Back to Journals » Journal of Inflammation Research » Volume 18
Psoriasis: Unraveling Disease Mechanisms and Advancing Pharmacological and Nanotechnological Treatments
Authors Miao M, Yan J, Sun Y, Liu J, Guo S
Received 12 November 2024
Accepted for publication 20 January 2025
Published 10 February 2025 Volume 2025:18 Pages 2045—2072
DOI https://doi.org/10.2147/JIR.S506103
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Miao Miao,1 Jiong Yan,2 Yujin Sun,3 Jia Liu,2 Shun Guo2
1Outpatient Department, Disease Prevention and Control Center of Tongshan District, Xuzhou, People’s Republic of China; 2Dermatology, Affiliated Hospital of Nanjing University of Chinese Medicine, Nanjing, People’s Republic of China; 3Dermatology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, People’s Republic of China
Correspondence: Jia Liu; Shun Guo, Affiliated Hospital of Nanjing University of Chinese Medicine, 155 Hanzhong Road, Qinhuai District, Nanjing, Jiangsu Province, 210029, People’s Republic of China, Email [email protected]; [email protected]
Abstract: Research into the pathogenesis of inflammatory skin diseases, including dermatitis and psoriasis, has yielded significant advancements in the last decades. The identification of age, gender, and genetic factors contributing to these complex conditions has been pivotal in developing novel pharmacological and technological treatments. This review delves into the molecular underpinnings of psoriasis, examining current therapies and promising investigational agents. We highlight the potential of nanotechnology to enhance drug delivery to affected skin areas, with microneedles emerging as a promising platform for psoriasis and other chronic inflammatory skin diseases.
Keywords: biodegradable nanoparticles, clinical trials, skin inflammatory diseases, psoriasis, microneedles
Introduction
Psoriasis is a chronic inflammatory skin disease primarily influenced by genetics and aging, though environmental factors like trauma (Koebner phenomenon), infections, and certain medications can also contribute. With a global prevalence of approximately 2%, psoriasis exhibits variations across populations, with higher rates observed in Caucasians and Scandinavians (up to 11%).1,2 The condition manifests in several forms, with plaque psoriasis being the most common (90%), characterized by itchy, scaly patches.3 Both innate and adaptive immune responses underlie psoriatic inflammation, although the innate response is more prominent in plaque psoriasis.4
One proposed mechanism for psoriasis involves the overexpression of antimicrobial peptides, such as LL-37, β-defensins, and S100 proteins, which trigger and sustain inflammation.5,6 Initially, a decline in LL-37 and other antimicrobial peptides occurs in keratinocytes following stress, like physical injury. Released LL-37 forms complexes with cellular DNA, stimulating toll-like receptor 9 on plasmacytoid dendritic cells, contributing to psoriasis pathogenesis.7 LL-37 induces type I interferon production, promoting dendritic cell maturation and driving Th1 and Th17 differentiation.6–8 Th17 cells, characterized by IL-17, IL-22, IL-21, TNF-α production and RORC expression, play a crucial role in psoriasis.9,10 Elevated RORC mRNA in psoriatic patients supports the involvement of Th17 cells in disease pathogenesis.11 RORγt, a key Th17 transcription factor, is encoded by the Rorg gene along with its isoform ROR. While sharing the same C-terminal region, they differ in their N-terminals.12 Beyond Th17 cells, RORγt influences cytokine production in CD8+Tc17 cells, invariant natural killer T cells, ILC3s, and γδ T cells, contributing to autoimmune inflammation.13 RORγ-deficient mice exhibit reduced Th17/IL-17 responses and protection from psoriasis-like inflammation.14 Targeting RORγt with small molecule inhibitors offers a potential therapeutic strategy to suppress the proinflammatory IL-17/IL-23 axis. As a nuclear hormone receptor, RORγt’s activity is ligand-dependent, making its ligand binding domain an attractive target for drug development. Several inhibitors have shown efficacy in preclinical models.15–19 Figure 1 illustrates the core hypothesis underlying plaque-type psoriasis pathogenesis.
 |
Figure 1 Plaque-type psoriasis pathogenesis principal hypothesis. |
The IL-17 receptor complex, upon interacting with ACT1, activates several intracellular kinases including ERK, p38 MAPK, TAK1, IKK, and GSK-3β. These kinases subsequently stimulate the production of proinflammatory cytokines, chemokines, and antimicrobial peptides. While Th1 and Th2 cytokine signaling is mediated by JAK-STAT pathways, Th17 responses involve ACT1 and NF-κB.20,21 Notably, γδ T cells can produce IL-17A independently of IL-23 stimulation.22 Pustular psoriasis, characterized by coalescing sterile pustules, differs markedly from plaque psoriasis. While adaptive immunity is central to plaque psoriasis pathogenesis, and therapies targeting these mechanisms are effective, pustular psoriasis primarily involves the innate immune system and responds less favorably to plaque psoriasis treatments.8,23–25 Although sharing some metabolic pathways, these psoriasis types exhibit distinct pathogenic mechanisms. Pustular psoriasis predominantly relies on keratinocytes, neutrophils, and monocytes.26 A mutation in the IL36RN gene leads to increased IL-1β, IL-36α, and IL-36γ expression, driving neutrophil accumulation in the epidermis.26–28 The elevated levels of neutrophil chemokines CXCL1, CXCL2, and CXCL8 (IL-8) support this pathogenic model.26
Generalized pustular psoriasis is an acute, rapidly progressing condition characterized by widespread redness and pus-filled blisters, often accompanied by systemic symptoms.27–29 In contrast, guttate psoriasis primarily affects children and adolescents, manifesting as small, red scaly patches. While its pathophysiology likely mirrors that of plaque psoriasis, a potential link to streptococcal superantigens has been suggested, involving molecular mimicry in HLA-Cw6 positive individuals.18,30–32 Inverse psoriasis, affecting skin folds, presents with erosive rather than scaly lesions. Although its underlying mechanisms remain similar to plaque psoriasis, a decreased number of CD161+ cells in affected areas, potentially due to microbial colonization, has been observed.33,34 Erythrodermic psoriasis, the most severe form, involves widespread inflammation and skin redness, without apparent deviations from the typical psoriasis pathophysiology. This review explores the molecular foundations of psoriasis, evaluating current treatments and promising new investigational therapies. We emphasize the role of nanotechnology in improving drug delivery to affected skin areas, highlighting microneedles as an emerging and promising platform for treating psoriasis and other chronic inflammatory skin conditions.
Clinical Presentation
Chronic Plaque
Psoriasis vulgaris, the most common form of chronic plaque psoriasis, is characterized by well-defined, salmon-pink plaques with silvery scales on white skin (Figure 2A) or gray plaques on black skin (Figure 2B), the condition often exhibits the Auspitz sign: pinpoint bleeding upon scale removal. Plaques vary widely in size and thickness, with smaller plaques indicating active disease and thinner plaques often responding better to phototherapy.35,36 Individual plaques expand outward, creating a central clearing with an annular appearance. Commonly affecting the knees, elbows, lumbosacral region, and scalp, psoriasis can involve any skin surface. Symmetrical plaque distribution is characteristic (Figure 2C). In severe cases, trauma or pressure can induce new lesions (Koebner phenomenon).37,38
Other Subtypes of Psoriasis
Guttate psoriasis, comprising 2% of cases (Figure 2D), is characterized by numerous, small and scaly papules. Often preceded by pharyngitis or tonsillitis, with elevated antistreptolysin O, anti-DNase B, or streptozyme titers in approximately half of patients, guttate psoriasis typically resolves spontaneously within weeks or months in children and young adults.39,40 However, approximately 40% progress to chronic plaque psoriasis. The role of antibiotics in management is unclear, while tonsillectomy may be beneficial for recurrent cases associated with tonsillitis. Factors determining disease resolution or persistence remain unknown.41,42
Erythroderma, a severe psoriasis condition characterized by widespread erythema and scaling, can lead to a range of complications including hypothermia, high-output cardiac failure, electrolyte imbalances, and intense itching and pain(Figure 2E).43,44 Pustular psoriasis, a group of rare psoriasis variants with distinct morphologies, includes generalized pustular psoriasis (von Zumbusch disease), palmoplantar pustulosis, and acrodermatitis continua of Hallopeau. Generalized pustular psoriasis (Figure 2F) is a potentially life-threatening autoimmune condition characterized by recurrent flares, sterile pustules, and fever.45,46 Unlike chronic plaque psoriasis, it affects women more frequently and can be triggered by corticosteroid withdrawal, hypocalcemia, pregnancy, or infection. Palmoplantar pustulosis (Figure 2G) primarily affects middle-aged female smokers, manifesting as sterile yellow pustules on palms and soles that resolve to red or brown macules.47,48 Approximately 20% of cases coexist with chronic plaque psoriasis. Acrodermatitis continua of Hallopeau, a rare condition, involves pustules on fingers and toes, sometimes leading to nail loss. Approximately 40% of individuals with this condition also have chronic plaque psoriasis.49,50
Psoriasis can also manifest in location-specific variants, such as inverse (flexural), palmoplantar, sebopsoriasis, and nail psoriasis, in addition to the more common guttate, erythrodermic, and pustular forms.51,52 Inverse psoriasis affects skin folds, presenting as shiny, red, non-scaling lesions (Figure 2H), often mimicking candidiasis or fungal infections.53,54 Sebopsoriasis affects the scalp, face, and upper chest, with its relationship to seborrheic dermatitis and dandruff remaining debated. Nail psoriasis, affecting approximately 50% of plaque psoriasis patients, presents with diverse manifestations, including pitting, onycholysis (Figure 2I), oil spots, and nail dystrophy.55,56 Onycholysis specifically doubles the risk of psoriatic arthritis and correlates with longer disease duration compared to psoriasis without nail involvement (Figure 2J).
Pathophysiology
Histological Features
Psoriatic histology demonstrates epidermal thickening (acanthosis) with elongated rete ridges, a reduced or absent granular layer, dilated capillaries, and suprapapillary thinning.57,58 The dermis and epidermis exhibit a T cell-predominant inflammatory infiltrate, often accompanied by neutrophil clusters within parakeratotic scales (Munro microabscesses). In pustular psoriasis, neutrophil clusters within pustules (Kogoj spongiform micropustules) serve as a diagnostic hallmark.59,60
Pathogenesis
Recent immunological and genetic research has illuminated critical immune pathways involved in psoriasis pathogenesis, particularly the roles of IL-17, IL-23, and TNFα. Psoriasis is characterized by a dysregulated immune response, where the interplay between the innate and adaptive immune systems results in chronic inflammation and epidermal hyperplasia.61 Central to this process is the interaction between keratinocytes and immune cells, such as T helper (Th) 17 cells.
