Back to Journals » Cancer Management and Research » Volume 17
Radiation-Induced Lymphopenia Prognosis and Risk Factors in Postmastectomy Radiotherapy Patients
Authors Ni W , Wang X, Wang Q, Ge Y, Mu X
Received 11 March 2025
Accepted for publication 16 May 2025
Published 28 May 2025 Volume 2025:17 Pages 1047—1058
DOI https://doi.org/10.2147/CMAR.S522807
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Bilikere Dwarakanath
Wenjie Ni, Xiunan Wang, Qin Wang, Yongqing Ge, Xiaofeng Mu
Department of Radiation Oncology, Beijing Shijitan Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Xiaofeng Mu, Email [email protected]
Objective: To investigate the effects of radiation-induced lymphopenia (RIL) on survival in postmastectomy radiotherapy (RT) patients and identify relevant RIL predictive factors.
Methods: Patients with breast cancer who received postmastectomy radiotherapy at the study hospital were enrolled over June 2016 to December 2022. The peripheral blood counts were obtained before and during treatment and at the first posttreatment follow-up. Lymphopenia was graded according to the degree of lymphocyte reduction. The Kaplan–Meier method was used to compare disease-free survival (DFS) and overall survival (OS) between grade 0– 2 (G0-2) and grade 3 (G3) lymphopenia, and the Log rank test was used to compare between-group differences. DFS prognostic factors were determined through Cox regression analysis, and G3 lymphopenia predictive factors were assessed through logistic regression analysis.
Results: 156 patients with a median RT duration of 5.0 weeks were enrolled. During treatment, 29 (18.6%), 36 (23.1%), 67 (42.9%), and 24 (15.4%) patients had G0, G1, G2, and G3 lymphopenia, respectively. Over RT duration, the absolute lymphocyte counts continued to decrease until they reached the nadir at week 5. The median follow-up duration was 45.5 months. The 1, 3-, and 5-year DFS rates were 97.0%, 90.3%, and 87.4% in the G0-2 group, respectively; they were higher than those in the G3 group (83.3%, 69.2%, and 39.5%, respectively; p < 0.001). Cox univariate and multivariate analyses revealed that pathological stage and lymphopenia degree were independent prognostic factors for DFS (both p < 0.001). Logistic regression analysis revealed that low body mass index (BMI), integrated RT, and high heart (Dmean ≥ 6 Gy) and sternum (Dmean ≥ 20Gy) exposure dose were associated with G3 lymphopenia (all p < 0.05).
Conclusion: G3 RIL led to poor DFS in postmastectomy radiotherapy patients. BMI, RT modality, and heart and sternum exposure dose were noted to be independent RIL risk factors.
Keywords: breast neoplasm, postoperative radiotherapy, lymphopenia, prognosis
Introduction
Breast cancer, the leading cancer in women and the second most common cancer globally,1 is typically treated through a multidisciplinary comprehensive methodology. Radiation therapy is an essential component of the treatment of breast cancer. Postmastectomy radiotherapy (RT) can reduce both local regional recurrence rate and breast cancer mortality in women with positive lymph nodes.2 Also, meta-analysis demonstrates that postoperative RT after breast-conserving surgery enhances local control rates and decreases breast cancer-specific mortality.3,4 Therefore, RT is the standard treatment after breast-conserving surgery and high-risk patients received mastectomy.5,6 However, it is reported that radiation may suppress host immunity, manifesting as lymphopenia.7,8 It is generally considered that lymphocytes are considered the most radiosensitive cells in humans, the counts of which decrease on the first post-RT day.9 Therefore, severe radiation-induced lymphopenia (RIL) often occurs during cancer treatment involving RT.10
A meta-analysis revealed that severe RIL can reduce survival rates associated with several solid tumors.11 According to recent studies, patients with G4 RIL during radical or postoperative adjuvant chemoradiotherapy of esophageal cancer have a poor OS.12,13 Another study on definitive concurrent chemoradiotherapy (CCRT) for esophageal cancer demonstrated that the occurrence of G4 RIL during radiotherapy was an independent prognostic factor for impaired OS rates, and this adverse prognosis persists even when lymphocyte counts return to normal or near-normal levels after treatment completion14. Kobzeva et al reported that the absolute levels of B-, T- and natural killer cells significantly reduced after RT regardless of whether the patients previously underwent chemotherapy courses in breast cancer.15 However, only a few studies have explored the relationship between RIL and survival in patients with breast cancer. The results of a recent post hoc analysis on 598 patients from a Phase III randomized clinical trial demonstrated significantly inferior 5-year disease-free survival (DFS) in breast cancer patients with a nadir-peripheral lymphocyte count (PLC)/pre-PLC ratio < 0.8 treated with mastectomy followed by adjuvant RT.16 However, the risk factors for RIL in patients with breast cancer remain unknown. Therefore, here, we investigated the relationship between RIL and survival rate in postmastectomy RT patients, as well as the related risk factors, specifically treatment-related risk factors such as radiation dosimetric factors and RT modalities.
