Back to Journals » International Journal of Nanomedicine » Volume 19
Recent Advancements and Trends of Topical Drug Delivery Systems in Psoriasis: A Review and Bibliometric Analysis
Authors An P , Zhao Q , Hao S, Wang X , Tian J , Ma Z
Received 5 February 2024
Accepted for publication 4 July 2024
Published 29 July 2024 Volume 2024:19 Pages 7631—7671
DOI https://doi.org/10.2147/IJN.S461514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Pingyu An,1 Qiyue Zhao,2 Siyu Hao,3 Xiaodong Wang,4 Jiangtian Tian,5,6 Zhiqiang Ma3
1Basic Medical College, Harbin Medical University, Harbin, People’s Republic of China; 2School of Nursing, Southern Medical University, Guangzhou, People’s Republic of China; 3Department of Dermatology, the Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 4Department of Ultrasound, Harbin Medical University Cancer Hospital, Harbin, People’s Republic of China; 5Department of Cardiology, the Second Affiliated Hospital of Harbin Medical University, Harbin, People’s Republic of China; 6The Key Laboratory of Myocardial Ischemia, Chinese Ministry of Education, Harbin, People’s Republic of China
Correspondence: Zhiqiang Ma, Department of Dermatology, The Second Affiliated Hospital of Harbin Medical University, No. 246, Xuefu Road, Nangang District, Harbin, 150081, People’s Republic of China, Tel +86 18845139619, Email [email protected]
Abstract: Psoriasis is an immune-mediated inflammatory skin disease where topical therapy is crucial. While various dosage forms have enhanced the efficacy of current treatments, their limited permeability and lack of targeted delivery to the dermis and epidermis remain challenges. We reviewed the evolution of topical therapies for psoriasis and conducted a bibliometric analysis from 1993 to 2023 using a predictive linear regression model. This included a comprehensive statistical and visual evaluation of each model’s validity, literature profiles, citation patterns, and collaborations, assessing R variance and mean squared error (MSE). Furthermore, we detailed the structural features and penetration pathways of emerging drug delivery systems for topical treatment, such as lipid-based, polymer-based, metallic nanocarriers, and nanocrystals, highlighting their advantages. This systematic overview indicates that future research should focus on developing novel drug delivery systems characterized by enhanced stability, biocompatibility, and drug-carrying capacity.
Keywords: psoriasis, bibliometric, drug delivery, topical treatment, nanoparticle, nanocarriers
Introduction
Psoriasis, a persistent and relapsing inflammatory autoimmune skin disease, impacts approximately 2%–5% of the global population, manifesting clinically as psoriasis vulgaris, psoriasis arthropathica, psoriasis erythrodermic and psoriasis pustulosa. Predominantly, psoriasis vulgaris represents the most common form, characterized by erythematous, scaly skin lesions with distinct borders.1 The pathogenesis involves a genetic predisposition (eg, HLA-C*06:02 risk allele), environmental triggers (such as streptococcal infections, stress, obesity, alcohol consumption, and smoking), and immune response abnormalities (eg, overactivation of the adaptive immune system). Psoriasis exhibits three major interconnected inflammatory loops: T helper (Th)17 cells and interleukin 17 (IL-17)-producing CD8+ T (Tc17) cells responses driven by IL-17, IL-23, and C-C motif chemokine ligand 20 (CCL20) feedback loops; type I and type II interferon (IFN) loops driven by plasmacytoid dendritic cells (pDCs) and IFN-γ-secreting T-cells (Th1 and Tc1); and IFN-γ-secreting T-cells (Th1 and Tc1) driven by IL-36. Treatment-induced remission is characterized by resolving psoriatic plaques containing tissue-resident memory cells (TRM), typically CD8⁺ IL-17-positive or CD8⁺ IFN-γ-positive cells. Concurrently, psoriasis is associated with multiple comorbidities, including cardiometabolic diseases, psoriatic arthritis, and depression.1,2
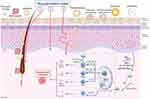
Topical treatments are commonly regarded as the initial choice for managing mild to moderate plaque psoriasis, and for patients with moderate to severe psoriasis, maintenance topical treatments exhibit the capacity to defer relapse.3 Anthralin (also referred to as hydroxyanthrone dithranol) was initially characterized in 1916 by Galewski and Unna, and served as a clinical agent for topically treating psoriatic skin lesions until the 1980s.4 Goeckerman demonstrated the scientific use of coal tar with ultraviolet radiation for treating psoriasis in 1925.5 In 1952, topical application of adrenocortical steroids was introduced, and in 1955, an emollient (liquid petrolatum, sodium chloride, and phenol) was first mentioned in the literature for treating scalp psoriasis.6,7 The exploration of novel drug delivery systems in dermatology dates back to the 1980s. Hermann RC et al prepared liposomes of cyclosporine and conducted in vitro tests in 1988.8 Although the results indicated that topical application of cyclosporine could not improve psoriasis symptoms, various drug delivery systems, including liposomes, have been progressively studied in psoriasis. Vitamin D analogues received approval for treating psoriasis in 1991 in the U.K.9 Tazarotene initiated clinical trials for psoriasis treatment in 1996, and in 1998, topical 0.3% tacrolimus (FK506) demonstrated efficacy in a clinical trial.10,11 The introduction of topical calcineurin inhibitors marked a significant non-steroidal advance in psoriasis treatment. In 2001, a mouse monoclonal antibody against human interleukin-8 cream was approved for psoriasis treatment in China.12 Tofacitinib began phase IIa clinical trials in 2013 but awaits formal approval for psoriasis treatment.13 Tapinarof, a natural aryl hydrocarbon receptor (AhR) agonist, exhibited a favorable anti-psoriasis effect in vivo and in vitro trials that commenced in 2017, leading to its approval in 2022.14,15 The PDE4 inhibitor 0.3% roflumilast completed phase IIb clinical trials in 2020 and is currently FDA-approved for use in patients with moderate-to-severe plaque psoriasis aged 12 years and older.16 Brepocitinib, a topical TYK2/JAK1 inhibitor, demonstrated good tolerability in phase IIb clinical trials in 2022.17 Tirbanibulin (KX01), a new Src kinase inhibitor that inhibits keratinocyte proliferation, is currently approved for treating actinic keratosis and has recently initiated a phase I clinical trial for psoriasis treatment.18 A timeline chart on the emergence of topicals is shown in Figure 1. As our understanding of psoriasis pathogenesis deepens, Figure 2 illustrates a diverse array of drug delivery systems emerging. Notably, new nano-delivery systems, encompassing lipid-based carriers, polymer-based carriers, metallic nanocarriers, and nanocrystals (NCs), offer advantages such as enhanced skin permeability, targeted accumulation at the site of interest, controlled drug release, favorable solubility, high encapsulation efficiency, diminished off-target effects, enhanced drug stability, and biocompatibility.19–21 These attributes collectively lead to a reduction in dosage and frequency of administration, consequently elevating therapeutic efficacy and fostering patient compliance.
 |
Figure 1 Timeline of topical therapies in psoriasis. The color text highlights the initial time of studies of novel drug delivery systems in psoriasis. |
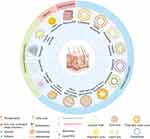 |
Figure 2 Schematic illustration of anatomical structure of the human skin and representative novel drug delivery system for the topical treatment of psoriasis. Created with BioRender.com. |
In recent decades, there has been a progressive exploration of drug delivery systems in the context of psoriasis. However, there is a dearth of literature systematically summarizing and consolidating these findings. Bibliometrics, a widely employed research analysis method, operates at a macro level within databases.22 This approach enables the examination of literature across various dimensions, unveiling the information network of publications. It offers researchers a comprehensive understanding of the field on a larger scale, facilitating grasping the knowledge structure within the research domain.23,24
Materials and Methods
Data Collection and Search Strategies
We conducted an advanced search in the Science Citation Index Expanded (SCI-EXPANDED) in the Web of Science Core Collection (WoSCC) database on 28 December 2022. We used the following search terms to identify publications primarily concerning psoriasis and drug delivery: TS= (“drug deliver*” OR “drug-delivery” OR “drug release*” OR “drug carr*” OR “pulsatile releas*” OR “transdermal deliver*” OR “drug delivery system*” OR “topical delivery” OR “skin delivery ”) AND TS= (psoriasis). Then, the document types were limited to articles and review articles, while the publication language was limited to English. Ultimately, 688 articles met the criteria for inclusion in this study, comprising 523 articles and 165 review articles. Publications meeting the inclusion criteria were exported as plain text files, adhering to the “Full Record and Cited References” format.
Data Analysis and Visualization
In this study, we employed five bibliometric analysis and visualization tools, ie, CiteSpace (6.2.R4), VOSviewer (version 1.6.19), the bibliometrix package in R software (10.1016/j.joi.2017.08.007), Scimago Graphica and Pajek 5.18 to focus on publications, citations, countries/regions and institutions, authors, journals, references, and keywords. Pajek can make the clustering distributions obtained in VOSviewer more rational and arranged. Scimago Graphica is used to map research collaborations between countries. Bibliometrix in conjunction with RStudio will be used to conduct statistics on impact indicators in bibliometrics. In addition to the common bibliometric data such as publication count, citation and co-citation frequency, and the impact factor (IF), our inquiry also introduces the betweenness centrality and the Hirsch index (h-index) has been introduced to provide a more comprehensive evaluation. Betweenness centrality can be used to identify potentially important nodes in a visualization network.25 The h-index is a quantitative measure used to assess an author’s scholarly output and its corresponding impact on citations within the scholarly community. Characterized by the apex value of h, the h-index signifies that an author has disseminated a minimum of h publications, each garnering no less than h citations.26–28 The application of the h-index examines the productivity and impact of an author, thus helping readers to identify more promising researchers. The detailed process of this study is outlined in Figure 3.
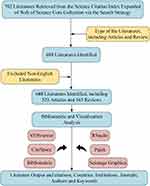 |
Figure 3 Schematic depiction outlining the literature search, screening, and analytical procedures employed in this investigation. |
Statistical Methods and Data Processing
Our analysis includes data up to October 2023, covering trends from 1993 to 2022 in annual publication volume and cumulative citations. Given the non-linear patterns in the raw data, we applied a log transformation to both metrics to stabilize variance and align more closely with the normality assumption of linear regression. We then fitted linear regression models using the year as the independent variable and the logarithms of annual publications and cumulative citations as dependent variables, respectively. Using ordinary least squares (OLS), we estimated positive coefficients indicating increasing trends: the publication model had a slope of 0.234 and an intercept of −463.96, while the citation model showed a slope of 0.238 with an intercept of −470.27 (Figure 4). Furthermore, we evaluated model efficacy using R-squared and Mean Squared Error (MSE). The R-squared values were 0.858 for publications and 0.9921 for citations, suggesting the models account for the most variability in the data. Additionally, the extremely low p-values for the year coefficients confirm the significant influence of time on the trends, reinforcing the robustness and credibility of our interpretations.
Results
Analysis of the Trend of Publications and Citations
The annual volume of publications and citation frequency can serve as indicators reflecting a research field’s developmental trajectory and prospects. Illustrated in Figure 4, the cumulative annual count of articles and citation frequency in this field has exhibited exponential growth since 1993. Although the annual publication count showed minor fluctuations until 2019, a discernible upward trend emerged thereafter, peaking in 2022 at 100 publications. Concurrently, the citation frequency has consistently risen from 1993 to 2022, signifying an increasing output of high-quality literature and breakthroughs in drug delivery systems research for psoriasis. In the statistical methods and data processing section, constructing predictive models for annual publications and cumulative citations suggests that psoriasis drug delivery systems represent significant research potential and are emerging academic hotspots warranting deeper investigation. The substantial research potential in drug delivery systems for psoriasis warrants the attention of the academic community, meriting further exploration.
Distribution of Countries/Regions and Institutions
This study encompasses 1871 institutions across 189 countries and regions. Table S1 details the leading ten countries and institutions in scholarly output. India ranks highest with 198 publications, surpassing most nations globally in citation frequency (5468), average citation frequency (27.62), and h-index (40). Jamia Hamdard University emerges as the most prolific research institution, with a citation frequency of 394. Notably, four Indian institutions feature among the top ten in academic output, underscoring India’s profound scientific prowess in the realm of drug delivery systems for psoriasis. Figure 5A illustrates the cooperation network diagram among the top 20 countries based on publication numbers. The dot size reflects the number of publications; the redder the dot, the stronger the collaboration with other nations. Connecting lines indicate the strength of cooperation between countries. Notably, while the United States exhibits a lower publication count than India and China, it demonstrates the closest collaboration with other countries, suggesting a broader future development outlook in this field. In Figure 5B, the top 20 countries are categorized by the extent of multi-country (red) and single-country (blue) publications. Figure 5C depicts the institutional cooperation network, where nodes of the same color represent a common cluster, emphasizing close collaborative relationships. The strength of the connecting line between nodes indicates the strength of cooperation between institutions. Chang Gung University stands out with the highest cooperation strength in this field. Figure 5D portrays a map of country publication numbers. Nodes of distinct colors correspond to different countries, with the size of each node reflecting the quantity of articles contributed by that specific country. The intensity of connections between nodes signifies the strength of collaboration among nations. Analyzing article publication and collaboration status provides a comprehensive understanding of drug delivery system research progress in psoriasis, facilitating deeper exploration in this domain. Meanwhile, this analysis lays the foundation for more detailed research. The factors contributing to the success of these high-yield regions and institutions warrant further investigation. Researchers may benefit from visiting and exchanging information with these entities, drawing on the strengths of their research models and policies. Additionally, synthesizing and analyzing data from various countries and institutions alongside other data in the text may yield valuable insights.
Analysis of Authors and Co-Cited Authors
In the realm of drug delivery in psoriasis, we scrutinized 3180 authors. Table S2 lists the top 10 authors based on publication count and co-citation frequency within drug delivery systems research for psoriasis. A higher co-citation frequency signifies closer collaboration with peers and underscores the significance of the author’s research contributions to field development.29 Figure S1A depicts the co-citation network of authors, with node size indicating co-citation frequency. Nodes with purple borders signify higher betweenness centrality (exceeding 0.1). Notably, Madhulika Pradhan from India holds the highest co-citation frequency. Figure S1B portrays an author co-occurrence network, showcasing collaborations among authors in the field. Gautam Singhvi from Birla Institute of Technology and Science leads in the number of publications, while Jia-You Fang from Chang Gung University stands out with the highest citation frequency and h-index. Through meticulous statistical analysis of scholarly publications and co-citations, we gain a more accurate understanding of the knowledge landscape in drug delivery systems for psoriasis, enabling the inference of research hotspots.
Analysis of Journals and Co-Cited Journals
To comprehensively explore the foundational and cutting-edge advancements in drug delivery systems for psoriasis, we conducted rigorous statistical and analytical evaluations of historical and co-cited journals. Table S3 enumerates the top ten journals based on both publication count and co-citation frequency. Figure 6A visualizes the co-citation network of journals, elucidating the types of journals serving as primary sources of knowledge in the field. Figure 6B illustrates the network of cited journals, encompassing 223 journal types. The top ten journals in Table S2 boast the highest publication volume and co-citation frequency. Notably, the International Journal of Pharmaceutics leads in publication volume and co-citation frequency, and all ten journals are recognized by the Journal Citation Reports (JCR). Furthermore, these journals, classified as Q1 in the JCR, underscore the growing academic attention towards studying drug delivery systems in psoriasis. Nine of the ten journals have an IF exceeding 5, signifying the increasing prominence of this research area and its potential as a future research hotspot.