The IL-23/Th17 axis has emerged as a key pathogenic pathway. IL-23, produced by antigen-presenting cells like dendritic cells, activates Th17 cells that subsequently secrete IL-17. This cytokine not only promotes keratinocyte proliferation and activation but also amplifies the inflammatory response by inducing the release of other pro-inflammatory cytokines, such as IL-6 and TNFα. The feedforward loop created by IL-17 induces a vicious cycle of continuous inflammation, which leads to the hallmark symptoms of psoriasis, including thickened, scaly plaques.62 TNFα, a potent pro-inflammatory cytokine, plays a critical role in the pathogenesis of psoriasis by enhancing the activation of both innate and adaptive immune responses. TNFα contributes to the development and maintenance of the disease through its effects on keratinocytes, endothelial cells, and immune cells. It further facilitates the dysregulation of the immune system, exacerbating inflammation and leading to the development of psoriasis lesions. Clinical evidence has shown that TNFα inhibitors significantly reduce disease activity, underscoring its pivotal role in the disease process.63 The genetic component of psoriasis also plays a crucial role, with several susceptibility loci identified through genome-wide association studies (GWAS). These loci, which include genes related to immune function such as HLA-Cw6, IL-12B, and IL-23R, provide valuable insights into the genetic underpinnings of psoriasis. Furthermore, genetic variants can influence the expression and activity of cytokines such as IL-17 and IL-23, contributing to individual differences in disease susceptibility and treatment response.64
To better understand and monitor these key immune markers in psoriasis, various molecular detection methods have been developed to track their expression and activity. For instance, Quantitative PCR (qPCR) assay allows the detection and quantification of mRNA expression of IL-17, IL-23, and TNFα in skin biopsies or peripheral blood, providing insight into the inflammatory status of the disease. Elevated levels of IL-17 and IL-23 have been consistently observed in the lesional skin of psoriasis patients, and these markers can be correlated with disease severity. By using specific antibodies against IL-17, IL-23, and TNFα, Immunohistochemistry (IHC) can visualize the spatial distribution and expression of these cytokines in psoriasis tissue samples.63 This method allows researchers and clinicians to observe the localization of inflammatory cells in the skin and helps assess the degree of immune cell infiltration in psoriatic lesions. Flow cytometry can be used to analyze T-cell populations, specifically Th17 cells, in peripheral blood or skin tissue. By staining for surface markers such as CD4 and intracellular cytokines like IL-17, researchers can quantify Th17 cell subsets and assess their activation status.64 This technique is also used in clinical trials to monitor the effect of targeted therapies that aim to modulate the IL-17/IL-23 axis. Enzyme-Linked Immunosorbent Assay (ELISA) is commonly employed to measure serum levels of TNFα, IL-23, and IL-17 in psoriasis patients, providing valuable biomarkers for assessing inflammation and monitoring therapeutic response. Elevated levels of these cytokines in serum have been associated with active disease, and their reduction can serve as a marker of therapeutic efficacy, particularly with biologic treatments.65
In conclusion, understanding the role of immune markers like TNFα, IL-23, and IL-17 in psoriasis pathogenesis is essential for developing targeted therapies. By utilizing molecular techniques such as qPCR, IHC, flow cytometry, and ELISA, clinicians and researchers can gain deeper insights into the disease mechanisms and improve the precision of diagnostics and treatment strategies. These advances will not only help elucidate the complexities of psoriasis but also enable more effective and personalized treatment approaches.
Genetic Contributions
Psoriasis has a strong genetic component, with monozygotic twins having a two- to threefold increased risk compared to dizygotic twins.65,66 Genome-wide association studies (GWAS) have identified over 80 genetic loci explaining approximately 30% of the disease’s heritability. While most variants have modest effects, they collectively illuminate the disease’s immunological underpinnings.67,68 HLA-C06:02 is a key genetic risk factor for early-onset psoriasis, increasing risk four- to fivefold and interacting with ERAP1 to further elevate susceptibility. Although not yet therapeutically actionable, HLA-C06:02 may inform clinical and therapeutic stratification.69,70 GWAS findings converge on innate and adaptive immune pathways, including type I interferons, NF-κB, IL-23, IL-17, and antigen presentation. These insights, coupled with immunological research, advance our understanding of psoriasis pathogenesis and inform therapeutic strategies.71
Triggers
Psoriasis expression is a result of complex gene-environment interactions, where environmental factors play a crucial role in triggering or exacerbating the disease in genetically predisposed individuals. While stress, infections (especially streptococcal), alcohol, smoking, and certain medications are well-established triggers, other factors, such as hormonal changes, allergies, irritants, and frequently consumed drugs, also contribute to the development or worsening of psoriasis. Each of these environmental triggers can influence immune system dysregulation, inflammatory pathways, or skin barrier function, which are central to psoriasis pathogenesis.72
Hormonal Changes
Hormonal fluctuations, particularly during puberty, pregnancy, and menopause, can significantly influence the onset and severity of psoriasis. Estrogen and progesterone levels, for instance, impact immune regulation and can alter the balance between pro-inflammatory and anti-inflammatory cytokines. During pregnancy, elevated estrogen levels can sometimes improve psoriasis symptoms, while in some cases, the disease may worsen postpartum due to rapid hormonal changes.73
For example, during pregnancy, elevated estrogen levels can sometimes improve psoriasis symptoms, while in some cases, the disease may worsen postpartum due to rapid hormonal changes. Besides, Menopause, with its decrease in estrogen, has been associated with a flare-up or onset of psoriasis in women who were previously asymptomatic. Androgens, which may rise in certain hormonal conditions (like polycystic ovary syndrome or hirsutism), may also influence psoriasis severity by stimulating keratinocyte proliferation and immune cell activation.74 Hormonal changes may modulate the immune response, particularly through the regulation of Th1 and Th17 pathways, both of which play crucial roles in psoriasis pathogenesis. Estrogen, for example, can downregulate the Th17 response, while a reduction in estrogen during menopause can enhance this pathway, triggering an inflammatory cascade in genetically susceptible individuals.75
Allergies and Allergic Responses
Psoriasis can also be influenced by allergic reactions or heightened allergic sensitization, particularly to environmental allergens like pollen, dust mites, fungi, or pet dander. Additionally, food allergies or sensitivities (eg, to dairy or gluten) have been implicated in some patients with psoriasis, potentially exacerbating inflammation. For instance, Contact dermatitis from allergens like nickel or certain fragrances can provoke localized flares of psoriasis in sensitive individuals.76 Type I hypersensitivity reactions (IgE-mediated) to airborne allergens might contribute to exacerbation of the disease through a systemic inflammatory response, particularly when the skin barrier is already compromised. Allergic reactions can trigger the release of histamine, prostaglandins, and cytokines such as IL-4, IL-5, and IL-13. These immune mediators can further activate T-helper cells, including Th2 cells, and promote the production of IL-17 and TNFα, both of which are key players in psoriasis pathogenesis.77
Irritants and Skin Trauma
Environmental irritants such as harsh soaps, chemicals, detergents, or abrasive fabrics can disrupt the skin barrier and trigger or worsen psoriasis lesions. Moreover, physical trauma (including cuts, scratches, sunburn, and even surgical procedures) can induce Koebner phenomenon, where new psoriatic lesions develop at sites of skin injury.78 Skin damage can activate keratinocytes and mast cells, leading to the release of pro-inflammatory cytokines like IL-1 and TNFα, which stimulate T-cell activation and exacerbate the inflammatory process. Additionally, the Koebner phenomenon is thought to involve the activation of the innate immune system, with dendritic cells presenting self-antigens to T-cells, thus triggering an autoimmune response.79
Commonly Consumed Drugs
Certain medications are known to be psoriasis triggers, either by directly inducing or exacerbating the disease. Commonly used for hypertension, beta-blockers can exacerbate psoriasis by increasing the activity of TNFα and IL-6, which promote inflammation. Besides, used to treat bipolar disorder, lithium can trigger psoriasis or worsen existing symptoms by disrupting immune cell signaling and increasing the expression of IL-2 and TNFα.80 In addition, Nonsteroidal anti-inflammatory drugs (NSAIDs), while effective for pain relief, may exacerbate psoriasis flares in some individuals by increasing the production of pro-inflammatory cytokines. Certain antibiotics, particularly penicillin and sulfonamides, can induce psoriatic flares, possibly due to their effect on the skin microbiome or immune system modulation. Medications like beta-blockers and lithium can influence immune cell function, especially by promoting the activity of T-helper (Th) 1/Th17 responses, which are central to psoriasis. These drugs may also alter the skin microbiome, increase systemic inflammation, or directly affect skin cell turnover, all contributing to disease exacerbation.81
Other Environmental Triggers
Infections, particularly streptococcal throat infections, can trigger guttate psoriasis in susceptible individuals. The immune system responds to the infection by activating the Th17 pathway, which can trigger or exacerbate psoriasis symptoms. Alcohol consumption, especially in excess, is known to provoke psoriasis flares, possibly by affecting immune function and increasing systemic inflammation.82 Alcohol can also interact with medications, reducing their efficacy in treating psoriasis. Cigarette smoke contains numerous toxins that can contribute to systemic inflammation. Smoking increases the risk of developing psoriasis, and it is associated with more severe disease outcomes by promoting the Th1/Th17 immune response, as well as increasing oxidative stress and inflammatory cytokine production.83
Innate and Adaptive Immune System and Feed-Forward Amplification
T cells have been recognized as central to psoriasis pathogenesis since the 1980s, as evidenced by ciclosporin’s efficacy.84 While T cells are pivotal, other immune cells (dendritic cells, neutrophils, keratinocytes) also contribute. Epidermal hyperproliferation and the production of antimicrobial proteins, growth factors, and chemokines are driven by intercellular communication via cytokines and keratinocyte activation.85 These factors perpetuate inflammation, inducing angiogenesis, neutrophil infiltration, and increased Th1 and Th17 cell numbers (Figure 3).
Tissue-resident memory T cells (TRM cells), non-circulating memory T cells residing in epithelial tissues, have garnered significant attention in recent years.86 While evolved for rapid pathogen defense, TRM cell dysregulation contributes to immune-mediated diseases like psoriasis. Triggered by autoantigens, including HLA-C*06:02-presented antigens, TRM cell activation drives psoriasis pathogenesis.87 The persistence of TRM cells in the skin explains disease characteristics like distinct lesion borders and recurrent involvement of previously affected sites.
Cytokine Circuits in Psoriasis: IL-23 and Th17 and Beyond
IL-23 and Th17 responses are central to psoriasis pathogenesis, as supported by GWAS and clinical trials. IL-23, a cytokine composed of p40 and p19 subunits, drives IL-17 production and T cell expansion.85–87 Sharing the p40 subunit (ustekinumab target) with IL-12, which promotes Th1 responses and IFN-γ production, IL-23 has been successfully targeted therapeutically, unlike IL-12.88–91
The IL-17 cytokine family, which includes six members (IL-17A-F), is involved in psoriasis pathogenesis, with IL-17A being the most prominent.92–94 IL-17C and IL-17F are also highly expressed, but their roles are less well-defined. Activated T cells and group 3 innate lymphoid cells are the primary sources of IL-17 in psoriatic plaques.95–97 IL-17 upregulates CCL20, a Th17 chemoattractant, leading to a positive feedback loop.
TNFα, a proinflammatory cytokine produced by dendritic cells, macrophages, and T cells, exerts diverse biological effects.98 These include inducing proinflammatory cytokine expression in dendritic cells and T cells, upregulating neutrophil chemoattractants, promoting vascular adhesion molecule expression for inflammatory cell influx, and amplifying cytokine effects, such as IL-17.99
IFN-γ, predominantly produced by T cells, was an early identified psoriasis plaque inflammatory mediator. IFN-γ, like type I interferons, exacerbates psoriasis by enhancing antigen processing, MHC class II expression on antigen-presenting cells, and the production of proinflammatory mediators, including the Th1 and Tc1 cell chemoattractants CXCL9 and CXCL10.100–103
The IL-36 cytokine family, consisting of IL-36α, IL-36β, IL-36γ, and IL-36 receptor antagonist (IL36RN), has recently gained attention.104 IL36RN gene mutations are associated with pustular psoriasis, particularly generalized pustular psoriasis. Primarily expressed by epithelial cells, IL-36 cytokine expression is induced by proinflammatory cytokines such as IL-17, TNFα, and IL-36 itself.104,105 This amplifies inflammation and neutrophil influx. Keratinocyte-secreted serine protease inhibitors, such as SERPINA3, regulate IL-36 activation. Loss-of-function SERPINA3 variants predispose to generalized pustular psoriasis.106 CARD14 and AP1S3 gene mutations are also associated with pustular psoriasis, with CARD14 mutations linked to familial pityriasis rubra pilaris.107
The interplay between IL-23, IL-17, interferon, and IL-36 cytokine circuits influences psoriasis clinical manifestations.108 In contrast to the predominance of IL-23 and IL-17 in plaque psoriasis, interferon responses are more pronounced in early-stage disease.109 IL-36 is more involved in pustular forms. These circuits exhibit complex interdependencies, with IFN-γ boosting IL-23 and Th17 responses, and IL-17 stimulating IL-36 expression, creating a self-perpetuating inflammatory cycle110 (Figure 4).