Materials and Methods
Patients
Breast cancer patients who received adjuvant RT after mastectomy at Beijing Shijitan Hospital Affiliated with Capital Medical University over June 2016 to December 2022 were enrolled. Peripheral blood counts were obtained before and during treatment and at the first posttreatment follow-up. We excluded patients who lacked complete blood count data or who paused RT for >3 consecutive days due to personal reasons during the RT period (Figure 1).
 |
Figure 1 Study flowchart. |
All patients received postmastectomy RT, with the clinical target volume including the supraclavicular area and chest wall, to which varied RT techniques were applied. The patients received either hybrid RT [involving three-dimensional conformal RT (CRT) or intensity-modulated RT (IMRT) to the supraclavicular area and two-dimensional (2D) electron-beam RT to the chest wall; Figure 2A] or integrated RT [involving IMRT or volume intensity-modulated arc RT (VMAT) to both the supraclavicular area and chest wall; Figure 2B]. Moreover, 50-Gy doses were delivered in 25 fractions.
 |
Figure 2 (A) Hybrid RT. (B) Integrated RT. |
Laboratory Data
The absolute lymphocyte counts (ALCs) of the included patients at different time points were collected. In particular, we included the ALCs at baseline (pre-ALC; within 1 month before RT), during RT (once a week during RT), and within 3 months after treatment (Figure 3). Lymphopenia was graded according to the Common Terminology Criteria for Adverse Events (version 4.03). The nadir ALC during the RT course was used to classify lymphopenia degrees: ALC ≥ 1.0 × 109/L, grade 0 (G0); ALC = (0.8–1.0) × 109/L, grade 1 (G1); ALC = (0.5–0.8) × 109/L, grade 2 (G2); ALC = (0.2–0.5) × 109/L, grade 3 (G3); and ALC < 0.2 × 109/L, grade 4 (G4).
 |
Figure 3 The typical time schedule of the RT and blood sample collection. |
Dose–Volume Parameters
The sternum was contoured with other organs at-risk (Figure 1). Moreover, the relative volume of normal tissues at risk of receiving x Gy (Vx) and mean dose (Dmean) were calculated from the dose–volume histogram.
Follow-up
After treatment, all patients were followed up every 3 months for the first 2 years, every 6 months for the next 2 years, and once a year thereafter. Recurrence was confirmed through diagnostic imaging or histopathology. Chest wall recurrence was considered local recurrence. Moreover, recurrence at axillary lymph nodes, internal mammary lymph nodes, and supraclavicular lymph nodes was considered recurrence at regional lymph nodes. The spread of a tumor to distant organs or nonregional lymph nodes was considered to indicate distant metastasis.
Statistical Analysis
DFS was defined as the period from the RT end date to the date of the first recurrence or death due to any cause or censorship. Overall survival (OS) was defined as the interval from the RT end date to death due to any cause or censorship. The Kaplan–Meier method was used to calculate DFS and OS, and the log-rank was used to determine the significance of between-group differences. Logistic regression analysis was used to identify the factors associated with G3 lymphopenia. Cox multivariate regression analysis was used to identify risk factors affecting DFS. Receiver operating characteristic (ROC) curves were used to determine the thresholds for G3 lymphopenia prevention.
All statistical analyses were performed on SPSS (version 23.0; IBM, Armonk, NY, USA). A two-tailed p value of <0.05 was considered to denote statistical significance.
Results
Patient Characteristics
We recruited a total of 156 breast cancer patients who received a mastectomy; of them, 59 patients received neoadjuvant chemotherapy, 107 patients received hybrid RT (15 IMRT vs 92 CRT cases in the supraclavicular area), and 49 patients received integrated RT (11 IMRT vs 38 VMAT cases). A total of 153 patients completed the prescribed dose of 50 Gy, while 3 patients received a reduced dose of 48 Gy (due to personal reasons resulting in incomplete treatment). Table 1 presents the characteristics of patients with varied degrees of lymphopenia. In total, 132 and 24 patients were assigned to the G0-2 and G3 groups, respectively. The ALCs before treatment were comparable between the G0-2 and G3 groups [(1.76 ± 0.56) × 109/L vs (1.62 ± 0.56) ×109/L; p = 0.293]. Moreover, 29 (18.6%), 36 (23.1%), 67 (42.9%), and 24 (15.4%) patients developed G0, G1, G2, and G3 lymphopenia during their RT course, respectively.
