpm for 10 minutes at 4°C. From the supernatant, 400 µL was evaporated under nitrogen and lyophilized. The residue was reconstituted in 100 µL of ultrapure water, filtered through a 0.22 µm nylon membrane, and stored at 4°C for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis. Procyanidin standards (0, 1.56, 3.13, 6.25, 12.5, 25, 50, and 100 µmol/L) were prepared, and their peak areas were measured by LC-MS/MS to generate a concentration-peak area calibration curve (y = 0.1659x + 0.2329, R² = 0.9981).
LC-MS/MS Instrumentation and Conditions
Mass spectrometry was performed using a Q Exactive Focus-Orbitrap MS (Thermo Fisher Scientific) with the following conditions: electrospray ionization (ESI) in positive mode, full MS scan; sheath gas flow rate, 35 L/min; auxiliary gas flow rate, 10 L/min; spray voltage, 4.5 kV; capillary temperature, 320°C; S-lens voltage, 60 V; auxiliary gas heater temperature, 300°C; scan range, 100–1500 m/z. Liquid chromatography was conducted on a Thermo Fisher Ultimate 3000+ system using a Waters Cortecs C18 column (2.1 mm × 100 mm, 1.6 μm). The mobile phases were 0.2% formic acid in acetonitrile (A) and 0.1% formic acid in water (B), with a flow rate of 0.2 mL/min. Gradient elution was as follows: 0–5 min, 60% B; 5–14 min, 60% to 30% B; 14–16 min, 30% to 60% B; 16–18 min, 60% B. The column temperature was 30°C, injection volume was 0.5 µL, and run time was 18 minutes.
Statistical Analysis
All results are expressed as mean ± SD and analyzed using GraphPad Prism 9.4.1. The “Brown-Forsythe test” was used to determine the homogeneity of the variances, Differences were analyzed using one-way analysis of variance for multiple groups, Differences between groups were analyzed using “Šídák’s multiple comparisons test”. P < 0.05 was considered statistically significant.
Ethics Approval and Consent to Participate
The protocol of this study was approved by the Ethical Committee of Qinghai University. All animal experiments conformed to the Guidelines for the Care and Use of Experimental Animals established by the Ministry of Science and Technology of the People’s Republic of China (Approved number: 2006–398).
Results
Procyanidin Directly Binds PDE5A by Forming Hydrogen Bonds
Procyanidin is a polyphenolic natural compound composed of catechin and epicatechin oligomers (Figure 1A).26 To investigate its interaction with PDE5A, we conducted MST experiment using recombinant PDE5A. MST, a biophysical technique used to measure molecular interactions, enables the determination of equilibrium dissociation constants (KD) in solution by monitoring molecular movement under a temperature gradient. A fixed concentration of fluorescently labeled PDE5A was titrated with procyanidin (ranging from 20 µmol/L to 600 nmol/L), loaded into capillary tubes, and analyzed using a Monolith system. The results showed a dose-dependent effect, with increasing procyanidin concentrations significantly altering PDE5A’s thermophoretic mobility. The KD for procyanidin binding to PDE5A was 7.77 ± 2.39 µmol/L (Figure 1B), while that of sildenafil was 0.153 ± 0.127 µmol/L. To elucidate the binding mode between procyanidin and PDE5A, molecular docking simulations were conducted. The docking revealed a binding energy of −7.5 kmol/L for procyanidin with PDE5A. Structural analysis showed that procyanidin formed three key hydrogen bonds with PDE5A residues GLN 817, MET 816, stabilizing the binding conformation essential for inhibiting PDE5A activity (Figure 1C). Notably, two hydrogen bonds involve GLN817, suggesting its pivotal role in the interaction. To assess binding stability, molecular dynamics (MD) simulations were performed on the procyanidin-PDE5A complex. As depicted in Figure 1D, the root mean square deviation (RMSD) remained stable throughout the simulation, indicating a robust and equilibrated complex. To further analyze the system, energy components—including solvation energy, buried solvent-accessible surface area, and interaction energies were evaluated, and steady-state trajectories were selected for Molecular Mechanics-Poisson Boltzmann Surface Area (MM-PBSA) calculations. Energy contributions to the binding affinity energy are summarized in Table 1. Analysis reveals that van der Waals energy (ΔEvdw) predominates in the binding of procyanidin to PDE5A, exceeding electrostatic interaction energy (ΔEele). The calculated binding free energy (ΔEBind) was −68.81 kJ/mol, indicating a strong binding affinity for PDE5A. These results suggest that procyanidin can directly bind to PDE5A.