Disease Associations
Psoriasis frequently co-occurs with other diseases, most notably psoriatic arthritis, affecting 10–40% of patients.111–113 Typically developing 10 years after psoriasis onset, psoriatic arthritis often presents asymmetrically, targeting distal interphalangeal joints, with potential axial involvement.114,115 Enthesitis and dactylitis also characterize psoriatic arthritis. While sharing pathogenic and immunological features, psoriasis and psoriatic arthritis represent distinct genetic, immunological, and therapeutic entities.116–118 While causality remains unclear, these conditions collectively increase morbidity in psoriasis patients. Lifestyle interventions, particularly weight management,119–121 may improve outcomes, as evidenced by reduced biologic therapy efficacy in obese psoriasis patients and beneficial effects of bariatric surgery on psoriasis.122–124
Management
Overall Treatment Strategy for Plaque Psoriasis
Psoriasis treatment strategies consider disease severity, psoriatic arthritis presence, comorbidities, and patient preferences.125,126 For patients with psoriatic arthritis, systemic treatments addressing both conditions are prioritized. Treatment selection also reflects psoriasis severity, location, and associated medical conditions.127,128
Patient education is crucial, with resources available from organizations like the Psoriasis Association and National Psoriasis Foundation.129 A holistic management approach emphasizes lifestyle modifications. Programs like PsoWell, incorporating motivational interviewing, have proven beneficial.130 While individual educational sessions are effective, resource limitations may hinder their widespread implementation.131
Treatment Goals
European guidelines typically stipulate that a body surface area involvement of more than 10%, a psoriasis area severity index (PASI) score of 10 or higher, or a dermatology life quality index score exceeding 10 is necessary.132,133 Alternatively, the condition can be defined by significant physical, social, or psychological impact, depression or anxiety, or treatment-resistant localized psoriasis with dysfunction.134–136 The International Psoriasis Council advocates a dual-therapy approach for moderate-to-severe psoriasis, with topical and systemic therapies as the two main options.137 Systemic therapy is indicated for body surface area involvement exceeding 10%, psoriasis affecting specific sites, or inadequate topical therapy response.138 While step therapy (phototherapy followed by oral agents and biologics) was previously common, current guidelines often consider biologics, oral agents, and phototherapies concurrently.139
In the UK, eligibility for biologic or oral small molecule therapies is based on the criteria set by the National Institute for Health and Care Excellence. Patients must have had plaque psoriasis for a minimum of six months, with a PASI score of 10 or higher, a DLQI score exceeding 10, and must have shown inadequate response to or intolerance of conventional systemic treatments such as methotrexate.137
Important and measurable disease endpoints for the management of psoriasis are continually developing, considering factors like disease severity, patient impact, and treatment response. A treat-to-target approach focusing on disease control and improved outcomes is gaining global momentum. Attaining a psoriasis area severity index (PASI) of 2 or lower, a physician’s global assessment indicating clear or nearly clear skin (which corresponds to a 90% reduction in PASI), and a dermatology life quality index (DLQI) score below 5 are achievable treatment objectives.138
Topical Therapy
Liberal emollient use is essential for all psoriasis patients. Localized psoriasis (affecting <3-5% of body surface) is typically managed with topical agents like corticosteroids, vitamin D3 analogs, and combination formulations. Targeted phototherapy is also an option. While crude coal tar and dithranol remain available in specialized settings, newer, more cosmetically appealing alternatives are preferred. Adherence to topical therapies often proves challenging.138
Phototherapy
Ultraviolet radiation has local immunosuppressive effects that specifically affect Langerhans cells, reduce epidermal hyperproliferation and angiogenesis, and promote T cell apoptosis. Various phototherapy options include narrowband ultraviolet B radiation (311–313 nm), broadband ultraviolet B radiation (280–320 nm), targeted phototherapy, and, though less frequently used due to the potential risk of skin cancer, oral psoralen ultraviolet A (PUVA). Narrowband ultraviolet B, administered two to three times weekly, is the most common modality, with home units gaining popularity. While effective for plaque psoriasis, narrowband ultraviolet B can cause burning and carries a lower photocarcinogenesis risk compared to PUVA. Some centers still combine narrowband ultraviolet B with crude coal tar or dithranol.139
Oral Systemic Therapies
Prior to the advent of biologics, oral systemic agents were the mainstay for treating moderate-to-severe plaque psoriasis. These medications exhibit a variety of mechanisms of action, effectiveness, and safety profiles (Table 1). Targeting ubiquitous intracellular targets, these small molecules often have broader effects than biologics. Frequently used oral agents comprise methotrexate, ciclosporin, acitretin, fumarates, and apremilast. Additionally, a TYK2 inhibitor is presently in the later stages of development.140
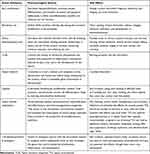 |
Table 1 Recognized Topical Psoriasis Therapies |
Methotrexate has been utilized in the treatment of psoriasis and psoriatic arthritis for more than 50 years. Its mechanism of action is thought to involve the inhibition of 5-aminoimidazole-4-carboxamide ribonucleotide transformylase and the production of adenosine, which suppresses lymphocyte activity.141 Administered weekly, methotrexate primarily causes bone marrow suppression, with liver fibrosis risk. Non-invasive techniques for assessing liver fibrosis include measuring serum levels of the amino propeptide fragment of procollagen III, elastography, and using algorithm-based risk assessments. Additional side effects may consist of nausea, vomiting, hair loss and teratogenic effects. Folic acid supplementation is recommended to mitigate hematological and gastrointestinal adverse effects.142
Subcutaneous methotrexate, increasingly replacing oral administration in some countries, offers potential advantages in efficacy, bioavailability, and gastrointestinal tolerability. While methotrexate can be combined with anti-TNFα biologics to reduce antidrug antibody development, this practice is more common in rheumatology than dermatology.143
Ciclosporin, a systemic calcineurin inhibitor, is indicated for short-term treatment of severe plaque psoriasis or for managing crises, in addition to serving as a bridge to long-term therapies. Rapid onset and robust efficacy characterize ciclosporin, but its use should not exceed one year due to nephrotoxicity risk. Other side effects may involve hypertension, increased risk of infections, nausea, excessive hair growth, gingival hyperplasia, interactions with other medications, and imbalances in electrolytes.144
Acitretin, a synthetic retinoid used systemically, treats severe plaque psoriasis by regulating keratinocyte proliferation and providing immunomodulatory effects. While also used for pustular psoriasis and palmoplantar psoriasis, acitretin’s use in plaque psoriasis has declined. Its use is not recommended for women of childbearing age because of its potential teratogenic effects.145
Fumaric acid esters are small molecules that inhibit the maturation of dendritic cells, promote T cell apoptosis, and disrupt leukocyte extravasation. They are commonly utilized in Germany for treating moderate to severe plaque psoriasis. Dimethyl fumarate, a formulation of a single ester, has received approval from the European Medicines Agency. Gradual dose escalation is necessary due to frequent flushing and diarrhea (affecting up to 40% of patients). Lymphocytopenia below 0.8 K/μL necessitates dose reduction to mitigate progressive multifocal leukoencephalopathy risk.146
Apremilast, which acts as a phosphodiesterase-4 inhibitor, is used to manage moderate to severe psoriasis and psoriatic arthritis. Its immunomodulatory properties reduce proinflammatory cytokines such as TNFα, IL-2, and IL-12, while enhancing the levels of the anti-inflammatory cytokine IL-10. Frequent side effects include nausea, diarrhea, and weight loss.147
Biological Treatment
In the past two decades, biologic therapies have significantly transformed the treatment of psoriasis and psoriatic arthritis. These therapies primarily consist of recombinant monoclonal antibodies or receptor fusion proteins, which can be fully human, humanized, or human–mouse chimeric, and are designed to target specific inflammatory mediators.148 While they are currently categorized as immunosuppressants, it is becoming increasingly clear that drugs targeting highly specific immune pathways cannot be simply classified as either suppressive or stimulatory.149 Therefore, a new classification system is needed to better describe the modulatory effects of these drugs, which is particularly important for identifying patients at increased risk of infections. All biologics for psoriasis, except infliximab, are administered via subcutaneous injection.150 Currently, there are 11 biologic treatments across four distinct classes—anti-TNFα, anti-IL-17, anti-IL-12p40 or IL-23p40, and anti-IL-23p19—used for managing moderate-to-severe psoriasis.151
Currently, four anti-TNFα agents are approved for the treatment of psoriasis: adalimumab, certolizumab pegol, etanercept, and infliximab. Etanercept is a fusion protein combining tumor necrosis factor receptor 2 (TNFRSF1B) with the Fc region of IgG1.152 Certolizumab pegol, on the other hand, is a monoclonal antibody fragment conjugated with polyethylene glycol, a modification that reduces immunogenicity, extends its half-life, and prevents it from crossing the placental barrier. Adalimumab is a fully human monoclonal antibody, while infliximab is a chimeric antibody.153 Additionally, a fifth anti-TNFα agent, golimumab, is approved for psoriatic arthritis but has not yet been approved for psoriasis treatment.
Three anti-IL-17 therapies have been approved: secukinumab, ixekizumab, and brodalumab. Secukinumab and ixekizumab both specifically target IL-17A, while brodalumab inhibits the IL-17 receptor A (IL-17RA), blocking IL-17A, IL-17F, and additional cytokines in the IL-17 family, including IL-17C and IL-17E (also known as IL-25). Bimekizumab, which targets both IL-17A and IL-17F, is currently undergoing Phase 3 clinical trials for psoriasis.154
Currently, four IL-23-targeting agents are approved for psoriasis treatment: ustekinumab, which inhibits the shared p40 subunit of IL-12 and IL-23, and guselkumab, risankizumab, and tildrakizumab, which specifically target the p19 subunit of IL-23.155 Another anti-IL-23p19 biologic, mirikizumab, is undergoing phase 3 clinical trials (NCT03482011 and NCT03535194). IL-23 plays a crucial role in sustaining and amplifying Th17 and Tc17 cell responses. The suppression of IL-17 activity is believed to be the primary mechanism driving the therapeutic effectiveness of these treatments.156
The highly targeted nature of biologic therapies can sometimes paradoxically trigger inflammatory responses, leading to disease worsening and shifts in clinical and immunological characteristics. A well-documented example of this phenomenon is the development of psoriasis or a worsening of existing psoriasis during anti-TNFα or anti-IL-6 therapy.157 This adverse reaction is more prevalent in women and often manifests as palmoplantar pustulosis. Another notable shift is the transformation of psoriasis into atopic dermatitis-like features, characterized by morphological changes, pruritus, and eosinophilia. This transformation has been observed with various biologics but is particularly prominent with anti-IL-17 and anti-IL23p19 therapies.158 Although highly effective for many, biologic therapies exhibit varying responses among patients. Some individuals may not respond at all (primary failure), while others may initially respond but subsequently lose efficacy (secondary failure). Factors such as non-adherence, low drug concentrations due to anti-drug antibody development, high body mass index, and sex differences have been implicated in these outcomes.155 Notably, men tend to have better initial responses and maintain therapeutic efficacy longer than women.156
The introduction of low-cost biosimilars following the expiration of biologics’ patents has made these treatments more accessible. Numerous biosimilars of infliximab, etanercept, and adalimumab are now available, offering significant cost savings in high-income countries while potentially enabling patients in low- and middle-income countries to access treatment.157 The true safety and, to a lesser extent, the efficacy of biologics for psoriasis can only be fully understood through real-world evidence gathered from long-term pharmacovigilance registries, such as the British Association of Dermatologists Biologics and Immunomodulators Register (UK), PsoBest (Germany), and BIOBADADERM (Spain).158 So far, the data are reassuring, demonstrating a generally favorable safety profile for biologics without major concerns regarding infection or cancer risk, provided that the required pretreatment and annual screening protocols are followed.159
Other Marketed Therapies
Psoriasis, a chronic condition, often necessitates long-term management. Treatment options vary widely based on disease severity, comorbidities, and individual responses. Psoriasis severity is graded using metrics like lesion severity, affected skin area, and quality of life.35 Psoriasis is classified into mild, moderate, and severe categories, taking into account factors such as the severity of lesions and the extent of skin involvement. Around 80% of individuals with psoriasis have mild to moderate forms, which are usually treated with topical therapies.36,37 Moderate cases often require a combination approach, including corticosteroids and other topical agents such as vitamin D derivatives.
