Back to Journals » Cancer Management and Research » Volume 17
Real-World Treatment Patterns and Survival Outcomes in Metastatic Hormone-Sensitive Prostate Cancer: Insights From a Retrospective Cohort Study
Authors Tashkandi E
Received 5 December 2024
Accepted for publication 25 February 2025
Published 1 March 2025 Volume 2025:17 Pages 419—428
DOI https://doi.org/10.2147/CMAR.S506423
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Emad Tashkandi
College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
Correspondence: Emad Tashkandi, Email [email protected]
Background: Real-world data on treatment patterns and survival outcomes in metastatic hormone-sensitive prostate cancer (mHSPC) remain limited. This study aims to characterize treatment sequencing, duration across lines of therapy, and survival outcomes in patients with mHSPC.
Methods: This single-center, retrospective, non-interventional study included men newly diagnosed with mHSPC at King Abdullah Medical City Cancer Center between 2016 and 2023. Treatment patterns, including sequencing and duration of therapy, were described. Kaplan–Meier methods were used to estimate overall survival (OS) from mHSPC diagnosis to death or censoring at the end of follow-up.
Results: Among 102 patients, the mean age was 70 years, BMI of 26, with 53% having a performance status of 2. Comorbidities included hypertension (51%), diabetes (45%), and cardiovascular disease (20.6%). Nearly half (48%) had a Gleason score of nine, with 62.7% presenting with bone metastases. Novel hormonal therapy (NHT) was the predominant first-line treatment (86%), with abiraterone used in 43% of cases. Second- and third-line treatments were received by 34% and 13% of patients, respectively. The median durations of first-, second-, and third-line therapies were 21, 5, and 2.6 months, respectively. Median OS from diagnosis was 24 months.
Conclusion: Despite the predominant use of novel hormonal therapy (NHT), patients in this cohort exhibited aggressive disease and poor survival outcomes. These findings highlight a critical need for more intensive and tailored treatment strategies for mHSPC.
Keywords: chemotherapy, metastatic hormone-sensitive prostate cancer, novel hormonal agents, overall survival, pattern, Prostate cancer
Introduction
Globally, the most common cancer in men is prostate cancer (PC), with an estimated 1.6 million cases and 366,000 deaths annually.1 According to the WHO Globocan Report, an estimated 693 new cases and 204 deaths were reported in Saudi Arabia in 2020.2 Saudi Arabia’s population exhibits unique demographic and lifestyle characteristics, including a relatively younger age distribution and increased genetic predispositions to certain cancers. Moreover, lifestyle factors such as dietary habits, physical activity levels, and smoking prevalence in Saudi Arabia may differ significantly from those in Western countries, potentially influencing both prostate cancer incidence and treatment outcomes.3 Age, family history, genetic predisposition, smoking, diet, and physical inactivity are risk factors for PC development.4 Despite an increase in incidence, not all mechanisms of onset have been elucidated.
Prostate cancer exhibits a wide spectrum of clinical behaviors, ranging from localized disease to advanced metastatic states, and treatment strategies have evolved significantly over time as treatments may include active surveillance, surgery, radiotherapy, hormone therapy, chemotherapy, targeted therapy, or bone health agents.
Our previous work provided a comprehensive analysis of treatment patterns and outcomes across the entire prostate cancer journey, including localized, advanced, and metastatic cases, irrespective of prior therapies or lines of treatment.5 However, mHSPC represents a distinct and aggressive subset of PC that warrants focused investigation due to its poor prognosis and unique therapeutic challenges. Building on the foundation of our prior study, the current work highlights the knowledge gap in real-world treatment patterns and survival outcomes specifically in patients with mHSPC.
Before 2015, the options for treating mHSPC were limited. Since 2015, numerous pivotal Phase III clinical trials have been published, with sufficient impact to change clinical practice because of the statistically significant improvements in survival in mHSPC, as clearly highlighted by Barata et al.6 These trials provided clinical evidence for docetaxel (CHAARTED7 and STAMPEDE8), abiraterone (LATITUDE9 and STAMPEDE10), apalutamide (TITAN11), and enzalutamide (ARCHES12 and ENZAMET13). Consequently, PC guidelines recommend the use of chemotherapy or novel hormonal therapy (NHT) for mHSPC.14,15
Recently, triplet therapy and intensified androgen deprivation therapy (ADT) combined with chemotherapy and hormonal therapy (abiraterone or darolutamide) proved to be effective and safe in the gold standard clinical trials PEACE-116 and ARASENS.17 International guidelines now recommend these two strategies as standard treatments for mHSPC.
