Back to Journals » Journal of Pain Research » Volume 18
Quantitative Analysis of the Minimum Clinically Important Difference in the Brief Pain Inventory After Total Knee Arthroplasty
Authors Wang S , Yao S, Xiao P , Shang L, Xu C, Ma J
Received 16 October 2024
Accepted for publication 10 February 2025
Published 20 February 2025 Volume 2025:18 Pages 803—813
DOI https://doi.org/10.2147/JPR.S501219
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Shunxing Wang,1,2,* Shuxin Yao,1,* Peng Xiao,3,* Lei Shang,4 Chao Xu,1,4,* Jianbing Ma1,*
1Department of Knee Joint Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’an, People’s Republic of China; 2Xi’an Medical University, Xi ‘An, People’s Republic of China; 3Department of Orthopedics (International Ward), Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, People’s Republic of China; 4Department of Health Statistics, Faculty of Preventive Medicine, the Air Force Military Medical University, Xi’an, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianbing Ma, Department of Knee Joint Surgery, Honghui Hospital, Xi’an Jiaotong University, No. 555 E.Youyi Road, Xi’an, 710061, People’s Republic of China, Email [email protected]
Purpose: Research on the Minimum Clinically Important Difference (MCID) of the Brief Pain Inventory (BPI) following Total Knee Arthroplasty (TKA) is limited. This study addresses this gap by determining the MCID of the BPI for patients undergoing primary TKA.
Patients and Methods: This study was designed to quantitatively determine the MCID of the BPI for patients undergoing primary TKA. A prospective cohort of 288 patients was evaluated, with the BPI administered both preoperatively and at a one-year follow-up. The MCID was calculated using two primary approaches: the anchor-based method, considered the gold standard, and the distribution-based method. Additionally, this study explored various calculation approaches within the distribution-based framework, benchmarking them against the anchor-based method. The distribution-based methods included calculations based on Standard Deviation (SD), Effect Size (ES), Standardized Response Mean (SRM), and Standard Error of Measurement (SEM). All statistical calculations were performed using established formulas.
Results: The anchor-based method determined the MCID for BPI pain severity to be 3.9 points, the pain interference dimension to be 5.8 points, and the total score to be 9.7 points. Comparisons with the gold standard highlighted the 0.65ES, 1.96SEM, and 0.5SRM methods as the most suitable approaches for distribution-based MCID estimation.
Conclusion: The MCID for BPI in TKA patients was established using both anchor-based and distribution-based methods. When anchor-based determination is impractical, the distribution-based methods— 0.65ES, 1.96SEM, and 0.5SRM—are recommended for MCID calculation.
Plain Language Summary: Understanding whether a treatment makes a meaningful difference to patients is crucial. This study focuses on determining the Minimum Clinically Important Difference (MCID) of the Brief Pain Inventory (BPI) for individuals undergoing Total Knee Arthroplasty (TKA). The MCID helps identify the smallest improvement in pain that patients perceive as beneficial and significant enough to influence their care. By analyzing BPI scores before and one year after surgery, the study used two methods: anchor-based (considered the gold standard) and distribution-based approaches. Various calculation techniques within the distribution-based method were compared to the anchor-based method. The results showed that when the anchor-based method is not feasible, specific distribution-based methods, such as 0.65ES, 1.96SEM, and 0.5SRM, can reliably estimate the MCID. These findings provide clinicians with clear benchmarks for interpreting pain outcomes, ensuring a patient-centered approach to post-TKA care.
Keywords: brief pain inventory, total knee arthroplasty, minimal clinically important difference, patient-related outcome, quality of life
Introduction
Total Knee Arthroplasty (TKA) is a highly effective surgical intervention widely used to treat advanced-stage knee osteoarthritis (OA).1 Each year, approximately 689,000 TKA procedures are performed in China.2 The Brief Pain Inventory (BPI) has been validated as a reliable tool for assessing the impact of knee OA and post-TKA interventions.3,4 Physical activity directly influences the central nervous system, modulating both pain perception and cognitive processing.5 This effect is especially significant in patients with knee OA, as mobility and walking are critical to their daily functioning and recovery process. The BPI focuses on pain severity and pain interference,6 addressing core symptoms of knee OA.
