Back to Journals » Nanotechnology, Science and Applications » Volume 18
Rapid Replenishment of Phylloquinone in the Plasma and Liver Using Hyaluronan-Based Nanocapsules Reverses Endothelial Dysfunction in Mice
Authors Kieronska-Rudek A , Kij A , Bar A, Sternak M, Paterek A, Rolski F, Czyzynska-Cichon I , Fedak FA, Wojnar-Lason K , Bednorz J , Janik-Hazuka M , Kostogrys RB, Franczyk-Zarow M, Czyżowska KZ, Michalkova L, Mączewski M , Zapotoczny S , Kus E , Chlopicki S
Received 30 January 2025
Accepted for publication 3 May 2025
Published 6 June 2025 Volume 2025:18 Pages 245—262
DOI https://doi.org/10.2147/NSA.S520030
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Replenishment of phylloquinone in the plasma and liver – Video S1 [520030]
Views: 80
Anna Kieronska-Rudek,1,2 Agnieszka Kij,1 Anna Bar,1 Magdalena Sternak,1 Aleksandra Paterek,3 Filip Rolski,3 Izabela Czyzynska-Cichon,1 Filip A Fedak,1,4 Kamila Wojnar-Lason,1,2 Justyna Bednorz,4,5 Małgorzata Janik-Hazuka,5 Renata B Kostogrys,6 Magdalena Franczyk-Zarow,6 Katarzyna Z Czyżowska,1 Lenka Michalkova,1 Michał Mączewski,3 Szczepan Zapotoczny,5 Edyta Kus,1 Stefan Chlopicki1,2
1Jagiellonian University, Jagiellonian Centre for Experimental Therapeutics (JCET), Krakow, 30-348, Poland; 2Jagiellonian University Medical College, Chair of Pharmacology, Faculty of Medicine, Krakow, 31-531, Poland; 3Centre of Postgraduate Medical Education, Department of Clinical Physiology, Warszawa, 01-813, Poland; 4Jagiellonian University, Doctoral School of Exact and Natural Sciences, Krakow, 30-348, Poland; 5Jagiellonian University, Faculty of Chemistry, Krakow, 30-387, Poland; 6University of Agriculture in Krakow, Department of Human Nutrition and Dietetics, Faculty of Food Technology, Krakow, 31-120, Poland
Correspondence: Edyta Kus, Jagiellonian Centre for Experimental Therapeutics (JCET), Michala Bobrzynskiego14, Krakow, 30-348, Poland, Email [email protected] Stefan Chlopicki, Jagiellonian Centre for Experimental Therapeutics (JCET), Michala Bobrzynskiego14, Krakow, 30-348, Poland, Email [email protected]
Introduction: As vitamin K1 (phylloquinone, PK) displays vasoprotective effect, low dietary intake and poor bioavailability of PK may result in insufficient systemic levels for maintaining vascular health. This study aimed to test whether PK in hyaluronan-based nanocapsules (PK-Oil-HyC12) improves PK pharmacokinetics and endothelial function compared to PK in oil emulsion (PK-Oil).
Methods: PK pharmacokinetics in plasma, liver and aorta were analysed after single, oral administration of PK (10 mg/kg) in oil (PK-Oil) or encapsulated in hyaluronan-based nanocapsules with oil core (PK-Oil-HyC12) in mice using liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization method. PK-Oil-HyC12 absorption and nanocapsules distribution in lymphatic system was determined using a cycloheximide-based chylomicron flow blockage and intravital confocal microscopy. The endothelial function was analyzed in vivo by MRI in mice with dietary PK deficiency after 7-day supplementation with PK-Oil or PK-Oil-HyC12 (0.5 mg PK/kg).
Results: After a single, oral dose of PK-Oil-HyC12 in mice total exposure of PK (AUC values) was 2– 4 times higher as compared to PK-Oil in plasma and liver, with no difference in PK content in the aorta. The efficient absorption and distribution of nanocapsules occurred mainly via a chylomicron-independent lymphatic route. Importantly, 7-day PK-Oil-HyC12 supplementation restored impaired endothelium-dependent vasodilation in the aorta of PK-deficient mice, while PK-Oil was ineffective.
Conclusion: The improved bioavailability of PK, when administered in the form of hyaluronan-based nanocapsules, afforded the rapid replenishment of systemic PK and the reversal of endothelial dysfunction induced by low PK levels.
Keywords: Vitamin K1, phylloquinone, hyaluronan-based nanocapsules, endothelial dysfunction, dietary phylloquinone deficiency
Introduction
Dietary vitamin K occurs in two biologically active forms: phylloquinone (PK; vitamin K1) present in various leafy green vegetables, fruits and plant oils, and a group of menaquinones (MKs; vitamin K2), differing in the length of their side chains and being mostly of bacterial origin, which are included in the human diet mainly through animal products and fermented food.1
Initially, the major role of vitamin K (PK or MKs) was attributed to the regulation of coagulation by the post-translational gamma-carboxylation of coagulation factors.2 However, subsequent studies have significantly broadened the pharmacological effects of vitamin K to include, among many others, anti-inflammatory effects, improvement of endothelial function, inhibition of vascular calcification, and protection against cardiovascular and neurodegenerative diseases.3–7
PK constitutes over 80% of the total vitamin K in the human diet; however, its absorption from green vegetables is very poor.8 PK is among the vitamins with the worst bioavailability (less than 20%) compared to other fat- and water-soluble vitamins provided through animal- or plant-based food.9 Recent evidence suggests that low plasma PK levels might contribute to an increased risk of cardiovascular disease. A prospective study involving over 50,000 participants (Danish Diet, Cancer and Health Study) demonstrated that the risk of atherosclerotic cardiovascular disease was inversely associated with the intake of dietary PK.10 These results are consistent with previous findings that low plasma PK levels were associated with higher cardiovascular risk.11 Accordingly, these clinical trials, together with several recently published reports, indicated that PK has beneficial cardiovascular activity.10,12 Therefore, increasing the plasma concentration of PK may be beneficial for cardiovascular health.11,13 This evidence also suggests that improving PK bioavailability would afford better vasoprotection by supplemented PK.
Given the low bioavailability of PK,14 it is plausible that the daily PK supplementation dose previously used in clinical trials may have resulted in insufficient plasma PK levels to achieve therapeutic effects. Numerous clinical studies showed negligible or no effects of PK supplementation15–17 that could be attributed at least partially to its low bioavailability and the low dose used. On the other hand, in the VitaVasK study,18 the authors used a dose ∼29 times higher than the daily recommended PK intake of 75 µg and observed a favourable vascular effect. Similarly, among the 55,545 participants of the Danish Diet, Cancer and Health Study cohort, only those with the highest intake of PK (⁓3 times the daily recommended dose) exhibited a significantly lower risk of aortic stenosis and subsequent complications or death attributed to cardiovascular disease.19
The present work assessed hyaluronan-based nanocapsules, representing a novel, effective approach to deliver hydrophobic, poorly bioavailable compounds.20–22 We sought to determine whether PK administration in the form of hyaluronan-based nanocapsules, compared to PK given in an oil emulsion, would significantly improve PK pharmacokinetics, effectively replenish systemic PK levels and reverse endothelial dysfunction induced by PK deficiency in mice.
Materials and Methods
Animals
All experiments were conducted on 8–14-week-old C57BL/6J male mice purchased from the Mossakowski Medical Research Centre at the Polish Academy of Sciences (Warszawa, Poland), the Center for Experimental Medicine at the Medical University of Bialystok (Poland) or the Central Laboratory of Experimental Animals (Warszawa, Poland). Throughout the experiment, animals were housed in individually ventilated cages with a 12 h light/dark cycle and unlimited access to water and a chow diet (unless otherwise stated).