Radiotherapy (RT) is gaining recognition as a potential treatment for mHSPC, particularly in patients with a low metastatic burden. The HORRAD and STAMPEDE trials demonstrated improved failure-free survival with RT, with the latter also showing an overall survival benefit in low-burden cases.18,19 The STOPCAP meta-analysis and real-world data by Morgan et al confirm RT’s efficacy, showing improved failure-free survival and survival rates in low-burden mHSPC when combined with ADT.20,21 These findings highlight RT as a promising adjunct in carefully selected patients with mHSPC.
Patients enrolled in clinical trials have better performance scores and organ function, as well as fewer comorbidities, than those in real-world practice, which can lead to some challenges when clinically applying these findings. Cohort studies have been conducted to address these limitations. However, the published literature on survival outcomes and current treatment patterns in patients with mHSPC is scarce outside the United States and Europe. Saudi Arabia’s healthcare system is a hybrid model, with a significant reliance on government-funded services, which influences access to care and treatment protocols. In contrast, Western healthcare systems often have a more diverse mix of public and private services. This study, focusing on prostate cancer, aims to address these differences and provide insights that could improve clinical outcomes and personalize treatment approaches, aligning with the goals of Saudi Vision 2030 to enhance healthcare services, standardize practices, and improve accessibility across the Kingdom of Saudi Arabia.22 Therefore, generating data on survival and treatment patterns across Saudi Arabia is imperative. This single-center, non-interventional study in patients with mHSPC to characterize treatment patterns, including treatment sequencing and duration of therapy across lines of therapy, as well as survival outcomes.
Materials and Methods
Study Design
This was a single institution, retrospective, non-interventional cohort study that included men diagnosed with mHSPC between January 1, 2016, and June 1, 2023, who had received at least one LOT, such as chemotherapy (docetaxel or cabazitaxel), NHT (abiraterone, enzalutamide, or apalutamide), or ADT, and had 12 months of follow-up data available.
Data Sources
This non-interventional study involved the retrospective collection of data from paper or electronic medical records (EMRs) of patients diagnosed with mHSPC. Data were collected retrospectively from the date of mHSPC diagnosis (index date) to the end of follow-up, that is, until death, the last medical record entry, or the date of data extraction, whichever was earliest. Data on the different types of treatment received by the patients and demographic and clinicopathological characteristics were extracted from the EMR of patients (alive or deceased) into a centrally designed electronic data capture system. The system underwent thorough validation for accuracy and reliability, with audits and cross-referencing of paper records to ensure data integrity.
Study Population
This study included men diagnosed with mHSPC between January 1, 2016, and June 1, 2023, as confirmed by pathology and/or imaging, and with at least 12 months of follow-up data available.
Inclusion Criteria
The inclusion criteria were: (1) mHSPC confirmed by biopsy, (2) evidence of metastatic disease on imaging studies, (3) availability of medical records with at least one LOT received in the mHSPC setting, and (4) at least 12 months of follow-up data from the index date (unless the patient died within the first 12 months after diagnosis).
Exclusion Criteria
Patients with a diagnosis of mHRPC or non-metastatic PC were excluded.
Outcomes
The outcome variables assessed included demographic characteristics, such as age; body mass index (BMI); comorbidities, including cardiovascular disease (CVS), hypertension (HTN), diabetes mellitus (DM), and osteoporosis; and the clinicopathological profile (at initial diagnosis or index date of mHSPC diagnosis, as applicable), including Eastern Cooperative Oncology Group (ECOG) performance status, Gleason score, and sites of metastases. We also assessed the proportion of patients receiving each LOT and its duration. An LOT was defined as one regimen administered from either the index diagnosis or disease progression until treatment failure or intolerance, disease progression, or death. Therapies that were paused and then restarted, including those at reduced doses without disease progression, were considered part of the same LOT rather than a new one. Overall survival (OS) was defined as the time from the index date until death from any cause or loss to follow-up.
