Back to Journals » Cancer Management and Research » Volume 17
Promotion Mechanisms of Stromal Cell-Mediated Lung Cancer Development Within Tumor Microenvironment
Received 20 November 2024
Accepted for publication 19 January 2025
Published 11 February 2025 Volume 2025:17 Pages 249—266
DOI https://doi.org/10.2147/CMAR.S505549
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Siyu Wu,1,* Yumeng Hu,1,* Bowen Sui2
1Heilongjiang University of Chinese Medicine, Harbin, People’s Republic of China; 2First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bowen Sui, Email [email protected]
Abstract: Lung cancer, with its high incidence and mortality rates, has garnered significant attention in the medical community. The tumor microenvironment (TME), composed of tumor cells, stromal cells, extracellular matrix, surrounding blood vessels, and other signaling molecules, plays a pivotal role in the development of lung cancer. Stromal cells within the TME hold potential as therapeutic targets for lung cancer treatment. However, the precise and comprehensive mechanisms by which stromal cells contribute to lung cancer progression have not been fully elucidated. This review aims to explore the mechanisms through which stromal cells in the tumor microenvironment promote lung cancer development, with a particular focus on how immune cells, tumor-associated fibroblasts, and endothelial cells contribute to immune suppression, inflammation, and angiogenesis. The goal is to provide new insights and potential strategies for the diagnosis and treatment of lung cancer.
Keywords: tumor microenvironment, lung cancer, stromal cells, immune cells, cancer-associated fibroblasts, endothelial cells
Lung cancer is one of the most burdensome malignancies worldwide, marked by its high incidence, mortality rate, and propensity for metastasis. As of 2020, it ranks second only to breast cancer in incidence, with approximately 2.2 million new cases annually, accounting for 11.4% of all cancer diagnoses. It remains the leading cause of cancer-related deaths, representing 18% of global cancer mortality.1 Furthermore, the prognosis for lung cancer is poor, with a five-year survival rate of less than 16%.2 Given these alarming statistics, it is critical to elucidate the pathogenesis of lung cancer and identify effective strategies for its prevention and treatment.
The tumor microenvironment (TME) is the “soil” in which tumor cells thrive, serving as a complex ecosystem that regulates tumor growth, invasion, and metastasis. Immune suppression, inflammation, and angiogenesis are well-established as key pro-cancer factors within the TME.3 The TME is highly structured, consisting primarily of stromal cells, extracellular matrix (ECM), and various cytokines and growth factors. Stromal cells reside within a complex network of ECM macromolecules, which includes, but is not limited to, immune cells (ICs), cancer-associated fibroblasts (CAFs), and endothelial cells (ECs). These components interact to shape tumor progression and modulate response to therapy.4 Stromal cells play a pivotal role in various processes such as cancer cell proliferation and invasion, ECM remodeling, immune evasion, and the initiation of angiogenesis and inflammation, all of which contribute to tumor formation and metastasis.5 By interacting with tumor cells, immune cells, and the ECM, stromal cells foster a supportive microenvironment that enables cancer cells to thrive and spread.6,7 Recent advances in single-cell and spatial transcriptomics have revealed the diverse characteristics of stromal cells in the TME, further highlighting their potential as therapeutic targets in cancer treatment.8 Therefore, understanding the intricate interplay between stromal cells and lung cancer development is paramount.
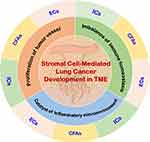
Despite these insights, the precise mechanisms by which stromal cells contribute to lung cancer development remain poorly understood. To address this gap, this review provides a comprehensive classification of stromal cells within the TME. It then explores the relationships between key TME components, including immune suppression, inflammation, and angiogenesis, in driving lung cancer progression. Ultimately, this review aims to enhance our understanding of stromal cell function in lung cancer and offer new perspectives for diagnosis and treatment (Figure 1).
Stromal Cells in the TME
The TME is a complex, highly structured system composed of surrounding blood vessels, stromal cells, extracellular matrix (ECM), and various signaling molecules. Stromal cells, as components of connective tissue, primarily include immune cells, fibroblasts, endothelial cells, and others. These cells play critical roles in biological processes such as immune suppression, inflammation, and angiogenesis, significantly influencing tumor initiation and progression (Figure 2). Below is an overview of the key stromal cell types within the TME.
Immune Cells
Immune cells and their associated factors are fundamental components of the TME.9 These cells act as “warriors” that eliminate cancer cells through immune surveillance and clearance. However, cancer cells can also manipulate immune cells to aid in immune evasion, creating a “fertile soil” that fosters tumor growth. Immune cells in the TME are primarily divided into innate and adaptive immune cells.10
Innate Immune Cells
Innate immune cells form the body’s first line of defense, providing broad and rapid initial protection against pathogens and abnormal cells. These cells include macrophages, neutrophils, natural killer cells, dendritic cells, and eosinophils, among others. Each type of immune cell has specific surface markers and secreted factors that enable them to perform distinct roles in the immune response (see Table 1).
 |
Table 1 Classification, Markers, Secreted Factors, and Functions of Innate Immune Cells |
Adaptive Immune Cells
Adaptive immune cells are highly specific and possess long-term memory. They are activated upon exposure to specific antigens and mount an immune response by “assessing” the threat using immunological memory. These cells mainly consist of T cells and B cells. T cells exhibit different subtypes and are key regulators of both cellular and humoral immunity (see Table 2).
 |
Table 2 Classification, Markers, Secreted Factors, and Functions of T Cells |
B cells are diverse (see Table 3) and can produce high-affinity antibodies, playing an important role in immune regulation and working in collaboration with T cells in the TME.
 |
Table 3 Classification, Markers, Secreted Factors, and Functions of B Cells |
Cancer-Associated Fibroblasts
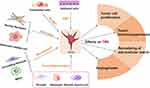
In the TME, cancer-associated fibroblasts are the most abundant stromal cells, constituting approximately 70% of the tumor tissue cells.11 CAFs act as communication hubs, facilitating extensive cross-talk between cells. They promote the formation of a tumor-permissive environment by generating immunosuppression, regulating epithelial-mesenchymal transition (EMT) in tumor cells, continuously remodeling the extracellular matrix (ECM), enhancing cancer stemness, and inducing angiogenesis.12,13
CAFs primarily originate from normal fibroblasts and quiescent stellate cells, which acquire the CAF phenotype upon activation.14 Additionally, epithelial and endothelial cells can contribute to CAFs through phenotypic transitions, while bone marrow-derived mesenchymal stem cells, pericytes, and adipocytes can interact with tumor cells to undergo transdifferentiation and serve as precursors to CAFs15–18 (see Figure 3 for the sources of fibroblasts).
Beyond their diverse origins, CAFs also exhibit significant heterogeneity, which is mainly reflected in differences in cell phenotype, including temporal and spatial characteristics. Temporally, CAF phenotypes are closely related to tumor progression stages, while spatial heterogeneity refers to variations in CAF phenotypes within different regions of the tumor tissue.19 Researchers have identified three distinct subtypes of CAFs in lung cancer:20 1. CAFs characterized by high expression of growth factors HGF and FGF7, which have a strong protective role. 2. CAFs with high expression of FGF7, providing moderate protection. 3. CAFs that produce inflammatory factors and recruit immune cells, but offer relatively low protective capacity.
Endothelial Cells
Endothelial cells are a single layer of flattened epithelial cells located on the inner membrane of blood vessels, forming a semipermeable barrier between the vascular compartment and the surrounding tissues. They serve as metabolic interfaces that control the entry and exit of substances and compound exchanges within the blood circulation. Under normal conditions, endothelial cells are polygonal and tightly connected. However, in the tumor microenvironment, tumor-associated endothelial cells (TECs) exhibit abnormal characteristics. Compared to normal endothelial cells, TECs are irregular in shape and size, proliferate and migrate faster, and may even display disordered endothelial networks and disrupted basal membranes.21,22 TECs, which form the inner layer of the tumor vasculature, have been definitively shown to play a crucial role in cancer development.23 A population structure map of TECs, constructed using single-cell RNA-seq data from 19 types of tumors, reveals their underestimated functional heterogeneity. These features and spatial characteristics form the basis for responses to anti-angiogenesis therapies and immunotherapies, making TECs a promising target for tumor intervention.24 According to single-cell sequencing, multiple subtypes of tumor endothelial cells exist in lung cancer, each playing roles in leukocyte recruitment, ECM remodeling, and vascular integrity.25 This review will further summarize the role of endothelial cells in lung cancer development.
Initiation of Lung Cancer: Imbalance of Immune Homeostasis
The immune system plays a pivotal role in recognizing, eliminating, and controlling tumor cells, thereby maintaining immune homeostasis. However, insufficient immune clearance and diminished immune surveillance can enable tumor cells to evade recognition and destruction, leading to immune evasion. This disruption of immune homeostasis culminates in the establishment of a tumor immune-suppressive microenvironment. Various stromal cells in the tumor microenvironment (TME) contribute to lung cancer initiation by undermining immune homeostasis, as discussed below.
