Back to Journals » International Journal of Nanomedicine » Volume 19
Recent Advancements of Nanomedicine in Breast Cancer Surgery
Authors Meng X, Wang X, Zhang Z, Song L , Chen J
Received 3 September 2024
Accepted for publication 28 November 2024
Published 31 December 2024 Volume 2024:19 Pages 14143—14169
DOI https://doi.org/10.2147/IJN.S494364
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. RDK Misra
Xiangyue Meng,1,2,* Xin Wang,1– 3,* Zhihao Zhang,1,2 Linlin Song,3,4 Jie Chen1,2
1Department of General Surgery, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 2Breast Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 3Laboratory of Integrative Medicine, Clinical Research Center for Breast, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University and Collaborative Innovation Center, Chengdu, Sichuan, 610041, People’s Republic of China; 4Department of Ultrasound, Laboratory of Ultrasound Medicine, West China Hospital, Sichuan University, Chengdu, Sichuan, 610041, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jie Chen, Division of Breast Surgery, Department of General Surgery, West China Hospital, Sichuan University, No. 37 Guo Xue Alley, Chengdu, Sichuan, 610041, People’s Republic of China, Email [email protected]
Abstract: Breast cancer surgery plays a pivotal role in the multidisciplinary approaches. Surgical techniques and objectives are gradually shifting from tumor complete resection towards prolonging survival, improving cosmetic outcomes, and restoring the social and psychological well-being of patients. However, surgical treatment still faces challenges such as inadequate sensitivity in sentinel lymph node localization, the need to improve intraoperative tumor boundary localization imaging, postoperative scar healing, and the risk of recurrence, necessitating other adjunct measures for improvement. To address these challenges, specificity-optimized nanomedicines have been introduced into the surgical therapeutic landscape of breast cancer. In particular, this review involves starting with an overview of breast structure and the composition of the tumor microenvironment and then introducing the guiding principle and foundation for the design of nanomedicine. Moreover, we will take the order process of breast cancer surgery diagnosis and treatment as the starting point, and adaptively propose the roles and advantages of nanomedicine in addressing the corresponding issues. Furthermore, we also involved the prospects of utilizing advanced technological approaches. Overall, this review seeks to uncover the sophisticated design and strategies of nanomedicine from a clinical standpoint, address the challenges faced in surgical treatment, and provide insights into this subject matter.
Keywords: breast cancer, surgery procedure, nanomedicine, wound healing, postoperative recurrence
Graphical Abstract:

Introduction
Breast cancer, its incidence rate is increasing year by year, has emerged as the top ranking diagnosed.1 According to data from the Cancer statistics 2024, approximately, 310,720 new cases were reported in 2024, occupied the first place of 32% in the total number of new cases of cancer in female, surpassing lung and colorectum.2 Exposure to factors (eg obesity and smoking) prompting tumorigenesis or abnormal occurrences in breast tissue may lead to the development of benign tumors or metastatic cancers.3 A significant contributor to decreased survival rates among breast cancer patients is the spread of cancer to diverse organs, such as the bone, liver, lung, brain, and lymph nodes. Breast cancer is categorized into various subtypes, including luminal A, luminal B, human epidermal growth factor receptor-2 (HER2)-positive, and triple-negative breast cancer (TNBC), among others.4 The classification of breast cancer into subtypes is crucial for the prognostication of patient response to treatment and the identification of pertinent interventions.
Throughout history, the treatment of breast cancer has gradually evolved to primarily include surgical treatment, complemented by chemotherapy, radiotherapy, endocrine therapy, targeted therapy, and immunotherapy as adjunctive comprehensive treatment approaches. Consequently, the surgical methods for breast cancer have also undergone revolutionary changes.5 A milestone event that laid the foundation for modern breast cancer treatment was the radical mastectomy proposed by William Stewart Halsted in 1894, establishing the core role of surgical treatment.6 He advocated for the extent of dissection of breast, pectoral muscles, and axillary lymph nodes. This marked the first successful practice of achieving R0 resection surgery for early-stage breast cancer in human history, significantly improving long-term patient survival, with the postoperative local recurrence rate dropping from 58%–85% to 6% and the 5-year overall survival rate reaching 30%. Subsequently, breast cancer surgery has gradually evolved to modified radical mastectomy, and now to the current breast-conserving surgery (BCS),7 further marking the transformation of breast cancer surgery from a one-size-fits-all approach to a mindset focused on seeking cure, preserving function, and pursuing aesthetic outcomes. It is worth mentioning that our team had developed a single-incision endoscope-assisted breast-conserving surgery (SINA-BCS), which yields excellent cosmetic outcomes and high patient satisfaction.8 Furthermore, robot-assisted breast-conserving surgery represents a burgeoning trend in breast surgery, exemplifying the application of modern technology in the medical field.9,10
However, surgical intervention can lead to severe pain and potential complications, such as delayed healing and infection, and the risk of disease recurrence.11 Additionally, the accuracy, false-negative rate, and safety of sentinel lymph node detection in breast cancer surgery are crucial factors that influence prognosis. However, the excessive use of blue dye in domestic practices can lead to rapid imaging of secondary lymph nodes and requires significant exposure of lymphatic vessels, resulting in greater trauma.12,13 Another key factor in influencing surgical outcomes is obtaining clear margins, ensuring that they are negative on frozen pathology at the anatomical tissue level. Thus, it is important to note that while the technology is still evolving, further research is necessary to optimize and assess the intraoperative tumor localization and integrity of tumor dissection.14 On the other hand, one significant advancement in surgery has transformed removing the cancer and reconstructing organ morphology and function, that is breast reconstruction, ultimately improving quality of life.15,16 Thus, promoting wound filling and healing and selecting the right prosthesis are crucial for enhancing incisional cosmetic effects and improving the psychological state of patients.
Recent advancements in nanomedicine, coupled with enhanced insights into cancer biology and nano-bio interactions, have facilitated the creation of a range of nanomaterials.17 Nanomedicine, first mentioned in research literature around 2000, utilized nanoscale or nanostructured materials in medical applications, leveraging nanotechnology to improve human health.18 Advancing beyond initial cancer nanomedicines, which focus on enhancing the accumulation of nanotherapeutics in solid tumors to minimize off-target effects through tissue-specific targeting, second-generation cancer nanomedicines aim to achieve specific and efficient internalization of nanotherapeutics into tumor cells via cell-specific targeting.19 These innovations seek to enhance the therapeutic effectiveness of encapsulated anticancer agents by targeting specific tumor tissues, cells, or organelles, through modifying nanocarriers with specific molecules like antibodies, peptides, carbohydrates, and small molecules.20 Hence, specially designed nanoparticles can be used as imaging contrast agents for their distinctive physicochemical and optoelectronic characteristics, exhibiting magnetic properties, and heat generation among other attributes, enabling highly sensitive and high-resolution tumor detection through tumor diagnosis.21,22 In addition, through rational design and manufacture of nanomedicine carriers, targeted drug delivery in tumor therapy can be realized.23 For instance, superparamagnetic iron oxide nanoparticles (SPIONs) have been employed as a contrast agent in magnetic resonance imaging (MRI) for highly sensitive cancer diagnosis.24,25 Recently, biomaterials can be designed and engineered to interact with biological systems in surgical procedures, such as tissue engineering, regenerative medicine, or medical devices.26–28 Accordingly, various categories of biomaterials have been created, encompassing naturally derived materials like collagen, hyaluronic acid, chitosan, as well as a range of synthetic polymers including hydrogels and polyethylene glycol (PEG).29,30 For example, short designer self-assembling peptide (dSAP) biomaterials offered surgeons a versatile and durable set of tools to integrate the wound microenvironment, providing a significantly safer and more effective hemostatic capacity compared to conventional materials and hemostatic agents.31
In this review, we will start from the shortcomings encountered in the diagnosis and treatment of breast cancer surgery procedure, then combined with the unique advantages of nanomedicine: (1) aiming to enhance the sensitivity and specificity in the diagnostic process, to achieve earlier and rapid diagnosis of tumors and primary prevention of tumors; (2) aiming to improve the localization of intraoperative tumors and metastatic lymph nodes, more accurately remove tumors, and reduce the risk of postoperative recurrence; (3) aiming to promote the healing of postoperative wounds, improve the patient-oriented cosmetic effect of wounds, and alleviate the psychological burden of patients (Figure 1). Finally, we would briefly introduce the progress of advanced technologies in the field of breast cancer surgery procedure, which is future trend and research direction.
 |
Figure 1 Application and advancement of nanomedicine during breast cancer surgery process. Created in BioRender. Xiangyue, M. (2025) https://BioRender.com/u57d024. Abbreviations: MRI, magnetic resonance imaging; Gold NP, gold nanoparticle; SLNs, sentinel lymph nodes; ROS, reactive oxygen species; CTCs, circulating tumor cells. |
General Overview of Breast Cancer and Relative Medicine
To better develop nanomaterials applicable during surgical procedures for breast cancer patients, lies in engineering them to efficiently target tumor tissues and effectively deliver therapeutic drugs. Therefore, the key to developing nanomedicines with high therapeutic efficacy, and understanding the anatomy of the breast and its tumor microenvironment (TME) is essential.
Anatomical Structure and Tumor Microenvironment
The majority of the breast extends downward from around the level of the 2nd or 3rd rib to the fold beneath the breast, which typically lies near the 6th or 7th rib. Horizontally, it spans from the edge of the breastbone to the front part of the armpit.32 Behind the breast lies portions of the fasciae of the pectoralis major, rectus abdominis muscles, external abdominal oblique, and serratus anterior.33 The breast primarily consists of fat tissue, known as adipose tissue.34 Female breasts are comprised of 12–20 lobes, further subdivided into smaller sections, connected by milk ducts.35 Breast cancer often manifests within the glandular epithelial tissue of the breast. A correct understanding of the anatomical structure of the breast is the foundation for performing breast tumor surgery.