Vitamin D3 analogs serve as the main topical therapy for plaque psoriasis and moderately severe cases affecting the scalp.38,39 The therapeutic advantages of vitamin D obtained from sunlight exposure in managing psoriasis have been acknowledged for many years.40–42 Nonetheless, the function of vitamin D in psoriasis and various other skin disorders is still intricate and subject to debate.43,44 Filoni et al helped shed light on this matter by demonstrating that psoriatic patients tend to have lower levels of vitamin D, which are associated with the duration of the disease.45 Lee et al further supported these findings by identifying decreased 25-hydroxyvitamin D (25OHD) levels, a marker of vitamin D stores, in psoriasis patients.46,47 Nevertheless, the correlation between increased 25OHD levels following phototherapy and improved psoriasis severity remains inconclusive.48
Vitamin D plays a crucial role in keratinocyte proliferation (Figure 5). Its precursor, 7-DHC, undergoes UV-induced conversion to cholecalciferol, subsequently transformed into the active form, calcitriol, via intermediate 25OHD.48–52 Calcitriol modulates keratinocyte differentiation, proliferation, and immune balance, exerting both stimulatory and inhibitory effects based on concentration.43 Through calcium regulation, calcitriol and its analogs demonstrate therapeutic potential. In vitro studies reveal their ability to reduce psoriatic S100A7 levels, modulate keratinocyte proliferation, and regulate glycoceramides. Calcitriol deficiency or receptor dysfunction disrupts epidermal homeostasis, leading to basal layer hyperproliferation.43 Vitamin D’s anti-inflammatory actions include suppressing IL-2 production. Additionally, calcitriol and novel vitamin D3 derivatives suppress NF-κB, a key inflammation mediator.41
Although multiple studies associate vitamin D receptor (VDR) polymorphisms with susceptibility to psoriasis, this connection is still a topic of controversy.43,53,54 The activated vitamin D receptor (VDR) forms a complex with the retinoid X receptor (RXR), which attaches to vitamin D response elements (VDREs) to modulate gene expression. This genomic action contrasts with vitamin D’s direct influence on signaling pathways (nongenomic action).38 VDR gene variations can impact treatment response, with VDR isoform A linked to improved outcomes in psoriatic patients.43 VDR ligands suppress T cell-derived proinflammatory cytokines, while 1α,25(OH)2D3 increases the expression of IL-10 in psoriatic lesions. Vitamin D3 analogs inhibit T cell-mediated immune responses.41 Additionally, CYP11A1-synthesized 20(OH)D3 exhibits anti-proliferative, pro-differentiation, and anti-inflammatory properties, comparable to or surpassing 25OHD. This metabolite holds promise as a novel therapy for hyperproliferative and inflammatory conditions, including psoriasis.55
Beyond the classic vitamin D activation pathway, alternative routes involving CYP11A1 have emerged. Traditionally linked to steroidogenesis in specific organs, CYP11A1 catalyzes cholesterol conversion to pregnenolone through sequential hydroxylations and cleavage.55 Recent findings expand CYP11A1’s substrate range to include 7-DHC, vitamins D2, and lumisterol.56 CYP11A1 initiates vitamin D metabolism, primarily producing 20(OH)D3, along with minor 22(OH)D3 and 17(OH)D3 metabolites.57 Subsequent CYP11A1-mediated hydroxylations generate dihydroxy and trihydroxy metabolites, including 20.23(OH)2D3 and 17,20,23(OH)3D3.58
Beyond the classic VDR-mediated genomic mechanism, vitamin D elicits rapid nongenomic effects through alternative binding sites. The VDR’s A-pocket and the membrane-bound receptor PDIA3 facilitate these responses. PDIA3, a rapid steroid binding protein, activates phospholipase C via a G protein-coupled mechanism, leading to IP3 and diacylglycerol production and subsequent calcium release.59 Additionally, vitamin D targets RORγ, another nuclear receptor, where hydroxyderivatives act as antagonists, influencing immune function and cerebellar development.59,60
Severe psoriasis often necessitates systemic therapy, sometimes in conjunction with topical treatments. To enhance topical efficacy, adjunctive therapies like penetrating enhancers or phototherapy can be considered.61 Monotherapy regimens, requiring inconsistent application, often hinder treatment adherence.62 Combination therapies, offering fixed dosing and reduced application frequency, represent a more effective approach to improving patient compliance.63,64
Topical treatments are fundamental in managing psoriasis, providing safety and good tolerability. Mild plaque psoriasis is often treated with a combination of vitamin D derivatives and betamethasone, whereas topical calcineurin inhibitors (TCIs) and vitamin D analogs are commonly recommended for different subtypes of psoriasis. TCIs serve as steroid-sparing agents in inverse psoriasis, and tazarotene excels as a maintenance therapy (Table 1). Innovative vehicle formulations enhance topical delivery, addressing adherence, penetration, and application site-specific needs, thereby improving patient compliance.78 Emerging target-based agents and nanotechnology promise to further optimize efficacy, adherence, and penetration while minimizing side effects. While topical therapy remains the mainstay for mild to moderate psoriasis, long-term data on these treatments remain limited. Severe psoriasis necessitates a range of treatments, from topical agents to systemic therapies, each with varying efficacy and safety profiles. All these systemic medications are approved for psoriasis. Studies consistently demonstrate superior PASI 90 rates with biologics compared to small-molecule therapies (Table 2).
 |
Table 2 Authorized Systemic Interventions |
Despite advancements in systemic psoriasis treatments, patient response variability and treatment limitations persist. Issues such as low drug absorption and potential toxicity hinder treatment efficacy and reproducibility. Ongoing research focuses on identifying novel drug candidates and biosimilars.100 Additionally, exploring alternative drug delivery methods, including microneedles and nanotechnology, is a growing area of interest.
Clinical Trials
Intensive research over the past decade has yielded significant advancements in psoriasis pharmacotherapy, encompassing both biologics and novel small molecules. A diverse pipeline of drugs, administered topically or orally, is currently undergoing clinical evaluation. These promising candidates have the potential to expand the therapeutic armamentarium for psoriasis.148 Notably, several new agents with various delivery methods are in late-stage development and may receive regulatory approval in the near future (Table 3).
 |
Table 3 Innovative Psoriasis Treatments Currently Being Tested in Clinical Trials |
Topical administration remains the primary route for psoriasis treatment, particularly in mild to moderate cases. Ongoing efforts focus on developing novel topical formulations and active ingredients to enhance therapeutic outcomes. Numerous topical drug candidates are currently under clinical investigation. While parenteral delivery, especially subcutaneous administration, has gained prominence for biologics, oral formulations are the predominant focus of current clinical trials.149
Nanotechnology Applications in Psoriasis Management
The past two decades have witnessed a nanotechnology revolution, with intense focus on developing novel medical treatments. The technology’s versatility enables the use of diverse materials with tailored properties. While nanoparticles often serve as drug carriers, biodegradable variants hold particular promise for psoriasis therapy.150
Polymeric Nanoparticles (PNPs)
Polymer-based nanoparticles (PNPs) excel as biomaterials due to their biocompatibility, versatile size and structure, biomimetic properties, and ease of modification. This versatility enables targeted drug delivery. The PNP family encompasses a wide range of structures beyond nanospheres and nanocapsules, including dendrimers and micelles.151
Nanospheres
Nanospheres encapsulate drugs within a polymer matrix.120 Whether biodegradable or non-biodegradable, their primary functions are to enhance drug solubility.126 Gel composition significantly influenced nanosphere dispersion, with METHOCEL™ K15M0 (HPMC) providing superior homogeneity compared to Carbopol. Optimal dispersion occurred at a 3 mg/mL tyrosphere concentration in HPMC, yielding ~40 nm particles. Diering et al explored tyrospheres for vitamin D3 delivery, achieving an average particle sizes between 64.3 and 73.4 nm relying on vitamin D3 concentration.127 Enhanced drug absorption with increased vitamin D3 levels was observed compared to controls.
Nanocapsules
Nanocapsules encapsulate drugs within a polymer shell. The core may contain an oily phase, a polymer matrix, or molecularly dispersed drug. These systems offer advantages including sustained release, improved drug selectivity, bioavailability, and reduced toxicity.128,129 Marchiori et al developed dexamethasone-loaded nanocapsules for topical delivery.130 A capric triglyceride core and polycaprolactone shell, embedded in Carbopol gel, yielded 201 nm particles with >95% encapsulation efficiency. In vitro studies demonstrated controlled drug release and stability, warranting further in vivo investigation.
Dendrimers
Dendrimers, spherical, monodisperse, multivalent macromolecules, offer unique properties including solubility, biocompatibility, and reactivity.131 Drug delivery can occur through encapsulation or covalent conjugation, protecting against degradation. Their structure allows for diverse release mechanisms.132 Agrawal et al investigated polypropyleneimine dendrimers for dithranol delivery, achieving 8 nm particles with drug loading upon dendrimer exposure to dithranol.133 Drug permeation increased from 35% to 95%, and skin irritation decreased compared to dithranol solution. These findings highlight dendrimers as promising drug delivery candidates.
Micelles
Micelles, formed by amphiphile self-assembly above the critical micelle concentration, possess a hydrophobic core and hydrophilic shell. This structure protects hydrophobic drugs, enhancing solubility, bioavailability, and circulation time.152 Micelles offer advantages like high drug loading, reduced degradation, and potential for decreased side effects, making them attractive for topical skin disease treatments. Lapteva et al investigated MPEG-dihex PLA micelles for tacrolimus delivery, achieving sub-50 nm particles.135 While tacrolimus’s poor water solubility made micelles promising, in vivo studies revealed skin surface retention without stratum corneum penetration, limiting their transdermal delivery efficacy compared to other systems.
Lipid-Based Nanoparticles
Lipid nanoparticles, made from natural physiological lipids, provide a safe and non-toxic option for various applications. Their unique structural composition brings numerous benefits, such as improved stability of the drugs they carry, extended circulation time within the body, and the ability to degrade naturally. Additionally, these nanoparticles possess targeting capabilities that allow for precise delivery of therapeutic agents to specific sites, while also facilitating a high drug loading capacity at competitive costs. This combination of features makes lipid nanoparticles an attractive choice in the development of effective treatment strategies.136,137
Liposomes
Liposomes are round vesicles formed by one or more layers of lipid bilayers that encapsulate water-filled compartments.136,138 Their ability to solubilize insoluble drugs, act as local drug depots, and enhance the penetration of poorly soluble molecules into the stratum corneum makes them a focus for dermatological treatments. Liposome physicochemical properties, including charge, size, permeability, and stability, are readily adjustable, offering versatility in therapeutic applications.
Wadhwa et al investigated fusidic acid-loaded liposomes for treating plaque psoriasis.139 Formulations exhibited varying physicochemical properties, with sizes ranging from 572.7 to 740.1 nm and entrapment efficiencies between 52.1% and 72.6%. These stable liposomes demonstrated enhanced anti-psoriatic efficacy compared to conventional treatments in a mouse tail model. The study highlights the potential of liposomes as effective drug delivery systems for skin disorders.
Lipospheres
Lipospheres are nanoparticles comprising a hydrophobic core enveloped by a phospholipid coating. These particles offer advantages over other lipid-based nanoparticles, including low cost, enhanced stability, simple production, controlled release, and excellent water dispersibility.140 Demonstrating versatility, lipospheres have been effectively administered via oral, intravenous, and transdermal routes for treating various diseases, including psoriasis.