Analysis of References and Co-Cited References
Visualization and statistical analysis of co-cited relationships in the literature enable the identification of influential publications in the domain of drug delivery systems for psoriasis. These publications exhibit temporal fluctuations that mirror the progress and shifts in the research frontiers of the field.30 Table S4 presents the top ten cited publications, with “Overcoming the Achilles’ heel of photodynamic therapy”, authored by Wenpei Fan et al and published in Chemical Society Reviews in 2016, emerging as the most frequently cited article. Figure 7A illustrates the co-citation network of the literature, while Figure 7B showcases the top 100 articles according to citation frequency. Figure 7C provides a visual network of co-cited literature clustered based on thematic directions, including novel colloidal carriers, skin disease treatment, transdermal drug delivery, clobitasol propionate, treatment modalities, psoriasis management, psoriasis treatment, topical therapy, and topical microRNA-directed therapy. Figure 7D displays co-cited reference clustering on a timeline diagram, offering insights into the evolution of the knowledge base in the field of drug delivery systems for psoriasis through changes in nodes over time. The initial cluster, denoted as #0 novel colloidal carrier, represents the embryonic research stage into drug delivery systems for psoriasis. It marks the inception of targeted and extensive investigations in this domain, initiated by Jia-You Fang’s 2004 contribution to the British Journal of Dermatology. This seminal work delineates and contrasts two resurfacing techniques—laser and microdermabrasion—that optimize the in vitro skin permeation of 5-aminolaevulinic acid (ALA).31 Recent research has identified #1 skin disease treatment and #2 transdermal drug delivery as pivotal areas of inquiry. In the #1 cluster, Xue Zhou’s 2022 article in Cell Death & Disease, succinctly outlines the pivotal role of keratinocytes as target cells in psoriasis therapy.32 In the #2 cluster, the latest contribution comes from Ruijie Chen and colleagues in 2022. It demonstrates the application of Alantolactone (ALT)-loaded chitosan/hyaluronic acid (HA) nanoparticles (CHALT) for psoriasis treatment. The study reveals the suppression of psoriasis through the deactivation of STAT3 hyperactivity within keratinocytes and the restriction of immune cell recruitment, offering a promising avenue for improving psoriasis symptoms.33
Analysis of Keywords
Following the exclusion of redundant keywords sharing search terms or demonstrating low relevance to the study, Table S5 presents the top 20 keywords arranged in descending order based on their frequency in titles and abstracts. Notably, “nanoparticles (NPs)” is the most frequently occurring keyword. In Figure 8A, the top 14 keywords are depicted over time, with a reddish period indicating concentrated bursts, reflective of hotspots in the research field during specific periods. The study identifies 14 influential terms in drug delivery systems for psoriasis, including percutaneous absorption, psoriasis, absorption, in vivo, liposomes, vesicles, mechanisms, stability, transdermal drug delivery, imiquimod-induced psoriasis, Box-Behnken design, inflammation, oxidative stress, and drug delivery system. Figure 8B illustrates the keyword co-occurrence network within the research area, while Figure 8C employs cluster visualization to categorize drug delivery system research in psoriasis into 12 directions. These encompass #0 topical transdermal delivery, #1 psoriasis physiopathology, #2 drug nail permeability, #3 using micellar nanocarrier, #4 in vivo microdialysis, #5 using electroporation, #6 passive diffusion, #7 cutaneous penetration studies, #8 human keratinocyte, #9 5-aminolaevulinic acid, #10 sampling technique, and #11 effective management. Further enhancing clarity, Figure 8D integrates a timeline analysis atop the clustering, providing a clearer depiction of directional changes over time. This approach also facilitates the observation of inter-cluster connections through node connectivity.34 The timeline analysis reveals that the current research frontiers encompass #0 topical transdermal delivery, #3 using micellar nanocarriers, #4 in vivo microdialysis, and #5 using electroporation.
Discussion
This study presents the inaugural bibliometric analysis of hotspots and trends within drug delivery systems in psoriasis research. Our investigation reveals a consistent, albeit fluctuating, increase in publications and annual citation frequency in this field since the close of the twentieth century. This trend signifies a growing scholarly emphasis and attention on studying drug delivery systems in psoriasis. An examination of countries, regions, and institutions identifies India as having the highest publication count, the United States boasts the highest average citation frequency and exhibits the closest collaboration with other nations. Notably, Jamia Hamdard University records the highest publication count, and Chang Gung University achieves the highest citation frequency. Among individual contributors, Gautam Singhvi claims the highest publication count, Madhulika Pradhan leads in co-citation frequency, and Jia-You Fang secures the highest citation frequency. The articles by Singhvi and Pradhan warrant meticulous examination. At the same time, Fang’s high citation frequency reflects scholarly recognition of the content and direction of their research—areas deserving of focused attention in future studies. Journal analysis underscores the International Journal of Pharmaceutics as the most prolific in terms of article count, with the European Journal of Pharmaceutics and Biopharmaceutics ranking highest in citation frequency. The International Journal of Pharmaceutics claims the top spot in co-citation frequency. Researchers are encouraged to prioritize literature within these journals for further study.
Topical treatments are the primary choice for mild to moderate plaque psoriasis.35 Maintenance topical therapy may delay relapse in patients with moderate to severe psoriasis. Noteworthy advantages of topical therapy encompass: 1. reduction of systemic side effects; 2. targeted concentration of the drug in the affected tissue; and 3. circumvention of first-pass metabolism. Commonly employed topical therapeutic agents in clinical practice comprise anthralin, coal tar, salicylic acid (SA), glucocorticoids, emollients, retinoic acid, vitamin D3 derivatives, calcium-modulated phosphatase inhibitors, and herbal medications.3
Over the past decades, nanotechnology has captivated the interest of researchers across diverse fields. Its application in topical therapy has proliferated, marking significant progress in drug delivery systems.19 Applications of nanocarriers in topical drug delivery systems for psoriasis are shown in Table 1. Nanocarriers confer several advantages: (1) heightened skin permeability; (2) optimal retention of active ingredients with subsequent sustained drug release; (3) binding of hydrophobic and hydrophilic active ingredients;36 (4) targeted delivery with high bioavailability; and (5) increased stability and protection of active ingredients from degradation.37 The skin’s hydrophobic nature and tightly packed stratum corneum (SC) are barriers against the penetration of toxic substances and active agents. Therefore, the efficacy of dermal therapy relies on a drug’s ability to overcome this skin barrier and penetrate the epidermis. Despite the effectiveness of current topical treatments for psoriasis in various forms (including gels, ointments, tinctures, emulsions, and applications), their limited penetration and lack of targeted enrichment within the dermis and epidermis restrict their application. Consequently, there is a pressing need to develop novel drug delivery systems that enhance topical drugs’ skin permeability and targeting capabilities, thereby improving their therapeutic efficacy.38 Figure 9 illustrates the mechanism of the novel drug delivery systems and their association with the pathogenesis of psoriasis.
 |
Table 1 Applications of Nanocarriers in Topical Drug Delivery Systems for Psoriasis |
Lipid-Based Carriers
Lipids are crucial in preserving skin hydration, integrity, and overall health. Lipid-based carriers encompass epidermal lipid-like components such as fatty acids, oils, polymers, and lipid derivatives, coupled with stabilizers (emulsifiers, surfactants, and cosurfactants). These carriers exhibit a notable ability to penetrate the deeper layers of the skin and demonstrate favorable biocompatibility as delivery systems.143 Simultaneously, they offer several advantages, including straightforward preparation, self-assembly capabilities, high bioavailability, the capacity to carry substantial payloads, and controllability.144–146 Lipid-based carriers primarily fall into four categories: emulsion systems (comprising multiple emulsions, microemulsions (MEs), and nanoemulsions (NEs), particulate systems (including solid lipid nanoparticles (SLNs), solid lipid microparticles, and Nanostructured lipid carriers (NLCs)), and NP systems (specifically liposomes, ethosomes (ETH), niosomes, transferosomes (TFs), and transmethylated liposomes), along with transmolecular systems (involving multiple emulsions, MEs, and NEs).144
Vesicular Systems
Liposomes
Liposomes, composed of phospholipids forming monolayer or multilayer vesicle structures, offer substantial pharmacokinetic advantages.147,148 They exhibit the capacity to transport and deliver hydrophilic, hydrophobic, and lipophilic drugs, as well as encapsulate both hydrophilic and lipophilic compounds within the same system.144,149 Modulations in NP size, surface charge, lipid composition, number of phospholipid layers, and surface modifications (with ligands or polymers) can influence the stability of liposomes.144,145,150 Combining many drugs with liposomes can enhance their physicochemical properties and facilitate drug absorption and retention in the skin, thereby demonstrating improved efficacy in psoriasis treatment.
Previously, the use of certain drugs in topical psoriasis treatments was constrained by poor water solubility, among other factors. However, liposome-related studies have enhanced drug solubility and penetration. Raju Saka et al optimized liposomes (67.8 ± 7.15 nm, PDI 0.26 ± 0.02) with an entrapment efficiency of over 90%, incorporating them into a gel loaded with bexarotene.44 In another study, quercetin-loaded liposomes were stabilized by hydroxypropyl-β-cyclodextrin (HPCD), which forms a stabilizing layer through hydrogen bonding with the liposome interface, enhancing stability.40 Additionally, Fan Yu developed peptide-modified liposomes, while Harsha Jain et al created formulations loaded with ibrutinib and co-loaded with curcumin, significantly improving transdermal curcumin efficiency.43,49
Liposome research on drugs commonly used for topical therapy is advancing rapidly. Sindhu Doppalapudi’s team investigated two liposomal formulations: cationic liposomes (DC-Chol and cholesterol) and anionic liposomes (egg lecithin, cholesterol, and tetramyristoyl cardiolipin), recording zeta potentials of +25.8mV and −28.5mV, and entrapment efficiencies of 75.12% and 60.08%, respectively. These studies demonstrated a fivefold increase in psoralen permeation using liposomal carriers, potentially enhancing the efficacy of topical PUVA treatments for psoriasis.46 Similarly, Manoj Walunj’s team developed cationic liposomes (DOTAP and cholesterol) loaded with cyclosporine.41 Fei Qu et al enhanced skin retention times to 132 hours and improved cellular internalization in vivo by integrating microneedles with 250 nm cationic liposomes. They found that dexamethasone-loaded cationic liposomes integrated with microneedles outperformed their anionic counterparts in a psoriasis-like animal model. Long Xi et al combined celastrol-loaded mannosylated liposomes with microneedles, inhibiting DC maturation markers (CD80, CD86, MHC-II).42 Wei Wang et al developed a nanotransdermal system (TCeO2-TRA-FNL-Gel) combining all-trans retinoic acid, triphenylphosphine-modified cerium oxide nanoparticles, and flexible nanoliposomes, showing enhanced skin retention and superior mitochondrial targeting related to scavenging reactive oxygen species (ROS) over free TRA.39 Lastly, formulations including liposomal fusidic acid and calcipotriol-loaded liposomes with one mol% PEG-DSPE, as well as a liposomal gel containing zedoary turmeric oil and tretinoin, have demonstrated superior efficacy compared to direct topical applications.45,47,48
Transferosomes
TFs, also known as transfersomes, are elastic liposomes primarily composed of phospholipids (eg, soya PC, egg PC, dipalmityl PC, etc). and surfactants (eg, Span®80, Tween®80, sodium cholate, etc). serving as edge activators.151–153 Their high deformability enables penetration into the skin through the self-extrusion of intracellular closed lipids along the SC.154 TFs exhibit a burst release effect during the initial 4 hours post-administration, followed by a gradual release. The bursting effect is linked to minor breaks in the lipid bilayer, and the drug within the nucleus is also partially released due to vesicle deformation during penetration.155
Recent studies have demonstrated the enhanced efficiency of TFs loaded with cyclosporine A (CyA/CsA) through membrane extrusion, in vitro skin diffusion studies revealed that the maximal flux (μg/cm²/h) of extruded transfersomes was twice as high as that of sonicated transfersomes. Additionally, extruded transfersomes exhibited a higher CyA concentration, reaching 445 ± 39 μg/mL.50 Wei Wang et al prepared TRA and BT dual-loaded flexible liposomes with an average particle diameter of ~70 nm and high drug encapsulation efficiency exceeding 98%. Compared to free drugs, these liposomes significantly enhanced drug penetration and retention in the skin. In vivo experiments showed optimal reduction in epidermal thickness and cytokine levels (TNF-α and IL-6).51 Mahdiyeh Bahramizadeh et al prepared methotrexate-entrapped TFs with a particle size of approximately 100 nm and an average zeta potential of −72.87 mV. These TFs exhibited an encapsulation efficiency greater than 85%, achieved 50% skin penetration, and resulted in a more significant reduction in skin thickness scores in mice compared to the group receiving injected methotrexate (MTX) in the in vivo experiments.52 Nilanchala Sahu et al formulated nanotransferosomes (NTF) using Phospholipon 90G, cholesterol, and sodium cholate for delivering Solanum xanthocarpum extract (SXE-NTF). The vesicles had an average diameter of 146.3 nm, a PDI of 0.2594, an EE of 82.24 ± 2.64%, a drug loading of 8.367 ± 0.07%, and a release rate of 78.86 ± 5.24%. Ex vivo permeation tests demonstrated that SXE-NTF gel significantly outperformed SXE gel, achieving a permeability of 82.86 ± 2.38% compared to 35.28 ± 1.62%.156 These findings substantiate the efficacy of the TFs delivery system.157
Ethosomes
ETHs consist of phospholipids, typically 2%–5% phosphatidylcholine (PC), along with 20%–45% ethanol and water (to 100%, ω/w).158 The vesicle size is adjustable within the nanometer to micrometer range, and vesicles can have single or multiple layers.159,160 The first stage in the penetration of ETH through the skin is termed the “ethanol effect”, wherein ethanol disrupts the conformation of SC lipids. Increased lipid mobility reduces the density of lipid multilayers, facilitating deeper vesicle penetration into the skin. This is followed by the “ETH effect”, during which the phospholipids within the SC fuse with ETH vesicles, enhancing drug delivery.161,162
In previous investigations, 30% alcohol plasmids loaded with tacrolimus (TAC) exhibited significantly heightened permeability in the epidermis and superior epidermal accumulation compared to traditional formulations.163 In recent research, hyaluronic acid-based tacrolimus ETH (HA-TAC-ETH) demonstrated nanometric vesicle sizes (315.7 ± 2.2 nm), a polydispersity index (PDI) of 0.472 ± 0.07, and high entrapment efficiency (88.3 ± 2.52%). These ETHs exhibited increased dermal flux and an enhancement ratio alongside sustained drug release. In a psoriasis mouse model, the HA-TAC-ETH gel effectively reduced erythema, edema, and skin thickness, offering a favorable safety profile compared to systemic drug administration.53 Yongtai Zhang et al developed a novel topical drug delivery system by covalently linking hyaluronic acid (HA) to propylene glycol-based ETHs (HA-ES). HA-ES significantly enhanced transdermal curcumin delivery compared to plain ethosomes (ES) and a curcumin propylene glycol solution (PGS). Specifically, the cumulative transdermal delivery and retention in the skin after 8 hours were 1.6 and 1.4 times greater with HA-ES than with ES and 3.1 and 3.3 times greater than with PGS, respectively. Furthermore, in vivo studies showed that the psoriatic skin retention of curcumin using HA-ES was 2.3 and 4.0 times higher than with ES and PGS, respectively.54 Yu Li et al developed curcumin-loaded composite phospholipid ETHs composed of a 1:1 ratio of unsaturated phosphatidylcholine (PC) to saturated hydrogenated phosphatidylcholine (HPC). These ETHs demonstrated optimal vesicle stability and flexibility compared to free curcumin. Additionally, their low uptake by HaCaT cells suggests reduced toxicity and enhanced penetration into deeper skin layers via the hair follicle pathway.55 In a comparative study, ETHs significantly outperformed liposomes in delivering psoralen, with transdermal flux and skin deposition rates of 38.89 ± 0.32 μg/cm²/h and 3.87 ± 1.74 μg/cm², respectively. These rates were 3.50 and 2.15 times higher than those achieved with liposomes.56 Liposomal and ethosomal gels designed for the topical delivery of anthralin demonstrated high drug encapsulation efficiencies, achieving ≥97.2% for liposomes and ≥77% for ETHs. Post-treatment, the mean PASI changes were −68.66% for liposomes and −81.84% for ETHs, indicating a statistically significant superior efficacy of ETHs.57