Immune Cells and Immune Homeostasis Imbalance
Dysfunction of immune cells is a major factor in disrupting immune homeostasis, creating an immune-suppressive microenvironment that facilitates lung cancer initiation. Among these cells, macrophages are the most abundant and functionally significant infiltrating immune cells in the TME. Their polarization from the M1 to the M2 phenotype influences the secretion of immune-related cytokines, promoting lung cancer progression.26 Multiple mechanisms regulate this polarization, including enhanced secretion of M-CSF,27 exosome-mediated signaling,28 induction of reactive oxygen species (ROS),29 and modulation of the transcription factor STAT6.30 Recent studies have identified POSTN, a highly mutated gene in lung adenocarcinoma, as a driver of macrophage polarization. By inhibiting lung cancer cell apoptosis and accelerating tumor progression, POSTN opens new avenues for targeted immunotherapy.31 Additionally, Li et al demonstrated that nanomedicine enhances anti-tumor immunity by modulating tumor-associated macrophages, suggesting that macrophage-targeted nanotherapeutics may offer innovative approaches for lung cancer treatment.32
NK cells, critical for the first line of defense against tumor formation, play a key role in improving the TME.33 However, reduced NK cell infiltration and functional impairment—marked by weakened cytotoxicity, decreased reactivity, and compromised survival—are strongly associated with lung cancer initiation.34 This dysfunction is driven by inhibited glucose metabolism and elevated levels of transforming growth factors.34,35 For instance, a decrease in sphingosine-1-phosphate receptor 1 and CX3CR1 expression, alongside an increase in CXCR5 and CXCR6, impairs NK cell migration. Additionally, elevated CTLA-4 and killer cell lectin-like receptor levels further suppress NK cell function within the lung cancer microenvironment.36 Ouyang et al highlighted that the traditional Chinese medicine Sophora alopecuroide-Taraxacum Decoction (STD) inhibits NK cell cytotoxicity and abundance, blocking lung cancer progression. These findings position STD as a promising immunotherapeutic target.37
Myeloid-derived suppressor cells (MDSCs) play a pivotal role in immune suppression within the TME, accelerating lung cancer progression.38 Mechanisms include metabolic regulation that inhibits T cell function, suppression of CD4+ and CD8+ T cell proliferation and activation via the STAT3 pathway, exosomal release of pro-inflammatory factors such as IL-13, and production of chemokines like CCL11.39
Dendritic cells (DCs), specialized antigen-presenting cells, represent approximately 2.1% of immune cells in the TME. However, their maturation and activation are inhibited in lung cancer, resulting in decreased immune surveillance and increased immune escape.40 In lung cancer, the exclusion of functional DCs from tumor lesions, downregulation of effector molecules, and induction of immunosuppressive pathways collectively impair immune clearance, disrupt homeostasis, and exacerbate disease progression.41
Tumor-infiltrating T lymphocytes, including CD4+ T cells (helper and regulatory subsets) and CD8+ T cells (cytotoxic subsets), are key mediators of anti-tumor immunity. During lung cancer progression, a Th1-to-Th2 immune response shift promotes immunosuppression and tumor immune escape.42 The Th1-specific transcription factor TBX21 drives lung cancer development by activating the TBX21-IL-4 signaling cascade.43 Th17 cells further contribute to disease progression by secreting IL-17, which increases extracellular matrix production.44 Zhang et al confirmed through single-cell transcriptomics and Mendelian randomization that regulatory T cells (Tregs) mediate the causal relationship between COPD and lung cancer, highlighting their potential as early intervention targets.45
B lymphocytes, derived from hematopoietic stem cells, play a dual role in immune homeostasis and lung cancer progression. By activating regulatory B cells (Bregs), they negatively regulate immune responses. Bregs secrete inhibitory cytokines like TGF-β, helping cancer cells evade immune surveillance.46 They also recruit CD49+ Tregs and suppress CD8+ T cell activity, promoting tumor growth.47 Furthermore, inhibiting high-density Bregs has been shown to significantly block lung cancer progression.48 Tousif et al identified elevated Bregs in lung cancer patients, where IL-10 secretion fosters an immunosuppressive microenvironment. Their study established Trp metabolites, such as L-Kyn, as therapeutic targets for regulating Bregs in lung cancer.47
CAFs and Immune Homeostasis Imbalance
Cancer-associated fibroblasts (CAFs) are pivotal stromal cells in lung cancer that foster an immunosuppressive microenvironment conducive to tumor growth. CAFs achieve this by secreting cytokines and metabolic products, reducing antigen presentation, modulating immune cell function, and inducing the proliferation of immunosuppressive cells.49
Recent advances in single-cell and spatial transcriptomics have identified a novel CAF subgroup, POSTN CAFs, which are closely associated with SPP1 macrophages and linked to T cell exhaustion and reduced T cell infiltration in non-small cell lung cancer (NSCLC). This subgroup highlights the significant role of CAFs in immune suppression.50 Additionally, Cords et al demonstrated that CAF phenotypes, analyzed using single-cell imaging mass spectrometry and flow cytometry (IMC), are strongly correlated with NSCLC prognosis. High-density myofibroblastic CAFs (mCAFs) were associated with low immune infiltration, driving fibrosis and preventing immune cells from reaching the tumor, thereby contributing to poor outcomes in lung cancer.51
CAFs also modulate immune responses by producing chemokines such as IL-1, IL-6, and IL-8, which inhibit the activation of T and B lymphocytes and promote tumor progression.52 Among these, CAF-derived IL-6, which is highly expressed, activates the JAK2/STAT3 pathway in lung cancer cells, upregulating vimentin while suppressing E-cadherin expression. This facilitates epithelial-mesenchymal transition (EMT), further driving tumor cell growth and proliferation.53
Another immunosuppressive mechanism involves the expression of tryptophan-2,3-dioxygenase (TDO) by CAFs isolated from lung cancer tissues. TDO degrades tryptophan into kynurenine, inhibiting dendritic cell antigen presentation and blocking T cell-mediated antitumor immunity.54
Transforming growth factor-β (TGF-β), a key factor in CAF recruitment and activation, is abundantly secreted by activated CAFs. TGF-β suppresses CD8+ T cell proliferation and cytotoxicity, weakens NK cell function, and polarizes macrophages towards the M2 phenotype, thereby reinforcing immune suppression and promoting lung cancer progression. TGF-β-dependent pathways have been shown to significantly enhance lung cancer growth and metastasis in preclinical models.55
Furthermore, CAFs recruit immune cells through the secretion of CC chemokine ligands (CCL), CXC chemokine ligands (CXCL), and colony-stimulating factors (CSF). For instance, CXCL2 secretion by CAFs upregulates PD-L1 expression in lung cancer cells, leading to immune system inactivation and reduced T cell functionality, which fosters an immunosuppressive TME.56 The CCL2-CCR2 signaling axis also contributes to lung cancer progression by recruiting immunosuppressive cells such as MDSCs and tumor-promoting monocytes, which inhibit CD8+ T cell proliferation and IFN-γ production.57
Recent findings further elucidate the regulatory mechanisms of CAFs. Sun et al reported that PRRX1 promotes CAF activation by recruiting H3K27ac and H3K4me3 to activate OLR1 expression, inducing immune evasion in lung cancer cells and advancing tumor progression.58 Moreover, Sun et al identified POSTN as a highly variable gene in CAFs. POSTN facilitates EMT, enhances macrophage recruitment, promotes M2 polarization, and inhibits lung cancer cell apoptosis, underscoring its critical role in lung cancer progression. These discoveries highlight the intricate interplay between CAFs and lung cancer cells and provide a foundation for advancing lung cancer immunotherapy.31
ECs and Immune Homeostasis Imbalance
Endothelial cells (ECs) are essential components of the tumor microenvironment (TME), forming tight junctions that act as barriers to restrict tumor cell infiltration and extravasation while preventing immune cells from entering tumor tissues. This structural integrity positions ECs as the first line of defense against effector immune cells.22 Interestingly, emerging evidence suggests that ECs share an evolutionary origin with traditional immune cells, earning them the designation of “novel immune cells”.59 Certain EC subtypes exhibit immune-like characteristics, such as recruiting immune cells, modulating immune functions, and presenting antigens in a semi-professional manner.60
Recent studies have further elucidated the immunoregulatory roles of ECs. Halima et al demonstrated that ECs influence macrophage phenotype transformation and the establishment of an immune-suppressive microenvironment through the secretion of CCL2. ECs can also present antigens to cytotoxic T cells via MHC class I and II molecules, highlighting their dual roles in immune modulation and antigen presentation. Single-cell RNA sequencing has identified ECs as potential therapeutic targets for optimizing tumor immunotherapy strategies.61
In the lungs, specialized ECs not only facilitate gas exchange but also participate in immune responses, protecting lung tissue from pathogens and pollutants.21 Mass cytometry analyses reveal that healthy lung ECs express high levels of HLA-DRA, comparable to immune cells, suggesting significant roles in immune regulation.25 Conversely, single-cell RNA sequencing data from non-small cell lung cancer (NSCLC) indicate that ECs suppress immune responses primarily by remodeling processes that reduce antigen presentation and immune activity, as well as diminishing their immune-stimulatory phenotype.62
The immune-regulatory functions of ECs are critical in lung cancer progression. Studies show that untreated lung cancer-associated ECs exhibit reduced expression of chemokines such as CCL2 and CCL18, cytokine IL-6, and HLA-I/HLA-II molecules, contributing to an immunosuppressive environment.63 Furthermore, aberrant ECs express inhibitory receptor molecules, including FasL, PD-L1, and CD73, which play direct roles in immune suppression. Activation of VEGF, PDGF-R, or FGFR pathways results in phosphorylation that suppresses AKT signaling, subsequently upregulating PD-L1 expression in ECs. This cascade reduces CD8+ T cell proliferation and activation while enhancing FoxP3+ Treg cell-mediated immunosuppressive functions. Ultimately, these processes establish an immune-suppressive microenvironment that supports lung cancer growth and progression.64
Inflammation as a Catalyst for Lung Cancer Development
In 1863, Rudolf Virchow first linked inflammation to cancer, initiating the exploration of the “inflammation-cancer” relationship. Chronic, persistent inflammation is now recognized as a major driver of tumorigenesis. With advances in research, inflammation has evolved from a “controllable” factor to an “uncontrollable” participant in tumor microenvironment (TME) formation, ultimately promoting tumor cell proliferation. As the “seventh hallmark” of cancer, inflammation influences every stage of tumor development.65
Inflammatory responses drive lung cancer progression by inducing immunosuppression, evading immune surveillance, causing oxidative stress, and promoting angiogenesis, thereby establishing a TME conducive to tumor growth.66 Below, we systematically explore how various stromal cells contribute to lung cancer progression through inflammation.