Chen et al evaluated curcumin-loaded liposphere gel for psoriasis treatment, comparing its efficacy to a solution formulation.141 Produced lipospheres, averaging 47 nm, were incorporated into a gel base. Enhanced drug permeation through skin layers was observed in a mouse model. The liposphere gel demonstrated superior therapeutic efficacy and histopathological improvement compared to the solution, accompanied by reduced TNF-α, IL-22, and IL-17 levels. These results emphasize the promise of lipospheres as an effective strategy for treating psoriasis.
Ethosomes
Ethosomes, ethanol-based phospholipid vesicles, excel in topical drug delivery due to their flexibility and softness. Their high ethanol content disrupts both ethosomal and stratum corneum lipid bilayers, enhancing skin penetration. This fluidization allows ethosomes to traverse the modified skin barrier and release their contents into deeper layers.
Zhang et al developed psoralen-loaded ethosomes with particle sizes varying from 56.71 to 159.07 nm, achieving optimal encapsulation efficiency at approximately 150 nm.143 Compared to ethanolic psoralen tincture, ethosomes demonstrated a sevenfold increase in drug deposition within rat skin. This enhanced permeation and penetration suggest reduced toxicity and improved therapeutic efficacy for long-term skin disorder management, including psoriasis.
Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are composed of physiological lipids and surfactants. Drug incorporation occurs either within the lipid matrix or as a surrounding layer.144 Solid lipid nanoparticles (SLNs) provide benefits including regulated drug release, decreased skin irritation, and enhanced protection for the drug when compared to other topical delivery systems. Their size facilitates enhanced drug permeation through close contact with the stratum corneum.145,146 These characteristics make SLNs promising candidates for topical psoriasis treatments.147
Pradhan et al investigated SLNs as a sustained-release delivery system for fluocinolone acetonide.148 Optimized SLNs, characterized by 107.4 nm particle size, 87% entrapment efficiency, 2% lipid, 1% surfactant, and 0.06% drug concentrations, exhibited controlled drug release following Higuchi kinetics. In contrast, the drug suspension displayed rapid zero-order release in vitro. SLNs demonstrated enhanced fluocinolone acetonide delivery to the epidermis compared to the suspension.149 These findings highlight the potential of SLNs for developing innovative psoriasis treatments.
Nanostructured Lipid Carriers
Nanostructured lipid carriers (NLCs) offer advantages over traditional formulations. NLCs enhance drug loading, control release, biocompatibility, and bioavailability, enabling diverse administration routes.150 Their superior penetration ability makes NLCs promising candidates for topical drug delivery systems.
Nanoemulsions of lipids (NLCs) demonstrate occlusive qualities that minimize transepidermal water loss and improve skin hydration by creating a lipid monolayer. These features, combined with their ability to deliver drugs effectively, have sparked interest in utilizing NLCs for treating skin conditions such as psoriasis.151
Avasatthi et al formulated a methotrexate-loaded nanostructured lipid carrier (NLC) nanogel.152 Optimized using Precirol ATO 5, the NLC exhibited a 278 nm particle size, 0.231 polydispersity index, and 22.3% entrapment efficiency. In vitro and in vivo studies demonstrated enhanced PASI scores and prolonged drug circulation following 48-hour application compared to controls. These findings underscore the potential of NLCs for developing novel psoriasis treatments.
Microneedles
Microneedles represent an innovative system for delivering drugs intradermally, consisting of a patch with vertical microneedles that penetrate the stratum corneum. Constructed from various materials, microneedles require sufficient strength and toughness for effective function.153 With lengths ranging from 25 to 2000 microns, microneedles penetrate the stratum corneum without activating pain receptors.
Microneedles enhance drug bioavailability by creating microchannels through the skin. This bypasses the initial skin barriers, facilitating drug deposition in the stratum corneum and improving permeability.154 Diverse microneedle types, varying in mechanism and material, have been developed to optimize this delivery system.
Microneedles are categorized into four types: solid, coated, dissolving, and hollow. While all types have potential applications, only dissolving microneedles have been studied for psoriasis treatment.155 Dissolving microneedles (DMNs) are biodegradable, dissolving upon skin insertion.155 The drug is incorporated into the polymer matrix. Material selection is crucial, requiring sufficient strength for skin penetration and subsequent biodegradation without toxicity. Given the body’s exposure to needle materials, biodegradability and metabolizability are essential.156 DMNs offer controlled drug release through material selection.
Tekko et al combined nanotechnology and microneedles by developing methotrexate disodium-loaded nanocrystal patches.157 After optimizing and characterizing the patches, in vitro release studies were conducted. Subsequent in vivo studies in Sprague Dawley rats compared the microneedle patch with oral methotrexate administration.158 The patch demonstrated sustained methotrexate release over 72 hours, attributed to skin retention. These findings support further investigation of microneedle-based methotrexate delivery in psoriasis models.159
Limited studies have explored microneedle-based drug delivery for psoriasis.160 Patients reported positive experiences, and PASI scores decreased. While these results demonstrate potential, larger studies with control groups are needed to confirm the findings.
Future Prospects
Translational medicine has revolutionized psoriasis care, but significant challenges persist. While genetics undeniably influence disease expression, the mechanisms underlying environmental triggers remain elusive. The microbiome, stress, and infections, including the lessons from COVID-19, likely play critical roles.161 A systems medicine approach, incorporating preventive, predictive, personalized, and participatory principles, aims to prevent severe disease and comorbidities.162 This involves stratifying patients based on integrated omics data to optimize treatment selection and dosage. Early intervention, initiated promptly after diagnosis, facilitates multimorbidity screening, patient education, and timely therapeutic initiation.163 Such strategies may modify disease course by preventing tissue-resident memory T cell accumulation. IL-17 and IL-23 remain central therapeutic targets, but identifying additional cytokines and intracellular signaling pathways is essential. The systemic inflammatory nature of psoriasis, including adipose inflammation, demands further exploration, especially regarding biologics’ potential in cardiovascular disease prevention.164
Psoriasis taxonomy is evolving as molecular and genomic insights refine clinical phenotypes. While high-income countries primarily drive treatment paradigms, psoriasis is a global burden.165 The Global Psoriasis Atlas, collaborating with WHO, International Psoriasis Council, International League of Dermatological Societies, and International Federation of Psoriasis Associations, is crucial for understanding global epidemiology and economic impact.166 Its goal of ensuring worldwide access to optimal psoriasis care is essential. Introducing low-cost biosimilars in low-income countries requires careful benefit-risk assessment.167 Topical therapies, though foundational, necessitate improvements in efficacy, durability, and aesthetics. Biologics and novel small molecules have transformed severe psoriasis management, but long-term registries are needed to assess real-world risk-benefit profiles. Identifying factors differentiating those with transient guttate psoriasis from those developing chronic plaque disease is crucial for future therapeutic strategies.168–170 This requires prospective analysis of immunological, genetic, and environmental factors.
Conclusions
While conventional oral and topical therapies have long been the cornerstone of psoriasis treatment, the advent of biologic therapies has fundamentally transformed the management of this chronic, inflammatory skin condition. Psoriasis, characterized by rapid skin cell turnover and the formation of thick, scaly plaques, has historically been managed through topical corticosteroids, vitamin D analogs, and oral systemic agents such as methotrexate and cyclosporine.171 While these therapies are effective for many patients, they are often limited by side effects, long-term use complications, and varying patient responses. The introduction of biologic therapies, which target specific immune system pathways involved in the disease’s pathophysiology, such as the interleukin-17 (IL-17), interleukin-23 (IL-23), and tumor necrosis factor (TNF) pathways, has provided a more precise and effective treatment option. These biologics offer dramatic improvements in patient outcomes, achieving better clearance of plaques, reducing inflammation, and improving quality of life.172
However, despite the substantial benefits biologics provide, challenges persist. One of the primary concerns is treatment failure, which can occur in two forms: primary failure, where the patient does not respond adequately to the biologic treatment, and secondary failure, where the patient initially responds but loses efficacy over time.173 These challenges highlight the complexity of psoriasis treatment and emphasize the need for continuous innovation in therapeutic approaches. Treatment resistance may arise due to a variety of factors, including patient-specific genetic and immunological differences, variations in drug absorption, and the development of neutralizing antibodies against biologic agents.174 Therefore, there is a critical need to explore alternative strategies that can address these issues and further optimize treatment outcomes.
In response to these challenges, researchers are focusing on developing novel drug delivery systems that enhance the effectiveness and precision of treatments. One promising avenue is the use of nanoparticles for targeted drug delivery. Nanoparticles, which are typically between 1 and 100 nanometers in size, offer several advantages over traditional drug delivery methods.175 By encapsulating therapeutic agents, nanoparticles can be designed to overcome the skin’s natural barrier, which is one of the primary obstacles in treating psoriasis topically. These particles can be engineered to target specific cells or tissues involved in the inflammatory process, allowing for localized drug delivery directly to the affected skin areas.176 This approach not only improves drug efficacy but also reduces the likelihood of systemic side effects. Moreover, lipid-based nanoparticles—which use lipid molecules as the carrier system—are gaining significant attention in dermatology due to their biocompatibility, ability to encapsulate poorly water-soluble drugs, and potential for sustained drug release.177 The lipid-based structure of these nanoparticles mimics the skin’s natural lipid layers, aiding in penetration through the skin’s barrier and ensuring that the therapeutic agents are delivered directly to the site of inflammation with minimal waste.
Another exciting development in psoriasis treatment is the microneedle technology, which offers a minimally invasive approach to drug delivery. Microneedles are tiny, needle-like structures, typically ranging from 25 to 100 micrometers in length, that can painlessly penetrate the skin’s outermost layer, the stratum corneum, to deliver drugs directly into the deeper layers of the skin. This innovative technology overcomes the limitations of conventional topical treatments that often struggle to reach the deeper layers of the skin where psoriasis lesions are located. Microneedles can be used to administer both biologic therapies and small molecules directly to the target site, offering precise control over the dosage and timing of drug release. This method has the potential to increase drug absorption, improve therapeutic efficacy, and significantly reduce the risk of systemic side effects. While dissolving microneedles—which dissolve upon insertion into the skin—have shown promising results in clinical trials, further research is necessary to evaluate the full potential of different microneedle designs, materials, and formulations. For instance, multi-layered or coated microneedles may allow for controlled and sustained release of drugs over an extended period, enhancing the therapeutic effect and reducing the need for frequent treatments.178
In conclusion, the combination of advanced biologic therapies with cutting-edge drug delivery systems, such as nanoparticles and microneedles, represents a promising future for the treatment of psoriasis. These novel approaches aim to address the limitations of conventional therapies, such as the risk of treatment failure and side effects, by improving the precision and effectiveness of drug delivery. As research in these areas continues to evolve, these innovative strategies could offer more effective, personalized, and tolerable treatment options for psoriasis patients. By overcoming the barriers associated with traditional treatments and optimizing drug delivery systems, these advancements have the potential to significantly improve patient outcomes and quality of life, offering new hope for individuals struggling with this chronic skin condition.
Data Sharing Statement
No data was used for the research described in the article.
Acknowledgments
The authors thank everyone who helped with this work.
