Back to Journals » Cancer Management and Research » Volume 17
Real-World Efficacy and Safety of Anti-PD-1 Antibody Plus Apatinib and Temozolomide for Advanced Acral Melanoma
Authors Zhang J, Tian H, Mao L, Li C, Wei X, Gu J, Wang X, Zhou L, Lian B, Tang B, Yan X, Li S, Cui C, Chi Z, Sheng X, Guo J, Si L
Received 5 February 2025
Accepted for publication 23 April 2025
Published 1 May 2025 Volume 2025:17 Pages 905—916
DOI https://doi.org/10.2147/CMAR.S520937
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Jiaran Zhang,1,* Huichun Tian,1,* Lili Mao,1 Caili Li,1 Xiaoting Wei,1 Junjie Gu,1 Xuan Wang,1 Li Zhou,2 Bin Lian,1 Bixia Tang,1 Xieqiao Yan,2 Siming Li,2 Chuanliang Cui,1 Zhihong Chi,1 Xinan Sheng,2 Jun Guo,1 Lu Si1
1Department of Melanoma and Sarcoma, Peking University Cancer Hospital & Institute, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing, 100142, People’s Republic of China; 2Department of Genitourinary Oncology, Peking University Cancer Hospital & Institute, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing, 100142, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lu Si, Peking University Cancer Hospital & Institute, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), 52# Fucheng Road, Haidian District, Beijing, 100142, People’s Republic of China, Email [email protected]
Purpose: The combination of programmed cell death-1 (PD-1) blockade camrelizumab plus apatinib (an antiangiogenic agent) and temozolomide has displayed promising therapeutic effects in patients with advanced acral melanoma (AM) in a non-randomized Phase II clinical trial (NCT04397770). The aim of this retrospective study was to evaluate the efficacy and safety of the triplet regimen for advanced AM in the real-world setting.
Methods: The data of patients with advanced AM who received anti-PD-1 antibody plus apatinib and temozolomide at Peking University Cancer Hospital and Institute between September 2019 and December 2023 were analyzed. The primary endpoint was the overall response rate (ORR). The secondary endpoints included progression-free survival (PFS), overall survival (OS), disease control rate (DCR), duration of response (DOR), and treatment-related adverse events (TRAEs).
Results: Overall, 250 patients were eligible for the analysis. The ORR was 38.1% and the DCR was 92.2%. The median PFS, OS, and DOR were 8.5, 18.0, and 13.2 months, respectively. When used as first-line treatment, the ORR was 48.1%, the median PFS was 12.0 months, and the median OS was 24.8 months. The number of lines of therapy (≥ 2 lines), elevated lactate dehydrogenase, and presence of brain or liver metastasis were negative predictors of survival. Overall, 92.4% and 45.2% of the patients experienced any-grade and grade 3– 4 TRAEs, respectively.
Conclusion: This study provides real-world evidence that support the effectiveness and safety of combined anti-PD-1 antibody, apatinib and temozolomide for treating advanced AM, demonstrating a considerable ORR and prolonged survival, as well as acceptable tolerability.
Keywords: acral melanoma, PD-1, anti-angiogenesis, apatinib, camrelizumab, temozolomide, objective response rate
Introduction
Acral melanoma (AM) is a highly aggressive tumor with a 5-year survival rate of approximately 50%.1 In the Asian population, AM is the most common melanoma subtype, with an incidence of around 40%.2 Genetically, AM differs significantly from cutaneous melanoma (CM), as it is characterized by lower rates of ultraviolet-induced mutations and a higher prevalence of structural variations and copy number alterations. Key driver mutations in AM include KIT (11.0%), BRAF (9.7%), and NRAS (23.4%).3
AM is less sensitive to immune checkpoint inhibitors (ICIs) than cutaneous melanoma (CM), with a median progression-free survival (mPFS) of 3.2–4.1 months and an objective response rate (ORR) of 14%–26%.4–7 Notably, the low prevalence of BRAF and NRAS mutations in AM limits the use of targeted therapies, unlike CM, where BRAF inhibitors have shown considerable efficacy.8 Despite these genetic differences, AM is often treated based on protocols developed for CM, indicating a gap in evidence-based guidelines specific to AM. AM is unique without specific treatment guidelines, patients with AM are generally treated based on the protocols developed for CM; however, the prognosis of advanced AM remains poor.