Immune Cells and Inflammation
Chronic inflammation exposes tissues to hypertonic, hypoxic, low-glucose, and acidic conditions, disrupting homeostasis and creating an inflammatory microenvironment.67 This environment reshapes the biological behavior of immune cells, accelerating lung cancer initiation and progression.
Neutrophils, which constitute 40–70% of peripheral white blood cells, dominate the inflammatory microenvironment. They play critical roles in infection resistance and homeostasis by balancing pro-apoptotic and anti-apoptotic signals to terminate inflammation.68 Recent findings by Qian et al reveal that IGF2BP2, an m6A modification regulator, is upregulated in lung cancer tissues and stabilizes LOX1 via m6A modification, facilitating neutrophil-mediated inflammatory responses and promoting lung cancer progression.69
In the TME, chronic inflammation induces the formation of neutrophil extracellular traps (NETs). NETs are significantly elevated in the plasma of lung cancer patients, with persistently high levels observed in tumor tissues even post-surgery, suggesting their role in cancer progression.70 NETs promote lung cancer by secreting neutrophil elastase (NE), which induces mitochondrial biogenesis and maintains mitochondrial homeostasis via the TLR4-p38-PGC-1α pathway.71,72 Furthermore, NETs impair the cytotoxic activity of NK cells and drive T cell exhaustion, exacerbating immune suppression in lung cancer.73 NETs also awaken dormant lung cancer cells by capturing circulating tumor cells, remodeling proteins, and activating integrin signaling pathways.74 Dimitrov et al demonstrated that prolonged NET exposure activates the Notch 1 signaling pathway, triggering EMT and enhancing lung cancer cell migration and invasion.75 Complementary research by Lu et al highlighted the anti–inflammatory effects of cryptotanshinone (CPT) and ginsenoside Rg1, which reduce neutrophil infiltration and NET formation in lung tissue, reversing NET-induced tumor-promoting effects.76
M2 macrophages play a role in suppressing immune clearance by inducing T cell dysfunction.77 The transformation of macrophages from the M1 to the M2 phenotype is closely related to inflammation. Inflammatory factors can activate the STAT6 signaling pathway and Toll-like receptor (TLR) signaling pathway, thereby promoting the conversion of M1-type tumor-associated macrophages (TAMs) to M2-type TAMs.78 Wang et al’s study demonstrated that nervonic acid alleviates lung inflammation and prevents lesions by targeting macrophage activation, underscoring the therapeutic potential of macrophage modulation.79
Inflammatory cytokines secreted by immune cells also promote lung cancer progression. For instance, IL-6, produced by activated T cells and macrophages, regulates immune and inflammatory responses and is strongly associated with lung cancer development.80 The SIRPα/IL-6 axis forms a positive feedback loop via STAT3 signaling, fostering an immunosuppressive environment that supports tumor growth.81 Moreover, Th22 cells, which increase with lung cancer severity, secrete IL-22. This cytokine activates the JAK-STAT3/MAPK/AKT pathways, further driving the formation of an immunosuppressive microenvironment and promoting lung cancer progression.82
CAFs and Inflammation
Inflammation is characterized by the persistent presence of leukocytes, unable to either repair or resolve. Studies have shown that fibroblasts contribute to leukocyte recruitment and retention by altering the stromal landscape, leading the body from a state of “homeostasis” to persistent inflammatory invasion. Inflammatory CAFs (iCAFs) represent a specific pro-inflammatory subtype, highlighting the critical role CAFs play in remodeling the inflammatory microenvironment within the lung cancer TME.83
Firstly, cancer-associated fibroblasts (CAFs), as tissue sentinel cells, engage in an interactive dialogue with inflammatory factors. These inflammatory factors are not only secreted by CAFs but can also activate the inflammatory profile of CAFs, creating a malignant feedback loop that promotes the formation of an inflammatory microenvironment.84 According to the study by Koppensteiner et al, IFNγ and TNFα can drive the inflammatory profile in CAFs derived from NSCLC, upregulating the secretion of inflammatory cytokines and chemokines, thereby promoting the development of lung cancer. IL-6, highly expressed in lung cancer and secreted by CAFs, cooperates with leukemia inhibitory factor (LIF) to modulate CAF invasion and promote lung cancer cell proliferation.85
Furthermore, CAFs induce the formation of immunosuppressive cells, which release inflammatory mediators to reshape the lung cancer inflammatory microenvironment. For instance, CAFs secrete IL-8, IL-6, and CCL2 to recruit and polarize pro-tumorigenic type 2 macrophages (TAM2) and pro-inflammatory Th2-type CD4 T cells, creating an inflammatory state that catalyzes lung cancer progression.86 Additionally, activated CAFs undergo metabolic reprogramming through glycolysis and lipid metabolism, which leads to the downregulation of caveolin-1 (Cav-1), promoting the remodeling of the lung cancer stroma. This metabolic shift stimulates the secretion of VEGF-A, CCL2, CXCL, and CXCL8, while lactate accumulation renders the lung cancer stroma acidic, further enhancing the inflammatory microenvironment and driving lung cancer development.87 Nicotinamide N-methyltransferase (NNMT) plays a pro-inflammatory role in cancer, and CAFs are the primary source of NNMT expression in solid tumors. According to a study by Yang et al, in A549 cells, NNMT promotes lung cancer cell proliferation and migration through a pro-inflammatory mechanism mediated by the STAT3/IL1β/PGE2 axis.88 Recently, the negative regulation of NNMT on genes in CAFs has also been confirmed to promote the occurrence and metastasis of lung adenocarcinoma.89
The inflammatory environment forms the basis for the interaction between CAFs and lung cancer, with inflammation signaling pathways exacerbating cancer progression. For example, the COX2 and PGE2-integrin signaling axis plays a key role in promoting lung cancer. Targeting cyclooxygenase-2 (COX2) in lung cancer has been shown to reduce the progression of squamous cell carcinoma, highlighting the therapeutic potential of inhibiting inflammatory pathways.90 The TLR4/MyD88/NF-κB signaling pathway has been widely reported in inflammatory diseases. Sun et al demonstrated through cellular experiments that exosome-derived miR-3124-5p from CAFs inhibits the expression of TOLLIP, thereby activating the TLR4/MyD88/NF-κB axis to promote malignant processes in NSCLC.91
ECs and Inflammation
ECs, located between blood and lung tissue, serve as a physical barrier, control metabolite exchange, and regulate inflammation.22 According to recent reports by Baris et al, the activation of nucleic acid sensors in endothelial cells has been shown to drive tumor-related inflammation. Specifically, activation of RIG-I reduces EC survival, angiogenesis, and triggers an inflammatory response. The researchers identified TYMP as a key mediator of RIG-I-induced EC dysfunction, suggesting that targeting TYMP could improve the inflammation driven by RIG-I activation in endothelial cells.92 Among various cell types, ECs are highly sensitive to hypoxic conditions, a hallmark of the inflammatory-to-cancer transition in the lungs. During this transition, lung tissue experiences sustained hypoxia, which disrupts the tightly connected vascular structures, increasing their permeability. This leads to upregulation of leukocyte adhesion molecules such as ICAM-1 and E-selectin, altering the function of red blood cells and inducing inflammation in lung ECs, ultimately triggering the recruitment and infiltration of inflammatory mediators.93 Studies suggest that hypoxia and inflammation create a mutually reinforcing environment, perpetuating a “hypoxia-inflammation-hypoxia” vicious cycle that accelerates lung cancer progression.94 ECs, as key participants and regulators of inflammatory responses, can inhibit the progression of lung cancer by modulating various signaling pathways.95 Targeting the Wnt/β-catenin signaling pathway may promote endothelial cell self-renewal in inflammation-induced lung injury. R-spondins (RSPOs), a highly conserved family of secreted glycoproteins, can enhance Wnt/β-catenin signaling. Recent studies by Zhang et al have shown that endothelial RSPO3 mediates lung endothelial regeneration after inflammatory vascular injury through β-catenin and ILK signaling pathways in an LGR4-dependent manner.96 ECs also directly secrete inflammatory factors that can accelerate lung cancer onset, such as IL-6 and IL-8, which foster a microenvironment niche that supports non-small cell lung cancer (NSCLC) cell proliferation and viability.97 In addition, IL-33, which is highly expressed in the vascular ECs of lung cancer patients, plays a crucial role in the inflammatory microenvironment. IL-33 can bind to the membrane-bound ST2 molecule (ST2L) on the IL-33R complex, inducing a Th2 immune response and promoting the polarization of M2 macrophages. Moreover, IL-33 acts as a transcriptional regulator that enhances the pro-inflammatory microenvironment in lung cancer.98