Transethosomes
Transethosomes (TEs) possess a structure comprising the fundamental constituents of ETH (20%–45% ethanol and phospholipids) and single-chain surfactants serving as edge activators (Tween®80, sodium taurocholate, oleic acid, or decyl methyl sulfoxide, etc).164 This surfactant amalgamates the features of TFs and ETHs, synergizing sudden release with robust permeability. Costanzo et al demonstrated that TEs, comprising polysorbate 80 (T80) loaded with cholecalciferol (vitamin D3), exhibited no adverse effects on keratinocyte survival. Within 2 hours of administration, these TEs penetrated keratinocytes, undergoing complete degradation and release within 24 hours. Hence, this vector emerges as a promising vehicle for the transdermal administration of vitamin D3.58 Rodríguez-Luna et al developed TEs -in-Carbopol® loaded with rosmarinic acid, wherein the TE incorporated sodium deoxycholate as a limbic activator. This formulation addressed the challenges of rosmarinic acid, characterized by poor water solubility, low permeability, and chemical instability. In a murine model of IMQ-induced psoriasiform dermatitis, notable improvement in skin edema was observed, accompanied by reduced levels of TNF-α and IL-6 at the skin lesions, affirming the efficacy of this delivery approach.59
Non-Ionic Surfactant Vesicles (Niosomes)
Niosomes (Nios), also recognized as nonionic surfactant vesicles, represent microscopic lamellar structures formed through the amalgamation of nonionic surfactants (eg, amino acid compounds, alkyl esters, alkyl amides, fatty acids, and alkyl ethers) followed by hydration.153,165,166 Their composition distinguishes Nios from liposomes, as the former contains less toxic nonionic surfactants than phospholipids. This choice imparts superior stability, enhanced antioxidant properties, and reduced production costs.167 Nonionic surfactants can modify the SC, rendering it more permeable and lax, consequently amplifying the residence time and local concentration of drugs in the SC and epidermis.168
Nio gel, laden with cyclosporine, demonstrated superior penetration compared to the suspension. Drug deposition studies and fluorescence microscopy revealed a 59-fold increase in drug deposition in the SC and the vivo epidermis/dermis (VED) layer. Concurrently, intervention using this drug delivery mode significantly reduced histopathological and PASI scores in the IMQ-induced psoriasis model, underscoring the formulation’s superiority.60 Gel formulations incorporating carbomer 980 into desoximetasone-loaded Nios exhibited a stable slow-release process in vitro skin permeation tests. After 24 hours, the niosomal gel released 9.75 ± 0.44 µg/cm² of desoximetasone, compared to 24.22 ± 4.29 µg/cm² from the reference gel. Drug deposition studies demonstrated that the Nios achieved superior skin deposition (30.88 ng/mg versus 26.01 ng/mg) than conventional gels, indicating enhanced drug penetration and retention in human skin.61 Meng et al synthesized Celastrol Nios using the thin film hydration method followed by probe sonication, utilizing a formulation of Span 20, Span 60, and cholesterol in a 3:1:1 weight ratio. These Nios, with an average particle size of approximately 147 nm, yielded up to 90%. They exhibited significantly enhanced water solubility and permeability alongside improved antipsoriatic activity in mice.62,169 Acitretin (Act) -loaded Nios (span 60: cholesterol molar ratio 1:1) demonstrated enhanced stability and reduced irritation. In vitro, skin penetration studies in rats revealed a significantly higher cumulative penetration of Act nio gel compared to Act gel after 30 hours. Moreover, Act nio gel exhibited notable antipsoriasis benefits in a topical application on a mouse tail model.63 Loaded with diacerein, Nios addressed low solubility, low bioavailability, a short half-life, and severe gastrointestinal adverse effects. Niosomes were prepared using 90 mg of Span 60 and 10 mg of cholesterol with a hydration time of 45 minutes. This formulation significantly enhanced the penetration of diacerein into the epidermal and dermal layers of rat skin, improving drug stability and supporting its use in psoriasis treatment.64
Cerosomes
Cerosomes represent a distinctive vesicular class, incorporating ceramide lipids as a crucial component in the lipid bilayer. The presence of ceramide sphingolipids, naturally occurring in the skin, imparts the vesicles prepared with ceramide with the capability to enhance drug penetration by disrupting the intercellular lipid organization of the skin.170
In tubulated vesicles, termed “cerosomes”, loaded with tazarotene, ceramides increased the encapsulation rate of tazarotene while reducing its release, concurrently enhancing its deposition in the skin. In a clinical trial involving 20 patients with plaque psoriasis, this delivery system significantly outperformed tazarotene gel, resulting in a more substantial reduction in PASI scores.65 Yang et al developed MTX/NIC cerosomes co-delivering MTX and nicotinamide (NIC). NIC effectively solubilizes MTX through the formation of hydrogen bonds. The cerosomes facilitate drug penetration and retention in rat skin. MTX/NIC cerosomes induce apoptosis and exhibit robust resistance to HaCaT cells, displaying potent antiproliferative effects.66 Sammar Fathy Elhabal et al developed positively charged cerosomes based on ceramide IIIB to co-deliver CsA and dithranol (DTH). The cerosomes exhibited an average particle size of 222.36 ± 0.36 nm, a polydispersity index of 0.415 ± 0.04, an entrapment efficiency of 96.91% ± 0.56, and a zeta potential of 29.36 ± 0.38 mV. Compared to a CsA/DTH solution, the cerosomes enhanced drug penetration by 66.7% and decreased PASI by 2.73-fold and 42.85%, respectively.67
Emulsion Drug Delivery Systems
Emulsions represent biphasic liquid systems, wherein the inner phase (dispersed phase) forms tiny droplets dispersed in the outer phase (continuous phase). The oil phase matrix typically comprises diacylglycerols, monoacylglycerols, triacylglycerols, and free fatty acids bound with loaded drugs and lipophilic surfactants. The aqueous phase consists mainly of water, surfactants, cosurfactants, emollients, and other components.171 Oil-in-water (O/W) emulsions consist of oil droplets dispersed in water, providing a vehicle for loading hydrophobic drugs into the oil core. Conversely, water-in-oil (W/O) emulsions possess the opposite structure, with water droplets dispersed in oil, making them a preferred carrier for hydrophilic drugs.172 The fundamental structure of W/O/W emulsions involves large oil droplets dispersed in an aqueous phase, containing water droplets as the dispersed phase. In the O/W/O emulsion system, water droplets containing the oil droplets are dispersed in the oil phase.171 These two multiple emulsion systems are suitable for loading hydrophilic and hydrophobic actives in the aqueous and oil phases. Emulsion Drug Delivery Systems primarily consist of two forms: miniemulsions and NEs.
Nanoemulsion (NE) gels containing TAC and using fish or flax oil as the oil phase with 1% Carbopol 934 significantly enhance penetration and skin retention. Compared to commercially available creams, these nanoemulsions achieve cumulative drug penetration into the skin, which is twice as effective and provides approximately 1.5 times greater penetration depth and skin retention. In IMQ-induced psoriasis mice, treatment with these NE gels resulted in significantly reduced levels of TNF-alpha and IL-6 in the skin, demonstrating superior antipsoriasis efficacy compared to cream administration.68 Sahu et al also developed a NE loaded with TAC, utilizing kalonji as the oil phase. In an in vitro mouse skin penetration assay, the NE gel with Caropol 934 displayed sustained release, achieving 4.33 times higher dermal bioavailability than commercially available cream.68,70 Wan et al devised and optimized a TAC-loaded microemulsion formulation using a binary surfactant mixture of d-α-tocopheryl polyethylene glycol succinate (TPGS) and Labrasol. The study results indicated complete solubilization of TAC in TPGS-ME, exhibiting significantly increased TPGS-ME uptake. The topical application of TAC-TPGS-ME for psoriasis treatment in a mouse model surpassed the efficacy of existing creams.69 Khatoon et al developed a NE gel co-loaded with curcumin (CRC), resveratrol, and thymoquinone. The optimized formulation featured a droplet size of 76.20 ± 1.67 nm, a PDI of 0.12 ± 0.05, a refractive index (RI) of 1.403 ± 0.007, and a viscosity of 137.9 ± 4.07 mPa·s. This formulation inhibited the A-431 cell line growth, displayed potent anti-angiogenic activity, and demonstrated therapeutic efficacy in an IMQ-induced mouse model psoriasis.71 Algahtani et al employed a novel method to optimize a NE loaded with CRC, transforming it into a nanoemulgel by adding Carbopol 934. The study results indicated a 4.87-fold increase in drug penetration in the nanoemulgel.72 Benigni et al developed microemulsions (MEs) loaded with cyclosporine, utilizing a high-viscosity system containing TPGS. This formulation showed significantly elevated skin accumulation and rapid absorption, achieving approximately six times the skin accumulation of cyclosporine compared to a control solution in propylene glycol.73 Pandey et al developed a microemulsion (ME) gel loaded with cyclosporine, using isopropyl myristate as the oil phase and Carbopol 940 as the gelling agent. This formulation demonstrated a significantly higher permeation rate than cyclosporine suspensions and exhibited substantial drug retention in skin tissue, achieving a retention rate of 38.92%.74 Tanghetti et al developed an O/W emulsion system that co-encapsulates halobetasol propionate and tazarotene within a polymeric matrix, incorporating moisturizing and hydrating ingredients. This formulation enhanced hydration and improved drug release, promoting deeper penetration into the dermis and reducing irritation. The 0.01% halobetasol propionate/0.045% tazarotene lotion, utilizing polymeric emulsion technology, achieved more effective delivery of active ingredients than either 0.05% halobetasol propionate or 0.1% tazarotene creams alone. Moreover, the synergistic effect of the combined drugs significantly increased efficacy in treating psoriasis compared to single-drug applications.75 El-Gogary et al formulated an Oleuropein ME formulation. The selected microemulsion formulation displayed a particle size of 30.25 ± 4.8 nm, zeta potential 0.15 ± 0.08 mV and polydispersity index 0.3 ± 0.08, with storage stability for 1 year in room temperature and total deposition in skin layers amounting to 95.67%. That, in an eight-week clinical trial involving 20 patients with plaque-type psoriasis, exhibited a more pronounced reduction in PASI scores than clobetasol propionate. Dermoscopic symptomatic improvement was more prominent with the Oleuropein ME formulation.76 Rashid et al developed a nanoemulsion gel (MTX NEG) containing MTX and olive oil, using sodium alginate as the gelling agent. The optimized MTX NEG displayed a particle size of 202.6 ± 11.59 nm, a PDI of 0.233 ± 0.01, and an average entrapment efficiency of 76.57 ± 2.48%. Over 24 hours, the gel facilitated the permeation of an average of 70.78 ± 5.8 μg/cm² of MTX, with a flux value of 2.078 ± 0.42 μg/cm²/h. This formulation achieved higher skin retention than both the MTX solution and the plain MTX gel, particularly in the deeper epidermal and dermal layers. In an IMQ-induced rat model, topical MTX NEG demonstrated superior therapeutic effects compared to oral MTX treatment.77 Guo et al designed a ME formulation loaded with salvianolic acid B, significantly improving IMQ-induced psoriasis lesions in mice. The formulation inhibited epidermal hyperplasia, increased skin moisture, and decreased IL-23/IL-17 cytokine expression.78
Lipid Nanoparticles
Lipid NPs are non-homogeneous systems comprising an inner lipid phase and an outer aqueous phase stabilized by one or two surfactants. Topically applied lipid NPs encompass SLNs and NLCs.103 The surface charge of lipid NPs is typically determined by the lipid headgroups, which exhibit positive, negative, or amphoterically charged characteristics. The surface potential, contingent on the surface charge density, governs inter-particle interactions and counteracts ion adsorption, thereby regulating NP stability.104,105,153 Lipid NPs augment drug penetration into the epidermis and mitigate systemic absorption by overcoming the stratum SC barrier through various mechanisms: (1) Forming a thin film on the skin surface enhances skin hydration and decreases water loss,173 facilitating improved drug penetration into the SC. (2) Inducing a controlled occlusion effect due to particle size enhances skin hydration in the SC and promotes active substance diffusion into deeper skin layers.106 (3) Contributing to rearranging SC lipids and aiding active substance penetration. (4) Facilitating the miscibility or mixing of NLC lipid components with SC lipids to enhance penetration. (5) Introducing surfactants to disrupt skin structure and improve absorption. (6) Significantly altering intercellular accumulation, reducing keratocyte accumulation, and widening the interstitial space between keratinocytes.174 Moreover, lipid NPs offer additional advantages such as non-toxicity and protection against light-sensitive, oxidizing, and hydrolyzing drugs.175
Solid Lipid Nanoparticles
SLNs represent a lipidic nanocarrier system designed to enhance drug penetration and overall efficacy, typically exhibiting a particle size ranging from 10 to 1000 nm, categorizing them as “first-generation lipid nanocarriers”.176 SLNs consist of solid lipids, forming a crystal structure akin to a “brick wall”, effectively preventing particle coalescence.177 This unique structure allows drug molecules to stay solubilized or dispersed within the lipid matrix, providing SLNs with superior physical stability compared to liposomes.178 The central lipid core of SLNs is enveloped by a surfactant (emulsifier) layer, with the selection of an appropriate lipid matrix and surfactant being crucial for the preparation of effective and stable SLNs.179 When delivered to the skin, SLNs exhibit an initial burst release from the NP surface, followed by a controlled release, enabling the drug to penetrate further into the epidermis and dermis, contingent on its lipophilicity.157 Furthermore, SLNs offer elevated drug loading, enhanced bioavailability, facile mass production, and obviate the need for organic solvents.180