Mice intended for ex vivo studies were fed a standard AIN-93M diet (ZooLab, Krakow, Poland), while mice intended for in vivo studies of endothelial dysfunction were fed modified AIN-93M diets (ZooLab, Krakow, Poland) for 5 weeks (the detailed composition of the diets and protocol of experiments are described below). The plasma, aorta and liver were collected after euthanasia using a mixture of ketamine and xylazine at doses of 100 and 10 mg/kg, respectively.
Experiments involving animals were performed after obtaining permission from the 2nd Local Ethical Committee on Animal Testing of the Institute of Pharmacology, Polish Academy of Sciences (Krakow, Poland; Permit Nos. 378/2021 and 268/2022) and the 2nd Local Ethical Committee for Animal Experiments of the Warsaw University of Life Sciences (Poland; Permit No. WAW2/004/2024). Studies were performed according to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes.
Collection of Plasma, Aorta and Liver
Blood samples were collected using K2-EDTA as an anticoagulant and subsequently centrifuged (664 × g, 12 min, 4 °C) to separate the plasma. Aortas were perfused with 0.9% sodium chloride (NaCl) and isolated, while surrounding tissues, including perivascular adipose tissue (PVAT), were removed. The livers were isolated after perfusion using 0.9% NaCl, and the right lobe was weighed and collected for further vitamin K analysis. Plasma, aorta, and liver lobe were immediately frozen and stored at −80 °C until further analyses. For ex vivo studies, the aorta was isolated, and subsequently, depending on the experimental set-up, the surrounding tissues and PVAT were removed, and the aorta and PVAT were incubated under the conditions described in the section “Studies of vascular uptake of encapsulated and non-encapsulated PK in the aorta ex vivo”.
Synthesis and Characterisation of Hyaluronan-Based Capsules with an Oil Core Containing PK
A hydrophobic derivative of hyaluronic acid (HyC12) was obtained following previously described protocols.20–23 To minimise PK delivery with the solvent (creating the oil core in capsules or an oil continuous phase in the emulsion without HyC12), the concentration of various forms of vitamin K, that is, PK, MK-4, and MK-7, was measured in sunflower, soybean and corn oils (Sigma-Aldrich, Poland). Corn oil was chosen for the nanocapsule’s core as it contained the lowest concentration of PK (0.443 mg/kg) in comparison to sunflower oil (18.811 mg/kg) or soybean oil (10.340 mg/kg), as measured by liquid chromatography-tandem mass spectrometry with atmospheric pressure chemical ionization (LC-APCI-MS/MS) technique.3,6 Nanocapsules (oil-in-water nanoemulsions) were prepared directly using an ultrasound-assisted emulsification process. An aqueous solution of HyC12 (1 g/L in 0.01 M PBS, Sigma-Aldrich, Poland) was mixed with an oil phase containing dissolved PK (Sigma-Aldrich, Poland) in a proportion of 1000:3 (v/v). The concentration of PK dissolved in corn oil was either 25 g/L (for in vivo experiments) or 77 g/L (for ex vivo experiments). To obtain a homogeneous mixture of capsules (PK-Oil-HyC12), the suspension was mixed using a vortex shaker and then sonicated. The emulsion of PK dissolved in corn oil (PK-Oil) was prepared using the procedure described above but without HyC12 or other stabilising agents.
Changes in the size and zeta potential of the PK-Oil-HyC12 and PK-Oil formulations were assessed using dynamic light scattering and analysed using a two-tailed Student’s t-test. A comparison of the initial volume-weighted hydrodynamic diameter distributions of PK-Oil-HyC12 (479±23 nm, −16.9±0.6 mV) and PK-Oil (410±29 nm, −17.7±0.9 mV) indicated similar particle sizes for both formulations. However, the variable size distributions obtained for PK-Oil showed that the emulsion was not stable, in contrast to the emulsion stabilised by HyC12. The stability of the capsules was assessed by analysing changes in size and zeta potential, which showed that these parameters remained stable not only for 7 days (the maximal treatment time, 504±17 nm, −18.8±0.9 mV) but also during long-term storage (59 days, 489±19 nm, −19.7±0.7 mV) at 4 °C, despite the fact that between the day of preparation and the 59th day of storage, and between the 7th and 59th days of storage, changes in zeta potential values were statistically significant (p < 0.05). The comparison of changes in capsule size during storage was not statistically significant (p > 0.05).
In vivo Pharmacokinetics of PK-Oil and PK-Oil-HyC12
The PK in oil (PK-Oil) or encapsulated within hyaluronan-based nanocapsules with an oil core (PK-Oil-HyC12) was administered to mice fed a standard chow diet. After a single oral administration of PK-Oil or PK-Oil-HyC12 at a dose of 10 mg/kg, animals were anaesthetised and sacrificed at the following time intervals: 0 min (before dosing), 5 min, 15 min, 30 min, 1 h, 2 h, 3 h, 5 h, 7 h and 24 h after PK administration. Plasma and aortas were collected as described above in the “Collection of plasma, aorta and liver” section of the Materials and methods above, while livers were collected, rinsed with PBS (pH 7.4), and immediately frozen at −80 °C until analysis. The plasma and liver PK levels and aortic PK and MK-4 concentrations were assessed by the LC-APCI-MS/MS-based method described below.
All data in the pharmacokinetic experiments were processed using Phoenix WinNonlin 6.3 software (Certara, St. Louis, MO, USA). A non-compartmental approach was applied to calculate basic pharmacokinetic parameters such as maximum concentration (Cmax), time to occurrence (Tmax), area under the concentration-time (AUC), apparent clearance (Cl/F) and apparent volume of distribution (V/F). Additionally, compartmental fitting of experimental data was performed to estimate the absorption rate constant (Ka).
Effect of Cycloheximide-Based Inhibition of Chylomicron-Dependent Transport on PK-Oil-HyC12 Absorption
The animals were fasted for 2 h prior to the experiment and then treated with either an intraperitoneal (i.p.) injection of cycloheximide (3 mg/kg)24 or an equal volume of saline (n=5–6 per group). One hour after the injection, the animals were given an oral gavage of PK in the form of hyaluronan-based nanocapsules, and the pharmacokinetic experiment was conducted as described above in the “In vivo pharmacokinetics of PK-Oil and PK-Oil-HyC12” section.
Intravital Visualisation of Hyaluronan-Based Nanocapsules Transport by the Lymphatic System and Liver Uptake in Mice
Intravital imaging of hyaluronan-based nanocapsules (HyC12-NCs) absorption and transport via the intestinal lymphatic system was performed according to Choe et al25 using a dual-mode intravital confocal and two-photon microscope IVM-CMS3 (IVIM Technology, South Korea). For imaging purposes, the HyC12-NCs were fluorescently labelled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil, Sigma-Aldrich, Poland). On the day of the experiment, mice were anaesthetised via the i.p. injection of ketamine with xylazine (100 mg/kg and 10 mg/kg, respectively). To visualise and distinguish lymphatic and blood vessels, the Alexa Fluor® 647 Anti-LYVE1 antibody (Abcam, UK) and a CD31 (PECAM-1) monoclonal antibody (390), Super Bright™ 436 (eBioscience™, Thermo Scientific, USA), were injected intravenously (i.v.) The abdomen was opened, approximately 8 cm of the proximal jejunum was exteriorised, and about 1.5 cm was cut open along the antimesenteric border. The exposed intestinal lumen was carefully wiped with a saline-moistened swab to remove the debris in the lumen, and the Dil-labelled HyC12-NCs were applied. Intravital imaging of the mesenteric blood and lymphatic vessels, as well as liver uptake, was performed. Throughout the intravital imaging process, the chamber temperature and body temperature of the anaesthetised mice were maintained at 37 °C. The vitality of the intestinal villi was confirmed by the flow of red blood cells in peripherally located blood vessels in the villi and peristaltic movements of the intestine (see Supplementary Movie 1).
