Back to Journals » International Journal of Nanomedicine » Volume 19
Progress and Challenges of Topical Delivery Technologies Meditated Drug Therapy for Osteoarthritis
Authors Shentu CY, Wang HB, Peng X, Xu DC, Qian LN, Chen Y, Peng LH
Received 11 April 2024
Accepted for publication 10 July 2024
Published 14 August 2024 Volume 2024:19 Pages 8337—8352
DOI https://doi.org/10.2147/IJN.S466437
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Cheng-Yu Shentu,1 Hao-Bin Wang,1 Xiao Peng,2 Dong-Chen Xu,1 Li-Na Qian,1 Yong Chen,1,2 Li-Hua Peng1– 3
1College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, People’s Republic of China; 2Jinhua Institute of Zhejiang University, Jinhua, Zhejiang, 321299, People’s Republic of China; 3State Key Laboratory of Quality Research in Chinese Medicine, Macau University of Science and Technology, Macau, Macau SAR, People’s Republic of China
Correspondence: Li-Hua Peng; Yong Chen, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Osteoarthritis (OA) is a degenerative disease commonly seen in middle-aged and elderly people. Multiple cytokines are involved in the local tissue damage in OA. Currently, non-pharmacologic and surgical interventions are the main conventional approaches for the treatment of OA. In terms of pharmaceutical drug therapy, NSAIDs and acetaminophen are mainly used to treat OA. However, it is prone to various adverse reactions such as digestive tract ulcer, thromboembolism, prosthesis loosening, nerve injury and so on. With the in-depth study of OA, more and more novel topical drug delivery strategies and vehicles have been developed, which can make up for the shortcomings of traditional dosage forms, improve the bioavailability of drugs, and significantly reduce drug side effects. This review summarizes the immunopathogenesis, treatment guidelines, and progress and challenges of topical delivery technologies of OA, with some perspectives on the future pharmacological treatment of OA proposed.
Keywords: topical drug delivery, immuno-therapy, cytokines, osteoarthritis
Introduction
Osteoarthritis (OA) is a common inflammatory joint disease occurring in middle-aged and elderly people, mainly manifested by articular cartilage degeneration, osteophyte formation, functional impairment and arthritis pain,1–4 which causes patients to experience clinical manifestations such as pain, stiffness, swelling, and limited joint function. As shown in Figure 1, the main diseased joint of OA lies in the cartilage of the patient, which leads to the subsequent degeneration of the joint, resulting in secondary synovitis. The patient’s bone hyperplasia leads to the appearance of osteophytes in joints. When the osteophytes are heavy, the patient’s cartilage and sub-bone will be seriously damaged. In the process of OA, cartilage structure is destroyed, which increases the sensitivity of cartilage to external stimuli. In the early stage of OA, the main pathological changes are the changes of subchondral bone trabecula led by bone remodeling. In the advanced stage of OA, the main pathological changes are subchondral osteosclerosis. A series of changes in the subchondral bone will aggravate the damage of articular cartilage. Due to the impaired repair ability of articular cartilage, the balance between synthesis and degradation is broken, and the structure and properties of articular cartilage are changed, which leads to the occurrence of OA.
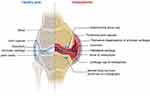 |
Figure 1 The pathology of OA. |
The causes of OA are numerous and complex, and it is currently believed that its pathogenesis is related to physical factors, age, genetics, immunity, hormone levels,5 with cytokines imbalance being the main pathological mechanism. They are involved in numerous physiological metabolic and functional regulations and maintain the normal structure and function of their tissues.6 Chondrocytes are targets for the action of cytokines, and conversely, chondrocyte regeneration may require locally high concentrations of cytokines. Therefore, it is of great interest to search for cytokines and signaling pathways that can alter endochondral homeostasis and to investigate how enhanced or suppressed levels of their expression affect the development of OA.
Currently, the treatment of OA is still limited to anti-inflammatory drugs, intra-articular injections into the knee joint, and joint replacement surgery, but these therapeutic strategies cannot completely prevent joint tissue damage or OA progression, and there are obvious side effects. Therefore, it is particularly important to explore an intervention that can effectively relieve OA and improve the condition of OA at an early stage. Given this, this review focuses on the latest therapeutic modalities related to cytokines and signaling pathways, integrates the mechanism of action of local drug delivery techniques and the latest research results, compares the clinical and basic experimental results of each therapeutic modality, and predicts the future direction of research based on the strengths and weaknesses of each therapeutic modality, which is important for the search for better OA treatment and bone tissue engineering research in the future. This review is of great significance in the search for better OA treatments and bone tissue engineering research. This review is intended to provide a basis for exploring new therapeutic targets and modalities and conducting related research in the future.
Immunoinflammatory Lesion Mechanisms in OA
Inflammatory cytokines are involved in a variety of physiological metabolic and functional regulation of the body, playing a vital role in maintaining organizational structure and function.7,8 Particularly, IL-1β plays an inflammatory role in disease mainly by activating a cascade of inflammatory responses.9 IL-1β can activate the NF-κB pathway and induce joint destruction. IL-1β can promote chondrocyte production of matrix metalloproteinases (MMPs).10 IL-1β can inhibit the expression of type II collagen with cartilage characteristics, and promote the production of type I collagen with fibroblast characteristics, thus destabilizing cartilage and causing the destruction of articular cartilage.11 IL-1β promotes the production of NO, which promotes the activation of MMPs, inhibits collagen and proteoglycan synthesis, promotes apoptosis. In addition, IL-1β promotes the synthesis and release of prostaglandin E2 (PGE2) from chondrocytes and synoviocytes to produce potent analgesic effects.12 TNF-α is an important pro-inflammatory factor.13 Inhibiting the expression of TNF-α helps ease the progression of OA.14,15 MMPs were significantly increased in synovium.16,17 TNF-α can promote the synthesis of polyproteinglycanase-7 (ADAMTS-7) through NF-κB signaling pathway, destroy cartilage matrix, mediate the synthesis and secretion of inflammatory mediators, and block cartilage repair.18 Studies have shown that targeted inhibition of IL-6 is effective in delaying the progression of OA in mouse experimental models.19,20 The increased IL-6 content in osteoblasts of OA patients secretes MMPs, which has a harmful effect on cartilage.21 IL-6 greatly increased the content of PGE2 and collagenase, and degraded articular cartilage.22,23 IL-6 stimulates synovial membrane to secrete RANKL, which promotes the maturation of osteoclasts and disrupts the balance between osteoblasts and osteoclasts. Synergizes with IL-1β and TNF-α to cause osteoporosis and aggravate joint damage. Transforming growth factor β (TGF-β) is an anti-inflammatory and anabolic cytokine that controls chondrocyte proliferation and differentiation as well as extracellular matrix deposition, and promotes proliferation of undifferentiated mesenchymal cells, endothelial cell chemotaxis, and angiogenesis. TGF-β has the ability to stimulate proteoglycan synthesis and inhibit hypertrophic chondrocyte differentiation.24 Alterations in its signaling or composition may therefore affect cartilage homeostasis and accelerate the course of OA. There is strong evidence to show that, in chondrocytes, TGF-β binds to TGFBR1 (ALK5) and TGFBR2, regulating gene transcription including the activation of chondrogenesis (Sox 9) and suppression of degeneration (MMP-13) and mineralization (ALP). TGF-β/ALK5 regulates articular cartilage homeostasis by up-regulating PRG4 expression through the PKA-CREB signaling pathway.25 Disrupting any step of this pathway such as deletion or mutation of the TGFBR and SMAD2/3 complex, leads to OA pathogenesis including chondrocyte hypertrophy, cartilage degradation, and excessive mineralization including osteophyte formation.26–28 At the onset of OA, TGF-β promotes chondrocyte MMP-13, ADAMT-5, and collagen type X (COL10) expression by upregulating the bone degeneration. In addition, TGF-β selectively inhibits collagenase or proteoglycanase, exerting protective effects. Leptin is a pro-inflammatory factor because it appears to act on a so-called low-grade inflammatory state in obese people.29,30 Leptin acts synergistically with other pro-inflammatory and catabolic factors to activate the MAPK pathway and the content of MMPs was increased, such as MMP-9 and MMP-13.31–34 Leptin induces chondrocyte apoptosis through the JAK2/STAT3 signaling pathway.35 Leptin upregulates the expression of IL-1β and IL-6 via the NF-κB pathway.36 Leptin activate nitric oxide synthase 2 (NOS2) through the PI3K, and MAPK pathways, thereby promoting cartilage apoptosis and joint degeneration.37 Leptin induces the production of inducible iNOS in human chondrocytes when acting synergistically with IL-1β in cartilage tissue.34 Large amounts of NO synthesized by iNOS genes induce tissue damage and accelerate inflammation.38 In addition, Leptin increases expression of ADAMTS-4, and ADAMTS-9 in human chondrocytes, leading to cartilage degeneration.39 Thus, Leptin induces chondrocytes to develop an Inflammatory environment that damages cartilage tissue. The pathogenesis of OA regulated by inflammatory cytokines is shown in Figure 2.