After introducing the anatomy of the breast, understanding the TME is crucial for subsequent selection of nanomaterials. The TME is an intricate milieu where dynamic interactions between cells and between cells and the ECM play a significant role in cancer initiation. Communication between stromal and immune cells triggers a series of events that promote tumor progression.36 Regarding the cellular composition, it consists of breast cancer cells and stromal cells, the latter including molecules of tumor-associated fibroblasts (CAFs), immune cells, vascular endothelial cells, and adipocytes.37 In addition, the ECM, a three-dimensional (3D) network composed of proteins such as collagen, laminin, and fibronectin, offers structural support and presents biomechanical and biochemical signals that enable cells to adhere, proliferate, and migrate.38,39 Changes in the ECM characteristics may influence cellular behavior and could potentially promote tumor progression. The variability among different tumors and the heterogeneity of tumor cells within breast cancer pose significant challenges for treatment. Hence, it is crucial to identify the alterations in cellular characteristics specific to each type and stage of breast tumors.
Design Principle of Nanomedicine
After introducing the breast anatomical structure and TME, it is necessary to understand the properties of nanomedicine. In 1986, Matsumura and Maeda first demonstrated that macromolecules within a specific molecular range tend to accumulate preferentially in solid tumors through a phenomenon known as the Enhanced Permeability and Retention (EPR) effect.40 This discovery has facilitated targeted delivery of macromolecular drugs and nanomedicines to tumor tissues. Nonetheless, a substantial portion (90% or more) of the therapeutic agent tends to accumulate in reticuloendothelial organs such as the liver and spleen, possibly due to uptake by mononuclear phagocytes, specific tumor types, intratumoral pressure, and vascularization.41,42 For instance, molecules smaller than with hydrodynamic diameters of 5–6 nm, which are below the renal clearance threshold, typically have brief circulatory lifespans. Conversely, nanoparticles larger than 200 nm often exhibit poor extravasation into tumors.43,44 Nanoparticles ranging in diameter from 50 to 150 nm are thus regarded as the ideal size for targeting tumors via the EPR effect.45
On the basis of that, active targeting strategies are utilized to guide nanoparticles towards receptors or surface membrane proteins that are overexpressed on target cells, facilitating internalization and initiating receptor-mediated endocytosis.46 Commonly employed engineered modifications encompass antibodies, peptides, and natural polysaccharides, granting them enhanced penetration and immune evasion capabilities.47–49 It is worth noting that breast cancers show increased expression of HER2, in which anti-HER2 monoclonal antibodies, such as Trastuzumab (Herceptin®) and Pertuzumab (Perjeta®), have been developed for directly killing cancer cells, delivering cytotoxic chemotherapy through antibody-drug conjugates (ADCs), and targeting nanosized drug delivery vehicles for cancer therapy.50 Alternatively, in the past decade, a natural biomimetic targeting approach has gained considerable interest. By cloaking nanocarriers with plasma membranes derived from cancer cells or blood cells, these nanocarriers acquire the homotypic or heterotypic adhesive properties of the source cells.51,52 Active targeting is poised as the future of cancer therapy, aligning with the principles of precision medicine.
Classification and Composition of Nanomedicine in Breast Cancer
After comprehensively introducing the characteristics of nanomedicine, we will then categorize nanomaterials into four types: organic, inorganic, biomimetic, and hybrid nanocarriers. Organic and inorganic nanomaterials are categorized according to their chemical composition, specifically whether they consist of carbon-based molecules.53 Organic components mainly consist of liposomes, polymers, and dendrimers, and inorganic components mainly consist of magnetic particles, cationic particles, gold nanoparticles (AuNPs), iron oxide nanoparticles and iron-gold nanoparticles.54–58 Furthermore, biomimetic nanoformulations integrate characteristics from biological systems and surfaces to actively interact with targets and improve their biocompatibility.59,60 These particles are engineered to replicate essential traits observed in nature, enabling them to closely mimic the behavior of natural systems.61 Due to the diversity of types and properties of nanomaterials, this paper only focuses on nanomaterials that can function in the surgical treatment of breast cancer (Figure 2).
 |
Figure 2 Classification and nanoformulations of nanomedicine applied in breast cancer surgery. Created in BioRender. Xiangyue, M. (2025) https://BioRender.com/e89b081. |
The organic nanomedicine has received significant attention due to their low toxicity and high biocompatibility with biological tissues.62–64 Liposomes are spherical vesicles characterized by an aqueous core enclosed within phospholipid bilayers, typically ranging in diameter from 50 nm to 1000 nm.65,66 For example, the initial FDA-approved nanomedicine, Doxil® (PEGylated liposomal doxorubicin), was now routinely used in clinical practice.67 Polymeric nanoparticles, created through polymer self-assembly or self-aggregation, achieve precise targeting and effective utilization of the EPR effect, leveraging their high molecular weight and structured nanoforms.68 Polyethylene glycol (PEG) and polylactic-co-glycolic acid (PLGA) are widely used in breast cancer therapy.69,70 For example, Lo et al designed a fucoidan/chitosan layered PLGA nanoparticles with enhanced delivery efficiency and increased TNBC immunogenic cell death.71 Furthermore, dendrimers, a type of hyperbranched polymeric macromolecules, were also widely utilized in drug delivery systems.72 Dendrimers offer distinct advantages due to their precise chemical structures and molecular weights, as well as the abundance and density of functional groups available for drug conjugation and targeting sites, which surpass those found in linear polymeric structures.73 A preclinical study demonstrated that Ma et al developed a PD-L1 antibody-conjugated PAMAM dendrimer nanosystem to be gene delivery vectors precisely with branched structure and cooperative multivalency.74
To mitigate the issue of postoperative residual tumor cells and promotion of wound healing, the mucoadhesive properties of nanomaterials are crucial, notably hydrogels. Hydrogels have emerged as highly competitive candidates for wound dressings due to their excellent hydrophilicity, biocompatibility, and 3D porous structure resembling the ECM and have attracted considerable interest from researchers.75 For example, Liu et al designed the biodegradable silk fibroin/perfluorocarbon hydrogel loaded with DOX demonstrated continuous oxygen release in physiological conditions to enhance hypoxia and radiotherapy sensitivity, while also effectively prevented tumor recurrence and reduced the progression of skin radiation damage. Stimuli-responsive hydrogels react to variations in the external environment (such as temperature, pH, or light), enabling them to undergo size or shape adjustments in response to these stimuli. These characteristics hold promising applications in the realm of wound dressing hydrogels.76
Inorganic materials have been utilized in the synthesis of nanostructured materials for diverse applications in drug delivery and imaging. These inorganic nanoparticles are carefully designed and can be tailored to exhibit a broad range of sizes, structures, and geometries.77 Carbon nanotubes (CNTs), known for their excellent thermal and electrical conductivity, possess a natural ability to absorb NIR light and convert it into heat through vibrational energy release. This heat induces cell death by mechanisms such as coagulation necrosis, cell membrane rupture, and protein denaturation.78,79 Gold nanoparticles (AuNPs) have free electrons on their surface that oscillate at frequencies determined by their size and shape, which confers upon them photothermal properties.80 Iron oxide is another frequently studied material used in the synthesis of inorganic nanoparticles, with iron oxide nanoparticles comprising the majority of FDA-approved inorganic nanomedicines.81 In December 2022, the FDA officially approved the Magtrace (consisted of iron oxide) and Sentimag magnetic localization system developed by Endomag to assist breast cancer patients in sentinel lymph node biopsy determining whether cancer has spread beyond the initial tumor site.82
Biomimetic nanomaterials mimic natural structures and processes, enhancing biocompatibility and reducing immune responses.83 Typical biomimetic nanomaterials encompass protein derivatives, predominantly albumin, and vesicles based on membranes, including cell membranes and exosomes. The most notable of albumin is Abraxane, an albumin-bound nanoparticle formulation of paclitaxel (PTX), which has received approval from the FDA.84 By utilizing the natural and unique transport mechanism of albumin, Abraxane facilitates a higher drug distribution within tumor tissues, and the formulation significantly reduces the occurrence of allergic reactions.85 The biomimetic membranes are separated from the cell membranes of natural cells and retain the biological recognition receptors (protein and carbohydrate molecules) completely on the membranes.86 By avoiding the recognition of immune cells, it realizes the “stealth” effect in the circulation system, thus prolonging the blood circulation time and improving the bioavailability of the nanomedicine. Biomimetic membranes include blood cell membranes (red blood cells (RBC) and platelets61,87), immune cell membranes (macrophages88), tumor cell membranes,89 exosomes,90,91 hybrid biological membranes and so on. For example, Li et al tailored a RBC membrane coated mesoporous titanium dioxide nanoparticles (mTNPs), termed as RBC-mTNPs@AQ4N.92 They utilized the RBC membranes to avoid the clearance of immune cells and extend circulation, with the tumor accumulation up to 1.8-fold higher than without RBC membranes coated after injected into the Balb/c mice for 24 h.