Comparison of Effects of Encapsulated and Non-Encapsulated PK in vivo on Endothelial Function in Mice Fed a PK-Deficient Diet
For 5 weeks, mice were fed a control diet containing sufficient PK, AIN-93M-RICE(+PK) or PK-deficient diet, namely AIN-93M-RICE(-PK) as described previously.26 Detailed composition of the diets is listed in Table 1.
 |
Table 1 Detailed Composition of the AIN-93M-RICE(+PK) and AIN-93M-RICE(-PK) |
Both AIN-93M-RICE(+PK) and AIN-93M-RICE(-PK) diet are based on the standard AIN-93M diet, but PK-rich ingredients have been replaced by ingredients containing lower PK amounts. Namely, soybean oil has been replaced by corn oil, and corn starch has been partially replaced by rice flakes (50%/50%). Additionally, the amount of casein has been reduced. The lack of PK in the AIN-93M-RICE(-PK) diet was confirmed by quantifying PK levels using an LC-APCI-MS/MS-based method.3,6 The AIN-93M-RICE(+PK) diet comprises the AIN-93M diet described above, supplemented with PK at a concentration of 0.75 mg/kg, resulting in an intake of around 90 µg/kg per day. Neither MK-4 nor MK-7 was detectable in either diet. Given similar pharmacological action of PK and MK3,7 the dose of PK (0.5mg/kg) to reverse the AIN-93M-RICE(-PK) diet phenotype was chosen based on our previous work demonstrating the effects of MK-7 on endothelial function in vivo.6 After 5 weeks on the AIN-93M-RICE(-PK) diet, various PK supplementation approaches were implemented to reverse PK deficiency, leading to the following experimental groups:
- AIN-93M-RICE(+PK): Based on the AIN-93M-RICE(-PK) diet supplemented with PK at an amount covering the daily demand dose (0.15 mg/kg); n=6
- AIN-93M-RICE(-PK): PK-deficient diet prepared without the addition of PK; n=6
- Two-day AIN-93M-RICE(-PK) + PK-Oil: Two administrations of PK-Oil with a PK dose of 0.5 mg/kg; n=6
- Two-day AIN-93M-RICE(-PK) + PK-Oil-HyC12: Two administrations of PK-Oil-HyC12 with a PK dose of 0.5 mg/kg; n=6
- Seven-day AIN-93M-RICE(-PK) + PK-Oil: Seven administrations of PK-Oil with a PK dose of 0.5 mg/kg; n=6
- Seven-day AIN-93M-RICE(-PK) + PK-Oil-HyC12: Seven administrations of PK-Oil-HyC12 with a PK dose of 0.5 mg/kg; n=6
All supplementations were administered via intragastric gavage (i.g.) once daily in 200 μL. After the final administration, aorta function was measured by magnetic resonance imaging (MRI). Then, mice were sacrificed, and the aorta, plasma and liver were collected within 22–26 h after the last PK administration as described above in the “Collection of plasma, aorta and liver” section of the Materials and methods. The samples were immediately frozen at −80 °C until analysis.
MRI-Based Assessment of Endothelial Function in Mice in vivo
To assess the functional impairment of endothelium-dependent vasodilation in the aorta in response to PK deficiency and analyse the effects of encapsulated and non-encapsulated PK on aorta function, MRI was performed using a 9.4 T scanner (BioSpec 94/20 USR, Bruker, Ettlingen, Germany), according to previous protocols.27–29 During the experiment, mice were under isoflurane anaesthesia (1.5% in a 1:2 mixture of oxygen and air). Body temperature, heart activity and respiration were constantly monitored by the Monitoring and Gating System (SA Inc., Stony Brook, NY, USA). Endothelial function in vivo was assessed as an endothelium-dependent response to acetylcholine (Ach; 16.6 mg/kg, i.p.; Sigma-Aldrich, Poznan, Poland), while vascular smooth muscle cell-dependent vasorelaxation was measured in response to sodium nitroprusside (SNP; 1 mg/kg, i.v.; Sigma-Aldrich, Poznan, Poland) in the thoracic and abdominal parts of the aorta. The dose of Ach and SNP used to assess vascular response in vivo in mice was chosen based on a previous studies.30–32 Images were acquired using the cine IntraGateTM FLASH 3D sequence and reconstructed using the IntraGate 1.2.b.2 macro-Bruker (Bruker, Ettlingen, Germany). The vasomotor responses were examined by comparing two, time-resolved 3D images of the vessels prior to and 30 minutes after Ach or SNP administration. Importantly, the endothelium-dependent response, induced by Ach, measured 30 minutes after injection, was independent of the effect of Ach on the heart and respiration, as described previously.30 Quantitative analysis was performed using ImageJ software, version 1.46r (NIH, Bethesda, MD, USA) and Matlab (MathWorks, Natick, MA, USA). Vascular responses were expressed as a percentage of changes in the vessel volume after Ach or SNP administration and defined as vasodilation and vasoconstriction for positive and negative values, respectively. A detailed analysis was described in our previous works.29,30
Studies of the Vascular Uptake of Encapsulated and Non-Encapsulated PK in the Aorta ex vivo
Isolated aorta without PVAT and isolated PVAT were incubated for 24 h with shaking in minimum essential medium (MEM; Gibco, Paisley, Scotland, UK) with 20% foetal bovine serum (FBS; Lonza, Basel, Switzerland) containing 5 μM of PK encapsulated in hyaluronan-based nanocapsules (PK-Oil-HyC12) or non-encapsulated PK dissolved in a tetrahydrofuran: H2O (9:1 v/v) mixture (PK). Aorta and PVAT were weighed, collected separately, and immediately frozen at −80 °C for LC-APCI-MS/MS-based MK-4 analysis.
The LC-APCI-MS/MS-Based Method for PK and MK-4 Level Analysis
To assess the status and uptake of PK, its concentration was measured in the plasma, aorta, PVAT and liver using an LC-APCI-MS/MS-based method described previously.3,6 Additionally, the level of endogenous MK-4 was assessed in the PVAT and aorta. Briefly, the aorta, PVAT and liver samples were homogenised in ethanol. Next, the plasma samples and homogenate supernatants were spiked with an internal standard (PK-d7) and extracted twice using hexane. The upper organic layer was collected in glass tubes and evaporated to dryness under a nitrogen stream. The dry residues were dissolved in isopropanol, and supernatants were injected into an LC-MS system consisting of an Ultimate 3000 UHPLC (Dionex, Sunnyvale, CA, USA) liquid chromatograph and a TSQ Quantum Ultra triple quadrupole mass spectrometer (Thermo Scientific, Waltham, MA, USA). The chromatographic separation and mass spectrometric detection were performed according to published protocols.3,6 The concentrations of PK and MK-4 were calculated based on the calibration curves plotted as the relationship between the peak area ratios of the analyte/internal standard (PK-d7) and the nominal concentration of the analyte. The tissue content was normalised to tissue weight.
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 9 (San Diego, CA, USA). Depending on the data distribution, the Shapiro–Wilk or D’Agostino–Pearson omnibus normality test was applied, and the results were presented as mean ± SD or median ± IQR. Statistical significance was defined in the post hoc tests, where only p-values <0.05 were considered significant.