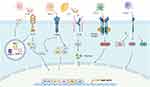 |
Figure 2 Major cytokines involved and their contributing mechanisms to OA. |
Non-Pharmacological Interventions
Exercise has been widely proven to have a pain-relieving effect on patients with OA.40 A small body of evidence supports aquatic exercise, which has been shown to have significant advantages on pain and objective measures of function.41 Scholars have noticed that weight loss will contribute to symptom or functional improvement in patients with OA.42 As shown in Figure 3, Tai Chi can help people feel better by reducing pain and stiffness.43,44 Hand orthoses can support the entire hand and may offer benefits by providing warmth and compression to the joints of the hand. Arthroscopic debridement significantly reduces inflammatory factors in synovial fluid, alleviates the destruction of articular cartilage, improves symptoms of OA, effectively avoids or delays total knee replacement, and relives the pain of patients.45 However, Arthroscopic debridement for OA is controversial.46 Numerous factors influence success rates.47–50 Osteotomy’s purpose is to transfer the mechanical axis from the pathologic area to the normal compartment. It is thought to improve physical activity by decreasing pain. Either unicompartmental knee arthroplasty (UKA) or total knee arthroplasty (TKA) can be used to treat severe knee arthritis. TKA is reserved as the final option for patients with OA. TKA has generally been recommended for older patients with intolerable knee pain.51
 |
Figure 3 Non-pharmacologic management of OA. |
Drug Therapy for OA
With the development of OA, lifestyle modification is no longer effective in controlling disease. At this time, drugs are needed in OA treatment. At present, the most commonly used drugs are acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs). They effectively reduce inflammation and relieve pain, playing a key role in symptom control. NSAIDs are commonly and frequently used for treating fever, pain, and inflammation.52 NSAIDs are the key drugs for OA, which can have anti-inflammatory functions in many ways. Firstly, NSAIDs can inhibit the activity of COX through the COX pathway, inhibiting PG. Secondly, NSAIDs can inhibit the production of inducible NO synthase and the transport of PG through the COX-independent pathway, blocking the conversion of arachidonic acid to PG and thus inhibiting inflammatory reactions.53,54 NSAIDs have significant analgesic effects. NSAIDs can reduce the inflammatory such as IL-1β, TNF-α, inhibiting the overexpression of inflammatory factors.55 What’s more, scholars found that NSAIDs has cartilage protective effect.56 However, once these drugs enter the blood circulation, they will not only face the phagocytosis of the reticuloendothelial system and the destruction of proteases, but also be distributed throughout the body, weakening the efficacy and increasing adverse reactions.57–61 Chondroitin sulfate (CS), is found in the cartilage and ECM. The main factor causing the inflammation of the OA is the activation of NF-κB. CS exerts anti-inflammatory effects by decreasing ERK1/2, p38 MAPK and JNK activation, decreasing NF-κB activation and nuclear translocation, and pro-inflammatory cytokines, protein hydrolases, and enzymes with pro-inflammatory activity (PLA2, COX-2, NOS-2) in chondrocytes and synovium.62 Glucocorticoids (GS) can inhibit the production of superoxide radicals in damaged cells, reduce the serum PGE2 level of patients, and block a variety of cell signaling pathways mediated by IL-1β, which can play a direct anti-inflammatory role. Glucosamine (GC) is a class of steroidal hormones. It can inhibit the production of some inflammation-related cytokines, such as PGE2, ILs, TNF-α, leukotrienes (LTs) and granulocyte colony-stimulating factor (G-CSF). GC inhibits the production of NO and adhesion molecules, reduces plasma exudation at the site of inflammation, and attenuates inflammatory symptoms. GC indirectly inhibits the function of cytokines and decreases cellular responsiveness to cytokines. However, GC reduces the body’s resistance and makes it susceptible to infection by a variety of pathogenic factors. Hyaluronic acid (HA) is produced by synoviocytes, fibroblasts, and chondrocytes and is a sulfate-free, naturally occurring, nonprotein glycosaminoglycan (GAG).63 Many studies have confirmed that HA has anti-inflammatory effect. HA produces chondroprotective effects by binding to CD44. HA-CD44 binding decreased the expression of IL-1β, resulting in decreased MMPs synthesis. The inhibition of multiple MMPs reduced the intra-articular cartilage partitioning dissimilatory metabolic enzyme activities.64,65 HA-CD44 binding further inhibits the inflammatory factors resulting in an anti-inflammatory effect.66 With intra-articular hyaluronic acid injections, Leptin-regulated osteophyte formation decreases, reducing the inflammatory response, pain relief, and functional limitations of the joint. In addition, saffronin derivatives can effectively reduce the level of NO secretion, which has anti-inflammatory effects.67
Topical Drug Delivery Strategies
At present, the main administration mode for OA is oral administration. However, Oral administration, as traditional administration, has a strong gastrointestinal response and low bioavailability.66 OA affects only a few parts of the joint, it can be treated locally joints, so it can be treated locally. As shown in Figure 4, in recent years, novel local delivery strategies have been developed. Intra-articular (IA) injections provide an effective route of drug delivery for OA therapy. However, free drug from IA injections is rapidly cleared, resulting in insufficient drug concentration in tissues. This challenges evidence the need to improve the drug bioavailability in the relevant tissues.68 Transdermal administration is a non-invasive way of drug delivery, which not only overcomes the disadvantages of gastrointestinal irritation and the first-pass effect of oral drug delivery but also has the advantage of prolonged and continuous drug delivery. It also has the advantage of prolonged and continuous drug delivery, better patient compliance, convenient drug delivery, and self-administration. The advantages and disadvantages of the topical drug delivery strategies are listed in Table 1.
 |
Table 1 Advantages and Disadvantages of Local Drug Delivery Strategies |
 |
Figure 4 Novel drug delivery platforms for OA. |
Intra-Articular Injection
IA injection can enhance the local action of drugs, improve the bioavailability of drugs and reduce the side effects of systemic administration.69,70 However, this does not mean that IA injection has no disadvantages. An obvious disadvantage of IA injection is the discomfort and pain they may cause and the possible risk of infection. And the drug is cleared rapidly from the joints because of the physiology of joints. GC and HA are available as IA injection formulations for the treatment of OA.71 Due to the rapid elimination in joints of traditional injection formulations, a novel drug delivery strategy needs to be developed that allows for a prolonged presence of active ingredients in the joint over several weeks or months and thus, a reduction of injection frequency.72 Safety, size and other aspects of the carrier should be taken into consideration.73
Micron Scaled Platforms for Sustained Drug Release
Microsphere is a skeleton spherical drug delivery system formed by dissolving or dispersing drugs in polymer materials.74 Arthritis drugs are made into microspheres and injected into the joint cavity, which not only protects the drugs from various factors in the body and effectively overcomes the shortcomings of small-molecule drugs injected into the bone IA, but also allows for the slow release of the drugs. Thus, the bioavailability of the drug is improved, which facilitates the delivery and release of IA injection drugs. Bone IA injection drug-carrying microspheres have outstanding clinical advantages, great potential, and broad market prospects.68,75 In some studies, tetramethylpyrazine sustained release microspheres were injected into the joint cavity of OA model rats, and compared with conventional tetramethylpyrazine injection. The number of administration was reduced, and the dosage of the drug was also reduced.