Endothelial inflammation can also be triggered by pyroptosis, a form of programmed cell death. Pyroptosis, initiated by the activation of NLRP3 and caspase-1, leads to cell membrane rupture and the release of intracellular contents, inducing endothelial inflammation. Activated caspase-1 releases active pro-inflammatory cytokines such as IL-1β and IL-18, which recruit inflammatory cells and amplify the inflammatory response.99 Pyroptosis-driven endothelial inflammation contributes to lung cancer progression through multiple mechanisms.100
Promoting Lung Cancer Development: Tumor Angiogenesis
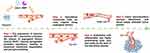
Angiogenesis accompanied by abnormal vasculature is a crucial component of the tumor microenvironment (TME). Angiogenesis is considered a hallmark of cancer formation, playing a key role in tumor proliferation and metastasis.101 Vascular endothelial growth factor (VEGF) and its receptors (VEGFR) play an essential role in inducing angiogenesis (as shown in Figure 4, the process of tumor vessel formation). The “angiogenesis switch” is a rate-limiting step in tumor development, dependent on the balance between pro-angiogenic and anti-angiogenic factors. Modulating this “angiogenesis switch” is a critical factor in promoting tumor angiogenesis, ensuring that the tumor acquires sufficient oxygen and nutrients for continuous growth within the TME.102
Tumor angiogenesis leads to the formation of physical barriers in the TME, where high vascular permeability and increased interstitial pressure induce an immune-suppressive microenvironment. The abnormal tumor vasculature is characterized by loose cell junctions and a low coverage of pericytes, which contributes to a hypoxic and acidic tumor environment.103 Notably, this immune suppression, hypoxia, and acidity in the TME provide a key setting for driving further angiogenesis. Thus, the TME and tumor angiogenesis interact and complement each other, creating a vicious cycle that promotes tumor progression. In the following, we will systematically review the correlation between various stromal cells in the TME and tumor angiogenesis.
Immune Cells and Angiogenesis
Angiogenesis is a critical process in lung cancer progression, and vascular endothelial growth factor (VEGF) is a key angiogenic protein that accelerates tumor development. According to recent reports, VEGF promotes lung cancer progression by inhibiting dendritic cell (DC) antigen presentation and the maturation of natural killer (NK) and T cells, while enhancing the immunosuppressive functions of regulatory T cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). This process fosters the creation of an immune-suppressive microenvironment that facilitates tumor growth and progression.104
As one of the most complex cellular populations within TME, immune cells not only contribute to immune surveillance but also actively promote angiogenesis. Macrophages are particularly important in this context, with Tie2-expressing macrophages (a subset of macrophages) secreting angiopoietin-2 (Ang-2) and VEGF to drive the formation of tumor vasculature.105 Studies have shown that both VEGF and Ang-2 are highly expressed in the serum of NSCLC patients. Together, these molecules synergistically promote endothelial cell differentiation and the formation of new blood vessels, thereby facilitating tumor growth.106,107
In addition to VEGF and Ang-2, macrophages secrete a variety of cytokines and pro-angiogenic factors, including TGF-β, TNF-α, arachidonic acid, IL-1α, VEGF-A, EGF, CXCL8, and bFGF2. These factors, along with regulatory molecules like urokinase plasminogen activator (uPA) and matrix metalloproteinases (MMPs), play a pivotal role in the angiogenic switch. They trigger the degradation of the basal membrane and extracellular matrix (ECM), destabilizing tumor vasculature, stimulating endothelial cell proliferation, and ultimately contributing to the formation of malignant tumor blood vessels.108 Furthermore, IL-37 has been shown to regulate macrophage polarization and enhance angiogenesis in lung cancer patients, further supporting the role of macrophages in shaping the tumor vasculature and driving malignancy.109
Myeloid-derived suppressor cells (MDSCs) also contribute to tumor vascularization by secreting MMP-9 and TGF-β1, which increase the bioavailability of VEGF, thereby enhancing vascular density. Additionally, MDSCs promote new blood vessel formation in tumors through activation of the STAT3 signaling pathway.106 Regulatory T cells (Tregs), critical components of the immunosuppressive landscape, secrete pro-angiogenic factors and cytotoxins that block anti-angiogenic signals from effector cells, regulate vascular permeability, and disrupt the stability and functionality of tumor blood vessels, further supporting angiogenesis and lung cancer progression.110
In a recent study, Yuan et al proposed a novel immunotherapy strategy for lung cancer, utilizing a protein targeting integrin αvβ3 (ProAgio). This approach reduces the number of CD4+ Tregs and MDSCs, while increasing the ratio of CD8+ T cells to M1/M2 macrophages, effectively depleting tumor angiogenesis. This study provides a promising therapeutic option for lung cancer patients with dense tumor masses and elevated angiogenesis.111
CAFs and Angiogenesis
Tumor angiogenesis, a hallmark of vascular-dependent diseases, is a critical factor in the development and progression of lung cancer. CAFs are the most abundant stromal cell component within TME and play a pivotal role in producing key growth factors that promote both tumor angiogenesis and proliferation. One of the first identified vascular growth factors, fibroblast growth factors (FGFs), is highly expressed in the nuclei of lung cancer cells.112 Recent findings by Lei et al have shown that overexpression of Glioma-associated oncogene 1 (Gli1) in lung cancer can promote angiogenesis. In this context, basic fibroblast growth factor (bFGF) has been identified as a critical regulator of Gli1-mediated angiogenic activity. Gli1 actively modulates bFGF protein levels by enhancing its transcriptional activity and reducing its protein degradation. This suggests that bFGF-based therapies could potentially reverse Gli1-mediated angiogenesis in lung cancer, offering a promising strategy to target tumor vasculature.113 Binding of FGF to its receptor (FGFR) induces phosphorylation, activating key signaling pathways such as PI3K, MAPK, and JNK, which mediate the growth and angiogenesis of NSCLC. Notably, FGFR fusion proteins have been shown to significantly inhibit angiogenesis.114
