Back to Journals » Infection and Drug Resistance » Volume 18
Rare Bloodstream Infection of Rhodococcus rhodochrous as the Prodromal Signal for Malignancy
Received 14 December 2024
Accepted for publication 6 April 2025
Published 18 April 2025 Volume 2025:18 Pages 1951—1959
DOI https://doi.org/10.2147/IDR.S512213
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Oliver Planz
Jia Liu,1 Xintong Wu2
1Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Department of Neurology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China
Correspondence: Xintong Wu, Department of Neurology, West China Hospital, Sichuan University, No. 37 Guoxue Alley, Wuhou District, Chengdu City, Sichuan Province, 610041, People’s Republic of China, Email [email protected]
Abstract: Rhodococcus-associated infections are extremely rare, and previous publications have indicated that such infections are primarily observed among individuals with HIV. Limited information is available regarding therapy, and no clear consensus has been reached to guide treatment. Here, we report the first case of bloodstream infection with Rhodococcus rhodochrous in a non-HIV patient with a viral intracranial infection. During follow-up, lymph node biopsy and bone marrow aspiration were performed because superficial lymphadenectasis had failed to regress as expected within 3 months. The patient was newly diagnosed with nodal T-follicular helper cell lymphoma, angioimmunoblastic-type. For cases of rare infection or co-infection, screening for pathogenic microorganisms is the priority, and several methods should be employed, such as microorganism culture, antigen and antibody detection, and metagenomic next-generation sequencing. In retrospect to integrated case management, our case indicated that early malignancy screening is significant for early diagnosis and treatment of occult cancer during patients with rare opportunistic infections.
Keywords: Rhodococcus rhodochrous, bloodstream infection, intracranial infection, nodal T-follicular helper cell lymphoma
Introduction
Rhodococcus-related infections are considered opportunistic. Zopf proposed the formation of the genus Rhodococcus in 1891, and there are now 43 species classified in this genus. Rhodococcus-related infections are mainly attributed to Rhodococcus equi among patients with acquired immune deficiency syndrome.1–4 To the best of our knowledge, only two human cases of Rhodococcus rhodochrous infection have been described in the literature. In 1988, Hart et al reported the isolation of R. rhodochrous from the sputum of patients with pulmonary infections that clinically resembled tuberculosis.5 In the same year, Gopaul et al described an older woman with chronic corneal ulcers infected with R. rhodochrous.6
According to the 5th edition of the World Health Organization guidelines published in 2022, nodal T-follicular helper cell lymphoma, angioimmunoblastic-type (nTFHL-AI) is the new term for angioimmunoblastic T cell lymphoma. nTFHL-AI is common in older individuals, with the median age being 62 to 67 years.7,8 The mechanisms underlying nTFHL-AI remain unclear. Most patients received antibiotic drugs or were infected by, for example, tuberculosis bacillus, Cryptococcus, human herpesvirus, or EB virus, before presentation. Approximately half of these patients develop skin lesions, which manifest as, for example, red rashes, nodular lesions, and urticaria. Furthermore, nearly 70% of patients have marrow invasion.8,9 Over 80% of patients with nTFHL-AI are diagnosed at an advanced stage (Ann Arbor III–IV),10 and the first-line treatments include CHOP, CHOEP, and EPOCH chemotherapy, leading to an overall survival of approximately 30%.10,11
Herein, we report a case of bloodstream infection caused by R. rhodochrous combined with a central nervous system viral infection in an immunocompromised patient without HIV. During follow-up visit and routine monitoring, enlarged lymph nodes on the neck and head failed to regress as expected. Three months after onset, the patient was finally diagnosed with nTFHL-AI (Ann Arbor IV) and received CHOP chemotherapy. We describe the detailed course of treatment and summarize the distinguishing characteristics of this case. Our findings highlight that screening for acquired immunodeficiency syndrome, solid tumors, and non-solid tumors in patients with rare infectious patterns is essential. Reviewing this case could provide a preliminary therapeutic approach for R. rhodochrous infections.