Patient-reported outcome measures (PROM) capture patients’ subjective experiences of their disease, including physical symptoms and emotional distress.7 In the interpretation of PROMs, it is crucial to distinguish between statistical significance and clinical relevance. While statistically significant changes in PROM scores may indicate a difference, they do not necessarily translate into meaningful clinical improvements for patients.8 For this reason, various metrics of clinical significance have been developed, including the Minimum Clinically Important Difference (MCID). The concept of MCID, introduced by Jaeschke et al in 1989, defines MCID as the smallest score difference perceived by patients as beneficial. This difference should prompt changes in patient management without causing burdensome side effects or excessive costs.9 MCID has since gained widespread acceptance and is now strongly recommended for use in clinical practice and research decision-making.10
In clinical practice, MCID is a valuable tool for evaluating the significance of changes in patients’ conditions before and after treatment.11 It helps healthcare professionals assess the clinical importance of observed changes in patient outcomes. In research, MCID is critical for estimating sample sizes in planning randomized clinical trials and interpreting changes in PROM.11
The applications of MCID are highly significant, yet research on the MCID of the BPI in patients following TKA remains limited. While studies have examined its MCID in conditions like low back pain and its MCID in advanced cancer with painful bone metastases,12,13 the MCID of the BPI in surgical populations, particularly in patients undergoing TKA, remains unexplored. Furthermore, our study utilizes a version of the BPI that has been culturally adapted and validated for use in TKA patients, distinguishing it from the original English version employed in previous research. To address this gap, our study aims to establish the MCID for the BPI in TKA patients. For patients who fail to achieve the MCID, orthopedic manual therapy has been shown to be an effective intervention for alleviating pain sensitization associated with chronic musculoskeletal pain, providing an additional therapeutic option for these individuals.14
Calculating MCID involves multiple approaches, but consensus on the optimal method is yet to be established. Currently, MCID calculation methods are broadly divided into anchor-based and distribution-based approaches.15 The anchor-based method evaluates the difference in average BPI score changes between patients reporting no improvement and those reporting slight improvement, based on an anchor question. In contrast, the distribution-based method relies on statistical indicators of score changes across the population. The absence of a standardized calculation method creates challenges in accurately capturing true patient changes due to the variability in approaches. Among these methods, the anchor-based approach is the most frequently reported and widely accepted for determining MCID.16
This study primarily aims to determine the MCID of the total BPI score and its components one year after TKA using both anchor-based and distribution-based methods. Additionally, it seeks to explore various calculation approaches within the distribution-based framework, benchmarking them against the anchor-based method as the gold standard. These findings aim to offer valuable insights and serve as a reference for applying distribution-based methods to determine MCID in future research.
Methods
Patients
Patients were recruited from a large orthopedic treatment center in Central China between January 10, 2022, and January 10, 2024. The inclusion criteria specified a diagnosis of primary knee OA and receipt of unilateral TKA. Exclusion criteria eliminated patients under the age of 50, those with post-traumatic osteoarthritis, inflammatory arthritis, a history of previous double knee surgery (except for meniscectomy), those undergoing revision TKA during the study period, and patients requiring a second TKA within the same period. Excluding patients under 50 years old mitigated potential bias in postoperative expectations, as this demographic represents a small proportion of TKA cases.17
The study adopted a one-year follow-up period after TKA surgery, informed by findings from a comprehensive study involving 27,000 TKA cases. This study demonstrated that patient satisfaction for uncorrected cases remained “significantly unchanged” one year post-surgery, with no notable fluctuations over time.18 Moreover, most patients undergoing TKA experience steady improvements in pain and functional outcomes within the first year, achieving near-complete resolution of pain and functional impairment by the end of this period.19
A STROBE-compliant flow diagram was utilized to illustrate the patient selection process, exclusions, and follow-up, aligning with the observational design of this study (Figure 1).
 |
Figure 1 STROBE flow diagram demonstrates the enrollment of patients, exclusion, and loss to follow-up for the study cohort. |
Two professional investigators underwent comprehensive training prior to the formal survey. This training covered the objectives and significance of the study, inclusion and exclusion criteria, and detailed instructions for administering the questionnaire. Before initiating the survey, the investigators provided a thorough explanation of the study’s objectives and guidelines for completing the scales to the patients. Participants were encouraged to seek clarification on any aspects they found unclear to ensure accurate understanding and responses. Subsequently, eligible patients participated in face-to-face surveys using electronic questionnaires, which were automatically coded upon completion.