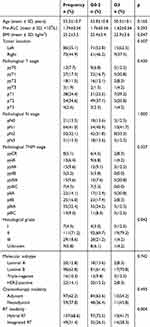 |
Table 1 Characteristics of Patients with Different Degrees of Lymphopenia |
ALC Changes
The median RT duration was 5.0 weeks. The ALC decreased gradually during treatment and reached the nadir in week 5 (Figure 4). In all patients, the ALC before RT was (1.74 ± 0.56) ×109/L. It reduced to (1.35 ± 0.41) × 109/L, (1.09 ± 0.33) × 109/L, (0.92 ± 0.29) × 109/L, (0.77 ± 0.27) × 109/L, and (0.72 ± 0.28) × 109/L after 1, 2, 3, 4, and 5 weeks of RT, respectively. Within 3 months after RT, the ALC was (1.20 ± 0.40) × 109/L.
 |
Figure 4 ALC distributions before, during, and after treatment. |
Lymphopenia–Survival Correlation
The date of the final follow-up was July 8, 2024, and the median follow-up duration was 45.5 months. Two G0-2 group patients died of cancer progression. The median OS was not achieved in the G0-2 and G3 groups. The 1-, 3-, and 5-year OS rates in the G0-2 group were 100%, 99.2%, and 98.3%, respectively. Moreover, the 1-, 3-, and 5-year OS rates in the G3 group were all 100% (p = 0.531). The median DFS in the G3 group was 54.0 months; however, this value was not obtained for the G0-2 group. The 1-, 3-, and 5-year DFS rates in the G3 group were 83.3%, 69.2%, and 39.5%, respectively; these values were lower than those in the G0-2 group [97.0%, 90.3%, and 87.4%, respectively; hazard ratio (HR) = 0.206, 95% confidence interval (CI) = 0.062–0.686, p < 0.001; Figure 5]. The Cox univariate and multivariate analysis results demonstrated that the pathological stage and lymphopenia degree were independent prognostic factors for DFS in patients with breast cancer (Table 2).
 |
Table 2 Multivariate Analysis of Prognostic Factors for DFS |
 |
Figure 5 DFS in patients with RIL. |
Lymphopenia Predictors
Table 3 presents the relationship between lymphopenia during treatment and different clinical characteristics. The age, tumor location, and chemotherapy modality of patients were nonsignificantly associated with G3 lymphopenia risk. In contrast, BMI and RT modality were significantly associated with G3 lymphopenia risk (both p < 0.05). Regarding the RT dosimetric predictors, the radiation doses at the sternum and heart were both associated with higher rates of G3 lymphopenia (all p < 0.05). Finally, our ROC curve analysis revealed the optimal cutoff points of the dosimetric variables significantly associated with G3 lymphopenia (Table 4).
 |
Table 3 Logistic Regression Analysis of Factors Associated with G3 Lymphopenia |
 |
Table 4 ROC Curve Cutoff Points for G3 Lymphopenia Prevention |
Discussion
This study revealed that lymphocytes exhibit exquisite radiosensitivity during postoperative radiotherapy for breast cancer, and G3 lymphopenia was significantly associated with impaired DFS. As we all know, lymphocytes are extremely sensitive to low doses of ionizing radiation. In the 1990s, in vitro studies reported that the entry of even a relatively low radiation dose into the bloodstream can significantly reduce lymphocyte counts; in particular, a lethal dose of only 2-Gy can reduce lymphocyte survival by 50%.17 Yovino et al18 designed a typical malignant glioma planning (8-cm tumor and 60-Gy radiation in 30 fractions) to monitor the radiation dose received by circulating blood cells. The results demonstrated that after a single dose of 2-Gy radiation, 5% of circulating blood cells received a 0.5-Gy dose, whereas 62%, 92%, and 99% of circulating blood cells received ≥0.5-Gy doses after 10, 20, and 30 fractions of 2-Gy radiation, respectively. In the present study, lymphocytes demonstrated extreme sensitivity to irradiation, and their counts gradually decreased as the RT course progressed, reaching their lowest value in RT week 5 and gradually recovering to baseline levels after the end of RT. This result is consistent with that of studies reporting lymphocyte decline after RT for esophageal cancer.13,14
RT during cancer treatment can influence tumor immunogenicity by increasing the expression of certain tumor-specific antigens. The immune system can process these antigens, stimulating the transformation of naive lymphocytes into tumor-specific lymphocytes. Lymphocytes are key effector cells in tumor immune responses; thus, a decrease in lymphocyte counts may reduce the immune system’s clearance efficiency of malignant tumor cells. Qiu et al19 identified a correlation of effective doses to immune cells with poor clinical outcomes and severe RIL, indicating that high doses to the immune system are related to cancer progression and death. Moreover, several studies have demonstrated that severe RIL is a negative prognostic factor for numerous cancers, such as brain tumor, pancreatic cancer, lung cancer, stomach cancer, cervical cancer, esophageal cancer, nasopharyngeal cancer, and breast cancer.11,13,14,16,20–24 A recent study reported that severe RIL can compromise survival benefits from durvalumab after CCRT for non–small-cell lung cancer.25 A study on CCRT for esophageal cancer demonstrated that the occurrence of G4 lymphopenia is an independent prognostic factor for worsened OS. Although lymphocyte counts may gradually recover to near-normal or normal levels after CCRT, the worsening of the OS cannot be reversed.14 Tseng et al26 also reported that inadequate lymphocyte recovery was significantly associated with worse OS and local recurrence-free survival in esophageal cancer patients who received RT. Therefore, maintaining a sufficient lymphocyte count during RT is crucial for cancer patient survival.