Applications of Nanomedicine in Diagnosis and Treatment in Breast Cancer Surgery
Nanomedicine for Increasing Diagnosis Sensitivity
Precise identification of the cancer is crucial, given its status as a pioneer in cancer treatment. Establishing a clinical diagnosis of breast cancer necessitates a comprehensive approach, including a detailed medical history, assessment of subjective symptoms and clinical signs, laboratory examination, imaging, and tissue biopsy, the latter being indispensable. By integrating imaging modalities such as ultrasound imaging, mammogram, CT-scan, and magnetic resonance imaging (MRI) to effectively locate pathological tissue within the body as an indispensable adjunct to clinical diagnosis.93–95 Then it is mandatory to perform a core needle biopsy (CNB) on suspicious solid masses with BIRADS IVa and beyond.96 However, the sensitivity of imaging examinations and the heterogeneity of tumors (including fat necrosis, fibrosis, and inflammatory cells among tumor cells) to some extent limit the accuracy of clinical diagnosis. In the following, we will elaborate on the unique role that nanomaterials can function, starting from different imaging diagnostic modalities. Since the diagnostic role of nanomedicine has been extensively discussed in the aforementioned article, only selected representative examples are presented in this paper.97,98
As for increasing ultrasound sensitivity, for example, Abdolahad et al fabricated a real-time in vivo biopsy guided probe (BGP) to realize real-time distinguish BIRADS III from IVa of breast tumors.99 The BGP was formed by electrostatically adhering positively charged multiwall carbon nanotube (MWCNT) powder onto steel needles coated with negatively charged silver paste. The BGP tracked the release of H2O2 from suspicious cancer cells during glycolysis using electrochemistry. In vivo results of the BGP demonstrated about 93% accuracy and 95% sensitivity, enabling differentiation between tumor cells (4T1 and MC4L2) and normal lesions. It is worth mentioning that besides utilizing tumor metabolism changes for early tumor detection with ultrasound, ultrasound can also be used for monitoring treatment responses. For instance, Sun et al developed a nano-ultrasonic contrast agent named Pt(IV)/CQ/PFH NPs-DPPA-1.100 Among that, perfluorohexane (PFH) acted as a stable imaging agent capable of undergoing phase transition to localize and image in response to an ultrasound therapeutic apparatus (US), which could monitor the therapeutic effect of NPs by responding to ultrasound.
To further enhance CT sensitivity while reducing contrast agents’ toxicity, Gkikas et al synthesized polyethylene glycol (PEG)-stabilized AuNPs (mPEG-S-AuNPs) linked with a fibronectin-mimetic peptide.101 It has been reported that the peptide could specifically recognize and bind to α5β1 integrins, which were overexpressed in MDA-MB-231 breast cancer cells.102 In addition, PEG, an FDA-approved compound, has been recognized as an outstanding surface modification agent for a wide range of nanoparticles because of high biocompatibility and biodegradable nature.103 In contrast to previous studies using 5–54 mg of Au for CT detection in mice, this study utilized only 0.5 mg of Au and successfully detected signals in tumors for up to 21 days. Furthermore, the specific accumulation of ligand-targeted NPs in tumors is observed, with minimal presence in other organs compared to the control group.
Nevertheless, as for MRI, their clinical utility is limited by extremely weak signals and reduced contrast in MRI scans, leading to challenges in distinguishing between normal tissues and diseased areas.104,105 Accordingly, Kulhari et al synthesized the manganese oxide nanocuboid system, surface modified with PEG and functionalized with biotin (Biotin-PEG-MNCs).106 In MRI investigations, Biotin-PEG-MNCs exhibited longitudinal and transverse relativity of approximately 0.091 and 7.66 mM−1 s−1, respectively at a 3.0 T MRI scanner. The studies clearly demonstrated the dual T1 and T2 contrast agent capabilities of Biotin-PEG-MNCs, positioning it as a promising nano-platform for diagnosis applications. To further increase precise and specific detection of cancer tissues, Whittaker et al introduced a new hybrid 19F MRI agent for pH-sensitive detection of breast cancer tissues, which is a composite system created by attaching a perfluoropolyether to the surface of manganese-incorporated layered double hydroxide (Mn-LDH@PFPE) nanoparticles.107 Following a 4-hour incubation period in various pH buffer solutions, no 19F NMR signal could be detected at pH 7.4, while the signal was “activated” upon lowering the pH below 6.5. This results from Mn2+ partial dissolution, reducing the paramagnetic relaxation effect. In MDA-MB-468 subcutaneous murine tumor models, after intravenously injected for 24 h, the 19F signal intensity originating from the tumor site represented 92.7% of the total signal from the entire mouse, while weak or absent in essential organs. The pH-activated Mn-LDH@PFPE nanoparticles utilizing paramagnetic relaxation offered novel prospects for highly specific and sensitive cancer detection.
On the basis of more sensitive and precise differentiation between tumors and normal tissues, distinguishing between different pathological types is also clinically necessary. Accordingly, there has been a growing interest in the precise and non-invasive fluorescence-guided imaging technology for analyzing membrane proteins.108 To differentiate HER-2 positive breast cancer cells, Shen et al presented a nano-probe consisting of HER-2 antibody-conjugated NIR emitting MnCuInS/ZnS quantum dots (QDs) encapsulated in bovine serum albumin (BSA), named the MnCuInS/ZnS@BSA-Anti-HER2.109 The nano-probe exhibited low non-specificity and cytotoxicity. After coincubation with SK-BR-3 cells (HER2 overexpression) of 3h, significant and plentiful fluorescence emission signals were observed from the SK-BR-3 cells through confocal microscopy imaging. In contrast, the control group showed no red fluorescence signal. The nano-probe offered a foundation for designing strategies to distinguish between different subtypes of breast cancer.
From the above examples, it can be seen that developing contrast agents with safety and high specificity of ultrasound, CT and MRI is clinically significant in preclinical studies. The corresponding content would be summarized in Table 1. In addition, the application of advanced techniques for the diagnosis of breast cancer will be detailed in Section 5.
 |
Table 1 Applications of Nanoformulations in Breast Cancer Surgery Procedure |
Nanomedicine for Enhancing Intraoperative Tumors and Lymph Nodes Localization
For intraoperative localization of breast tumors, wire localization has been the standard method for preoperative localization in breast imaging for decades. Recently, the available options have greatly expanded to include nonwire devices such as radioactive and magnetic seeds, radar reflectors, and radiofrequency identification tags.131,132 While the current recommended gold standard for intraoperative localization of sentinel lymph nodes (SLN) in clinical practice is the dual tracers combining dye and radionuclide.133 The use of 99mTc radioactive colloid and methylene blue dual tracing technique in SLNB achieved a higher SLN identification rate (>90%) and lower false-negative rate (FNR) (<5–10%), as well as a lower axillary recurrence rate.134 However, it also faces disadvantages such as the risk of allergic reactions, radiation exposure, and insufficient targeting. To address the above-mentioned issues and offer a precise delineation of tumor boundaries and enable an accurate assessment of tumor margins, one can amplify the imaging agent’s visualization signal at the tumor site or increase the probe’s sensitivity to capture signals. Focusing on enhancing tumor imaging signals, nano-radiopharmaceuticals and fluorescence imaging both offer methods for accurate real-time surgical navigation.135,136 In the following, we will proceed to provide specific examples separately.
Involving nano-radiopharmaceuticals, for example, Derya et al developed an innovative nano-radiopharmaceutical, technetium-99m-labeled trastuzumab-decorated methotrexate-loaded human serum albumin nanoparticles, abbreviated as [99mTc]-TRZ-MTX-HAS.137 The cellular binding percentage of [99mTc]-TRZ-MTX-HSA nanoparticles in MCF-7 cells (breast cancer cell lines) varied from 95.21 ± 3.25% at 30 minutes to 97.54 ± 2.16% at 120 minutes, which suggested higher cellular uptake compared with control groups. In addition, [99mTc]-TRZ-MTX-HSA nanoparticles demonstrated a 3-fold higher uptake in MCF-7 and 4T1 breast cancer cell lines compared to healthy cells (p < 0.05). Moreover, to further enhance the targeting ability, antibody conjugation modification is a feasible solution. Seyedeh et al synthesized 99mTc-dendrimer-anti-VEGF (Figure 3Ai), among that VEGF referred to vascular endothelial growth factor overexpressed on the surface of tumors.138 In comparison to the aforementioned examples, this study validated the accumulation of tumors in vivo. After intravenously administration with 37 MBq, the in vivo biodistribution mainly accumulated in the tumor sites and excreted through the hepatobiliary system (Figure 3Aii). The MTT cytotoxicity assay indicated no toxicity in normal cells and demonstrated dose-dependent toxicity in cancerous cells.