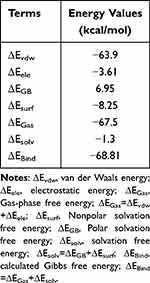 |
Table 1 The Calculated Binding Free Energy (kJ/Mol) for the Procyanidin and the PDE5A Complexes |
Procyanidin Increases the Level of cGMP in CCSM Cells
CCSM cells, comprising 40% to 52% cavernous tissue, are critical to erectile function.27 cGMP, a direct substrate of PDE5A enzyme, activates protein kinase G (PKG), reducing intracellular calcium in CCSM cells. This signaling process leads to cellular relaxation and enhances blood flow into the cavernous sinusoidal space to facilitate erection.28,29 To ensure safe drug concentrations for cellular studies, we first evaluated the cytotoxicity of procyanidin on CCSM cells using the CCK-8 assay. Procyanidin exhibited a mild, dose-dependent inhibitory effect, with cell viability remaining above 80% at a maximum concentration of 100 µM, indicating an IC50 exceeding 100 µM (Figure 2A). To minimize cytotoxicity, a 40 µM concentration was selected for subsequent experiments. CCSM cells were then treated with 40 µM procyanidin, using sildenafil (5 µM) as a positive control. Procyanidin significantly increased cGMP levels compared to the control (P = 0.0242), an effect consistent with sildenafil (P = 0.0002) (Figure 2B).
Procyanidin Reaches Maximal Absorption within 1 hour Post- Gavage in Rats
Understanding the absorption of procyanidin is crucial for its application as PDE5I in treating erectile dysfunction. We assessed its absorption in rats by collecting blood samples at 0, 0.5, 1, 2, 4, 6, 8, and 10 hours post-gavage. Samples were analyzed using liquid chromatography-tandem mass spectrometry (LC-MS/MS) to quantify the fragmentation peaks of procyanidin at each time point (Figure 3A).30 Two distinct absorption peaks of procyanidin were observed at 1 hour and 6 hours within the 0–10 hour timeframe, with the maximal peak at 1 hour (Figure 3B). The secondary peak at 6 hours may result from enterohepatic circulation.31 Based on these findings, we propose that procyanidin reaches maximal absorption within 1 hour post-gavage in rats, providing a temporal reference for optimizing ICP measurements in subsequent animal studies.
Procyanidin Increases Penile Intracavernous Pressure by Maintaining High cGMP Levels in vivo
The nitric oxide (NO)–cGMP signaling pathway mediates smooth muscle relaxation, enabling penile erection.5 NO, released by penile nerves and endothelial cells, activates guanylate cyclase to produce cGMP, which lowers intracellular calcium levels, inducing arterial and smooth muscle relaxation. These changes trigger the relaxation of arterial and smooth muscle, resulting in arteriolar dilation, venoconstriction, and ultimately, penile erection.4–6 PDE5A, the predominant phosphodiesterase isoform in the corpus cavernosum, degrades cGMP to GMP. PDE5Is, such as sildenafil, exert therapeutic effects by blocking cGMP degradation.6,14 To evaluate the therapeutic potential of procyanidin as PDE5I in vivo, we conducted experiments in SD rats. Informed by prior absorption studies indicating peak procyanidin levels at 1 hour post-gavage, we measured ICP and MAP at this time point, aligning with sildenafil’s clinical onset (~1 hour).32 Procyanidin significantly increased the maximum ICP-to-MAP ratio (ICPMax/MAP) compared to controls (P = 0.0429), an effect consistent with sildenafil (P = 0.0076) (Figure 4A–D). As cGMP is a key mediator in PDE5I-mediated ED treatment, we quantified its levels in penile cavernous tissue immediately following ICP measurements. Procyanidin significantly elevated cGMP content compared to the control group (P = 0.0295), mirroring sildenafil’s effect (P = 0.0189) (Figure 4E). These results suggest that procyanidin enhance erectile function in vivo by sustaining elevated cGMP levels in penile tissue, thereby augmenting ICP.