A posterior-stabilized, fully cemented total knee prosthesis (Legion, Smith & Nephew, Memphis, Tennessee, USA) was uniformly used in all cases.
Over the study period, 288 patients met the inclusion criteria and completed preoperative and postoperative evaluations. This observational study did not constitute an interventional trial and was therefore exempt from registration in a clinical trial registry. Ethical approval for the study was obtained from the Medical Ethics Committee of Honghui Hospital (No. 202201017). All participants provided informed consent in accordance with the Declaration of Helsinki. The minimum sample size required to determine MCID followed the recommendations of the Consensus-based Standards for the selection of health Measurement Instruments (COSMIN). COSMIN guidelines define a sample size exceeding 100 as excellent.20 This robust sample size strengthens the reliability and generalizability of the study’s findings.
Prom
The BPI is a self-reported questionnaire originally developed for cancer pain assessment, but it has since become a widely used tool for evaluating chronic pain in various conditions. This study uses the Chinese version of the BPI Short Form questionnaire, which has been validated for the Chinese knee OA population, as the primary assessment tool.3 The BPI questionnaire consists of two key components: the pain severity score and the pain interference score. Both scores use a numerical rating scale ranging from 0 to 10. The pain severity score is based on four items assessing the “worst”, “least”, and “average” pain experienced in the past 24 hours, as well as current pain. Ratings range from 0 (no pain) to 10 (worst pain imaginable). The pain interference score is derived from seven sub-items evaluating the impact of pain on “life enjoyment”, “general activities”, “walking ability”, “emotions”, “sleep”, “normal work”, and “relationships with others.” Each sub-item is scored from 0 (no interference) to 10 (complete interference). The total BPI score is calculated by combining these two components.21 This comprehensive evaluation captures both the severity of pain and its impact on various aspects of patients’ lives.
Gold Standard by Anchor‑based Method
Given that pain is the primary symptom of knee OA and profoundly affects patients’ quality of life, we evaluated improvements in quality of life by asking patients, “To what extent has knee arthroplasty improved your quality of life?” Respondents indicated their answers on a Likert scale with five options: “much better”, “better”, “about the same”, “slightly worse”, and “much worse.” This question served as an anchor for determining the Minimum Clinically Important Effect, using a well-established technique.15
To improve precision, we excluded responses of “much better” or “much worse” from the analysis, as these outcomes represented extreme values.22 The remaining responses were grouped into the “improvement group” (those who answered “better”) and the “no improvement group” (those who answered “about the same” or “slightly worse”). Within each group, the average difference between pre-operative and post-operative BPI scores was calculated. The MCID of BPI scores was then determined by comparing the average score changes between the “improvement group” and the “no improvement group.” This approach offers a robust framework for calculating the MCID in BPI scores, using the perceived improvement in quality of life as a key anchor point.
Distribution‑based methods
The distribution-based method for calculating MCID relies on statistical parameters of the data, providing a clear and straightforward formula. This method incorporates four approaches—Standard Deviation (SD), Effect Size (ES), Standardized Response Mean (SRM), and Standard Error of Measurement (SEM)—to determine MCID, with specific calculation methods detailed in Table 1. For the ES and SRM methods, differences of 0.2, 0.5, and 0.8 are categorized as low, medium, and high, respectively.23 In the SEM method, differences of 1, 1.96, and 2.77 are classified as low, medium, and high, respectively.24
 |
Table 1 Calculation Formulas for Distribution-Based Methods |
Statistical analyses were performed using Statistical Product and Service Solutions (SPSS) 24.0 software (IBM Corp, New York). A significance level of p < 0.05 was adopted.