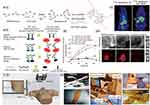 |
Figure 3 Nanomedicine for enhancing intraoperative tumors and lymph nodes localization. (A) 99mTc-Anionic dendrimer targeted vascular endothelial growth factor as a novel nano-radiotracer for in-vivo breast cancer imaging: (i) Schematic illustration of citric acid second generation dendrimer and conjugation with vascular endothelial growth factor antagonist (anti-VEGF); (ii) SPECT images. Arrow showed the accumulation of 99mTc-dendrimer-anti-VEGF in the tumor site. Reproduced from Ebrahimi A et al 99mTc- Anionic dendrimer targeted vascular endothelial growth factor as a novel nano-radiotracer for in-vivo breast cancer imaging. Bioorg Chem. 2022, 128, 106085. Reprinted from Bioorg Chem, volume 128, Ebrahimi A, Pirali Hamedani M, Mohammadzadeh P, et al (99m)Tc-Anionic dendrimer targeted vascular endothelial growth factor as a novel nano-radiotracer for in-vivo breast cancer imaging. 106085, copyright 2022, with permission from Elsevier.138 (B) Image-guided surgery using near-infrared Turn-On fluorescent nanoprobes for precise detection of tumor margins: (i) Scheme illustration of Turn-On probes; (ii) Representative fluorescence intensity. Data represent mean ± SD (n = 3); (iii) Surgical field representative images of the surgery performed under P-GFLG-Cy5 guidance. Dotted circles refers to the surgical field. Adapted from Blau R; Epshtein et al Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics 2018, 8 (13), 3437–3460, http://creativecommons.org/licenses/by/4.0/.139 (C) A fiber optoacoustic guide with augmented reality: (i) Principle of using a fiber optoacoustic guide (FOG) and an AR system to locate the FOG tip and provide the visual guidance on the AR display. The green sphere refers to the breast tumor area. The red and black arrow show the wave emission. (ii) Illustration of using a biopsy clip in breast cancer patient using the system. The FOG tip (red circle) is close to the implanted biopsy clip (green circle). The green arrow shows the FOG tip, and the red arrow shows the biopsy clip. Reproduced from Lan L, Xia Y et al A fiber optoacoustic guide with augmented reality for precision breast-conserving surgery. Light Sci Appl 2018, 7, 2, http://creativecommons.org/licenses/by/4.0/.140 Abbreviations: PEG, polyethylene glycol; DCC, N,N-Dicy-clohexylcarbodiimide; EDC, 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide; NHS, N-Hydroxysuccinimide; DMSO, Dimethyl sulfoxide; DMF, Dimethylformamide; VEGF, vascular endothelial growth factor; HPMA, N-(2-hydroxypropyl)-methacrylamide; PGA, poly-L-glutamic acid; GFLG, Gly-Phe-Leu-Gly; CTSB, cathepsin B; FOG, fiber optoacoustic guide; AR, augmented reality. |
Accurate assessment of margins during surgery can lower re-excision rates, and currently, pathological examination is considered the gold standard for evaluating tumor margins.141 However, it is time-consuming and prone to discrepancies. To visualize tumor margins, Zhang et al explored NIR-II fluorescence imaging guided surgery and synthesized Gd-coated and tetrasulfide bonds doped mesoporous virus-like SiO2 nanoprobe with surface modification of folic acid and encapsulated with indocyanine green, named as VGd@ICG-FA.142,143 The nanoparticles are virus-like morphology facilitated strong adhesion to tumor cells and improved cellular uptake efficiency. Following intravenous injection into tumor-bearing mice, it could be accurately localized at the tumor site, resulting in a higher tumor-to-background ratio (TBR) compared to that of ICG alone (8.26 ± 0.83 vs 1.76 ± 0.06, p < 0.0001). Moreover, the probe effectively assisted in the accurate resection of 4T1-Luc residual and 4T1-bearing tumor models under NIR-II fluorescence imaging, ensuring the complete removal of solid tumors with a volume of at least 1 mm3. Similarly, Yuan et al developed a smart fibronectin-targeting and metalloproteinase-activatable imaging probe CREKA-GK8-QC.144 In vivo experiments showed that NIR-I fluorescence imaging could detect primary and micro-metastatic lesions (approximately 1 mm). It was mentioned that Kaplan-Meier analysis showed that, in comparison to conventional white light surgery, the survival rate increased from 0% to 80% within 80 days after CREKA-GK8-QC guided fluorescence surgery.
Intraoperative fluorescence imaging provides advantages such as strong differentiation and sensitivity, affordability, simplicity, safety, and the ability to observe cells and tissues both in vitro and in vivo.145,146 However, the present obstacle in NIR fluorescence-guided surgery lies in developing agents with exceptional tumor specificity and distinct imaging capabilities. In pursuit of this goal, Ronit et al designed polymeric Turn-ON probes, that was “smart probes”, triggered by the cathepsin enzymatic degradation that overexpressed at the tumor sites, resulting in the production of a fluorescence signal.139 “Smart” probes became active specifically at the tumor location, remaining inert elsewhere in the body. The three Turn-ON probes were HPMA copolymer-GFLG-Cy5 (P-GFLG-Cy5), PGA-Cy5 (PC), and PGA-Cy5-Quencher (PCQ) (Figure 3Bi). Within P-GFLG-Cy5, enzymatic cleavage of the Gly-Phe-Leu-Gly (GFLG) tetrapeptide linker led to the liberation of the dye, while the polymeric structure remained undisturbed. As for the others, the enzymes broke down the polymeric backbone composed of poly-glutamic acid (Figure 3Bii). These processes ultimately facilitated the release of the free dyes, enabling them to emit fluorescence and activated accordingly. As for the in vivo characterization and surgical guidance, the P-GFLG-Cy5 probe showed the highest accumulation after 4 h post-intravenous administration in the breast cancer model (Figure 3Biii). At this stage, the tumor’s position could be identified without the need for invasive procedures.
Despite the above radioactive and light-guided lumpectomy can located the tumor accurately, the approaches had still faced limited qualitative measure and dependent on the depth to some extent. To increase the probe’s sensitivity to capture signals, for example, Cheng et al presented a fiber optic acoustic guiding system (FOG) integrated with augmented reality (AR) to precisely locate tumors at sub-millimeter scales and provide intuitive surgical navigation with minimal disruption (Figure 3Ci).140 The FOG was surgically implanted into the tumor prior to the procedure. When exposed to external pulsed light, the FOG emitted acoustic waves in all directions due to the optoacoustic effect facilitated by a specifically engineered nano-composite layer at its tip. An AR setup utilizing a tablet gauges the ultrasound sensors’ coordinates and converted the position of the FOG tip into visual guidance with precision exceeding 1 millimeter (Figure 3Cii). This assisted surgeons in directly observing the tumor’s location and conducting swift and precise tumor resections, leading to a notable decrease in reoperation rates and shortened surgical durations.
Nanomedicine for Inducing Postoperative Wound Healing
Considering both tumor staging and patient preference, breast mastectomy and/or breast reconstruction involves preserving the breast skin, removing the subcutaneous gland and fat, and placing an implant under the skin to achieve a visually and tactilely symmetrical appearance, still holding an important position.147 However, significant surgical trauma would result in persistent wounds failing to heal posed by infection, and the fibrous layer encasing the implants constricts, leading to discomfort and swelling.148 In recent times, nanomedicine have gained extensive utilization in wound recovery attributed to their remarkable abilities in absorption, drug incorporation, and antimicrobial functions.149 Presently, novel nanomedicine dressings such as hydrogels, nanofibers, and films are seeing widespread application. Various influencing factors and treatment strategies have been previously addressed in published reviews,150 that can be categorized into hemostasis, antimicrobial inhibition, inflammation, etc. Next, we will enumerate representative examples to support the role of nanomedicine in promoting wound healing in breast cancer surgery procedure.
After breast cancer surgery, the significant skin deficiency, combined with the microenvironment surrounding residual tumors, creates an environment conducive to bacterial colonization, leading to complications associated with infections.151 Additionally, vascular damage hinders drug transportation to the remaining microtumors at the surgery site. Consequently, this disrupts the incision recovery of postoperative breast cancer. To exert antimicrobial activity, Chandrakant K. et al synthesized ZnO nanoparticles with biotemplate of Locust Bean Gum (LBG), a galactomannan polysaccharide.152 Exposing bacterial cells to ZnO nanoparticle results in the disruption of the cell membrane, proteins, and genome, ultimately leading to cell demise.153 Its wide-ranging antibacterial efficacy is primarily credited to its capacity to stimulate the production of surplus reactive oxygen species (ROS). The study showed that substantial cell mortality at concentrations of 500 μg/mL of nanoparticles in breast cancer cell lines. Wound healing assays revealed that the nanoparticles effectively impeded cell migration at concentrations as low as 62.5 μg/mL in breast cancer cell lines. Furthermore, continued refinement of the nanomedicine characteristics and introduction of biomimetic nanomaterials will position it as a compelling contender in the realm of nanobiotechnology and bioengineering. Accordingly, Yao et al fabricated a nano-sensor system, Van-ICG@PLT, consisting of the innate tropism of platelets (PLT) membrane to delivery vancomycin (Van) and indocyanine green (ICG) (Figure 4Ai).154 This study capitalized on the potential of phototherapy and antibiotic treatment to address the dual challenge of bacterial infection. The Van-ICG@PLT nano-sensor was drawn to surgical wounds through a mechanism that mimics the role of platelets as immediate responders to vascular damage.155 Upon exposure to laser light, the membrane permeability would increase, and bacterial sensitivity to Van would be facilitated (Figure 4Aii). Moreover, it had been observed to notably increase ROS generation, effectively impeding the growth of Staphylococcus aureus (S. aureus). In the S. aureus subcutaneously abscess model, the combination of Van-ICG@PLT with laser treatment resulted in the eradication of nearly 100% of the bacteria while the Van group (55.1%) and the ICG + laser group (59.5%) (Figure 4Aiii and Aiv). Above all, the nano-sensor showed high safety and great potential in managing postoperative wound infection.
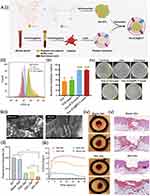 |
Figure 4 Nanomedicine for inducing postoperative wound healing. (A) Platelet-mimetic nano-sensor for combating postoperative recurrence and wound infection of TNBC: (i) Scheme illustration of Van-ICG@PLT; (ii) Evaluation of 4T1 intracellular ROS generation; (iii) Bacterial inhibition rate. Data represent mean ± SD (n = 5), $$p < 0.01 vs Van, ##p < 0.01 vs ICG + laser; (iv) Photographs of various treatments. Reproduced from Liu Y, Qi Y et al. Platelet-mimetic nano-sensor for combating postoperative recurrence and wound infection of triple-negative breast cancer. J Control Release 2023, 362, 396–408. Reprinted from J Control Release, volume 362, Liu Y, Qi Y, Chen C, et al. Platelet-mimetic nano-sensor for combating postoperative recurrence and wound infection of triple-negative breast cancer. 396–408, copyright 2023, with permission from Elsevier.154 (B) Nano oxygen chamber by cascade reaction for hypoxia mitigation and ROS scavenging in wound healing: (i) SEM images. Scale bar: 300 μm; (ii) Quantitative assessment of SOD. Data represent mean ± SD (n = 3), *: p < 0.05, **: p < 0.01; (iii) Intracellular hypoxia levels; (iv) Digital photos and (v) H&E imaging of wounds. Yellow arrow: newly generated epidermis; Green arrow: granulation tissue; Black arrow: newly born glands. Reproduced from Han X, Ju L; Saengow, C et al. Irudayaraj, J Nano oxygen chamber by cascade reaction for hypoxia mitigation and reactive oxygen species scavenging in wound healing. Bioact Mater. 2024, 35, 67–81, http://creativecommons.org/licenses/by/4.0/.156 Abbreviations: Van, vancomycin; ICG, indocyanine green; PLT, platelets; SOS, superoxide scavenging; OGC, oxygen-generating catalyst. |
In the array of factors leading to insufficient wound healing, excessive inflammation and hypoxia also emerge as central contributing factors.157,158 An increased level of ROS in wounds with inadequate healing would trigger oxidative pressure and worsen the inflammatory reaction, thus maintaining macrophages in the M1 state and significantly upregulated pro-inflammatory factors.159 Accordingly, Joseph et al designed a cascade enzymatic reaction-based oxygen-delivering nano chamber, embedded within an alginate hydrogel, called Mix-Gel (Figure 4Bi).156 The nano chamber consisted of two types of oxygen bubbles: one for scavenging superoxide (SOS) and another for generating oxygen (OGC). These bubbles were enclosed within a glycosylated protein complex composed of dextran-conjugated superoxide dismutase (SOD) and catalase (CAT) (Figure 4Bii). Mechanically, the sequential enzymatic mechanism propelled by SOS prompting the transformation of superoxide anions into hydrogen peroxide, subsequently efficiently converted into oxygen and water by catalase within OGC (Figure 4Biii). In the rat model of full-thickness wounds, the nano oxygen chamber demonstrated a remarkable ability to accelerate wound closure without resulting in scar formation (Figure 4Biv). As for the in vivo wound healing efficacy, they utilized day 3 post-surgery porcine full-thickness wound models, and the Mix-Gel significantly increased wound closure rates compared to the OGC-Gel and Blank-Gel groups (Figure 4Bv).
