Back to Journals » International Journal of Nanomedicine » Volume 19
Progress of Nanomaterials Based on Manganese Dioxide in the Field of Tumor Diagnosis and Therapy
Authors Liang L, Jia M, Zhao M, Deng Y, Tang J, He X, Liu Y, Yan K, Yu X, Yang H, Li C, Li Y, Li T
Received 7 May 2024
Accepted for publication 10 August 2024
Published 29 August 2024 Volume 2024:19 Pages 8883—8900
DOI https://doi.org/10.2147/IJN.S477026
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Lijuan Liang,1,* Ming Jia,2,3,* Min Zhao,4,* Yiping Deng,5,* Jun Tang,5 Xinghui He,2 Yilin Liu,2 Kexin Yan,2 Xin Yu,6 Hong Yang,7 Chunhong Li,2 Yao Li,2,8 Tao Li4
1Department of Pharmacy, Hejiang County People’s Hospital, Luzhou, Sichuan, People’s Republic of China; 2Department of Pharmaceutical Sciences, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 3Nanchong Institute for Food and Drug Control, Nanchong, Sichuan, People’s Republic of China; 4Key Laboratory of Medical Electrophysiology of Ministry of Education, Institute of Cardiovascular Research, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 5Analysis and Testing Center, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 6Chinese Pharmacy Laboratory, School of Pharmacy, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 7Department of Pediatrics, The Affiliated Hospital of Southwest Medical University, Luzhou, Sichuan, People’s Republic of China; 8Science and Technology department, Southwest Medical University, Luzhou, Sichuan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yao Li; Tao Li, Email [email protected]; [email protected]
Abstract: As a pivotal transition metal oxide, manganese dioxide (MnO2) has garnered significant attention owing to its abundant reserves, diverse crystal structures and exceptional performance. Nanosizing MnO2 results in smaller particle sizes, larger specific surface areas, optimized material characteristics, and expanded application possibilities. With the burgeoning research efforts in this field, MnO2 has emerged as a promising nanomaterial for tumor diagnosis and therapy. The distinctive properties of MnO2 in regulating the tumor microenvironment (TME) have attracted considerable interest, leading to a rapid growth in research on MnO2-based nanomaterials for tumor diagnosis and treatment. Additionally, MnO2 nanomaterials are also gradually showing up in the regulation of chronic inflammatory diseases. In this review, we mainly summarized the recent advancements in various MnO2 nanomaterials for tumor diagnosis and therapy. Furthermore, we discuss the current challenges and future directions in the development of MnO2 nanomaterials, while also envisaging their potential for clinical translation.
Keywords: manganese dioxide nanoparticles, tumors, inflammation, biomedicine, research progress
Introduction
Manganese (Mn), the twelfth most common element on Earth, ranks as the third most prevalent transition element following iron and titanium. Mn2+ serves as a crucial metal cofactor in numerous enzymes with varied functions and constitutes one of the pivotal functional components in plant oxygenation.1 As one of the main sources of Mn, manganese dioxide (MnO2) stands as a significant transition metal oxide, garnering considerable attention due to its ample reserves, varied crystalline structures, and exceptional properties.2 With the advancements in nanotechnology and molecular biology, the exploration and development of multifunctional nanomaterials have profoundly impacted contemporary medical practice.3 Nanoscale MnO2 boasts a larger specific surface area, superior properties, and diverse functionalities, holding considerable promise across various domains, particularly in tumor diagnosis and therapy.4
Over the past decade, there has been continuous development and investigation of MnO2 nanomaterials with diverse morphologies and structures. Concerning preparation methods, these nanomaterials are primarily fabricated and produced using various approaches such as chemical precipitation, solid-phase synthesis, and template methods (Table 1).5,6 In terms of microscopic morphology and structure, nanoscale MnO2 encompasses one-dimensional materials such as nanowires and nanofibres, two-dimensional materials like nanosheets, and multidimensional materials consisting of nanocubes, nanorods, and nanoflowers (Figure 1).6 Functionally, MnO2-based nanomaterials exhibit capabilities including drug loading/controlled release, T1-weighting and imaging, Fenton-like reactions, redox reactions, and enzyme-like catalytic activities.6,7 However, agglomeration and biocompatibility issues of nanomaterials can affect the efficiency and performance of the materials.8,9 Therefore, the surface of nanomaterials is often modified to distribute them uniformly in composites and to mitigate the adverse effects of the materials.10,11
 |
Table 1 Preparation of MnO2 Nanomaterials |
 |
Figure 1 Classification of MnO2 nanomaterials according to the microscopic morphology and structure. |
With continuous research and stormy development, these nanomaterials have emerged as the “star players” in the realm of tumor diagnosis and therapy. Consequently, this review systematically summarized the relevant applications of different manganese dioxide nanomaterials in the tumor diagnosis and therapy area, among which we pay special attention to their applications in bioimaging and therapy. In addition to this, the problems and developmental directions of MnO2 nanomaterials are discussed and their future clinical translation prospects are envisioned.
Application of MnO2 Nanoparticle (MnO2 NP) in Tumor Diagnosis and Therapy
MnO2 nanoparticle (MnO2 NP) is defined as three-dimensional solid spherical particles of 1–100 nm, offering advantages such as straightforward preparation, cost-effectiveness, and environmental friendliness.12 The simplest preparation method is the redox method. The specific preparation method is to take potassium permanganate dissolved in deionized water, dispersed homogeneously and then add appropriate amount of reducing agent (n-butanol, polyethyleneimine) for the reaction, and finally get MnO2 NP. As a new type of inorganic nanomaterials, MnO2 NP can be used for loading drugs, imaging, alleviating hypoxia, and amplifying oxidative stress, and its application in biomedical therapy primarily centers around tumor-related or inflamed-related diseases.13
A biomimetic MnO2 NP was designed by employing bovine serum albumin (BSA) as carrier, and results showed that the preparation could relieve oxidative stress in osteoarthritis and promote chondrocyte proliferation.14 Similarly, Wen et al synthesized hydrogen-peroxide-responsive protein biomimetic nanoparticles (MnO2-ICG@BSA) by loading indocyanine green (ICG) into a bovine serum albumin-manganese dioxide complex. It could serve as a good candidate material of PTT-PDT for melanoma treatment.15 Based on a similar strategy, the researchers obtained MnO2/Se-BSA nanoparticles (SMB NPs) by adding selenite to MnO2/BSA nanoparticle, in which MnO2 and selenite endowed the SMB NPs with acid-responsive and catalytic as well as antioxidant properties, respectively. Exploiting these features allowed them to overcome the limitations of TME and provided an opportunity to design high-performance, comprehensive reagents for tumor therapy.16 However, its clinical efficacy is constrained by the immunosuppressive microenvironment within the tumor, as well as severe oxygen depletion and deprivation. To address these limitations, Zhou et al constructed a nanosystem with oxygen regulation and PD-1/PD-L1 axis cascade disruption capabilities. The nanosystem encapsulated the mitochondria-associated oxidative phosphorylation disruption agent Butformin and the PDT drug methylene blue with programmed cell death protein 1 (PD-1) inhibition capacity in MnO2 NPs. It could selectively and responsively release two drugs in the tumors, synergistically reversing tumor hypoxia by inhibiting oxygen depletion with buguanidine and promoting oxygen generation with MnO2 NP. Methylene blue exhibited enhanced reactive oxygen species (ROS) generation due to reversed tumor hypoxia. Moreover, the PD-1/PD-L1 axis was significantly disrupted, thus reversing the immunosuppressive microenvironment.17
Similarly, in the tumor microenvironment (TME), hypoxia affects the ability of natural killer cells, an essential “killer member” of the immune system, to undergo overt metastasis. Leveraging this phenomenon, Murphy et al encapsulated MnO2 NP in poly (lactic-co-glycolic acid) (PLGA) to catalyze for the degradation of H2O2 in tumors and generate O2 to promote adoptive transfer of natural killer cells.18 In addition to natural killer cells, MnO2 NP can also affect the phenotype of tumor-associated macrophages (TAMs). The elevated concentration of glutathione (GSH) in TME facilitated the decomposition of MnO2 NP, and releasing Mn2+, which increases the iNOS expression level of TAMs.19 A dual cascade activatable nano-enhancer based on MnO2 NP for deep tumor immunotherapy was prepared. The results demonstrated that the catabolically released Mn2+ could help to up-regulate the stimulator of interferon genes (STING) and thus activated the anti-tumor immune response (Figure 2).20 Building upon this same immune activation mechanism, MnO2 NP had also been reported to be used in the development of vaccine adjuvant/delivery systems. A tumor vaccine was constructed using MnO2 NP piggybacking on the antigen ovalbumin, and it showed that MnO2 NP could induce cellular immune responses by delivering antigens into the cytoplasm of dendritic cells in several ways. In addition, as an advanced adjuvant depot in dendritic cells, it could enhance immune responses by influencing the STING pathway through the sustained release of Mn2+.21
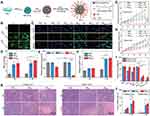 |