The synergistic effect of chemotherapy with anti-angiogenesis agent and PD-1 inhibitor in AM has been evaluated. The recent CAP 03 phase II non-randomized clinical trial demonstrated the promising efficacy of first-line combination treatment with camrelizumab (the PD-1 inhibitor) plus apatinib (the anti-angiogenesis inhibitor) and temozolomide for advanced AM. This combination regimen showed an ORR of 64.0% and a mPFS of 18.4 months, while the median overall survival (mOS) was not reached.9 However, the real-world application of these results is limited due to differences in patient characteristics and clinical practice settings, highlighting the need for real-world evidence to validate these outcomes. The results of the CAP 03 trial displayed notable improvements over historical treatments, and the triple regime is being further investigated in an ongoing multicenter, randomized Phase III trial (NCT05789043).
However, clinical trials often do not accurately reflect actual clinical practice and patient outcomes due to their strict inclusion and exclusion criteria. Therefore, it remains unclear whether this triple regimen can prolong survival, and its tolerability in a broader population remains to be clarified. We conducted this retrospective analysis to evaluate the effectiveness and safety of anti-PD-1 antibody plus apatinib and temozolomide combination therapy in the real-world setting for patients with advanced AM, as well as identifying the potential prognostic factors affecting survival associated with this treatment regimen.
Patients and Methods
Study Design and Patients
This retrospective study included consecutive patients diagnosed with unresectable stage III and IV AM and were treated with PD-1 inhibitor plus apatinib and temozolomide as first-line therapy or above from September 2019 to December 2023 at Peking University Cancer Hospital and Institute. The study protocol was approved on January 27, 2024 (Approval Number: 2025YJZ09). Eligible patients had histologically confirmed acral melanoma (AM) with molecular profiling to identify key mutations (eg, BRAF, NRAS, KIT) and an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2. Patients were required to have adequate organ function and no severe comorbid conditions that could interfere with the treatment.
Inclusion criteria: (1)Histologically confirmed acral melanoma (AM); (2)Unresectable stage III or IV disease; (3)ECOG performance status of 0–2; (4)Adequate organ function; (5)Molecular profiling data available; (6)No prior enrollment in CAP 03 or ongoing phase III trials.
Exclusion criteria: (1)Active infection or severe comorbidity; (2)Enrollment in related clinical trials; (3)Incomplete baseline data.
Baseline characteristics, including demographic information, performance status, tumor staging, prior treatment history, and genetic mutation profiles, were systematically collected from medical records. To address potential confounding factors, we performed multivariate analyses to account for variables such as prior treatments and genetic mutations.
Treatment
Patients were treated with the combination of anti-PD-1 antibody plus apatinib and temozolomide. For anti-PD-1 antibodies, camrelizumab (200 mg intravenous every 2 weeks), pembrolizumab (200 mg intravenous every 3 weeks), or toripalimab (240 mg intravenous every 2 weeks; Jiangsu Hengrui Pharmaceuticals, Jiangsu, China) were used in real-world practice. The dose of apatinib was 250 mg orally once daily, and the dose of temozolomide was 200 mg/m2 intravenous once daily on days 1–5 of every 28-day cycle.
Outcome
The primary endpoint was the ORR, defined as the percentage of patients who achieved a complete response (CR) or a partial response (PR) as the best response. The secondary endpoints were the disease control rate (DCR), duration of response (DOR), PFS, OS, and TRAEs. DCR was defined as the percentage of patients with a CR, PR, or stable disease (SD). DOR was calculated as the duration from the date of a tumor achieving a CR or a PR until progression. PFS was defined as the duration between the start of triple therapy and the date of progression or all-cause death. OS was defined as the duration from the start of triple therapy to all-cause death. Tumor responses were assessed according to the Response Evaluation Criteria in Solid Tumor (RECIST) version 1.1 guidelines based on computed tomography or magnetic resonance imaging. Radiographic evaluation was generally performed every 8 weeks during treatment. Adverse events (AEs) were graded based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
Statistical Analysis
Baseline variables are presented as descriptive statistics. Survival outcomes were estimated using the Kaplan‒Meier method and were compared using the Log rank test. The 95% confidence intervals (CIs) of the ORR and DCR were determined using the Clopper–Pearson method. Univariate and multivariate Cox proportional-hazards regression models were used to identify prognostic factors influencing PFS and OS, for which the results are shown as hazard ratios (HRs) and 95% CIs. Factors with a univariate p value <0.1 were added to the multivariate analysis. A p value <0.05 was considered statistically significant. All statistical analyses were performed using R version 4.3.0.
Results
Patient Characteristics
Between September 2019 and December 2023, 783 patients with advanced AM who attended Peking University Cancer Hospital and Institute were screened. After excluding 533 patients, 250 patients were enrolled in the final analysis. The study flowchart is shown in Supplementary Figure S1. The safety analysis was assessed in all 250 patients, who each received at least one cycle of treatment. The efficacy analysis was assessed in 244 patients. Among the included patients, 159 (63.6%) and 91 (36.4%) patients were treated with the triple regimen as first-line and later-line therapy, respectively.