In order to achieve better adipose tissue filling and reduce chronic inflammation caused by fillers and to promote wound healing and recovery, Morimoto et al reported that poly-L-lactic acid (PLLA) mesh implants including collagen sponge (CS) were substituted with in vivo autologous adipose tissue regeneration.160 The results showed that the PLLA implants experienced internal space collapse after 6 months and the higher regeneration, while the remaining after 12 months. However, preclinical trials still faced many obstacles transforming into clinical trials. It was worth mentioning that a retrospective clinical trial compared the utilization of round nano-surface Ergonomix in capsular contracture rate (0.9%), with better performance of wound healing and patient satisfaction.161 In conclusion, promoting incision healing and reducing scarring are crucial for restoring the prognosis and improving the appearance.
Nanomedicine for Preventing from Postoperative Recurrence
Surgery incompletely eradication leaving behind residual microtumors, leading circulating tumor cells (CTCs) counts increased postoperatively, which often result in recurrence and metastasis. 162 According to clinical guidelines, following breast-conserving surgery (BCS), patients typically undergo radiotherapy to reduce the risk of local recurrences and improve long-term survival outcomes. Nevertheless, the ionizing radiation administered increases the likelihood of mortality from radiation-induced cardiac disease and secondary malignancies.163 Recently, in 2020, CTC testing was included in the CSCO guidelines that post-treatment CTCs could predict the prognosis of patients, especially suffering recurrence and metastasis patients after the completion of radical breast cancer surgery.164,165 Due to the low content of CTC, it is necessary to develop a technology that combines enrichment and separation technology with high sensitivity and specificity detection.166 It is worth mentioning that the TUMORFISHER method had sufficient clinical validation data and had been incorporated into clinical expert consensus, which could be used to predict the efficacy of PD-L1 drugs and effectively monitor the efficacy after medication.110,167 However, the current CTC testing technology is still expensive, and the search for new more sensitive and cheaper technology platforms is still needed in the clinic.
Currently, to address the above problems, the implantation of hydrogel systems following resection has demonstrated efficacy as a promising therapeutic approach with significant clinical promise. Injectable hydrogels exhibit significant promise in drug delivery owing to their consistent three-dimensional (3D) porous architecture and remarkable capacity for encapsulation upon formation in situ.111,112 Also, photothermal tumor ablation is a burgeoning treatment approach leveraging the heat produced by specific nanoparticles when exposed to NIR light, aiming to eliminate cancer cells without causing radiation-related harm and with exceptional specificity towards tumors.168,169 Finally, CTCs based strategy combined with nanomedicine could provide robust and high-throughput platforms for CTCs isolation and analysis, which facilitate the development of personalized medicine approaches. In the following, we would discuss specific instances of strategy applications.
Injectable Gels Strategy
To eradicate residual tumor cells, utilizing the gel–sol transition characteristics with the temperature/magnetic responsiveness of hydrogels, Zhang et al designed a shear-thinning injectable magnetic supramolecular hydrogel (MSH) self-assembled using PEGylated iron oxide (Fe3O4) nanoparticles and a-cyclodextrin (a-CD) through complex formation loaded with paclitaxel (PTX) and doxorubicin (DOX) to prevent post-operative recurrence.170 Mechanically, MSH was uniformly administered into the postoperative wound, rendering it flexible and seamlessly conforming to irregular cavities without any inaccessible areas. Then exposed to the alternating current magnetic field (ACMF), the MSH exhibited gel–sol transition and sustained-released dual drugs with local precise. Apart from lingering tumor cells, the immunosuppressive tumor microenvironment (TME) following surgery stands out as another significant driver of tumor recurrence and metastasis.171 Accordingly, Ye et al devised an injectable hydrogel system for localized drug administration, aiming to bolster immune response while thwarting tumor relapse.113 They introduced poly-(sulfobetaine methacrylate) (PSBMA) hydrogels to load DOX, copper peroxide nanoparticles (CuO2) coated with macrophage membranes and 2′,3′-cGAMP, that was the stimulator of interferon genes (STING) agonist, named Gel@M/CuO2/DOX/STING (Figure 5Ai and Aii). Among that, the 2′,3′-cGAMP initially diffused out of the hydrogel to trigger the STING pathway, then the dendritic cells developed, presented tumor antigens to T cells, and enhanced tumor-specific CD8+ T cell responses, which this process reshaped the TME (Figure 5Aiii and Aiv). In addition, the CuO2 reacted to the TME acidic conditions, initiating the generation of hydroxyl radicals (˙OH), thereby facilitating self-sustained hydrogen peroxide (H2O2) production for chemodynamic therapy (CDT), that was introduced tumor cell death with ˙OH.114 In the subcutaneous tumor recurrence model of 4T1 breast cancer, the incidence of local tumor recurrence markedly declined following Gel@M/CuO2/DOX/STING therapy, with a complete suppression rate of 100% observed in residual 4T1 tumors (Figure 5Av and Avi). Accordingly, the Tregs in 4T1 breast cancer decreased from 19.9% to 8.8% treated with Gel@M/CuO2/DOX/STING. However, regarding temporal regulation, prolonged antigen exposure and immune memory effects were considered more efficacious for preventing tumor relapse. To address the problem, Chen et al introduced a single-dose injectable nano-in-gel vaccine (NIGel-Vax) for postoperative breast cancer treatment, which consisted with protein antigens and polyethylenimine-benzoimidazole-4-carboxylic acid-2-isothiocyanatoethyl-α-D-mannopyranoside (PEI-4BImi-Man) adjuvant, then encapsulated within hydrogel (Figure 5Bi).172 Following subcutaneous injection administration, the NIGel-Vax was released slowly and migrated to the lymph nodes, thus eliciting immune responses specific to the antigens (Figure 5Bii). The flow cytometry analysis exhibited Cy5-labeled dendritic cells and macrophages kept an increase by day 21, and the draining lymph nodes had higher fluorescence intensity at day 21 then at day 14 (Figure 5Biii). Utilizing the tumor-associated antigen trophoblast cell-surface antigen 2 (TROP2) protein as the immunogen, NIGel-Vax attained a 96% inhibition rate of tumor growth and a 50% remission rate in models of TNBC (Figure 5Biv).
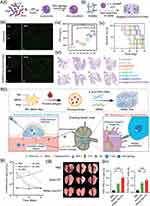 |
Figure 5 Nanomedicine for preventing from postoperative recurrence through injectable gels strategy. (A) Injectable hydrogel with tumor remodeling and antitumor recurrence: (i) and (ii) Scheme illustration of Gel@M/CuO2/DOX/STING; (iii) Immunofluorescence staining image. Scale bars represent 100 μm; (iv) Level of IFN-γ; Data are presented as mean ± SD (n = 5): *p < 0.05; **p < 0.01; ***p < 0.001. (v) Survival curves; (vi) H&E staining of lung metastatic nodules. Among that, (1) - (8) represent the following meanings: untreated, blank gel, Gel@STING, Gel@DOX, Gel@CuO2, Gel@CuO2/DOX, Gel@M/CuO2/DOX and Gel@M/CuO2/DOX/STING. Reprinted (adapted) with permission fromFang YL, Huang SS, et al. Injectable Zwitterionic Physical Hydrogel with Enhanced Chemodynamic Therapy and Tumor Microenvironment Remodeling Properties for Synergistic Anticancer Therapy. Acs Nano 2023, 17 (24), 24,883–24,900. Copyright 2023 American Chemical Society.113 (B) Injectable nano-in-gel vaccine for breast cancer postsurgical therapy: (i) Schematic illustration of NIGel-Vax and action mechanism; (ii) Quantitative analysis of fluorescence intensity in draining lymph nodes at day 7, 14 and 21; (iii) The number of visible metastatic lung lesions; (iv) The proportion of CD4+ IFN-γ+ and CD8+ IFN-γ+ T cells in the peripheral blood. Results are presented as means ± SD (n = 4): *P < 0.05; ***P < 0.001; ****P < 0.0001. Reprinted(adapted) with permission from Liu T, Si X et al. Injectable Nano-in-Gel Vaccine for Spatial and Temporal Control of Vaccine Kinetics and Breast Cancer Postsurgical Therapy. ACS Nano 2024, 18 (4), 3087–3100. Copyright 2024 American Chemical Society.172 Abbreviations: PSBMA, poly-(sulfobetaine methacrylate); DOX, doxorubicin; CuO2, copper peroxide nanoparticles; ODEX, oxidized dextran. |
In summary, hydrogels are highly deformable, capable of high drug loading, and biocompatible materials that can perfectly conform to the shape of an incision, maximally inhibiting tumor growth. Moreover, when combined with chemotherapy drugs and immune adjuvants, they can enhance their therapeutic effects, offering significant potential for development and utilization.