Results
In this study, a total of 288 patients were included, with an average age of 65.0 years. The detailed demographic and clinical characteristics of the participants are presented in Table 2.
 |
Table 2 Baseline Characteristics of the Sample |
After excluding patients who reported “much better” or “much worse” in response to the anchoring question, the remaining 144 patients were divided into the “improvement” group (n=85) and the “no improvement” group (n=59) based on their responses at the 1-year follow-up. The changes in BPI scores for patients with different responses to the anchoring question are shown in Table 3.
 |
Table 3 Classification of Quality of Life Improvement After TKA for 1 year |
MCID Calculated by Anchor‑based Method
The changes in BPI scores between the improvement and non-improvement groups are summarized in Table 4. In the improvement group, the average change in BPI pain severity was 18.9 (6.2), compared to 15.0 (6.7) in the non-improvement group. Based on these values, the MCID for BPI pain severity, determined using the anchor method, was calculated as 3.9.
 |
Table 4 MCID of the BPI Determined by Anchor-Based Method (n = 144) |
Similarly, the average change in BPI pain interference was 34.9 (9.8) in the improvement group and 29.1 (9.3) in the non-improvement group, establishing an MCID of 5.8 for the pain interference dimension. Finally, the average change in the total BPI score was 53.8 (13.0) in the improvement group and 44.1 (12.9) in the non-improvement group, resulting in an MCID of 9.7 for the total BPI score using the anchor method.
MCID Calculated by Distribution‑based Methods
The results were calculated using various formulas outlined in Table 1. Using the SD method, the MCID values for pain severity, pain interference, and total BPI scores were 2.9, 5.1, and 7.3, respectively. For an ES of 0.2, the MCID values for the two dimensions and total BPI score were 1.2, 2.0, and 2.9, respectively. With an ES of 0.5, the MCID values increased to 3.0, 5.1, and 7.3, while an ES of 0.8 resulted in MCID values of 4.7, 8.2, and 11.7.
Using the SRM method, for an SRM of 0.2, the MCID values were 1.3, 2.3, and 3.3 for pain severity, pain interference, and total BPI scores, respectively. For an SRM of 0.5, the MCID values rose to 3.4, 5.8, and 8.3, while an SRM of 0.8 yielded values of 5.4, 9.3, and 13.2.
The Cronbach alpha coefficients for pain severity, pain interference, and total BPI scores were 0.856, 0.835, and 0.885, respectively. Using the SEM method, for an SEM of 1, the MCID values were 2.2, 4.1, and 5.0. For an SEM of 1.96, the values increased to 4.4, 8.1, and 9.7, while an SEM of 2.77 produced values of 6.2, 11.5, and 13.7. Detailed results are presented in Tables 5–7.
 |
Table 5 MCID of the BPI Determined by SD Method and ES Method |
 |
Table 6 MCID of the BPI Determined by SRM Method |
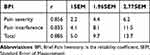 |
Table 7 MCID of the BPI Determined by SEM Method |
Comparisons of MCID by Anchor‑based and Distribution‑based Methods
The MCID of the BPI scale was calculated using various formulas in the distribution-based method and compared with the anchor-based method. Results indicated that the MCID derived from the SD method was smaller than that obtained from the anchor-based method. For the ES method, the MCID calculated using an ES of 0.5 was smaller, whereas an ES of 0.8 yielded a larger value. Notably, an ES of 0.65 produced a result consistent with the anchor-based method.
In the SRM method, the MCID calculated with an SRM of 0.2 was smaller, while an SRM of 0.8 resulted in a larger value. An SRM of 0.5 closely aligned with the anchor-based method. Similarly, in the SEM method, the MCID calculated using an SEM of 1.96 closely matched the anchor-based result.
Considering the stability of these findings, we recommend calculating MCID using the ES method with an ES of 0.65, the SEM method with an SEM of 1.96, or the SRM method with an SRM of 0.5 for each domain. These recommendations are summarized in Table 8.
 |
Table 8 MCID Calculated Based on the Anchored Method and the Distribution-Based Method |
Discussion
PROMs are rigorously validated instruments that enable patients to directly convey their health status, offering valuable insights into their lived experiences and individual perspectives. Notably, a statistically significant improvement in a PROM score does not necessarily reflect a clinically meaningful change or one that justifies therapeutic intervention. The MCID addresses this limitation by defining the smallest change in score that patients perceive as beneficial and sufficiently impactful to inform clinical decision-making, provided it is achieved without undue side effects or excessive costs. The findings of this study hold substantial clinical relevance, particularly in the assessment and management of patients undergoing TKA. By establishing the MCID for the BPI, this research delivers a practical framework for interpreting patient-reported outcomes, empowering clinicians to discern between statistical significance and patient-centered clinical relevance, thereby promoting a more individualized and meaningful approach to care.