The median age of the patients was 57 years (range 20–89). The majority of the patients (97.2%) had an Eastern Cooperative Oncology Group (ECOG) performance score of 0–1. Almost two thirds of the patients (61.6%) had a primary tumor on the sole of the foot, while 10% and 28.4% had primary tumors on the palm and subungual space, respectively. Baseline lactate dehydrogenase (LDH) was elevated in nearly half of the patients (120/250, 48%), of which 27 had an LDH level above twice the upper limit of normal (ULN). In terms of the key driver genes for melanoma, 226 patients were available for next-generation sequencing, and the results revealed that 9.7%, 23.4%, and 11.0% of the patients had BRAF, NRAS, and CKIT mutations, respectively. During treatment, 42.4% of the patients (106/250) received adjuvant therapy (interferon-α, PD-1 inhibitor, and dacarbazine-based regimen). The baseline characteristics of the patients are summarized in Table 1.
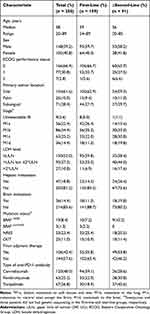 |
Table 1 Baseline Characteristics of the Patients |
Of the 91 patients with prior systemic therapy, 55 (60.4%) and 36 (39.6%) patients had treatment failure after one or more regimens, respectively. The details of previous treatments are shown in Supplementary Table S1. Fifty patients (55.0%) had previously been treated with PD-1 inhibitor, and 20 patients (22.0%) progressed from prior anti-angiogenesis agents plus PD-1 inhibitor.
Response Rate
Overall, 244 patients were assessed for clinical response (Table 2). The ORR was 38.1%, the DCR was 92.2%, and the median DOR was 13.2 months. Among the 156 treatment-naïve patients, the ORR was 48.1%, the DCR was 97.4%, and the median DOR was 16.3 months. Among the 88 pretreated patients, the ORR was 20.5%, the DCR was 83.0%, and the DOR was 7.9 months.
 |
Table 2 Tumor Response |
The antitumor activity of the combination regimen was evaluated in the different subgroups (Figure 1). Treatment-naïve patients had a significantly greater ORR than pretreated patients (48.1% vs 20.5%, p < 0.001). Moreover, patients carrying KIT mutations responded better than those with wild-type KIT (56.0% vs 32.3%, p = 0.019). The ORR showed no significant differences among the primary AM sites (p = 0.690). Of the 50 patients who previously received PD-1 inhibitor in the later-line group, the ORR was 20.0%.
Of the 17 patients with BRAFV600 mutation in the efficacy analysis, eight (47.1%) received the triple regimen as first-line therapy, and seven (41.2%) received first-line BRAF/MEK inhibitors (dabrafenib plus trametinib). Among the eight treatment-naïve patients with BRAFV600 mutations, the ORR to the triple regimen as the first-line therapy was 75% (6/8). Among the seven pre-treated patients, the ORR was 71.4% (5/7) to first-line BRAF/MEK inhibitors.
To enhance the interpretation of our findings, we compared the ORR, PFS, and OS from our study with results from previous real-world studies and clinical trials on advanced acral melanoma (AM) (Table 3). Our study demonstrated an ORR of 38.1%, a median PFS of 8.5 months, and a median OS of 18.0 months, which are lower than those reported in the CAP 03 trial but higher than outcomes from anti-PD-1 monotherapy and the POLARIS-01 trial.
 |
Table 3 Comparison of Efficacy Outcomes from Current Study and Previous Clinical Trials or Real-World Studies on Advanced Acral Melanoma |
Survival Outcomes and Prognostic Factors for Survival
At data cut-off, the median follow-up was 23.9 months (range 1.1–45.8 months). For the entire cohort, the mPFS and the mOS were 8.5 months (95% CI 7.3–11.0; Figure 2A) and 18.0 months (95% CI 16.5–21.9; Figure 2B), respectively. The mPFS was 12.0 months (95% CI 10.3–14.9) for patients receiving triple regimen as the first-line treatment and 5.8 months (95% CI 4.3–6.4) for those who received triple regimen as the later-line treatment (p < 0.001; Figure 3A). Treatment-naïve patients also experienced a significantly longer mOS than patients who had received prior treatments (24.8 months vs 12.0 months, p < 0.001; Figure 3B).
 |
Figure 2 Kaplan‒Meier estimates of (A) progression-free survival and (B) overall survival in the overall population. |
 |
Figure 3 Kaplan‒Meier estimates of (A) progression-free survival and (B) overall survival in patients with 1 line or ≥2 lines of therapy. |
Survival outcomes of the combination regimen was evaluated in the different subgroups of age, gender, ECOG, stage, LDH, liver metastasis, brain metastasis, BRAF/NRAS/KIT status, prior anti-PD-1 therapy, and prior adjuvant therapy (Supplementary Table S2). Patients who failed to respond to anti-PD-1 antibody-based regimens still benefited from the triple combination therapy, with a mPFS of 5.4 months (95% CI 4.2–6.2) and a mOS of 12.3 months (95% CI 8.7–15.5).