Moreover, CAF-derived vascular endothelial growth factor (VEGF) plays a crucial role in promoting angiogenesis and is essential for the progression of lung adenocarcinoma.112,115 According to Fu et al, CAFs secrete stromal cell-derived factor 1 (SDF-1/CXCL12), which recruits endothelial progenitor cells (EPCs) to the TME, remodeling the extracellular matrix (ECM). Additionally, CAFs directly chemotax blood endothelial cells (ECs) and secrete matrix metalloproteinases (MMPs) to degrade the ECM, facilitating the formation of new blood vessels and significantly contributing to tumor angiogenesis and proliferation.116 Research also indicates that long non-coding RNAs (lncRNAs) promote tumor formation through their vascular-active properties. For instance, lncRNA ZEB1-AS1, which is specifically overexpressed in CAFs, enhances lung adenocarcinoma occurrence and invasion by promoting angiogenesis via the miR-505-3p/VEGFA axis. This promotes the growth of new blood vessel branches and their elongation.117,118 Furthermore, exosomal microRNA-20a derived from CAFs plays a crucial regulatory role in immune response, angiogenesis, and cancer progression. The upregulation of miR-20a in NSCLC patients has been linked to enhanced angiogenic activity. Recent studies have shown that CAF-derived exosomal miR-20a inhibits the PTEN/PI3K-AKT pathway, promoting the progression of NSCLC.119
Periostin (POSTN), a non-structural ECM protein synthesized by both cancer cells and CAFs, is one of the key factors significantly influencing the process of angiogenesis. Research indicates that the high expression of POSTN promotes NSCLC progression via angiogenesis.120 Single-cell RNA sequencing of lung cancer has revealed elevated expression of FHL2 in fibroblast subtypes. In surgically resected lung adenocarcinoma specimens, FHL2-positive expression correlated significantly with microvascular density. Further investigation showed that FHL2 expressed by CAFs promotes lung adenocarcinoma progression by enhancing angiogenesis and metastasis.121
ECs and Angiogenesis
In normal physiological conditions, ECs are organized in a structured manner, tightly connected with a high coverage of pericytes and an intact basement membrane, which helps maintain vascular homeostasis by minimizing leakage and sprouting.122 However, when ECs are activated by local environmental stimuli, metabolic reprogramming occurs, driving tumor angiogenesis. Tumor vascularization is a critical step in tumor development, with tumor angiogenesis being one of the primary forms of this process.123
The metabolic reprogramming of ECs involves alterations in the biosynthesis and metabolism of glucose, fatty acids, and amino acids.124–126 Upregulation of VEGF signaling enhances glycolysis in ECs, leading to excessive lactate accumulation. This lactate is then utilized by ECs, activating hypoxia-inducible factor 1-alpha (HIF-1α), which promotes sprouting and the formation of pathological blood vessels.127
As a hallmark of tumor progression, angiogenesis plays a pivotal role in lung cancer development, and increasing evidence suggests that ECs significantly contribute to tumor progression by driving angiogenesis. Recent research identified microRNA-186-5p (miR-186) expression in ECs within non-small cell lung cancer (NSCLC) tissues. Through both in vitro and in vivo studies, researchers demonstrated that downregulation of miR-186 in ECs led to upregulation of protein kinase Cα, which mediates angiogenesis in NSCLC.128
Moreover, in NSCLC patients, neutrophil extracellular traps (NETs) can damage endothelial cells, converting them into a pro-coagulant phenotype. In collaboration with platelets, this conversion promotes a hypercoagulable state that disrupts vascular function and accelerates lung cancer progression.129 Further studies by Santio et al uncovered the central role of reactive capillary endothelial cells (rCap) in lung cancer progression and metastasis. rCaps are enriched in angiogenic and inflammatory pathways, with PIM3, activated by the JAK-STAT pathway, acting as a key regulator. Inhibition of PIM3 increases vascular leakage and metastatic colonization while compromising endothelial cell barriers, suggesting PIM3 as a potential therapeutic target for preventing lung cancer metastasis.130
VEGF specifically binds to its receptors (VEGFR1, VEGFR2, VEGFR3) on ECs, leading to the downregulation of microRNA-1 (miR-1) in lung ECs, which promotes angiogenesis and contributes to lung cancer progression.131 In NSCLC, Wnt proteins bind to their receptor Frizzled, activating the Wnt/β-catenin signaling pathway, which regulates endothelial cell proliferation and further drives angiogenesis.132 Additionally, extracellular vesicles (EVs) transfer YAP from lung adenocarcinoma H1975 cells to ECs, modulating their role in vascular formation.64
Summary and Outlook
Stromal cells infiltrating TME—including ICs, CAFs, and ECs—are active drivers of lung cancer initiation and progression. Immune suppression, inflammation, and angiogenesis are the primary factors within the TME that facilitate the advancement of lung cancer. This review integrates these concepts and delves into the roles and specific mechanisms by which various stromal cells contribute to lung cancer pathogenesis. It highlights how stromal cells initiate lung cancer by recruiting immune-suppressive cells, creating an immunosuppressive microenvironment, and catalyzing tumorigenesis through inflammatory responses, thereby fostering the development of an inflammatory milieu. Furthermore, by promoting tumor angiogenesis, stromal cells enhance tumor progression, thus serving as key contributors to lung cancer development. This review advances our understanding of how stromal cells influence the TME and provides insights into lung cancer pathogenesis, which is crucial for both diagnosis and treatment strategies.
Currently, remodeling the TME to restore homeostasis remains a major focus in medical research. While significant progress has been made in lung cancer therapies, including immune-modulatory therapies, anti-angiogenic treatments, and targeted approaches, challenges persist, including limited therapeutic options, suboptimal efficacy, and poor prognosis. This review underscores the interconnected roles of various stromal cells as critical components of the TME that promote lung cancer progression.
Looking ahead, the development of therapies targeting more specific stromal cell pathways holds promise. For example, immune-modulatory drugs could be designed not only to target immune cells but also CAFs, ECs, and the complex interplay between angiogenesis and immune suppression. Future research into the role of stromal cells, coupled with the development of combination treatment strategies to restore TME homeostasis and inhibit lung cancer initiation, presents an exciting avenue for advancing lung cancer therapies.
Acknowledgment
The work is supported by the The Fifth Batch of National Traditional Chinese Medicine Excellent Clinical Talents Training Project (Announcement from the Personnel and Education Department of the National Administration of Traditional Chinese Medicine. No. 2022-1).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li N, Tan FW, Chen WQ, et al. One-off low-dose CT for lung cancer screening in China: a multicentre, population-based, prospective cohort study. Lancet Respir Med. 2022;10(4):378–391. doi:10.1016/S2213-2600(21)00560-9
2. Wu FY, Wang L, Zhou CC. Lung cancer in China: current and prospect. Curr Opin Oncol. 2021;33(1):40–46. doi:10.1097/CCO.0000000000000703
3. Zhang CY, Tang BX, Hu JP, et al. Neutrophils correlate with hypoxia microenvironment and promote progression of non-small-cell lung cancer. Bioengineered. 2021;12(1):8872–8884. doi:10.1080/21655979.2021.1987820
4. de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41(3):374–403. doi:10.1016/j.ccell.2023.02.016
5. Saha S, Pradhan N, Neha B, Mahadevappa R, Minocha S, Kumar S. Cancer plasticity: investigating the causes for this agility. Semi Cancer Biol. 2023;88:138–156.
6. Lin W, Noel P, Borazanci EH, et al. Single-cell transcriptome analysis of tumor and stromal compartments of pancreatic ductal adenocarcinoma primary tumors and metastatic lesions. Genome Med. 2020;12(1). doi:10.1186/s13073-020-00776-9
7. Li D, Yu HS, Hu JJ, et al. Comparative profiling of single-cell transcriptome reveals heterogeneity of tumor microenvironment between solid and acinar lung adenocarcinoma. J Transl Med. 2022;20(1):423.