In a recent study, Rahmanian-Devin P et al developed SLN encapsulating Noscapine (SLN-NOS) using a central composite design, modified high-shear homogenization, and ultrasound methods. Precirol® was chosen as the optimal lipid base. Over 72 hours, SLN-NOS released 83.23% of Noscapine at pH 5.8 and 58.49% at pH 7.4. Franz diffusion cell experiments showed that skin absorption of Noscapine from SLN was 46.88%, significantly higher than the 13.5% from cream formulations, effectively improving symptoms in a psoriasis mouse model. The formulation also decreased IL-17, TNF-α, and transformed growth factor-β levels while increasing IL-10.79 Alhelal HM et al developed leflunomide (LEF)-loaded solid lipid nanoparticles (SLNs) integrated with hydrogels, achieving an entrapment efficiency of 65.25 ± 0.95% and a total drug content of 93.12 ± 1.72%. These formulations exhibited enhanced stability and anti-inflammatory activity compared to pure drug solutions. Additionally, modifications to the preparation method significantly reduced skin irritation.80 For the topical delivery of apremilast (API), Rapalli et al developed SLNs, which achieved delayed release of API over 18 hours. The dispersion of API in these SLNs led to a twofold reduction in TNF-α miRNA expression compared to the free drug. This formulation enhanced API’s skin penetration and retention, offering slow release, non-toxicity, reduced systemic absorption, and improved efficacy in treating psoriasis.81 Pitzanti et al developed SLNs incorporating the penetration enhancer Transcutol® P (TRC) to deliver 8-methoxy psoralen (8-MOP). The study showed that SLNs with 4% TRC enhanced the accumulation of 8-MOP in each skin layer compared to formulations with 2% and 0% TRC. Additionally, including TRC in SLNs increased cellular nanoparticle uptake without increasing cytotoxicity.82 Pradhan M et al developed SLNs encapsulating triamcinolone acetonide (TA), which exhibited prolonged drug release, closely following Higuchi release kinetics with an R² value of 0.9909. Furthermore, the sustained-release profile of these TA-loaded SLNs and their preferential accumulation in the epidermis significantly reduced undesirable systemic absorption and associated side effects.83 Sonawane, R. et al developed solid lipid nanoparticles (CT-BD-SLNs) loaded with betamethasone dipropionate (BD) and clobetasol propionate (CT), incorporated into a carbopol gel matrix. Compared to Daivobet ointment, this SLN gel formulation reduced the systemic absorption of CT and BD, exhibited non-irritating properties, and increased dermal bioavailability. This enhanced distribution within skin layers and demonstrated improved anti-psoriatic effects in a mouse psoriasis model.84 Essaghraoui, A. et al formulated SLNs based on Softisan® 649, a commonly used cosmetic ingredient, for localized CsA delivery. These SLNs exhibit a remarkable 95-fold increase in the local bioavailability of drugs by enhancing their water solubility.85 In a parallel approach, Silva et al developed an oleogel nanoformulation incorporating CsA-loaded SLNs, demonstrating improved drug stability and enhanced skin penetration.86 Additionally, Trombino, zaS.et al enriched SLN carriers with naringenin and linolenic acid, both possessing potent anti-inflammatory activity, for the topical administration of CsA. The researchers explored SLNs as a vehicle for CsA topical administration, incorporating various lipophilic gelling agents to enhance local drug release.87,181 Debarati Maiti et al engineered SLNs loaded with MTX. The results indicated that MTX was gradually released, achieving 80.36% skin permeation and effectively inhibiting keratinocyte growth with reduced cytotoxicity. Furthermore, this formulation’s flux and permeation rates surpassed those of existing marketed and standard preparations.88 Kang J. et al investigated thermosensitive SLNs encapsulating TAC with various shell surfactants to facilitate rapid drug release into surrounding tissues for enhanced therapeutic effects.89 Ferreira, M. et al encapsulated MTX in etanercept-coupled SLNs within carboplatin hydrogels. This formulation significantly increased MTX’s dermal bioavailability and allowed for sustained drug release through the carboplatin hydrogel. In vitro studies showed that these SLNs provided a sustained release of methotrexate over 8 hours. The hydrogel’s mucosal adhesion properties also extended MTX retention in the skin.90
Nanostructured Lipid Carriers
NLCs, recognized as “second-generation lipid nanocarriers” and structurally akin to SLNs, are typically synthesized through various inorganic or organic solvent methods. Their superior loading capacity and long-term stability position them as more favorable nanocarriers than SLNs. NLCs consist of solid and liquid lipids, particularly unsaturated ones, resulting in a minimal crystalline matrix.182,183 Moreover, the reduced water content in NLC particle suspensions minimizes drug release during storage compared to SLNs. The irregular structure of NLCs facilitates the creation of distinct compartments in the lipid matrix, enhancing drug loading capacity and preventing premature release during storage.178 Due to their high skin adhesion, controlled occlusion properties, which enhance skin hydration and bioavailability, and stable drug encapsulation capability, NLCs find extensive use in topical drug delivery.183
Recent investigations have leveraged luteolin (Lut) as an anti-psoriasis drug in the formulation of Lut-NLC. The prepared nanoparticles exhibited sustained drug release up to 24h and enhanced the skin deposition of Lut by 3.4-fold higher in stratum corneum, epidermis and dermis compared to Lut suspension with minimum transdermal delivery. This administration mode heightened the anti-psoriasis efficacy of Lut compared to the free drug.91 A newly developed nanogel based on TAC and thymoquinone-co-loaded NLCs demonstrated sustained drug release over 24 hours, showcasing superior penetration depth in suspension gel with dose-dependent toxicity.92 The optimized NLC loaded with riluzole exhibited optimal properties for dermal application, inhibiting keratinocyte proliferation and enabling sustained drug delivery.93 Madan, J.R.et al formulated NLCs by a cold homogenization technique using Compritol 888ATO, oleic acid, Tween 80 and Span 20, and Transcutol P as a solid lipid, liquid lipid, surfactant mixture, and penetration enhancer, respectively. Containing Apremilast (APM) for topical application. ex vivo skin permeation results showed low drug diffusion, sustained drug release, and 60.1% skin deposition.94 In a parallel effort, Rapalli, V.K.et al developed APM-loaded NLCs characterized by their lack of cytotoxicity and skin irritation. Notably, these formulations achieved a threefold increase in ear skin retention in vitro compared to conventional gel formulations. In a psoriasis mouse model, the APM-loaded NLC dispersions were more effective than the free drug, particularly in reducing TNF-α levels.95 Agrawal, Y.O.et al developed NLCs optimized using 32 full factorial designs loaded with MTX by a solvent diffusion technique. Significantly higher deposition of MTX was found in HCS from MTX NLC gel (71.52 ± 1.13%) as compared to MTX plain gel (38.48 ± 0.96%). In a mouse psoriasis model, the application of MTX NLC gel led to a noteworthy reduction in the PASI score and decreased expression of inflammatory cytokines. It mitigated local side effects.96 Pradhan, M. et al developed NLCs loaded with fluocinolone acetonide (FA) and successfully integrated them into carbopol 934 gel bases containing salicylic acid (SA), resulting in an FA-loaded NLC + plain SA gel formulation (FSG). These FA-loaded NLCs demonstrated selective localization in the epidermis and deep dermis. In a psoriasis mouse model, the FSG treatment was more effective than the PFSG (plain FA and SA gel) group.97 Pradhan, M., D. Singh, and M.R. Singh developed optimized NLCs loaded with TA, which enhanced drug solubility. In vitro studies demonstrated selective drug deposition in the epidermis and reduced adverse side effects associated with systemic exposure compared to TA suspension.98 Viegas, J.S.R.et al designed NLCs to co-deliver TAC and TNF-α siRNA. Experimental results demonstrated slow TAC release over 24 hours, efficient siRNA translocation across the stratum corneum (SC), and enhanced TAC retention in the skin. In a psoriasis mouse model, NLCs loaded with TAC and TNF-α siRNA significantly reduced TNF-α expression in skin tissue compared to controls.99 Rapalli, V.K. et al formulated NLCs loaded with CRC exhibiting extended in vitro release for up to 48 hours. The formulation demonstrated a 3.24-fold improvement in penetration and skin retention compared to free CRC gel.100 Sathe, P. et al prepared NLC, particle size below 300 nm, polydispersity index (PDI) below 0.3 and percentage entrapment efficiency of ∼100% gels loaded with dithranol, showcasing extended drug release and protection from oxidation. The NLC gel loaded with dithranol led to a more pronounced reduction in IL-17, IL-22, and IL-23 levels in the skin tissues of IMQ-induced psoriasis mice compared to commercially available creams.101
Liquid Crystalline Nanoparticles
Liquid Crystalline Nanoparticles (LCNs) represent bulk liquid crystalline arrays between liquid and solid crystalline states, deconstructed into NPs characterized by anisotropic structures composed of amphiphilic lipids.101 LCNs, derived from MO, exhibit the capacity to modulate and disrupt lipid phases within the SC barrier. Their bioadhesive properties, resembling biofilm-like structures, and the pro-osmotic effects of related substances facilitate drug uptake.184
In the most recent investigation, Apremilast-loaded lyotropic LCNs were developed by researchers, incorporating non-toxic excipients in the formulation. The in-vitro drug release showed the prolonged-release for 18h. The ex-vivo studies revealed that LCNs formulation exhibited drug retention up to 3.2 and 11.9-fold higher, in stratum corneum and viable epidermis compared to conventional gel preparation. The dermatokinetic study revealed the AUC0-24 of the LCNs loaded gel was 8.4 fold higher in epidermis and 2.06 fold in dermis, respectively compared to plain gel.102 Furthermore, Silvestrini et al developed triptolide-loaded LCNs coupled with small interfering RNAs (siRNAs) targeting TNF-α and IL-6. In vitro permeation studies revealed an increase of more than 20-fold in the distribution of triptolide (TP) through the porcine epidermis/dermis was achieved after the application of LCN-TP or LCN TP in hydrogel. In cell culture, the formulation showcased good compatibility and rapid internalization.103 Rapamycin (RAPA), an immunosuppressant that inhibits the mammalian target of RAPA complex 1 (mTORC1), is proposed for treating psoriasis. RAPA is encapsulated in phytantriol-based liquid crystalline nanoparticles (NPs) stabilized with pluronic F127. This formulation exhibits over 95% encapsulation efficiency, provides sustained drug release for 14 days in vitro, and demonstrates antiproliferative activity against natural killer cells.104 TAC-loaded LCNs, prepared by Thapa and Yoo, resulted in a six-fold increase in drug penetration into mouse skin after 24 hours of application compared to TAC dissolved in propylene glycol. This application significantly reduced PASI scores and the number of inflammatory cells in skin tissues.105 Berberine (BBR) is recognized as a highly promising natural plant-derived drug for future psoriasis treatment due to its diverse biological effects, including antiproliferative, anti-inflammatory, and antioxidant properties.173 However, BBR needs help with poor solubility in aqueous solutions and low skin permeability. Freag and Torky’s group addressed this issue by preparing monoolein-based BBR-oleate-loaded (BBR-OL) LCNs. The optimized BBR-OL-LCNs functioned as a liquid crystalline nanosuppository, Berberine (Brb)-OL-LCNPs showed a threefold increase in the drug accumulated within rat skin and around tenfold increase in the drug permeation compared with crude Brb. In vivo studies revealed that topical application of Brb-OL-LCNPs hydrogel significantly alleviated psoriasis symptoms and reduced the levels of psoriatic inflammatory cytokines.106
Polymer-Based Carriers
Polymer-based carriers typically comprise eco-friendly polymers within the 10–1000 nm size range. Therapeutic components can either be internally encapsulated or surface-bound to the nanocarrier.185 These carriers are effective delivery vehicles due to their tunable size, high stability, surface ligand modification, controlled drug release, ease of preparation, and targeting capabilities. Drug release can be modulated through adjustments in the drug-to-polymer ratio and the polymer’s composition and molecular weight.184
Dendrimers
Dendrimers, intricate drug delivery systems, comprise highly branched molecules with a precisely controlled, spherical, reactive three-dimensional structure and many controllable peripheral functions. Drug molecules are either physically enveloped or covalently linked to functional groups, giving rise to drug-dendrimer couplings.186 Various dendrimer types encompass glycodendrimers, peptide dendrimers, and lysine core dendrimers.174 Different types of dendrimers have the potential to modulate drug solubility, penetration, and retention, offering diverse avenues for achieving effective topical psoriasis therapy.
Jebbawi, R. et al discovered that a poly(phosphorhydrazone) dendrimer, which features anionic aza bisphosphonate groups and is referred to as an ABP dendrimer, demonstrates anti-psoriatic therapeutic potential in a mouse model of psoriasis. RNA interference operates at the genomic level, with siRNA acting post-transcriptionally to down-regulate protein production, a crucial mechanism for managing and mitigating diverse diseases. Furthermore, Pandi P. et al investigated the impact of a poly(amidoamine) dendrimer (PAMAM) on human immune cells in vitro. They demonstrated control over clinical and histopathological scores and macrophage infiltration in the skin of treated mice within corresponding mouse models.175 PAMAM dendrimers and liposomes were utilized for the targeted delivery of anti-TNF-α siRNA in a mouse model of psoriasis. The dendriplex particles displayed a size of 99.80 ± 1.80 nm, a zeta potential of 13.40 ± 4.84 mV, and an entrapment efficiency of 98.72 ± 2.02%. Both treatment groups demonstrated significant improvements in clinical phenotype and histopathology, accompanied by reduced levels of IL-6, TNF-α, IL-17, and IL-22 in the skin lesions.107 Tripathi, P.K. et al investigated the potential of PAMAM dendrimers loaded with dithranol (DIT) for topical application in a microsponge-based gel. The formulation yielded a 66.28% return, with encapsulation efficiencies ranging from 71.33% to 49.21% and particle sizes varying from 28 ± 1.12 μm to 130 ± 1.01 μm. The optimized formulation demonstrated enhanced stability and non-irritation to the skin of experimental animals, increased DIT solubility, improved skin permeability, and reduced the rate of anthralin autoxidation.108 Agrawal U. et al explored the application of polypropylene imine (PPI) dendrimers for the topical administration of DIT. DIT-PPI demonstrated a significantly enhanced permeation rate constant of 11.61 ± 1.80 μg/cm²/h and reduced skin irritation, rated at 1.0, compared to the plain DIT solution, which showed a rate of 2.72 ± 0.31 μg/cm²/h and an irritation score of 2.3. Additionally, DIT-PPI was found to effectively penetrate the sebaceous gland area of hair follicles.109 Dhanikula, R.S., investigated MTX-loaded polyester-co-polyether dendrimers, noting their enhanced biocompatibility and significant encapsulation capacity, with MTX loading as high as 24.5% w/w. Increases in polyethylene glycol branches and expansions of hydrophobic regions within the dendrimers moderated the rapid drug release, suggesting the formulation’s potential as a targeted sustained-release carrier.110
Micelle
A micelle is a self-assembled nanocarrier comprised of amphiphilic compounds, exhibiting a size ranging from 5 to 100 nm.187 Comprising a hydrophobic core and a hydrophilic shell, the micelle carries lipophilic and hydrophilic drugs in its shell. The modes of drug release vary depending on the loading method and drug location.111 Micelles offer advantages such as high bioavailability, substantial drug loading capacity, a low drug degradation rate, minimal side effects, and enhanced skin permeability.184
Mycophenolic acid (MPA), CsA, and MTX are conventional immunosuppressive drugs employed in psoriasis. However, their limited water solubility significantly hampers their topical application. Supasena et al synthesized poloxamer 407 (P407)-MPA micelles by coupling Kolliphor® 407 with MPA, resulting in a conjugate that displayed enhanced micellization properties, achieving over a 12-fold reduction in critical micelle concentration compared to P407 alone. These micelles exhibited a sustained release profile of MPA and demonstrated antiproliferative effects on TNF-α-induced HaCaT cells.111 Guo, D.et al designed a hydrophilic nanocarrier for MTX using micelles. This formulation ensured efficient loading of MTX when dispersed in an aqueous solution.113 Lapteva, M. et al developed TAC polymer micelles using MPEG-dihex-PLA, achieving an optimal 0.1% micelle formulation that remained stable at 4°C for seven months. This formulation enhanced TAC deposition in the skin, delivering 1.50 ± 0.59 μg/cm² compared to 0.47 ± 0.20 μg/cm² for Protopic (0.1% w/w TAC ointment).114 Silibinin (SL), the principal component of milk thistle (Silybum marianum), exhibits antioxidant and antitumor activities, along with inhibitory effects on STAT3 and cell proliferation.188 Chavoshy, F. et al synthesized SL-loaded polymeric micelles. The optimized batch exhibited a mean particle size of 18.3 ± 2.1 nm and an entrapment efficiency of 75.8 ± 5.8%. These SL-loaded micelles reduced the Psoriasis Area Index by over 78% after 14 days. This suggests topical STAT-3 inhibitors may represent a novel strategy in psoriasis treatment.115
Hydrogel
Hydrogel, a self-assembled supramolecular aggregate, excels at water retention. Its mesh size, degradation rate, and drug interactions make it a potent drug delivery vehicle.189 Hydrogel offers an alternative to traditional creams and ointments in treating psoriasis. Its high water content and non-greasy texture help maintain skin moisture, minimize transdermal water loss, enhance drug penetration and sustained release, and provide a cooling effect via surface evaporation.