Author Contributions
SG and JL designed and conceptualized the report. MM wrote the first draft of the manuscript. JY and YS reviewed and revised the manuscript. All authors have read and approved the article. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was supported by the National Natural Science Foundation of China (81960658) and Jiangsu Provincial Hospital for Traditional Chinese Medicine Faculty-Level Project (Y24054).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Samotij D, Nedoszytko B, Bartosi´nska J, et al. Pathogenesis of psoriasis in the “omic” era. Part I. Epidemiology, clinical manifestation, immunological and neuroendocrine disturbances. Postepy Dermatol Alergol. 2020;37:135–153. doi:10.5114/ada.2020.94832
2. Rendon A, Schäkel K. Psoriasis pathogenesis and treatment. Int J mol Sci. 2019;20(1475):1475. doi:10.3390/ijms20061475
3. Ortonne J, Chimenti S, Luger T, Puig L, Reid F, Trüeb RM. Scalp psoriasis: European consensus on grading and treatment algorithm. J Eur Acad Dermatol Venereol JEADV. 2009;23:1435–1444. doi:10.1111/j.1468-3083.2009.03372.x
4. Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun. 2015;64:66–73. doi:10.1016/j.jaut.2015.07.008
5. Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22:2959–2966. doi:10.1016/S0142-9612(01)00042-4
6. Morizane S, Gallo RL. Antimicrobial peptides in the pathogenesis of psoriasis. J Dermatol. 2012;39:225–230. doi:10.1111/j.1346-8138.2011.01483.x
7. Morizane S, Yamasaki K, Mühleisen B, et al. Cathelicidin antimicrobial peptide LL-37 in psoriasis enables keratinocyte reactivity against TLR9 ligands. J Investig Dermatol. 2012;132:135–143. doi:10.1038/jid.2011.259
8. Liang Y, Sarkar MK, Tsoi LC, Gudjonsson JE. Psoriasis: a mixed autoimmune and autoinflammatory disease. Curr Opin Immunol. 2017;49:1–8. doi:10.1016/j.coi.2017.07.007
9. Girolomoni G, Strohal R, Puig L, et al. The role of IL-23 and the IL-23/T(H) 17 immune axis in the pathogenesis and treatment of psoriasis. J Eur Acad Dermatol Venereol JEADV. 2017;31:1616–1626. doi:10.1111/jdv.14433
10. Fotiadou C, Lazaridou E, Sotiriou E, Ioannides D. Targeting IL-23 in psoriasis: current perspectives. Psoriasis. 2018;8:1–5. doi:10.2147/PTT.S98893
11. Mansouri M, Mansouri P, Raze AA, Jadali Z. The potential role of Th17 lymphocytes in patients with psoriasis. An Bras Dermatol. 2018;93:63–66. doi:10.1590/abd1806-4841.20186123
12. Ruan Q, Kameswaran V, Zhang Y, et al. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi:10.1084/jem.20110462
13. Zhang Y, Luo X-Y, Wu D-H, Xu Y. ROR nuclear receptors: structures, related diseases, and drug discovery. Acta Pharm Sin. 2015;36:71–87. doi:10.1038/aps.2014.120
14. Ecoeur F, Weiss J, Kaupmann K, Hintermann S, Orain D, Guntermann C. Antagonizing retinoic acid-related-orphan receptor gamma activity blocks the T helper 17/Interleukin-17 pathway leading to attenuated pro-inflammatory human keratinocyte and skin responses. Front Immunol. 2019;10:10. doi:10.3389/fimmu.2019.00010
15. Cyr P, Bronner SM, Crawford JJ. Recent progress on nuclear receptor RORγ modulators. Bioorganic Med Chem Lett. 2016;26:4387–4393. doi:10.1016/j.bmcl.2016.08.012
16. Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J mol Sci. 2018;19:530. doi:10.3390/ijms19020530
17. Hänsel A, Günther C, Ingwersen J, et al. Human slan (6-sulfo LacNAc) dendritic cells are inflammatory dermal dendritic cells in psoriasis and drive strong TH17/TH1 T-cell responses. J Allergy Clin Immunol. 2011;127:787–794.e9. doi:10.1016/j.jaci.2010.12.009
18. Diluvio L, Vollmer S, Besgen P, Ellwart JW, Chimenti S, Prinz JC. Identical TCR beta-chain rearrangements in streptococcal angina and skin lesions of patients with psoriasis vulgaris. J Immunol. 2006;176:7104–7111. doi:10.4049/jimmunol.176.11.7104
19. Georgescu SR, Tampa M, Caruntu C, et al. Advances in understanding the immunological pathways in psoriasis. Int J mol Sci. 2019;20:739. doi:10.3390/ijms20030739
20. Matsuzaki G, Umemura M. Interleukin-17 family cytokines in protective immunity against infections: role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. Microbiol Immunol. 2018;62:1–13. doi:10.1111/1348-0421.12560
21. Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi:10.1038/nri2586
22. Lee JS, Tato CM, Joyce-Shaikh B, et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity. 2015;43:727–738. doi:10.1016/j.immuni.2015.09.003
23. Schmitt J, Rosumeck S, Thomaschewski G, Sporbeck B, Haufe E, Nast A. Efficacy and safety of systemic treatments for moderate-to-severe psoriasis: meta-analysis of randomized controlled trials. Br J Dermatol. 2014;170:274–303. doi:10.1111/bjd.12663
24. Leonardi CL, Romiti R, Tebbey PW. Ten years on: the impact of biologics on the practice of dermatology. Dermatol Clin. 2015;33:111–125. doi:10.1016/j.det.2014.09.009
25. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2012;67:279–288. doi:10.1016/j.jaad.2011.01.032
26. Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–120. doi:10.1016/j.jaci.2016.08.056
27. Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432–437. doi:10.1016/j.ajhg.2011.07.022
28. Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620–628. doi:10.1056/NEJMoa1013068
29. Navarini AA, Burden AD, Capon F, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol JEADV. 2017;31:1792–1799. doi:10.1111/jdv.14386
30. Ko HC, Jwa SW, Song M, Kim MB, Kwon KS. Clinical course of guttate psoriasis: long-term follow-up study. J Dermatol. 2010;37:894–899. doi:10.1111/j.1346-8138.2010.00871.x
31. Leung DY, Travers JB, Giorno R, et al. Evidence for a streptococcal superantigen-driven process in acute guttate psoriasis. J Clin Investig. 1995;96:2106–2112. doi:10.1172/JCI118263
32. Johnston A, Gudjonsson JE, Sigmundsdottir H, Love TJ, Valdimarsson H. Peripheral blood T cell responses to keratin peptides that share sequences with streptococcal M proteins are largely restricted to skin-homing CD8(+) T cells. Clin. Exp Immunol. 2004;138:83–93. doi:10.1111/j.1365-2249.2004.00600.x
33. Micali G, Verzì AE, Giuffrida G, Panebianco E, Musumeci ML, Lacarrubba F. Inverse psoriasis: from diagnosis to current treatment options. Clin Cosmet Invest Dermatol. 2019;12:953–959. doi:10.2147/CCID.S189000
34. Syed ZU, Khachemoune A. Inverse psoriasis: case presentation and review. Am J Clin Dermatol. 2011;12:143–146. doi:10.2165/11532060-000000000-00000
35. Mrowietz U, Kragballe K, Reich K, et al. Definition of treatment goals for moderate to severe psoriasis: a European consensus. Arch Dermatol Res. 2011;303:1–10. doi:10.1007/s00403-010-1080-1
36. Torsekar R, Gautam MM. Topical Therapies in Psoriasis. Indian Dermatol Online J. 2017;8:235–245. doi:10.4103/2229-5178.209622
37. Stein Gold LF. Topical therapies for psoriasis: improving management strategies and patient adherence. Semin Cutan Med Surg. 2016;35:S36–S44.
38. Kim WB, Jerome D, Yeung J. Diagnosis and management of psoriasis. Can Fam Physician. 2017;63:278–285.
39. Psomadakis CE, Han G. New and emerging topical therapies for psoriasis and atopic dermatitis. J Clin Aesthetic Dermatol. 2019;12:28–34.
40. Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323:1945–1960. doi:10.1001/jama.2020.4006
41. Piotrowska A, Wierzbicka J, Zmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63:17–29. doi:10.18388/abp.2015_1104
42. Wadhwa B, Relhan V, Goel K, Kochhar AM, Garg VK. Vitamin D and skin diseases: a review. Indian J Dermatol Venereol Leprol. 2015;81:344–355. doi:10.4103/0378-6323.159928
43. Barrea L, Savanelli MC, Di Somma C, et al. Vitamin D and its role in psoriasis: an overview of the dermatologist and nutritionist. Rev Endocr Metab Disord. 2017;18:195–205. doi:10.1007/s11154-017-9411-6
44. Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: an update. J Dermatol. 2016;43:507–514. doi:10.1111/1346-8138.13305
45. Filoni A, Vestita M, Congedo M, Giudice G, Tafuri S, Bonamonte D. Association between psoriasis and vitamin D: duration of disease correlates with decreased vitamin D serum levels: an observational case-control study. Medicine. 2018;97:e11185. doi:10.1097/MD.0000000000011185
46. Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43:529–535. doi:10.1111/ced.13381
47. Giustina A, Bouillon R, Binkley N, et al. Controversies in vitamin D: a statement from the third international conference. JBMR Plus. 2020;4:e10417. doi:10.1002/jbm4.10417
48. Hambly R, Kirby B. The relevance of serum vitamin D in psoriasis: a review. Arch Dermatol Res. 2017;309:499–517. doi:10.1007/s00403-017-1751-2
49. Umar M, Sastry KS, Al Ali F, Al-Khulaifi M, Wang E, Chouchane AI. Vitamin D and the pathophysiology of inflammatory skin diseases. Ski Pharmacol Physiol. 2018;31:74–86. doi:10.1159/000485132
50. Jones G. The discovery and synthesis of the nutritional factor vitamin D. Int J Paleopathol. 2018;23:96–99. doi:10.1016/j.ijpp.2018.01.002
51. Jarrett P, Scragg R. A short history of phototherapy, vitamin D and skin disease. Photochem Photobiol Sci off J Eur Photochem Assoc Eur Soc Photobiol. 2017;16:283–290.
52. Juzeniene A, Grigalavicius M, Juraleviciute M, Grant WB. Phototherapy and vitamin D. Clin Dermatol. 2016;34:548–555. doi:10.1016/j.clindermatol.2016.05.004
53. Lee YH. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: an updated meta-analysis. Clin Exp Dermatol. 2019;44:498–505. doi:10.1111/ced.13823
54. Liu J, Wang W, Liu K, et al. Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp Dermatol. 2020;29:1186–1190. doi:10.1111/exd.14202
55. Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem mol Biol. 2014;144(Pt A):28–39. doi:10.1016/j.jsbmb.2013.10.012
56. Lang PO, Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (Not) understood. Clin Ther. 2017;39:930–945. doi:10.1016/j.clinthera.2017.04.004
57. Jenkinson C. The vitamin D metabolome: an update on analysis and function. Cell Biochem Funct. 2019;37:408–423. doi:10.1002/cbf.3421
58. Tuckey RC, Li W, Shehabi HZ, et al. Production of 22-hydroxy metabolites of vitamin d3 by cytochrome p450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos Biol Fate Chem. 2011;39:1577–1588. doi:10.1124/dmd.111.040071
59. Wierzbicka J, Piotrowska A, Zmijewski MA. The renaissance of vitamin D. Acta Biochim Pol. 2014;61:679–686.
60. Gao C, Liao MZ, Han LW, Thummel KE, Mao Q. Hepatic transport of 25-Hydroxyvitamin D(3) conjugates: a mechanism of 25-Hydroxyvitamin D(3) delivery to the intestinal tract. Drug Metab Dispos Biol Fate Chem. 2018;46:581–591. doi:10.1124/dmd.117.078881
61. Farahnik B, Patel V, Beroukhim K, et al. Combining biologic and phototherapy treatments for psoriasis: safety, efficacy, and patient acceptability. Psoriasis. 2016;6:105–111. doi:10.2147/PTT.S98952
62. Young M, Aldredge L, Parker P. Psoriasis for the primary care practitioner. J Am Assoc Nurse Pract. 2017;29:157–178. doi:10.1002/2327-6924.12443
63. Perrone V, Sangiorgi D, Buda S, Degli Esposti L. Topical medication utilization and health resources consumption in adult patients affected by psoriasis: findings from the analysis of administrative databases of local health units. ClinicoEconomics Outcomes Res. 2017;9:181–188. doi:10.2147/CEOR.S126975
64. Pathak SN, Scott P, West CE, Feldman SR. Self-management in patients with psoriasis. Psoriasis Targets Ther. 2014;4:19–26.