Statistical Analysis
Data analysis was conducted using SPSS Statistics Software (version 25; IBM, Chicago, Illinois, USA). Categorical variables are presented as counts and percentages, and continuous variables as mean ± standard deviation. Overall survival was defined as the time from mHSPC diagnosis to death or censoring at the end of follow-up. Survival probabilities and curves were estimated using the Kaplan–Meier method, with the Log rank test employed to compare survival between groups. Patients lost to follow-up, comprising less than 1% of the cohort, were censored at their last known contact date. The significance level for the Log rank test was set to α = 0.05, and all tests were two-tailed. Given the study design, no multivariate models such as Cox proportional hazards regression were applied. Missing data were managed using a complete-case analysis.
Results
Baseline Characteristics
Data were collected from 102 patients. The patient demographics and clinical characteristics are shown in Table 1. At initial diagnosis, the mean age was 70 years, and the median BMI was 26.2. More than half of the participants (54 [52.9%]) had a performance status of two. In terms of comorbidities, 52 (51%) had HTN, 46 (45%) had DM, and 21 (20.6%) had CVS. Most patients (49 [48%]) had a Gleason score of nine, and 64 (62.7%) had bone metastases.
 |
Table 1 Baseline Demographic and Clinical Characteristics |
mHSPC Treatment, LOTs, and Duration
The mHSPC treatments are listed by LOT in Table 2. As first-line treatment, 88 (86.2%) patients received NHTs (abiraterone, enzalutamide, or apalutamide), 2 (1.9%) received docetaxel, and 12 (11.8%) received ADT alone due to advanced age or multiple comorbidities. The most common first-line treatments were abiraterone (44 [43.1%]), enzalutamide (24 [23.5%]), apalutamide (20 [19.6%]), and docetaxel (2 [1.9%]). The most common second-line treatments were enzalutamide (20 [19.6%]) and abiraterone (14 [13.7%]). The most common third-line treatments were docetaxel (13 [10.8%]), enzalutamide (1 [1%]), and abiraterone (1 [1%]).
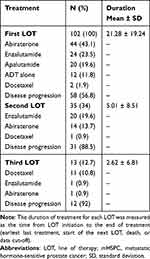 |
Table 2 mHSPC Treatments by Lines of Therapies |
Among those who received first-line treatment, 35 (34%) subsequently received a second LOT, and 13 (12.7%) received a third LOT. The average duration of first-, second-, and third-line treatments was 21.2, 5, and 2.6 months, respectively.
Survival
The overall mortality rate was 41% (N=42). Median survival from mHSPC diagnosis was 24 months (95% confidence interval, 21.6–32.2 months) (Figure 1).
 |
Figure 1 Overall survival from mHSPC diagnosis. Abbreviation: CI, confidence interval. |
Discussion
In this analysis, the average age at diagnosis was 70 years, which is younger than the 73 years reported by two large cohorts, the USA SEER program and the Danish Prostate Cancer Registry.23 This discrepancy may be explained by the higher BMI in our cohort, as Popovici et al observed that the median diagnostic age decreases with increasing BMI.24
Our study found that more than half of the patients had a performance status score of two, which is associated with a higher mortality risk.25 This may be attributed to the presence of comorbidities: more than half had HTN, close to half had DM, and a quarter had CVS. Moreover, we observed that almost half of the patients had a Gleason score of nine, indicating a poor prognosis.26 Notably, the presence of comorbidities and poor performance status are associated with poorer OS.27
Bone is the most common site of distant metastasis in prostate cancer, aligning with global trends;28 therefore, early detection and tailored treatment plans for bone health are imperative. Bone metastases in prostate cancer lead to skeletal-related events (SREs) such as pain, fractures, and spinal cord compression, severely impacting patients’ quality of life. Bone-modifying agents (BMAs) like zoledronic acid and denosumab are used to reduce SREs and improve bone health. Zoledronic acid helps alleviate pain and improve bone mineral density (BMD), but its impact on overall survival is inconsistent. Denosumab, which targets RANKL, has shown superior efficacy in reducing SREs compared to zoledronic acid in clinical trials.29 Although BMAs are well-established in metastatic castration-resistant prostate cancer (mCRPC), their role in metastatic hormone-sensitive prostate cancer (mHSPC) is less clear. Emerging treatments like OsteoDex, a polybisphosphonate with cytotoxic properties, offer potential in targeting bone metastases more effectively. Furthermore, extending BMA dosing intervals may reduce adverse effects like osteonecrosis of the jaw while maintaining efficacy. Advances in understanding bone metastasis mechanisms are driving the development of novel therapies, enhancing management strategies for prostate cancer patients with bone metastases.