Figure 2 (A) Schematic illustration of the fabrication of NPMCA. (B) Fluorescence images of 4T1 cells in PBS-, NPMC- and NPMCA-treated groups without or with US treatment in the presence of ROS probes. (C) Immunofluorescence CRT staining images of 4T1 cells after different treatments. (D) MDA levels for 4T1 cells in PBS-, NPMC- and NPMCA-treated groups without or with US treatment. (E) Released ATP levels for 4T1 cells in various treatment groups. (F) Extracellular HMGB1 levels for 4T1 cells in PBS-, NPMC- and NPMCA-treated groups without or with US treatment. (G) Relative tumor volume of chicken breast tissue-covered 4T1 primary tumors in PBS-, NPMC- and NPMCA-treated mice without or with US treatment. (H) Relative tumor volume of 4T1 distant tumors in PBS-, NPMC- and NPMCA-treated mice without or with US treatment. (I) Total weight of primary and distant tumors in PBS-, NPMC- and NPMCA-treated mice without or with US treatment. (J) Tumor inhibition efficacy analysis. (K) Images of H&E stained primary and distant tumors in PBS-, NPMC- and NPMCA-treated mice without or with US treatment. The data are presented as the means ± SDs. The p values are calculated using two-tailed unpaired t test. *p < 0.05, **p < 0.01, and ***p < 0.001. Adapted from Zhan M, Wang F, Liu Y, et al. Dual-cascade activatable nanopotentiators reshaping adenosine metabolism for sono-chemodynamic-immunotherapy of deep tumors. Adv Sci. 2023;10(10):e2207200. Creative Commons.20 Abbreviations: ADA, adenosine deaminase; Ce6, chlorin e6; NPMCA, the nanopotentiator constructed through crosslinking ADA with Ce6-conjugated MnO2 NPs via a ROS-cleavable linker; NPMC, conjugating sonosensitizer Ce6 onto BSA-MnO2 NPs; US, ultrasound; MDA, malondialdehyde. |
In addition to influencing the body’s immune response to tumors, Mn2+ serves as a valuable T1 contrast agent capable of reducing unnecessary toxicity and improving the accuracy of cancer detection. The conjugates prepared by Hong et al exhibited rapid reduction of permanganate to MnO2 NP, demonstrating excellent aqueous dispersion and stability under physiological conditions, and effectively responding to TME, thereby utilizing the generated Mn2+ for magnetic resonance imaging (MRI).22 This coupling of hyaluronic acid modified with a histidine derivative enhanced the stability of MnO2 nanomaterials and avoided their agglomeration effect.23 In the pursuit of novel therapeutic diagnostic for MRI and targeted therapy of osteosarcoma, Ju and co-workers reported an Phytic acid (PA)-modified MnO2 NPs (MnO2@PA NPs). They were effectively internalized by tumor cells and generated Mn2+ and O2 in an acidic microenvironment for MRI, achieving targeted therapy of osteosarcoma.24 In order to overcome the limitations of single diagnostic imaging modalities and chemotherapeutic methods, Luo et al had integrated multimodal imaging function based on MnO2 NP and developed a hybrid NP, which was composed of superparamagnetic iron oxide, MnO2 and doxorubicin, achieving efficient T2-T1 MRI and switchable photoacoustic imaging (PAI).25
Chemotherapy holds a pivotal role in the clinical treatment of cancer, yet hypoxia-mediated chemoresistance remains a significant hurdle to effective tumor chemotherapy. It’s helpful to address this obstacle by harnessing the oxygen-producing capacity of MnO2 NPs within the weakly acidic and high H2O2 environment of TME. On this basis, researchers rationally designed a new class of tLyP-1-modified dopamine (DOPA)-β-cyclodextrin (CD)-coated paclitaxel (PTX)- and manganese dioxide (MnO2)-loaded NPs (tLyP-1-CD-DOPA-MnO2@PTX) for glioma chemotherapy.26 They could efficiently across the blood-brain barrier to accumulate at the tumor site and enhance chemotherapy. The NPs delivered to the tumor site undergo decomposition in the weak acid environment and excessive H2O2 in the TME, leading to systemic collapse generating Mn2+ and O2 to relieve the hypoxia of tumor tissue and produce ROS to induce apoptosis. In a rat model of intracranial glioma for promoting apoptosis, the nanoparticle could inhibit tumor cell proliferation and achieve real-time tumor monitoring via MRI. This therapeutic and diagnostic-based approach would provide a prospective strategy to simultaneously enhance chemotherapy and enable real-time imaging of glioma treatment processes.26 In addition, MnO2 NP is widely utilized to increase radiotherapy (RT), chemodynamic therapy (CDT), sonodynamic therapy (SDT), and PDT due to its Fenton-like reaction.27–30 Researchers synthesized novel polymer-lipid manganese dioxide NPs (PLMDs) by incorporating MnO2 NP into myristic acid solid polymeric lipid NPs. Their bioactivity was exploited to remodel the tumor immune microenvironment by increasing local oxygen levels and extracellular pH and enhancing radiation-induced immunogenic cell death. The preparation could down-regulate programmed death ligand 1 and promote anti-tumor CD8 T cells and M1 macrophages to infiltrate into the tumor site, which could improve the therapeutic effect of radio-resistant and immunosuppressive solid tumors.31 Furthermore, Zhang et al reported a smart nanoplatform coloaded with Au and MnO2 NP for dual-mode computed tomography (CT) /MRI-guided tumor sensitization of RT throughout the treatment process. Utilizing the Fenton-like reaction of Mn2+, this platform efficiently generated ROS, leading to the distribution of the cell cycle towards the deeply radiosensitive G2/M phase before X-ray irradiation. Co-loading of Au NP sensitized cancer cells to RT under X-ray, resulting in significant DNA damage.32
To counter the enormous challenge of crossing physiological barriers, biomimetic technology research is dedicated to the optimization and development of innovative nanomedicines. For instance, Du et al prepared a C6 cell membrane-coated doxorubicin conjugated manganese dioxide biomimetic nanomedicine system (MnO2-DOX-C6) for glioma therapy. The selection of C6 cell membranes allowed homologous targeting of MnO2 NP and modulation of drug release rate.33 Moreover, due to macrophage membrane protein-mediated recognition of cell adhesion molecules overexpressed on the damaged vascular endothelium, NPs could accumulate rapidly to the damaged brain. On this basis, macrophage membranes can be camouflaged on the surface of drug-carrying MnO2 NPs and thus traverse the blood brain barrier (BBB) to rescue neuronal damage (Figure 3).34 According to this principle, in another work, Xiao et al also utilized macrophage membranes to camouflage multifunctional polymer nanogels based on MnO2 NP to endow them a longer blood circulation time and the ability to cross the blood-brain barrier.35 In addition, a MnO2 NP-doped nanovesicles carrying an adenosine 2A receptor agonist were developed to affect the F-actin and tight junction effects on endothelial cells, and they could temporarily increase the permeability of the BBB, thereby helping MnO2 NP to cross the BBB.36 These cell membrane-encapsulated MnO2 nanomaterials can be modified and functionalized by self-recognition under the camouflage of the membrane to be biocompatible and immune evasive for better therapeutic effects.37
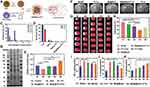 |
Figure 3 (A) Scheme of the preparation process of Ma@(MnO2+FTY) NPs. (B) Proteins in macrophage membrane, macrophage membrane vesicles and Ma@(MnO2+FTY), analyzed with SDS-PAGE. (C) H2O2 scavenging behavior of MnO2 nanospheres with multiple concentration over 8 hours. (D) H2O2 scavenging behavior of different formulations. (E) Quantification of the relative fluorescence intensity of CD206 versus CD16/32. (F) The infarct area of tMCAO/R rats treated with different drugs, monitored by MRI at 24 h post reperfusion. (G) The rescue ability of NPs on ischemic penumbra, brain sections were stained with TTC. (H) The quantified results of TTC staining. (I-K) Treatment with Ma@(MnO2+FTY) NPs reversed the proinflammatory microenvironment. Data are presented as means ± SD, n = 3, *P < 0.05, **P < 0.01, ***P < 0.001. Adapted from Li C, Zhao Z, Luo Y, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci. 2021;8(20):e2101526. Creative Commons.34 Abbreviations: FTY, fingolimod; Ma@(MnO2+FTY), a macrophage-disguised honeycomb MnO2 nanosphere loaded with FTY. |
In addition, studies about synthesis of chiral (L/D)-MnO2 NP using threonine molecule as a chiral ligand was reported. The preparation exhibited different chirality-based effects on tumor cell internalization and tumor cell ablation, and it was notably that the D-MnO2 NP being more therapeutically effective than L-MnO2 NP.38
Application of Hollow Mesoporous MnO2 NP (HMMnO2 NP) in Tumor Diagnosis and Therapy
In comparison with MnO2 NP, Hollow mesoporous MnO2 NP (HMMnO2 NP) has been reported to have faster degradation, higher specific surface area, improved colloidal dispersion and enhanced drug piggybacking ability.39 HMMnO2 NPs are mainly prepared by the template method, using mesoporous silicon, metals, carbon nanotubes and polymers as templates, and finally etching the core using different reagents (eg, HCl, Na2CO3 aqueous solution) to obtain them. However, the elimination of templating agents during the synthesis of nanomaterials by the template method may lead to the collapse of some of the pore structures, affecting the product properties, and therefore different methods (eg, surface modification) need to be investigated to improve the filling capacity. In addition, the poor stability of some template agents is easily affected by the synthesis system, so there are certain requirements for the synthesis system.40 Therefore, it is necessary to control the reaction conditions, including the reaction temperature, time and the choice of template, to avoid the chemical reaction between the assembled medium and the template, so that the stability of the template is maintained during the assembly process.