Only a few studies have assessed the relationship between RIL and breast cancer prognosis. Kobzeva et al15 reported that RT led to significant reductions in the absolute counts of B, T, and natural killer cells in all breast cancer patients, regardless of whether they had previously undergone chemotherapy. Another study revealed that RIL after breast-conserving surgery can affect prognosis.27 Sun et al16 assessed the relationship between RIL and survival after mastectomy and noted that the 5-year DFS rate was 71.8% in patients with nadir-PLC/pre-PLC ratio < 0.8, which was significantly lower than that in patients with nadir-PLC/pre-PLC ratio ≥ 0.8 (82.6%; p = 0.01); no such between-group difference was noted in the patients’ OS rate. Similarly, the current results indicated that G3 lymphopenia after postmastectomy RT is associated with poor DFS in patients with breast cancer.
The causal relationship between RIL and poor prognosis in cancer patients remains unclear. Naive T cells can be categorized into helper T (Th) cells (CD3+CD4+) and cytotoxic Tcyt cells (CD3+CD8+).28 Tcyt cells can directly kill abnormal cells.29 Regulatory T (Treg) cells (CD4+CD25+Foxp3+)—a subset of Th cells28—are involved in immune suppression.30 Muroyama et al31 found that after exposure to 10-Gy radiation, the number of Treg cells in the tumor microenvironment increased in tumor-bearing mice. Oweida et al32,33 reported that RT combined with Treg-cell inhibitors can inhibit tumor growth. Because of the relative resistance of Treg cells to radiation, surviving Treg cells may be able to inhibit effector T-cell recovery during lymphocyte recovery.34 A clinical study also indicated that an increased proportion of CD8+T/Treg cells predicts improved cancer prognosis.35 Therefore, the effects of RIL on survival might be indicated by changes in the circulating T-cell numbers and subpopulations during RT. In the current study, Cox multivariate analysis revealed that the pathological stage and lymphopenia degree were independent prognostic factors for DFS in our patients—consistent with the results of Sun et al.16
In the present study, low BMI, integrated RT use, and increased heart and sternum exposure were associated with G3 lymphopenia. The lymphocyte counts may have decreased mainly because irradiation reduced circulating mature lymphocyte numbers, as well as diminished lymphocyte production in the hematopoietic organs. In the human body, the blood volume accounts for 7%–8% of body weight. Patients with a higher BMI have larger blood volumes, indicating the presence of a richer reserve of mature lymphocytes in peripheral blood; as such, RT has less impact on lymphocytes. Therefore, high BMI may be a protective factor for lymphocyte depletion. Low BMI increases RIL risk in patients with esophageal cancer and breast cancer.16,36 However, the underlying mechanism warrants further research. Modern radiation techniques in which a large volume of tissue is irradiated with low doses of radiation can increase lymphopenia risk.
The heart is rich in blood, and the sternum is an adult hematopoietic organ; both are located near the radiation field used for breast cancer. Therefore, compared with hybrid RT, integrated RT demonstrates a larger low-dose area distribution; this results in an increase in the radiation doses delivered to the heart and sternum, accelerating lymphocytopenia development. Studies have reported that radiation doses to the heart, lungs, sternum, thoracic vertebrae, and spleen are predictive factors for RIL in cancer patients receiving RT.13,37–42 Therefore, low-dose irradiation of structures containing large amounts of blood or demonstrating high-velocity blood flow may be associated with RIL development. Hence, optimizing treatment plans to decrease radiation doses to immune cells is essential for improving relevant clinical outcomes.