In the univariate analysis, the number of lines of therapy, liver metastasis, brain metastasis, LDH level, and adjuvant therapy were potential prognostic variables influencing the PFS (all p < 0.1). The multivariate analysis identified ≥2 lines of therapy (HR 2.03, 95% CI 1.44–2.87, p < 0.001), LDH elevation (HR 1.89, 95% CI 1.36–2.63, p < 0.001), and prior adjuvant therapy (HR 1.48, 95% CI 1.06–2.06, p = 0.021) as predictors of shorter PFS (Supplementary Table S3).
The univariate analysis demonstrated that the number of lines of therapy, sex, primary tumor location, liver metastasis, brain metastasis, LDH level, BRAF status, and adjuvant therapy were potential prognostic variables influencing OS (all p < 0.1). The multivariate analysis demonstrated that the following four factors were independent prognostic factors for OS: ≥2 lines of therapy (HR 2.43, 95% CI 1.62–3.66, p < 0.001), LDH elevation (HR 2.04, 95% CI 1.34–3.11, p < 0.001), liver metastasis (HR 1.80, 95% CI 1.14–2.83, p = 0.011), and brain metastasis (HR 1.69, 95% CI 1.03–2.79, p = 0.039) (Supplementary Table S4).
Safety
The safety profile of the triple regimen in our study was generally consistent with the expected toxicities of the individual agents. Most treatment-related adverse events (TRAEs) were mild to moderate (grade 1–2), and the most frequent grade 3–4 TRAEs included gamma-glutamyltransferase elevation (14.4%), decreased platelet count (13.2%), transaminase elevation (12.8%), and hypertension (11.2%).
Unexpected toxicities included two cases of immune-related enteritis and one case of immune-related myositis, which were not commonly observed in prior studies using similar combinations. Dose adjustments and supportive care measures successfully managed most severe toxicities without compromising efficacy.
Of the 250 patients, 231 (92.4%) experienced any-grade TRAEs and 113 (45.2%) had grade 3–4 AEs (Table 4). The most common any-grade TRAE was decreased white blood cell count (61.6%), followed by hyperbilirubinemia (47.2%), transaminase elevation (46.0%), decreased neutrophil count (40.4%), gamma-glutamyltransferase elevation (36.4%), and decreased platelet count (35.6%), most of which were grade 1–2. The most frequent grade 3–4 TRAEs were gamma-glutamyltransferase elevation (14.4%), decreased platelet count (13.2%), transaminase elevation (12.8%), and hypertension (11.2%). TRAEs resulting in treatment discontinuation occurred in 13 patients (5.2%), including decreased platelet count, transaminase elevation, hyperbilirubinemia, and immune-related enteritis. Treatment-related deaths were not observed.
 |
Table 4 Treatment-Related Adverse Events |
Impact of Prior Therapy on Outcomes
The impact of prior therapies, particularly PD-1 inhibitors and anti-angiogenesis agents, was evaluated to understand their influence on subsequent responses. Patients who had previously received PD-1 inhibitors exhibited a lower ORR (20.0%) compared to treatment-naïve patients (48.1%). Similarly, those treated with anti-angiogenesis agents prior to the triple regimen demonstrated a reduced response rate and shorter PFS.
Discussion
This study provides real-world evidence to verify the effectiveness and safety of anti-PD-1 antibody plus apatinib and temozolomide for treating advanced AM. The triple regimen used in this study consists of an anti-PD-1 antibody (eg, camrelizumab, pembrolizumab, toripalimab), apatinib, and temozolomide. Anti-PD-1 antibodies work by blocking the interaction between PD-1 and PD-L1, thereby reactivating T-cell-mediated immune responses against tumor cells. Apatinib, a potent inhibitor of VEGFR-2, reduces tumor vascularization by blocking angiogenesis, leading to tumor hypoxia and cell death.10 Temozolomide is an alkylating agent that induces DNA damage (especially O6-methylguanine lesions), triggering cell cycle arrest and apoptosis.11 Moreover, temozolomide may modulate the tumor microenvironment by reducing regulatory T cells (Tregs) and enhancing CD8+ T cell infiltration, thereby amplifying the efficacy of immune checkpoint blockade.12 To our knowledge, this is the first real-world analysis to report on the efficacy of PD-1 inhibitor plus anti-angiogenesis and chemotherapy in patients with melanoma. The ORR was 38.1%, the mPFS was 8.5 months, and the mOS was 18.0 months in the overall population. For treatment-naïve patients, the ORR was 48.1%, the mPFS was 12.0 months, and the mOS was 24.8 months. Patients in the later-line group also achieved considerable clinical outcomes. Based on these results, we suggest that this triple regimen is useful for advanced AM, especially when used as the first-line therapy. Few clinical trials have explored therapeutic regimens specific to AM; therefore, this real-world study fills this knowledge gap and provides guidance for clinical application.