Qiu et al prepared NPs that could target activated macrophages, remove ROS, and exert anti-inflammatory effects by coating dextran sodium sulfate (DSS) on HMnO2 NP.41 In order to improve the efficacy of cisplatin (CDDP) in thyroid cancer, Liu et al designed CDDP-loaded HMMnO2 NP by modifying with polydopamine (PDA) and Cy5.5, namely MnO2/[email protected] NPs. Under acidic or redox conditions, the NPs rapidly released CDDP by inducing the breakdown of PDA and MnO2 to generate the MRI contrast agent Mn2+, showing favorable diagnostic and therapeutic functions.42 To further improve its targeting and immune escape, Wu et al used biomimetic nanotechnology to encapsulate HMMnO2 NPs by breast cancer cell membranes, which resulted in excellent homologous targeting and immune escape properties, as well as efficient in-situ generation of O2 to alleviate tumor hypoxia.43 Utilizing similar structures and methods as above, Zhu et al designed a biomimetic system for synergistically enhanced radiotherapy, which consisted by an internal camptothecin (CPT)-loaded hollow MnO2 core and an external tumor cell membrane. The tumor cell membrane endowed the system with prominent tumor targeting ability for improving hypoxia and enhancing radiotherapy sensitivity.44 Moreover, encapsulation of HMMnO2 NP with human umbilical cord mesenchymal stem cell (hUC-MSC) membranes inherited the active targeting ability. It also demonstrated the great potential of HMMnO2 NP as a nucleus-targeting nanocarrier for cancer chemo-immunotherapy.45 HMMnO2 NP encapsulated by cancer cell membranes had the dual advantages of immune escape and improved targeting, which could simplify the complexity of surface modification. However, its homologous targeting was based on the adhesion properties of cancer cells, and the degree of specific targeting against cancer cells was weaker than the specific binding modality of receptor-ligand.46 In addition to coating by K7M2 cell membrane on the surface of HMMnO2 NP, it had been reported that the surface modification of alendronate sodium (ALD), a bone targeting ligand with simple structure and high bone-affinity, could show enhanced bone tumor targeting and tumor homing ability.47
HMMnO2 NP can also be dissociated by low pH and high levels of GSH in tumor cells, generating Mn2+ for therapeutic strategies in different pathways.48 For example, a polyethylene glycol (PEG)-modified hollow mesoporous manganese dioxide (HMnO2@PEG) NPs were designed for tumor treatment, and they significantly improved efficacy through excellent tumor microenvironment responsiveness, while cleverly integrating diagnosis and treatment.49 Likewise, a hollow mesoporous manganese dioxide-based (H-MnO2) multifunctional nanoplatform, H-MnO2@AFIPB@PDA@Ru-NO@FA (MAPRF NPs), was constructed for synergistic tumor treatment.50 The MAPRF NPs showed TME-responsive properties of depletion of GSH synergizing with Mn2+-catalyzed CDT to significantly enhance NO-based gas therapy. In order to further help HMMnO2 NPs to be “invisible” under physiological conditions and “visible” in disease environments, thus realizing precise drug delivery at disease sites and reducing cargo loss, a hollow manganese dioxide nanoparticle (HMDN) functionalized with polyethyleneimine (PEI) and citraconic anhydride (cit) functionalized poly-L-lysine (PLL (cit)) was designed. This preparation had a multi-stimuli responsive ability, and the charge reversal properties of the nanoplatforms conferred invisibility to normal cells and enzyme-catalyzed activation in tumor cells, thus effectively synergizing DOX-mediated chemotherapy and Mn2+-mediated CDT.51 Likewise, a H-MnO2-based NPs (HMDN) coated with the pH-sensitive polymer PEI-PEG were prepared by loading gallic acid-ferrous (GA-Fe) nanodots that was fabricated from gallic acid (GA) and ferrous ion (Fe2+), in which the polymer PEI-PEG acted as a dynamic protection against drug leakage. The GA-Fe@HMDN-PEI-PEG synergized for effective CDT via GSH depletion, Mn2+ and Fe2+ mediated Fenton reactions, and transformation of GA and metal ions.52 This means by preventing premature cargo leakage by encapsulating HMMnO2 NP with macromolecules had also been reported in other studies, eg, the use of polydopamine (PDA) as a shell to encapsulate HMMnO2 NP to form a responsive controlled-release layer with adjustable thickness to prevent premature cargo release (Figure 4).53,54 The ability to ameliorate hypoxia using HMMnO2 NP also promoted the enhancement of PDT, which was particularly significant in tumors treatment.55
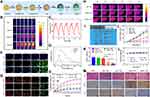 |
Figure 4 (A) Schematic illustration of the synthesis procedures of AHTPR NPs. (B) The infrared thermal images and time-dependent temperature elevation of AHTPR at various concentrations under 1.0 W cm−2 808 nm laser irradiation. (C) Photothermal stability curve of AHTPR (120 µg mL−1) NPs following on/off NIR laser irradiation (2.0 W cm−2) for 5 cycles. (D) UV–vis spectra of AIBI, HTPR and AHTPR. (E) The release profiles of AIBI from AHTPR in PBS with different pH values without or with NIR irradiation (808 nm, 1.0 W cm−2, 10 min). (F) The generation of alkyl radicals in MNNG/HOS cells after corresponding treatment. (G) Detection of mitochondrial membrane potential (ΔΨm). (H) In vivo thermal images and temperature variation curves of tumor region recorded by using the NIR thermal camera during NIR laser irradiation (1.0 W cm−2). (I) The photographs of all tumors taken from mice after various treatments. (J) Tumor volume growth curves of tumor-bearing mice during the administration of different formulations. (K) Tumor weights were measured after various treatments. (L) Body weight changes of MNNG/HOS tumor-bearing mice in different groups during the treatment. (M) H&E and immunohistochemical (TUNEL and Ki-67) analyses of tumor slices in different groups. (*P<0.05, **P<0.01, ***P<0.001). Adapted from Hu H, Deng X, Song Q, et al. Mitochondria-targeted accumulation of oxygen-irrelevant free radicals for enhanced synergistic low-temperature photothermal and thermodynamic therapy. J Nanobiotechnol. 2021;19(1):390. Creative Commons.53 Abbreviations: TPP, triphenylphosphonium; AIBI, 2,2′-azobis[2-(2-imidazolin-2-yl) propane] dihydrochloride; AHTPR, a core–shell nanoplatform composed of RGD functioned PDA as a shell and a TPP modified H-MnO2 as a core with the encapsulation of AIBI; HTPR, AHTPR without AIBI. |
The Mn2+ released from HMMnO2 NP can be used to enhance T1 MRI signals and monitor cargoes in real time. Wei et al reported a small octopod-shaped HMMnO2 NP as a stimulus-responsive T1-activated nanoplatform, which could be decomposed into paramagnetic Mn2+ to monitor in vivo cargo deliveries in the tumor’s weakly acidic microenvironmental conditions or in lysosomes.56 Zhuang et al even demonstrated a special yolk-shell-like nanostructure composed of a large cavity based on the HMMnO2 shell and accompanied by a core of small NPs, and it could generate Mn2+ to enhance tumor-specific T1-MR imaging.57 Similar studies have been competently reported by Li’s study.58
In addition to making HMMnO2 NP invisible in the blood system and enhancing target cell uptake, how to make HMMnO2 NP that reaches outside the tumor tissue effectively and consistently penetrate the dense tumor stroma and enter the tumor core is another key to disintegrate and damage tumor cells. With the ability to ablate the collagen component of the tumor extracellular matrix, bromelain was modified on the surface of HMMnO2 NP, which could be used to promote its accumulation in the deeper tumor tissues and significantly enhance the therapeutic efficacy.59 More interestingly, inspired by micro/nano-motors, Ou et al developed a self-propelled biodegradable nanomotor system based on HMMnO2 NP, which provided efficient cargo drag and effective tumor penetration even with low concentrations of H2O2 fuel due to the large surface area, high catalytic activity, and mesoporous structure of HMMnO2 NP.60 However, for some specific diseases, delivery of HMMnO2 NP also requires overcoming the barrier between blood and tissue. Surface modification of HMMnO2 NP using Opca protein, an outer membrane invasion protein of Neisseria meningitidis, could effectively overcome the BBB and catalyze the decomposition of excess H2O2 in tumor tissues to produce O2, alleviating tumor hypoxia thereby treating malignant glioma.61 Similarly, Li et al developed chitosan-modified HMMnO2 NP to penetrate blood-spinal cord barrier (BSCB) to assist in the treatment of spinal cord injury (SCI). In vitro and ex vivo studies demonstrated that it could significantly alleviate oxidative stress by decreasing the levels of ROS, MDA, and SOD (superoxide dismutase), and increasing the level of GSH.62
Chronic inflammatory diseases such as rheumatoid arthritis (RA) also produce large amounts of ROS. In our previous work, we similarly used a combination of HMMnO2 NP and macrophage-derived microvesicles to simulate SOD and CAT enzyme activity to help restore O2- and H2O2 metabolism in inflammatory activated macrophages.63
Most of the previously reported MnO2 nanostructures may not be ideal for achieving the most efficient drug loading and precisely controlling the release of therapeutic payloads.64,65 It is indeed this hollow mesoporous structure that gives HMMnO2 unique advantages compared with other morphological structures, and it has great research and application value in the field of multi-functional carrier carrying and transporting cargo, especially drug delivery, and also can achieve the purpose of accurately controlling the release of goods by adjusting the shell structure or coating.66,67
Application of MnO2 Nanosheet (MnO2 NS) in Tumor Diagnosis and Therapy
Nanosheets are two-dimensional nanostructures with a large specific surface area and a thickness ranging from 1 to 100 nm, where collisions and contacts between substrates and their active sites can easily occur.68 MnO2 NS consists of MnO6 octahedra with shared edges, ie, manganese ions occupy the center of the octahedra, and as a transition metal-oxide base layer nanomaterial, it has not only strong absorptive and reactive properties but also excellent mechanical properties.5 Currently, the hydrothermal method is preferred for the preparation of MnO2 NS. Under mechanical stirring, potassium permanganate is added to the corresponding solvent (eg, deionized water, Sodium dodecyl sulfate). Subsequently, the above solution is obtained by reacting it at high temperature and pressure. It is more environmentally friendly compared to the traditional template method.69 However, it is more dangerous than other synthesis methods due to the high temperature and pressure synthesis environment. In addition, the temperature and the size of the reactor liner are the most important factors affecting the convection, and they will have a large impact on the morphology and structure of the reaction products, and need to be screened to obtain the optimal preparation conditions.