Several studies have focused on RIL prevention during RT. Proton or carbon-ion RT, which can protect organs at-risk because of its physical advantages, is gradually being applied in cancer treatment. Compared with photon RT, proton RT can effectively reduce G3 or G4 RIL occurrence.43–50 Similarly, carbon-ion RT can reduce severe RIL development in patients with locally advanced pancreatic cancer compared with photon RT.51 However, the suitability of proton or carbon-ion RT as postoperative adjuvant RT for patients with breast cancer remains unclear. The RT segmentation method can also affect the degree of RIL during RT. McLaughlin et al52 reported that treatment with stereotactic RT was associated with a low degree of RIL in patients with early non–small-cell lung cancer. Sun et al16 also reported that RIL risk after hypofractionated RT was lower than that after conventional fractionated RT. McCullum et al53 used dynamic four-dimensional blood flow simulations to predict RIL severity in individual proton RT patients by varying dose rates and fractionation. Their results indicated increasing the dose rate at constant fractionation can reduce ALC depletion more significantly than reducing the number of fractions. Moreover, when shortening the fractionation regimen, higher dose rates are associated with increased lymphocyte sparing, particularly in high-risk patients with radiosensitive lymphocytes. However, relatively few studies have focused on the role of chemotherapeutic drugs in preventing or reversing RIL. Zheng et al54 reported that cinnamon effectively reversed T-cell subpopulation imbalance and promoted effective anticancer immunity by increasing Th1-cell proliferation and inhibiting Th17- and Treg-cell expansion in a mouse lung melanoma model after a single low-dose whole-body irradiation. In addition, other animal studies have demonstrated that administering exogenous interleukin (IL) 7 can not only restore lymphocyte counts but also enhance RT’s anticancer effects. Exogenous IL-7 can aid in overcoming lymphocyte depletion, and in combination with RT, it can improve treatment efficacy.55 However, these findings require validation through further clinical research.
In the current study, we analyzed the relationship between RIL and survival in patients who received postmastectomy RT and, for the first time, elucidated the relationship between dosimetric parameters of organs at-risk and RIL development. The major limitations of this study are the retrospective design, small sample size (mainly because of few eligible patients opting for routine complete blood analysis), and relatively short follow-up duration. Moreover, we included patients who received adjuvant or neoadjuvant chemotherapy. Therefore, the current results may be validated by additional prospective studies with larger sample sizes and longer follow-up periods.
In summary, RIL, commonly occurring during RT, influences cancer prognosis during and after treatment. However, the causal relationship of RIL with poor survival and the underlying mechanisms remain unclear and warrant further research. Strategies to reduce RIL risk include using hypofractionated RT, increasing dose rate, optimizing RT for new critical organs such as the heart and sternum, using particle RT, and applying other procedures to reduce the integral radiation dose.
Conclusions
G3 RIL was noted to be a crucial prognostic predictor of postmastectomy RT in patients with breast cancer. Significant predictors in the prediction model of G3 lymphopenia during RT were identified to be BMI, RT modality, and heart and sternum exposure dose. The current findings highlight the need for RT dose optimization and planning to minimize RIL risks. Thus, the sternum adjacent to the heart should also be considered a routine organ for RIL risk evaluation during RT planning for patients with breast cancer.
Data Sharing Statement
The raw data can be available on reasonable request with the consent of all authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Clinical Research Ethics Committee of Beijing Shijitan Hospital (Number: IIT2024-012-001). The ethics committee granted an exemption from obtaining written informed consent for this research due to the following reasons: The study involves retrospective analysis of anonymized data collected for non-research purposes originally, where re-identification of participants is impossible; The research poses minimal risk to participants, as no additional interventions or data collection were performed beyond routine practices; Requiring individual consent would render the study impracticable without compromising its scientific validity, given the large-scale/de-identified nature of the dataset. All procedures adhered to the ethical standards of the Declaration of Helsinki and relevant institutional regulations. Participant confidentiality was rigorously protected through data anonymization and secure handling protocols throughout the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Youth Fund of Beijing Shijitan Hospital [grant number 2020-q12].
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
2. Mcgale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi:10.1016/S0140-6736(14)60488-8
3. Early Breast Cancer Trialists Collaborative Group. Effects of radiotherapy and surgery in early breast cancer — an overview of the randomized trials. N Engl J Med. 1995;333(22):1444–1455. doi:10.1056/NEJM199511303332202
4. Darby S, Mcgale P, Correa C, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi:10.1016/S0140-6736(11)61629-2
5. 中国抗癌协会乳腺癌专业委员会. 中国抗癌协会乳腺癌诊治指南与规范(2021年版). 中国癌症杂志, 2021; 31(10):954–1040. doi:10.19401/j.cnki.1007-3639.2021.10.013. Breast Cancer Committee of China Anti-Cancer Association. Guidelines and Standards for the Diagnosis and Treatment of Breast Cancer by China Anti-Cancer Association (2021 Edition). China Oncology. 2021;31(10):954-1040. doi: 10.19401/j.cnki.1007-3639.2021.10.013.