Results
Comparison of PK Pharmacokinetics After a Single Dose Administration in Hyaluronan-Based Nanocapsules (PK-Oil-HyC12) or Oil (PK-Oil) to Mice
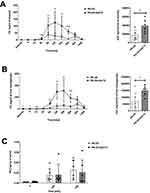
The pharmacokinetics of PK was based on measurement of PK concentrations in plasma, liver and aorta in PK-Oil and PK-Oil-HyC12 groups. As shown in Figure 1, the pharmacokinetic profiles of PK (10 mg/kg) administered in hyaluronan-based nanocapsules (PK-Oil-HyC12) differed significantly from those of PK administered in oil (PK-Oil). Administration of PK-Oil-HyC12 resulted in a markedly higher PK concentration in the plasma (Figure 1A) and liver (Figure 1B) compared with PK-Oil administration, while the content of PK in the aorta increased similarly 120–180 min after PK administration independently of the PK formulation (Figure 1C). The calculated exposure parameters (both maximal concentration Cmax and total exposure AUC) were significantly higher in the plasma and liver after the administration of encapsulated PK, indicating increased bioavailability for PK-Oil-HyC12. The observed concentration-time profiles for both formulations showed similar circulation time, with the same last measurable time point (24 h). The elimination half-life (T ½) for PK-Oil-HyC12 was shorter compared to PK-Oil formulation (4 vs 8.9 h) (Table 2).
 |
Table 2 Basic Pharmacokinetic Parameters of PK-Oil and PK-Oil-HyC12 Following Their Gavage Administration to C57BL/6J Mice |
To assess relative differences in bioavailability, compartmental modeling was employed. The best fit to experimental data was achieved with a one-compartment model with lag time. The estimated relative bioavailability ratio (F) was 4:1 for PK-Oil-HyC12: PK-Oil, indicating a four-fold higher bioavailability for the encapsulated form of PK. Importantly, the observed difference in bioavailability between groups was not related with nanocapsules properties, as the estimated parameters for clearance (Cl) and volume of distribution (V) using the compartmental model were similar for both formulations. The estimated absorption lag time (Tlag) for both formulations was also similar – approximately 0.5 h. A notable difference was however observed in the absorption rate constant (Ka). The absorption rate constant for PK-Oil formulation was very high (Ka = 37.98 h−1), indicating rapid but brief absorption, consistent with the lower Tmax. In contrast, for PK-Oil-HyC12 the absorption rate constant was lower (Ka = 2.7 h−1), suggesting prolonged absorption for this formulation (Table 2).
Intravital Visualisation of Fluorescently Labelled Hyaluronan-Based Nanocapsules Absorption and Transport by the Lymphatic System
PK is a fat-soluble vitamin absorbed along with dietary fat from the small intestine and carried by chylomicrons via the lymphatic transport system to the systemic circulation and liver, the main site of PK metabolism.33–35 To test whether hyaluronan-based nanocapsules are also absorbed via the lymphatic transport system and whether this transport is mediated in a chylomicron-dependent manner, the cycloheximide chylomicron flow blocking method and intravital visualisation of the mesenteric blood and lymphatic vessels and liver were performed.
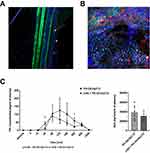
Dil-labeled HyC12NCs were applied to the luminal surface of the jejunum, which had previously been opened along the antimesenteric border in anaesthetised mice. As shown in Figure 2, within minutes of application, Dil-HyC12-NCs could be found in the mesenteric lymphatic collecting vessels, while they were absent in the mesenteric veins and arteries (Figure 2A). Abundant localisation of Dil-HyC12-NCs in the liver was visible as soon as 30 min after their application, indicating the liver as a major target for Dil-HyC12-NCs (Figure 2B).
In the presence of cycloheximide injected into mice 1 h prior to oral PK-Oil-HyC12 gavage, PK-Oil-HyC12 was absorbed to an extent similar to that in the absence of cycloheximide, suggesting a chylomicron-independent pathway for PK-Oil-HyC12 absorption (Figure 2C).
Comparison of the Pharmacological Effects of PK Administered in Hyaluronan-Based Nanocapsules (PK-Oil-HyC12) or Oil (PK-Oil) on Endothelial Function in vivo Measured by MRI in Mice with a Dietary PK Deficiency
To test whether improved systemic PK bioavailability via the chylomicron-independent absorption of hyaluronan-based nanocapsules (PK-Oil-HyC12) would result in improved pharmacological effects, after 7 days of treatment with a low dose of PK (0.5 mg/kg), we compared the effects of PK-Oil-HyC12 and PK-Oil on endothelial function in the thoracic (TA) and the abdominal (AA) aorta in a murine model of dietary PK deficiency.
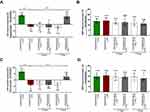
Mice fed the AIN-93M-RICE(-PK) diet for 5 weeks displayed impaired endothelium-dependent Ach-induced vasodilation in the TA (Figure 3A) and AA (Figure 3C), as evidenced in vivo by MRI-based measurements (−1.83% and −3.81%, respectively). Endothelium-independent vasodilation in response to SNP administration remained unchanged in the TA and AA (Figure 3B and D), confirming specifically endothelial dysfunction in AIN-93M-RICE(-PK)-fed mice.
The 2-day administration of either PK-Oil-HyC12 or PK-Oil to the mice fed the AIN-93M-RICE(-PK) diet was ineffective in reversing the PK deficiency-induced impairment of Ach-induced responses in the TA (Figure 3A) and AA (Figure 3C). The SNP-induced response also remained unchanged.
Extending PK therapy to 7 days with PK-Oil-HyC12 resulted in the reversal of Ach-induced vasoconstriction in the TA (Figure 3A) and AA (Figure 3B) to Ach-induced vasodilation. In the TA, Ach-induced vasodilation was fully achieved to the level observed in AIN-93M-RICE(+PK)-fed mice (6.21% vs 7.12%), while in the AA, the impairment of Ach-induced vasodilation was only partially reversed (2.66% vs 6.75%). SNP-induced responses remained unaltered, confirming the specific effect of PK-Oil-HyC12 treatment on endothelium-dependent vasodilation. In contrast to PK-Oil-HyC12, when PK was delivered in corn oil for 7 days (PK-Oil), Ach still induced vasoconstriction in both the TA and AA. Furthermore, the magnitude of Ach-induced vasoconstriction in PK-Oil-treated AIN-93M-RICE (-PK)-fed mice was similar to that in untreated AIN-93M-RICE (-PK)-fed mice (−2.72% vs −1.83% in the TA and −4.84% vs −3.81% in the AA), demonstrating the lack of effect of PK-Oil on endothelial function after 7 days of treatment, in contrast to PK-Oil-HyC12 treatment.
The treatment with PK-Oil-HyC12 or PK-Oil did not affect the basal diameter of the aorta, as there were no differences in basal values of the AA or TA volume between all studied groups, as measured in vivo by MRI (data not shown).
Profiling of Systemic and Vascular PK Levels After 7 days of PK Treatment in Hyaluronan-Based Nanocapsules (PK-Oil-HyC12) or Oil (PK-Oil)
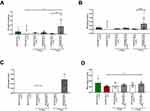
To verify whether the improved pharmacological effects of 7-day PK treatment on endothelial dysfunction when given in hyaluronan-based nanocapsules (PK-Oil-HyC12) compared to PK-Oil could be ascribed to increased systemic PK bioavailability, the concentration of PK in the plasma (Figure 4A), liver (Figure 4B) and aorta (Figure 4C) was measured. In mice with a dietary PK deficiency displaying endothelial dysfunction (achieved after 5 weeks on the AIN-93M-RICE(-PK) diet), the PK content in the plasma, liver and aorta decreased to levels below the detection limit of the LC-APCI-MS/MS method, whereby in mice fed the AIN-93M-RICE(+PK) diet, the PK was quantified in the plasma and liver (Figure 4A–C). Supplementation with PK-Oil resulted in detectable levels of PK in the plasma and liver but not in the aorta. In the PK-Oil-HyC12-treated group, the concentration of PK in the plasma and liver was much higher than that in PK-Oil-treated mice after 7 days of treatment (Figure 4A and B). Interestingly, after 7 days of treatment, 2/6 mice in the PK-Oil-HyC12 group showed a relatively high concentration of PK in the aorta (Figure 4C). The MK-4 analysis in the aorta showed that a PK-deficient diet (AIN-93M-RICE(-PK)) had a tendency to lower MK-4 levels in the aorta, while in the PK-Oil-HyC12-treated group, a significantly increased MK-4 level in the aorta was noted (Figure 4D).