Inflammation of the synovium and joint capsule is a major factor in OA joint pain. Triamcinolone acetonide (TAA) is a classic corticosteroid that temporarily reduces synovitis and pain.73 In the study, at 37°C, an initial burst of TAA release from PBS buffer occurred on the first day, with a gradual increase in TAA release after 60 days, reaching a cumulative release of 50%.76 It strongly proved that PEA-based microspheres are promising for controlled release strategies in intra-articular drug delivery systems. In another study, Rac1 inhibitor NSC23766 coated with chitosan microspheres containing HA was injected into OA knee joint to achieve the effect of slow release and lubrication,77 which may provide a new option for OA treatment. However, the disadvantage is that the controlled release system only has 3 days of sustained release.
Nano-Osmosis Delivery Systems
Compared with microspheres, nanoparticles (NPs) have the characteristics of small particle size and stable structure, and have better application potential. NPs are tiny materials that have specific physicochemical properties different from bulk materials of the same composition and such properties make them very attractive for medical development. Bone IA injection drug-carrying NPs can increase the drug residence time in IA and avoid the rapid removal of water-soluble drugs and bioactive molecules, improving the therapeutic effects. NPs as a drug delivery system for bone IA injection are of great significance for OA treatment. NPs as a drug delivery system for bone IA injection are of great significance for OA treatment. Polymeric micellar NPs bound to TGF-α, a potent EGFR ligand, have been reported to be stable and non-toxic, with long joint retention time, and strong penetration ability.78 Peng79 investigated solid lipid NPs (SLN) as a novel carrier for piroxicam sustained release and transdermal delivery (Pir-SLN). Pir-SLN inhibits inflammation by lowering the level of PGE 2, which has great potential for anti-inflammatory applications. Chen et al80 developed superlubricated nanospheres. Bionic nanospheres can form a tough hydration layer around the amphiphilic ionic charge of polymer brushes to enhance lubrication and enable local delivery of drug using nanocarriers. Lubrication properties and slow release properties of the nanospheres were improved. In addition, super-lubricated drug-loaded nanospheres treat OA by regulating the relationship between synthesis and metabolism. The nanospheres have the characteristics of lubrication and continuous administration, and may be an effective intra-articular nanomedicine. A study has developed a nanoreactor (ZFVO) effectively promotes angiogenesis by releasing cobalt ions and hydroxyl radicals, targeting injury and infection sites, with the potential for precise treatment of trauma and orthopedic diseases.81
Exosomes are small in size, uniform in distribution and wide in source. They are one of the materials used as nanoscale carriers and can be used as a drug delivery system. Exosomes may become a new type of transporter for the treatment of OA by using the stability of non-degradation after exosomes encapsulation to accurately target delivery by loading drugs or functioning miRNAs. A research team reports that exosomes act as vectors to deliver miR-140 into chondrocytes as a novel treatment for OA.82 Compared to unlabeled exosome vesicles, cap-exosomes can deliver cargo to chondrocytes in vitro. Targeted delivery of KGN to synovial fluid-derived mesenchymal stem cells (SF-MSCs) via engineered exosomes has been reported to result in uniform dispersion of KGN in the cytoplasm, increase its effective concentration in cells, and strongly promote cartilage formation in SF-MSCs in vitro and in vivo. Exosome-delivered KGN enables SF-MSCs to have in situ chondrogenic function, and transplantation is expected to be an advanced stem cell therapy for OA.
In addition, exosomes can be used in the treatment of OA. It was confirmed that bone marrow mesenchymal stem (BMSC) exosomes could alleviate OA by reducing cartilage damage and synovial macrophage infiltration.83 The expression of IL-1β was inhibited in synovial fluid, and the release of IL-10 was increased. Exosome-treated macrophages maintain chondroblast properties. These results suggest that BMSC-exos may alleviate OA by transforming of synovial macrophage M1 into M2 phenotype.
Hydrogels offer better biosafety than NPs.84–86 Hydrogel is a kind of three-dimensional polymer network structure colloid containing a lot of water inside, which has a high degree of hydrophilicity, swelling and porosity.87,88 Hydrogels have become a research object in recent years because of their excellent stability, drug loading ability and low irritability to the environment.89 For OA treatment, injection of hydrogel through bone IA can realize the increase of drug residence time in OA and controlled release of the drug, and controlled release of the drug.90 A novel formulation of betamethasone dipropionate has been developed based on hollow silver hydrogel microcapsules.91 Encapsulation of betamethasone dipropionate into silver alginate microcapsules allowed to enhance its bioavailability and effectiveness which was demonstrated in vitro on mouse fibroblasts. The proposed approach provides a new promising strategy for improving OA therapy by reducing the side effects of glucocorticoid drugs. Kartogenin (KGN) is a small molecule that induces differentiation of SF-MSCs to chondrocytes in vitro and in vivo. However, KGN forms intracellular precipitates, resulting in low effective concentrations, thus limiting its chondrogenic activity. KGN forms precipitates within cells, resulting in low effective concentrations, which limits its chondrogenic activity.92 An experiment to prepare KGN particles and KGN hydrogels by covalent cross-linking showed that KGN hydrogels significantly prolonged the release time of KGN drugs compared to KGN particles.93 In vivo, cartilage repair experiments demonstrated that KGN hydrogels injected into bone IA could inhibit the development of OA and protect chondrocytes more effectively than KGN particles and HA hydrogels. It proves that bone IA injection of KGN hydrogel has a good effect on OA treatment.
Liposomes have excellent targeting and biodegradability compared to hydrogels. Biodegradability, the use of liposomes for bone IA injection in OA therapy is of great Significance. Liposomes are microscopic vesicles formed by encapsulating a drug within a lipid-like bilayer. Liposomes are biocompatible, targeted, biodegradable, and non-toxic. In recent years, the use of colloidal lipid dispersions as nanoparticulate drug carriers has gained more attention. Further, the development of lipid NPs using solid lipids instead of liquid oils has come out with better results. Thus, solid lipid NPs were introduced as an alternative carrier system for drug delivery which offers the potential for sustained or controlled drug release by immobilization of the drug within a solid matrix.94 Chondroitin sulfate modified diacerein carrying solid lipid NPs (ChS-DC-SLN) were prepared to take advantage of the synergistic function of these drugs in OA treatment. Experiments prove that this system can extend release from 4 h up to 16 h.95 Therefore, ChS-DC-SLN has great potential in treating OA.
In vitro cell viability studies, scholars found that the injectable system developed was non-cytotoxic and had excellent cell viability in chondrocytes and osteoblasts. Thus, they can be candidates for the treatment of OA.
Transdermal Drug Delivery
Skin is the largest organ of the human body.96 As the first barrier of the human body, skin plays the main defense role against foreign invasion, which can prevent the loss of water in the body, inhibit the invasion of external substances, regulate body temperature and other functions.97 The penetration of drugs through the skin is the basis of transdermal drug delivery.98 The stratum corneum is the main barrier that prevents drugs from entering the deep skin tissue.98 When there is a significant first-pass effect, the drug may be metabolized prematurely. Transdermal injections also have many advantages over subcutaneous injections, which can be very painful for patients.99
The epidermis is composed of the stratum basale, spinosum, granulosum, and stratum corneum. These cells, called keratinizing cells, are constantly dividing in the basal layer and moving toward the surface. In a process known as keratinization, the keratinizing cells produce precursors of barrier components, such as keratin, filaggrin, and lipids, which ultimately seal the surface of the skin. The end product of this process, the stratum corneum, is the skin’s permeable barrier to most substances.100–103 It is difficult for hydrophilic or macromolecular drugs to pass through the skin barrier, so it is necessary to use penetration enhancers to assist their delivery.
To make the strategy of transdermal delivery of drugs to the articular cavity site more effective, more drugs need to be allowed to overcome the stratum corneum barrier to be effective. As shown in Figure 5, a variety of physical and chemical pro-osmotic means are currently available for the attempted transdermal delivery of osteoarthritic drugs.