Using MnO2 NS as an enzyme-like active material for superoxide dismutase and catalase could remove H2O2 and thus provide antioxidant support.70 While Li et al further took advantage of the enzyme-like activity of MnO2 NS by integrating it into the same system with glucose oxidase via mesoporous PDA thereby undergoing an efficient synergistic cascade effect for amplified enhancement of photothermal therapy (PTT), PDT as well as starvation therapy for the treatment of cancer.71 However, it is important to take into account that the non-specific distribution of photo-sensitizers may cause damage to normal tissues upon application of light. In order to achieve safer and more efficient PDT, Yin et al loaded gold nanoclusters (AuNCs) that with PDT effect into mesoporous silica and encapsulated them with MnO2 NS to obtain AuNCs@mSiO2@MnO2. It could respond to H2O2 in the acidic tumor environment to achieve the smart on/off release of drugs, and simultaneously enhance both MRI and PDT (Figure 5).72
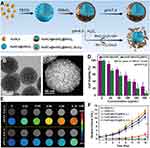 |
Figure 5 (A) Synthesis process of AuNCs@mSiO2@MnO2 nanozyme for H2O2-responsive “off/on” modulation. (B and C) TEM, STEM of AuNCs@mSiO2@MnO2. (D) Viability of MDA-MB-435 cells irradiated by a 635 nm laser (0.1 W/cm2) for 15 min. (E) Intracellular T1-weighted MR imaging incubated with AuNCs@mSiO2@MnO2 at different concentrations. (F) Tumor volume change curves of different groups. Data are presented as means ± SD, n = 3, **P < 0.01. Adapted withpermissionfrom Yin Z, Ji Q, Wu D, et al. H(2)O(2)-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for”Off/On” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl Mater Interface. 2021;13(13):14928–14937. Copyright 2021 American Chemical Society.72 Abbreviations: AuNCs, gold nanoclusters; AuNCs@mSiO2@MnO2, a nanozyme loaded with AuNCs in mesoporous silica and wrapped with MnO2 nanosheets on the outer surface; STEM, scanning transmission electron microscopy. |
In addition to using H2O2 as an “on/off“ trigger for drug release, MnO2 NS can also use overexpressed GSH in TME as an alternative redox release trigger. A core-shell “loading-type” nanomaterials (termed as MSNs@MnO2) were constructed by using mesoporous silica NPs (MSNs) as core and a two-dimensional manganese dioxide nanosheets (MnO2) as outer layer.73 Different from Yin’s study, their MnO2 NS design not only blocked R6G leakage in MSNs, but also acted as a reservoir for nucleic acid compounds. After entering cells, the specific redox reaction between MnO2 NS and GSH triggered the leakage of R6G in MSNs and the release of nucleic acid compounds on MnO2 NS nanosheets. However, inorganic nanomaterials with large specific surface area and high surface free energy are more easily recognized and cleared by the monocyte-endothelial reticulum system as foreign visitors in the blood system. Based on this dilemma, PEG-modified MnO2 NS may be a better choice for drug delivery, which can help MnO2 NS evade clearance by the blood system.74 To further exploit the ability to target cancer, Hao et al prepared a FA-PEG-modified MnO2 NS drug delivery system, which could effectively target tumor sites and precisely regulated drug release through a slightly acidic environment and elevated concentration of GSH.75 It is worth noting that Fan et al introduced a creative idea using transferrin and dihydroartemisinin coupled with DSPE-PEG-COOH to double modify MnO2 NS and piggyback on antisense oligonucleotide sequences for GSH depletion, ROS generation and GPx downregulation. This preparation produced transitional lipid peroxides (LPO) and promote ferroptosis in cancer cells, which is undoubtedly enlightening.76 However, repeated injections of PEGylated nanomaterials triggered an accelerated blood clearance effect (ABC effect) that resulted in the loss of recognition shielding and long circulation properties. Modification of MnO2 NS with hyperbranched poly-L-lysine (HBPL) greatly improved the stability of MnO2 NS in physiological environment.77 As a further optimization, a novel gene-carrier system CM@MnO2-PEI-NLS-ss/p53 (M@MPNs/p53) was constructed by using B16F10 cell membrane to encapsulate p53 gene-loaded manganese dioxide (MnO2) nanosheets.78 Due to tumor cell membrane coating endowed NPs with homotypic targeting and immune escape capabilities, the NPs effectively reduced GSH in tumor tissues to significantly improve p53 gene-mediated anti-tumor therapy.
Interestingly, MnO2 NS also has been reported to possess photothermal properties with near-infrared absorption, in addition to enzyme-like activity and redox properties. Wang et al prepared ultrathin MnO2 NS by a simple hydrothermal method for the first time, which possessed excellent photothermal conversion properties and could be used in PTT therapy of cancer.79 On this basis, the ultrathin MnO2 NS was further modified by polyethylene glycol-cyclic arginine-glycine-aspartic acid tripeptide (PEG-cRGD) to piggyback on Ce6, which could be utilized for targeted cancer PTT and PDT synergistic therapies.80
MnO2 NS for fabricating biosensors with high sensitivity and good stability is superior compared to other morphological structures. The two-dimensional structure based on MnO2 NS facilitates fast electron transfer between layers, which improves the electrochemical performance. This good conductivity and electrochemical activity can facilitate the electron transfer process and improve the electrochemical signal of the biosensor, thus enhancing the detection sensitivity. This will be of great significance for monitoring cancer health and for cancer prevention, early diagnosis and precision treatment.
Application of MnO2 Nanoflower (MnO2 NF) in Tumor Diagnosis and Therapy
MnO2 NF is a three-dimensional structure of manganese dioxide nanorods formed by clustering of flaky manganese dioxide.81 Preparation of MnO2 NF by chemical precipitation is a simpler option. It is prepared by adding potassium permanganate solution to a certain precipitating agent (ammonium persulfate, hydrogen peroxide) and then sonicated until a dark brown precipitate is formed.82 However, during the reaction process, the concentration, temperature, and pH will have some effects on the particle size, crystal shape, yield, purity, and surface properties of the products. Besides, chemical precipitation method is prone to agglomeration of nanoparticles, which needs to be controlled by appropriate dispersion techniques for different agglomeration mechanisms.83
As a unique nutrient-responsive immunogenic cell death (ICD) inducer with intrinsic immunomodulatory properties, MnO2 NF can directly regulate immune surveillance of tumor cells. Based on this, Yang et al synthesized MnO2 NF by a one-pot self-assembly method, which could effectively induce ICD in cancer cells under nutrient-deficient conditions.82 However, due to the complexity of tumor pathogenesis and the heterogeneity of the TME, it is difficult to obtain satisfactory efficacy with single pathway therapy. CDT therapy based on ROS generation is a promising strategy for tumor treatment. Xiao et al developed an efficient nanotherapeutic agent for CDT in collaboration with PTT based on Fe2O3, MnO2, hyaluronic acid (HA) and doxorubicin. In the acidic microenvironment of cancer cells, it could decompose and release Mn2+ to triggering the Fenton-like reaction to generate ·OH, while alleviating tumor hypoxia for assisting its own photothermal properties to further accelerate ROS generation.84 Similarly, a HA-modified ruthenium nanoaggregate (RuNA) and glucose oxidase (GOD)-loaded MnO2 NF (MRG@HA) have been prepared. It could produce O2 in TME to alleviate hypoxia in tumor tissue. At the same time, RuNA is a good photothermic agent which could perform high-temperature ablation of solid tumors under infrared laser irradiation.85
It should be noted that the strong dependence of phototherapy on oxygen status/external stimulation is one of the main reasons for its limited efficacy. Based on this bottleneck, Wang et al prepared bimetallic PtCo NPs using a simple one-pot method, and then guided the growth of MnO2 NF and self-assembly into highly ordered PtCo@MnO2 NF by adjusting the ratio of PtCo NPs and KMnO4. Among them, PtCo NPs could catalyze the oxidation reaction cascade and induce intracellular oxidative damage. At the same time, MnO2 component had inherent Cat-like activity, which could induce the decomposition of H2O2 to O2 quickly and efficiently. Both in vitro and in vivo experiments demonstrated that PtCo@MnO2 NF could significantly induce intracellular oxidative damage and inhibit tumor growth in xenograft mice under different oxygen tension. This strategy did not require external input of energy or oxygen, and CDT could be readily achieved by utilizing only endogenous chemical energy to generate ROS (Figure 6).86 In addition to the PtCo generation pathway, MnO2 NF was also accurately grown after coating functional polyphosphonitrile on Fe3O4 nanoclusters.87 This NF had the characteristics of H2O2/pH/GSH multi-stage response, which realized the specific drug release in the tumor to make the maximum synergistic therapeutic effect. Ma et al also demonstrated a versatile nanoplatform based on 2,2 ‘- azobis [2-(2 imidazolin-2-yl) propane] dihydrochloride (AIPH) loaded MnO2 NF that was able to promote PDT/PTT/TDT through self-supply of O2 and consumption of GSH.88 Once MnO2 NF internalized into the tumor, it could catalyze the conversion of endogenous H2O2 into O2 to enhance PDT. In addition, MnO2 NF could also promote GSH oxidation and amplify oxidative stress to further enhance cancer cell killing. In short, the NF can provide continuous oxygen, consume glutathione and combine phototherapy with TDT, and it will serve as an example of improving anti-tumor efficiency.
 |
Figure 6 (A) Schematic illustration showing the self-assembly of nanozymes into well-defined nanoflowers and schematic representation of the generation mechanism of ROS and cytotoxicity by MnO2@PtCo nanoflowers under different oxygen tensions. (B) TEM image of MnO2@PtCo nanoflowers. Scale bars are 100 nm. (C) Fluorescence spectra of hydroethidine incubated with MnO2@PtCo nanoflowers in the hypoxic H2O2 (100 μM) condition. (D) Fluorescence spectra of TA incubated with MnO2@PtCo nanoflowers in the hypoxic H2O2 (100 μM) condition. (E) ESR spectra of BMPO/•OOH adducts from different groups in the hypoxic H2O2 (100 μM) condition upon addition of DMSO. (F) ESR spectra of BMPO/•OH adducts were collected from different samples in the hypoxic H2O2 (100 μM) condition upon addition of SOD. (G) The ability of MnO2@PtCo nanoflowers on mitigating hypoxia, generating ROS and cytotoxicity in MCTS. (H) Changes of MDA content in 4T1 cells following treatment with different formulations. Asterisks indicate significantly differences, analyzed by unpaired Student’s two-sided t test. Data were presented as means ± SD, n = 3, *P < 0.05, **P < 0.01. (I) GSH/GSSH ratios of 4T1 cells treated with different concentrations of MnO2@PtCo nanoflowers. (J) JC-1 analysis of 4T1 cells as a measure of mitochondrial depolarization after treatment with different formulation. (K) Photos of the tumors extracted from mice in different groups at the end of treatments (day 14). (L) Representative immunofluorescence images of tumor slices after hypoxia staining. Scale bars are 100 μm. Data were presented as mean ± S.D. (n = 5). Adapted from Wang Z, Zhang Y, Ju E, et al. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat Commun. 2018;9(1):3334. . Creative Commons.86 Abbreviations: PtCo, atomic of Pt/Co; MnO2@PtCo, high-ordered nanoflowers composed of PtCo NPs and MnO2 NPs; TA, terephthalic acid; ESR, electron spin resonance; MCTS, multicellular tumor spheroids. |
Compared to other structures, MnO2 NF has a larger specific surface area due to its multibranched and concave structure, which can provide more active sites and increase the reaction contact area, thus improving the reaction efficiency and catalytic performance and CAT, SOD enzyme activity of MnO2 for more efficient therapeutic and diagnostic effects.