Photothermal Assisted Strategy
Photothermal therapy (PTT) damages tumor cells by increasing the local temperature as the photosensitizer absorbs energy and transitions to an excited state upon exposure to specific wavelengths of light. The excited photosensitizer then converts the light energy into heat, affecting surrounding molecules and inducing tumor ablation.115 The elevated temperature also affects the permeability of biofilms.116 Significant research has focused on developing nanoplatforms for imaging-guided PTT, with various photothermal nanomaterials being developed and functionalized for enhanced imaging properties, enabling treatment visualization.117 Metallic nanoparticles and carbon nanotubes, designed to absorb electromagnetic radiation in the NIR or infrared (IR) spectrum, create localized heating that can lead to temperatures up to 113 °F. When these nanoparticles are targeted to cancerous regions and irradiated, they induce cell death through necrosis.118 For example, chitosan modified molybdenum selenide (MoSe2) had been proved to have excellent photothermal conductivity under NIR irradiation and prevent the aggregation of nanoparticles.119
To prove the feasibility of PTT as the adjuvant approach followed BCS, Lu et al utilized PEGylated gold nanobipyramids (GBP-PEG) postoperation to evaluate local recurrence rates in orthotopic mouse models of breast cancer (Figure 6Ai).120 The studies demonstrated that the acquired GBP-PEG exhibited greater efficiency in converting light into heat compared to nanorods, which the tumor temperature rose by approximately 10°C lowering the likelihood of dermal injuries surrounding the surgical cut (Figure 6Aii). The Kaplan–Meier curves revealed that by day 18 after treatment, the occurrence of local recurrences was 60% in the surgical cohort, while 10% in the surgery combined with PTT cohort (Figure 6Aiii–Av).
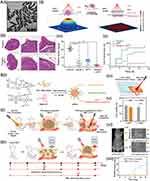 |
Figure 6 Nanomedicine for preventing from postoperative recurrence through photothermal assisted strategy. (A) Adjuvant photothermal therapy inhibits local recurrences after breast-conserving surgery: (i) TEM images. Scale bars represent 100 nm; (ii) Illustration scheme of laser energy distribution; (iii) H&E staining; (iv) Survival curves and (v) H&E staining of lung metastatic nodules with various treatments. P values <0.001 are denoted with three asterisks. Reprinted (adapted) with permission from Wang S, Ma X et al. Adjuvant Photothermal Therapy Inhibits Local Recurrences after Breast-Conserving Surgery with Little Skin Damage. ACS Nano 2018, 12 (1), 662–670. Copyright 2018 American Chemical Society.120 (B) Afterglow/photothermal bifunctional polymeric nanoparticle for early recurrence theranostic: (i)-(iii) Schematic illustration of PPV-PEG-cRGD; (iv)-(v) Scheme illustration of in vitro deep tissue phototherapeutic ability and cell ability; (vi) Afterglow imaging and bioluminescence of recurrence tumors and scars distinction. Red dashed circle: tumor. White dashed circle: scar; (vii) Relative tumor signals. Results are presented as means ± SD: *** < 0.001. Reprinted (adapted) with permission from Qu R, He, D et al. Afterglow/Photothermal Bifunctional Polymeric Nanoparticles for Precise Postbreast-Conserving Surgery Adjuvant Therapy and Early Recurrence Theranostic. Nano Lett 2023, 23 (10), 4216–4225, Copyright 2023 American Chemical Society.122 Abbreviations: cPTT, conventional photothermal therapy; aPTT, adjuvant photothermal therapy; BCS, breast-conserving surgery; APPN, afterglow/photothermal bifunctional polymeric nanoparticle; cRGD, cyclic arginine-glycine-aspartate; PEG, peptide-conjugated poly(ethylene glycol); MRI, magnetic resonance imaging; NIR, near-infrared. |
Besides the metal nanomaterials, semiconducting polymer nanoparticles (SPNs) have been confirmed to exhibit outstanding NIR-II photothermal capabilities and afterglow luminescence, making them valuable for NIR-II photoacoustic imaging and PTT.121 For instance, Zhen et al presented an afterglow/photothermal bifunctional polymeric nanoparticle (APPN) utilized for in vivo PTT and early recurrence theranostics in orthotopic breast cancer mouse models.122 The APPN comprised a targeted component (PPV-PEG-cRGD), incorporating a NIR dye serving as an initiator (NCBS) and a NIR-II light-absorbing semiconducting polymer acting as a photothermal converter (PBBTOT) (Figure 6Bi–Biii). The therapeutic studies showed that following 10 minutes of 1064 nm laser exposure, the temperature elevation of the APPN solution reached 22.9°C with a tissue depth of 5 mm, which sufficient to heat ablation of tumor cells (Figure 6Biv). Following a 15-day treatment period, the occurrence of local recurrence was 100% in both the BCS group and the PTT group, whereas in the BCS + PTT treatment group, it was 0% (Figure 6Bv). In addition, it was surprising that clear afterglow signals were observed in the breast area on day 6 following BCS, with the signal-to-background ratio (SBR) reaching 41.0, which was 5.0 times higher than that on day 0 after BCS (Figure 6Bvii). The afterglow signals from the APPN aligned closely with the bioluminescence signals of 4T1 tumor cells, demonstrating the effective identification of localized tumor recurrence on day 6 post-BCS (Figure 6Bvii).
In summary, PTT can serve as an alternative to postoperative radiotherapy. When combined with suitable nanomaterials, it can circumvent the drawbacks of local thermal damage. Additionally, when integrated with real-time imaging modalities such as ultrasound or MRI,123 it enables the continuous monitoring of residual tumors, offering significant potential for clinical translation.
Circulating Tumor Cells Based Strategy
Sites of early metastasis, along with shrinking tumors, are extremely small, measuring only a few micrometers and cannot be seen with current medical imaging technology.124 Due to these constraints, scientists have redirected their focus to the initial phase of metastasis and are concentrating on identifying CTCs directly.125 For example, G. Brolo et al presented a CTC identification method through direct visualization labeled metallic nanoshells (Au-Thy nanoshells).126 Cells labeled with antibodies and Au-Thy nanoshells displayed heightened brightness at lower levels of magnification. A clear differentiation was made between the positive and negative cells with a small quantity of nanoshells attaching to the negative cells (less than 6), while multiple nanoshells (more than 25) labeled the positive cells. This facilitated rapid sample screening and enabled the effortless visual identification of individual CTCs. However, the restrictions and difficulties associated with capturing CTCs have significantly decreased the likelihood of targeted elimination of CTCs in the blood of patients according to treatment protocols. Accordingly, Abdolahad et al chose to disable the intrusive capability of CTCs, while they moved through the blood vessels by subjecting the entire blood sample to a form of stimulation involving a uniform positive electrostatic charge stimulation (PPECS).127 The primary mechanism of this chip could involve the alteration of cellular surface charge, leading to an uneven distribution of ions within cancer cells and disruption the ion channels. The study restrained the spread of cancer to the lungs and prolonged the lifespan of the mice (up to 40 days) that had received intravenous by electrostatically deactivated 4T1 breast cancer CTCs. Investigating the impact of PPECS on blood components indicated that there were no significant alterations in the functional characteristics of white blood cells. Only a minimal portion (approximately 10%) was affected during this procedure. Overall, the detection of CTCs levels and the development of stratified treatment strategies based on postoperative breast cancer patients are crucial for improving their disease-free survival (DFS).128 It is an important field where nanomaterials can have tremendous potential and deserve close attention.
Clinical Research Status of Breast Cancer Surgery
Currently, evolved in breast cancer surgery, accurately assessing the axillary status after neoadjuvant chemotherapy (NAC) and exploring the feasibility and oncological safety of avoiding axillary lymph node dissection (ALND) is an important issue.129 Targeted axillary dissection (TAD) refers to the placement of metal markers in lymph nodes confirmed as metastatic foci through ultrasound-guided core needle biopsy prior to NAC.130 A clinical trials demonstrated the false-negative rate (FNR) of SLNB using single or dual tracing methods was over 10%, whereas the FNR using the TAD method was further reduced to 1.4%.173 A recent clinical study published in the New England Journal of Medicine further confirmed that for breast cancer patients with clinically negative axillary lymph nodes and 1 or 2 positive sentinel lymph nodes, ALND could be omitted after breast-conserving surgery (BCS) or mastectomy.174 The 5-year recurrence-free survival rate was sentinel-node biopsy only (89.7%) vs completion ALND (88.7%).
In addition, contralateral prophylactic mastectomy (CPM) is also a hotly debated issues in recent clinical discussions, but guidelines lack clear implement recommendations.175 Germline mutations in the BRCA gene are one of the major risk factors. It was mentioning that Xie et al had developed and validated the first risk prediction model, called BRCA-CRisk.176 BRCA-CRisk could accurately predict the 10-year absolute cumulative risk (ROC, 0.702) of contralateral breast cancer in BRCA mutation carriers and serves as an important reference tool for clinicians and patients in making decisions regarding the need for CPM. Additionally, clinical studies involving the surgical diagnosis and treatment processes of breast cancer have been summarized in Table 2.
 |
Table 2 Clinical Trials Evolved in Breast Cancer Surgery Procedure |
Summary and Prospect
Both preclinical and clinical research indicated that cancer nanomedicines could enable earlier diagnosis, stimulated the immune system against tumors, disrupted the TME support for neoplastic cells, and fulfilled other roles that may be even more effective or applicable than traditional drug carriers.177 These expanded functionalities are likely to revolutionize and expand the application of cancer nanomedicines in clinical settings. Yet, cancer nanomedicine research is evolving into a more genuinely interdisciplinary field, integrating tools and advancements from various other disciplines. In the upcoming sections, we will explore future directions in cancer diagnosis and therapeutic applications.