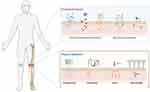 |
Figure 5 Skin penetration enhancement. |
Chemical Enhancers
Chemical penetration enhancers can enter the stratum corneum and interact with its components, temporarily lowering the penetration barrier without causing significant cellular damage.104 The enhancers include hydrocarbons, alcohols, acetals, amines, carboxylic acids, esters, amides, and sulfoxides if we use the main functional group to classify them. However, the challenge is to make penetration enhanced, which is often associated with increased skin irritation. Among the chemical penetration enhancement methods, nanocarriers have been extensively studied.105 When used alone, nanocarriers mainly deliver drugs to the skin and can be used to treat skin diseases. For effective transdermal drug delivery, nanocarriers should be used in combination with physical methods as they act synergistically to enhance drug penetration.
In one study, nanostructures containing NPs were prepared by coupling with the hydrophobic polymer polylactic acid (PLGA), solving the major problem of high hydrophilicity and low permeability of glucosamine as a transdermal delivery system.106 The outer shell is hydrophobic PLGA and the inner core is hydrophilic Glc, which enables the nanocarriers to penetrate the skin lipid membrane more effectively and release Glc continuously for 48 hours. PLGA-Glc nanostructures exhibit a better penetration profile through in vitro transdermal penetration through human skin, with the shortest delay time and high flux value. Peng107 induced a percutaneous photo-TRAIL procedure for the treatment of cutaneous melanoma using αvβ3 integrin-targeting peptide ligands and transgenic E. coli derived outer membrane vesicles modified with indocyanine green (I-P-OMV). I-P-OMV activated TRAIL-induced apoptosis of disseminated tumor cells leading to complete eradication of the melanoma, with enhanced anti-tumor properties and a high safety profile.
Chemical enhancers avoid the hepatic first-pass effect and gastrointestinal inactivation that may occur with oral administration, improve therapeutic efficacy and reduce the side effects of gastrointestinal administration. The role of chemical enhancers in transdermal drug delivery systems is very important, but there are shortcomings, for example, it promotes the permeation of small molecules. For some large-molecule drugs, chemical enhancers should be combined with other physical osmotic promotion methods. Recent studies have found that bio-permeable agents such as permeation-enhancing peptides, ceramides, and their analogs are also effective in promoting transdermal absorption of drugs.108,109 Compared with chemical permeation enhancers, bio-permeable agents have lower toxicity, a wider range of permeation enhancement, and better biocompatibility. However, the current understanding of bio-permeable enhancers is still very limited, and its related issues such as drug-forming properties and safety still need to be further investigated.
Iontophoresis
Iontophoresis is a non-invasive penetration promotion technique that enhances the penetration of charged drugs into tissues by applying a low intensity current.110 Iontophoresis can be used for local or systemic administration, and is one of the most promising directions for percutaneous and transmucosal administration. The main advantage of ion introduction is that it provides control over the kinetics of drug input and the ability to tailor the rate of drug input, which can be optimized for a given patient.111 In clinical practice, iontophoresis is often used for the rapid delivery of lidocaine under local anesthesia112 and sweat-inducing pilocarpine as part of a diagnostic test for cystic fibrosis.113 Fukuta114 demonstrated for the first time that iontophoresis can also be used as a transdermal delivery of macromolecular drugs, such as antibodies and anti-cytokine therapeutics, and that this non-invasive and effective drug delivery is expected to improve the quality of life for more patients. In addition, traditional Chinese medicine iontophoresis therapy is also often used in the treatment of OA, which can promote the dilation of blood vessels, accelerate local blood circulation, make the drug directly act on the patient’s lesion site, and thus play a better curative effect. However, iontophoresis cannot deliver fat-soluble drugs, which requires high pressure to the electrode to allow the drug to penetrate from the skin surface, which can easily cause skin damage.
Ultrasound
Fellinger K et al115 reported for the first time the treatment of multiple digital arthritis with hydrocortisone and ultrasound promoting infiltration technology. Experiments showed that hydrocortisone injection combined with ultrasonic “massage” was more effective than hydrocortisone injection alone in the treatment of bursitis. Cameron116 reported the successful use of cadocaine ultrasound in the treatment of closed Kohler fractures. Studies have shown that ultrasound enhances the transdermal absorption of methyl and ethyl nicoate by altering the lipid structure in the stratum corneum. The experimental results of Levy et al117 showed that the effect of 3–5 minutes of ultrasound could increase the transdermal penetration of mannitol and physical amine into the skin of hairless rats by 15 times. David’s research group118 applied the ultrasound machine to 90 knee OA patients. They found that prolonged low-intensity ultrasound relieved pain in patients with OA and had a better therapeutic effect. It was revealed that low intensity ultrasound played an effective role in the treatment of OA. In addition to the therapeutic effect of ultrasound itself, it can also be used to assist delivery of drugs. As ultrasound passes through the tissue, bubbles expand and contract due to rectification diffusion and bubble aggregation.119 The bubbles oscillate and break on the surface of the skin, creating local shock waves and microjets of liquid flowing toward the stratum corneum.120 The vibration of the cavitation bubble can cause a large amount of water to penetrate into the disordered lipid region to form water-based channels, and the diffusion of drugs through these channels is much faster than normal lipid channels.121 In addition to cavitation, ultrasound can also produce thermal effects, which play a key role by increasing skin permeability coefficient.122,123 For example, Masterson124 reported that the current study demonstrated ultrasound therapy using a 1% diclofenac ultrasound patch applied sam in a human skin simulant. The data showed increased temperature, local vasodilation, increased oxygenation and drug exchange, as well as increased drug penetration due to increased skin porosity and acoustic flow. Overall, all of these properties improve targeted drug delivery, which is critical for localized pathologies such as arthritis. However, ultrasonic penetration also has disadvantages, such as expensive equipment, difficult to popularize in individual families, difficult for ordinary patients to bear and there are some risks and complications.
Heat
In a study, scholars use the heating system to improve isotretinoin delivery into human skin.125 They found that the system significantly increased the total delivery of isotretinoin from the optimized vehicle into the skin. These data suggest that the use of short-term heating combined with chemical penetration enhancers provides a valuable strategy for improving the delivery of drugs such as isotretinoin to the skin. What’s more, the researchers studied the function of temperature on in vitro by measuring the transdermal reflux of fentanyl at 32 °C and 37 °C.126 For every 5 °C increase in temperature, the return flow of drug approximately doubled. It has also been shown that a change of 5 °C is required to induce measurable enhancement of intercellular permeability and drug transport.
Microneedles
Microneedles can be administered via a transdermal route and are simple and inexpensive to use.127,128 Henry et al129 first proposed the application of microneedles for transdermal drug delivery, and the results showed that the microneedles were inserted into the skin, which can promote the penetration of drugs. Liposomes are spherical carrier preparations formed by encapsulating drugs in lipid-like bilayer films. Liposomes can load hydrophilic and hydrophobic drugs, and have the advantages of good biocompatibility and delaying drug release.130,131 Triptolide (TP), is a toxic and insoluble drug with poor oral bioavailability. To expand the prospect of its application in OA treatment, scholars developed a liposomal carrying dissolved microneedles (DMN) system that can efficiently deliver poorly water-soluble TP. In vivo drug distribution indicated that TP-Lipo-DMNs had a sustained release effect compared to IA injection.132 TP-Lipo-DMNs can significantly reduce the level of inflammatory cytokines and effectively reduce cartilage damage. Although microneedle patches have been very successful in sustained-release drugs, there are still some challenges. The small size of the microneedle patch and the limited dose of loaded drugs will limit the practical application of microneedle to some extent. Most of the slow-release microneedles are generally PLA or PLGA, which have potential safety hazards when they stay in the skin for a long time.