Application of Other MnO2 Nanomaterials in Tumor Diagnosis and Therapy
As a one-dimensional structure with unique properties and a size controlled below 100 nm, nanowire (NW) is one of the crucial members in the field of nano-biomedicine.89 In order to obtain one-dimensional MnO2 NW with controllable structure and unique electron transfer properties more conveniently, Han et al synthesized a novel glucose biosensor M13-E4@MnO2 NWs by uniformly coating MnO2 crystals on the surface of phage (M13-E4). The biosensor could catalyze the electrooxidation of H2O2 under neutral pH condition, and further combined with glucose oxidase.90 In addition, due to its good repeatability between and within measurements and desirable storage stability, it has broad application prospects in electrocatalysis, electrochemical sensors and supercapacitors. As an important intracellular antioxidant, GSH can be used as an effective endogenous molecule for diagnosis and tumor microenvironment activation therapy.91,92 MnO2 NW has been used as an oxidant, quencher and recognition unit for GSH determination.93 With the help of MnO2 NW, the GSH sensor was constructed by mixing thiamine (VB1) and Rhodamine B (RhB). MnO2 NW could not only effectively quench the fluorescence of RhB through the internal filtration effect (IFE), but also oxidize non-fluorescent VB1 to blue fluorescent thiochrome (oxVB1). After interacting with GSH, the quenching RhB fluorescence could recover, while oxVB1 fluorescence decreased. In other words, the fluorescence intensity of the RhB and VB1 mixture could be simultaneously adjusted by MnO2 NW, making it a sensitive diagnostic method. In addition to its diagnosis, using MnO2 NW for can also be used for treatment. He et al directly synthesized MnO2 NW under alkaline conditions (pH = 10) using catechol moiety-rich melanin as a biological template. By cross-linking agent with glucose oxidase, the preparation could achieve self-oxygenation/hyperthermia dual-enhanced hunger therapy guided by MRI/PAI dual-mode imaging.94 MnO2 NW has suitable stability and high catalytic efficiency in a complex physiological environment, which is conducive to the synergistic therapy of self-replicating O2.
With high surface area, porous structure, excellent mechanical properties, and tunable degradability, nanofibers (NFB) are available physical barriers and drug carriers and has great potential in spinal oncology by preventing postoperative tissue adhesion and reducing tumor recurrence.95,96 Qian et al prepared a multifunctional NFB doped with MnO2 by selecting water-in-oil (W/O) emulsion droplets to form ultra-fine DOX@BSA/PCL nanofibers with a core/shell structure, further depositing MnO2 on the outermost layer of the membrane. The Fenton-like reaction between MnO2 and GSH in the outermost layer of NFB promoted the first-order release of Mn2+. In addition, the cascade release of DOX regulated the inflammatory tumor microenvironment, improved tumor elimination efficiency through synergistic chemotherapy/CDT, and inhibited the recurrence of spinal tumors. More interestingly, magnetic resonance and photoacoustic bimodal imaging enables the visualization of tumor treatment and material degradation in the body, enabling rapid pathological analysis and diagnosis.97 The NFB has a multifunctional cascade therapy with significant spinal anti-adhesion properties, which provides a potential material for improving therapeutic efficacy and preventing spinal tumor recurrence. Postoperative tissue adhesion is a common and persistent complication that may trigger peripheral inflammatory responses and even increase the risk of tumor recurrence. Among the existing anti-adhesion strategies, nonwoven pads or fabrics of nanofibers can mimic the structure of the skin and be used to cover and protect wounds and promote wound healing.
Due to their larger accessible surface area with a flat surface than other materials, nanocubes (NC) are favored for a variety of applications in various disease therapy.98 Tapeinos et al formed a hybrid NC with Fe3O4 and MnO2, and then coated U-251 MG cell-derived membranes on its surface to obtain CM-NC. After entering the cell, CM-NC could remove excess H2O2 due to the presence of MnO2, and the exothermic reaction of H2O2 clearance led to the increasing of intracellular temperature and the O2 concentration level, so as to be used in the multifunctional treatment of glioblastoma.99 However, since their anisotropy resulting in instability, how to quickly and efficiently prepare a large number of NC by convenient methods is still a challenge.
Besides, some heterostructures are also used for cancer therapy. A piezoelectric nanoplatform (M-BOC NSs) for enhancing the anticancer effect of SDT was obtained by loading heterostructured manganese oxide (MnOx) on the surface of piezoelectric bismuth oxychloride nanosheets (BiOCl NSs).100 When exposed to ultrasound (US) irradiation, the generation of ROS in SDT was enhanced. This anticancer nanoplatform using piezoelectric platforms provided a feasible way to improve SDT. Based on the above experiments, Yue et al fabricated a piezoelectric sonarsensitizer BTO@M NPs by coating heterostructured MnO2 on BaTiO3 NPs.101 The NPs could catalyze the generation of O2 from overexpressed H2O2 in TME to supplement the gas source in SDT and deplete GSH for TME remodeling, which promoted the development of SDT.
Conclusions and Prospects
MnO2-based nanomaterials possess some general properties including enzyme-like activity (eg catalytic reaction against H2O2), redox activity (eg unique reaction with GSH), high sensitivity to pH, and imaging capability after degradation into Mn2+.102,103 More importantly, its personalized function also depends on its own structural changes such as light and heat conversion.5 Based on these factors, MnO2-based nanomaterials have been widely used in the construction of biosensors, imaging and disease therapy.
Although MnO2-based nanomaterials offer greater opportunities to facilitate the generation of new diagnostic and therapeutic technologies and are constantly being explored and developed, their research in clinically relevant therapeutics is still in the “nascent stage”. There are a number of thorny issues to be resolved: 1) Clinical translation of most inorganic nanomaterials like MnO2 has been low, which mainly attributed to their ambiguous toxicity profiles and unclear biosafety.4 For example, whether multiple repeated injections cause aggregation toxicity or changes in nanomaterial morphology and size further affect toxicity and degradation. In addition, its long-term toxicity has not been adequately evaluated, and it is important to reduce its toxicity and accelerate its biodegradation. Unfortunately, issues regarding the toxicity of MnO2 nanomaterials are frequently overlooked in most studies. Therefore, a “personalized” and detailed biosafety assessment of MnO2 and its nanocomposites should be carried out in future studies. 2) MnO2-based composite nanosystems are often accompanied by multicomponent and complex synthesis steps, and whether a more rigorous and complex assessment of the different substances in the same system interact with each other should be carried out.104 For example, polycationic electrolytes are often used as reducing agents in the preparation of MnO2, which results in severe agglomeration of the prepared MnO2 NPs due to electrostatic interactions.105,106 Decreased catalytic ability due to ultrafine particle size and agglomeration is also a pressing issue. 3) Most oxygen self-producing nanosystems similar to MnO2-based nanomaterials need to face the same challenge, ie sustainability of oxygen production. While MnO2 nanomaterials can mimic enzyme activity, other components of the oxidoreductase family also have irreplaceable functions in many cases.106 Stable and long-lasting oxygen release is beneficial to perform better therapeutic or adjuvant therapeutic roles, however, to date, this critical issue has not been significantly addressed yet. 4) Compared to animals, human diseases are heterogeneous with many mutations, especially on tumors, therefore MnO2-based nanomaterials often face great challenges due to they are occupying the majority of research applications in oncological diseases. Although animal models can provide some valuable preclinical data, it does not mean that they can be well interpreted and applied to human patients. Therefore, animal models that are closer to human diseases should be continuously explored and established for better design and evaluation.
In addition, the preparation methods for MnO2 nanomaterials need to be further explored. The design and preparation of new templates of multidimensionally ordered with excellent performance and suitable for mass production to explore the innovative type of MnO2 nanostructure will be the hotspots of future research. For example, by modifying or partially covering the templates, MnO2 nanomaterials can be grown in specific parts to appear different structures, and even the structure of MnO2 nanomaterials can be corrected or adjusted by etching the specified parts. Moreover, it is necessary to start from modifying the surface of the mesoporous body and increasing the driving force of the precursor itself to completely fill the mesoporous templates.
Therefore, the future research direction of MnO2 nanomaterials should be devoted to solving the obvious problems at present. As enzymes-like or oxygen-producing materials, combining abiotic nanomaterials with the active center structure of biomolecules may have unexpected effects on improving biocatalysis and oxygen-producing efficiency; As the loading material, it is also helpful to control leakage and release of cargo by using responsive surface finishing and adjusting aperture size. As the diagnostic material, multi-element doping may be more useful to improve the diagnostic signal; As the therapeutic material, the use of “green” synthesis and the design of composite materials with the help of body operation rules and biological characteristics in different states can help solve the problem of high toxicity and low efficiency of exogenous materials. Meanwhile, it is also necessary to open up more applications of MnO2 nanomaterials, for example, including but not limited to the use of its antibacterial and immunomodulatory capabilities in the field of regenerative medicine and wound healing.107
It is undeniable that MnO2 nanomaterials are multifunctional and modifiable, bringing a ray of light and becoming an important player in the development of nanomedicine to address a variety of complex diseases. In addition, they are expected to become new clinical disease therapeutic tools by subsequent continuous refinement and clarification of long-term toxicity and metabolism, as well as the exploration and ensuring of reliable clinical-grade fabrication-quality methods.
