Back to Journals » Cancer Management and Research » Volume 17
Rare Site of an Inflammatory Myofibroblastic Tumor at the Epididymis: A Case Report
Authors Lange A , Dominiak N, Petros FG
Received 16 August 2024
Accepted for publication 13 January 2025
Published 28 January 2025 Volume 2025:17 Pages 193—196
DOI https://doi.org/10.2147/CMAR.S481940
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Alyssa Lange,1 Nicole Dominiak,2 Firas G Petros1
1Department of Urology, College of Medicine and Life Sciences, The University of Toledo, Toledo, OH, USA; 2Department of Pathology, College of Medicine and Life Sciences, The University of Toledo, Toledo, OH, USA
Correspondence: Firas G Petros, Department of Urology, The University of Toledo College of Medicine and Life Sciences, 3000 Arlington Ave. Mail Stop 1091, Toledo, Ohio, 43614-2598, USA, Tel +1(419).383.3584, Fax +1(419).383.3785, Email [email protected]
Background: Inflammatory myofibroblastic tumors (IMT) are uncommon with an estimated 150– 200 people being diagnosed in the US annually. We describe a healthy adult male who presented with an enlarging, painless scrotal mass. Based on current literature, only nine epididymal inflammatory myofibroblastic tumors have been reported and in this case report we describe the tenth.
Case Presentation: A 40-year-old Caucasian male presented with an enlarging mass near his right testicle with no associative symptoms or obvious etiology. Scrotal ultrasound showed a solid heterogenous mass with internal vascularity. Tumor markers were unremarkable for lactate dehydrogenase (LDH), beta-human chorionic gonadotropin (b-hCG), and alpha-fetoprotein (AFP). A right inguinal approach was performed. A 2– 3 cm round mass adjacent to the tail of the epididymis was excised with clear margins and sent to pathology. Histology confirmed a 2.4 cm inflammatory myofibroblastic tumors with scattered positivity for smooth muscle actin, negative pancytokeratin, and negative anaplastic lymphoma kinase. Patient recovered well with no reoccurrence at this time.
Conclusion: Inflammatory myofibroblastic tumors recurrence rate is < 2%; however, some literature suggests higher depending on location and immunohistochemical profile. The expertise of pathologists, utilization of morphology, and immunohistological profile are all crucial in accurate diagnoses of these lesions. Literature reports some lesions have demonstrated metastatic tendencies and therefore complete excision of the mass is the recommended therapy of choice. This case highlights the increasing need to include IMT in differential diagnoses for patients presenting with painless lumps even in unlikely locations. While there is little data on epididymal tail mass recurrence rate potential, we report no current recurrence after complete excision of the mass.
Keywords: paratesticular tumor, IMT, epididymis, intrascrotal mass
Introduction
Inflammatory myofibroblastic tumors (IMT) are uncommon with an estimated 150–200 people being diagnosed in the US annually.1 It is no surprise that there is broad terminology to describe these tumors including inflammatory pseudotumor, plasma cell granuloma, lymphoid hamartoma, myxoid hamartoma, and most recently IMT, likely due to the uncertainty in the etiology of these lesions.2 The clouded understanding of IMT origination and pathology demonstrates the need for precise diagnoses as these tumors have the potential to reoccur or become unresectable.
We describe a healthy adult male who presented with an enlarging, painless scrotal mass. Based on the current literature, only nine epididymal IMT have been reported and in this case report we describe the tenth.3 This case serves to add to our understanding of these uncommon tumors and underscores the importance of considering IMTs in the differential diagnosis of scrotal masses, particularly in cases where traditional etiological factors are not apparent.
Case Presentation
A relatively healthy 40-year-old Caucasian male presented to the UTMC Urology Clinic after noticing a lump near his right testicle. The lump appeared within the past year and has slowly grown to the size of a marble without causing pain or discomfort. The patient denied any lower urinary tract symptoms, hematuria, prior STDs, scrotal trauma, infection, or dysuria.
A scrotal ultrasound showed a solid heterogenous 1.7 × 1.5 × 1.9 cm lesion adjacent to the medial aspect of the right testicle with internal vascularity. At the time, it was unclear the level of involvement into the epididymis or testicle.
Patient underwent serum testing for tumor markers, which included lactate dehydrogenase (LDH), beta-human chorionic gonadotropin (b-hCG), and alpha-fetoprotein (AFP). All markers came back unremarkable.
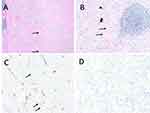
After discussion of the patient’s risk factors, the patient elected for right inguinal exploration. During the procedure, a 2 cm marble-like mass adjacent to the tail of the epididymis was identified and completely excised (Figure 1). On gross pathologic examination, the mass was isolated from the epididymal tail and surrounding structures appeared unaffected. Histology revealed a 2.4 cm IMT (Figure 2A and B) confirmed by immunohistochemistry (IHC) staining with scattered positivity of smooth muscle actin (Figure 2C), negative pancytokeratin, and negative anaplastic lymphoma kinase (ALK) (Figure 2D). Surgical margins were negative for involvement. The patient recovered well and had no evidence of recurrence at 6 months follow-up.