Discussion
ED, one of the most common male sexual dysfunctions, profoundly impacts patients’ quality of life and may signal underlying cardiovascular disease. Oral PDE5Is, including sildenafil, tadalafil, vardenafil, and avanafil, remain the primary treatment. However, a growing number of patients show limited or no response to these therapies and may experience adverse effects, underscoring the urgent need for safer, more effective therapeutic alternatives.33
Procyanidin, previously identified as a PDE5I, lacks detailed characterization of its binding affinity and mode. Our analysis revealed that procyanidin directly bind to the catalytic site of PDE5A with moderate affinity (KD=7.77 µM). Molecular docking and dynamics simulations further confirmed its binding mode and affinity to PDE5A.
Subsequently, we observed a significant elevation in intracellular cGMP levels following co-incubation of procyanidin with CCSM cells. However, it is less effective as the positive drug sildenafil in increasing intracellular cGMP levels in CCSM, which may be related to its moderate affinity for PDE5A.
To better elucidate the efficacy of procyanidin as a new PDE5I for treating erectile dysfunction, further studies in vivo are conducted. Initially, to determine the optimal timing for the procyanidin efficacy, we conducted blood concentration measurements following gavage. It was found that procyanidin reaches the maximum absorption around 1 hour post-gavage. This finding served as a valuable reference for determining the appropriate timing to measure ICP in rats. Meanwhile, we observed a secondary absorption peak at 6 hours post-gavage, suggesting the presence of enterohepatic circulation for procyanidin. Following metabolism by intestinal microorganisms, the metabolites of procyanidin may re-enter systemic circulation through enterohepatic circulation, which can influence the bioavailability and therapeutic efficacy of procyanidin. Moreover, this process may extend the duration of procyanidin activity within the body. These findings could significantly impact the dosing regimen of procyanidin in clinical settings, necessitating adjustments in administration frequency to manage drug concentrations effectively and prevent potential toxicity from drug accumulation.
Measuring alterations of ICP is internationally recognized and widely employed for determining whether a drug has a therapeutic effect on ED. Here, we found that procyanidin significantly elevated the ICP in rats. Additionally, procyanidin effectively inhibited the degradation of cGMP in the penile cavernous tissue. The efficacy of procyanidin is comparatively diminished relative to that of sildenafil. These findings indicate that procyanidin exerts its therapeutic effects by inhibiting PDE5A activity, akin to the mechanism of sildenafil. However, the disparity in dosing between procyanidin and sildenafil underscores the significant differences in potency and therapeutic potential of these two compounds. This discrepancy can be attributed to the multi-targeting nature of procyanidins and should be carefully considered in both study design and clinical application to optimize therapeutic strategies and enhance therapeutic efficacy. Consequently, future research should focus on dosage form and delivery system, such as nanotechnology or targeted delivery systems that can improve the bioavailability and targeting of procyanidin. Notably, as a natural product, the pleiotropic efficacy of procyanidin (eg, anti-inflammatory, antioxidant, and cardiovascular protective effects) may be beneficial in the treatment ED.