It is crucial to acknowledge the absence of a consensus on the optimal method for calculating the MCID.10 Various approaches, including anchor-based methods, distribution-based methods, and expert opinion methods, are available, each with distinct advantages and limitations. The anchor-based method emphasizes the patient’s perspective and uses an external anchor, such as a global rating of change, to estimate the MCID. However, selecting an appropriate anchor can be challenging and may introduce memory bias.25 Conversely, the distribution-based method derives the MCID from statistical characteristics, such as SD or ES, providing a straightforward calculation framework. Nevertheless, this method may not fully reflect the patient’s experience.26 The expert opinion method, based on the insights of clinicians and experts, offers valuable professional judgment but lacks balance and is seldom applied in clinical practice.
Although there is no universally accepted method for calculating the MCID, it is widely recommended to combine distribution-based and anchor-based methods to achieve complementary results.27,28 A systematic review on MCID research revealed that nearly half of the studies employed both methods, while the anchor-based method was approximately seven times more frequently utilized than the distribution-based method when only one approach was adopted.29 Ideally, the MCID should be determined by integrating the outcomes from both distribution-based and anchor-based methods. However, if a single method must be chosen, the anchor-based method is generally preferred due to its clear clinical significance derived from the selected anchor.30 In this study, the anchor-based method is regarded as the gold standard, and various computational techniques within the distribution-based method are analyzed to provide a foundation for applying the distribution-based approach.
The anchor-based approach utilizes patient-reported changes in health status, as measured by PROMs, to define clinically meaningful differences.31 This approach relies on anchoring questions, which query patients about their perceived changes in health status following treatment. These questions serve as an external criterion for comparing changes in PROM scores. Anchoring questions typically offer a discrete set of response options, often presented as a transition grade scale.32 While the number of options can vary, odd numbers, such as five or seven, are commonly employed.28 These options typically represent a spectrum of change, such as “much better”, “better”, “about the same”, “slightly worse”, and “much worse.” Typically, the conversion level associated with a “little” improvement in the anchoring problem is used to determine the MCID of the PROM.28
The Oxford Knee Score (OKS), Knee Society Score (KSS), and Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) are commonly used outcome measures to evaluate the effectiveness of TKA surgery. Studies have reported the MCID for these scores. For the OKS, Clement et al reported an MCID of 5/48 points, which accounts for approximately 10.4% of the total possible value.33 For the KSS, Alejandro et al reported an MCID of 9/100 for the KSS knee score and 10/100 for the KSS function score.34 For the WOMAC, Nicholas et al reported an MCID of 11/100 for the WOMAC pain severity, 9/100 for the WOMAC function dimension, and 10/100 for the total WOMAC score.35 In this study, the MCID of the BPI pain severity was determined to be 3.9/40, the BPI pain interference dimension was 5.8/70, and the BPI total score was 9.7/110. These values represent 9.3%, 9.3%, and 9.2% of the possible score range, respectively. These findings are comparable to the MCID values reported for related scales such as the OKS, KSS, and WOMAC. This suggests that the BPI can be a useful tool for assessing pain severity and pain interference outcomes in patients undergoing TKA.
While MCID remains a straightforward and practical measure for assessing clinical significance in real-world settings, alternative approaches offer greater psychometric and conceptual advantages. Latent Class Analysis (LCA), for example, identifies subgroups within a population and tracks their distinct progressions over time, providing features absent in the MCID method. These include falsifiability, independence from arbitrary cut points, control over random measurement error, and an accounting for measurement levels. However, LCA does not address intervention risks or costs, which can limit its applicability in certain clinical situations. Similarly, the Smallest Worthwhile Effect (SWE) measures the minimum improvement patients consider worthwhile, incorporating factors such as treatment cost, side effects, and benefits. Despite its strengths, SWE may be susceptible to biases in the order of scale and lacks grounding in patients’ real treatment experiences, complicating its direct application in clinical practice.