Our study demonstrated the prevalent use of NHTs and ADT alone, which is comparable to findings from the recent large Ipsos Global Oncology Monitor database in the USA, Europe, and Asia.30 These results deviate from the current guidelines31 recommending the use of triplet therapy or intensified strategies. The most common first-line treatments were abiraterone, enzalutamide, and apalutamide, which suggests an increased use of NHTs and a decline in the use of chemotherapy, likely because of patient preferences and frailty in this population. These findings are consistent with those of Yang et al.32
Our study revealed that 34% of patients received a second-line therapy, and 13% received a third, which is less than the recently published 46% and 15%, respectively, in a community oncology practice setting.33 In addition, the mean duration of first-, second-, and third-line treatments were 21, 5, and 2.6 months, respectively. In contrast, Conner et al found that the mean durations of first- and second-line treatments were 12 (11.8%) and 10 (11.1%) months, respectively. This may be explained by the fact that our population was older, had worse performance, multiple comorbidities, and declined chemotherapy until the third line; thus, cross-resistance may have occurred when using NHTs as first- and second-line treatments.34
In our study, the median survival was 24 months, which is lower than the 31.6 months reported by Geynisman et al35 in a similar real-world study. These conflicting results may be because they only included abiraterone and docetaxel, unlike this study which included all NHTs. Additionally, our population had more aggressive disease and poorer prognosis. While our earlier research,5 provided a comprehensive overview of prostate cancer across all stages and treatment histories, the current study offers a focused analysis of mHSPC, highlighting real-world survival outcomes and treatment patterns specific to this aggressive subset. This distinction is critical, as mHSPC patients present unique therapeutic challenges that are under-represented in broader studies. This study has several strengths, including its robust data collection that allowed us to address the current real-world treatment patterns and survival outcomes in Saudi Arabia, which can serve as a guide and benchmark for future treatment decision making. However, this study was limited by its retrospective, single-center design and small sample size. Furthermore, the EMR may have missing information, affecting the quality of our data. Therefore, the findings should be interpreted with caution, as unmeasured confounding variables may exist. Moreover, the treatments captured in our study were limited to those available at our center, as other therapies, such as cabazitaxel, theranostics, and Poly (ADP-ribose) polymerase (PARP) inhibitors, were not accessible during the data collection period. Over the past decade, significant advances in prostate cancer genomics have emphasized the critical role of genetic testing, particularly in mHSPC. A broad genetic panel, including BRCA1, BRCA2, mismatch repair (MMR) genes, and potentially ATM, plays an essential role in guiding treatment and predicting prognosis.36 Germline mutations, particularly in BRCA1/2, have been shown to predict better responses to PARP inhibitors.37 Germline testing is vital not only for optimizing therapy, but also for assessing familial cancer risks, and current guidelines recommend it for high-risk localized and metastatic prostate cancer to improve patient care and inform early detection strategies in relatives.
Raising awareness to emphasize early diagnosis, treatment, and prevention of chronic comorbidities with timely interventions is imperative in populations with more aggressive disease and poorer prognoses. Furthermore, effective management of the most common comorbidities is important because NHTs and ADTs can induce and exacerbate existing comorbidities, leading to a poorer prognosis. Additionally, potential targets for the prevention and treatment of bone metastases may be identified via osteoporosis evaluation during ADTs, as neglected awareness of bone health leads to serious skeletal-related events.