Discussion and Outlook
At present, the molecular mechanisms of OA are not completely disclaimed yet. With the progress of medicine, the research on the pathogenesis of OA will be more in-depth, so as to explore more therapeutic targets for OA.133 Therefore, this article summarizes the pathological and molecular mechanisms of OA, hoping to be helpful for further research. Lifestyle modification is still the most effective way in the treatment of OA. Adopting good lifestyle can slow down the progression of osteoarthritis. Meanwhile, it is also essential to develop novel therapeutic protocols for treating OA. Currently, the clinical treatment of OA primarily involves the oral administration of NSAIDs. However, Oral administration can cause severe gastrointestinal adverse reactions and low bioavailability due to first pass effect. IA injections are invasive and require frequent dosing due to the rapid removal of the drug from the joint. How to deliver drugs into cartilage is a challenge. As a result, various drug delivery systems (DDSs) have been gradually developed to fill this gap. It would increase patient compliance, and reduce the risk of infection.
Since OA is only a localized disease, transdermal delivery would be well suited for the treatment of OA if the existing problems can be solved. However, how to cross the skin cuticle barrier and the dense collagen network in the joint cavity is a great challenge for transdermal drug delivery. Therefore, a new drug delivery system needs to be developed to address these problems. To promote transdermal penetration, there is an increasing tendency towards the combined use of multiple permeation-promoting techniques, such as microneedle-electroporation, ion introduction-ultrasound coupling and so on. Huang combined microneedle and electroporation techniques to effectively deliver DNA and siRNA into mouse skin. This electroporation protocol assisted by pre-perforation using a microneedle roller not only enables nucleic acid delivery under low-voltage conditions but also has a higher safety profile compared with treatment using electroporation only.133 Lanke et al134 investigated in vitro transdermal administration of low molecular weight heparin (LMWH). The experimental results showed that the combined use of microneedles and iontophoresis had a synergistic effect on LMWH transport, and the flux increased 14.7 times and 5.4 times compared with that of iontophoresis alone. Mojeiko found that using a combination of microneedles and microemulsions for topical breast skin drug delivery further improved transdermal drug penetration.134 Le et al135 studied the synergistic effect of ultrasound and iontophoresis on the transdermal transport of heparin. Ultrasonic iontophoresis enhanced the heparin flux by 56 times, which was significantly greater than the sum of ion stimulation alone (15 times) and ultrasound stimulation (3 times). Therefore, it is an inevitable trend to combine the existing methods. To explore the synergistic effect of the combination has become a hot spot in the study of transdermal drug delivery. However, these also pose challenge to drug delivery devices, and the ideal drug transdermal device should be safe, effective, portable, and simple to operate. With the advancement of science and the continuous development of various fields, transdermal delivery systems are expected to treat more diseases.
Given the unique advantages of topical-delivered drug strategies, it is believed that with the continuous development of drug carrier technology as well as pharmacology and other fields, there is a bright future in the route of drug delivery. Novel locally delivered drugs are constantly being developed, and it is increasingly recognized that by improving the absorption efficiency of drugs, new routes of use will be given to some therapeutically effective drugs that cannot be taken orally. In the future, we should consider not only how to deliver drugs to the target efficiently, but also how to adapt the properties of locally delivered drugs to the needs of joint resorption, for which more efforts and in-depth studies will be necessary in the future.
Acknowledgments
This review was supported by China’s National Key Research and Development Program (82374043, 2022YFC3501904, 2021YFC1712805), Zhejiang province commonweal projects (TGY23H090038, LGF22H280001), and the Macau Science and Technology Development Fund, Macau Special Administrative Region, China.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Karsdal MA. et al. Disease-modifying treatments for osteoarthritis (DMOADs) of the knee and Hip: lessons learned from failures and opportunities for the future. Osteoarthritis Cartilage. 2016;24(12):2013–2021. doi:10.1016/j.joca.2016.07.017
2. Lotz MK, Caramés B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nat Rev Rheumatol. 2011;7(10):579–587. doi:10.1038/nrrheum.2011.109
3. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
4. Hunter DJ. Pharmacologic therapy for osteoarthritis-the era of disease modification. Nat Rev Rheumatol. 2011;7(1):13–22. doi:10.1038/nrrheum.2010.178
5. Berenbaum F, Wallace IJ, Lieberman DE, et al. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018;14(11):674–681. doi:10.1038/s41584-018-0073-x
6. Sanchez-Lopez E, et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18(5):258–275. doi:10.1038/s41584-022-00749-9
7. Ayral X, Pickering EH, Woodworth TG, et al. Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis -- results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage. 2005;13(5):361–367. doi:10.1016/j.joca.2005.01.005
8. Pozgan U, Caglič D, Rozman B, et al. Expression and activity profiling of selected cysteine cathepsins and matrix metalloproteinases in synovial fluids from patients with rheumatoid arthritis and osteoarthritis. Biol Chem. 2010;391(5):571–579. doi:10.1515/bc.2010.035
9. Akdis M, Burgler S, Crameri R, et al. Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immun. 2011;127(3):701–770. doi:10.1016/j.jaci.2010.11.050
10. Shiozawa S, Tsumiyama K. Pathogenesis of rheumatoid arthritis and c-Fos/AP-1. Cell Cycle. 2009;8(10):1539–1543. doi:10.4161/cc.8.10.8411
11. Santangelo KS, Nuovo GJ, Bertone AL. In vivo reduction or blockade of interleukin-1 beta in primary osteoarthritis influences expression of mediators implicated in pathogenesis. Osteoarthr Cartilage. 2012;20(12):1610–1618. doi:10.1016/j.joca.2012.08.011
12. Kulkarni P, et al. Pathophysiological landscape of osteoarthritis. In:Adv Clin Chem. Makowski GS. editor. 2021;100:37–90. doi:10.1016/bs.acc.2020.04.002
13. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11(9):372–377. doi:10.1016/S0962-8924(01)02064-5
14. Zhao Y-P, Liu B, Tian Q-Y, et al. Progranulin protects against osteoarthritis through interacting with TNF-alpha and beta-Catenin signalling. Ann Rheum Dis. 2015;74(12):2244–2253. doi:10.1136/annrheumdis-2014-205779
15. Liacini A, et al. Induction of matrix metalloproteinase-13 gene expression by TNF-alpha is mediated by MAP kinases, AP-1, and NF-kappaB transcription factors in articular chondrocytes. Exp Cell Res. 2003;288(1):208–217. doi:10.1016/S0014-4827(03)00180-0
16. Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7(1):33–42. doi:10.1038/nrrheum.2010.196
17. Zhao B. TNF and bone remodeling. Curr Osteoporos Rep. 2017;15(3):126–134. doi:10.1007/s11914-017-0358-z
18. Lai Y, Bai X, Zhao Y, et al. ADAMTS-7 forms a positive feedback loop with TNF-alpha in the pathogenesis of osteoarthritis. Ann Rheum Dis. 2014;73(8):1575–1584. doi:10.1136/annrheumdis-2013-203561
19. Nasi S, So A, Combes C, et al. Interleukin-6 and chondrocyte mineralisation act in tandem to promote experimental osteoarthritis. Ann Rheum Dis. 2016;75(7):1372–1379. doi:10.1136/annrheumdis-2015-207487
20. Latourte A, Cherifi C, Maillet J, et al. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann Rheum Dis. 2017;76(4):748–755. doi:10.1136/annrheumdis-2016-209757
21. Sakao K, Takahashi KA, Arai Y, et al. Osteoblasts derived from osteophytes produce interleukin-6, interleukin-8, and matrix metalloproteinase-13 in osteoarthritis. J Bone Miner Metab. 2009;27(4):412–423. doi:10.1007/s00774-009-0058-6
22. Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependent NF-kappaB activation. Am J Physiol-Cell Ph. 2010;298(6):C1445–1456. doi:10.1152/ajpcell.00508.2009
23. Liu XH, et al. The role of the interleukin-6/gp130 signaling pathway in bone metabolism. Vitam Horm. 2006;74:341–355 doi:10.1016/S0083-6729(06)74014-6.