6. 中国医师协会放射肿瘤治疗医师分会. 乳腺癌放射治疗指南(中国医师协会2020版). 中华放射肿瘤学杂志, 2021; 30(04):321–342. doi:10.3760/cma.j.cn113030-20210107-00010. Radiation Oncology Physicians Branch of Chinese Medical Doctor Association. Guidelines for Radiotherapy of Breast Cancer (Chinese Medical Doctor Association 2020 Edition). Chin J Radiat Oncol. 2021;30(4):321-342. doi: 10.3760/cma.j.cn113030-20210107-00010.
7. Santin AD, Hermonat PL, Ravaggi A, et al. Effects of concurrent cisplatinum administration during radiotherapy vs. Radiotherapy alone on the immune function of patients with cancer of the uterine cervix. Int J Radiat Oncol Biol Phys. 2000;48(4):997–1006. doi:10.1016/S0360-3016(00)00769-0
8. Grossman SA, Ye X, Lesser G, et al. Immunosuppression in patients with high-grade gliomas treated with radiation and temozolomide. Clin Cancer Res. 2011;17(16):5473–5480. doi:10.1158/1078-0432.CCR-11-0774
9. 周湛明, 徐慆, 舒琦. 乳腺癌锁骨上区不同放疗技术甲状腺剂量学比较及其对生存期和淋巴细胞亚群的影响. 中国医学物理学杂志, 2021; 38(2):143–147. doi:10.3969/j.issn.1005-202X.2021.02.003. Zhou ZM, Xu T, Shu Q. Thyroid dosimetric comparison of different techniques for supraclavicular radiotherapy of breast cancer and their effects on survival time and lymphocyte subsets. Chin J Med Phys. 2021,38(2):143-147. doi: 10.3969/j.issn.1005-202X.2021.02.003.
10. Venkatesulu BP, Mallick S, Lin SH, Krishnan S. A systematic review of the influence of radiation-induced lymphopenia on survival outcomes in solid tumors. Crit Rev Oncol Hematol. 2018;123:42–51. doi:10.1016/j.critrevonc.2018.01.003
11. Damen P, Kroese TE, van Hillegersberg R, et al. The influence of severe radiation-induced lymphopenia on overall survival in solid tumors: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys. 2021;111(4):936–948. doi:10.1016/j.ijrobp.2021.07.1695
12. Davuluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99(1):128–135. doi:10.1016/j.ijrobp.2017.05.037
13. Ni W, Xiao Z, Zhou Z, et al. Severe radiation-induced lymphopenia during postoperative radiotherapy or chemoradiotherapy has poor prognosis in patients with stage IIB-III after radical esophagectomy: a post hoc analysis of a randomized controlled trial. Front Oncol. 2022;12. doi:10.3389/fonc.2022.936684
14. Deng W, Xu C, Liu A, et al. The relationship of lymphocyte recovery and prognosis of esophageal cancer patients with severe radiation-induced lymphopenia after chemoradiation therapy. Radiother Oncol. 2019;133:9–15. doi:10.1016/j.radonc.2018.12.002
15. Kobzeva I, Astrelina T, Suchkova Y, et al. Effect of radiation therapy on composition of lymphocyte populations in patients with primary breast cancer. J Pers Med. 2023;13(9):1399. doi:10.3390/jpm13091399
16. Sun G, Wang S, Song Y, et al. Radiation-induced lymphopenia predicts poorer prognosis in patients with breast cancer: a post hoc analysis of a randomized controlled trial of postmastectomy hypofractionated radiation therapy. Int J Radiat Oncol Biol Phys. 2020;108(1):277–285. doi:10.1016/j.ijrobp.2020.02.633
17. Nakamura N, Kusunoki Y, Akiyama M. Radiosensitivity of CD4 or CD8 positive human t-lymphocytes by an in vitro colony formation assay. Radiat Res. 1990;123(2):224–227. doi:10.2307/3577549
18. Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E. The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest. 2013;31(2):140–144. doi:10.3109/07357907.2012.762780
19. Qiu J, Lin H, Ke D, et al. Higher radiation dose on immune cells is associated with radiation-induced lymphopenia and worse prognosis in patients with locally advanced esophageal squamous cell carcinoma. Front Immunol. 2023;14:1066255. doi:10.3389/fimmu.2023.1066255
20. Upadhyay R, Venkatesulu BP, Giridhar P, et al. Risk and impact of radiation related lymphopenia in lung cancer: a systematic review and meta-analysis. Radiother Oncol. 2021;157:225–233. doi:10.1016/j.radonc.2021.01.034
21. Kim N, Lee J, Shin H, et al. Nomogram for radiation-induced lymphopenia in patients receiving intensity-modulated radiotherapy based-chemoradiation therapy for newly diagnosed glioblastoma: a multi-institutional study. Clin Transl Radiat Oncol. 2024;47:100799. doi:10.1016/j.ctro.2024.100799