Author contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Sichuan Science and Technology Program (grant numbers 2023NSFSC0620, 2022YFS0614, 2022YFS0622, 2022YFS0627), the Luzhou Municipal People’s Government-Southwest Medical University Joint Scientific Research Project (grant number 2023LZXNYDHZ003), the Open fund for Key Laboratory of Medical Electrophysiology of Ministry of Education (grant numbers KeyME-2023-07), the Youth Science Foundation Project of Southwest Medical University (grant numbers 2023QN075, 2022QN025), the Southwest Medical University Science and Technology Project (No.2021ZKMS034), the Hejiang County People’s Hospital-Southwest Medical University Joint Scientific Research Project (grant numbers 2023HJXNYD03, 2022HJXNYD03, 2022HJXNYD14), Chinese student innovation and entrepreneurship project (202310632027).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Wu M, Hou P, Dong L, et al. Manganese dioxide nanosheets: from preparation to biomedical applications. Int J Nanomed. 2019;14:4781–4800. doi:10.2147/ijn.S207666
2. Sun K, Li S, Waigi MG, Huang Q. Nano-MnO(2)-mediated transformation of triclosan with humic molecules present: kinetics, products, and pathways. Environ Sci Pollut Res Int. 2018;25(15):14416–14425. doi:10.1007/s11356-018-1637-7
3. Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The history of nanoscience and nanotechnology: From chemical-physical applications to nanomedicine. Molecules. 2019;25(1):112. doi:10.3390/molecules25010112
4. Zhang Z, Ji Y. Nanostructured manganese dioxide for anticancer applications: preparation, diagnosis, and therapy. Nanoscale. 2020;12(35):17982–18003. doi:10.1039/d0nr04067c
5. Chen J, Meng H, Tian Y, et al. Recent advances in functionalized MnO(2) nanosheets for biosensing and biomedicine applications. Nanoscale Horiz. 2019;4(2):321–338. doi:10.1039/c8nh00274f
6. Guan S, Li W, Ma J, et al. A review of the preparation and applications of MnO2 composites in formaldehyde oxidation. J Ind Eng Chem. 2018;66:126–140. doi:10.1016/j.jiec.2018.05.023
7. Zhu D, Zhu XH, Ren SZ, Lu YD, Zhu HL. Manganese dioxide (MnO(2)) based nanomaterials for cancer therapies and theranostics. J Drug Target. 2021;29(9):911–924. doi:10.1080/1061186x.2020.1815209
8. Setyawati MI, Zhao Z, Ng KW. Transformation of nanomaterials and its implications in gut nanotoxicology. Small. 2020;16(36):e2001246. doi:10.1002/smll.202001246
9. Hu X, Sun A, Kang W, Zhou Q. Strategies and knowledge gaps for improving nanomaterial biocompatibility. Environ Int. 2017;102:177–189. doi:10.1016/j.envint.2017.03.001
10. Gong H, Ma X, Meng S, Wang B, Cui Z. Study on the influence of the agglomeration effect of composite nanoparticles on the photothermal properties of nanofluids. Sol Energy. 2024;270:112406. doi:doi:10.1016/j.solener.2024.112406
11. Kyriakides TR, Raj A, Tseng TH, et al. Biocompatibility of nanomaterials and their immunological properties. Biomed Mater. 2021;16(4):042005. doi:10.1088/1748-605X/abe5fa
12. Zhang P, Wang J, Chen H, et al. Tumor microenvironment-responsive ultrasmall nanodrug generators with enhanced tumor delivery and penetration. J Am Chem Soc. 2018;140(44):14980–14989. doi:10.1021/jacs.8b09396
13. Chernyak V, Fowler KJ, Kamaya A, et al. Liver imaging reporting and data system (LI-RADS) Version 2018: Imaging of hepatocellular carcinoma in At-Risk Patients. Radiology. 2018;289(3):816–830. doi:10.1148/radiol.2018181494
14. Zhou T, Ran J, Xu P, et al. A hyaluronic acid/platelet-rich plasma hydrogel containing MnO(2) nanozymes efficiently alleviates osteoarthritis in vivo. Carbohydr Polym. 2022;292:119667. doi:10.1016/j.carbpol.2022.119667
15. Wen L, Hyoju R, Wang P, et al. Hydrogen-Peroxide-Responsive Protein Biomimetic Nanoparticles for Photothermal-Photodynamic Combination Therapy of Melanoma. Laser Surg Med. 2021;53(3):390–399. doi:10.1002/lsm.23292
16. Yu Y, Fan P, Li J, Wang S. Preparation of biocompatible manganese selenium-based nanoparticles with antioxidant and catalytic functions. Molecules. 2023;28(11):4498. doi:10.3390/molecules28114498
17. Zhou Z, Liu Y, Song W, et al. Metabolic reprogramming mediated PD-L1 depression and hypoxia reversion to reactivate tumor therapy. J Control Rel. 2022;352:793–812. doi:10.1016/j.jconrel.2022.11.004
18. Murphy DA, Cheng H, Yang T, Yan X, Adjei IM. Reversing Hypoxia with PLGA-Encapsulated manganese dioxide nanoparticles improves natural killer cell response to tumor spheroids. Mol Pharmaceut. 2021;18(8):2935–2946. doi:10.1021/acs.molpharmaceut.1c00085
19. Lim JW, Son HY, Huh YM, Haam S. Cationic poly(amino acid) surface functionalized manganese nanoparticles for nitric oxide-based immunotherapy and magnetic resonance imaging. J Mater Chem B. 2022;10(28):5402–5409. doi:10.1039/d2tb00794k
20. Zhan M, Wang F, Liu Y, et al. Dual-cascade activatable nanopotentiators reshaping adenosine metabolism for sono-chemodynamic-immunotherapy of deep tumors. Adv Sci. 2023;10(10):e2207200. doi:10.1002/advs.202207200
21. Song T, Liao Y, Zuo Q, Liu N, Liu Z. MnO(2) nanoparticles as a minimalist multimode vaccine adjuvant/delivery system to regulate antigen presenting cells for tumor immunotherapy. J Mater Chem B. 2022;10(18):3474–3490. doi:10.1039/d1tb02650j
22. Hong JY, Lim YG, Song YJ, Park K. Tumor microenvironment-responsive histidine modified-hyaluronic acid-based MnO(2) as in vivo MRI contrast agent. Int J Biol Macromol. 2023;226:121–131. doi:10.1016/j.ijbiomac.2022.12.033
23. Buffa R, Odstrčilová L, Šedová P, Basarabová I, Novotný J, Velebný V. Conjugates of modified hyaluronic acid with amino compounds for biomedical applications. Carbohydr Polym. 2018;189:273–279. doi:10.1016/j.carbpol.2018.02.048
24. Ju Q, Huang R, Hu R, et al. Phytic acid-modified manganese dioxide nanoparticles oligomer for magnetic resonance imaging and targeting therapy of osteosarcoma. Drug Delivery. 2023;30(1):2181743. doi:10.1080/10717544.2023.2181743
25. Luo M, Lv Y, Luo X, et al. Developing smart nanoparticles responsive to the tumor micro-environment for enhanced synergism of thermo-chemotherapy with PA/MR bimodal imaging. Front Bioeng Biotechnol. 2022;10:799610. doi:10.3389/fbioe.2022.799610
26. Jiang S, Li X, Zhang F, et al. Manganese dioxide-based nanocarrier delivers paclitaxel to enhance chemotherapy against orthotopic glioma through hypoxia relief. Small Methods. 2022;6(7):e2101531. doi:10.1002/smtd.202101531
27. Wei P, Chen S, Shi J, Du J. Oxygen-generating polymer vesicles for enhanced sonodynamic tumor therapy. J Control Rel. 2023;353:975–987. doi:10.1016/j.jconrel.2022.12.023
28. Zhong W, Guo F, Chen F, et al. A multifunctional oxidative stress nanoamplifier with ROS amplification and GSH exhaustion for enhanced chemodynamic therapy. Front Pharmacol. 2022;13:1044083. doi:10.3389/fphar.2022.1044083
29. Min JS, Hong JY, Lim YG, Ahn JW, Park K. Oxygen-generating glycol chitosan-manganese dioxide nanoparticles enhance the photodynamic effects of chlorin e6 on activated macrophages in hypoxic conditions. Int J Biol Macromol. 2021;184:20–28. doi:10.1016/j.ijbiomac.2021.06.036
30. Ren H, Yang Q, Yong J, et al. Mitochondria targeted nanoparticles to generate oxygen and responsive-release of carbon monoxide for enhanced photogas therapy of cancer. Biomater Sci. 2021;9(7):2709–2720. doi:10.1039/d0bm02028a
31. Zetrini AE, Lip H, Abbasi AZ, et al. Remodeling tumor immune microenvironment by using polymer-lipid-manganese dioxide nanoparticles with radiation therapy to boost immune response of castration-resistant prostate cancer. Research. 2023;DC(6):0247. doi:10.34133/research.0247
32. Zhang C, Tu W, Chen X, et al. Intelligent design of polymer nanogels for full-process sensitized radiotherapy and dual-mode computed tomography/magnetic resonance imaging of tumors. Theranostics. 2022;12(7):3420–3437. doi:10.7150/thno.70346
33. Du J, Sun J, Liu X, et al. Preparation of C6 cell membrane-coated doxorubicin conjugated manganese dioxide nanoparticles and its targeted therapy application in glioma. Eur J Pharm Sci. 2023;180:106338. doi:10.1016/j.ejps.2022.106338