While LCA and SWE present scientifically rigorous alternatives to MCID, both methods still face limitations. In our future research, we will focus on comparing these two approaches with traditional MCID calculations, providing deeper insights to inform clinical practice.
Limitations
Our study has contributed valuable insights into the clinical application of the MCID. However, there are certain limitations that need to be acknowledged. Firstly, we focused exclusively on patients with primary osteoarthritis, excluding those with other arthritis etiologies. This restriction improves the internal validity of our findings but may limit their generalizability to populations with different arthritis causes. Future studies could address this limitation by including more diverse patient groups.
Secondly, we utilized quality-of-life anchoring questions to calculate the MCID for the BPI. While these anchoring questions serve as a practical reference for identifying clinically meaningful score changes, they may not fully encompass the broader spectrum of clinical changes experienced by patients. This could introduce a bias toward outcomes more aligned with quality-of-life improvements, potentially overlooking other dimensions of patient-reported outcomes.
Potential biases related to patient recall and subjectivity in responding to anchoring questions were mitigated by ensuring comprehensive preoperative and follow-up evaluations, minimizing memory bias and promoting consistency in data collection. Furthermore, statistical analyses were rigorously performed to ensure robustness in interpreting the results.
Despite these limitations, the study provides significant clinical value by establishing the MCID for the BPI, offering a meaningful tool for clinicians to interpret patient-reported outcomes more effectively. These findings have broader implications for improving patient-centered care, particularly in tailoring interventions to address pain and functionality in patients undergoing TKA. Future research should explore the application of these findings across diverse clinical settings and populations, enhancing their utility and generalizability. Additionally, future investigations could examine the potential predictive value of pre- and postoperative blood biomarkers for assessing and managing pain outcomes in TKA patients,36 providing objective metrics to complement patient-reported measures and further optimize personalized postoperative care.
Conclusion
The main findings of this study demonstrate that the MCID for the pain severity of the BPI in knee OA was calculated to be 3.9, the MCID for the pain interference dimension was 5.8, and the MCID for the total BPI score was 9.7, using the anchor-based method. Furthermore, various distribution-based calculation methods were compared with the anchor-based method as the gold standard. The results indicated that the MCID calculated using the 0.65ES method was the closest to that derived from the anchor-based method. Additionally, the 1.96SEM method and the 0.5SRM method also yielded results that were reasonably similar to the anchor-based approach. Given that the conditions for using the anchor-based method can be relatively strict, these three distribution-based methods—ES 0.65, SEM 1.96, and SRM 0.5—serve as viable alternatives, particularly in cases where a reliable anchor point is not available or when the sample size is too small to implement the anchor-based method effectively.
Abbreviations
MCID, Minimum Clinically Important Difference; BPI, Brief Pain Inventory; TKA, Total Knee Arthroplasty; SD, Standard Deviation; ES, Effect Size; SRM, Standardized Response Mean; SEM, Standard Error of Measurement; OA, osteoarthritis; PROM, Patient-Reported Outcome Measures; COSMIN, Consensus-based Standards for the selection of health Measurement Instruments; OKS, Oxford Knee Score; KSS, Knee Society Score; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; LCA, Latent Class Analysis; SWE, Smallest Worthwhile Effect.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval and Consent to Participate
The study was approved by the Medical Ethics Committee of Honghui Hospital (202201017), and informed consent was obtained.
Funding
(CX) has received funding from Key Research and Development Program of Shaanxi Province (2023-YBSF-464) and Cultivation Project for General Project of Xi’an Health Commission (2024ms10). (LS) has received funding from National Natural Science Foundation of China (82173627).
Disclosure
Shunxing Wang, Shuxin Yao and Peng Xiao are co-first authors for this study. Chao Xu and Jianbing Ma contributed equally to this work as co-correspondence authors. The authors report no conflicts of interest in this work.