22. Wang J, Shao H, Yan J, et al. Neoadjuvant chemoradiotherapy induced lymphopenia in gastric cancer and associations with spleen dosimetry and survival outcomes. Clin Transl Radiat Oncol. 2023;40:100617. doi:10.1016/j.ctro.2023.100617
23. Cao H, Yan H, Bai S, Gu B. Radiation-induced lymphopenia and the survival of women with cervical cancer: a meta-analysis. J Obstet Gynaecol. 2023;43(1):2194991. doi:10.1080/01443615.2023.2194991
24. Xie X, Gong S, Jin H, et al. Radiation-induced lymphopenia correlates with survival in nasopharyngeal carcinoma: impact of treatment modality and the baseline lymphocyte count. Radiat Oncol. 2020;15(1):65. doi:10.1186/s13014-020-01494-7
25. Jing W, Xu T, Wu L, et al. Severe radiation-induced lymphopenia attenuates the benefit of durvalumab after concurrent chemoradiotherapy for NSCLC. JTO Clin Res Rep. 2022;3(9):100391. doi:10.1016/j.jtocrr.2022.100391
26. Tseng I, Ai D, Chen Y, et al. Lymphocyte recovery from radiation-induced lymphopenia in locally advanced esophageal squamous cell carcinoma: correlations with prognosis and lymphocyte-related organs. Radiat Oncol. 2023;18(1). doi:10.1186/s13014-023-02354-w
27. Yoon CI, Hwang J, Kim D, et al. Prognostic impact of radiotherapy-induced-lymphopenia in patients treated with breast-conservative surgery. Sci Rep. 2023;13(1). doi:10.1038/s41598-023-41301-3
28. Paganetti H. A review on lymphocyte radiosensitivity and its impact on radiotherapy. Front Oncol. 2023;13:1201500. doi:10.3389/fonc.2023.1201500
29. Newell EW, Sigal N, Bendall SC, Nolan GP, Davis MM. Cytometry by time-of-flight shows combinatorial cytokine expression and virus-specific cell niches within a continuum of CD8+ t cell phenotypes. Immunity. 2012;36(1):142–152. doi:10.1016/j.immuni.2012.01.002
30. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi:10.1038/nrc3245
31. Muroyama Y, Nirschl TR, Kochel CM, et al. Stereotactic radiotherapy increases functionally suppressive regulatory t cells in the tumor microenvironment. Cancer Immunol Res. 2017;5(11):992–1004. doi:10.1158/2326-6066.CIR-17-0040
32. Oweida A, Hararah MK, Phan A, et al. Resistance to radiotherapy and PD-l1 blockade is mediated by TIM-3 upregulation and regulatory t-cell infiltration. Clin Cancer Res. 2018;24(21):5368–5380. doi:10.1158/1078-0432.CCR-18-1038
33. Oweida AJ, Darragh L, Phan A, et al. STAT3 modulation of regulatory t cells in response to radiation therapy in head and neck cancer. J Natl Cancer Inst. 2019;111(12):1339–1349. doi:10.1093/jnci/djz036
34. Baba J, Watanabe S, Saida Y, et al. Depletion of radio-resistant regulatory t cells enhances antitumor immunity during recovery from lymphopenia. Blood. 2012;120(12):2417–2427. doi:10.1182/blood-2012-02-411124
35. Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating t cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21(Suppl 3):S414–S421. doi:10.1245/s10434-014-3584-y
36. van Rossum P, Deng W, Routman DM, et al. Prediction of severe lymphopenia during chemoradiation therapy for esophageal cancer: development and validation of a pretreatment nomogram. Pract Radiat Oncol. 2020;10(1):e16–e26. doi:10.1016/j.prro.2019.07.010
37. van Rossum P, Juan-Cruz C, Stam B, et al. Severe radiation-induced lymphopenia during concurrent chemoradiotherapy for stage III non-small cell lung cancer: external validation of two prediction models. Front Oncol. 2023;13:1278723. doi:10.3389/fonc.2023.1278723
38. Terrones-Campos C, Ledergerber B, Forbes N, et al. Prediction of radiation-induced lymphopenia following exposure of the thoracic region and associated risk of infections and mortality. Clin Oncol. 2023;35(7):e434–e444. doi:10.1016/j.clon.2023.04.003
39. Chen F, Yu H, Zhang H, et al. Risk factors for radiation induced lymphopenia in patients with breast cancer receiving adjuvant radiotherapy. Ann Transl Med. 2021;9(16):1288. doi:10.21037/atm-21-2150