In a recent study, Lin Li et al developed hydrogels loaded with indirubin NCs mixed with HA, improving indirubin delivery and skin accumulation and enhancing efficacy against psoriatic inflammation.116 Su, H. et al formulated a kaempferol hydrogel with controlled-release properties that scavenged over 90% of free radicals at specific concentrations, also inhibiting HaCaT cell proliferation without significant cytotoxicity; in mouse models, it markedly reduced psoriasis symptoms and pro-inflammatory cytokines TNF-α, IL-6, and IL-17A.120 Sunita Thakur et al created a tazarotene-CT-loaded nanofiber and carbopol-based hydrogel film that biodegraded within two weeks, achieving nearly 96% drug release in 72 hours with anti-psoriatic solid effects in a mouse model.121 Rana, K. et al used a cholic acid-dipeptide conjugate for self-assembly in a betamethasone-loaded hydrogel, enhancing the steroid’s solubility and release properties.125 Kumar, S., M. Prasad, and R. Rao developed a cyclodextrin nanosponge-based hydrogel loaded with clobetasol propionate, which decreased oxidative stress markers and improved anti-psoriatic effects in a mouse tail model.126 Gabriel, D. et al employed mPEGhexPLA-based nanocarriers in a carbopol-based hydrogel for enhanced dermal delivery of TAC in a psoriasis mouse model, showing superior efficacy to the commercial cream Protopic.127 Finally, Qiu, F. et al demonstrated that their Cel Niosome hydrogel significantly inhibited pro-inflammatory markers and improved histological outcomes compared to topical TAC in skin models.130
There is a notable focus on hydrogel studies involving MTX. Shu, Y. et al developed a thermo-responsive hydrogel with an ionic liquid ME for MTX delivery, which exhibits inherent antimicrobial properties and enhances MTX solubility for temperature-controlled release.119 Bernardes, M.T.C.P.et al prepared alkylated carbomer-based hydrogels loaded with MTX, which reduced inflammation, modulated epidermal thickness, and decreased COX-2, myeloperoxidase activity, and TNF-α levels in a psoriasis mouse model.122 Asad, M.I. et al optimized chitosan hydrogels loaded with MTX-NPs, retaining most of the drug in the epidermis within 24 hours and demonstrating 73% sustained drug release over 48 hours. This formulation outperformed commercially available TAC cream and free MTX hydrogel in improving psoriasis symptoms, significantly reducing TNF-α and IL-6 levels in the skin.123 Xu, J. et al introduced a multifunctional composite hydrogel modified with nanomicelles and ZnO/Ag NPs, which suppressed pro-inflammatory pathways in macrophages and keratinocytes while optimizing MTX release and enhancing transdermal delivery. This hydrogel demonstrated superior immunomodulatory effects and reduced cytokine expression in a psoriasis mouse model.124
Research on curcumin hydrogels is advancing across multiple teams. Fernández-Romero, A. et al created an Epichlorohydrin-β-Cyclodextrin/CRC Binary System embedded in a Pluronic®/Hyaluronate Hydrogel, which increased CRC solubility and permeation, showed anti-inflammatory solid effects in HaCaT cells, and notably decreased IL-6 expression.128 Similarly, Filippone, A. et al engineered a choline-calix[4]arene-based nanohydrogel loaded with CRC that normalized the distribution of tight junction proteins ZO1 and occludin and effectively reduced TNF-α and inducible nitric oxide synthase levels.129
Pérez-García L et al developed supramolecular hydrogels employing low-molecular-weight gelators to explore a range of psoriasis therapeutics, including gemcitabine hydrochloride, MTX sodium salt, TAC, betamethasone 17-valerate, and triamcinolone acetonide. These gelators, typically amphiphilic compounds, self-assemble via diverse supramolecular interactions such as electrostatic, hydrophobic, and π-π interactions. Supramolecular hydrogels combine the properties of traditional hydrogels with those of nanoparticulate systems containing amphiphiles, often incorporating ethanol to enhance permeation. Consequently, these hydrogels can increase drug penetration and retention in the skin by up to 15 times compared to conventional formulations at equivalent drug concentrations.117,118
Hybrid Nanoparticles
Lipid-polymer hybrid nanoparticles present clear advantages over lipid- or polymer-based systems, encompassing enhanced drug loading, exceptional colloidal stability, sustained release profiles, and heightened cellular uptake.190,191
In a recent study, investigators crafted a self-assembled gallic acid-loaded lecithin-chitosan hybrid nanostructured gel for psoriasis treatment. This formulation exhibits superior drug release and deeper penetration levels compared to standard gel systems. Moreover, it mitigates irritation, reduces PASI scores, and addresses splenomegaly.132 Pukale, S.S., A. Mittal, and D. Chitkara formulated monolithic lipid-polymer hybrid nanoparticles (VD3/LPHNPs) loaded with vitamin D3 and developed them into a topical gel containing VD3/LPHNPs. This formulation exhibited marked improvement in histopathology and PASI scores in a psoriasis mouse model, surpassing the antipsoriasis activity of free VD3 gel.133 Pukale, S.S.et al additionally formulated monolithic lipid-polymer hybrid nanoparticles loaded with clobetasol (CP/LPNs) and incorporated carbopol 974P to create hydrogels. This formulation exhibited sustained release and stability, with CP/LPNs demonstrating substantial growth inhibition of HaCaT cells. Compared to commercial creams, the CP/LPNs gel significantly improved PASI scores, reducing skin damage and hyperplasia in rat models of psoriasis.134 Suzuki et al developed a siRNA delivery system utilizing hybrid polymer-lipid nanoparticles (PLNs). This system efficiently encapsulates TPPS2a and complexed siRNA to enhance the endosomal escape of TNFα siRNA into the cytoplasm. In murine models, the combination of PLN-TPPS2a and complexed siRNA demonstrated enhanced endosomal escape of TNFα siRNA into the cytoplasm.135 Fereig, S.A.et al developed lecithin-chitosan hybrid NPs loaded with TAC. The study showed that particles containing Tween 80 released 79.98% of the drug within 48 hours, faster than those containing olive oil. These hybrid NPs significantly improved PASI scores and skin histopathology in mouse models. Moreover, the skin deposition of these NPs exceeded that of commercially available products.136
Metallic Nanocarriers
Metallic nanocarriers, derived from elements like gold, silver, platinum, zirconium, copper, palladium, iron, selenium, and strontium, can assume various arrangements such as nanopores, nanotubes, nanorods, nanoclusters, and nanostars.192 Nanopores, synthesized through chemical or organic materials in a process known as green synthesis or biosynthesis, offer a cost-effective and environmentally safe approach.179 These nanocarriers boast advantages such as small size, a relatively large surface area, facile modification of surface functional groups, and inherent anti-inflammatory effects.185 Gold nanoparticles (AuNPs) within the 1–100 nm size range offer versatile advantages as drug carriers, presenting heightened stability, enhanced transmembrane permeability, and intracellular targeting.
Studies have revealed that localized application of AuNPs enables penetration through the SC and further into the epidermis and dermis, augmenting their efficacy in transdermal drug delivery.193 Han et al innovatively designed NPs with a gold core and a 1000 Da polyethylene glycol shell, modified with octadecyl chains. These nanoparticles efficiently penetrated the stratum corneum and selectively targeted keratinocytes when applied to imiquimod-induced psoriasis in mice without an excipient. In a psoriasis mouse model, this formulation demonstrated antipsoriatic activity comparable to that of steroids and vitamin D analogs when used independently.194 Nirmal GR et al developed a nanoformulation that induces hyperthermia-mediated apoptosis for treating psoriasis, activated by near-infrared (NIR) irradiation. This formulation incorporates gold nanorods (GNRs) and isatin—an anti-inflammatory agent—to enhance antipsoriatic efficacy within a poly(lactic-co-glycolic acid) (PLGA) matrix, creating effective nanocomplexes. Upon NIR exposure, these nanocomplexes penetrate the keratinocyte cytoplasm, reducing keratinocyte viability by approximately 60%, offering a novel therapeutic strategy against psoriasis.137 Fratoddi et al synthesized AuNPs functionalized with 3-mercapto-1-propanesulfonate (AuNPs-3MPS) and loaded them with MTX. Topical application of AuNPs-3MPS@MTX in a mouse model of psoriasis led to reduced epidermal thickness, decreased PASI scores, and alterations in immunohistochemistry, specifically lower Ki67, K6, CD3, and CD8 staining.138 HSP70, critical in maintaining homeostasis and exhibiting cytokine-like and immunomodulatory properties, is a vital target in psoriasis pathogenesis. Raghuwanshi et al developed AuNPs loaded with an ethanolic extract of Woodfordia fruticosa flowers, encapsulated in a Carbopol® 934 gel to target HSP70-1. In a rat model of psoriasis, this formulation significantly reduced serum cytokine levels, attenuated epidermal thickness, keratotic dyskeratosis, and keratinocyte hyperproliferation.139
Nanocrystals
NCs are submicron particles, ranging from 100 to 1000 nm, consisting of 100% drugs in a crystalline state, typically encased by a stabilizing layer.195 Unlike conventional carriers, NCs lack additional materials, maximizing their drug loading capacity to 100%. Notably, they exhibit prolonged stability with neutral pH and can be produced through solvent-free and easily scalable techniques.196 NCs significantly improve the concentration gradient between the formulation and the skin. This, in turn, leads to heightened drug skin penetration and increased bioavailability by enhancing poorly soluble drugs’ dissolution rate and saturated solubility.197
Recently, Shahine et al developed a diosmin-loaded NC gel, a formulation that enhances the drug’s bioavailability. This gel was demonstrated to preserve the balance between T helper 17 and T regulatory cells. Furthermore, it modulates TLR7/8/NF-κB, miRNA-31, AKT/mTOR/P70S6K, and upregulates TNFAIP3/A20 expression in psoriatic skin tissues, presenting a promising approach for psoriasis treatment.140 The saturation solubility of NCs increased twofold compared to micronized apremilast. Skin penetration studies demonstrated superior penetration for nanoformulations compared to standard formulations. Consequently, apremilast NC-based formulations facilitate selective delivery into viable layers by overcoming the SC barrier.141 The researchers utilized NC and dissolving MN technologies to prepare MTX NC. This formulation enables sustained in vitro drug release for over 72 hours, reducing systemic drug exposure. The skin deposition is higher than oral administration, while blood concentration is only 40% of that observed with the oral route. This delivery approach proves effective as a topical method for treating psoriasis.142 Döge et al prepared NCs loaded with dexamethasone, facilitating rapid drug release and significantly accelerating its penetration. Dex became detectable in eluates after just 6 hours when NCs were applied to intact skin.198
Limitations
In our bibliometric analysis, we primarily used the Web of Science database. This approach may have omitted some data, prompting manual searches for articles by critical authors to enrich our dataset. Future research will consider employing multiple databases for comparative analyses and engaging industry experts to enhance data accuracy and completeness. Although our annual publication and cumulative citation models appeared robust visually, the Durbin-Watson statistic indicated residual autocorrelation in annual citation frequency models, suggesting incomplete capture of data patterns. Future studies should consider more complex time series models or include additional explanatory variables such as research field activity, financial support, and team size. While the h-index is a prevalent metric for assessing academic impact, it may favor researchers with longer careers and more opportunities to accumulate citations. Moreover, it needs to reflect citation quality.199 To address this, our paper pairs the h-index with total citation counts for a more comprehensive evaluation.
Conclusion
Currently, topical therapy is the first choice for treating and maintaining mild to moderate psoriasis, and we summarized the timeline of the development of topical therapy. However, problems such as skin permeability and drug targeting affect the therapeutic effect. Novel drug delivery systems were born for this purpose and became a hot research topic. We pioneered the use of bibliometrics to track the research hotspots in this field, and according to the frequency of keywords, solid lipid nanoparticles and nanostructured lipid carriers are the most intensively and extensively researched drug delivery modes in lipid-based carriers. The former has a small particle size and large surface area, and its solid lipid layer can enhance the stability of the loaded drug. At the same time, the nanoscale dimensions can help to change its biodistribution, which is conducive to carrying the drug through the skin barrier and realizing precise delivery.200 As the second generation of lipid nanocarriers to make up for the disadvantages of the former, such as easy aggregation and low loading of certain drugs, NLC has an irregular structure. Its biocompatibility can reduce its toxicity, and the property of no recrystallization after cooling can effectively prolong the drug’s release time, thus increasing its efficacy.201 The two drugs MTX and CsA are also hot research topics in various carriers. While both drugs are usually taken orally for systemic therapy, more and more scholars have turned their attention to the development of topical delivery of both drugs, aiming to achieve therapeutic efficacy while reducing the toxic side effects of the drugs. The topical delivery of MTX needs to overcome the unique physicochemical properties, as well as hydrophilicity, which affects the residence time of the drug in the skin.142 In animal experiments, MTX in TFs, emulsion drug delivery systems, SLNs, NLCs, dendrimers, micelles, hydrogels, metallic nanocarriers, NCs, and other carriers assisted by localized drug delivery has been achieved with good results, among which hydrogels, is one of the more frequently used modalities for MTX. The high molecular weight and lipophilicity of CyA limit the application of topical drug delivery, so our development of its nanocarriers mainly focuses on these two points. SLNs for CyA can be used as the primary vehicle for local drug delivery, which can improve the drug’s stability and reduce the drug’s systemic absorption through the skin while increasing the dermal delivery of the drug.
In response to the discussion of the current research progress in this field, we can see that lipid-based nanodelivery systems have more substantial skin penetration and drug-carrying capacity, which can achieve targeted drug release and improve local drug action while reducing the side effects of drug therapy. However, lipid nanodelivery systems are less stable and water-soluble, limiting their application. Compared with lipid-based nanodelivery systems, polymer-based carriers, metallic nanocarriers, and nanocrystals have broader application prospects due to their controllable particle size, easy modification, and better drug loading ability. However, compared with the above nanodelivery systems, NCs, due to their strong stability and biocompatibility, can effectively change the permeability of the skin at the treatment site as well as the local drug accumulation capacity, thereby improving the local therapeutic effect of psoriasis.
In the future, for the development of new drug delivery systems in the field of psoriasis treatment, on the one hand, we can choose more suitable drug carriers for the physicochemical properties of commonly used topical therapeutic drugs. This includes using carriers to improve skin permeability, ensure continuous drug release in skin lesions, prolong drug retention time in the skin, protect the drug’s structure within the carrier, stabilize the drug’s nature, and achieve targeted transport of the drug for effective local treatment. The bioavailability of the drug is improved by using the carrier to realize the targeted transportation of the drug. On the other hand, the choice of new drugs, from the original oral to external application of the drug delivery method of innovation, as well as new ingredients or even new drugs and old drugs, co-carrying the innovation of the drug composition. All will help the development of localized psoriasis treatment, thus reducing the chance of psoriasis recurrence, reducing the adverse effects of treatment, and improving the quality of patients’ prognosis and survival.
Acknowledgments
Figures 2 and 9 were created with BioRender.com.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffiths CEM, Armstrong AW, Gudjonsson JE, Barker J. Psoriasis. Lancet. 2021;397(10281):1301–1315. doi:10.1016/s0140-6736(20)32549-6
2. Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: a Review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
3. Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84(2):432–470. doi:10.1016/j.jaad.2020.07.087
4. Schön MP, Boehncke WH. Psoriasis. N Engl J Med. 2005;352(18):1899–1912. doi:10.1056/NEJMra041320
5. Thami G, Sarkar R. Coal tar: past, present and future. Clin Exp Dermatol. 2002;27(2):99–103. doi:10.1046/j.1365-2230.2002.00995.x
6. Baden HP. THE TREATMENT OF PSORIASIS. N Engl J Med. 1963;269:907–909. doi:10.1056/nejm196310242691707
7. Sulzberger MB, Obadia J. A modified liquid petrolatum preparation; its use in the management of certain common dermatoses of the scalp. AMA Arch Derm. 1956;73(4):373–375. doi:10.1001/archderm.1956.01550040067009
8. Hermann RC, Taylor RS, Ellis CN, et al. Topical ciclosporin for psoriasis: in vitro skin penetration and clinical study. Skin Pharmacol. 1988;1(4):246–249. doi:10.1159/000210782
9. Berth-Jones J, Hutchinson PE. Vitamin D analogues and psoriasis. Br J Dermatol. 1992;127(2):71–78. doi:10.1111/j.1365-2133.1992.tb08035.x
10. Chandraratna RAS. Tazarotene: the first receptor-selective topical retinoid for the treatment of psoriasis. J Am Acad Dermatol. 1997;37(2):S12–7. doi:10.1016/S0190-9622(97)80395-7
11. Remitz R, Reitamo R, Erkko E, Granlund G, Lauerma L. Tacrolimus ointment improves psoriasis in a microplaque assay. Br J Dermatol. 1999;141(1):103–107. doi:10.1046/j.1365-2133.1999.02927.x
12. Yuanyuan Z, Xue M, Xinying W, et al. Study on transdermal absorption of mouse monoclonal antibody against human interleukin-8 cream for the treatment of psoriasis. Chin J Hospital Pharm. 2023;43(20):2264–2269. doi:10.13286/j.1001-5213.2023.20.05
13. Ports WC, Khan S, Lan S, et al. A randomized phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169(1):137–145. doi:10.1111/bjd.12266
14. Smith SH, Jayawickreme C, Rickard DJ, et al. Tapinarof Is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137(10):2110–2119. doi:10.1016/j.jid.2017.05.004
15. Larkin HD. Nonsteroidal Topical Treatment for Plaque Psoriasis Is Approved. JAMA. 2022;328(1):11. doi:10.1001/jama.2022.10352
16. Kircik LH, Alonso-Llamazares J, Bhatia N, et al. Once-daily roflumilast foam 0.3% for scalp and body psoriasis: a randomized, double-blind, vehicle-controlled phase IIb study. Br J Dermatol. 2023;189(4):392–399. doi:10.1093/bjd/ljad182
17. Landis MN, Smith SR, Berstein G, et al. Efficacy and safety of topical brepocitinib cream for mild-to-moderate chronic plaque psoriasis: a phase IIb randomized double-blind vehicle-controlled parallel-group study. Br J Dermatol. 2023;189(1):33–41. doi:10.1093/bjd/ljad098
18. Hong JB, Wu PY, Qin A, et al. Topical Tirbanibulin, a Dual Src Kinase and Tubulin Polymerization Inhibitor, for the Treatment of Plaque-Type Psoriasis: phase I Results. Pharmaceutics. 2022;14(10). doi:10.3390/pharmaceutics14102159
19. Goyal R, Macri LK, Kaplan HM, Kohn J. Nanoparticles and nanofibers for topical drug delivery. J Control Release. 2016;240:77–92. doi:10.1016/j.jconrel.2015.10.049
20. Shen Q, Liu R, Tan S, Xu X, Fang J, Li R. Advances in pathogenesis and nanoparticles (NPs)-mediated treatment of psoriasis. Front Immunol. 2022;13:1089262. doi:10.3389/fimmu.2022.1089262
21. Sun L, Liu H, Ye Y, et al. Smart nanoparticles for cancer therapy. Signal Transduct Target Ther. 2023;8(1):418. doi:10.1038/s41392-023-01642-x
22. Kokol P, Blažun Vošner H, Završnik J. Application of bibliometrics in medicine: a historical bibliometrics analysis. Health Info Libr J. 2021;38(2):125–138. doi:10.1111/hir.12295
23. Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2022;11(3):173–176. doi:10.1007/s40037-021-00695-4
24. Thompson DF, Walker CK. A descriptive and historical review of bibliometrics with applications to medical sciences. Pharmacotherapy. 2015;35(6):551–559. doi:10.1002/phar.1586
25. Synnestvedt MB, Chen C, Holmes JH. CiteSpace II: visualization and knowledge discovery in bibliographic databases. AMIA Annu Symp Proc. 2005;2005:724–728.