8. Du Y, Shi J, Wang J, et al. Integration of pan-cancer single-cell and spatial transcriptomics reveals stromal cell features and therapeutic targets in tumor microenvironment. Cancer Res. 2024;84(2):192–210. doi:10.1158/0008-5472.CAN-23-1418
9. Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–R5. doi:10.1016/j.cub.2020.06.081
10. Fu T, Dai L-J, Wu S-Y, et al. Spatial architecture of the immune microenvironment orchestrates tumor immunity and therapeutic response. J hematol oncol. 2021;14(1):98. doi:10.1186/s13045-021-01103-4
11. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2018;18(2):99–115. doi:10.1038/s41573-018-0004-1
12. Hu DD, Li ZQ, Zheng B, et al. Cancer-associated fibroblasts in breast cancer: challenges and opportunities. Cancer Commun. 2022;42(5):401–434. doi:10.1002/cac2.12291
13. Delinassios JGG, Hoffman RMM. The cancer-inhibitory effects of proliferating tumor-residing fibroblasts. Biochim Biophys Acta. 2022;1877(1):188673. doi:10.1016/j.bbcan.2021.188673
14. Friedman G, Levi-Galibov O, David E, et al. Cancer-associated fibroblast compositions change with breast cancer progression linking the ratio of S100A4 and PDPN CAFs to clinical outcome. Nat Cancer. 2020;1(7):692. doi:10.1038/s43018-020-0082-y
15. Saijo A, Goto H, Nakano M, et al. Bone marrow-derived fibrocytes promote stem cell-like properties of lung cancer cells. Cancer Lett. 2018;421:17–27. doi:10.1016/j.canlet.2018.02.016
16. Krzysiek-Maczka G, Targosz A, Szczyrk U, Strzalka M, Brzozowski T, Ptak-Belowska A. Involvement of epithelial-mesenchymal transition-inducing transcription factors in the mechanism of -induced fibroblasts activation. J Physiol Pharmacol. 2019;70(5). doi:10.26402/jpp.2019.5.08
17. Ning XF, Zhang HR, Wang C, Song XQ. Exosomes released by gastric cancer cells induce transition of pericytes into cancer-associated fibroblasts. Med Sci Monit. 2018;24:2350–2359. doi:10.12659/MSM.906641
18. Bochet L, Lehuédé C, Dauvillier S, et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–5668. doi:10.1158/0008-5472.CAN-13-0530
19. De Wever O, Van Bockstal M, Mareel M, Hendrix A, Bracke M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semi Cancer Biol. 2014;25:33–46. doi:10.1016/j.semcancer.2013.12.009
20. Hu HC, Piotrowska Z, Hare PJ, et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell. 2021;39(11):1531. doi:10.1016/j.ccell.2021.09.003
21. Mammoto A, Mammoto T. Vascular niche in lung alveolar development, homeostasis, and regeneration. Front Bioeng Biotechnol. 2019;7. doi:10.3389/fbioe.2019.00318
22. Augustin HG, Koh GY. A systems view of the vascular endothelium in health and disease. Cell. 2024;187(18):4833–4858. doi:10.1016/j.cell.2024.07.012
23. Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18(8):527–540. doi:10.1038/s41571-021-00496-y
24. Li J, Wang D, Tang F, Ling X, Zhang W, Zhang Z. Pan-cancer integrative analyses dissect the remodeling of endothelial cells in human cancers. Natl Sci Rev. 2024;11(9):nwae231. doi:10.1093/nsr/nwae231
25. Goveia J, Rohlenova K, Taverna F, et al. An integrated gene expression landscape profiling approach to identify lung tumor endothelial cell heterogeneity and angiogenic candidates. Cancer Cell. 2020;37(1):21–36.e13. doi:10.1016/j.ccell.2019.12.001
26. Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22(13):6995. doi:10.3390/ijms22136995
27. Hamidzadeh K, Belew AT, El-Sayed NM, Mosser DM. The transition of M-CSF-derived human macrophages to a growth-promoting phenotype. Blood Adv 2020;4(21):5460–5472. doi:10.1182/bloodadvances.2020002683
28. Shen HQ, Cao DW, Zhang XR. Advances in exosome research in the management of lung cancer. Curr Top Med Chem. 2023;23(10):921–930. doi:10.2174/1568026623666230504101208
29. Zhou SY, Lan Y, Li YQ, Li ZX, Pu JD, Wei LP. Hypoxic tumor-derived exosomes induce M2 macrophage polarization via PKM2/AMPK to promote lung cancer progression. Cell Transplant. 2022;31: 09636897221106998.
30. Shi JH, Liu LN, Song DD, et al. TRAF3/STAT6 axis regulates macrophage polarization and tumor progression. Cell Death Differ. 2023;30(8):2005–2016. doi:10.1038/s41418-023-01194-1
31. Sun D, Lu J, Tian H, et al. The impact of POSTN on tumor cell behavior and the tumor microenvironment in lung adenocarcinoma. Int Immunopharmacol. 2025;145:113713. doi:10.1016/j.intimp.2024.113713
32. Li R, Huang J, Wei Y, et al. Nanotherapeutics for macrophage network modulation in tumor microenvironments: targets and tools. Int J Nanomed. 2024;19:13615–13651. doi:10.2147/IJN.S491573
33. Tripathy DK, Panda LP, Biswal S, Barhwal K. Insights into the glioblastoma tumor microenvironment: current and emerging therapeutic approaches. Front Pharmacol. 2024;15: 1355242.
34. Russick J, Joubert PE, Gillard-Bocquet M, et al. Natural killer cells in the human lung tumor microenvironment display immune inhibitory functions. J Immuno Ther Cancer. 2020;8(2):e001054. doi:10.1136/jitc-2020-001054
35. Schuijs MJ, Png S, Richard AC, et al. ILC2-driven innate immune checkpoint mechanism antagonizes NK cell antimetastatic function in the lung. Nat Immunol. 2020;21(9):998. doi:10.1038/s41590-020-0745-y
36. Dokhanchi M, Javaherdehi AP, Raad M, et al. Natural killer cells in cancers of respiratory system and their applications in therapeutic approaches. Immun Inflamm Dis. 2024;12(11):e70079. doi:10.1002/iid3.70079
37. Xiaohu O, Wang J, Qiu X, et al. Sophora alopecuroide - Taraxacum decoction (STD) inhibits non-small cell lung cancer via inducing ferroptosis and modulating tumor immune microenvironment. Heliyon. 2024;10(20):e39564. doi:10.1016/j.heliyon.2024.e39564
38. Wu YZ, Yi M, Niu MK, Mei Q, Wu KM. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022;21(1). doi:10.1186/s12943-022-01657-y
39. De Cicco P, Ercolano G, Ianaro A, Qiu L, Zhu L. The new era of cancer immunotherapy: targeting myeloid-derived suppressor cells to overcome immune evasion. Front Immunol. 2020;11:11. doi:10.3389/fimmu.2020.00011
40. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi:10.1158/0008-5472.CAN-18-3962
41. Matsuo K, Yoshie O, Kitahata K, Kamei M, Hara Y, Nakayama T. Recent progress in dendritic cell-based cancer immunotherapy. Cancers. 2021;13(10):2495. doi:10.3390/cancers13102495
42. Liu C, Wu SK, Meng XY, et al. Predictive value of peripheral regulatory T cells in non-small cell lung cancer patients undergoing radiotherapy. Oncotarget. 2017;8(26):43427–43438. doi:10.18632/oncotarget.15238
43. Zhao ST, Shen WZ, Yu JY, Wang LH. TBX21 predicts prognosis of patients and drives cancer stem cell maintenance via the TBX21-IL-4 pathway in lung adenocarcinoma. Stem Cell Res Ther. 2018;9(1). doi:10.1186/s13287-018-0820-6
44. Salazar Y, Zheng X, Brunn D, et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J Clin Investig. 2020;130(7):3560–3575. doi:10.1172/JCI124037
45. Zhang D, Liu H, Zhao F, et al. Exploring the relationship between treg-mediated risk in COPD and lung cancer through Mendelian randomization analysis and scRNA-seq data integration. BMC Cancer. 2024;24(1):453. doi:10.1186/s12885-024-12076-1
46. Murakami Y, Saito H, Shimizu S, et al. Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep. 2019;9(1). doi:10.1038/s41598-019-49581-4
47. Tousif S, Wang Y, Jackson J, et al. Indoleamine 2, 3-dioxygenase promotes aryl hydrocarbon receptor-dependent differentiation of regulatory B cells in lung cancer. Front Immunol. 2021;12:747780.