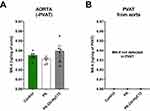
To further determine whether PK can be taken up by the aorta and converted to MK-4 and to clarify whether the difference in the pharmacological effects of PK-Oil-HyC12 and PK-Oil on endothelial function could be linked to a difference in the direct aortic uptake of encapsulated vs nonencapsulated PK, the isolated aorta without PVAT and isolated PVAT were both incubated for 24 h in the absence or the presence of 5 µM PK given in encapsulated or non-encapsulated form (PK-Oil-HyC12 and PK, respectively). Aortic MK-4 levels were similar after 24 h of incubation with the encapsulated or non-encapsulated forms, suggesting similar aortic uptake of PK delivered in vitro regardless of formulation (Figure 5A). Notably, MK-4 was not detectable in PVAT isolated from the aorta after incubation with PK, independently of whether PK was encapsulated or not (Figure 5B), excluding the involvement of PVAT in PK-MK-4 conversion and storage.
Discussion
In the present work, we demonstrated that PK administered in the form of hyaluronan-based nanocapsules with an oil core (PK-Oil-HyC12) had a higher systemic bioavailability than PK given in an oil emulsion (PK-Oil). Most importantly, PK encapsulated in hyaluronan-based nanocapsules efficiently restored systemic PK deficiency and reversed endothelial dysfunction induced by a dietary PK deficit within a period as short as 7 days, whereas PK delivered in oil did not.
In the current work, we took advantage of a previously described experimental model of endothelial dysfunction induced by short-term dietary PK deficiency.26 In this model, 5 weeks of a PK-deficient diet did not affect blood coagulation but markedly impaired endothelium-dependent vasodilation in vivo in mice, which was fully reversed by subsequent dietary PK supplementation for 5 weeks.26 Here, using the murine model of dietary PK deficiency, we showed that systemic levels of PK may be replenished within as little as 7 days using a relatively low dose of 0.5 mg/kg/day of PK encapsulated in hyaluronan-based nanocapsules. Of note, the PK dose of 0.5 mg/kg/day in mice reflects a human dose of ⁓2 mg/day.36
Taken together, our results indicate that PK encapsulated in hyaluronan-based nanocapsules (or possibly any other PK formulation ensuring increased PK bioavailability) may afford the rapid replenishment of inadequate PK levels using a relatively low dose of PK and short-term treatment, resulting in the reversal of endothelial dysfunction driven by insufficient PK levels.
In the current study, we showed that cycloheximide, a chylomicron assembly formation inhibitor,24 displayed minor effects, if any, on PK-Oil-HyC12 absorption, indicating the involvement of a chylomicron-independent absorption pathway in the case of nanocapsules compared to dietary PK, which is absorbed in a chylomicron-dependent manner.37 Given that there are two possible pathways for intestinal lymphatic transport, namely via chylomicrons in enterocytes and via the microfold cells (M cells) in Peyer’s patches, our observation of the chylomicron-independent lymphatic transport of PK-Oil-HyC12 suggests that the intestinal absorption of PK encapsulated in hyaluronan-based nanocapsules was mainly mediated by M cells, as previously reported for a number of polymeric nanoparticles.38–41
Taking advantage of in situ confocal imaging, we demonstrated that within a short time after application (5–10 min), hyaluronan-based nanocapsules were observed in mesenteric lymphatics but not in mesenteric blood veins and arteries, pointing out to lymphatic transport of hyaluronan-based nanocapsules. Of note, PK was administered in the form of hyaluronan-based nanocapsules with an approximate size of 450 nm, thereby precluding the possibility of direct absorption into the bloodstream due to the restrictive nature of the tight junctions of endothelial cells.42 In contrast, the lymphatic capillaries, characterized by their fenestrated structure with openings typically ranging from tens to hundreds of nanometers, facilitate the drainage of macromolecules and relatively big particulate matter.43 Moreover, our pharmacokinetic data demonstrate a prolonged absorption of PK-Oil-HyC12 in the systemic circulation as compared to PK-Oil providing further support for chylomicron-independent lymphatic transport for PK encapsulated in hyaluronan-based nanocapsules, in contrast to the chylomicron-dependent lymphatic transport observed with dietary PK.
In the current study, we demonstrated that the liver was the target organ of PK encapsulated in hyaluronan-based nanocapsules. In the organism, dietary PK is absorbed alongside lipids in the small intestine and transported to the liver which serves as a primary storage site for PK.33–35,44 In turn, PK transport out of the liver occurs via the intermediation of very low-density lipoprotein (VLDL). Thus, the extrahepatic transport of dietary PK including vascular delivery seems to be strongly linked to VLDL, metabolised into intermediate-density lipoproteins (IDL) and low-density lipoproteins (LDL).34,35 Our data show effective delivery of PK in form of nanocapsules to the liver that might result in an increased bioavailability of PK and increased delivery to the targeted tissues (including vasculature) most likely by the same pathways as for dietary PK. However, to confirm if the PK targeted to liver by nanocapsules is more actively transported via LDL to the vasculature further studies are needed.
It was suggested that liver uptake of hyaluronan-based nanocapsules may be mediated by hyaluronan receptors which high expression has been reported in liver endothelial sinusoidal cells (LSECs), Kupffer cells and hepatocytes.45–47 Moreover, previous studies demonstrated that liver uptake of hyaluronan-based nanocapsules48 was dramatically enhanced in endotoxemia, which was ascribed to the upregulation of hyaluronan receptor expression in inflammation.49 Still, more detailed studies are needed to confirm the involvement of hyaluronan receptor in hyaluronan-based nanocapsules uptake to the liver.
The key finding of this study was that PK encapsulated in hyaluronan-based nanocapsules effectively restored systemic PK levels and reversed endothelial dysfunction caused by a dietary PK deficiency within just 7 days, whereas PK administered in oil was not effective. To assess the functional effect of the therapy, we examined endothelium-dependent vasodilation using an in vivo MRI approach, which is a well-validated method to measure endothelial function that has been used in a number of our previous studies.29,50–52 This approach represents a reliable and sensitive method to detect alterations in the nitric oxide-dependent function of the endothelium.28 Importantly, in our study, only PK-Oil-HyC12 but not PK-Oil reversed Ach-induced vasoconstriction in mice fed a PK-deficient diet to Ach-induced vasodilation, consistent with improved endothelial function.53 The effects of PK on endothelial function observed here stay in line with previous reports showing improvement of endothelial function in vivo by MK or PK.6,7,26 At the same time, the novelty of this study was the demonstration that hyaluronan-based nanocapsules with an oil core are an efficient carrier for PK delivery increasing the bioavailability of PK, that resulted in the rapid reversal of impaired endothelial function induced by a dietary PK deficiency.
The effects of PK-Oil-HyC12 was studied in the aorta but not in other vessels, that present a limitation of this study. However, in our previous studies, effects of vitamin K on endothelial function were detected not only in the aorta but also in the femoral artery,26 brachiocephalic artery and left common carotid artery7 indicative of the systemic effects of vitamin K treatment on conduit-type vessels. Further studies are needed to test whether 7 days PK therapy with PK-Oil-HyC12 would result in the reversal of PK-deficient diet-induced endothelial dysfunction in other types of conduit vessels and in smaller vessels. Furthermore, in the current study we used only male mice which is justified by their vulnerability to developing endothelial dysfunction and cardiovascular diseases,54–56 but obviously studies in female mice should be carried out to confirm the similar efficiency of encapsulated PK to reversed endothelial dysfunction induced by PK dietary deficiency.