In summary, procyanidin presents potential for treating ED as a PDE5I. However, its moderate binding affinity and efficacy, relative to current clinically approved PDE5Is, present significant challenges for clinical translation. These limitations may impact its potency, optimal dosing, and overall therapeutic potential, necessitating further optimization and rigorous studies before procyanidin can be considered viable candidates for clinical use in managing ED.
Conclusion
Our study demonstrates that procyanidin binds to the catalytic site of PDE5A, resulting in elevated cGMP levels and improved erectile function in vitro and in vivo. These findings highlight the potential of procyanidin as a natural PDE5I.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (22467019) and Project of the Qinghai Science and Technology Department (2023-ZJ-964J).
Disclosure
The authors declare that they have no competing financial interests or personal relationships that may have influenced the research reported in this study.
References
1. McCabe MP, Sharlip ID, Atalla E, et al. Definitions of sexual dysfunctions in women and men: a consensus statement from the fourth international consultation on sexual medicine 2015. J Sex Med. 2016;13(2):135–143. doi:10.1016/j.jsxm.2015.12.019
2. McKinlay JB. The worldwide prevalence and epidemiology of erectile dysfunction. Int J Impot Res. 2000;12(4):S6–S11. doi:10.1038/sj.ijir.3900567
3. Ayta IA, McKinlay JB, Krane RJ. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU Int. 1999;84(1):50–56. doi:10.1046/j.1464-410x.1999.00142.x
4. Irwin GM. Erectile Dysfunction. Prim Care. 2019;46(2):249–255. doi:10.1016/j.pop.2019.02.006
5. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol. 2017;219(1):22–96. doi:10.1111/apha.12646
6. Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–165. doi:10.1016/s0140-6736(12)60520-0
7. Grover SA, Lowensteyn I, Kaouache M, et al. The prevalence of erectile dysfunction in the primary care setting: importance of risk factors for diabetes and vascular disease. Arch Intern Med. 2006;166(2):213–219. doi:10.1001/archinte.166.2.213
8. Saigal CS, Wessells H, Pace J, Schonlau M, Wilt TJ. Predictors and prevalence of erectile dysfunction in a racially diverse population. Arch Intern Med. 2006;166(2):207–212. doi:10.1001/archinte.166.2.207
9. Montorsi P, Ravagnani P, Galli S, et al. The triad of endothelial dysfunction, cardiovascular disease, and erectile dysfunction: clinical implications. Eur Urol Suppl. 2009;8(2):58–66. doi:10.1016/j.eursup.2008.10.010
10. Randrup E, Baum N, Feibus A. Erectile dysfunction and cardiovascular disease. Postgrad Med. 2015;127(2):166–172. doi:10.1080/00325481.2015.992722
11. Konstantinos G, Petros P. Phosphodiesterase-5 inhibitors: future perspectives. Curr Pharm Des. 2009;15(30):3540–3551. doi:10.2174/138161209789206953
12. Brant WO, Bella AJ, Lue TF. Treatment options for erectile dysfunction. Endocrinol Metab Clin North Am. 2007;36(2):465–479. doi:10.1016/j.ecl.2007.02.001
13. Corbin JD. Mechanisms of action of PDE5 inhibition in erectile dysfunction. Int J Impot Res. 2004;16(1):S4–S7. doi:10.1038/sj.ijir.3901205
14. Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl. 2008;29(1):3–14. doi:10.2164/jandrol.107.003483
15. Karakus S, Burnett AL. The medical and surgical treatment of erectile dysfunction: a review and update. Can J Urol. 2020;27(S3):28–35.