Recent evidence highlights the emerging role of radiotherapy (RT) in the management of oligometastatic HSPC. The HORRAD trial,19 one of the pioneering RCTs, demonstrated the feasibility of adding RT to ADT in mHSPC, though no significant OS benefit was observed. Importantly, the trial suggested that patients with a low metastatic burden might derive the greatest benefit from this approach. Supporting these findings, the STAMPEDE trial,18 a larger RCT, showed that RT improved failure-free survival and demonstrated an OS benefit in patients with a low metastatic burden, without significant adverse effects. These results underscore the potential of RT as a valuable addition to systemic therapies in selected subgroups of patients, particularly those with limited metastatic disease.38,39 Further research is needed to refine patient selection criteria and to assess the impact of advanced imaging modalities in better defining oligometastatic disease, which could expand the applicability of RT in this setting.
Differences in guideline evolution and adoption, access to care, availability of novel drugs, and regulatory approval can be optimized, which may explain some of the differences found in our study. Moreover, larger multicenter international studies can provide more applicable data to empower the current results and practices. At present, oncologists extrapolate from international clinical results; thus, we propose initiating or enrolling patients in multicenter national and international studies to better understand the effectiveness and safety of NHTs in our population.
Cancer care is expensive, and the intensification of treatment through triplet therapy compounds both the financial burden and potential for increased toxicity. Careful patient selection is critical, as triplet therapy demonstrates the greatest efficacy and safety in patients with high metastatic burdens.16,17 With the availability of multiple LOTs, oncologists must balance clinical factors, such as disease burden and comorbidities, with practical considerations like cost, treatment availability, and safety—especially in resource-limited settings where healthcare constraints often restrict access to advanced therapies.
The financial burden of treating mHSPC necessitates evaluation within a health economics framework. While triplet therapies combining ADT, NHT, and chemotherapy provide substantial survival benefits (eg, PEACE-1, ARASENS), they are not cost-effective when analyzed through quality-adjusted survival metrics.40 Chemotherapy is substantially more cost-effective than NHT,41 and cost-effectiveness varies significantly depending on drug pricing and country-specific payer perspectives.42 High-income countries with universal healthcare systems incorporate metrics like quality-adjusted life years (QALYs) in cost-effectiveness analyses, enabling broader access to these therapies. However, middle-income regions face financial constraints and competing healthcare priorities, limiting the adoption of intensive regimens.
The high cost of agents such as abiraterone and darolutamide remains a significant barrier despite their efficacy. Patient selection is critical for optimizing outcomes and managing costs, as triplet therapies are most effective in patients with high metastatic burdens but carry risks of toxicity and financial strain. Tailored cost-effectiveness analyses can help balance efficacy and affordability, particularly in resource-constrained settings. Strategies such as staggered therapy initiation or extended treatment intervals may offer cost-efficient alternatives without compromising outcomes.
This study highlights the urgent need for region-specific health economics research in Saudi Arabia and similar settings to evaluate the feasibility of triplet therapies. Such efforts can inform evidence-based policymaking, optimize resource allocation, and enhance access to life-extending treatments. Addressing these challenges is essential to translating clinical trial success into real-world benefits while ensuring equitable access to advanced therapies. Streamlining regulatory approvals and reimbursement for innovative treatments, such as triplet therapy and PARP inhibitors, alongside developing localized, evidence-based guidelines, would enable consistent, context-specific decision-making in Saudi oncology care. Given the high prevalence of comorbidities like hypertension, diabetes, and cardiovascular diseases, integrating routine comorbidity management into mHSPC treatment plans is vital to reduce toxicities and improve survival outcomes. Collaborative multicenter and global clinical trials should be prioritized to evaluate newer therapies while generating region-specific data. Additionally, cost-effectiveness analyses tailored to the Saudi healthcare system are essential to optimize resource allocation and support the adoption of high-value treatments.
In conclusion, this study highlights the treatment patterns and survival outcomes of mHSPC patients in Saudi Arabia, revealing gaps in adopting intensified strategies and addressing unique challenges such as aggressive disease and high comorbidity rates. Optimizing care requires region-specific policies to improve access to novel therapies, streamline regulatory processes, and develop localized guidelines.
Future efforts should prioritize collaborative clinical trials, health economics research, and the integration of genetic testing and advanced imaging to refine treatment strategies. Addressing these gaps through targeted initiatives and resource optimization can improve survival outcomes and set a model for enhancing cancer care in similar resource-constrained settings.
Data Sharing Statement
The datasets generated and analyzed in the current study are available upon request.