References
1. Mota RE, Tarricone R, Ciani O. et al. Determinants of demand for total Hip and knee arthroplasty: a systematic literature review. Bmc Health Serv Res. 2012;12(1):225. doi:10.1186/1472-6963-12-225
2. Wang Q, Hunter S, Lee RL, et al. Mobile rehabilitation support versus usual care in patients after total Hip or knee arthroplasty: study protocol for a randomised controlled trial. Trials. 2022;23(1):553. doi:10.1186/s13063-022-06269-x
3. Wang S, Yao S, Shang L, et al. Validation of the Chinese version of the brief pain inventory in patients with knee osteoarthritis. J Orthop Surg Res. 2023;18(1):720. doi:10.1186/s13018-023-04218-1
4. Wang S, Yao S, Wei J, et al. Psychometric properties of the brief pain inventory among patients with osteoarthritis undergoing total knee arthroplasty surgery. J Arthroplasty. 2024;39(3):672–676. doi:10.1016/j.arth.2023.08.072
5. Gonzalez-Alvarez ME, Sanchez-Romero EA, Turroni S, et al. Correlation between the altered gut microbiome and lifestyle interventions in chronic widespread pain patients: a systematic review. Medicina (Kaunas). 2023;60(1):59. doi:10.3390/medicina60010059
6. Poquet N, Lin C. The Brief Pain Inventory (BPI). J Physiother. 2016;62(1):52. doi:10.1016/j.jphys.2015.07.001
7. Frid S, Fuentes EM, Grau-Corral I, et al. Successful Integration of EN/ISO 13606-standardized extracts from a patient mobile app into an electronic health record: description of a methodology. Jmir Med Inform. 2022;10(10):e40344. doi:10.2196/40344
8. Orr MN, Klika AK, Piuzzi NS. Patient reported outcome measures: challenges in the reporting! Ann Surg Open. 2021;2(3):e070. doi:10.1097/AS9.0000000000000070
9. Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi:10.1016/0197-2456(89)90005-6
10. Copay AG, Chung AS, Eyberg B, et al. Minimum clinically important difference: current trends in the orthopaedic literature, part i: upper extremity: a systematic review. Jbjs Rev. 2018;6(9):e1. doi:10.2106/JBJS.RVW.17.00159
11. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342–1343. doi:10.1001/jama.2014.13128
12. Raman S, Ding K, Chow E, et al. Minimal clinically important differences in the EORTC QLQ-C30 and brief pain inventory in patients undergoing re-irradiation for painful bone metastases. Qual Life Res. 2018;27(4):1089–1098. doi:10.1007/s11136-017-1745-8
13. Song CY, Chen CH, Chen TW, et al. Assessment of low back pain: reliability and minimal detectable change of the brief pain inventory. Am J Occup Ther. 2022;76.
14. Martínez-Pozas O, Sánchez-Romero EA, Beltran-Alacreu H, et al. Effects of orthopedic manual therapy on pain sensitization in patients with chronic musculoskeletal pain: an umbrella review with meta-meta-analysis. Am J Phys Med Rehabil. 2023;102(10):879–885. doi:10.1097/PHM.0000000000002239
15. Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi:10.1016/S0895-4356(03)00044-1
16. Adindu EK, Singh D, Geck M, et al. Minimal clinically important difference and patient-acceptable symptom state in orthopaedic spine surgery: a review. Jbjs Rev. 2023;11.