Patients with AM achieve limited benefit with anti-PD-1 antibody monotherapy; therefore, combination therapy is a promising approach. However, the effects of anti-angiogenesis plus anti-PD-1 antibody failed to meet our expectations. Indeed, the combination of chemotherapy plus anti-angiogenesis and ICI has become a novel treatment option, and good efficacy has been observed in treating advanced esophageal squamous cell carcinoma and lung cancer.13,14 Patients with AM have a severe immunosuppressive state,15 and studies have suggested that temozolomide may modify the tumor microenvironment via depletion of regulatory T-cells, enrichment of CD8+ T-cells, and improvement of CD8+ T-cell infiltration into melanoma tumors, thereby enhancing the efficacy of immunotherapy.16,17
The efficacy of the triple therapy in this real-world cohort was lower than that of the CAP 03 trial, and several factors might have contributed to this discrepancy. First, in our study, 36.4% of the patients had previously been treated. Receiving the triple regimen as second-line therapy or beyond was a negative prognostic factor in our multivariate analysis of PFS and OS. Second, patients in the present study showed worse baseline characteristics than those in the CAP 03 trial. More patients in our study had LDH elevation (48.0% vs 32.0%), which is considered a poor prognostic factor for most regimens and displays negative association with prognosis in patients with melanoma.18 According to the M-stage, in the CAP 03 trial, more than two thirds of the patients had only soft tissue/ lymph node or lung invasion (M1a or M1b, 72%), which is considered a positive predictor of response to therapy.19,20 The lower percentage of patients with M1a/M1b in our study (56.8%) might explain the poorer efficacy. Third, our study included patients with baseline brain metastasis. The prognosis of patients with melanoma brain metastasis is considered to be poor, and it is significantly worse than those with no brain involvement.21
For patients with progression following PD-1 inhibitor therapy, the ORR was 20% and the PFS was 5.4 months, both of which were higher than the ORR and PFS of other combination regimens reported in AM subgroup analyses of PD-1-refractory melanoma.22,23
An interesting finding is that patients carrying KIT mutation tended to benefit more from this triple regimen, which is consistent with the CAP 03 study.9 The KIT gene encodes a receptor tyrosine kinase involved in cell signaling pathways, including MAPK and PI3K/AKT.24 Mutations in KIT lead to constitutive activation of these pathways, promoting tumor proliferation and survival. In melanomas, especially acral and mucosal subtypes, KIT mutations are associated with aggressive behavior and poor prognosis. Notably, patients with KIT mutations responded better to the triple regimen (ORR 56%) compared to those with wild-type KIT, possibly due to the synergistic effect of antiangiogenic therapy with PD-1 inhibition.25 The BRAFV600 mutation results in constitutive activation of the MAPK pathway, promoting uncontrolled cell growth.26 While BRAF/MEK inhibitors (eg, dabrafenib plus trametinib) are effective in BRAFV600-mutant melanoma, our study found that the triple regimen as first-line therapy also achieved high response rates (ORR 75%), suggesting that combining chemotherapy and immunotherapy remains an effective alternative. Although MEK inhibitors have shown partial efficacy, the triple regimen demonstrated a favorable ORR of 42.3% in patients with mutations, highlighting its potential as a treatment option in this subset of melanoma.
In our study, patients with KIT mutation had a higher ORR than their wild-type counterparts (56.0% vs 32.3%), but the two groups showed no significant differences in PFS or OS, probably due to the limited number of KIT-mutated patients. However, how KIT mutation contributed to the improved outcomes with PD-1 inhibitor therapy plus apatinib and temozolomide in this study is unclear, so further studies are required for elucidating this.
KIT mutations exhibit a significant association with poor prognosis in patients with melanoma,27 suggesting the importance of exploring effective treatment options in this group. In previous studies, the KIT inhibitors imatinib and nilotinib had ORR of 29.2% and 26.2%, respectively.28,29 The ORR of patients with KIT mutation to the triple regimen in this study was higher than those of targeted therapies.