Data Access and Responsibility
Emad Tashkandi had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Ethics Statement
The study protocol was approved by the Institutional Review Board of KAMC, Makkah, Saudi Arabia (IRB no. 22-965). The need for informed consent was waived because de-identified data was used. All procedures were performed in accordance with the principles outlined in the Declaration of Helsinki.
Acknowledgments
The author would like to thank Mr. Maher Alhazmi and Ms. Doaa Mohorjy for their support with database creation and data management. This study was conducted using data from the Cancer Center at King Abdullah Medical City (KAMC) in Makkah, Saudi Arabia. The study protocol was approved by the Institutional Review Board (IRB no. 22-965) at KAMC. As Umm Al-Qura University does not have a hospital or cancer center for clinical research, IRB approval from KAMC was essential to access patient data and conduct this study. The author acknowledges the support of KAMC’s Cancer Center in facilitating this research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The author declares no conflicts of interest.
References
1. Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 Cancer Groups, 1990 to 2015: a systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3(4):524–548. doi:10.1001/JAMAONCOL.2016.5688
2. “Saudi Arabia: the global cancer observatory. international agency for research on cancer. globocan 2020 - google search.” Available from: https://www.google.com/search?q=Saudi+Arabia%3A+The+Global+Cancer+Observatory.+International+Agency+for+Research+on+Cancer.++Globocan+2020&sca_esv=419ad3eaf305d86b&sca_upv=1&rlz=1C1YTUH_arSA1024SA1024&sxsrf=ADLYWIIddHwRxrMVqtlgUoz_Q49lERhjcw%3A1725266379517&ei=y3nVZt-TH-uP9u8PwbCX-As&ved=0ahUKEwifpcaN7qOIAxXrh_0HHUHYBb8Q4dUDCBA&uact=5&oq=Saudi+Arabia%3A+The+Global+Cancer+Observatory.+International+Agency+for+Research+on+Cancer.++Globocan+2020&gs_lp=Egxnd3Mtd2l6LXNlcnAiaFNhdWRpIEFyYWJpYTogVGhlIEdsb2JhbCBDYW5jZXIgT2JzZXJ2YXRvcnkuIEludGVybmF0aW9uYWwgQWdlbmN5IGZvciBSZXNlYXJjaCBvbiBDYW5jZXIuICBHbG9ib2NhbiAyMDIwSABQAFgAcAB4AZABAJgBAKABAKoBALgBA8gBAPgBAfgBApgCAKACAJgDAJIHAKAHAA&sclient=gws-wiz-serp.
3. Al-Muftah M, Al-Ejeh F. Cancer incidence and mortality estimates in Arab countries in 2018: a GLOBOCAN data analysis. Cancer Epidemiol Biomarkers Prev. 2023;32(12):1738. doi:10.1158/1055-9965.EPI-23-0520
4. Bergengren O, Pekala KR, Matsoukas K, et al. 2022 update on prostate cancer epidemiology and risk factors—a systematic review. Eur Urol. 2023;84(2):191–206. doi:10.1016/j.eururo.2023.04.021
5. Khizanah RA, Tashkandi E, Jaffal M, et al. Real-world treatment patterns and clinical outcomes in patients with prostate cancer.: a single institution experience in Saudi Arabia. Saudi Med J. 2024;45(6):639–642. doi:10.15537/SMJ.2024.45.6.20240042
6. Barata PC, Leith A, Ribbands A, et al. Real-world treatment trends among patients with metastatic castration-sensitive prostate cancer: results from an international study. Oncologist. 2023;28(9):780–789. doi:10.1093/ONCOLO/OYAD045
7. Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746. doi:10.1056/NEJMOA1503747/SUPPL_FILE/NEJMOA1503747_DISCLOSURES.PDF
8. James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177. doi:10.1016/S0140-6736(15)01037-5
9. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377(4):352–360. doi:10.1056/NEJMOA1704174
10. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377(4):338–351. doi:10.1056/NEJMOA1702900
11. Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med. 2019;381(1):661. doi:10.1056/NEJMOA1903307
12. Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: a randomized, phase iii study of androgen deprivation therapy with enzalutamide or placebo in men with metastatic hormone-sensitive prostate cancer. J Clin Oncol. 2019;37(32):2974–2986. doi:10.1200/JCO.19.00799
13. Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with standard first-line therapy in metastatic prostate cancer. N Engl J Med. 2019;381(2):121–131. doi:10.1056/NEJMOA1903835
14. Lowrance WT, Breau RH, Chou R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline PART I. J Urol. 2021;205(1):14–21. doi:10.1097/JU.0000000000001375
15. Virgo KS, Rumble RB, de Wit R, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. 2021;39(11):1274–1305. doi:10.1200/JCO.20.03256
16. Fizazi K, Foulon S, Carles J, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): a multicentre, open-label, randomised, Phase 3 study with a 2 × 2 factorial design. Lancet. 2022;399(10336):1695–1707. doi:10.1016/S0140-6736(22)00367-1
17. Smith MR, Hussain M, Saad F, et al. Darolutamide and survival in metastatic, hormone-sensitive prostate cancer. N Engl J Med. 2022;386(12):1132–1142. doi:10.1056/NEJMOA2119115/SUPPL_FILE/NEJMOA2119115_DATA-SHARING.PDF
18. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353–2366. doi:10.1016/S0140-6736(18)32486-3
19. Boevé LMS, Hulshof MCCM, Vis AN, et al. Effect on survival of androgen deprivation therapy alone compared to androgen deprivation therapy combined with concurrent radiation therapy to the prostate in patients with primary bone metastatic prostate cancer in a prospective randomised clinical trial: data from the HORRAD trial. Eur Urol. 2019;75(3):410–418. doi:10.1016/J.EURURO.2018.09.008
20. Burdett S, Boevé LM, Ingleby FC, et al. Prostate radiotherapy for metastatic hormone-sensitive prostate cancer: a STOPCAP systematic review and meta-analysis. Eur Urol. 2019;76(1):115–124. doi:10.1016/J.EURURO.2019.02.003
21. Morgan SC, Holmes OE, Craig J, Grimes S, Malone S. Long-term outcomes of prostate radiotherapy for newly-diagnosed metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021;24(4):1041–1047. doi:10.1038/s41391-021-00339-y
22. Suleiman AK, Ming LC. Transforming healthcare: Saudi Arabia’s vision 2030 healthcare model. J Pharm Policy Pract. 2025;18(1). doi:10.1080/20523211.2024.2449051
23. Helgstrand JT, Røder MA, Klemann N, et al. Trends in incidence and 5-year mortality in men with newly diagnosed, metastatic prostate cancer-A population-based analysis of 2 national cohorts. Cancer. 2018;124(14):2931–2938. doi:10.1002/CNCR.31384
24. Popovici D, Stanisav C, Pricop M, Dragomir R, Saftescu S, Ciurescu D. Associations between body mass index and prostate cancer: the impact on progression-free survival. Medicina. 2023;59(2):289. doi:10.3390/MEDICINA59020289
25. Assayag J, Kim C, Chu H, Webster J. The prognostic value of Eastern Cooperative Oncology Group performance status on overall survival among patients with metastatic prostate cancer: a systematic review and meta-analysis. Front Oncol. 2023;13. doi:10.3389/FONC.2023.1194718
26. Egevad L, Micoli C, Samaratunga H, et al. Prognosis of Gleason score 9–10 prostatic adenocarcinoma in needle biopsies: a nationwide population-based study. Eur Urol Oncol. 2024;7(2):213–221. doi:10.1016/J.EUO.2023.11.002
27. Lehtonen M, Heiskanen L, Reinikainen P, Kellokumpu-Lehtinen PL. Both comorbidity and worse performance status are associated with poorer overall survival after external beam radiotherapy for prostate cancer. BMC Cancer. 2020;20(1). doi:10.1186/S12885-020-06812-6
28. Coleman R, Hadji P, Body -J-J, et al. Bone health in cancer: ESMO clinical practice guidelines. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(12):1650–1663. doi:10.1016/J.ANNONC.2020.07.019