Case Presentation
A 62-year-old male patient presented to the emergency department complaining of headache and fever that persisted for 10 days. The patient had a history of erythema nodosum confirmed by a lower-limb skin biopsy 3 months prior to presentation. Two months prior to presentation, the patient complained of a painful rash and blisters on the skin, which were confined to the left upper limb and dorsal scapular region and was admitted to an external general hospital with a diagnosis of herpes zoster. Ten days prior to presentation, the patient experienced sudden onset hyperpyrexia (>103° F) with occasional expectoration of yellow phlegm. Laboratory results showed a high white blood cell count (11.84 × 109/L), of which 82% were neutrophilic granulocytes, along with rising levels of procalcitonin (0.08 ng/mL), cytokines IL-6 (151 pg/mL), and C-reactive protein (58.96 mg/L). A computed tomography scan of the chest revealed scattered ground-glass opacities in the right middle lobe. Intravenous ceftriaxone was administered as an anti-infective therapy. The patient had parietal and occipital lobe headaches, so a lumbar puncture was performed on the sixth day after the fever. First cerebrospinal fluid (CSF) analysis highly indicated intracranial infection, as shown in Table 1. The next-generation sequencing assay found eight reads of varicella-zoster virus and 439 reads of torque teno virus in the CSF.
 |
Table 1 CSF Component Alterations During the Disease Course |
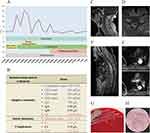
Considering the severity of the disease, the patient visited our emergency department seeking further medical intervention. Subsequently, the patient was transferred to the neurology department for advanced evaluation and treatment. Physical examination of the nervous system showed a normal level of consciousness and no apparent cranial nerve abnormality. The right pupil was round, the left pupil was oval, bilateral pupils with a diameter of approximately 2.5 mm, normal light reflexes, the activities of both eyes were in place in all directions, and no nystagmus was induced. The muscle strength of the upper limbs was 5-/5, the muscle strength of the lower limbs was 5/5, and muscle tone was normal; the knee reflexes were hyper-reflexive on both sides; and the sensory system was symmetrical, negative for pathological reflexes, and positive for meningeal irritation sign with nuchal rigidity. In summary, there was some evidence to suspect intracranial infection, and we prescribed acyclovir for antiviral therapy, mannitol and glycerin fructose for dehydration, piperacillin and tazobactam for antimicrobial therapy. The clinical workup was extensive, assessing potential bacterial, fungal, and viral infections. Enhanced magnetic resonance imaging (MRI) of the brain and a lumbar puncture were performed. The temperature curve is illustrated in Figure 1A. However, even after receiving β-lactam antibiotics, the peak temperature increased. Auxiliary tests demonstrated an immunocompromised status, which was characterized by impaired adaptive immunity (Figure 1B). On review of his MRI findings, we observed swelling from the C7 to T8 spinal cord, clear enhancement of the segment of adjacent spinal meninges, protrusion of the C3-C6 intervertebral disc, and suspected spinal cord compression at the level of the C5-C6 intervertebral disc (Figure 1C–F).
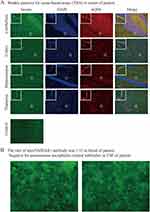
On the fifth day at our institution, a blood culture of R rhodochrous was obtained, as shown in Figure 1G and H. R. rhodochrous is a gram-positive, obligate aerobic coccobacillus that produces fungus-like mycelia that can quickly break off into rods and spheres; the appearance of spheres or rods under the microscope is associated with the species, type of specimen, growing environment, and growth stage. Our antibiotic susceptibility tests revealed that R. rhodochrous was sensitive to ceftriaxone, ciprofloxacin, linezolid, and imipenem (Table 2). The second CSF analysis showed high levels of protein and low levels of glucose; the cytological classification of CSF showed that 70% of the karyocytes were lymphocytes and the other cells were monocytes (Table 1). A work-up to distinguish other illnesses was performed; the tissue-based assay for blood was weakly positive, and the titer of anti-NMDAR1 antibody was 1:32, as shown in Figure 2A and B. Then we switched to ceftriaxone as anti-infective therapy. Human immunoglobulin was administered for 5 days to modulate immunity and intervene with the inflammatory response. Over the following 3 days, we failed to observe signs supporting clear alleviation; the clinical condition seemed to worsen because the patient gradually developed somnolence. After consulting an infectiologist, we changed the dosage of ceftriaxone to 2 g every 12 h, and the patient received an 80 mg dose of methylprednisolone daily. The third CSF sample still showed elevated levels of protein and low levels of glucose (Table 1). We observed a gradually reducing temperature peak, and the patient became awake and alert. The patient was transferred to the Department of Rehabilitation for further rehabilitation exercises and sequential anti-infection therapy.