24. Ryu J-H, Yang S, Shin Y, et al. Interleukin-6 plays an essential role in hypoxia-inducible factor 2 alpha-induced experimental Osteoarthritic Cartilage Destruction in Mice. Arthritis Rheumatol US. 2011;63(9):2732–2743. doi:10.1002/art.30451
25. Wang Q, Tan QY, Xu W, et al. Cartilage-specific deletion of Alk5 gene results in a progressive osteoarthritis-like phenotype in mice. Osteoarthr Cartilage. 2017;25(11):1868–1879. doi:10.1016/j.joca.2017.07.010
26. Shen J, Li J, Wang B, et al. Deletion of the transforming growth factor β receptor type II gene in articular chondrocytes leads to a progressive osteoarthritis-like phenotype in mice. Arthritis Rheumatol US. 2013;65(12):3107–3119. doi:10.1002/art.38122
27. Chen CG, Thuillier D, Chin EN, et al. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheumatol US. 2012;64(10):3278–3289. doi:10.1002/art.34566
28. Harradine KA, Akhurst RJ. Mutations of TGFbeta signaling molecules in human disease. Ann Med. 2006;38(6):403–414. doi:10.1080/07853890600919911
29. Lago F, Gómez R, Gómez-Reino JJ, et al. Adipokines as novel modulators of lipid metabolism. Trends Biochem Sci. 2009;34(10):500–510. doi:10.1016/j.tibs.2009.06.008
30. Ben-Eliezer M, Phillip M, Gat-Yablonski G. Leptin regulates chondrogenic differentiation in ATDC5 cell-line through JAK/STAT and MAPK pathways. Endocrine. 2007;32(2):235–244. doi:10.1007/s12020-007-9025-y
31. Richter M, Trzeciak T, Owecki M, et al. The role of adipocytokines in the pathogenesis of knee joint osteoarthritis. Int Orthop. 2015;39(6):1211–1217. doi:10.1007/s00264-015-2707-9
32. Koskinen A, Vuolteenaho K, Nieminen R, et al. Leptin enhances MMP-1, MMP-3 and MMP-13 production in human osteoarthritic cartilage and correlates with MMP-1 and MMP-3 in synovial fluid from OA patients. Clin Exp Rheumatol. 2011;29(1):57–64 doi:10.1186/ar3384.
33. Toussirot E, Streit G, Wendling D. The contribution of adipose tissue and adipokines to inflammation in joint diseases. Curr Med Chem. 2007;14(10):1095–1100. doi:10.2174/092986707780362826
34. Vuolteenaho K, Koskinen A, Kukkonen M, et al. Leptin Enhances Synthesis of Proinflammatory Mediators in Human Osteoarthritic Cartilage—Mediator Role of NO in Leptin-Induced PGE 2, IL-6, and IL-8 Production. Mediators Inflammation. 2009;2009:345838 doi:10.1155/2009/345838.
35. Zhang ZM, Shen C, LI H, et al. Leptin induces the apoptosis of chondrocytes in an in vitro model of osteoarthritis via the JAK2-STAT3 signaling pathway. Mol Med Rep. 2016;13(4):3684–3690. doi:10.3892/mmr.2016.4970
36. Azamar-Llamas D, et al. Adipokine contribution to the pathogenesis of osteoarthritis. Mediators Inflammation. 2017;1:2017 doi:10.1155/2017/5468023.
37. Otero M, Reino JJG, Gualillo O. Synergistic induction of nitric oxide synthase type II - In vitro effect of leptin and interferon-gamma in human chondrocytes and ATDC5 chondrogenic cells. Arthritis Rheumatol US. 2003;48(2):404–409. doi:10.1002/art.10811
38. Yang C, Gao J, Niu J, et al. Synthesis, characterization and inhibitory effects of crocetin derivative compounds in cancer and inflammation. Biomed Pharmacother. 2018;98:157–164. doi:10.1016/j.biopha.2017.12.018
39. Yaykasli KO, Hatipoglu OF, Yaykasli E, et al. Leptin induces ADAMTS-4, ADAMTS-5, and ADAMTS-9 genes expression by mitogen-activated protein kinases and NF-kappa B signaling pathways in human chondrocytes. Cell Biol Int. 2015;39(1):104–112. doi:10.1002/cbin.10336
40. Vignon E, Valat J-P, Rossignol M, et al. Osteoarthritis of the knee and Hip and activity: a systematic international review and synthesis (OASIS). Joint Bone Spine. 2006;73(4):442–455. doi:10.1016/j.jbspin.2006.03.001
41. Bartels EM, et al. Aquatic exercise for the treatment of knee and Hip osteoarthritis. Cochrane Database Syst Rev. 2016;3:Cd005523 doi:10.1002/14651858.CD005523.pub2.
42. Messier SP, Resnik AE, Beavers DP, et al. Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthrit Care Res. 2018;70(11):1569–1575. doi:10.1002/acr.23608
43. Chen YW, Hunt MA, Campbell KL, et al. The effect of Tai Chi on four chronic conditions-cancer, osteoarthritis, heart failure and chronic obstructive pulmonary disease: a systematic review and meta-analyses. Br J Sport Med. 2016;50(7):397–407. doi:10.1136/bjsports-2014-094388
44. Ghandali E, Moghadam ST, Hadian MR, et al. The effect of Tai Chi exercises on postural stability and control in older patients with knee osteoarthritis. J Bodyw Mov Ther. 2017;21(3):594–598. doi:10.1016/j.jbmt.2016.09.001
45. Rychel JK. Diagnosis and treatment of osteoarthritis. Top Companion Anim Med. 2010;25(1):20–25. doi:10.1053/j.tcam.2009.10.005
46. Feeley BT, Gallo RA, Sherman S, et al. Management of osteoarthritis of the knee in the active patient. American Academy of Orthopaedic Surgeon. 2010;18(7):406–416. doi:10.5435/00124635-201007000-00003
47. Dervin GF, Stiell IG, Rody K, et al. Effect of arthroscopic débridement for osteoarthritis of the knee on health-related quality of life. J Bone Joint Surg Am. 2003;85(1):10–19. doi:10.2106/00004623-200301000-00003
48. Fond J, Rodin D, Ahmad S, et al. Arthroscopic debridement for the treatment of osteoarthritis of the knee: 2- and 5-year results. Arthroscopy. 2002;18(8):829–834. doi:10.1053/jars.2002.36225
49. Hanssen AD, Stuart MJ, Scott RD, et al. Surgical options for the middle-aged patient with osteoarthritis of the knee joint. Instr Course Lect. 2001;50:499–511 doi:10.2106/00004623-200012000-00011.
50. Vance CG, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: a randomized controlled trial. Phys Ther. 2012;92(7):898–910. doi:10.2522/ptj.20110183
51. Dieppe P, Lim K, Lohmander S. Who should have knee joint replacement surgery for osteoarthritis? Int J Rheum Dis. 2011;14(2):175–180. doi:10.1111/j.1756-185X.2011.01611.x
52. Bacchi S, Palumbo P, Sponta A, et al. Clinical pharmacology of non-steroidal anti-inflammatory drugs: a review. Antiinflamm Antiallergy Agents Med Chem. 2012;11(1):52–64. doi:10.2174/187152312803476255
53. Shivaprakash G, Rao JP, Pallavi LC. Unlocking the mystery of paracetamol mechanism of action and existance of cox-3-A review. Int J Univers Pharm Bio Sci. 2013;1:1.
54. Botting RM. Vane’s discovery of the mechanism of action of aspirin changed our understanding of its clinical pharmacology. Pharmacol Rep. 2010;62(3):518–525. doi:10.1016/S1734-1140(10)70308-X
55. Argoff C. Mechanisms of pain transmission and pharmacologic management. Curr Med Res Opin. 2011;27(10):2019–2031. doi:10.1185/03007995.2011.614934
56. Gunson MJ, Arnett GW, Milam SB. Pathophysiology and pharmacologic control of osseous mandibular condylar resorption. journal of Oral and Maxillofacial Surgery. 2012;70(8):1918–1934. doi:10.1016/j.joms.2011.07.018
57. Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: a current perspective. Biochem Pharmacol. 2020;180:114147. doi:10.1016/j.bcp.2020.114147
58. García-Rayado G, Navarro M, Lanas A. NSAID induced gastrointestinal damage and designing GI-sparing NSAIDs. Expert Rev Clin Phar. 2018;11(10):1031–1043. doi:10.1080/17512433.2018.1516143
59. Tai FWD, McAlindon ME. NSAIDs and the small bowel. Curr Opin Gastroenterol. 2018;34(3):175–182. doi:10.1097/MOG.0000000000000427
60. Bjarnason I. Gastrointestinal safety of NSAIDs and over-the-counter analgesics. Int J Clin Pract Suppl. 2013(178):37–42. doi:10.1111/ijcp.12048
61. Ghosh R, Alajbegovic A, Gomes AV. NSAIDs and cardiovascular diseases: role of reactive oxygen species. Oxid Med Cell Longev. 2015;2015:536962. doi:10.1155/2015/536962
62. du Souich P, García AG, Vergés J, et al. Immunomodulatory and anti-inflammatory effects of chondroitin sulphate. J Cell Mol Med. 2009;13(8A):1451–1463. doi:10.1111/j.1582-4934.2009.00826.x
63. Litwiniuk M, Krejner A, Speyrer MS, et al. Hyaluronic acid in inflammation and tissue regeneration. Wounds. 2016;28(3):78–88.