Finally, although we provide evidence that PK therapy, when delivered in hyaluronan-based nanocapsules, rapidly improves endothelial function, the precise mechanism behind remains unclear. Specifically, it remains uncertain whether the effects are due to PK itself or the MK-4 formed from PK within the vascular wall.
Several cells and organs can synthesise MK-4, including macrophages, bacteria, the intestinal epithelium, kidneys, the brain and the endothelium.3,57–60 In the present study, we detected increased MK-4 levels in the aorta after incubation with PK, confirming that MK-4 synthesis could also occur in the vascular wall, compatible with our previous report,7 but not in the PVAT, in which MK-4 was not detectable. In our previous study, we demonstrated that the incubation of an isolated aorta with PK resulted in a substantial increase in intracellular PK levels associated with a modest increase in MK-4 content.7 These data confirm the notion that the increased systemic bioavailability of PK could contribute to increased MK-4 content in the vascular wall. In the present work, we did not provide direct evidence for the metabolism of PK into MK-4 in the vascular wall; however, this might be related to the dynamic turnover of PK and MK-4.61,62
In the current study, we assessed whether PK delivered in hyaluronan-based nanocapsules would be taken up in the isolated aorta better than PK incubated in its free form. We observed no difference, suggesting that nanocapsule does not constitute a superior vascular delivery system in vitro. These results however, again suggest that the improvement of endothelial function after supplementation with encapsulated PK could be ascribed to an increased and prolonged exposure of vasculature to PK and possibly also to increased PK bioavailability in the liver, the major reservoir for PK, from which PK is then delivered to extrahepatic tissues by lipoprotein-based transport, providing an abundant substrate for vascular MK-4 synthesis.7 In this context, a robust uptake of PK encapsulated in hyaluronan-based nanocapsules in the liver might play an important role in the vascular delivery of PK. In the current study, the PK-deficient diet reduced PK levels in plasma and liver below the detection limit and caused endothelial dysfunction. Accordingly, the systemic and liver levels of PK determined the endothelial functional status and thus seemed to be the major player in the maintenance of vascular vitamin K-dependent function.
The details of canonical or non-canonical mechanisms of PK- and MK- regulated endothelial function are still not well understood63,64 and require further studies. Irrespective of the mechanisms involved, PK and MK have been demonstrated to exert comparable vasoprotective effects.7 Consequently, both may support vascular homeostasis in relation to their availability as substrates for vitamin K–dependent processes in the vascular wall.
Taken together, although our study shows that hyaluronan-based nanocapsules enhance the efficacy of PK deficiency reversal and improve endothelial function, several questions remain to be addressed in future studies, including the mechanisms of action of vitamin K in the endothelium, effective lymphatic pathway uptake, increased liver content and increased plasma bioavailability, which seem to represent the major factors responsible for the apparent pharmacological efficacy of PK administered in hyaluronan-based nanocapsules compared to PK in oil in reversing endothelial dysfunction.
PK delivery in hyaluronan-based nanocapsules would offer a promising approach for the rapid replenishment of vitamin K in cases of low vitamin K levels to improve vascular health. Several clinical trials showed either a lack of effect or only modest effects of PK supplementation, most likely because of low PK bioavailability and inadequate dosage and treatment time.15–17 Therefore, using hyaluronan-based nanocapsules or other delivery systems that enhance PK bioavailability may allow for lower dosages and shorter treatment periods to achieve therapeutic efficacy of PK. This approach could be particularly beneficial for patients with clinically significant dietary PK deficiencies, such as those with chronic kidney failure undergoing hemodialysis65,66 or older individuals who represent a population with increased cardiovascular risk and relative PK deficiency.11 Clinical studies are warranted to translate our assumptions into the clinical setting.
Conclusion
In summary, this study provides evidence that PK delivered in the form of hyaluronan-based nanocapsules (PK-Oil-HyC12) offers enhanced bioavailability compared to nonencapsulated PK. Most importantly, the short-term supplementation of encapsulated PK at a relatively low dose resulted in the full restoration of impaired endothelial function in mice fed a PK-deficient diet, indicating that rapid systemic replenishment of PK in the plasma and liver using hyaluronan-based nanocapsules translates into better endothelial function in mice. Given that low systemic PK levels were linked to increased cardiovascular risk,26 the rapid systemic replenishment of PK using hyaluronan-based nanocapsules provides a novel approach to support vascular health.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was performed in line with guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. All experiments involving animals were conducted after obtaining permission from the 2nd Local Ethical Committee on Animal Testing of the Institute of Pharmacology, Polish Academy of Sciences (Krakow, Poland; Permit Nos. 378/2021 and 268/2022) and the 2nd Local Ethical Committee for Animal Experiments of the Warsaw University of Life Sciences (Poland; Permit No. WAW2/004/2024).
Consent for Publication
The present manuscript does not contain data from human subjects; therefore, consent for publication is not required.
Funding
This work was supported by the following Polish National Science Centre (NCN) grants: PRELUDIUM No. 2018/31/N/NZ7/03900 to Anna Kieronska-Rudek and OPUS No. 2018/29/B/NZ7/01684 to Stefan Chlopicki. Anna Kieronska-Rudek also acknowledges the support of the National Centre for Research and Development (InterDokMed Project No. POWR.03.02.00-00-I013/16). Justyna Bednorz gratefully acknowledges the financial support of the Polish Ministry of Education and Science within the “Industrial Doctoral Programme I” (Agreement No. DWD/5/0402/2021).
Disclosure
Szczepan Zapotoczny serves as a consultant for the CHDE company, and Justyna Bednorz and Małgorzata Janik-Hazuka are employees of CHDE Polska S.A., which holds Patent No. WO2017014655 for the nanocapsules used in the study. Anna Kieronska-Rudek, Agnieszka Kij, Anna Bar, Justyna Bednorz, Małgorzata Janik-Hazuka, Szczepan Zapotoczny and Stefan Chlopicki are authors of a patent application (PCT/PL2024/000056) on the reversal of vitamin K deficiency with the use of the described nanocapsule-vitamin K formulation. Magdalena Sternak, Filip A Fedak, Izabela Czyzynska-Cichon, Kamila Wojnar-Lason, Renata B Kostogrys, Magdalena Franczyk-Zarow, Aleksandra Paterek, Filip Rolski, Katarzyna Z Czyżowska, Lenka Michalkova, Michał Mączewski and Edyta Kus have no relevant financial or non-financial interests to disclose.