34. Li C, Zhao Z, Luo Y, et al. Macrophage-disguised manganese dioxide nanoparticles for neuroprotection by reducing oxidative stress and modulating inflammatory microenvironment in acute ischemic stroke. Adv Sci. 2021;8(20):e2101526. doi:10.1002/advs.202101526
35. Xiao T, He M, Xu F, et al. Macrophage membrane-camouflaged responsive polymer nanogels enable magnetic resonance imaging-guided chemotherapy/chemodynamic therapy of orthotopic glioma. ACS nano. 2021;15(12):20377–20390. doi:10.1021/acsnano.1c08689
36. Meng L, Wang C, Lu Y, et al. targeted regulation of blood-brain barrier for enhanced therapeutic efficiency of hypoxia-modifier nanoparticles and immune checkpoint blockade antibodies for glioblastoma. ACS Appl Mater Interfaces. 2021;13(10):11657–11671. doi:10.1021/acsami.1c00347
37. Gao W, Zhang L. Coating nanoparticles with cell membranes for targeted drug delivery. J Drug Target. 2015;23(7–8):619–626. doi:10.3109/1061186x.2015.1052074
38. Gao F, Sun M, Zhang J, et al. Fenton-like reaction and glutathione depletion by chiral manganese dioxide nanoparticles for enhanced chemodynamic therapy and chemotherapy. J Colloid Interface Sci. 2022;616:369–378. doi:10.1016/j.jcis.2022.02.060
39. Greene A, Hashemi J, Kang Y. Development of MnO(2) hollow nanoparticles for potential drug delivery applications. Nanotechnology. 2021;32(2):025713. doi:10.1088/1361-6528/abb626
40. Rumplecker A, Kleitz F, Salabas E-L, Schüth F. Hard templating pathways for the synthesis of nanostructured porous Co3O4. Chem Mater. 2007;19(3):485–496. doi:10.1021/cm0610635
41. Qiu H, Gong H, Bao Y, Jiang H, Tong W. Reactive oxygen species-scavenging hollow MnO(2) nanozymes as carriers to deliver budesonide for synergistic inflammatory bowel disease therapy. Biomater Sci. 2022;10(2):457–466. doi:10.1039/d1bm01525g
42. Liu J, Guo C, Li C, et al. Redox/pH-responsive hollow manganese dioxide nanoparticles for thyroid cancer treatment. Front Chem. 2023;11:1249472. doi:10.3389/fchem.2023.1249472
43. Wu M, Chen T, Wang L, et al. The strategy of precise targeting and in situ oxygenating for enhanced triple-negative breast cancer chemophototherapy. Nanoscale. 2022;14(23):8349–8361. doi:10.1039/d2nr00985d
44. Zhu D, Lyu M, Jiang W, Suo M, Huang Q, Li K. A biomimetic nanozyme/camptothecin hybrid system for synergistically enhanced radiotherapy. J Mater Chem B. 2020;8(24):5312–5319. doi:10.1039/d0tb00676a
45. Xie L, Zhang C, Liu M, et al. Nucleus-targeting manganese dioxide nanoparticles coated with the human umbilical cord mesenchymal stem cell membrane for cancer cell therapy. ACS Appl Mater Interfaces. 2023;15(8):10541–10553. doi:10.1021/acsami.3c01176
46. Xia J, Cheng Y, Zhang H, Li R, Hu Y, Liu B. The role of adhesions between homologous cancer cells in tumor progression and targeted therapy. Expert Rev Anticancer Ther. 2017;17(6):517–526. doi:10.1080/14737140.2017.1322511
47. Fu L, Zhang W, Zhou X, Fu J, He C. Tumor cell membrane-camouflaged responsive nanoparticles enable MRI-guided immuno-chemodynamic therapy of orthotopic osteosarcoma. Bioact Mater. 2022;17:221–233. doi:10.1016/j.bioactmat.2022.01.035
48. Deng X, Song Q, Zhang Y, Liu W, Hu H, Zhang Y. Tumour microenvironment-responsive nanoplatform based on biodegradable liposome-coated hollow MnO(2) for synergistically enhanced chemotherapy and photodynamic therapy. J Drug Target. 2022;30(3):334–347. doi:10.1080/1061186x.2021.1999961
49. Zhang M, Jia C, Zhuang J, et al. GSH-responsive drug delivery system for active therapy and reducing the side effects of bleomycin. ACS Appl Mater Interfaces. 2022;14(1):417–427. doi:10.1021/acsami.1c21828
50. Zhang HL, Wang Y, Tang Q, Ren B, Yang SP, Liu JG. A mesoporous MnO(2)-based nanoplatform with near infrared light-controlled nitric oxide delivery and tumor microenvironment modulation for enhanced antitumor therapy. J Inorg Biochem. 2023;241:112133. doi:10.1016/j.jinorgbio.2023.112133
51. Xu X, Duan J, Liu Y, et al. Multi-stimuli responsive hollow MnO(2)-based drug delivery system for magnetic resonance imaging and combined chemo-chemodynamic cancer therapy. Acta Biomater. 2021;126:445–462. doi:10.1016/j.actbio.2021.03.048
52. Duan J, Liao T, Xu X, Liu Y, Kuang Y, Li C. Metal-polyphenol nanodots loaded hollow MnO(2) nanoparticles with a “dynamic protection” property for enhanced cancer chemodynamic therapy. J Colloid Interface Sci. 2023;634:836–851. doi:10.1016/j.jcis.2022.12.088
53. Hu H, Deng X, Song Q, et al. Mitochondria-targeted accumulation of oxygen-irrelevant free radicals for enhanced synergistic low-temperature photothermal and thermodynamic therapy. J Nanobiotechnol. 2021;19(1):390. doi:10.1186/s12951-021-01142-6
54. Deng M, Wu Y, Ren Y, et al. Clickable and smart drug delivery vehicles accelerate the healing of infected diabetic wounds. J Control Release. 2022;350:613–629. doi:10.1016/j.jconrel.2022.08.053
55. Zhang Y, Lv F, Cheng Y, et al. Pd@Au bimetallic nanoplates decorated mesoporous MnO(2) for Synergistic Nucleus-Targeted NIR-II Photothermal and Hypoxia-Relieved Photodynamic Therapy. Adv Healthcare Mater. 2020;9(2):e1901528. doi:10.1002/adhm.201901528
56. Wei R, Gong X, Lin H, et al. Versatile Octapod-Shaped Hollow Porous Manganese(II) Oxide Nanoplatform for Real-Time Visualization of Cargo Delivery. Nano Lett. 2019;19(8):5394–5402. doi:10.1021/acs.nanolett.9b01900
57. Zhuang H, Zhao M, Ding S, et al. Multifunctional smart yolk-shell nanostructure with mesoporous MnO(2) Shell for enhanced cancer therapy. ACS Appl Mater Interfaces. 2020;12(35):38906–38917. doi:10.1021/acsami.0c08389
58. Li Y, Du L, Li F, Deng Z, Zeng S. Intelligent nanotransducer for deep-tumor hypoxia modulation and enhanced dual-photosensitizer photodynamic therapy. ACS Appl Mater Interfaces. 2022;14(13):14944–14952. doi:10.1021/acsami.1c24172
59. Zhu X, Wang M, Wang H, et al. Multifunctional Hollow MnO(2) @Porphyrin@Bromelain Nanoplatform for Enhanced Photodynamic Therapy. Small. 2022;18(52):e2204951. doi:10.1002/smll.202204951
60. Ou J, Tian H, Wu J, et al. MnO(2)-Based Nanomotors with Active Fenton-like Mn(2+) Delivery for Enhanced Chemodynamic Therapy. ACS Appl Mater Interfaces. 2021;13(32):38050–38060. doi:10.1021/acsami.1c08926
61. Dong CY, Huang QX, Cheng H, et al. Neisseria meningitidis Opca Protein/MnO(2) hybrid nanoparticles for overcoming the blood-brain barrier to treat glioblastoma. Adv Mater. 2022;34(12):e2109213. doi:10.1002/adma.202109213
62. Li Y, Zou Z, An J, et al. Chitosan-modified hollow manganese dioxide nanoparticles loaded with resveratrol for the treatment of spinal cord injury. Drug delivery. 2022;29(1):2498–2512. doi:10.1080/10717544.2022.2104957
63. Jia M, Ren W, Liu Y, et al. Messenger nanozyme for reprogramming the microenvironment of rheumatoid arthritis. ACS Appl Mater Interfaces. 2023;15(1):338–353. doi:10.1021/acsami.2c16458
64. Prasad P, Gordijo CR, Abbasi AZ, et al. Multifunctional albumin-MnO2 nanoparticles modulate solid tumor microenvironment by attenuating hypoxia, acidosis, vascular endothelial growth factor and enhance radiation response. ACS Nano. 2014;8(4):3202–3212. doi:10.1021/nn405773r
65. Abbasi AZ, Prasad P, Cai P, et al. Manganese oxide and docetaxel co-loaded fluorescent polymer nanoparticles for dual modal imaging and chemotherapy of breast cancer. J Control Release. 2015;209:186–196. doi:10.1016/j.jconrel.2015.04.020
66. Chen Y, Meng Q, Wu M, et al. Hollow mesoporous organosilica nanoparticles: a generic intelligent framework-hybridization approach for biomedicine. J Am Chem Soc. 2014;136(46):16326–16334. doi:10.1021/ja508721y
67. Li Y, Shi J. Hollow-structured mesoporous materials: chemical synthesis, functionalization and applications. Adv Mater. 2014;26(20):3176–3205. doi:10.1002/adma.201305319
68. Zhang S, Sunami Y, Hashimoto H Mini Review: Nanosheet Technology towards biomedical application. Nanomaterials (Basel, Switzerland). 2017;7(9)doi:10.3390/nano7090246 246
69. Chen Y, Cong H, Shen Y, Yu B. Biomedical application of manganese dioxide nanomaterials. Nanotechnology. 2020;31(20):202001. doi:10.1088/1361-6528/ab6fe1