 |
Table 2 The Antibiotic Susceptibility Test for R. rhodochrous |
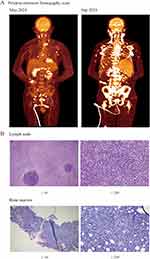
During follow-up visits, the patient complained of recurrent cutaneous erythema in their head and trunk. The superficial lymph nodes gradually enlarged, especially in the neck and inguinal area. To systemically evaluate changes in illness, we re-examined the patient’s positron emission tomography-computed tomography (PET-CT) results. The PET-CT showed diffuse lymphadenopathy distributed at the neck, hilar, abdomen, and inguinal area (maximum SUV 13.01); the enlarged node was about 20×18 mm in size in the right inguinal area. The lymphadenopathy had evidently advanced in 4 months. The image suggested that the number and size of nodes was increasing. The most enlarged node in the inguinal area was approximately 47×34 mm in size, and the hypermetabolic state was aggravated (maximum SUV 22.57) compared with 4 months previously (Figure 3A). Hence, a lymph node biopsy in the inguinal area was arranged. The morphological features were lymphoid tissue hyperplasia, angiogenesis, and clustered histological cells (Figure 3B). The immunohistochemistry staining showed CD3 expression in all cells, CD5 expression in most cells, 60–70% Ki-67 expression, and partial expression of CD30, PD-1, and EBER. The clonal amplification of IgH and TCRG was detected in B cells, and RHOA mutation (c.50G > T,p.Gly17Val) was found. In addition, the pathological evidence indicated nodal T-follicular helper cell lymphoma in the bone marrow (Figure 3B). Consequently, the patient was newly diagnosed with nodal T-follicular helper cell lymphoma, angioimmunoblastic-type (Ann Arbor IV), and CHOP chemotherapy was selected for the initial therapy.
Discussion
Rhodococcus belongs to the family Nocardiaceae. Among the 43 species in the genus, those related to medical field are R. equi,4 R. rhodnii,12,13 R. erythropolis,14–18 R. rhodochrous, and R. bronchialis.19–22 R. equi attacks lung macrophages and is a rare contributor to cavitary pneumonia. Regarding the general understanding of R. rhodochrous in the medical field, few reports have investigated infection caused by R. rhodochrous in humans. To the best of our knowledge, only two cases reported >30 years ago have described the isolation of R. rhodochrous separately from phlegm and corneal ulcers,5,6 and rifampicin was suggested as an effective antibiotic agent for rhodococcal infection. Previous studies have mainly elucidated the promising role of R. rhodochrous in producing synthetic organic substances, whole-cell biocatalysts in organic chemistry,23 and plastic degradation.24 With regards to antibiotic susceptibility testing, we found that R. rhodochrous was sensitive to ceftriaxone, ciprofloxacin, linezolid, and imipenem. Initially, the infusion dosage of ceftriaxone was 2 g daily; however, clinical deterioration may have been caused by a poor response to anti-bacterial therapy, particularly the aggravative disturbance of consciousness. Subsequently, the ceftriaxone dosage was changed to 2 g every 12 h, and dynamic alterations in laboratory tests and peak temperatures indicated clinical alleviation. The dosage of treatment for Rhodococci-related bloodstream infection is not standardized, and our case revealed that 2 g of ceftriaxone every 12 h may be a more suitable choice for initial therapy. Therapy recommendations were absent for R. rhodochrous infections at present, further researches into the optimal dosage in whether individuals with different comorbidities or infection in disparate organs, and studies on possible mechanism of drug resistance in R. rhodochrous may prevent poor prognosis.