In addition, the triple regimen exerted potential antitumor activity in patients with NRAS or BRAF mutations. In patients with NRAS mutation, the ORR (42.3%) was similar to the ORR (42.9%) in a phase II trial with the MEK inhibitor tunlametinib,30 suggesting that in patients with NRAS-mutated AM, the triple combination is a viable treatment option. A follow-up analysis of a phase II trial assessed the efficacy of BRAF/MEK inhibitors (dabrafenib plus trametinib) in patients with melanoma with BRAFV600 mutation, the ORR was 83.3% in the AM subgroup.31 In our study, patients with BRAFV600 mutation had an ORR of 75% to the triple regimen when used as first-line therapy. It seems that targeted therapies might be the preferred strategy for AM with BRAF mutations, but no head-to-head studies have been conducted in these patients; therefore, this hypothesis should be explored in future studies.
We also explored how the primary site of AM affects the ORR to the triple regimen. Our results are consistent with the observations of the CAP 03 trial. No statistically significant difference was found in ORR among the primary sites of AM. Many other studies similarly revealed no association between the anatomic site and ORR, including a large Japanese cohort of 325 patients with advanced AM treated with ICIs.7,32 However, previous studies have yielded controversial results, showing that different primary sites in patients with AM might be associated with different prognoses.33 Although clinicopathological features and TMB across the primary lesions are important, more complicated mechanisms may contribute to patient outcomes.1,34
Our findings align with previous real-world studies, indicating that the combination regimen offers a feasible and potentially advantageous therapeutic option compared to monotherapies or dual therapies. Despite variations in outcomes between clinical trials and real-world settings, our study highlights the importance of considering patient heterogeneity and prior treatment history when interpreting efficacy results.
In this study, patients showed good tolerability to anti-PD-1 antibody plus apatinib and temozolomide. The types of TRAE were similar to those reported in the CAP 03 trial.9 Most of the TRAEs were grade 1–2. The main grade 3–4 TRAEs were liver injury and hematologic toxicity, which were alleviated by dose adjustment and symptomatic treatment. Furthermore, few patients experienced treatment discontinuation. In this study, patients exposed to the PD-1 inhibitor experienced new AEs that were not observed in our previous studies.9,35 Two patients had immune-related enteritis and one had immune-related myositis. Previous studies have also reported enteritis and myositis with the use of immunotherapies in melanoma.36,37
This study has several limitations that should be considered. There might be potential selection bias due to its retrospective design. Moreover, this study did not include the baseline characteristic of PD-L1 expression. However, the CAP 03 study did not identify PD-L1 expression as having prognostic significance in patients treated with the triple regimen.
Conclusions
This study provides real-world evidence that anti-PD-1 antibody plus apatinib and temozolomide is an effective therapeutic strategy for advanced AM, especially when used as the first-line therapy. Moreover, the safety profile of this triple regimen was acceptable Compared with clinical trial results, the real-world efficacy observed in our study was relatively lower, which may be attributed to differences in patient characteristics and treatment settings. This highlights the challenges in translating clinical trial outcomes to real-world practice.
To further validate our findings, prospective studies are warranted. Additionally, identifying predictive biomarkers will help to optimize patient selection and improve treatment efficacy. Developing personalized therapy strategies based on individual molecular profiles could also enhance outcomes and reduce toxicity. A 3-arm, multicenter, randomized controlled study is currently in progress for further validating the efficacy of this triple regimen in patients with AM.
Data Sharing Statement
The datasets generated during the present study are available from the corresponding author upon reasonable request.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Peking University Cancer Hospital & Institute (2025YJZ09). Written informed consent requirement was waived by the Ethics Committee due to the use of anonymized retrospective medical records, which did not involve direct patient interaction. All patient data were handled with strict confidentiality, and personal identifying information was excluded to protect patient privacy.
Acknowledgments
We wish to thank all the patients, family members and staff from all the units that participated in the study.
Funding
This study was supported by National Natural Science Foundation of China (82372869, 82272676, and 82073011), Beijing Municipal Administration of Hospitals’ Ascent Plan (DFL20220901, QML20231107), National Key Research and Development Program (2023YFC2506404), Beijing Natural Science Foundation (7242021), and Beijing Xisike Clinical Oncology Research Foundation (Y-SY201901-0270).
Disclosure
Jun Guo serves in consulting/advisory roles at Merck Sharp & Dohme, Roche, Bayer, Novartis, Simcere Pharmaceutical Group, Shanghai Junshi Biosciences and Oriengene. Lu Si has received speakers’ honoraria from MSD, Roche, Novartis, Shanghai Junshi Biosciences and Oriengene. For the remaining authors, there are no conflicts of interest.