70. Savchak OK, Wang N, Ramos-Docampo MA, et al. Manganese dioxide nanosheet-containing reactors as antioxidant support for neuroblastoma cells. J Mater Chem B. 2022;10(24):4672–4683. doi:10.1039/d2tb00393g
71. Li S, Lin K, Hu P, et al. A multifunctional nanoamplifier with self-enhanced acidity and hypoxia relief for combined photothermal/photodynamic/starvation therapy. Int J Pharm. 2022;611:121307. doi:10.1016/j.ijpharm.2021.121307
72. Yin Z, Ji Q, Wu D, et al. H(2)O(2)-Responsive Gold Nanoclusters @ Mesoporous Silica @ Manganese Dioxide Nanozyme for ”Off/On” Modulation and Enhancement of Magnetic Resonance Imaging and Photodynamic Therapy. ACS Appl Mater Interface. 2021;13(13):14928–14937. doi:10.1021/acsami.1c00430
73. Lu LL, Zhang Q, Gu Y, Li XL, Xie JJ. Core-shell ”loading-type” nanomaterials towards: simultaneous imaging analysis of glutathione and microRN. Anal Chim Acta. 2022;1196:339551. doi:10.1016/j.aca.2022.339551
74. Shi M, Wang S, Zheng S, et al. Activatable MRI-monitoring gene delivery for the theranostic of renal carcinoma. Colloids Surf B. 2020;185:110625. doi:10.1016/j.colsurfb.2019.110625
75. Hao Y, Wang L, Zhang B, et al. Multifunctional nanosheets based on folic acid modified manganese oxide for tumor-targeting theranostic application. Nanotechnology. 2016;27(2):025101. doi:10.1088/0957-4484/27/2/025101
76. Fan S, Yang Q, Song Q, et al. Multi-pathway inducing ferroptosis by MnO(2)-based nanodrugs for targeted cancer therapy. Chem Commun. 2022;58(45):6486–6489. doi:10.1039/d2cc02134j
77. Tu C, Lu H, Zhou T, et al. Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials. 2022;286:121597. doi:10.1016/j.biomaterials.2022.121597
78. Fang Z, Zhang M, Kang R, Cui M, Song M, Liu K. A cancer cell membrane coated nanoparticles-based gene delivery system for enhancing cancer therapy. Int J Pharm. 2022;629:122415. doi:10.1016/j.ijpharm.2022.122415
79. Wang L, Guan S, Weng Y, et al. Highly Efficient Vacancy-Driven Photothermal Therapy Mediated by Ultrathin MnO(2) Nanosheets. ACS Appl Mater Interfaces. 2019;11(6):6267–6275. doi:10.1021/acsami.8b20639
80. Zeng D, Wang L, Tian L, Zhao S, Zhang X, Li H. Synergistic photothermal/photodynamic suppression of prostatic carcinoma by targeted biodegradable MnO(2) nanosheets. Drug delivery. 2019;26(1):661–672. doi:10.1080/10717544.2019.1631409
81. Cheng H, Scott K. Carbon-supported manganese oxide nanocatalysts for rechargeable lithium–air batteries. J Power Sources. 2010;195(5):1370–1374. doi:10.1016/j.jpowsour.2009.09.030
82. Yang Y, Gu Z, Tang J, et al. MnO(2) Nanoflowers Induce Immunogenic Cell Death under Nutrient Deprivation: enabling an Orchestrated Cancer Starvation-Immunotherapy. Adv Sci. 2021;8(4):2002667. doi:10.1002/advs.202002667
83. Hachem K, Ansari MJ, Saleh RO, et al. Methods of Chemical Synthesis in the Synthesis of Nanomaterial and Nanoparticles by the Chemical Deposition Method: a Review. BioNanoScience. 2022;12(3):1032–1057. doi:10.1007/s12668-022-00996-w
84. Xiao HF, Yu H, Wang DQ, et al. Dual-Targeted Fe₃O₄@MnO2 Nanoflowers for Magnetic Resonance Imaging-Guided Photothermal-Enhanced Chemodynamic/Chemotherapy for Tumor. J Biomed Nanotechnol. 2022;18(2):352–368. doi:10.1166/jbn.2022.3254
85. Kang H, Chen L, Li Q, Chen H, Zhang L. Dual-Oxygenation/Dual-Fenton Synergistic Photothermal/Chemodynamic/Starvation Therapy for Tumor Treatment. ACS Appl Mater Interfaces. 2023;15(12):15129–15139. doi:10.1021/acsami.2c22578
86. Wang Z, Zhang Y, Ju E, et al. Biomimetic nanoflowers by self-assembly of nanozymes to induce intracellular oxidative damage against hypoxic tumors. Nat Commun. 2018;9(1):3334. doi:10.1038/s41467-018-05798-x
87. Jing X, Xu Y, Liu D, et al. Intelligent nanoflowers: a full tumor microenvironment-responsive multimodal cancer theranostic nanoplatform. Nanoscale. 2019;11(33):15508–15518. doi:10.1039/c9nr04768a
88. Ma D, Chen W, Wang L, Han R, Tang K. O(2) self-sufficient and glutathione-depleted nanoplatform for amplifying phototherapy synergistic thermodynamic therapy. Colloids Surf B. 2023;222:113060. doi:10.1016/j.colsurfb.2022.113060
89. Wang Y, He Y, Lai Q, Fan M. Review of the progress in preparing nano TiO2: an important environmental engineering material. J Environ Sci. 2014;26(11):2139–2177. doi:10.1016/j.jes.2014.09.023
90. Han L, Shao C, Liang B, Liu A. Genetically engineered phage-templated mno2 nanowires: synthesis and their application in electrochemical glucose biosensor operated at neutral pH condition. ACS Appl Mater Interfaces. 2016;8(22):13768–13776. doi:10.1021/acsami.6b03266
91. Wang S, Zhang L, Zhao J, He M, Huang Y, Zhao S. A tumor microenvironment-induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci Adv. 2021;7(12). doi:10.1126/sciadv.abe3588
92. Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M. Role of Glutathione in Cancer: from Mechanisms to Therapies. Biomolecules. 2020;10(10):1429. doi:10.3390/biom10101429
93. Fan Y, Wang X, Huang H, et al. A visual ratiometric fluorescence sensor for glutathione response based on MnO(2) nanowires as an oxidant, quencher and recognition unit. Anal Methods. 2023;15(4):419–429. doi:10.1039/d2ay01812h
94. He T, Xu H, Zhang Y, et al. Glucose oxidase-instructed traceable self-oxygenation/hyperthermia dually enhanced cancer starvation therapy. Theranostics. 2020;10(4):1544–1554. doi:10.7150/thno.40439
95. Qian J, Lin Z, Liu Y, et al. Functionalization strategies of electrospun nanofibrous scaffolds for nerve tissue engineering. Smart Mater Med. 2021;2:260–279. doi:doi:10.1016/j.smaim.2021.07.006
96. Huang A, Ma Y, Peng J, et al. Tailoring the structure of silicon-based materials for lithium-ion batteries via electrospinning technology. eScience. 2021;1(2):141–162. doi:doi:10.1016/j.esci.2021.11.006
97. Qian J, Su L, He J, et al. Dual-modal imaging and synergistic spinal tumor therapy enabled by hierarchical-structured nanofibers with cascade release and postoperative anti-adhesion. ACS nano. 2022;16(10):16880–16897. doi:10.1021/acsnano.2c06848
98. Margulis K, Zhang X, Joubert LM, et al. Formation of polymeric nanocubes by self-assembly and crystallization of dithiolane-containing triblock copolymers. Angew Chem. 2017;56(51):16357–16362. doi:10.1002/anie.201709564
99. Tapeinos C, Tomatis F, Battaglini M, et al. Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ros scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv Healthcare Mater. 2019;8(18):e1900612. doi:10.1002/adhm.201900612
100. Zhao Y, Huang T, Wang S, et al. Manganese oxide-modified bismuth oxychloride piezoelectric nanoplatform with multiple enzyme-like activities for cancer sonodynamic therapy. J Colloid Interface Sci. 2023;640:839–850. doi:10.1016/j.jcis.2023.03.008
101.. Yue Z, Zhao Q, Wang S, et al.. Manganese dioxide coated piezoelectric nanosonosensitizer for cancer therapy with tumor microenvironment remodeling and multienzyme-like catalysis. Small Methods. 2024:e2400018. doi:10.1002/smtd.202400018
102. Zhang W, Li S, Liu X, et al. Oxygen-generating mno2 nanodots-anchored versatile nanoplatform for combined chemo-photodynamic therapy in hypoxic cancer. Adv Funct Mater. 2018;28(13):1706375. doi:doi:10.1002/adfm.201706375
103. Pi F, Deng X, Xue Q, et al. Alleviating the hypoxic tumor microenvironment with MnO(2)-coated CeO(2) nanoplatform for magnetic resonance imaging guided radiotherapy. J Nanobiotechnol. 2023;21(1):90. doi:10.1186/s12951-023-01850-1
104. Wen J, Yang K, Sun S. MnO(2)-Based nanosystems for cancer therapy. Chem Commun. 2020;56(52):7065–7079. doi:10.1039/d0cc02782k
105. Zhao Q, Song A, Ding S, et al. Preintercalation strategy in manganese oxides for electrochemical energy storage: review and prospects. Adv Mater. 2020;32(50):e2002450. doi:10.1002/adma.202002450
106. Liu Q, Zhang A, Wang R, Zhang Q, Cui D. A review on metal- and metal oxide-based nanozymes: properties, mechanisms, and applications. Nano-Micro Lett. 2021;13(1):154. doi:10.1007/s40820-021-00674-8
107. Sisakhtnezhad S, Rahimi M, Mohammadi S. Biomedical applications of MnO(2) nanomaterials as nanozyme-based theranostics. Biomed Pharmacother. 2023;163:114833. doi:10.1016/j.biopha.2023.114833
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