26. Shi S, Lv J, Chai R, et al. Opportunities and challenges in cardio-oncology: a bibliometric analysis from 2010 to 2022. Curr Probl Cardiol. 2023;48(8):101227. doi:10.1016/j.cpcardiol.2022.101227
27. Bornmann L, Daniel H-D. What do we know about the h index? J Am Soc Inf Sci Technol. 2007;58(9):1381–1385. doi:doi:10.1002/asi.20609
28. Hirsch JE. Does the H index have predictive power? Proc Natl Acad Sci U S A. 2007;104(49):19193–19198. doi:10.1073/pnas.0707962104
29. Cheng P, Tang H, Dong Y, Liu K, Jiang P, Liu Y. Knowledge mapping of research on land use change and food security: a visual analysis using citespace and VOSviewer. Int J Environ Res Public Health. 2021;18(24). doi:10.3390/ijerph182413065
30. Zhang X, Zhou Y, Wei N, et al. A bibliometric analysis of heart failure with preserved ejection fraction from 2000 to 2021. Curr Probl Cardiol. 2022;47(9):101243. doi:10.1016/j.cpcardiol.2022.101243
31. Fang JY, Lee WR, Shen SC, Fang YP, Hu CH. Enhancement of topical 5-aminolaevulinic acid delivery by erbium:YAG laser and microdermabrasion: a comparison with iontophoresis and electroporation. Br J Dermatol. 2004;151(1):132–140. doi:10.1111/j.1365-2133.2004.06051.x
32. Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81. doi:10.1038/s41419-022-04523-3
33. Chen R, Zhai YY, Sun L, et al. Alantolactone-loaded chitosan/hyaluronic acid nanoparticles suppress psoriasis by deactivating STAT3 pathway and restricting immune cell recruitment. Asian J Pharm Sci. 2022;17(2):268–283. doi:10.1016/j.ajps.2022.02.003
34. Shou X, Wang Y, Duan C, et al. Knowledge Domain and Emerging Trends of Glucagon-Like Peptide 1 Receptor Agonists in Cardiovascular Research: a Bibliometric Analysis. Curr Probl Cardiol. 2023;48(8):101194. doi:10.1016/j.cpcardiol.2022.101194
35. Rapalli VK, Waghule T, Gorantla S, Dubey SK, Saha RN, Singhvi G. Psoriasis: pathological mechanisms, current pharmacological therapies, and emerging drug delivery systems. Drug Discov Today. 2020;25(12):2212–2226. doi:10.1016/j.drudis.2020.09.023
36. Santos AC, Rodrigues D, Sequeira JAD, et al. Nanotechnological breakthroughs in the development of topical phytocompounds-based formulations. Int J Pharm. 2019;572:118787. doi:10.1016/j.ijpharm.2019.118787
37. Paiva-Santos AC, Silva AL, Guerra C, et al. Ethosomes as nanocarriers for the development of skin delivery formulations. Pharm Res. 2021;38(6):947–970. doi:10.1007/s11095-021-03053-5
38. Xie J, Huang S, Huang H, et al. Advances in the application of natural products and the novel drug delivery systems for psoriasis. Front Pharmacol. 2021;12:644952. doi:10.3389/fphar.2021.644952
39. Wang W, Xu X, Song Y, et al. Nano transdermal system combining mitochondria-targeting cerium oxide nanoparticles with all-trans retinoic acid for psoriasis. Asian J Pharm Sci. 2023;18(5):100846. doi:10.1016/j.ajps.2023.100846
40. Zhang Y, Gong S, Liu L, et al. Cyclodextrin-coordinated liposome-in-gel for transcutaneous quercetin delivery for psoriasis treatment. ACS Appl Mater Interfaces. 2023;15(34):40228–40240. doi:10.1021/acsami.3c07582
41. Walunj M, Doppalapudi S, Bulbake U, Khan W. Preparation, characterization, and in vivo evaluation of cyclosporine cationic liposomes for the treatment of psoriasis. J Liposome Res. 2020;30(1):68–79. doi:10.1080/08982104.2019.1593449
42. Xi L, Lin Z, Qiu F, et al. Enhanced uptake and anti-maturation effect of celastrol-loaded mannosylated liposomes on dendritic cells for psoriasis treatment. Acta Pharm Sin B. 2022;12(1):339–352. doi:10.1016/j.apsb.2021.07.019
43. Yu F, Zhang Y, Yang C, Li F, Qiu B, Ding W. Enhanced transdermal efficiency of curcumin-loaded peptide-modified liposomes for highly effective antipsoriatic therapy. J Mater Chem B. 2021;9(24):4846–4856. doi:10.1039/d1tb00557j
44. Saka R, Jain H, Kommineni N, Naveen C, Khan W. Enhanced penetration and improved therapeutic efficacy of bexarotene via topical liposomal gel in imiquimod induced psoriatic plaque model in BALB/c mice. J Drug Delivery Sci Technol. 2020;58:101691. doi:10.1016/j.jddst.2020.101691
45. Wadhwa S, Singh B, Sharma G, Raza K, Katare OP. Liposomal fusidic acid as a potential delivery system: a new paradigm in the treatment of chronic plaque psoriasis. Drug Deliv. 2016;23(4):1204–1213. doi:10.3109/10717544.2015.1110845
46. Doppalapudi S, Jain A, Chopra DK, Khan W. Psoralen loaded liposomal nanocarriers for improved skin penetration and efficacy of topical PUVA in psoriasis. Eur J Pharm Sci. 2017;96:515–529. doi:10.1016/j.ejps.2016.10.025
47. Knudsen N, Rønholt S, Salte RD, et al. Calcipotriol delivery into the skin with PEGylated liposomes. Eur J Pharm Biopharm. 2012;81(3):532–539. doi:10.1016/j.ejpb.2012.04.005
48. Chen J, Ma Y, Tao Y, et al. Formulation and evaluation of a topical liposomal gel containing a combination of zedoary turmeric oil and tretinoin for psoriasis activity. J Liposome Res. 2021;31(2):130–144. doi:10.1080/08982104.2020.1748646
49. Jain H, Geetanjali D, Dalvi H, Bhat A, Godugu C, Srivastava S. Liposome mediated topical delivery of Ibrutinib and Curcumin as a synergistic approach to combat imiquimod induced psoriasis. J Drug Delivery Sci Technol. 2022;68:103103. doi:10.1016/j.jddst.2022.103103
50. Carreras JJ, Tapia-Ramirez WE, Sala A, Guillot AJ, Garrigues TM, Melero A. Ultraflexible lipid vesicles allow topical absorption of cyclosporin A. Drug Deliv Transl Res. 2020;10(2):486–497. doi:10.1007/s13346-019-00693-4
51. Wang W, Shu GF, Lu KJ, et al. Flexible liposomal gel dual-loaded with all-trans retinoic acid and betamethasone for enhanced therapeutic efficiency of psoriasis. J Nanobiotechnology. 2020;18(1):80. doi:10.1186/s12951-020-00635-0
52. Bahramizadeh M, Bahramizadeh M, Kiafar B, et al. Development, characterization and evaluation of topical methotrexate-entrapped deformable liposome on imiquimod-induced psoriasis in a mouse model. Int J Pharm. 2019;569:118623. doi:10.1016/j.ijpharm.2019.118623
53. Dadwal N, Amisha, Singh D, Singh A. Quality-by-Design approach for investigating the efficacy of tacrolimus and hyaluronic acid-loaded ethosomal gel in dermal management of psoriasis: in vitro, ex vivo, and in vivo evaluation. AAPS Pharm Sci Tech. 2023;24(8):220. doi:10.1208/s12249-023-02678-6
54. Zhang Y, Xia Q, Li Y, et al. CD44 assists the topical anti-psoriatic efficacy of curcumin-loaded hyaluronan-modified ethosomes: a new strategy for clustering drug in inflammatory skin. Theranostics. 2019;9(1):48–64. doi:10.7150/thno.29715
55. Li Y, Xu F, Li X, et al. Development of curcumin-loaded composite phospholipid ethosomes for enhanced skin permeability and vesicle stability. Int J Pharm. 2021;592:119936. doi:10.1016/j.ijpharm.2020.119936
56. Zhang YT, Shen LN, Wu ZH, Zhao JH, Feng NP. Comparison of ethosomes and liposomes for skin delivery of psoralen for psoriasis therapy. Int J Pharm. 2014;471(1–2):449–452. doi:10.1016/j.ijpharm.2014.06.001
57. Fathalla D, Youssef EMK, Soliman GM. Liposomal and ethosomal gels for the topical delivery of anthralin: preparation, comparative evaluation and clinical assessment in psoriatic patients. Pharmaceutics. 2020;12(5). doi:10.3390/pharmaceutics12050446
58. Costanzo M, Esposito E, Sguizzato M, et al. Formulative study and intracellular fate evaluation of ethosomes and transethosomes for vitamin D3 delivery. Int J Mol Sci. 2021;22(10). doi:10.3390/ijms22105341
59. Rodríguez-Luna A, Talero E, Ávila-Román J, et al. Preparation and in vivo evaluation of rosmarinic acid-loaded transethosomes after percutaneous application on a psoriasis animal model. AAPS Pharm Sci Tech. 2021;22(3):103. doi:10.1208/s12249-021-01966-3
60. Pandey SS, Shah KM, Maulvi FA, et al. Topical delivery of cyclosporine loaded tailored niosomal nanocarriers for improved skin penetration and deposition in psoriasis: optimization, ex vivo and animal studies. J Drug Delivery Sci Technol. 2021;63:102441. doi:10.1016/j.jddst.2021.102441
61. Shah P, Goodyear B, Dholaria N, Puri V, Michniak-Kohn B. Nanostructured non-ionic surfactant carrier-based gel for topical delivery of desoximetasone. Int J Mol Sci. 2021;22(4). doi:10.3390/ijms22041535
62. Meng S, Sun L, Wang L, et al. Loading of water-insoluble celastrol into niosome hydrogels for improved topical permeation and anti-psoriasis activity. Colloids Surf B Biointerfaces. 2019;182:110352. doi:10.1016/j.colsurfb.2019.110352
63. Abu H, Abo El-Magd NF, El-Sheakh AR, Hamed MF, El-Gawad AEH A. Pivotal role of Acitretin nanovesicular gel for effective treatment of psoriasis: ex vivo-in vivo evaluation study. Int J Nanomed. 2018;13:1059–1079. doi:10.2147/ijn.S156412
64. Moghddam SR, Ahad A, Aqil M, Imam SS, Sultana Y. Formulation and optimization of niosomes for topical diacerein delivery using 3-factor, 3-level Box-Behnken design for the management of psoriasis. Mater Sci Eng C Mater Biol Appl. 2016;69:789–797. doi:10.1016/j.msec.2016.07.043
65. Abdelgawad R, Nasr M, Moftah NH, Hamza MY. Phospholipid membrane tubulation using ceramide doping ”Cerosomes”: characterization and clinical application in psoriasis treatment. Eur J Pharm Sci. 2017;101:258–268. doi:10.1016/j.ejps.2017.02.030
66. Yang X, Tang Y, Wang M, et al. Co-delivery of methotrexate and nicotinamide by cerosomes for topical psoriasis treatment with enhanced efficacy. Int J Pharm. 2021;605:120826. doi:10.1016/j.ijpharm.2021.120826
67. Elhabal SF, Abdelaal N, Al-Zuhairy SAS, et al. Revolutionizing psoriasis topical treatment: enhanced efficacy through ceramide/phospholipid composite cerosomes co-delivery of cyclosporine and dithranol: in-vitro, ex-vivo, and in-vivo studies. Int J Nanomed. 2024;19:1163–1187. doi:10.2147/ijn.S443812
68. Mittal S, Ali J, Baboota S. Enhanced anti-psoriatic activity of tacrolimus loaded nanoemulsion gel via omega 3 - Fatty acid (EPA and DHA) rich oils-fish oil and linseed oil. J Drug Delivery Sci Technol. 2021;63:102458. doi:10.1016/j.jddst.2021.102458
69. Wan T, Pan J, Long Y, et al. Dual roles of TPGS based microemulsion for tacrolimus: enhancing the percutaneous delivery and anti-psoriatic efficacy. Int J Pharm. 2017;528(1–2):511–523. doi:10.1016/j.ijpharm.2017.06.050
70. Sahu S, Katiyar SS, Kushwah V, Jain S. Active natural oil-based nanoemulsion containing tacrolimus for synergistic antipsoriatic efficacy. Nanomedicine (Lond). 2018;13(16):1985–1998. doi:10.2217/nnm-2018-0135
71. Khatoon K, Ali A, Ahmad FJ, et al. Novel nanoemulsion gel containing triple natural bio-actives combination of curcumin, thymoquinone, and resveratrol improves psoriasis therapy: in vitro and in vivo studies. Drug Deliv Transl Res. 2021;11(3):1245–1260. doi:10.1007/s13346-020-00852-y
72. Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulsion loaded polymeric hydrogel for topical delivery of curcumin in psoriasis. J Drug Delivery Sci Technol. 2020;59:101847. doi:10.1016/j.jddst.2020.101847
73. Benigni M, Pescina S, Grimaudo MA, Padula C, Santi P, Nicoli S. Development of microemulsions of suitable viscosity for cyclosporine skin delivery. Int J Pharm. 2018;545(1–2):197–205. doi:10.1016/j.ijpharm.2018.04.049
74. Pandey SS, Maulvi FA, Patel PS, et al. Cyclosporine laden tailored microemulsion-gel depot for effective treatment of psoriasis: in vitro and in vivo studies. Colloids Surf B Biointerfaces. 2020;186:110681. doi:10.1016/j.colsurfb.2019.110681
75. Tanghetti EA, Stein Gold L, Del Rosso JQ, Lin T, Angel A, Pillai R. Optimized formulation for topical application of a fixed combination halobetasol/tazarotene lotion using polymeric emulsion technology. J DermatolTreat. 2021;32(4):391–398. doi:10.1080/09546634.2019.1668907
76. El-Gogary RI, Ragai MH, Moftah N, Nasr M. Oleuropein as a novel topical antipsoriatic nutraceutical: formulation in microemulsion nanocarrier and exploratory clinical appraisal. Expert Opin Drug Deliv. 2021;18(10):1523–1532. doi:10.1080/17425247.2021.1932813
77. Rashid SA, Bashir S, Naseem F, Farid A, Rather IA, Hakeem KR. Olive oil based methotrexate loaded topical nanoemulsion gel for the treatment of imiquimod induced psoriasis-like skin inflammation in an animal model. Biology (Basel). 2021;10(11). doi:10.3390/biology10111121
78. Guo J-W, Cheng Y-P, Liu C-Y, et al. Salvianolic acid B in microemulsion formulation provided sufficient hydration for dry skin and ameliorated the severity of imiquimod-induced psoriasis-like dermatitis in mice. Pharmaceutics. 2020;12(5):457. doi:10.3390/pharmaceutics12050457
79. Rahmanian-Devin P, Askari VR, Sanei-Far Z, et al. Preparation and characterization of solid lipid nanoparticles encapsulated noscapine and evaluation of its protective effects against imiquimod-induced psoriasis-like skin lesions. Biomed Pharmacother. 2023;168:115823. doi:10.1016/j.biopha.2023.115823
80. Alhelal HM, Mehta S, Kadian V, et al. Solid Lipid nanoparticles embedded hydrogels as a promising carrier for retarding irritation of leflunomide. Gels. 2023;9(7). doi:10.3390/gels9070576
81. Rapalli VK, Sharma S, Roy A, Alexander A, Singhvi G. Solid lipid nanocarriers embedded hydrogel for topical delivery of apremilast: in-vitro, ex-vivo, dermatopharmacokinetic and anti-psoriatic evaluation. J Drug Delivery Sci Technol. 2021;63:102442. doi:10.1016/j.jddst.2021.102442