29. Zhou W, Zhang W, Yan S, et al. Novel therapeutic targets on the horizon: an analysis of clinical trials on therapies for bone metastasis in prostate cancer. Cancers. 2024;16(3):627. doi:10.3390/CANCERS16030627/S1
30. Goebell PJ, Raina R, Chen S, et al. Real-world treatment of metastatic hormone-sensitive prostate cancer in the USA, Europe and Asia. Future Oncol. 2024;20(14):903–918. doi:10.2217/FON-2023-0814/ASSET/C463BEF7-8CDD-480C-BC75-2C3CEC9F2E18/ASSETS/GRAPHIC/IFON_A_12367183_F0005.JPG
31. Raval AD, Lunacsek O, Korn MJ, Littleton N, Constantinovici N, George DJ. Real-world intensification beyond androgen deprivation therapy (ADT) in metastatic hormone sensitive prostate cancer (mHSPC) in the United States 2017–2023: an administrative claims database study. J Clin Oncol. 2024;42(16_suppl):e17082. doi:10.1200/JCO.2024.42.16_SUPPL.E17082
32. Yang X, Tan YG, Gatsinga R, et al. Far from the truth: real-world treatment patterns among newly diagnosed metastatic prostate cancer in the era of treatment intensification. Int J Urol. 2023;30(11):991–999. doi:10.1111/IJU.15243
33. Conner T, Appukkuttan S, Kong S, et al. Utilization of metastatic hormone sensitive prostate cancer (mHSPC) treatment in a community oncology practice setting. J Clin Oncol. 2023;41(6_suppl):86. doi:10.1200/JCO.2023.41.6_SUPPL.86
34. Simon I, Perales S, Casado-Medina L, et al. Cross-Resistance to Abiraterone and Enzalutamide in Castration Resistance Prostate Cancer Cellular Models Is Mediated by AR Transcriptional Reactivation. Cancers. 2021;13(6):1483. doi:10.3390/CANCERS13061483
35. Geynisman DM, Correa AF, Ramamurthy C, Beck JR, Handorf EA. Real-world survival of men with metastatic hormone-sensitive prostate cancer (mHSPC) treated with Abiraterone acetate (Abi) or docetaxel (Doc) and comparison with clinical trial outcomes. J Clin Oncol. 2021;39(6_suppl):53. doi:10.1200/JCO.2021.39.6_SUPPL.53
36. Zannini G, Facchini G, De Sio M, et al. BRCA1 and BRCA2 mutations testing in prostate cancer: detection in formalin fixed paraffin embedded (FFPE) and blood samples. Pathol Res Pract. 2025;266:155803. doi:10.1016/J.PRP.2024.155803
37. Chanza NM, Tkint De Roodenbeke M, Desmyter L, et al. Prevalence and clinical impact of BRCA1/2 mutations in patients with de novo metastatic hormone-sensitive prostate cancer (mHSPC). J Clin Oncol. 2020;38(6_suppl):44. doi:10.1200/JCO.2020.38.6_SUPPL.44
38. Cardili L, Bastos DA, Ilario EN, et al. Tumor regression after neoadjuvant hormonal therapy in high risk prostate cancer: pathological outcomes from a randomized Phase II trial. World J Urol. 2024;42(1):618. doi:10.1007/S00345-024-05323-4
39. Ferro M, Crocetto F, Lucarelli G, Lievore E, Barone B. Radiotherapy to the primary tumor: the first step of a tailored therapy in metastatic prostate cancer. Diagnostics. 2022;12(8):1981. doi:10.3390/DIAGNOSTICS12081981
40. Sathianathen NJ, Lawrentschuk N, Konety B, et al. Cost effectiveness of systemic treatment intensification for metastatic hormone-sensitive prostate cancer: is triplet therapy cost effective? Eur Urol Oncol. 2024;7(4):870–876. doi:10.1016/J.EUO.2023.11.013
41. Ramamurthy C, Handorf EA, Correa AF, Beck JR, Geynisman DM. Cost-effectiveness of Abiraterone versus docetaxel in the treatment of metastatic hormone naïve prostate cancer. Urol Oncol. 2019;37(10):688–695. doi:10.1016/J.UROLONC.2019.05.017
42. Lester-Coll NH, Benjamin DJ, Ribault H, Foulon S, Fizazi K, Rezazadeh A. Cost-effectiveness of triplet therapies in metastatic hormone-sensitive prostate cancer used in PEACE-1 and ARASENS. J Clin Oncol. 2024;42(4_suppl):222. doi:10.1200/JCO.2024.42.4_SUPPL.222
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