65. Dattola A, Silvestri M, Bennardo L, et al. A novel vehicle for the treatment of psoriasis. Dermatol Ther. 2020;33:e13185. doi:10.1111/dth.13185
66. Pinzon MI, Garcia OR, Villa CC. The influence of aloe vera gel incorporation on the physicochemical and mechanical properties of banana starch-chitosan edible films. J Sci Food Agric. 2018;98:4042–4049. doi:10.1002/jsfa.8915
67. Fluhr JW, Cavallotti C, Berardesca E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin Dermatol. 2008;26:380–386. doi:10.1016/j.clindermatol.2008.01.015
68. Nola I, Kostovi´c K, Kotrulja L, Lugovi´c L. The use of emollients as sophisticated therapy in dermatology. Acta Dermatovenerol Croat Adc. 2003;11:80–87.
69. Arbiser JL, Govindarajan B, Battle TE, et al. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Investig Dermatol. 2006;126:1396–1402. doi:10.1038/sj.jid.5700276.
70. Lebwohl M. The role of salicylic acid in the treatment of psoriasis. Int J Dermatol. 1999;38:16–24. doi:10.1046/j.1365-4362.1999.00500.x
71. Wang C, Lin A. Efficacy of topical calcineurin inhibitors in psoriasis. J Cutan Med Surg. 2014;18:8–14. doi:10.2310/7750.2013.13059
72. Duvic M, Asano AT, Hager C, Mays S. The pathogenesis of psoriasis and the mechanism of action of tazarotene. J Am Acad Dermatol. 1998;39:S129–S133. doi:10.1016/S0190-9622(98)70309-3
73. Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760–767. doi:10.1067/mjd.2003.103
74. McGill A, Frank A, Emmett N, Turnbull DM, Birch-Machin MA, Reynolds NJ. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J off Publ Fed Am Soc Exp Biol. 2005;19:1012–1014.
75. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi:10.1016/j.jaad.2005.01.010
76. Van De Kerkhof PCM, Berth-Jones J, Griffiths CEM, et al. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br J Dermatol. 2002;146:414–422. doi:10.1046/j.1365-2133.2002.04567.x
77. Lee CS, Li K. A review of acitretin for the treatment of psoriasis. Expert Opin Drug Saf. 2009;8:769–779. doi:10.1517/14740330903393732
78. Smith D. Fumaric acid esters for psoriasis: a systematic review. Ir J Med Sci. 2017;186:161–177. doi:10.1007/s11845-016-1470-2
79. Dogra S, Mahajan R, Narang T, et al. Systemic cyclosporine treatment in severe childhood psoriasis: a retrospective chart review Systemic cyclosporine treatment in severe childhood psoriasis: a retrospective chart review. J Dermatol Treat. 2017;2017:6634.
80. Greb JE, Goldminz AM, Gottlieb AB. Insights on methotrexate in psoriatic disease. Clin Immunol. 2016;172:61–64. doi:10.1016/j.clim.2016.07.008
81. Keating GM. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2017;77:459–472. doi:10.1007/s40265-017-0709-1
82. Azevedo A, Torres T. Tofacitinib: a new oral therapy for psoriasis. Clin Drug Investig. 2018;38:101–112. doi:10.1007/s40261-017-0596-y
83. Dapavo P, Vujic I, Fierro MT, Quaglino P, Samlorenzo M. The infliximab biosimilar in the treatment of moderate to severe plaque psoriasis. Pract Nurs. 2008;19:560–565.
84. Leonardi CL, Powers JL, Matheson RT, et al. Etanercept as monotherapy in patients with psoriasis. N Engl J Med. 2003;349:2014–2022. doi:10.1056/NEJMoa030409
85. Berends MAM, Driessen RJB, Langewouters AMG, Boezeman JB, Van De Kerkhof PCM, De Jong EMGJ. Etanercept and efalizumab treatment for high-need psoriasis. Effects and side effects in a prospective cohort study in outpatient clinical practice. J DermatolTreat. 2007;18:76–83. doi:10.1080/09546630601121086
86. Alwawi EA, Mehlis SL, Gordon KB. Treating psoriasis with Adalimumab. Ther Clin Risk Manag. 2008;4:345–351. doi:10.2147/TCRM.S1265
87. Chimenti MS, Saraceno R, Chiricozzi A, Giunta A, Chimenti S, Perricone R. Profile of certolizumab and its potential in the treatment of psoriatic arthritis. Open Access Rheumatol Res Rev. 2013;6:7–13.
88. Fleischmann R, Vencovsky J, Van Vollenhoven RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68:805–811. doi:10.1136/ard.2008.099291
89. Leonardi CL, Kimball AB, Papp KA. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (Phoenix 1). Lancet. 2008;24:34.
90. Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326–338. doi:10.1056/NEJMoa1314258
91. Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991–1001. doi:10.1111/bcp.13185
92. Reich K. Anti-interleukin-17 monoclonal antibody ixekizumab in psoriasis. N Engl J Med. 2012;367:274–275.
93. Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345–356. doi:10.1056/NEJMoa1512711
94. Krueger JG, Kricorian G, Aras G, et al. Brodalumab, an anti–interleukin-17–receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi:10.1056/NEJMoa1109017
95. Papp K, Thaçi D, Reich K, et al. Tildrakizumab (MK-3222), an anti-interleukin-23p19 monoclonal antibody, improves psoriasis in a phase IIb randomized placebo-controlled trial. Br J Dermatol. 2015;173:930–939. doi:10.1111/bjd.13932
96. Nakamura M, Lee K, Jeon C, et al. Guselkumab for the treatment of psoriasis: a review of Phase III trials. Dermatol Ther. 2017;7:281–292. doi:10.1007/s13555-017-0187-0
97. Sofen H, Smith S, Matheson RT, et al. Guselkumab (an IL-23-specific mAb) demonstrates clinical and molecular response in patients with moderate-to-severe psoriasis. J Allergy Clin Immunol. 2014;133:1032–1040. doi:10.1016/j.jaci.2014.01.025
98. Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650–661. doi:10.1016/S0140-6736(18)31713-6
99. Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized Phase II study. Br J Dermatol. 2019;181:88–95. doi:10.1111/bjd.17628
100. Iannone LF, Bennardo L, Palleria C, et al. Safety profile of biologic drugs for psoriasis in clinical practice: an Italian prospective pharmacovigilance study. PLoS One. 2020;15:e0241575. doi:10.1371/journal.pone.0241575
101. Boston Pharmaceuticals. Study to Evaluate the Safety, Tolerability, and Pharmacokinetics of Single and Repeat Topical Administration of BOS-475 in Healthy Subjects and Patients With Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2020.
102. AbbVie. A Study to Evaluate the Pharmacokinetics, Safety and Tolerability of ABBV-157 in Healthy Volunteers and in Participants With Chronic Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
103. Celgene. A Safety Study of CC-92252 in Healthy Adult Subjects and Adult Subjects With Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
104. Evelo Biosciences I. A Study of EDP1066 in Healthy Participants and Participants With Mild to Moderate Psoriasis and Atopic Dermatitis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
105. Affibody. A Study to Evaluate ABY-035 in Subjects With Moderate-to-Severe Plaque Psoriasis (AFFIRM-35). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
106. Arcutis Biotherapeutics, I. Safety, Pharmacokinetics and Efficacy of ARQ-151 Cream in Adults With Mild to Moderate Chronic Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
107. Boehringer I. A Study to Test. How Well Patients With Plaque Psoriasis Tolerate BI 730357 Over a Longer Period and How Effective It Is. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
108. Santalis Pharmaceuticals Inc. A Trial of a Botanical Drug (EISO) for Treatment of Mild-to-Moderate Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
109. Akros Pharma Inc. Study to Evaluate the Efficacy and Safety of JTE-451 in Subjects With Moderate to Severe Plaque Psoriasis (IMPACT-PS). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
110. Bond Avillion. 2 Development LP. A Phase 2b Study of the Efficacy, Safety, and Tolerability of M1095 in Subjects With Moderate to Severe Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2017.
111. Pfizer. Dose Ranging Study to Assess. Efficacy, Safety and Tolerability of PF-06700841 Topical Cream in Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
112. Pfizer. A Study to Evaluate Safety and Efficacy of PF-06826647 for Moderate to Severe Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
113. Jiangsu Hengrui Medicine Company Ltd. A Clinical Study of SHR-1314 Injection in the Treatment of Moderate to Severe Plaque Psoriasis in Adults. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
114. Squibb B-M. An Investigational Study to Evaluate Experimental Medication BMS-986165 Compared to Placebo and a Currently Available Treatment in Participants With Moderate-to-Severe Plaque Psoriasis (POETYK-PSO-2). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
115. Biocad. Clinical Study of Efficacy and Safety of BCD-085 (Monoclonal Anti-IL-17 Antibody) in Psoriatic Arthritis (PATERA). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
116. Boehringer I. The VOLTAIRE-X Trial Looks at the Effect of Switching Between Humira® and BI 695501 in Patients With Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2017.
117. Can-Fite BioPharma Ltd. CF101 Therapy in Patients With Moderate-to-Severe Plaque Psoriasis. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2017.
118. Coherus Biosciences, Inc. Comparison of CHS-1420 Versus Humira in Subjects With Chronic Plaque Psoriasis (Psosim). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
119. Gilead Sciences. Study to Evaluate the Efficacy and Safety of Filgotinib in Participants With Active Psoriatic Arthritis Who are Naive to Biologic DMARD Therapy (PENGUIN 1). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
120. Pradhan M, Alexander A, Singh MR, Singh D, Saraf S, Saraf S. Understanding the prospective of nanoformulations towards the treatment of psoriasis. Biomed Pharmacother. 2018;107:447–463. doi:10.1016/j.biopha.2018.07.156
121. Menlo Therapeutics Inc. Study of the Long Term Safety of Serlopitant for the Treatment of Pruritus (Itch). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
122. Dermavant Sciences GmbH. Long Term Extension Study of Tapinarof for Plaque Psoriasis in Adults. Silver Spring, MD, USA: U.S. Food and Drug Administration; 2019.
123. Sun Pharma Global Fze. A Study to Evaluate the Efficacy and Safety/Tolerability of Subcutaneous Tildrakizumab (SCH 900222/MK-3222) in Participants With Moderate-to-Severe Chronic Plaque Psoriasis Followed by a Long-Term Extension Study (MK-3222-011) (Resurface 2). Silver Spring, MD, USA: U.S. Food and Drug Administration; 2018.
124. AbbVie. A Study Comparing Upadacitinib (ABT-494) to Placebo in Participants With Active Psoriatic Arthritis Who Have a History of Inadequate Response to at Least One Biologic Disease Modifying Anti-Rheumatic Drug (SELECT—PsA 2). MD, USA: U.S. Food and Drug Administration: Silver Spring; 2017.
125. Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:3702518. doi:10.1155/2019/3702518
126. Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: formulation optimization and in vitro and in vivo skin distribution studies. J Control Release. 2011;149:159–167. doi:10.1016/j.jconrel.2010.10.005
127. Diering. Polymeric nanospheres for topical delivery of vitamin D3. Physiol Behav. 2018;176:139–148.
128. Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385:113–142. doi:10.1016/j.ijpharm.2009.10.018
129. Barbosa TC, Nascimento LED, Bani C, et al. Development, cytotoxicity and eye irritation profile of a new sunscreen formulation based on benzophenone-3-poly(epsilon-caprolactone) nanocapsules. Toxics. 2019;7:51. doi:10.3390/toxics7040051
130. Marchiori ML, Lubini G, Dalla Nora G, et al. Hydrogel containing dexamethasone-loaded nanocapsules for cutaneous administration: preparation, characterization, and in vitro drug release study. Drug Dev Ind Pharm. 2010;36:962–971. doi:10.3109/03639041003598960
131. Mignani S, El Kazzouli S, Bousmina M, Majoral JP. Expand classical drug administration ways by emerging routes using dendrimer drug delivery systems: a concise overview. Adv Drug Deliv Rev. 2013;65:1316–1330. doi:10.1016/j.addr.2013.01.001
132. Sikwal DR, Kalhapure RS, Govender T. An emerging class of amphiphilic dendrimers for pharmaceutical and biomedical applications: janus amphiphilic dendrimers. Eur J Pharm Sci. 2017;97:113–134. doi:10.1016/j.ejps.2016.11.013
133. Agrawal U, Mehra NK, Gupta U, Jain NK. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J Drug Target. 2013;21:497–506. doi:10.3109/1061186X.2013.771778
134. Damiani G, Pacifico A, Linder DM, et al. Nanodermatology-based solutions for psoriasis: state-of-the art and future prospects. Dermatol Ther. 2019;32:1–15. doi:10.1111/dth.13113
135. Lapteva M, Mondon K, Möller M, Gurny R, Kalia YN. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11:2989–3001. doi:10.1021/mp400639e
136. Teixeira MC, Carbone C, Souto EB. Beyond liposomes: recent advances on lipid based nanostructures for poorly soluble/poorly permeable drug delivery. Prog Lipid Res. 2017;68:1–11. doi:10.1016/j.plipres.2017.07.001
137. Doktorovova S, Kovacevic AB, Garcia ML, Souto EB. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–252. doi:10.1016/j.ejpb.2016.08.001
138. Clares B, Calpena AC, Parra A, et al. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: effect on skin permeation. Int J Pharm. 2014;473:591–598. doi:10.1016/j.ijpharm.2014.08.001
139. Wadhwa S, Singh B, Sharma G, Raza K, Katare OP. Liposomal fusidic acid as a potential delivery system: a new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016;23:1204–1213. doi:10.3109/10717544.2015.1110845
140. Hua S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front Pharmacol. 2015;6:2011–2015. doi:10.3389/fphar.2015.00219
141. Jain A, Doppalapudi S, Domb AJ, Khan W. Tacrolimus and curcumin co-loaded liposphere gel: synergistic combination towards management of psoriasis. J Control Release. 2016;243:132–145. doi:10.1016/j.jconrel.2016.10.004
142. Ainbinder D, Paolino D, Fresta M, Touitou E. Drug delivery applications with ethosomes. J Biomed Nanotechnol. 2010;6:558–568. doi:10.1166/jbn.2010.1152
143. Zhang YT, Shen LN, Zhao JH, Feng NP. Evaluation of psoralen ethosomes for topical delivery in rats by using in vivo microdialysis. Int J Nanomed. 2014;9:669–678. doi:10.2147/IJN.S57314
144. Mueller RH, Mehnert W, Souto EB. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers for dermal delivery. Adv Drug Deliv Rev. 2006;37.
145. Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59:478–490. doi:10.1016/j.addr.2007.04.007
146. Teeranachaideekul V, Souto EB, Muller RH, Junyaprasert VB. Physicochemical characterization and in vitro release studies of ascorbyl palmitate-loaded semi-solid nanostructured lipid carriers (NLC gels). J Microencapsul. 2008;25:111–120. doi:10.1080/02652040701817762
147. Fernandes AR, Martins-Gomes C, Santini A, Silva AM, Souto EB. Chapter 9—psoriasis vulgaris—pathophysiology of the disease and its classical treatment versus new drug delivery systems. In: Grumezescu AM, editor. Design of Nanostructures for Versatile Therapeutic Applications. Norwich, NY, USA: William Andrew Publishing; 2018:379–406.
148. Pradhan M, Singh D, Singh MR. Development characterization and skin permeating potential of lipid based novel delivery system for topical treatment of psoriasis. Chem. Phys. Lipids. 2015;186:9–16. doi:10.1016/j.chemphyslip.2014.11.004
149. Shimojo AAM, Fernandes ARV, Ferreira NRE, Sanchez-Lopez E, Santana MHA, Souto EB. Evaluation of the influence of process parameters on the properties of resveratrol-loaded NLC using 2(2) full factorial design. Antioxidants. 2019;8:272. doi:10.3390/antiox8080272
150. Souto EB, Doktorovova S. Chapter 6—solid lipid nanoparticle formulations pharmacokinetic and biopharmaceutical aspects in drug delivery. Methods Enzym. 2009;464:105–129.
151. Shrotriya SN, Ranpise NS, Vidhate BV. Skin targeting of resveratrol utilizing solid lipid nanoparticle-engrossed gel for chemically induced irritant contact dermatitis. Drug Delivery Trans Res. 2017;7:37–52. doi:10.1007/s13346-016-0350-7
152. Avasatthi V, Pawar H, Dora CP, Bansod P, Gill MS, Suresh S. A novel nanogel formulation of methotrexate for topical treatment of psoriasis: optimization, in vitro and in vivo evaluation. Pharm Dev Technol. 2016;21:554–562. doi:10.3109/10837450.2015.1026605
153. Donnelly RF, Raghu T, Singh R, Woolfson AD. Microneedle-based drug delivery systems: microfabrication, drug delivery, and safety. Drug Deliv. 2010;17:187–207. doi:10.3109/10717541003667798
154. Larrañeta E, Lutton REM, Woolfson AD, Donnelly RF. Microneedle arrays as transdermal and intradermal drug delivery systems: materials science, manufacture and commercial development. Mater Sci Eng R Rep. 2016;104:1–32. doi:10.1016/j.mser.2016.03.001
155. Zhao Z, Chen Y, Shi Y. Microneedles: a potential strategy in transdermal delivery and application in the management of psoriasis. RSC Adv. 2020;10:14040–14049. doi:10.1039/D0RA00735H
156. Ishihara T, Kubota T, Choi T, et al. Polymeric nanoparticles encapsulating betamethasone phosphate with different release profiles and stealthiness. Int J Pharm. 2009;375:148–154. doi:10.1016/j.ijpharm.2009.04.001
157. Ourique AF, Pohlmann AR, Guterres SS, Beck RCR. Tretinoin-loaded nanocapsules: preparation, physicochemical characterization, and photostability study. Int J Pharm. 2008;352:1–4. doi:10.1016/j.ijpharm.2007.12.035
158. Pandi P, Jain A, Kommineni N, Ionov M, Bryszewska M, Khan W. Dendrimer as a new potential carrier for topical delivery of siRNA: a comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int J Pharm. 2018;550:240–250. doi:10.1016/j.ijpharm.2018.08.024
159. Lapteva M, Santer V, Mondon K, et al. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J Control Release. 2014;196:9–18. doi:10.1016/j.jconrel.2014.09.021
160. Nagle A, Goyal AK, Kesarla R, et al. Efficacy study of vesicular gel containing methotrexate and menthol combination on parakeratotic rat skin model Efficacy study of vesicular gel containing methotrexate and menthol combination on parakeratotic rat skin model. J Liposome Res. 2011;2011:2104.
161. Kumar R, Dogra S, Amarji B. Efficacy of novel topical liposomal formulation of cyclosporine in mild to moderate stable plaque psoriasis: a randomized clinical trial. JAMA Dermatol. 2016;152:807–815. doi:10.1001/jamadermatol.2016.0859
162. Østergaard N, Jorgensen L, Hansen J, Vermehren C, Frokjaer S, Foged C. Targeting of liposome-associated calcipotriol to the skin: effect of liposomal membrane fluidity and skin barrier integrity. Int J Pharm. 2011;416:478–485. doi:10.1016/j.ijpharm.2011.03.014
163. Chen M, Kumar S, Anselmo AC, et al. Topical delivery of cyclosporine A into the skin using SPACE-peptide. J Control Release. 2015;199:190–197. doi:10.1016/j.jconrel.2014.11.015
164. Li G, Fan Y, Fan C, et al. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82:49–57. doi:10.1016/j.ejpb.2012.05.011
165. Agrawal U, Gupta M, Vyas SP. Capsaicin delivery into the skin with lipidic nanoparticles for the treatment of psoriasis. Artif Cells Nanomed Biotechnol. 2015;43:33–39. doi:10.3109/21691401.2013.832683
166. Sonawane R, Harde H, Katariya M, et al. Solid lipid nanoparticlesloaded topical gel containing combination drugs: an approach to offset psoriasis Solid lipid nanoparticles-loaded topical gel containing combination drugs: an approach to offset psoriasis. Expert Opin Drug Deliv. 2014;2014:5247.
167. Doktorovová S, Araújo J, Garcia ML, Rakovský E, Souto EB. Formulating fluticasone propionate in novel PEG-containing nanostructured lipid carriers (PEG-NLC). Colloids Surf B Biointerfaces. 2010;75:538–542. doi:10.1016/j.colsurfb.2009.09.033
168. Lin Y-K. Combination of calcipotriol and methotrexate in nanostructured lipid carriers for topical delivery. Int J Nanomed. 2010;5:117–128.
169. Tekko IA, Permana AD, Vora L, Hatahet T, McCarthy HO, Donnelly RF. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: potential for enhanced treatment of psoriasis. Eur J Pharm Sci. 2020;152:105469. doi:10.1016/j.ejps.2020.105469.
170. Lee JH, Jung YS, Kim GM, Bae JM. A hyaluronic acid-based microneedle patch to treat psoriatic plaques: a pilot open trial. Br J Dermatol. 2018;178:e24–e25. doi:10.1111/bjd.15779
171. Merlo G, Cozzani E, Burlando M, et al. Effects of TNFα inhibitors in patients with psoriasis and metabolic syndrome: a preliminary study. G Ital Dermatol Venereol. 2020;155(1):14–18. doi:10.23736/S0392-0488.17.05621-8
172. Marcianò I, Randazzo MP, Panagia P, et al. Real-world use of biological drugs in patients with psoriasis/psoriatic arthritis: a retrospective, population-based study of years 2010-2014 from Southern Italy. G Ital Dermatol Venereol. 2020;155(4):441–451. doi:10.23736/S0392-0488.18.05753-X
173. Bednarski IA, Lesiak A, Salińska M, Woźniacka A, Narbutt J. Serum levels of tumor necrosis factor-alpha in patients with psoriasis before, during and after narrow-band UVB phototherapy: a reply. G Ital Dermatol Venereol. 2020;155(2):249–250. doi:10.23736/S0392-0488.18.05996-5
174. Cannizzaro MV, Marchetti E, Babino G, et al. Association between psoriasis and periodontitis, and efficacy of anti-TNF-α therapy. G Ital Dermatol Venereol. 2020;155(4):508–511. doi:10.23736/S0392-0488.18.06045-5
175. Goyal A, Dey AK, Chaturvedi A, et al. Chronic stress-related neural activity associates with subclinical cardiovascular disease in psoriasis: a prospective cohort study. JACC Cardiovasc Imaging. 2020;13(2 Pt 1):465–477. doi:10.1016/j.jcmg.2018.08.038
176. Mazloom SE, Yan D, Hu JZ, et al. TNF-α inhibitor-induced psoriasis: a decade of experience at the Cleveland Clinic. J Am Acad Dermatol. 2020;83(6):1590–1598. doi:10.1016/j.jaad.2018.12.018
177. Odorici G, Paganelli A, Peccerillo F, et al. Moderate to severe psoriasis: a single-center analysis of gender prevalence. Ital J Dermatol Venerol. 2021;156(2):226–230. doi:10.23736/S2784-8671.18.06200-4
178. Pithadia DJ, Reynolds KA, Lee EB, Wu JJ. Psoriasis-associated itch: etiology, assessment, impact, and management. J DermatolTreat. 2020;31(1):18–26. doi:10.1080/09546634.2019.1572865
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Microneedle Patch Delivery of Methotrexate-Loaded Albumin Nanoparticles to Immune Cells Achieves a Potent Antipsoriatic Effect
Wang H, Zhao Z, Wu C, Tong X, Shi Y, Chen S
International Journal of Nanomedicine 2022, 17:3841-3851
Published Date: 1 September 2022