64. Brun P, Zavan B, Vindigni V, et al. In vitro response of osteoarthritic chondrocytes and fibroblast-like synoviocytes to a 500–730 kDa hyaluronan amide derivative. J Biomed Mater Res B. 2012;100B(8):2073–2081. doi:10.1002/jbm.b.32771
65. Pohlig F, Guell F, Lenze U, et al. Hyaluronic acid suppresses the expression of metalloproteinases in osteoarthritic cartilage stimulated simultaneously by interleukin 1β and mechanical load. PLoS One. 2016;11(3):e0150020. doi:10.1371/journal.pone.0150020
66. Chang -C-C, Hsieh M-S, Liao S-T, et al. Hyaluronan regulates PPAR gamma and inflammatory responses in IL-1 beta-stimulated human chondrosarcoma cells, a model for osteoarthritis. Carbohyd Polym. 2012;90(2):1168–1175. doi:10.1016/j.carbpol.2012.06.071
67. Wang M-Z, et al. Synthesis of crocetin derivatives and their potent inhibition in multiple tumor cells proliferation and inflammatory property of macrophage. BMC Complementary Med Ther. 2020;20(1):1 doi:10.1186/s12906-020-2831-y.
68. Maudens P, Jordan O, Allémann E. Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug Discov Today. 2018;23(10):1761–1775 doi:10.1016/j.drudis.2018.05.023 doi:.
69. Emami A, Tepper J, Short B, et al. Toxicology evaluation of drugs administered via uncommon routes: intranasal, intraocular, intrathecal/intraspinal, and intra-articular. Int J Toxicol. 2018;37(1):4–27. doi:10.1177/1091581817741840
70. Evans CH, Kraus VB, Setton LA. Progress in intra-articular therapy. Nat Rev Rheumatol. 2014;10(1):11–22. doi:10.1038/nrrheum.2013.159
71. Zhang Y, Chen X, Tong Y, et al. Development and prospect of intra-articular injection in the treatment of osteoarthritis: a review. J Pain Res. 2020;13:1941–1955. doi:10.2147/JPR.S260878
72. Bari, Hitesh. A prolonged release parenteral drug delivery system -- an overview. J Pharm Pharm Sci. 2010;1:1.
73. Burt HM, Tsallas A, Gilchrist S, et al. Intra-articular drug delivery systems: overcoming the shortcomings of joint disease therapy. Expert Opin Drug Deliv. 2009;6(1):17–26. doi:10.1517/17425240802647259
74. Uyen NTT, Hamid ZAA, Tram NXT, et al. Fabrication of alginate microspheres for drug delivery: a review. Int J Biol Macromol. 2020;153:1035–1046. doi:10.1016/j.ijbiomac.2019.10.233
75. Zhang X, Shi Y, Zhang Z, et al. Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis. Asian J Pharm Sci. 2018;13(3):229–238. doi:10.1016/j.ajps.2017.12.007
76. Rudnik-Jansen I, Colen S, Berard J, et al. Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform. J Control Release. 2017;253:64–72. doi:10.1016/j.jconrel.2017.03.014
77. Zhu S, Lu P, Liu H, et al. Inhibition of Rac1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann Rheum Dis. 2015;74(1):285–293. doi:10.1136/annrheumdis-2013-203901
78. Wei Y, Luo L, Gui T, et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;13(576). doi:10.1126/scitranslmed.abb3946.
79. Peng L-H, Wei W, Shan Y-H, et al. Sustained release of piroxicam from solid lipid nanoparticle as an effective anti-inflammatory therapeutics in vivo. Drug Dev Ind Pharm. 2017;43(1):55–66. doi:10.1080/03639045.2016.1220563
80. Chen H, Sun T, Yan Y, et al. Cartilage matrix-inspired biomimetic superlubricated nanospheres for treatment of osteoarthritis. Biomaterials. 2020;242:119931. doi:10.1016/j.biomaterials.2020.119931
81. Yuan T, Wang H, Tan M, et al. ZIF@VO 2 as an Intelligent Nano-Reactor for On-Demand Angiogenesis and Disinfection. Adv Healthc Mater. 2023;12(1). doi:10.1002/adhm.202201608.
82. Liang Y, et al. Chondrocyte-targeted microrna delivery by engineered exosomes toward a cell-free osteoarthritis therapy. Acs Appl Mater Inter. 2020. doi:10.1021/acsami.0c10458
83. Zhang J, Rong Y, Luo C, et al. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging. 2020;12(24). doi:10.18632/aging.104110.
84. Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr Cartilage. 2013;21(1):16–21. doi:10.1016/j.joca.2012.11.012
85. Yue K, Trujillo-de Santiago G, Alvarez MM, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi:10.1016/j.biomaterials.2015.08.045
86. Zhang J, Muirhead B, Dodd M, et al. An injectable hydrogel prepared using a peg/vitamin e copolymer facilitating aqueous-driven gelation. Biomacromolecules. 2016;17(11):3648–3658. doi:10.1021/acs.biomac.6b01148
87. Klouda L, Mikos AG. Thermoresponsive hydrogels in biomedical applications. Eur J Pharm Biopharm. 2008;68(1):34–45. doi:10.1016/j.ejpb.2007.02.025
88. Van Tomme SR, Storm G, Hennink WE. In situ gelling hydrogels for pharmaceutical and biomedical applications. Int J Pharm. 2008;355(1–2):1–18. doi:10.1016/j.ijpharm.2008.01.057
89. Zhang Z, Huang G. Micro- and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J Nanotechnol. 2011;2012(1687–9503):11 doi:10.1155/2012/748909.
90. He Z, Wang B, Hu C, et al. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloid Surf B. 2017;154:33–39. doi:10.1016/j.colsurfb.2017.03.003
91. David J, et al. Osteoarthritis. Lancet. 2019;1:1 doi:10.1016/S0140-6736(19)30417-9.
92. Xiao X, et al. Exosome-mediated delivery of kartogenin for chondrogenesis of synovial fluid-derived mesenchymal stem cells and cartilage regeneration. Biomaterials. 2020;1:1 doi:10.1016/j.biomaterials.2020.120539.
93. Kang ML, Se-Yeong J, Im GI. Hyaluronic acid hydrogel functionalized with self-assembled micelles of amphiphilic pegylated kartogenin for the treatment of osteoarthritis. Osteoarthr Cartilage. 2017;25:S76–S76. doi:10.1016/j.joca.2017.02.766
94. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. doi:10.1016/S0169-409X(01)00105-3
95. Jain A, Mishra SK, Vuddanda PR, et al. Targeting of diacerein loaded lipid nanoparticles to intra-articular cartilage using chondroitin sulfate as homing carrier for treatment of osteoarthritis in rats. Nanomedicine. 2014;10(5):1031–1040. doi:10.1016/j.nano.2014.01.008
96. Brown MB, Williams AC Theoretical Aspects of Transdermal and Topical Drug Delivery. The Art and Science of Dermal Formulation Development. 2019 doi:10.1201/9780429059872-2.
97. Yin K, Smith AG. Nuclear receptor function in skin health and disease: therapeutic opportunities in the orphan and adopted receptor classes. Cell Mol Life Sci. 2016;73(20):3789–3800. doi:10.1007/s00018-016-2329-4
98. Hadgraft J, Lane ME. Skin permeation: the years of enlightenment. Int J Pharm. 2005;305(1–2):2–12 doi:10.1016/j.ijpharm.2005.07.014.