Artificial Intelligence (AI)
The initial advancements in AI for breast cancer detection set the stage for broader applications in diagnostic tasks like tumor classification and cancer detection.178 Over the past decade, AI-based diagnostic tools have been continually refined, demonstrating diagnostic performance in many cases that matches or exceeds that of human experts across various cancer types. This success has prompted the evaluation of AI approaches for more intricate decision-making roles.179 For instance, by employing AI techniques in breast imaging, quantitative information regarding tumor size, shape, morphology, texture, and kinetics can be derived in early screening and diagnosis.180–183 Recently, the breast cancer mammography screening AI system exceeded the performance of all five full-time breast-imaging specialists, with an average sensitivity enhancement of 14%.184 Additionally, Ji et al designed a completely digital platform that merged label-free stimulated Raman scattering (SRS) microscopy with weakly supervised learning. This approach allowed for swift and automated cancer diagnosis on unlabeled breast core needle biopsies (CNB) reaching 95% diagnosis accuracy.185 The deep-learning based algorithms created a clarity and visual representation to perform benign/malignant area segmentation in histologically heterogeneous breast CNB.186
Machine learning techniques applied to genomic, transcriptomic, and other data modalities hold promise for forecasting prognosis and patient survival.187–189 For example, the most prevalent method for survival prediction is the Cox proportional hazards regression model (Cox-PH), which is a multivariate linear regression model that identifies correlations between survival time and predictor variables.190 In addition, a deep learning model based on 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG-PET-CT) imaging signals can predict the EGFR mutation status of patients with non-small cell lung cancer, thereby enabling the selection of more precise treatment strategies, which is of paramount importance for clinical decision-making.191 This strategy evaluates the potential risks and clinical implications of numerous nanoparticles to facilitate their practical application, enabling the tailoring of patient-specific treatment regimes.
Three-Dimensional Bioprinting (3D Bioprinting)
3D printing is a method of additive manufacturing where precursor materials are deposited layer by layer to create intricate 3D shapes based on computer-aided designs.192 The advancement of medical 3D printing has been rapid, catering to diverse medical requirements and showcasing a broad spectrum of applications. These include the fabrication of patient-specific implants, prosthetics, surgical instruments, drug delivery systems, organ models, and bioprinting of living cells and biomolecules, which have been reviewed in detail.193,194 Its functionality has evolved from solely addressing repair needs to encompassing disease treatment and tissue regeneration.195
The development of 3D printing technology also enables more realistic simulation of changes under physiological conditions in vitro.196 Utilizing 3D printing technology, Mak et al presented a novel thick collagen network that closely resembled the structure of the extracellular matrix (ECM) in vivo, particularly mirroring the characteristics of human skin scars.197 The elongated and undulating nature of these thickened collagen bundles contributed to their overall flexibility. This collagen structure imposed fewer physical barriers and facilitated the spread of tumor cells. On the basis of the 3D-printed scaffold system, the incorporation of continuous drug release can exert a postoperative local anti-inflammatory and anti-metastatic effect on the wound.198,199 Accordingly, Song et al reported a 3D-printed scaffold for a local drug delivery platform termed CCNACA, incorporating carbon dots-curcumin nano-drug release (CCNPs) enabled visualization of drug release.200 To sum up, CCNACA structures demonstrated robust mechanical integrity and hold promise as skin alternatives for repairing damaged skin with the elastic moduli of 42.51 Kpa located in the skin tissue range.201 The in vitro drug release assessment over a 14-day period revealed that CCNACA scaffolds achieved a 73.77 ± 1.68% inhibition rate in breast cancer cells (MCF-7). Consequently, advancing 3D printing technologies and the development of printable biomaterials should be a focal point of future endeavors, as they are pivotal for achieving substantial advancements in cancer treatment.
Conclusion
We focus on the application of nanomaterials in breast cancer surgery from a clinical perspective. From practical clinical practice needs to expand to the function of nanomaterials, we aim to provide research direction for the subsequent development of nanomaterials. With limitless possibilities and a promising future, this area warrants further investigation. As technology continues to advance, it is hoped that significant milestones will be reached in the treatment of cancer.
Acknowledgments
All original images in this article were created through www.biorender.com, for which we would like to express our gratitude. Graphical abstract is created in BioRender. Xiangyue, M. (2025) https://BioRender.com/u57d024.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (32371539), the Key Research and Development Program of Sichuan Province Grant (2023YFG0125), Clinical Research Program for West China Hospital, Sichuan University (2022HXFH021).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
3. Coughlin SS. Epidemiology of Breast Cancer in Women. Adv Exp Med Biol. 2019;1152:9–29. doi:10.1007/978-3-030-20301-6_2
4. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast cancer. Lancet. 2021;397(10286):1750–1769. doi:10.1016/s0140-6736(20)32381-3
5. Wyld L, Audisio RA, Poston GJ. The evolution of cancer surgery and future perspectives. Nat Rev Clin Oncol. 2015;12(2):115–124. doi:10.1038/nrclinonc.2014.191
6. Osborne MP. William Stewart Halsted: his life and contributions to surgery. Lancet Oncol. 2007;8(3):256–265. doi:10.1016/s1470-2045(07)70076-1
7. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575–580. doi:10.1136/bmj.301.6752.575
8. Lu S, Yang J, Wei T, et al. Single-incision endoscope-assisted breast-conserving surgery and sentinel lymph node biopsy: prospective SINA-BCS cohort study. Br J Surg. 2023;110(9):1076–1079. doi:10.1093/bjs/znad059
9. Toesca A, Park HS, Ryu JM, et al. Robot-assisted mastectomy: next major advance in breast cancer surgery. Br J Surg. 2023;110(4):502–503. doi:10.1093/bjs/znad006
10. Lai H-W, Toesca A, Sarfati B, et al. Consensus Statement on Robotic Mastectomy—Expert Panel From International Endoscopic and Robotic Breast Surgery Symposium (IERBS) 2019. Ann Surg. 2020;271(6):1005–1012. doi:10.1097/sla.0000000000003789
11. Angelos P. Ethics and surgical innovation: challenges to the professionalism of surgeons. Int J Surg. 2013;11 Suppl 1:S2–5. doi:10.1016/s1743-9191(13)60003-5
12. Woods RW, Camp MS, Durr NJ, Harvey SC. A Review of Options for Localization of Axillary Lymph Nodes in the Treatment of Invasive Breast Cancer. Acad Radiol. 2019;26(6):805–819. doi:10.1016/j.acra.2018.07.002
13. Ahmed M, Purushotham AD, Douek M. Novel techniques for sentinel lymph node biopsy in breast cancer: a systematic review. Lancet Oncol. 2014;15(8):e351–362. doi:10.1016/s1470-2045(13)70590-4
14. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10(9):507–518. doi:10.1038/nrclinonc.2013.123
15. Walton L, Ommen K, Audisio RA. Breast reconstruction in elderly women breast cancer: a review. Cancer Treat Rev. 2011;37(5):353–357. doi:10.1016/j.ctrv.2011.02.001
16. Cordeiro PG. Breast reconstruction after surgery for breast cancer. N Engl J Med. 2008;359(15):1590–1601. doi:10.1056/NEJMct0802899
17. Fan D, Cao Y, Cao M, Wang Y, Cao Y, Gong T. Nanomedicine in cancer therapy. Signal Transduct Target Ther. 2023;8(1):293. doi:10.1038/s41392-023-01536-y
18. Kim BY, Rutka JT, Chan WC. Nanomedicine. N Engl J Med. 2010;363(25):2434–2443. doi:10.1056/NEJMra0912273
19. Youn YS, Bae YH. Perspectives on the past, present, and future of cancer nanomedicine. Adv Drug Deliv Rev. 2018;130:3–11. doi:10.1016/j.addr.2018.05.008
20. Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi:10.1016/j.addr.2013.11.009
21. Lammers T. Nanomedicine Tumor Targeting. Adv Mater. 2024;36(26):e2312169. doi:10.1002/adma.202312169
22. Siddique S, Chow JCL. Recent Advances in Functionalized Nanoparticles in Cancer Theranostics. Nanomaterials. 2022;12(16). doi:10.3390/nano12162826
23. Siddique S, Chow JCL. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials. 2020;10(9). doi:10.3390/nano10091700
24. Hu Y, Mignani S, Majoral JP, Shen M, Shi X. Construction of iron oxide nanoparticle-based hybrid platforms for tumor imaging and therapy. Chem Soc Rev. 2018;47(5):1874–1900. doi:10.1039/c7cs00657h
25. Chow JCL. Chapter 13 - Magnetic nanoparticles in magnetic resonance imaging: principles and applications. In: Wu K, Wang J-P, editors. Magnetic Nanoparticles in Nanomedicine. Woodhead Publishing; 2024:371–399.