48. Bruno TC, Ebner PJ, Moore BL, et al. Antigen-presenting intratumoral B cells affect CD4 TIL phenotypes in non-small cell lung cancer patients. Cancer Immunol Res. 2017;5(10):898–907. doi:10.1158/2326-6066.CIR-17-0075
49. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi:10.1038/nrc.2016.73
50. Chen C, Guo Q, Liu Y, et al. Single-cell and spatial transcriptomics reveal POSTN(+) cancer-associated fibroblasts correlated with immune suppression and tumour progression in non-small cell lung cancer. Clin Transl Med. 2023;13(12):e1515. doi:10.1002/ctm2.1515
51. Cords L, Engler S, Haberecker M, et al. Cancer-associated fibroblast phenotypes are associated with patient outcome in non-small cell lung cancer. Cancer Cell. 2024;42(3):396–412.e5. doi:10.1016/j.ccell.2023.12.021
52. Tran E, Chinnasamy D, Yu ZY, et al. Immune targeting of fibroblast activation protein triggers recognition of multipotent bone marrow stromal cells and cachexia. J Exp Med. 2013;210(6):1125–1135. doi:10.1084/jem.20130110
53. Wang LM, Cao LM, Wang HM, et al. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget. 2017;8(44):76116–76128. doi:10.18632/oncotarget.18814
54. Hsu YL, Hung JY, Chiang SY, et al. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget. 2016;7(19):27584–27598. doi:10.18632/oncotarget.8488
55. Derynck R, Turley SJ, Akhurst RJ. TGFβ biology in cancer progression and immunotherapy. Nat Rev Clin Oncol. 2021;18(1):9–34. doi:10.1038/s41571-020-0403-1
56. Teramoto K, Igarashi T, Kataoka Y, et al. Clinical significance of PD-L1-positive cancer-associated fibroblasts in pN0M0 non-small cell lung cancer. Lung Cancer. 2019;137:56–63. doi:10.1016/j.lungcan.2019.09.013
57. Vennin C, Mélénec P, Rouet R, et al. CAF hierarchy driven by pancreatic cancer cell p53-status creates a pro-metastatic and chemoresistant environment via perlecan. Nat Commun. 2019;10(1). doi:10.1038/s41467-019-10968-6
58. Sun Y, Ying K, Sun J, et al. PRRX1-OLR1 axis supports CAFs-mediated lung cancer progression and immune suppression. Cancer Cell Int. 2024;24(1):247. doi:10.1186/s12935-024-03436-9
59. Amersfoort J, Eelen G, Carmeliet P. Immunomodulation by endothelial cells — partnering up with the immune system? Nat Rev Immunol. 2022;22(9):576–588. doi:10.1038/s41577-022-00694-4
60. Georganaki M, van Hooren L, Dimberg A. Vascular targeting to increase the efficiency of immune checkpoint blockade in cancer. Front Immunol. 2018;9. doi:10.3389/fimmu.2018.03081
61. Alnaqbi H, Becker LM, Mousa M, et al. Immunomodulation by endothelial cells: prospects for cancer therapy. Trends Cancer. 2024;10(11):1072–1091. doi:10.1016/j.trecan.2024.08.002
62. Zhang J, Song C, Tian Y, Yang X. Single-cell RNA sequencing in lung cancer: revealing phenotype shaping of stromal cells in the microenvironment. Front Immunol. 2021;12:802080. doi:10.3389/fimmu.2021.802080
63. Lambrechts D, Wauters E, Boeckx B, et al. Phenotype molding of stromal cells in the lung tumor microenvironment. Nature Med. 2018;24(8):1277. doi:10.1038/s41591-018-0096-5
64. Wang Y, Dong LY, Zhong H, et al. Extracellular vesicles (EVs) from lung adenocarcinoma cells promote human umbilical vein endothelial cell (HUVEC) angiogenesis through yes kinase-associated protein (YAP) transport. Int J Bio Sci. 2019;15(10):2110–2118. doi:10.7150/ijbs.31605
65. Briukhovetska D, Doerr J, Endres S, Libby P, Dinarello CA, Kobold S. Interleukins in cancer: from biology to therapy. Nat Rev Cancer. 2021;21(8):481–499. doi:10.1038/s41568-021-00363-z
66. Feng Q, Zhang Y, Fang Y, et al. Research progress of exosomes as drug carriers in cancer and inflammation. J Drug Target. 2023;31(4):335–353. doi:10.1080/1061186X.2022.2162059
67. Solta A, Ernhofer B, Boettiger K, et al. Small cells - big issues: biological implications and preclinical advancements in small cell lung cancer. Mol Cancer. 2024;23(1). doi:10.1186/s12943-024-01953-9
68. Klopf J, Brostjan C, Eilenberg W, Neumayer C. Neutrophil extracellular traps and their implications in cardiovascular and inflammatory disease. Int J Mol Sci. 2021;22(2):559. doi:10.3390/ijms22020559
69. Qian L, Ji Z, Mei L, Zhao J. IGF2BP2 promotes lung adenocarcinoma progression by regulating LOX1 and tumor-associated neutrophils. Immunol Res. 2024;73(1):16. doi:10.1007/s12026-024-09563-9
70. Masucci MT, Minopoli M, Del Vecchio S, Carriero MV, Zhu L. The emerging role of neutrophil extracellular traps (NETs) in tumor progression and metastasis. Front Immunol. 2020;11:11.
71. Wang YZ, Wang YC, Liu W, et al. TIM-4 orchestrates mitochondrial homeostasis to promote lung cancer progression ANXA2 /PI3K/AKT/OPA1 axis. Cell Death Dis. 2023;14(2):141.
72. Li J, Chen J, Sun J, Li K, Manu KA. The formation of NETs and their mechanism of promoting tumor metastasis. J Oncol. 2023;2023:1–8.
73. Yang SF, Zou XM, Li JC, et al. Immunoregulation and clinical significance of neutrophils/NETs-ANGPT2 in tumor microenvironment of gastric cancer. Front Immunol. 2022;13:1010434.
74. Bordon Y. NETs awaken sleeping cancer cells. Nat Rev Immunol. 2018;18(11):665. doi:10.1038/s41577-018-0081-8
75. Dimitrov J, Maddalena M, Terlizzi C, et al. Dynamic roles of neutrophil extracellular traps in cancer cell adhesion and activation of Notch 1-mediated epithelial-to-mesenchymal transition in EGFR-driven lung cancer cells. Front Immunol. 2024;15:1470620. doi:10.3389/fimmu.2024.1470620
76. Lu K, Xia Y, Cheng P, et al. Synergistic potentiation of the anti-metastatic effect of a Ginseng-Salvia miltiorrhiza herbal pair and its biological ingredients via the suppression of CD62E-dependent neutrophil infiltration and NETformation. J Adv Res. 2024. doi:10.1016/j.jare.2024.10.036
77. Zou GY, Zhang XT, Wang L, et al. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics. 2020;10(15):6839–6853. doi:10.7150/thno.43622
78. Yi H, Zhang Y, Yang X, et al. Hepatitis B core antigen impairs the polarization while promoting the production of inflammatory cytokines of M2 macrophages via the TLR2 pathway. Front Immunol. 2020;11:11.
79. Wang C, Wu Y, Liu C, et al. Nervonic acid alleviates radiation-induced early phase lung inflammation by targeting macrophages activation in mice. Front Immunol. 2024;15:1405020. doi:10.3389/fimmu.2024.1405020
80. Hung C-H, Wu S-Y, Yao C-ID, et al. Defective N-glycosylation of IL6 induces metastasis and tyrosine kinase inhibitor resistance in lung cancer. Nat Commun. 2024;15(1). doi:10.1038/s41467-024-51831-7
81. Wang B, Pan LY, Chen MJ, et al. SIRP-alpha-IL-6 axis induces immunosuppressive macrophages in non-small-cell lung cancer. Biochem Biophys Res Commun. 2023;682:386–396. doi:10.1016/j.bbrc.2023.10.035
82. Yao YA, Yang GD, Lu GH, et al. Th22 cells/IL-22 serves as a protumor regulator to drive poor prognosis through the JAK-STAT3/MAPK/AKT signaling pathway in non-small-cell lung cancer. J Immunol Res. 2022;2022:1–12.
83. Davidson S, Coles M, Thomas T, et al. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat Rev Immunol. 2021;21(11):704–717. doi:10.1038/s41577-021-00540-z
84. Biffi G, Oni TE, Spielman B, et al. IL1-induced JAK/STAT signaling is antagonized by TGFβ to shape CAF heterogeneity in pancreatic ductal adenocarcinoma. Cancer Discovery. 2019;9(2):282–301. doi:10.1158/2159-8290.CD-18-0710
85. Healthcare Engineering Jo. Retracted: impact of HSP90α, CEA, NSE, SCC, and CYFRA21‐1 on lung cancer patients. J Healthc Eng. 2023;2023(1):6929971.
86. Toledo B, Picon-Ruiz M, Marchal JA, Perán M. Dual role of fibroblasts educated by tumour in cancer behavior and therapeutic perspectives. Int J Mol Sci. 2022;23(24). doi:10.3390/ijms232415576
87. Varveri A, Papadopoulou M, Papadovasilakis Z, et al. Immunological synapse formation between T regulatory cells and cancer-associated fibroblasts promotes tumour development. Nat Commun. 2024;15(1). doi:10.1038/s41467-024-49282-1
88. Yang C, Wang T, Zhu S, et al. Nicotinamide N-methyltransferase remodeled cell metabolism and aggravated proinflammatory responses by activating STAT3/IL1β/PGE(2) pathway. ACS Omega. 2022;7(42):37509–37519. doi:10.1021/acsomega.2c04286
89. Wang P, Wang G, Li H, et al. Nicotinamide N-methyltransferase negatively regulates metastasis-promoting property of cancer-associated fibroblasts in lung adenocarcinoma. Cancer Commun. 2024. doi:10.1002/cac2.12633
90. Zhang FS, Ma YS, Li DQ, et al. Cancer associated fibroblasts and metabolic reprogramming: unraveling the intricate crosstalk in tumor evolution. J hematol oncol. 2024;17(1):80.