17. Klit J, Jacobsen S, Rosenlund S, et al. Total knee arthroplasty in younger patients evaluated by alternative outcome measures. J Arthroplasty. 2014;29(5):912–917. doi:10.1016/j.arth.2013.09.035
18. Robertsson O, Dunbar M, Pehrsson T, et al. Patient satisfaction after knee arthroplasty: a report on 27,372 knees operated on between 1981 and 1995 in Sweden. Acta Orthop Scand. 2000;71(3):262–267. doi:10.1080/000164700317411852
19. Dumenci L, Perera RA, Keefe FJ, et al. Model-based pain and function outcome trajectory types for patients undergoing knee arthroplasty: a secondary analysis from a randomized clinical trial. Osteoarthritis Cartilage. 2019;27(6):878–884. doi:10.1016/j.joca.2019.01.004
20. Terwee CB, Roorda LD, Dekker J, et al. Mind the MIC: large variation among populations and methods. J Clin Epidemiol. 2010;63(5):524–534. doi:10.1016/j.jclinepi.2009.08.010
21. Stanhope J. Brief Pain Inventory review. Occup Med (Lond). 2016;66(6):496–497. doi:10.1093/occmed/kqw041
22. Revicki D, Hays RD, Cella D, et al. Recommended methods for determining responsiveness and minimally important differences for patient-reported outcomes. J Clin Epidemiol. 2008;61(2):102–109. doi:10.1016/j.jclinepi.2007.03.012
23. Mattos JL, Schlosser RJ, Mace JC, et al. Establishing the minimal clinically important difference for the questionnaire of olfactory disorders. Int Forum Allergy Rhinol. 2018;8(9):1041–1046. doi:10.1002/alr.22135
24. Rai SK, Yazdany J, Fortin PR, et al. Approaches for estimating minimal clinically important differences in systemic lupus erythematosus. Arthritis Res Ther. 2015;17(1):143. doi:10.1186/s13075-015-0658-6
25. Brigden A, Parslow RM, Gaunt D, et al. Defining the minimally clinically important difference of the SF-36 physical function subscale for paediatric CFS/ME: triangulation using three different methods. Health Qual Life Outcomes. 2018;16:202. doi:10.1186/s12955-018-1028-2
26. Su F, Allahabadi S, Bongbong DN, et al. Minimal clinically important difference, substantial clinical benefit, and patient acceptable symptom state of outcome measures relating to shoulder pathology and surgery: a systematic review. Curr Rev Musculoskelet Med. 2021;14(1):27–46. doi:10.1007/s12178-020-09684-2
27. de Vet HC, Ostelo RW, Terwee CB, et al. Minimally important change determined by a visual method integrating an anchor-based and a distribution-based approach. Qual Life Res. 2007;16(1):131–142. doi:10.1007/s11136-006-9109-9
28. King MT. A point of minimal important differe nce (MID): a critique of terminology and methods. Expert Rev Pharmacoecon Outcomes Res. 2011;11(2):171–184. doi:10.1586/erp.11.9
29. Jayadevappa R, Cook R, Chhatre S. Minimal important difference to infer changes in health-related quality of life-a systematic review. J Clin Epidemiol. 2017;89:188–198. doi:10.1016/j.jclinepi.2017.06.009
30. de Vet HC, Terwee CB, Ostelo RW, et al. Minimal changes in health status questionnaires: distinction between minimally detectable change and minimally important change. Health Qual Life Outcomes. 2006;4(1):54. doi:10.1186/1477-7525-4-54
31. Sedaghat AR. Understanding the Minimal Clinically Im portant Difference (MCID) of patient-reported outcome measures. Otolaryngol Head Neck Surg. 2019;161(4):551–560. doi:10.1177/0194599819852604
32. Guyatt GH, Norman GR, Juniper EF, et al. A critical look at transition ratings. J Clin Epidemiol. 2002;55(9):900–908. doi:10.1016/S0895-4356(02)00435-3
33. Ares O, Castellet E, Maculé F, et al. (2013) Translation and validation of ‘The Knee Society Clinical Rating System’ into Spanish. Knee Surg Sports Traumatol Arthrosc. 2006;21(11):2618–2624. doi:10.1007/s00167-013-2412-4
34. Lizaur-Utrilla A, Gonzalez-Parreño S, Martinez-Mendez D, et al. Minimal clinically important differences and substantial clinical benefits for Knee Society Scores. Knee Surg Sports Traumatol Arthrosc. 2020;28(5):1473–1478. doi:10.1007/s00167-019-05543-x
35. Clement ND, Bardgett M, Weir D, et al. What is the minimum clinically important difference for the WOMAC Index After TKA? Clin Orthop Relat Res. 2018;476(10):2005–2014. doi:10.1097/CORR.0000000000000444
36. Sánchez-Romero EA, Battaglino A, Campanella W, et al. Impact on blood tests of lower limb joint replacement for the treatment of osteoarthritis: hip and knee[J]. Top Geriatric Rehabil. 2021;37(4):227–229. doi:10.1097/TGR.0000000000000337
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