82. Pitzanti G, Rosa A, Nieddu M, et al. Transcutol(®) P containing SLNs for improving 8-methoxypsoralen skin delivery. Pharmaceutics. 2020;12(10). doi:10.3390/pharmaceutics12100973
83. Pradhan M, Singh D, Singh MR. Influence of selected variables on fabrication of triamcinolone acetonide loaded solid lipid nanoparticles for topical treatment of dermal disorders. Artif Cells Nanomed Biotechnol. 2016;44(1):392–400. doi:10.3109/21691401.2014.955105
84. Sonawane R, Harde H, Katariya M, Agrawal S, Jain S. Solid lipid nanoparticles-loaded topical gel containing combination drugs: an approach to offset psoriasis. Expert Opin Drug Deliv. 2014;11(12):1833–1847. doi:10.1517/17425247.2014.938634
85. Essaghraoui A, Belfkira A, Hamdaoui B, Nunes C, Lima SAC, Reis S. Improved dermal delivery of cyclosporine a loaded in solid lipid nanoparticles. Nanomaterials. 2019;9(9). doi:10.3390/nano9091204
86. Silva MI, Barbosa AI, Costa Lima SA, Costa P, Torres T, Reis S. Freeze-dried softisan(®) 649-based lipid nanoparticles for enhanced skin delivery of cyclosporine A. Nanomaterials (Basel). 2020;10(5). doi:10.3390/nano10050986
87. Trombino S, Servidio C, Laganà AS, Conforti F, Marrelli M, Cassano R. Viscosified solid lipidic nanoparticles based on naringenin and linolenic acid for the release of cyclosporine A on the skin. Molecules. 2020;25(15). doi:10.3390/molecules25153535
88. Maiti D, Naseeruddin Inamdar M, Almuqbil M, et al. Evaluation of solid-lipid nanoparticles formulation of methotrexate for anti-psoriatic activity. Saudi Pharm J. 2023;31(6):834–844. doi:10.1016/j.jsps.2023.04.007
89. Kang JH, Chon J, Kim YI, et al. Preparation and evaluation of tacrolimus-loaded thermosensitive solid lipid nanoparticles for improved dermal distribution. Int J Nanomed. 2019;14:5381–5396. doi:10.2147/ijn.S215153
90. Ferreira M, Barreiros L, Segundo MA, et al. Topical co-delivery of methotrexate and etanercept using lipid nanoparticles: a targeted approach for psoriasis management. Colloids Surf B Biointerfaces. 2017;159:23–29. doi:10.1016/j.colsurfb.2017.07.080
91. Hatem S, El-Kayal M. Novel anti-psoriatic nanostructured lipid carriers for the cutaneous delivery of luteolin: a comprehensive in-vitro and in-vivo evaluation. Eur J Pharm Sci. 2023;191:106612. doi:10.1016/j.ejps.2023.106612
92. Alam M, Rizwanullah M, Mir SR, Amin S. Statistically optimized tacrolimus and thymoquinone co-loaded nanostructured lipid carriers gel for improved topical treatment of psoriasis. Gels. 2023;9(7). doi:10.3390/gels9070515
93. Llorente X, Esteruelas G, Bonilla L, et al. Riluzole-loaded nanostructured lipid carriers for hyperproliferative skin diseases. Int J Mol Sci. 2023;24(9). doi:10.3390/ijms24098053
94. Madan JR, Khobaragade S, Dua K, Awasthi R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol Ther. 2020;33(3):e13370. doi:10.1111/dth.13370
95. Rapalli VK, Sharma S, Roy A, Singhvi G. Design and dermatokinetic evaluation of Apremilast loaded nanostructured lipid carriers embedded gel for topical delivery: a potential approach for improved permeation and prolong skin deposition. Colloids Surf B Biointerfaces. 2021;206:111945. doi:10.1016/j.colsurfb.2021.111945
96. Agrawal YO, Mahajan UB, Mahajan HS, Ojha S. Methotrexate-Loaded Nanostructured Lipid Carrier Gel Alleviates Imiquimod-Induced Psoriasis By Moderating Inflammation: Formulation, Optimization, Characterization, in-vitro and in-vivo studies. Int J Nanomed. 2020;15:4763–4778. doi:10.2147/ijn.S247007
97. Pradhan M, Yadav K, Singh D, Singh MR. Topical delivery of fluocinolone acetonide integrated NLCs and salicylic acid enriched gel: a potential and synergistic approach in the management of psoriasis. J Drug Delivery Sci Technol. 2021;61:102282. doi:10.1016/j.jddst.2020.102282
98. Pradhan M, Singh D, Singh MR. Fabrication, optimization and characterization of triamcinolone acetonide loaded nanostructured lipid carriers for topical treatment of psoriasis: application of box behnken design, in vitro and ex vivo studies. J Drug Delivery Sci Technol. 2017;41:325–333. doi:10.1016/j.jddst.2017.07.024
99. Viegas JSR, Praça FG, Caron AL, et al. Nanostructured lipid carrier co-delivering tacrolimus and TNF-α siRNA as an innovate approach to psoriasis. Drug Deliv Transl Res. 2020;10(3):646–660. doi:10.1007/s13346-020-00723-6
100. Rapalli VK, Kaul V, Waghule T, et al. Curcumin loaded nanostructured lipid carriers for enhanced skin retained topical delivery: optimization, scale-up, in-vitro characterization and assessment of ex-vivo skin deposition. Eur J Pharm Sci. 2020;152:105438. doi:10.1016/j.ejps.2020.105438
101. Sathe P, Saka R, Kommineni N, Raza K, Khan W. Dithranol-loaded nanostructured lipid carrier-based gel ameliorate psoriasis in imiquimod-induced mice psoriatic plaque model. Drug Dev Ind Pharm. 2019;45(5):826–838. doi:10.1080/03639045.2019.1576722
102. Rapalli VK, Tomar Y, Sharma S, Roy A, Singhvi G. Apremilast loaded lyotropic liquid crystalline nanoparticles embedded hydrogel for improved permeation and skin retention: an effective approach for psoriasis treatment. Biomed Pharmacother. 2023;162:114634. doi:10.1016/j.biopha.2023.114634
103. Silvestrini AVP, Garcia Praça F, Leite MN, De Abreu Fantini MC, Frade MAC, Badra Bentley MVL. Liquid crystalline nanoparticles enable a multifunctional approach for topical psoriasis therapy by co-delivering triptolide and siRNAs. Int J Pharm. 2023;640:123019. doi:10.1016/j.ijpharm.2023.123019
104. Ramalheiro A, Paris JL, Silva Bruno FB, Pires LR. Rapidly dissolving microneedles for the delivery of cubosome-like liquid crystalline nanoparticles with sustained release of rapamycin. Int J Pharm. 2020;591:119942. doi:10.1016/j.ijpharm.2020.119942
105. Thapa RK, Yoo BK. Evaluation of the effect of tacrolimus-loaded liquid crystalline nanoparticles on psoriasis-like skin inflammation. J DermatolTreat. 2014;25(1):22–25. doi:10.3109/09546634.2012.755250
106. Freag MS, Torky AS, Nasra MM, Abdelmonsif DA, Abdallah OY. Liquid crystalline nanoreservoir releasing a highly skin-penetrating berberine oleate complex for psoriasis management. Nanomedicine (Lond). 2019;14(8):931–954. doi:10.2217/nnm-2018-0345
107. Pandi P, Jain A, Kommineni N, Ionov M, Bryszewska M, Khan W. Dendrimer as a new potential carrier for topical delivery of siRNA: a comparative study of dendriplex vs. lipoplex for delivery of TNF-α siRNA. Int J Pharm. 2018;550(1–2):240–250. doi:10.1016/j.ijpharm.2018.08.024
108. Tripathi PK, Gorain B, Choudhury H, Srivastava A, Kesharwani P. Dendrimer entrapped microsponge gel of dithranol for effective topical treatment. Heliyon. 2019;5(3):e01343. doi:10.1016/j.heliyon.2019.e01343
109. Agrawal U, Mehra NK, Gupta U, Jain NK. Hyperbranched dendritic nano-carriers for topical delivery of dithranol. J Drug Target. 2013;21(5):497–506. doi:10.3109/1061186x.2013.771778
110. Dhanikula RS, Hildgen P. Influence of molecular architecture of polyether-co-polyester dendrimers on the encapsulation and release of methotrexate. Biomaterials. 2007;28(20):3140–3152. doi:10.1016/j.biomaterials.2007.03.012
111. Supasena W, Muangnoi C, Thaweesest W, et al. Enhanced antipsoriatic activity of mycophenolic acid against the TNF-α-induced HaCaT cell proliferation by conjugated poloxamer micelles. J Pharm Sci. 2020;109(2):1153–1160. doi:10.1016/j.xphs.2019.11.010
112. Lapteva M, Santer V, Mondon K, et al. Targeted cutaneous delivery of ciclosporin A using micellar nanocarriers and the possible role of inter-cluster regions as molecular transport pathways. J Control Release. 2014;196:9–18. doi:10.1016/j.jconrel.2014.09.021
113. Guo D, Shi C, Wang L, Ji X, Zhang S, Luo J. A rationally designed micellar nanocarrier for the delivery of hydrophilic methotrexate in psoriasis treatment. ACS Appl Bio Mater. 2020;3(8):4832–4846. doi:10.1021/acsabm.0c00342
114. Lapteva M, Mondon K, Möller M, Gurny R, Kalia YN. Polymeric micelle nanocarriers for the cutaneous delivery of tacrolimus: a targeted approach for the treatment of psoriasis. Mol Pharm. 2014;11(9):2989–3001. doi:10.1021/mp400639e
115. Chavoshy F, Zadeh BSM, Tamaddon AM, Anbardar MH. Delivery and anti-psoriatic effect of silibinin-loaded polymeric micelles: an experimental study in the psoriatic skin model. Curr Drug Deliv. 2020;17(9):787–798. doi:10.2174/1567201817666200722141807
116. Li L, Liu C, Fu J, et al. CD44 targeted indirubin nanocrystal-loaded hyaluronic acid hydrogel for the treatment of psoriasis. Int J Biol Macromol. 2023;243:125239. doi:10.1016/j.ijbiomac.2023.125239
117. Limón D, Talló Domínguez K, Garduño-Ramírez ML, Andrade B, Calpena AC, Pérez-García L. Nanostructured supramolecular hydrogels: towards the topical treatment of Psoriasis and other skin diseases. Colloids Surf B Biointerfaces. 2019;181:657–670. doi:10.1016/j.colsurfb.2019.06.018
118. Limón D, Gil-Lianes P, Rodríguez-Cid L, et al. Supramolecular hydrogels consisting of nanofibers increase the bioavailability of curcuminoids in inflammatory skin diseases. ACS Appl Nano Mater. 2022;5(10):13829–13839. doi:10.1021/acsanm.2c01482
119. Shu Y, Xue R, Gao Y, Zhang W, Wang J. A thermo-responsive hydrogel loaded with an ionic liquid microemulsion for transdermal delivery of methotrexate. J Mater Chem B. 2023;11(24):5494–5502. doi:10.1039/d2tb02189g
120. Su H, Liu Z, Zhang Z, Jing X, Meng L. Development of a deep eutectic solvent-assisted kaempferol hydrogel: a promising therapeutic approach for psoriasis-like skin inflammation. Mol Pharm. 2023;20(12):6319–6329. doi:10.1021/acs.molpharmaceut.3c00729
121. Thakur S, Anjum MM, Jaiswal S, et al. Novel synergistic approach: tazarotene-calcipotriol-loaded-PVA/PVP-nanofibers incorporated in hydrogel film for management and treatment of psoriasis. Mol Pharm. 2023;20(2):997–1014. doi:10.1021/acs.molpharmaceut.2c00713
122. Bernardes M, Agostini SBN, Pereira GR, et al. Preclinical study of methotrexate-based hydrogels versus surfactant based liquid crystal systems on psoriasis treatment. Eur J Pharm Sci. 2021;165:105956. doi:10.1016/j.ejps.2021.105956
123. Asad MI, Khan D, Rehman AU, Elaissari A, Ahmed N. Development and in vitro/in vivo evaluation of pH-sensitive polymeric nanoparticles loaded hydrogel for the management of psoriasis. Nanomaterials (Basel). 2021;11(12). doi:10.3390/nano11123433
124. Xu J, Chen H, Chu Z, et al. A multifunctional composite hydrogel as an intrinsic and extrinsic coregulator for enhanced therapeutic efficacy for psoriasis. J Nanobiotechnology. 2022;20(1):155. doi:10.1186/s12951-022-01368-y
125. Rana K, Pani T, Jha SK, et al. Hydrogel-mediated topical delivery of steroids can effectively alleviate psoriasis via attenuating the autoimmune responses. Nanoscale. 2022;14(10):3834–3848. doi:10.1039/d1nr06001e
126. Kumar S, Prasad M, Rao R. Topical delivery of clobetasol propionate loaded nanosponge hydrogel for effective treatment of psoriasis: formulation, physicochemical characterization, antipsoriatic potential and biochemical estimation. Mater Sci Eng C Mater Biol Appl. 2021;119:111605. doi:10.1016/j.msec.2020.111605
127. Gabriel D, Mugnier T, Courthion H, et al. Improved topical delivery of tacrolimus: a novel composite hydrogel formulation for the treatment of psoriasis. J Control Release. 2016;242:16–24. doi:10.1016/j.jconrel.2016.09.007
128. Fernández-Romero AM, Maestrelli F, García-Gil S, et al. Preparation, characterization and evaluation of the anti-inflammatory activity of epichlorohydrin-β-cyclodextrin/curcumin binary systems embedded in a pluronic(®)/hyaluronate hydrogel. Int J Mol Sci. 2021;22(24). doi:10.3390/ijms222413566
129. Filippone A, Consoli GML, Granata G, et al. Topical delivery of curcumin by choline-calix[4]arene-based nanohydrogel improves its therapeutic effect on a psoriasis mouse model. Int J Mol Sci. 2020;21(14). doi:10.3390/ijms21145053
130. Qiu F, Xi L, Chen S, Zhao Y, Wang Z, Zheng Y. Celastrol niosome hydrogel has anti-inflammatory effect on skin keratinocytes and circulation without systemic drug exposure in psoriasis mice. Int J Nanomed. 2021;16:6171–6182. doi:10.2147/ijn.S323208
131. Mo C, Lu L, Liu D, Wei K. Development of erianin-loaded dendritic mesoporous silica nanospheres with pro-apoptotic effects and enhanced topical delivery. J Nanobiotechnology. 2020;18(1):55. doi:10.1186/s12951-020-00608-3
132. Hazari SA, Sheikh A, Abourehab MAS, Tulbah AS, Kesharwani P. Self-assembled Gallic acid loaded lecithin-chitosan hybrid nanostructured gel as a potential tool against imiquimod-induced psoriasis. Environ Res. 2023;234:116562. doi:10.1016/j.envres.2023.116562
133. Pukale SS, Mittal A, Chitkara D. Topical application of vitamin D(3)-loaded hybrid nanosystem to offset imiquimod-induced psoriasis. AAPS Pharm Sci Tech. 2021;22(7):238. doi:10.1208/s12249-021-02116-5
134. Pukale SS, Sharma S, Dalela M, et al. Multi-component clobetasol-loaded monolithic lipid-polymer hybrid nanoparticles ameliorate imiquimod-induced psoriasis-like skin inflammation in Swiss albino mice. Acta Biomater. 2020;115:393–409. doi:10.1016/j.actbio.2020.08.020
135. Suzuki IL, de Araujo MM, Bagnato VS, Bentley M. TNFα siRNA delivery by nanoparticles and photochemical internalization for psoriasis topical therapy. J Control Release. 2021;338:316–329. doi:10.1016/j.jconrel.2021.08.039