References
1. Jung HJ, Kweon SS, Lee JB, Lee SC, Yun SJ. A clinicopathologic analysis of 177 acral melanomas in Koreans: relevance of spreading pattern and physical stress. JAMA Dermatol. 2013;149(11):1281–1288. doi:10.1001/jamadermatol.2013.5853
2. Fujisawa Y, Yoshikawa S, Minagawa A, et al. Classification of 3097 patients from the Japanese melanoma study database using the American joint committee on cancer eighth edition cancer staging system. J Dermatol Sci. 2019;94(2):284–289. doi:10.1016/j.jdermsci.2019.04.003
3. Tod BM, Schneider JW, Bowcock AM, Visser WI, Kotze MJ. The tumor genetics of acral melanoma: what should a dermatologist know? JAAD International. 2020;1(2):135–147. doi:10.1016/j.jdin.2020.07.004
4. van Not OJ, de Meza MM, van den Eertwegh AJM, et al. Response to immune checkpoint inhibitors in acral melanoma: a nationwide cohort study. Eur J Cancer. 2022;167:70–80. doi:10.1016/j.ejca.2022.02.026
5. Si L, Zhang X, Shu Y, et al. A phase Ib study of pembrolizumab as second-line therapy for Chinese patients with advanced or metastatic melanoma (KEYNOTE-151). Transl Oncol. 2019;12(6):828–835. doi:10.1016/j.tranon.2019.02.007
6. Tang B, Chi Z, Chen Y, et al. Safety, efficacy, and biomarker analysis of toripalimab in previously treated advanced melanoma: results of the POLARIS-01 multicenter phase II trial. Clin Cancer Res. 2020;26(16):4250–4259. doi:10.1158/1078-0432.CCR-19-3922
7. Bhave P, Ahmed T, Lo SN, et al. Efficacy of anti-PD-1 and ipilimumab alone or in combination in acral melanoma. J Immunother Cancer. 2022;10(7):e004668. doi:10.1136/jitc-2022-004668
8. Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545(7653):175–180. doi:10.1038/nature22071
9. Mao L, Lian B, Li C, et al. Camrelizumab plus apatinib and temozolomide as first-line treatment in patients with advanced acral melanoma: the CAP 03 Phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9(8):1099–1107. doi:10.1001/jamaoncol.2023.1363
10. Zhang J, Liu P, Zhang Z, et al. Apatinib-loaded nanoparticles inhibit tumor growth and angiogenesis in a model of melanoma. Biochem Biophys Res Commun. 2020;521(2):296–302. doi:10.1016/j.bbrc.2019.10.084
11. Kubitschek J, Takhaveev V, Mingard C, et al. Single-nucleotide-resolution genomic maps of O6-methylguanine from the glioblastoma drug temozolomide. Nucleic Acids Res. 2025;53(2). doi:10.1093/nar/gkae1320
12. Karachi A, Yang C, Dastmalchi F, et al. Modulation of temozolomide dose differentially affects T-cell response to immune checkpoint inhibition. Neuro-Oncology. 2019;21(6):730–741. doi:10.1093/neuonc/noz015
13. Lu S, Wu L, Jian H, et al. Sintilimab plus bevacizumab biosimilar IBI305 and chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer who progressed on EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): first interim results from a randomised, double-blind, multicentre, Phase 3 trial. Lancet Oncol. 2022;23(9):1167–1179. doi:10.1016/S1470-2045(22)00382-5
14. Zhang B, Qi L, Wang X, et al. Phase II clinical trial using camrelizumab combined with apatinib and chemotherapy as the first-line treatment of advanced esophageal squamous cell carcinoma. Cancer Commun. 2020;40(12):711–720. doi:10.1002/cac2.12119
15. Zhang C, Shen H, Yang T, et al. A single-cell analysis reveals tumor heterogeneity and immune environment of acral melanoma. Nat Commun. 2022;13(1):7250. doi:10.1038/s41467-022-34877-3
16. Iversen TZ, Brimnes MK, Nikolajsen K, et al. Depletion of T lymphocytes is correlated with response to temozolomide in melanoma patients. Oncoimmunology. 2013;2(2):e23288. doi:10.4161/onci.23288
17. Tan KW, Evrard M, Tham M, et al. Tumor stroma and chemokines control T-cell migration into melanoma following Temozolomide treatment. Oncoimmunology. 2015;4(2):e978709. doi:10.4161/2162402X.2014.978709
18. Diem S, Kasenda B, Spain L, et al. Serum lactate dehydrogenase as an early marker for outcome in patients treated with anti-PD-1 therapy in metastatic melanoma. Br J Cancer. 2016;114(3):256–261. doi:10.1038/bjc.2015.467