References
1. Mladěnka P, Macáková K, Kujovská Krčmová L, et al. Vitamin K – sources, physiological role, kinetics, deficiency, detection, therapeutic use, and toxicity. Nutr Rev. 2022;80(4):677–698. doi:10.1093/nutrit/nuab061
2. Dam H, Schønheyder F. The occurrence and chemical nature of vitamin K. Biochem J. 1936;30(5):897–901. doi:10.1042/bj0300897
3. Kieronska-Rudek A, Kij A, Kaczara P, et al. Exogenous Vitamins K exert anti-inflammatory effects dissociated from their role as substrates for synthesis of endogenous MK-4 in murine macrophages cell line. Cells. 2021;10(7):1571. doi:10.3390/cells10071571
4. Pan MH, Maresz K, Lee PS, et al. Inhibition of TNF-α, IL-1α, and IL-1β by pretreatment of human monocyte-derived macrophages with menaquinone-7 and cell activation with TLR agonists in vitro. J Med Food. 2016;19(7):663–669. doi:10.1089/jmf.2016.0030
5. Halder M, Petsophonsakul P, Akbulut AC, et al. Vitamin K: double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int J Mol Sci. 2019;20(4):896. doi:10.3390/ijms20040896
6. Bar A, Kus K, Manterys A, et al. Vitamin K2-MK-7 improves nitric oxide-dependent endothelial function in ApoE/LDLR−/− mice. Vascul Pharmacol. 2019;122–123:106581. doi:10.1016/j.vph.2019.106581
7. Kieronska-Rudek A, Kij A, Bar A, et al. Phylloquinone improves endothelial function, inhibits cellular senescence, and vascular inflammation. GeroScience. 2024;46(5):4909–4935. doi:10.1007/s11357-024-01225-w
8. Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food: effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30(6):298–307. doi:10.1159/000054147
9. Chungchunlam SMS, Moughan PJ. Comparative bioavailability of vitamins in human foods sourced from animals and plants. Crit Rev Food Sci Nutr. 2024;64(31):11590–11625. doi:10.1080/10408398.2023.2241541
10. Bellinge JW, Dalgaard F, Murray K, et al. Vitamin K intake and atherosclerotic cardiovascular disease in the danish diet cancer and health study. J Am Heart Assoc. 2021;10(16):e020551. doi:10.1161/JAHA.120.020551
11. Shea MK, Booth SL, Weiner DE, et al. Circulating vitamin K is inversely associated with incident cardiovascular disease risk among those treated for hypertension in the health, aging, and body composition study (Health ABC). J Nutr. 2017;147(5):888–895. doi:10.3945/jn.117.249375
12. Erkkilä AT, Booth SL, Hu FB, et al. Phylloquinone intake as a marker for coronary heart disease risk but not stroke in women. Eur J Clin Nutr. 2005;59(2):196–204. doi:10.1038/sj.ejcn.1602058
13. Shea MK, O’Donnell CJ, Hoffmann U, et al. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89(6):1799–1807. doi:10.3945/ajcn.2008.27338
14. Park JE, Kim KE, Choi YJ, Park YD, Kwon HJ. The stability of water- and fat-soluble vitamin in dentifrices according to pH level and storage type. Biomed Chromatogr. 2016;30(2):191–199. doi:10.1002/bmc.3535
15. Shishavan NG, Gargari BP, Jafarabadi MA, Kolahi S, Haggifar S, Noroozi S. Vitamin K1 supplementation did not alter inflammatory markers and clinical status in patients with rheumatoid arthritis. Int J Vitam Nutr Res. 2018;88(5–6):251–257. doi:10.1024/0300-9831/a000276
16. Bolton-Smith C, McMurdo MET, Paterson CR, et al. Two-year randomized controlled trial of vitamin K1 (Phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res. 2007;22(4):509–519. doi:10.1359/jbmr.070116
17. Rasekhi H, Karandish M, Jalali MT, et al. The effect of vitamin K1 supplementation on sensitivity and insulin resistance via osteocalcin in prediabetic women: a double-blind randomized controlled clinical trial. Eur J Clin Nutr. 2015;69(8):891–895. doi:10.1038/ejcn.2015.17
18. Saritas T, Reinartz S, Krüger T, et al. Vitamin K1 and progression of cardiovascular calcifications in hemodialysis patients: the VitaVasK randomized controlled trial. Clin Kidney J. 2022;15(12):2300–2311. doi:10.1093/ckj/sfac184
19. Schultz CJ, Dalgaard F, Bellinge JW, et al. Dietary Vitamin K1 intake and incident aortic valve stenosis. Arterioscler Thromb Vasc Biol. 2024;44(2):513–521. doi:10.1161/ATVBAHA.123.320271
20. Czyzynska-Cichon I, Janik-Hazuka M, Szafraniec-Szczęsny J, et al. Low dose curcumin administered in hyaluronic acid-based nanocapsules induces hypotensive effect in hypertensive rats. Int J Nanomed. 2021;16:1377–1390. doi:10.2147/IJN.S291945
21. Janik‐Hazuka M, Kamiński K, Kaczor‐Kamińska M, et al. Hyaluronic acid‐based nanocapsules as efficient delivery systems of garlic oil active components with anticancer activity. Nanomaterials. 2021;11(5):1354. doi:10.3390/nano11051354
22. Janik-Hazuka M, Szafraniec-Szczęsny J, Kamiński K, Odrobińska J, Zapotoczny S. Uptake and in vitro anticancer activity of oleic acid delivered in nanocapsules stabilized by amphiphilic derivatives of hyaluronic acid and chitosan. Int J Biol Macromol. 2020;164:2000–2009. doi:10.1016/j.ijbiomac.2020.07.288
23. Szafraniec-Szczęsny J, Janik-Hazuka M, Odrobińska J, Zapotoczny S. Polymer capsules with hydrophobic liquid cores as functional nanocarriers. Polymers. 2020;12(9):1–25. doi:10.3390/polym12091999
24. Dahan A, Hoffman A. Evaluation of a chylomicron flow blocking approach to investigate the intestinal lymphatic transport of lipophilic drugs. Eur J Pharm Sci. 2005;24(4):381–388. doi:10.1016/j.ejps.2004.12.006
25. Choe K, Jang JY, Park I, et al. Intravital imaging of intestinal lacteals unveils lipid drainage through contractility. J Clin Invest. 2015;125(11):4042–4052. doi:10.1172/JCI76509
26. Kij A, Kieronska-Rudek A, Bar A, et al. Low phylloquinone intake deteriorates endothelial function in normolipidemic and dyslipidaemic mice. J Nutr Biochem. 2025;140:109867. doi:10.1016/j.jnutbio.2025.109867
27. Kij A, Bar A, Przyborowski K, et al. Thrombin inhibition prevents endothelial dysfunction and reverses 20-HETE overproduction without affecting blood pressure in angiotensin ii-induced hypertension in mice. Int J Mol Sci. 2021;22(16):8664. doi:10.3390/ijms22168664
28. Bar A, Kieronska-Rudek A, Proniewski B, et al. In vivo magnetic resonance imaging-based detection of heterogeneous endothelial response in thoracic and abdominal aorta to short-term high-fat diet ascribed to differences in perivascular adipose tissue in mice. J Am Heart Assoc. 2020;9(21):e016929. doi:10.1161/JAHA.120.016929
29. Bar A, Targosz-Korecka M, Suraj J, et al. Degradation of glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/low-density lipoprotein receptor-deficient mice. J Am Heart Assoc. 2019;8(6). doi:10.1161/JAHA.118.011171
30. Bar A, Skórka T, Jasiński K, et al. Retrospectively gated MRI for in vivo assessment of endothelium-dependent vasodilatation and endothelial permeability in murine models of endothelial dysfunction. NMR Biomed. 2016;29(8):1088–1097. doi:10.1002/nbm.3567
31. Pośpiech E, Bar A, Pisarek-Pacek A, Karaś A, Branicki W, Chlopicki S. Epigenetic clock in the aorta and age-related endothelial dysfunction in mice. GeroScience. 2024;46(4):3993–4002. doi:10.1007/s11357-024-01086-3
32. Phinikaridou A, Andia ME, Protti A, et al. Noninvasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation. 2012;126(6):707–719. doi:10.1161/CIRCULATIONAHA.112.092098
33. Allison AC. The possible role of vitamin K deficiency in the pathogenesis of Alzheimer’s disease and in augmenting brain damage associated with cardiovascular disease. Med Hypotheses. 2001;57(2):151–155. doi:10.1054/mehy.2001.1307
34. Mosler K, von Kries R, Vermeer C, Saupe J, Schmitz T, Schuster A. Assessment of vitamin K deficiency in CF - How much sophistication is useful? J Cyst Fibros. 2003;2(2):91–96. doi:10.1016/S1569-1993(03)00025-0
35. Kohlmeier M, Salomon A, Saupe J, Shearer MJ. Transport of vitamin K to bone in humans. J Nutr. 1996;126(4 SUPPL):1192S–6S. doi:10.1093/jn/126.suppl_4.1192s
36. Department of Health and Human Services, Administration F and D. Guidance for industry on estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. 2005. Available from: https://www.fda.gov/media/72309/download.