40. Xie X, Lin SH, Welsh JW, et al. Radiation-induced lymphopenia during chemoradiation therapy for non-small cell lung cancer is linked with age, lung v5, and XRCC1 rs25487 genotypes in lymphocytes. Radiother Oncol. 2021;154:187–193. doi:10.1016/j.radonc.2020.09.002
41. Abravan A, Faivre-Finn C, Kennedy J, Mcwilliam A, van Herk M. Radiotherapy-related lymphopenia affects overall survival in patients with lung cancer. J Thorac Oncol. 2020;15(10):1624–1635. doi:10.1016/j.jtho.2020.06.008
42. Ma Y, Kong Y, Zhang S, et al. The relationship between splenic dose and radiation-induced lymphopenia. J Radiat Res. 2024;65(3):337–349. doi:10.1093/jrr/rrae023
43. Hsieh RC, Lee CH, Huang HC, et al. Clinical and dosimetric results of proton or photon radiation therapy for large (>5 cm) hepatocellular carcinoma: a retrospective analysis. Int J Radiat Oncol Biol Phys. 2024;118(3):712–724. doi:10.1016/j.ijrobp.2023.09.049
44. Ebrahimi S, Lim G, Liu A, et al. Radiation-induced lymphopenia risks of photon versus proton therapy for esophageal cancer patients. Int J Part Ther. 2021;8(2):17–27. doi:10.14338/IJPT-20-00086
45. Kim N, Myoung Noh J, Lee W, et al. Proton beam therapy reduces the risk of severe radiation-induced lymphopenia during chemoradiotherapy for locally advanced non-small cell lung cancer: a comparative analysis of proton versus photon therapy. Radiother Oncol. 2021;156:166–173. doi:10.1016/j.radonc.2020.12.019
46. Routman DM, Garant A, Lester SC, et al. A comparison of grade 4 lymphopenia with proton versus photon radiation therapy for esophageal cancer. Adv Radiat Oncol. 2019;4(1):63–69. doi:10.1016/j.adro.2018.09.004
47. Fang P, Shiraishi Y, Verma V, et al. Lymphocyte-sparing effect of proton therapy in patients with esophageal cancer treated with definitive chemoradiation. Int J Part Ther. 2018;4(3):23–32. doi:10.14338/IJPT-17-00033.1
48. Liu C, Bhangoo RS, Sio TT, et al. Dosimetric comparison of distal esophageal carcinoma plans for patients treated with small‐spot intensity‐modulated proton versus volumetric‐modulated arc therapies. J Appl Clin Med Phys. 2019;20(7):15–27. doi:10.1002/acm2.12623
49. Shiraishi Y, Fang P, Xu C, et al. Severe lymphopenia during neoadjuvant chemoradiation for esophageal cancer: a propensity matched analysis of the relative risk of proton versus photon-based radiation therapy. Radiother Oncol. 2018;128(1):154–160. doi:10.1016/j.radonc.2017.11.028
50. Mohan R, Liu AY, Brown PD, et al. Proton therapy reduces the likelihood of high-grade radiation-induced lymphopenia in glioblastoma patients: Phase II randomized study of protons vs photons. Neuro Oncol. 2021;23(2):284–294. doi:10.1093/neuonc/noaa182
51. Yang G, Koom WS, Lee BM, et al. Reduced risk of severe radiation-induced lymphopenia in carbon ion radiation therapy for locally advanced pancreatic cancer: a comparative analysis of carbon versus photon therapy. Int J Radiat Oncol Biol Phys. 2024. doi:10.1016/j.ijrobp.2024.04.003
52. Mclaughlin MF, Alam M, Smith L, Ryckman J, Lin C, Baine MJ. Stereotactic body radiation therapy mitigates radiation induced lymphopenia in early stage non-small cell lung cancer. PLoS One. 2020;15(11):e0241505. doi:10.1371/journal.pone.0241505
53. Mccullum L, Shin J, Xing S, et al. Predicting severity of radiation induced lymphopenia in individual proton therapy patients for varying dose rate and fractionation using dynamic 4-dimensional blood flow simulations. Int J Radiat Oncol Biol Phys. 2023;116(5):1226–1233. doi:10.1016/j.ijrobp.2023.01.054
54. Zheng XM, Guo YM, Wang LM, et al. Recovery profiles of t-cell subsets following low-dose total body irradiation and improvement with cinnamon. Int J Radiat Oncol Biol Phys. 2015;93(5):1118–1126. doi:10.1016/j.ijrobp.2015.08.034
55. Byun HK, Kim KJ, Han SC, Seong J. Effect of interleukin-7 on radiation-induced lymphopenia and its antitumor effects in a mouse model. Int J Radiat Oncol Biol Phys. 2021;109(5):1559–1569. doi:10.1016/j.ijrobp.2020.12.004
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