16. Corona G, Rastrelli G, Burri A, Jannini EA, Maggi M. The safety and efficacy of avanafil, a new 2(nd) generation PDE5i: comprehensive review and meta-analysis. Expert Opin Drug Saf. 2016;15(2):237–247. doi:10.1517/14740338.2016.1130126
17. Hemnes AR, Champion HC. Sildenafil, a PDE5 inhibitor, in the treatment of pulmonary hypertension. Expert Rev Cardiovasc Ther. 2006;4(3):293–300. doi:10.1586/14779072.4.3.293
18. Jin F, Gong QH, Xu YS, et al. Icariin, a phosphodiesterase-5 inhibitor, improves learning and memory in APP/PS1 transgenic mice by stimulation of NO/cGMP signalling. Int J Neuropsychopharmacol. 2014;17(6):871–881. doi:10.1017/s1461145713001533
19. Aherne SA, O’Brien NM. Dietary flavonols: chemistry, food content, and metabolism. Nutrition. 2002;18(1):75–81. doi:10.1016/s0899-9007(01)00695-5
20. Aron PM, Kennedy JA. Flavan-3-ols: nature, occurrence and biological activity. mol Nutr Food Res. 2008;52(1):79–104. doi:10.1002/mnfr.200700137
21. Chen J, Zhong K, Jing Y, et al. Procyanidin B2: a promising multi-functional food-derived pigment for human diseases. Food Chem. 2023;420:136101. doi:10.1016/j.foodchem.2023.136101
22. Ma Y, Zhang F, Zhong Y, et al. A label-free LC/MS-based enzymatic activity assay for the detection of PDE5A inhibitors. Front Chem. 2023;11:1097027. doi:10.3389/fchem.2023.1097027
23. Zhang N, Zhao H. Enriching screening libraries with bioactive fragment space. Bioorg Med Chem Lett. 2016;26(15):3594–3597. doi:10.1016/j.bmcl.2016.06.013
24. Van Der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ. GROMACS: fast, flexible, and free. J Comput Chem. 2005;26(16):1701–1718. doi:10.1002/jcc.20291
25. Zhao S, Kang R, Deng T, et al. Comparison of two cannulation methods for assessment of intracavernosal pressure in a rat model. PLoS One. 2018;13:e0193543. doi:10.1371/journal.pone.0193543
26. Ou K, Gu L. Absorption and metabolism of proanthocyanidins. J Funct Foods. 2014;7:43–53. doi:10.1016/j.jff.2013.08.004
27. Moreland RB, Traish A, McMillin MA, Smith B, Goldstein I, Saenz de Tejada I. PGE1 suppresses the induction of collagen synthesis by transforming growth factor-beta 1 in human corpus cavernosum smooth muscle. J Urol. 1995;153(3 Pt 1):826–834. doi:10.1016/S0022-5347(01)67730-9
28. Minhas S, Cartledge J, Eardley I, Joyce AD, Morrison J. The interaction of nitric oxide and prostaglandins in the control of corporal smooth muscle tone: evidence for production of a cyclooxygenase-derived endothelium-contracting factor. BJU Int. 2001;87:882–888. doi:10.1046/j.1464-410x.2001.02178.x
29. Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32(4):379–95,v. doi:10.1016/j.ucl.2005.08.007
30. Kuchta K, Cameron S, Lee M, Cai SQ, Shoyama Y. Which East Asian herbal medicines can decrease viral infections? Phytochem Rev. 2022;21(1):219–237. doi:10.1007/s11101-021-09756-2
31. Okusanya O, Forrest A, DiFrancesco R, et al. Compartmental pharmacokinetic analysis of oral amprenavir with secondary peaks. AAC. 2007;51(5):1822–1826. doi:10.1128/aac.00570-06
32. Walker DK, Ackland MJ, James GC, et al. Pharmacokinetics and metabolism of sildenafil in mouse, rat, rabbit, dog and man. Xenobiotica. 1999;29(3):297–310. doi:10.1080/004982599238687
33. Cayetano-Alcaraz AA, Tharakan T, Chen R, Sofikitis N, Minhas S. The management of erectile dysfunction in men with diabetes mellitus unresponsive to phosphodiesterase type 5 inhibitors. Andrology. 2023;11(2):257–269. doi:10.1111/andr.13257
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