19. Robert C, Ribas A, Hamid O, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36(17):1668–1674. doi:10.1200/JCO.2017.75.6270
20. Ochenduszko S, García Sanchez J, Fita MJJ, et al. Characteristics and outcomes of advanced melanoma patients with complete response and elective discontinuation of first-line anti-programmed death-1 monotherapy: a real-world multicentre observational cohort study. Pigm Cell Melanoma Res. 2023;36(5):388–398. doi:10.1111/pcmr.13093
21. Zhang D, Wang Z, Shang D, Yu J, Yuan S. Incidence and prognosis of brain metastases in cutaneous melanoma patients: a population-based study. Melanoma Res. 2019;29(1):77–84. doi:10.1097/CMR.0000000000000538
22. Wang X, Xu W, Chi Z, et al. Chemotherapy combined with antiangiogenic drugs as salvage therapy in advanced melanoma patients progressing on PD-1 immunotherapy. Transl Oncol. 2021;14(1):100949. doi:10.1016/j.tranon.2020.100949
23. Zhou L, Yang Y, Si L, et al. Phase II study of apatinib combined with temozolomide in patients with advanced melanoma after failure of immunotherapy. Melanoma Res. 2022;32(3):142–149. doi:10.1097/CMR.0000000000000809
24. Corrales E, Levit-Zerdoun E, Metzger P, et al. PI3K/AKT signaling allows for MAPK/ERK pathway independency mediating dedifferentiation-driven treatment resistance in melanoma. Cell Communication Signaling. 2022;20(1):187. doi:10.1186/s12964-022-00989-y
25. Caraban BM, Aschie M, Deacu M, et al. A narrative review of current knowledge on cutaneous melanoma. Clinics Pract. 2024;14(1):214–241. doi:10.3390/clinpract14010018
26. Bharti J, Gogu P, Pandey SK, et al. BRAF V600E in cancer: exploring structural complexities, mutation profiles, and pathway dysregulation. Exp Cell Res. 2025;446(1):114440. doi:10.1016/j.yexcr.2025.114440
27. Bai X, Kong Y, Chi Z, et al. MAPK pathway and TERT promoter gene mutation pattern and its prognostic value in melanoma patients: a retrospective study of 2793 cases. Clin Cancer Res. 2017;23(20):6120–6127. doi:10.1158/1078-0432.CCR-17-0980
28. Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol. 2013;31(26):3182–3190. doi:10.1200/JCO.2012.47.7836
29. Guo J, Carvajal RD, Dummer R, et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: final results from the global, single-arm, phase II TEAM trial. Ann Oncol. 2017;28(6):1380–1387. doi:10.1093/annonc/mdx079
30. Wei X, Zou Z, Zhang W, et al. A phase II study of efficacy and safety of the MEK inhibitor tunlametinib in patients with advanced NRAS-mutant melanoma. Eur J Cancer. 2024;202:114008. doi:10.1016/j.ejca.2024.114008
31. Mao L, Ding Y, Bai X, et al. Overall survival of patients with unresectable or metastatic BRAF V600-mutant acral/cutaneous melanoma administered dabrafenib plus trametinib: long-term follow-up of a multicenter, single-arm phase IIa Trial. Front Oncol. 2021;11:720044. doi:10.3389/fonc.2021.720044
32. Teramoto Y, Keim U, Gesierich A, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German central malignant melanoma registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178(2):443–451. doi:10.1111/bjd.15803
33. Wei X, Wu D, Li H, et al. The clinicopathological and survival profiles comparison across primary sites in acral melanoma. Ann Surg Oncol. 2020;27(9):3478–3485. doi:10.1245/s10434-020-08418-5
34. Newell F, Wilmott JS, Johansson PA, et al. Whole-genome sequencing of acral melanoma reveals genomic complexity and diversity. Nat Commun. 2020;11(1):5259. doi:10.1038/s41467-020-18988-3
35. Wang X, Wu X, Yang Y, et al. Apatinib combined with camrelizumab in advanced acral melanoma patients: an open-label, single-arm phase 2 trial. Eur J Cancer. 2023;182:57–65. doi:10.1016/j.ejca.2022.12.027
36. Yang J, Lagana SM, Saenger YM, Carvajal RD. Dual checkpoint inhibitor-associated eosinophilic enteritis. J Immunother Cancer. 2019;7(1):310. doi:10.1186/s40425-019-0743-5
37. Serra-Bellver P, Versluis JM, Oberoi HK, et al. Real-world outcomes with ipilimumab and nivolumab in advanced melanoma: a multicentre retrospective study. Eur J Cancer. 2022;176:121–132. doi:10.1016/j.ejca.2022.09.004
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