136. Fereig SA, El-Zaafarany GM, Arafa MG, Abdel-Mottaleb MMA. Self-assembled tacrolimus-loaded lecithin-chitosan hybrid nanoparticles for in vivo management of psoriasis. Int J Pharm. 2021;608:121114. doi:10.1016/j.ijpharm.2021.121114
137. Nirmal GR, Lin ZC, Tsai MJ, Yang SC, Alalaiwe A, Fang JY. Photothermal treatment by PLGA-gold nanorod-isatin nanocomplexes under near-infrared irradiation for alleviating psoriasiform hyperproliferation. J Control Release. 2021;333:487–499. doi:10.1016/j.jconrel.2021.04.005
138. Fratoddi I, Benassi L, Botti E, et al. Effects of topical methotrexate loaded gold nanoparticle in cutaneous inflammatory mouse model. Nanomedicine. 2019;17:276–286. doi:10.1016/j.nano.2019.01.006
139. Raghuwanshi N, Yadav TC, Srivastava AK, Raj U, Varadwaj P, Pruthi V. Structure-based drug designing and identification of Woodfordia fruticosa inhibitors targeted against heat shock protein (HSP70-1) as suppressor for Imiquimod-induced psoriasis like skin inflammation in mice model. Mater Sci Eng C Mater Biol Appl. 2019;95:57–71. doi:10.1016/j.msec.2018.10.061
140. Shahine Y, El-Aal SAA, Reda AM, et al. Diosmin nanocrystal gel alleviates imiquimod-induced psoriasis in rats via modulating TLR7,8/NF-κB/micro RNA-31, AKT/mTOR/P70S6K milieu, and Tregs/Th17 balance. Inflammopharmacology. 2023;31(3):1341–1359. doi:10.1007/s10787-023-01198-w
141. Parmar PK, Bansal AK. Novel nanocrystal-based formulations of apremilast for improved topical delivery. Drug Deliv Transl Res. 2021;11(3):966–983. doi:10.1007/s13346-020-00809-1
142. Tekko IA, Permana AD, Vora L, Hatahet T, McCarthy HO, Donnelly RF. Localised and sustained intradermal delivery of methotrexate using nanocrystal-loaded microneedle arrays: potential for enhanced treatment of psoriasis. Eur J Pharm Sci. 2020;152:105469. doi:10.1016/j.ejps.2020.105469
143. Puri A, Loomis K, Smith B, et al. Lipid-based nanoparticles as pharmaceutical drug carriers: from concepts to clinic. Crit Rev Ther Drug Carrier Syst. 2009;26(6):523–580. doi:10.1615/critrevtherdrugcarriersyst.v26.i6.10
144. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
145. Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286. doi:10.3389/fphar.2015.00286
146. Fonseca-Santos B, Gremião MP, Chorilli M. Nanotechnology-based drug delivery systems for the treatment of Alzheimer’s disease. Int J Nanomed. 2015;10:4981–5003. doi:10.2147/ijn.S87148
147. Wang J, Li P, Yu Y, et al. Pulmonary surfactant-biomimetic nanoparticles potentiate heterosubtypic influenza immunity. Science. 2020;367(6480). doi:10.1126/science.aau0810
148. Tang S, Davoudi Z, Wang G, et al. Soft materials as biological and artificial membranes. Chem Soc Rev. 2021;50(22):12679–12701. doi:10.1039/d1cs00029b
149. Sarfraz M, Afzal A, Yang T, et al. Development of dual drug loaded nanosized liposomal formulation by a reengineered ethanolic injection method and its pre-clinical pharmacokinetic studies. Pharmaceutics. 2018;10(3). doi:10.3390/pharmaceutics10030151
150. Sedighi M, Sieber S, Rahimi F, et al. Rapid optimization of liposome characteristics using a combined microfluidics and design-of-experiment approach. Drug Deliv Transl Res. 2019;9(1):404–413. doi:10.1007/s13346-018-0587-4
151. Paul A, Cevc G, Bachhawat BK. Transdermal immunisation with an integral membrane component, gap junction protein, by means of ultradeformable drug carriers, transfersomes. Vaccine. 1998;16(2–3):188–195. doi:10.1016/s0264-410x(97)00185-0
152. Cevc G, Blume G. New, highly efficient formulation of diclofenac for the topical, transdermal administration in ultradeformable drug carriers, Transfersomes. Biochim Biophys Acta. 2001;1514(2):191–205. doi:10.1016/s0005-2736(01)00369-8
153. Grimaldi N, Andrade F, Segovia N, et al. Lipid-based nanovesicles for nanomedicine. Chem Soc Rev. 2016;45(23):6520–6545. doi:10.1039/c6cs00409a
154. Fernández-García R, Lalatsa A, Statts L, Bolás-Fernández F, Ballesteros MP, Serrano DR. Transferosomes as nanocarriers for drugs across the skin: quality by design from lab to industrial scale. Int J Pharm. 2020;573:118817. doi:10.1016/j.ijpharm.2019.118817
155. Omar MM, Hasan OA, El Sisi AM. Preparation and optimization of lidocaine transferosomal gel containing permeation enhancers: a promising approach for enhancement of skin permeation. Int J Nanomed. 2019;14:1551–1562. doi:10.2147/ijn.S201356
156. Sahu N, Alam P, Ali A, et al. Optimization, in vitro and ex vivo assessment of nanotransferosome gels infused with a methanolic extract of Solanum xanthocarpum for the topical treatment of psoriasis. Gels. 2024;10(2). doi:10.3390/gels10020119
157. Sala M, Diab R, Elaissari A, Fessi H. Lipid nanocarriers as skin drug delivery systems: properties, mechanisms of skin interactions and medical applications. Int J Pharm. 2018;535(1–2):1–17. doi:10.1016/j.ijpharm.2017.10.046
158. Romero EL, Morilla MJ. Highly deformable and highly fluid vesicles as potential drug delivery systems: theoretical and practical considerations. Int J Nanomed. 2013;8:3171–3186. doi:10.2147/ijn.S33048
159. Touitou E, Dayan N, Bergelson L, Godin B, Eliaz M. Ethosomes - novel vesicular carriers for enhanced delivery: characterization and skin penetration properties. J Control Release. 2000;65(3):403–418. doi:10.1016/s0168-3659(99)00222-9
160. Verma P, Pathak K. Therapeutic and cosmeceutical potential of ethosomes: an overview. J Adv Pharm Technol Res. 2010;1(3):274–282. doi:10.4103/0110-5558.72415
161. Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23(9):3319–3329. doi:10.1080/10717544.2016.1177136
162. Yang L, Wu L, Wu D, Shi D, Wang T, Zhu X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int J Nanomed. 2017;12:3357–3364. doi:10.2147/ijn.S134708
163. Li G, Fan Y, Fan C, et al. Tacrolimus-loaded ethosomes: physicochemical characterization and in vivo evaluation. Eur J Pharm Biopharm. 2012;82(1):49–57. doi:10.1016/j.ejpb.2012.05.011
164. Touitou E, Natsheh H. Topical administration of drugs incorporated in carriers containing phospholipid soft vesicles for the treatment of skin medical conditions. Pharmaceutics. 2021;13(12). doi:10.3390/pharmaceutics13122129
165. Handjani-Vila RM, Ribier A, Rondot B, Vanlerberghie G. Dispersions of lamellar phases of non-ionic lipids in cosmetic products. Int J Cosmet Sci. 1979;1(5):303–314. doi:10.1111/j.1467-2494.1979.tb00224.x
166. Marianecci C, Di Marzio L, Rinaldi F, et al. Niosomes from 80s to present: the state of the art. Adv Colloid Interface Sci. 2014;205:187–206. doi:10.1016/j.cis.2013.11.018
167. Marianecci C, Rinaldi F, Mastriota M, et al. Anti-inflammatory activity of novel ammonium glycyrrhizinate/niosomes delivery system: human and murine models. J Control Release. 2012;164(1):17–25. doi:10.1016/j.jconrel.2012.09.018
168. Sinico C, Fadda AM. Vesicular carriers for dermal drug delivery. Expert Opin Drug Deliv. 2009;6(8):813–825. doi:10.1517/17425240903071029
169. Chen SR, Dai Y, Zhao J, Lin L, Wang Y, Wang Y. A mechanistic overview of triptolide and celastrol, natural products from tripterygium wilfordii hook F. Front Pharmacol. 2018;9:104. doi:10.3389/fphar.2018.00104
170. El-Zaafarany GM, Nasr M. Insightful exploring of advanced nanocarriers for the topical/transdermal treatment of skin diseases. Pharm Dev Technol. 2021;26(10):1136–1157. doi:10.1080/10837450.2021.2004606
171. Dinshaw IJ, Ahmad N, Salim N, Leo BF. Nanoemulsions: a review on the conceptualization of treatment for psoriasis using a ‘green’ surfactant with low-energy emulsification method. Pharmaceutics. 2021;13(7). doi:10.3390/pharmaceutics13071024
172. Wilson RJ, Li Y, Yang G, Zhao C-X. Nanoemulsions for drug delivery. Particuology. 2022;64:85–97. doi:10.1016/j.partic.2021.05.009
173. Müller K, Ziereis K, Gawlik I. The antipsoriatic mahonia aquifolium and its active constituents; II. antiproliferative activity against cell growth of human keratinocytes. Planta Med. 1995;61(1):74–75. doi:10.1055/s-2006-958005
174. Cloninger MJ. Biological applications of dendrimers. Curr Opin Chem Biol. 2002;6(6):742–748. doi:10.1016/s1367-5931(02)00400-3
175. Jebbawi R, Oukhrib A, Clement E, et al. An anti-inflammatory poly(PhosphorHydrazone) dendrimer capped with azabisphosphonate groups to treat psoriasis. Biomolecules. 2020;10(6). doi:10.3390/biom10060949
176. Katari O, Jain S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics for the treatment of psoriasis. Expert Opin Drug Deliv. 2021;18(12):1857–1872. doi:10.1080/17425247.2021.2011857
177. Mirchandani Y, Patravale VB. Solid lipid nanoparticles for hydrophilic drugs. J Control Release. 2021;335:457–464. doi:10.1016/j.jconrel.2021.05.032
178. Jensen LB, Petersson K, Nielsen HM. In vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur J Pharm Biopharm. 2011;79(1):68–75. doi:10.1016/j.ejpb.2011.05.012
179. Biswasroy P, Pradhan D, Kar B, Ghosh G, Rath G. Recent advancement in topical nanocarriers for the treatment of psoriasis. AAPS Pharm Sci Tech. 2021;22(5):164. doi:10.1208/s12249-021-02057-z
180. Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47(2–3):165–196. doi:10.1016/s0169-409x(01)00105-3
181. Salehi B, Fokou PVT, Sharifi-Rad M, et al. The therapeutic potential of naringenin: a review of clinical trials. Pharmaceuticals (Basel). 2019;12(1). doi:10.3390/ph12010011
182. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
183. Hemrajani C, Negi P, Parashar A, et al. Overcoming drug delivery barriers and challenges in topical therapy of atopic dermatitis: a nanotechnological perspective. Biomed Pharmacother. 2022;147:112633. doi:10.1016/j.biopha.2022.112633
184. Li N, Qin Y, Dai D, et al. Transdermal delivery of therapeutic compounds with nanotechnological approaches in psoriasis. Front Bioeng Biotechnol. 2021;9:804415. doi:10.3389/fbioe.2021.804415
185. Pradhan M, Alexander A, Singh MR, et al. Understanding the prospective of nano-formulations towards the treatment of psoriasis. Biomed Pharmacother. 2018;107:447–463. doi:10.1016/j.biopha.2018.07.156
186. Liu M, Fréchet JM. Designing dendrimers for drug delivery. Pharm Sci Technol Today. 1999;2(10):393–401. doi:10.1016/s1461-5347(99)00203-5
187. Tang M, Hu P, Zheng Q, et al. Polymeric micelles with dual thermal and reactive oxygen species (ROS)-responsiveness for inflammatory cancer cell delivery. J Nanobiotechnology. 2017;15(1):39. doi:10.1186/s12951-017-0275-4
188. Hung CF, Lin YK, Zhang LW, Chang CH, Fang JY. Topical delivery of silymarin constituents via the skin route. Acta Pharmacol Sin. 2010;31(1):118–126. doi:10.1038/aps.2009.186
189. Li Y, Rodrigues J, Tomás H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41(6):2193–2221. doi:10.1039/c1cs15203c
190. Gajbhiye KR, Salve R, Narwade M, Sheikh A, Kesharwani P, Gajbhiye V. Lipid polymer hybrid nanoparticles: a custom-tailored next-generation approach for cancer therapeutics. Mol Cancer. 2023;22(1):160. doi:10.1186/s12943-023-01849-0
191. Zhang W, Jiang Y, He Y, et al. Lipid carriers for mRNA delivery. Acta Pharm Sin B. 2023;13(10):4105–4126. doi:10.1016/j.apsb.2022.11.026
192. Fereig SA, El-Zaafarany GM, Arafa MG, Abdel-Mottaleb MMA. Tackling the various classes of nano-therapeutics employed in topical therapy of psoriasis. Drug Deliv. 2020;27(1):662–680. doi:10.1080/10717544.2020.1754527
193. Gupta R, Rai B. Effect of size and surface charge of gold nanoparticles on their skin permeability: a molecular dynamics study. Sci Rep. 2017;7:45292. doi:10.1038/srep45292
194. Han R, LWC H, Bai Q, et al. Alkyl-Terminated Gold Nanoparticles as a Self-Therapeutic Treatment for Psoriasis. Nano Lett. 2021;21(20):8723–8733. doi:10.1021/acs.nanolett.1c02899
195. Junghanns JU, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295–309. doi:10.2147/ijn.s595
196. McGuckin MB, Wang J, Ghanma R, et al. Nanocrystals as a master key to deliver hydrophobic drugs via multiple administration routes. J Control Release. 2022;345:334–353. doi:10.1016/j.jconrel.2022.03.012
197. Cláudia Paiva-Santos A, Gama M, Peixoto D, et al. Nanocarrier-based dermopharmaceutical formulations for the topical management of atopic dermatitis. Int J Pharm. 2022;618:121656. doi:10.1016/j.ijpharm.2022.121656
198. Döge N, Hönzke S, Schumacher F, et al. Ethyl cellulose nanocarriers and nanocrystals differentially deliver dexamethasone into intact, tape-stripped or sodium lauryl sulfate-exposed ex vivo human skin - assessment by intradermal microdialysis and extraction from the different skin layers. J Control Release. 2016;242:25–34. doi:10.1016/j.jconrel.2016.07.009
199. Hirsch JE. An index to quantify an individual’s scientific research output. Proc Natl Acad Sci U S A. 2005;102(46):16569–16572. doi:10.1073/pnas.0507655102
200. Huang L, Huang XH, Yang X, et al. Novel nano-drug delivery system for natural products and their application. Pharmacol Res. 2024;201:107100. doi:10.1016/j.phrs.2024.107100
201. Viegas C, Patrício AB, Prata JM, Nadhman A, Chintamaneni PK, Fonte P. Solid lipid nanoparticles vs. nanostructured lipid carriers: a comparative review. Pharmaceutics. 2023;15(6). doi:10.3390/pharmaceutics15061593
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.