37. Blomstrand R, Forsgren L. Vitamin K1-3H in man. Its intestinal absorption and transport in the thoracic duct lymph. Int J Vitam Res. 1968;38(1):45–64.
38. Cai S, Xie Y, Bagby TR, Cohen MS, Forrest ML. Intralymphatic chemotherapy using a hyaluronan-cisplatin conjugate. J Surg Res. 2008;147(2):247–252. doi:10.1016/j.jss.2008.02.048
39. Van Der Lubben IM, Verhoef JC, Van Aelst AC, Borchard G, Junginger HE. Chitosan microparticles for oral vaccination: preparation, characterization and preliminary in vivo uptake studies in murine Peyer’s patches. Biomaterials. 2001;22(7):687–694. doi:10.1016/S0142-9612(00)00231-3
40. Khan AA, Mudassir J, Mohtar N, Darwis Y. Advanced drug delivery to the lymphatic system: lipid-based nanoformulations. Int J Nanomed. 2013;8:2733–2744. doi:10.2147/IJN.S41521
41. Managuli RS, Raut SY, Reddy MS, Mutalik S. Targeting the intestinal lymphatic system: a versatile path for enhanced oral bioavailability of drugs. Expert Opin Drug Deliv. 2018;15(8):787–804. doi:10.1080/17425247.2018.1503249
42. Wang X, Wang N, Yuan L, Li N, Wang J, Yang X. Exploring tight junction alteration using double fluorescent probe combination of lanthanide complex with gold nanoclusters. Sci Rep. 2016;6. doi:10.1038/srep32218
43. Zhang Z, Lu Y, Qi J, Wu W. An update on oral drug delivery via intestinal lymphatic transport. Acta Pharm Sin B. 2021;11(8):2449–2468. doi:10.1016/j.apsb.2020.12.022
44. Kindberg CG, Suttie JW. Effect of various intakes of phylloquinone on signs of vitamin K deficiency and serum and liver phylloquinone concentrations in the rat. J Nutr. 1989;119(2):175–180. doi:10.1093/jn/119.2.175
45. Siew A, Le H, Thiovolet M, Gellert P, Schatzlein A, Uchegbu I. Enhanced oral absorption of hydrophobic and hydrophilic drugs using quaternary ammonium palmitoyl glycol chitosan nanoparticles. Mol Pharm. 2012;9(1):14–28. doi:10.1021/mp200469a
46. Mortimer BC, Redgrave TG, Spangler EA, Verstuyft JG, Rubin EM. Effect of human ApoE4 on the clearance of chylomicron-like lipid emulsions and atherogenesis in transgenic mice. Arterioscler Thromb Vasc Biol. 1994;14(10):1542–1552. doi:10.1161/01.atv.14.10.1542
47. Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240(4852):622–630. doi:10.1126/science.3283935
48. Crainie M, Belch AR, Mant MJ, Pilarski LM. Overexpression of the receptor for hyaluronan-mediated motility (RHAMM) characterizes the malignant clone in multiple myeloma: identification of three distinct RHAMM variants. Blood. 1999;93(5):1684–1696. doi:10.1182/blood.v93.5.1684
49. Szafraniec J, Błazejczyk A, Kus E, et al. Robust oil-core nanocapsules with hyaluronate-based shells as promising nanovehicles for lipophilic compounds. Nanoscale. 2017;9(47):18867–18880. doi:10.1039/c7nr05851a
50. Bar A, Skorka T, Jasinski K, Chlopicki S. MRI-based assessment of endothelial function in mice in vivo. Pharmacol Rep. 2015;67(4):765–770. doi:10.1016/j.pharep.2015.05.007
51. Bar A, Olkowicz M, Tyrankiewicz U, et al. Functional and biochemical endothelial profiling in vivo in a murine model of endothelial dysfunction; comparison of effects of 1-methylnicotinamide and angiotensin-converting enzyme inhibitor. Front Pharmacol. 2017;10(8):183. doi:10.3389/fphar.2017.00183
52. Sternak M, Bar A, Adamski MG, et al. The deletion of endothelial sodium channel α (αENaC) impairs endothelium-dependent vasodilation and endothelial barrier integrity in endotoxemia in vivo. Front Pharmacol. 2018;9(APR 10):178. doi:10.3389/fphar.2018.00178
53. Gaertner S, Auger C, Farooq MA, et al. Oral intake of EPA:DHA 6:1 by middle-aged rats for one week improves age-related endothelial dysfunction in both the femoral artery and vein: role of cyclooxygenases. Int J Mol Sci. 2020;21(3):920. doi:10.3390/ijms21030920
54. Zhang TY, Zhao BJ, Wang T, Wang J. Effect of aging and sex on cardiovascular structure and function in wildtype mice assessed with echocardiography. Sci Rep. 2021;11. doi:10.1038/s41598-021-02196-0
55. Hamm TE, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48(3):221–233. doi:10.1016/0021-9150(83)90040-0
56. Patten RD. Models of gender differences in cardiovascular disease. Drug Discov Today Dis Model. 2007;4(4):227–232. doi:10.1016/j.ddmod.2007.11.002
57. Harshman SG, Kyla Shea M, Fu X, et al. Atorvastatin decreases renal menaquinone-4 formation in C57BL/6 Male mice. J Nutr. 2019;149(3):416–421. doi:10.1093/jn/nxy290
58. Okano T, Shimomura Y, Yamane M, et al. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283(17):11270–11279. doi:10.1074/jbc.M702971200
59. Hirota Y, Tsugawa N, Nakagawa K, et al. Menadione (vitamin K3) is a catabolic product of oral phylloquinone (vitamin K1) in the intestine and a circulating precursor of tissue menaquinone-4 (vitamin K2) in rats. J Biol Chem. 2013;288(46):33071–33080. doi:10.1074/jbc.M113.477356
60. Conly JM, Stein K, Worobetz L, Rutledge‐Harding S. The contribution of Vitamin K2 (Menaquinones) produced by the intestinal microflora to human nutritional requirements for Vitamin K. Am J Gastroenterol. 1994;89(6):915–923. doi:10.1111/j.1572-0241.1994.tb03181.x
61. Gijsbers BLMG, Jie K-SG, Vermeer C. Effect of food composition on vitamin K absorption in human volunteers. Br J Nutr. 1996;76(2):223–229. doi:10.1079/bjn19960027
62. Chatron N, Hammed A, Benoît E, Lattard V. Structural insights into phylloquinone (Vitamin K1), menaquinone (MK4, MK7), and menadione (vitamin K3) binding to VKORC1. Nutrients. 2019;11(1):67. doi:10.3390/nu11010067
63. Dowd P, Ham SW, Naganathan S, Hershline R. The mechanism of action of vitamin K. Annu Rev Nutr. 1995;15(1):419–440. doi:10.1146/annurev.nu.15.070195.002223
64. Welsh JE, Bak MJ, Narvaez CJ. New insights into vitamin K biology with relevance to cancer. Trends Mol Med. 2022;28(10):864–881. doi:10.1016/j.molmed.2022.07.002
65. Fusaro M, D’Alessandro C, Noale M, et al. Low vitamin K1 intake in haemodialysis patients. Clin Nutr. 2017;36(2):601–607. doi:10.1016/j.clnu.2016.04.024
66. Cheema A, Singh T, Kanwar M, et al. Chronic kidney disease and mortality in implantable cardioverter-defibrillator recipients. Cardiol Res Pract. 2010;2010:1–6. doi:10.4061/2010/989261
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.