99. Prausnitz MR, Langer R. Transdermal drug delivery. Nat Biotechnol. 2008;26(11):1261–1268. doi:10.1038/nbt.1504
100. Menon GK, Kligman AM. Barrier functions of human skin: a holistic view. Skin Pharmacol Phys. 2009;22(4):178–189. doi:10.1159/000231523
101. Proksch E, Brandner JM, Jensen JM. The skin: an indispensable barrier. Exp Dermatol. 2008;17(12):1063–1072 doi:10.110.1111/j.1600-0625.2008.00786.x111.
102. Elias PM. Skin barrier function. current Allergy and Asthma Reports. 2008;8(4):299–305. doi:10.1007/s11882-008-0048-0
103. Bouwstra JA, Ponec M. The skin barrier in healthy and diseased state. Biochim Biophys Acta. 2006;1758(12):2080–2095 doi:10.1016/j.chemphyslip.2009.06.136.
104. Kováčik A, Kopečná M, Vávrová K. Permeation enhancers in transdermal drug delivery: benefits and limitations. Expert Opin Drug Deliv. 2020;17(2):145–155. doi:10.1080/17425247.2020.1713087
105. Dragicevic N, Maibach H. Combined use of nanocarriers and physical methods for percutaneous penetration enhancement. Adv Drug Deliv Rev. 2018;127:58–84 doi:10.1016/j.addr.2018.02.003.
106. Marimuthu M, Bennet D, Kim S. Self-assembled nanoparticles of PLGA-conjugated glucosamine as a sustained transdermal drug delivery vehicle. Polym J. 2013;45(2):202–209. doi:10.1038/pj.2012.103
107. Peng L-H, Wang M-Z, Chu Y, et al. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma. Sci Adv. 2020;6(27). doi:10.1126/sciadv.aba2735.
108. Campos PM, et al. Liquid crystalline nanodispersion functionalized with cell-penetrating peptides improves skin penetration and anti-inflammatory effect of lipoic acid after in vivo skin exposure to UVB radiation. Drug Deliv Transl Re. 2020;10(6):1810–1828 doi:10.1007/s13346-020-00840-2.
109. Veryser L, Boonen J, Taevernier L, et al. The influence of the acyl chain on the transdermal penetration-enhancing effect of synthetic phytoceramides. Skin Pharmacol Phys. 2015;28(3):124–136. doi:10.1159/000365730
110. Pikal MJ. The role of electroosmotic flow in transdermal iontophoresis. Adv Drug Deliv Rev. 2001;46(1–3):281–305 doi:10.1016/S0169-409X(00)00138-1.
111. Kalia YN, Naik A, Garrison J, et al. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56(5):619–658. doi:10.1016/j.addr.2003.10.026
112. Zempsky WT, Sullivan J, Paulson DM, et al. Evaluation of a low-dose lidocaine iontophoresis system for topical anesthesia in adults and children: a randomized, controlled trial. Clin Ther. 2004;26(7):1110–1119. doi:10.1016/S0149-2918(04)90183-X
113. Beauchamp M, Lands LC. Sweat-testing: a review of current technical requirements. Pediatr Pulm. 2005;39(6):507–511. doi:10.1002/ppul.20226
114. Fukuta T, Oshima Y, Michiue K, et al. Non-invasive delivery of biological macromolecular drugs into the skin by iontophoresis and its application to psoriasis treatment. J Control Release. 2020;323:323–332. doi:10.1016/j.jconrel.2020.04.044
115. Fellinger K, Schmid J. Pathophysiology of rheumatism in the light of modern rheumatism therapy. Wien Klin Wochenschr. 1954;66(11):183–189.
116. Cameron BM. Ultra sound enhanced local anesthesia. Am J Orthop. 1966;8(3):47.
117. Levy D, Kost J, Meshulam Y, et al. Effect of ultrasound on transdermal drug delivery to rats and Guinea-pigs. J Clin Invest. 1989;83(6):2074–2078. doi:10.1172/JCI114119
118. Draper DO, Klyve D, Ortiz R, et al. Effect of low-intensity long-duration ultrasound on the symptomatic relief of knee osteoarthritis: a randomized, placebo-controlled double-blind study. J Orthop Surg Res. 2018;13(1):257. doi:10.1186/s13018-018-0965-0
119. Ter Haar G. Ultrasound bioeffects and safety. P I Mech Eng H. 2010;224(2):363–373. doi:10.1243/09544119JEIM613
120. Stride EP, Coussios CC. Cavitation and contrast: the use of bubbles in ultrasound imaging and therapy. P I Mech Eng H. 2010;224(2):171–191 doi:10.1243/09544119JEIM622.
121. Paliwal S, Menon GK, Mitragotri S. Low-frequency sonophoresis: ultrastructural basis for stratum corneum permeability assessed using quantum dots. J Invest Dermatol. 2006;126(5):1095–1101. doi:10.1038/sj.jid.5700248
122. Canavese G, et al. Nanoparticle-assisted ultrasound: a special focus on sonodynamic therapy against cancer. Chem Eng J. 2018;340:155–172 doi:10.1016/j.cej.2018.01.060.
123. Park D, Song G, Jo Y, et al. Sonophoresis using ultrasound contrast agents: dependence on concentration. PLoS One. 2016;11(6):e0157707. doi:10.1371/journal.pone.0157707
124. Masterson J, Kluge B, Burdette A, et al. Sustained acoustic medicine; sonophoresis for nonsteroidal anti-inflammatory drug delivery in arthritis. Ther Deliv. 2020;11(6):363–372. doi:10.4155/tde-2020-0009
125. Farah HA, Brown MB, McAuley WJ. Heat enhanced follicular delivery of isotretinoin to the skin. Pharmacol Res. 2019;36(8):124. doi:10.1007/s11095-019-2659-7
126. Lee H, Song C, Baik S, et al. Device-assisted transdermal drug delivery. Adv Drug Deliv Rev. 2018;127:35–45. doi:10.1016/j.addr.2017.08.009
127. Kennedy J, Larrañeta E, McCrudden MTC, et al. In vivo studies investigating biodistribution of nanoparticle-encapsulated rhodamine B delivered via dissolving microneedles. J Control Release. 2017;265:57–65. doi:10.1016/j.jconrel.2017.04.022
128. Hs DJ, Kong WH, Sung DK, et al. Nanographene oxide-hyaluronic acid conjugate for photothermal ablation therapy of skin cancer. Acs Nano. 2014;8(1):260–268. doi:10.1021/nn405383a
129. Henry S, et al. Microfabricated microneedles: a novel approach to transdermal drug delivery (vol 87, pg 922, 1998). J Pharm Sci-Us. 1999;88(9):948 doi:10.1021/js980042+.
130. Vanniasinghe AS, Bender V, Manolios N. The potential of liposomal drug delivery for the treatment of inflammatory arthritis. Semin Arthritis Rheu. 2009;39(3):182–196. doi:10.1016/j.semarthrit.2008.08.004
131. Szoka F Jr, Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Annu Rev Biophys Bioeng. 1980;9(1):467–508. doi:10.1146/annurev.bb.09.060180.002343
132. Zhou P, Chen C, Yue X, et al. Strategy for osteoarthritis therapy: improved the delivery of triptolide using liposome-loaded dissolving microneedle arrays. Int J Pharmaceut. 2021;609:121211. doi:10.1016/j.ijpharm.2021.121211
133. Rahmati M, Nalesso G, Mobasheri A, et al. Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. doi:10.1016/j.arr.2017.07.004
134. Lanke SSS, Kolli C, Strom J, et al. Enhanced transdermal delivery of low molecular weight heparin by barrier perturbation. Int J Pharmaceut. 2009;365(1–2):26–33. doi:10.1016/j.ijpharm.2008.08.028
135. Le L, Kost J, Mitragotri S. Combined effect of low-frequency ultrasound and iontophoresis: applications for transdermal heparin delivery. Pharm Res-Dordr. 2000;17(9):1151–1154. doi:10.1023/A:1026426317413
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