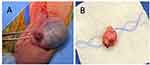 |
Figure 1 (A) Intraoperative view of right testicle with mass on epididymal tail. (B) Complete excision of right testicle epididymal tail mass. |
Discussion
IMTs have been found in a variety of anatomical sites including the lung, bladder, spleen, breast, pancreas, liver, colon, and soft tissue to name a few.4 Interestingly, the occurrence of IMTs in the epididymal region is exceptionally rare, with only a handful of cases reported, numbering as few as nine in literature. Proposed hypotheses include delayed response to trauma, infectious etiologies including Epstein Barr virus, herpes virus 8, and mycobacterium avium intracellulare, however, no clear cause has been established.3
IMT histological features are important in diagnosis with biomarkers used for additional diagnostic support. Histologically IMTs are described with spindle myofibroblastic cell proliferation and lymphocytic and inflammatory infiltration.3,5,6 Approximately half of IMTs are negative for ALK, and these ALK-negative variants may exhibit a more aggressive behavior, potentially predisposing to increased metastatic occurrences compared to ALK-positive IMTs.5,6 Recent investigations into the oncogenic mechanisms underlying IMTs have unveiled a prevalence of kinase fusions in the majority of cases, offering prospects for targeted therapies in unresectable tumors.6 Such discoveries illuminate a promising avenue for the development of novel therapeutic modalities tailored to the molecular profile of IMTs.
Traditionally perceived as benign reactive lesions, certain variants of IMTs, particularly those found in the abdomen and pelvis, exhibit recurrence rates of 85%.5 It is important to note the previous study did not have negative surgical margins for all patients, and one-third of the patients were treated with combination treatments including surgery plus chemotherapy, surgery plus radiation, and surgery plus chemoradiation.5 Another study was done on ALK-negative IMT on the kidney which had 0% recurrence rate after following their eight patients from one to seventeen years.7 While IMT pathophysiology remains not well understood, surgery is the first choice for diagnostic and therapeutic roles with a recurrence rate of <2% after complete resection.8 Although data specific to recurrence rates and metastatic potential in epididymal tail mass IMTs are scarce, it is noteworthy that other variants have demonstrated metastatic tendencies, underscoring the importance of identifying and monitoring these lesions. Our patient had a negative surgical resection and continues to have no evidence of disease.
Conclusion
There are a variety of differential diagnoses with IMT requiring the expertise of pathologists. Crucial aspects of diagnosis include immunohistochemical profile and general morphology when discovering these tumors in unlikely locations including the epididymal tail. Due to reoccurrence rate of IMT, complete excision of the mass is recommended as the therapy of choice. As more awareness is brought to IMT, an increased demand for research is warranted including treatments for unresectable IMT, standardized follow-up protocols, and treatment of recurrence disease. The immunochemistry from biopsied samples will be another research aspect looking into ALK-positive vs ALK-negative IMT specific treatments. Long-term follow-up of such patients is essential to identify IMT recurrence as early as possible, allowing for prompt and potentially more effective treatment interventions to be initiated.
Abbreviations
IMT, Inflammatory myofibroblastic tumors; LDH, lactate dehydrogenase; b-hCG, Beta-human chorionic gonadotropin; AFP, alpha-fetoprotein; IHC, immunohistochemistry; ALK, anaplastic lymphoma kinase; H&E, Hematoxylin & Eosin.
Consent for Publication
A written informed consent was obtained from the patient for publication of this case report and any accompanying images. Institutional approval was not required.
Funding
Frank Stranahan Foundation for Oncological Research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Inflammatory Myofibroblastic Tumor. National Cancer Institute: My Pediatric and Adult Rare Tumor Network. 2019. Available From:https://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/rare-soft-tissue-tumors/inflammatory-myofibroblastic-tumor.
2. Palaskar S, Koshti S, Maralingannavar M, et al. Inflammatory myofibroblastic tumor. Contemp Clin Dent. 2011;2(4):274–277. doi:10.4103/0976-237X.91787
3. Dangle PP, Wang WP, Pohar KS. Inflammatory myofibroblastic tumor of epididymis: a case report and review of literature. World J Surg Oncol. 2008;6:119. doi:10.1186/1477-7819-6-119
4. Kovach SJ, Fischer AC, Katzman PJ, et al. Inflammatory myofibroblastic tumors. J Surg Oncol. 2006;94(5):385–391. doi:10.1002/jso.20516
5. Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31(4):509–520. doi:10.1097/01.pas.0000213393.57322.c7
6. Lovly CM, Gupta A, Lipson D, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov. 2014;4(8):889–895. doi:10.1158/2159-8290.CD-14-0377
7. Kapusta LR, Weiss MA, Ramsay J, et al. Inflammatory myofibroblastic tumors of the kidney: a clinicopathologic and immunohistochemical study of 12 cases. Am J Surg Pathol. 2003;27(5):658–666. doi:10.1097/00000478-200305000-00009
8. Carrasco Rodríguez R, García Fontán EM, Blanco Ramos M, et al. Inflammatory pseudotumor and myofibroblastic inflammatory tumor. Diagnostic criteria and prognostic differences. Cir Esp. 2022;100(6):329–335. doi:10.1016/j.ciresp.2021.03.009
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.