91. Sun T, Song Q, Liu H. CAF-derived exosome-miR-3124-5p promotes malignant biological processes in NSCLC via the TOLLIP/TLR4-MyD88-NF-κB pathway. Oncol Res. 2025;33(1):133–148. doi:10.32604/or.2024.054141
92. Baris A, Fraile-Bethencourt E, Eubanks J, Khou S, Anand S. Thymidine phosphorylase facilitates retinoic acid inducible gene-I induced endothelial dysfunction. Cell Death Dis. 2023;14(4):294. doi:10.1038/s41419-023-05821-0
93. Jin QY, Su H, Yang R, et al. C1q/TNF-related protein-9 ameliorates hypoxia-induced pulmonary hypertension by regulating secretion of endothelin-1 and nitric oxide mediated by AMPK in rats. Sci Rep. 2021;11(1). doi:10.1038/s41598-021-90779-2
94. McGettrick AF, O’Neill LAJ. The Role of HIF in Immunity and Inflammation. Cell Metab. 2020;32(4):524–536.
95. Guo W, Zhou BL, Bie FL, et al. Single-cell RNA sequencing analysis reveals transcriptional heterogeneity of multiple primary lung cancer. Clin Trans Med. 2023;13(10). doi:10.1002/ctm2.1453
96. Zhang H, Liu D, Xu QF, et al. Endothelial RSPO3 mediates pulmonary endothelial regeneration by LGR4-dependent activation of β-catenin and ILK signaling pathways after inflammatory vascular injury. Int J Biol Macromol. 2024;269(Pt 2):131805. doi:10.1016/j.ijbiomac.2024.131805
97. Paluskievicz CM, Cao XF, Abdi R, Zheng P, Liu Y, Bromberg JS. T regulatory cells and priming the suppressive tumor microenvironment. Front Immunol. 2019;10:10. doi:10.3389/fimmu.2019.00010
98. Casciaro M, Gangemi S, Caramori G, Nucera F, Tuccari G, Ieni A. IL-33 immunohistochemical pattern of expression in neoplastic and nonneoplastic peripheral lung tissues of stage 1 o 2 lung adenocarcinoma. Pathol Res Pract. 2024;255:155208.
99. Miao R, Jiang C, Chang WY, et al. Gasdermin D permeabilization of mitochondrial inner and outer membranes accelerates and enhances pyroptosis. Immunity. 2023;56(11):2523–41.e8. doi:10.1016/j.immuni.2023.10.004
100. Gao WT, Wang XY, Zhou Y, Wang XQ, Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct Target Ther. 2022;7(1):196.
101. Jiang X, Wang J, Deng X, et al. The role of microenvironment in tumor angiogenesis. J Exp Clin Cancer Res. 2020;39(1):204. doi:10.1186/s13046-020-01709-5
102. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248–1264. doi:10.1016/j.cell.2019.01.021
103. Wang B, Zhao Q, Zhang YY, et al. Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy. J Exp Clin Cancer Res. 2021;40(1):1–6.
104. Genova C, Dellepiane C, Carrega P, et al. Therapeutic implications of tumor microenvironment in lung cancer: focus on immune checkpoint blockade. Front Immunol. 2021;12:799455. doi:10.3389/fimmu.2021.799455
105. Thapa K, Khan H, Kaur G, Kumar P, Singh TG. Therapeutic targeting of angiopoietins in tumor angiogenesis and cancer development. Biochem Biophys Res Commun. 2023;687:149130.
106. Jirjees S, Htun ZM, Aldawudi I, Katwal PC, Khan S. Role of morphological and hemodynamic factors in predicting intracranial aneurysm rupture: a review. Cureus J Med Sci. 2020;12(7):1.
107. Zhang J, Can A, Lai PMR, et al. Vascular geometry associated with anterior communicating artery aneurysm formation. World Neurosurg. 2021;146:E1318–E25. doi:10.1016/j.wneu.2020.11.160
108. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77(9):1745–1770. doi:10.1007/s00018-019-03351-7
109. Duthie MS, Reed SG. Skin tests for the detection of infections: achievements, current perspectives, and implications for other diseases. Appl Microbiol Biotechnol. 2021;105(2):503–508. doi:10.1007/s00253-020-11062-4
110. Gao XD, Sui HS, Zhao S, Gao XM, Su YP, Qu P. Immunotherapy targeting myeloid-derived suppressor cells (MDSCs) in tumor microenvironment. Front Immunol. 2021;11:585214.
111. Yuan Y, Mishra F, Li B, et al. Modulating tumor immunity by targeting tumor fibrotic stroma and angiogenic vessels for lung cancer treatment. Cancers. 2024;16(13):2483. doi:10.3390/cancers16132483
112. Cuzziol CI, Castanhole-Nunes MMU, Pavarino ÉC, Goloni-Bertollo EM. MicroRNAs as regulators of and in cancer. Gene. 2020;759:144944.
113. Lei X, Li Z, Huang M, et al. Gli1-mediated tumor cell-derived bFGF promotes tumor angiogenesis and pericyte coverage in non-small cell lung cancer. J Exp Clin Cancer Res. 2024;43(1):83. doi:10.1186/s13046-024-03003-0
114. Zheng LL, Liu H, Chen LF, et al. Expression and purification of FGFR1-Fc fusion protein and its effects on human lung squamous carcinoma. Appl Biochem Biotechnol. 2024;196(1):573–587. doi:10.1007/s12010-023-04542-6
115. Schmittnaegel M, Rigamonti N, Kadioglu E, et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci, trans med. 2017;9(385). doi:10.1126/scitranslmed.aak9670
116. Fu HT, Deng C, Teng LP, Cai Z, Chen JH, Lu GZ. Effect of heparan sulfate mimetics from K5 polysaccharide on SDF-1/CXCL12-induced endothelial progenitor cells in vitro. Int J Biol Macromol. 2018;107:2492–2500. doi:10.1016/j.ijbiomac.2017.10.132
117. Zhou Q, Wu X, Wang X, et al. The reciprocal interaction between tumor cells and activated fibroblasts mediated by TNF-α/IL-33/ST2L signaling promotes gastric cancer metastasis. Oncogene. 2019;39(7):1414–1428. doi:10.1038/s41388-019-1078-x
118. Al-Jomah N, Al-Mohanna FH, Aboussekhra A. Tocilizumab suppresses the pro-carcinogenic effects of breast cancer-associated fibroblasts through inhibition of the STAT3/AUF1 pathway. Carcinogenesis. 2021;42(12):1439–1448. doi:10.1093/carcin/bgab102
119. Shi L, Zhu W, Huang Y, et al. Cancer-associated fibroblast-derived exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to promote the progression and chemoresistance of non-small cell lung cancer. Clin Transl Med. 2022;12(7):e989. doi:10.1002/ctm2.989
120. Wasik A, Podhorska-Okolow M, Dziegiel P, et al. Correlation between periostin expression and pro-angiogenic factors in non-small-cell lung carcinoma. Cells. 2024;13(17):1406. doi:10.3390/cells13171406
121. Kanzaki R, Reid S, Bolivar P, et al. FHL2 expression by cancer-associated fibroblasts promotes metastasis and angiogenesis in lung adenocarcinoma. Int, J, Cancer. 2025;156(2):431–446. doi:10.1002/ijc.35174
122. Jin KT, Yao JY, Fang XL, Di H, Ma YY. Roles of lncRNAs in cancer: focusing on angiogenesis. Life Sci. 2020;252:117647.
123. Kim JY, Kim YM. Tumor endothelial cells as a potential target of metronomic chemotherapy. Arch Pharmacal Res. 2019;42(1):1–13. doi:10.1007/s12272-018-01102-z
124. Du W, Ren L, Hamblin MH, Fan YB. Endothelial cell glucose metabolism and angiogenesis. Biomedicines. 2021;9(2):147. doi:10.3390/biomedicines9020147
125. Ye Y, Sun XT, Lu YT, Fimia GM, Ciccosanti F. Obesity-related fatty acid and cholesterol metabolism in cancer-associated host cells. Front Cell Develop Biol. 2020;8:8. doi:10.3389/fcell.2020.00008
126. Reina-Campos M, Diaz-Meco MT, Moscat J. The complexity of the serine glycine one-carbon pathway in cancer. J Cell Biol. 2020;219(1). doi:10.1083/jcb.201907022
127. Saleh A, ElFayoumi HM, Youns M, Barakat W. Rutin and orlistat produce antitumor effects via antioxidant and apoptotic actions. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018;392(2):165–175. doi:10.1007/s00210-018-1579-0
128. Becker V, Yuan X, Boewe AS, et al. Hypoxia-induced downregulation of microRNA-186-5p in endothelial cells promotes non-small cell lung cancer angiogenesis by upregulating protein kinase C alpha. Mol Ther Nucleic Acids. 2023;31:421–436. doi:10.1016/j.omtn.2023.01.015
129. Tong D, Gao Y, Sun W, et al. Neutrophil extracellular traps, platelets and endothelial cells cooperatively contribute to hypercoagulability in non-small cell lung cancer. Thromb Haemost. 2024. doi:10.1055/a-2493-2499
130. Santio NM, Ganesh K, Kaipainen PP, et al. Endothelial Pim3 kinase protects the vascular barrier during lung metastasis. Nat Commun. 2024;15(1):10514. doi:10.1038/s41467-024-54445-1
131. Tang PY, Sun DJ, Xu W, Li H, Chen LX. Long non-coding RNAs as potential therapeutic targets in non-small cell lung cancer (Review). Int J Mol Med. 2023;52(2). doi:10.3892/ijmm.2023.5271
132. Bats ML, Peghaire C, Delobel V, Dufourcq P, Couffinhal T, Duplàa C. Wnt/frizzled signaling in endothelium: a major player in blood-retinal- and blood-brain-barrier integrity. Cold Spring Harb Perspect Med. 2022;12(4). doi:10.1101/cshperspect.a041219
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.