CSF culture, viral and bacterial polymerase chain reactions, and blood culture are crucial laboratory tests for suspected intracranial infections. The positive rate of organisms in CSF culture is generally unsatisfactory. Beyond CSF culture, blood culture is recommended to identify pathogenic organisms, particularly when the body temperature is >101.3° F. A distinguishing feature of our patient was the high level of protein and extremely low level of glucose in the CSF, which was distinct from a typical viral infection. The isolation of R. rhodochrous using blood cultures may partly explain the aberrant CSF results. Poor therapeutic effectiveness of anti-viral and anti-bacterial treatments or clinical worsening was considered; thus, tissue-based assay of autoimmune encephalitis-related antibodies in both blood and CSF should be performed whenever possible. Reviewing the temperature curve, we observed that additional infusion of human immunoglobulin and methylprednisolone was favorable for clinical remission. Reviewing the residential treatment, the therapeutic response only represented individual efficacy, due to the overlapping infection of R. rhodochrous and virus, the description and evaluation of clinical characteristics about R. rhodochrous was inadequate. Overall, timely evaluation of the therapeutic effects and identification of possible comorbidities and subsequent diseases are important for tailoring therapeutic schedules and promoting recovery.
Tumors are strongly associated with opportunistic infections. Patients with cancer are vulnerable to opportunistic infections, the pneumonia is more common than bloodstream infection and central nervous system infection. Opportunistic infections in patients with cancer that are primarily treatment-driven (such factors include immunocompromised states like neutropenia, and allogeneic hematopoietic cell transplantation) but may also be malignancy-driven.25 Neutropenia has been recognized as a major risk factor for infections in patients with cancer receiving chemotherapy. In our case, the patient initially manifested a complex infection involving R. rhodochrous in the blood and a virus in the brain. Drug-taking, AIDS, uncontrolled diabetes mellitus, glucocorticoid use, and cancers are the main risk factors for immune disorders and rare infections. Finally, the pathological results of the patient indicated that the principal diagnosis was nTFHL-AI, a hematologic malignancy. Our case suggested that not only we should alert to opportunistic infections in patients with cancer, but also early cancer screening are significant for uncovering the occult malignancies in patients with rare opportunistic infection.
Ethics Approval and Informed Consent
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. The case report was approved by the Ethics Committee of the West China Hospital, Sichuan University.
Acknowledgments
The authors thank the patient and her family for placing their trust in us.
Funding
This research was supported by grants from Science & Technology Department of Sichuan Province (No.2021YFC2401204 and No.2022YFC2503805), and Science & Technology Department (No.2023YFQ0109).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Ayoade F, Vaqar S, Alam MU. Rhodococcus equi. StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC; 2024.
2. Li N, Wu C, Cao P, Chen D, Chen F, Shen X. Multiple systemic infections caused by Rhodococcus equi: a case report. Access Microbiol. 2024;6(2). doi:10.1099/acmi.0.000600.v4
3. Li P, Zhang L, Li X, Zhang X. Rhodococcus equi and Brucella pulmonary mass in immunocompetent: a case report and literature review. Open Life Sci. 2024;19(1):20220888. doi:10.1515/biol-2022-0888
4. Ranganath N, Mendoza MA, Stevens R, Kind D, Wengenack N, Shah A. Rhodococcus infection: a 10-year retrospective analysis of clinical experience and antimicrobial susceptibility profile. J Clin Microbiol. 2024;62(3):e0153723. doi:10.1128/jcm.01537-23
5. Hart DH, Peel MM, Andrew JH, Burdon JG. Lung infection caused by Rhodococcus. Aust NZ J Med. 1988;18(6):790–791. doi:10.1111/j.1445-5994.1988.tb00182.x
6. Gopaul D, Ellis C, Maki A, Joseph MG. Isolation of Rhodococcus rhodochrous from a chronic corneal ulcer. Diagn Microbiol Infect Dis. 1988;10(3):185–190. doi:10.1016/0732-8893(88)90039-9
7. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124–4130.
8. Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–4470. doi:10.1182/blood-2007-08-105759
9. Lunning MA, Vose JM. Angioimmunoblastic T-cell lymphoma: the many-faced lymphoma. Blood. 2017;129(9):1095–1102. doi:10.1182/blood-2016-09-692541
10. Federico M, Rudiger T, Bellei M, et al. Clinicopathologic characteristics of angioimmunoblastic T-cell lymphoma: analysis of the international peripheral T-cell lymphoma project. J Clin Oncol. 2013;31(2):240–246. doi:10.1200/JCO.2011.37.3647
11. Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi:10.1182/blood-2016-01-643569
12. Tobias NJ, Eberhard FE, Guarneri AA. Enzymatic biosynthesis of B-complex vitamins is supplied by diverse microbiota in the Rhodnius prolixus anterior midgut following Trypanosoma cruzi infection. Comput Struct Biotechnol J. 2020;18:3395–3401. doi:10.1016/j.csbj.2020.10.031
13. Batista KKS, Vieira CS, Figueiredo MB, et al. Influence of Serratia marcescens and Rhodococcus rhodnii on the humoral immunity of Rhodnius prolixus. Int J mol Sci. 2021;22(20):10901. doi:10.3390/ijms222010901
14. Baba H, Nada T, Ohkusu K, Ezaki T, Hasegawa Y, Paterson DL. First case of bloodstream infection caused by Rhodococcus erythropolis. J Clin Microbiol. 2009;47(8):2667–2669. doi:10.1128/JCM.00294-09
15. Bagdure SR, Fisher MA, Ryan ME, Khasawneh FA. Rhodococcus erythropolis encephalitis in patient receiving rituximab. Emerg Infect Dis. 2012;18(8):1377–1379. doi:10.3201/eid1808.110434
16. Grant LM, Seville MT, Graf EH, Vikram HR. Rhodococcus erythropolis septic arthritis. BMJ Case Rep. 2023;16(10):e256864. doi:10.1136/bcr-2023-256864
17. Park SD, Uh Y, Jang IH, Yoon KJ, Kim HM, Bae YJ. Rhodococcus erythropolis septicaemia in a patient with acute lymphocytic leukaemia. J Med Microbiol. 2011;60(Pt 2):252–255. doi:10.1099/jmm.0.015412-0
18. Roy M, Sidhom S, Kerr KG, Conroy JL. Case report: Rhodococcus erythropolis osteomyelitis in the toe. Clin Orthopaedics Related Res. 2009;467(11):3029–3031. doi:10.1007/s11999-009-0901-z
19. Alnajjar M, Mudawi D, Cherif H, et al. Central catheter-related Gordonia bronchialis bacteremia in an immunocompromised patient: a case report, and literature review. IDCases. 2023;32:e01738. doi:10.1016/j.idcr.2023.e01738
20. Pino-Rosa S, Medina-Pascual MJ, Carrasco G, et al. Focusing on Gordonia infections: distribution, antimicrobial susceptibilities and phylogeny. Antibiotics. 2023;12(11):1568. doi:10.3390/antibiotics12111568
21. Quita R, Ferraz B, Silva B, Faria N, Dias C, Cruz H. A unique case of Gordonia bronchialis pneumonia. Archivos de Bronconeumologia. 2023;59(2):114–115. doi:10.1016/j.arbres.2022.10.010
22. Zivkovic Zaric R, Canovic P, Zaric M, et al. Antimicrobial treatment in invasive infections caused by Gordonia bronchialis: systematic review. Front Med. 2024;11:1333663. doi:10.3389/fmed.2024.1333663
23. Busch H, Hagedoorn PL, Hanefeld U. Rhodococcus as a versatile biocatalyst in organic synthesis. Int J mol Sci. 2019;20(19):4787. doi:10.3390/ijms20194787
24. Zampolli J, Orro A, Vezzini D, Di Gennaro P. Genome-based exploration of Rhodococcus species for plastic-degrading genetic determinants using bioinformatic analysis. Microorganisms. 2022;10(9):1846. doi:10.3390/microorganisms10091846
25. Baden LR, Swaminathan S, Almyroudis NG, et al. Prevention and treatment of cancer-related infections, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. J Natl Comprehens Cancer Network. 2024;22(9):617–644. doi:10.6004/jnccn.2024.0056
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.