26. Liu S, Yu JM, Gan YC, et al. Biomimetic natural biomaterials for tissue engineering and regenerative medicine: new biosynthesis methods, recent advances, and emerging applications. Mil Med Res. 2023;10(1):16. doi:10.1186/s40779-023-00448-w
27. Qi C, Yan X, Huang C, Melerzanov A, Du Y. Biomaterials as carrier, barrier and reactor for cell-based regenerative medicine. Protein Cell. 2015;6(9):638–653. doi:10.1007/s13238-015-0179-8
28. Whitaker R, Hernaez-Estrada B, Hernandez RM, Santos-Vizcaino E, Spiller KL. Immunomodulatory Biomaterials for Tissue Repair. Chem Rev. 2021;121(18):11305–11335. doi:10.1021/acs.chemrev.0c00895
29. Montazerian H, Davoodi E, Baidya A, et al. Engineered Hemostatic Biomaterials for Sealing Wounds. Chem Rev. 2022;122(15):12864–12903. doi:10.1021/acs.chemrev.1c01015
30. Zheng M, Wang X, Chen Y, et al. A Review of Recent Progress on Collagen-Based Biomaterials. Adv Healthc Mater. 2023;12(16):e2202042. doi:10.1002/adhm.202202042
31. Yang Z, Chen L, Liu J, et al. Short Peptide Nanofiber Biomaterials Ameliorate Local Hemostatic Capacity of Surgical Materials and Intraoperative Hemostatic Applications in Clinics. Adv Mater. 2023;35(39):e2301849. doi:10.1002/adma.202301849
32. Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clin Oncol. 2009;6(12):718–730. doi:10.1038/nrclinonc.2009.166
33. McGhee DE, Steele JR. Breast Biomechanics: what Do We Really Know? Physiology. 2020;35(2):144–156. doi:10.1152/physiol.00024.2019
34. Aronson KJ, Miller AB, Woolcott CG, et al. Breast adipose tissue concentrations of polychlorinated biphenyls and other organochlorines and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9(1):55–63.
35. Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50(1):33. doi:10.1186/s40659-017-0140-9
36. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
37. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21(3):309–322. doi:10.1016/j.ccr.2012.02.022
38. Bahcecioglu G, Basara G, Ellis BW, Ren X, Zorlutuna P. Breast cancer models: engineering the tumor microenvironment. Acta Biomater. 2020;106:1–21. doi:10.1016/j.actbio.2020.02.006
39. Farach-Carson MC, Warren CR, Harrington DA, Carson DD. Border patrol: insights into the unique role of perlecan/heparan sulfate proteoglycan 2 at cell and tissue borders. Matrix Biol. 2014;34:64–79. doi:10.1016/j.matbio.2013.08.004
40. Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392.
41. Pietersz GA, Wang X, Yap ML, Lim B, Peter K. Therapeutic targeting in nanomedicine: the future lies in recombinant antibodies. Nanomedicine. 2017;12(15):1873–1889. doi:10.2217/nnm-2017-0043
42. Xu X, Ho W, Zhang X, Bertrand N, Farokhzad O. Cancer nanomedicine: from targeted delivery to combination therapy. Trends Mol Med. 2015;21(4):223–232. doi:10.1016/j.molmed.2015.01.001
43. Choi HS, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25(10):1165–1170. doi:10.1038/nbt1340
44. Noguchi Y, Wu J, Duncan R, et al. Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues. Jpn J Cancer Res. 1998;89(3):307–314. doi:10.1111/j.1349-7006.1998.tb00563.x
45. Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi:10.1038/nrd2614
46. Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles, pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–187. doi:10.1016/j.jconrel.2011.09.063
47. Yu MK, Park J, Jon S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics. 2012;2(1):3–44. doi:10.7150/thno.3463
48. Byrne JD, Betancourt T, Brannon-Peppas L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv Drug Deliv Rev. 2008;60(15):1615–1626. doi:10.1016/j.addr.2008.08.005
49. Jiang Z, Guan J, Qian J, Zhan C. Peptide ligand-mediated targeted drug delivery of nanomedicines. Biomater Sci. 2019;7(2):461–471. doi:10.1039/c8bm01340c
50. Dhritlahre RK, Saneja A. Recent advances in HER2-targeted delivery for cancer therapy. Drug Discov Today. 2021;26(5):1319–1329. doi:10.1016/j.drudis.2020.12.014
51. Fang RH, Kroll AV, Gao W, Zhang L. Cell Membrane Coating Nanotechnology. Adv Mater. 2018;30(23):e1706759. doi:10.1002/adma.201706759
52. Chen L, Hong W, Ren W, Xu T, Qian Z, He Z. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct Target Ther. 2021;6(1):225. doi:10.1038/s41392-021-00631-2
53. Chauhan VP, Jain RK. Strategies for advancing cancer nanomedicine. Nat Mater. 2013;12(11):958–962. doi:10.1038/nmat3792
54. Nel J, Elkhoury K, Velot É, et al. Functionalized liposomes for targeted breast cancer drug delivery. Bioact Mater. 2023;24:401–437. doi:10.1016/j.bioactmat.2022.12.027
55. Moghaddam FD, Heidari G, Zare EN, et al. Carbohydrate polymer-based nanocomposites for breast cancer treatment. Carbohydr Polym. 2023;304:120510. doi:10.1016/j.carbpol.2022.120510
56. Fu S, Li G, Zang W, Zhou X, Shi K, Zhai Y. Pure drug nano-assemblies: a facile carrier-free nanoplatform for efficient cancer therapy. Acta Pharm Sin B. 2022;12(1):92–106. doi:10.1016/j.apsb.2021.08.012
57. Santiago CA, Chow JCL. Variations in Gold Nanoparticle Size on DNA Damage: a Monte Carlo Study Based on a Multiple-Particle Model Using Electron Beams. Appl Sci. 2023;13:4916.
58. Chow JCL, Santiago CA. DNA Damage of Iron-Gold Nanoparticle Heterojunction Irradiated by kV Photon Beams: a Monte Carlo Study. Appl Sci. 2023;13:8942.
59. Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi:10.1200/jco.2005.04.937
60. Li X, Yu Y, Chen Q, et al. Engineering cancer cell membrane-camouflaged metal complex for efficient targeting therapy of breast cancer. J Nanobiotechnol. 2022;20(1):401. doi:10.1186/s12951-022-01593-5
61. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi:10.1038/nrd3499
62. Cao H, Dan Z, He X, et al. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano. 2016;10(8):7738–7748. doi:10.1021/acsnano.6b03148
63. Mohammadpour-Haratbar A, Zare Y, Rhee KY. Electrochemical biosensors based on polymer nanocomposites for detecting breast cancer: recent progress and future prospects. Adv Colloid Interface Sci. 2022;309:102795. doi:10.1016/j.cis.2022.102795
64. Brinkman AM, Chen G, Wang Y, et al. Aminoflavone-loaded EGFR-targeted unimolecular micelle nanoparticles exhibit anti-cancer effects in triple negative breast cancer. Biomaterials. 2016;101:20–31. doi:10.1016/j.biomaterials.2016.05.041
65. Li M, Du C, Guo N, et al. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi:10.1016/j.ejmech.2019.01.007
66. Moosavian SA, Bianconi V, Pirro M, Sahebkar A. Challenges and pitfalls in the development of liposomal delivery systems for cancer therapy. Semin Cancer Biol. 2021;69:337–348. doi:10.1016/j.semcancer.2019.09.025
67. He H, Liu L, Morin EE, Liu M, Schwendeman A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc Chem Res. 2019;52(9):2445–2461. doi:10.1021/acs.accounts.9b00228
68. Jin H, Huang W, Zhu X, Zhou Y, Yan D. Biocompatible or biodegradable hyperbranched polymers: from self-assembly to cytomimetic applications. Chem Soc Rev. 2012;41(18):5986–5997. doi:10.1039/c2cs35130g
69. Huang T, Zhang Q, Yi J, et al. PEG-Sheddable Nanodrug Remodels Tumor Microenvironment to Promote Effector T Cell Infiltration and Revise Their Exhaustion for Breast Cancer Immunotherapy. Small. 2023;19(38):e2301749. doi:10.1002/smll.202301749
70. Bowerman CJ, Byrne JD, Chu KS, et al. Docetaxel-Loaded PLGA Nanoparticles Improve Efficacy in Taxane-Resistant Triple-Negative Breast Cancer. Nano Lett. 2017;17(1):242–248. doi:10.1021/acs.nanolett.6b03971
71. Yen YW, Lee YL, Yu LY, et al. Fucoidan/chitosan layered PLGA nanoparticles with melatonin loading for inducing intestinal absorption and addressing triple-negative breast cancer progression. Int J Biol Macromol. 2023;250:126211. doi:10.1016/j.ijbiomac.2023.126211
72. Ren JM, McKenzie TG, Fu Q, et al. Star Polymers. Chem Rev. 2016;116(12):6743–6836. doi:10.1021/acs.chemrev.6b00008
73. Majoros IJ, Williams CR, Becker A, Baker Jr JR. Methotrexate delivery via folate targeted dendrimer-based nanotherapeutic platform. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1(5):502–510. doi:10.1002/wnan.37
74. Zhang P, Li Z, Cao W, et al. A PD-L1 Antibody-Conjugated PAMAM Dendrimer Nanosystem for Simultaneously Inhibiting Glycolysis and Promoting Immune Response in Fighting Breast Cancer. Adv Mater. 2023;35(41):e2305215. doi:10.1002/adma.202305215
75. Ahmed EM. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(2):105–121. doi:10.1016/j.jare.2013.07.006
76. Liang Y, He J, Guo B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano. 2021;15(8):12687–12722. doi:10.1021/acsnano.1c04206
77. Wang F, Li C, Cheng J, Yuan Z. Recent Advances on Inorganic Nanoparticle-Based Cancer Therapeutic Agents. Int J Environ Res Public Health. 2016;13:12. doi:10.3390/ijerph13121182
78. Guo Q, Shen XT, Li YY, Xu SQ. Carbon nanotubes-based drug delivery to cancer and brain. J Huazhong Univ Sci Technolog Med Sci. 2017;37(5):635–641. doi:10.1007/s11596-017-1783-z
79. Senapati S, Mahanta AK, Kumar S, Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct Target Ther. 2018;3:7. doi:10.1038/s41392-017-0004-3
80. Wang J, Potocny AM, Rosenthal J, Day ES. Gold Nanoshell-Linear Tetrapyrrole Conjugates for Near Infrared-Activated Dual Photodynamic and Photothermal Therapies. ACS Omega. 2020;5(1):926–940. doi:10.1021/acsomega.9b04150
81. Chen D, Tang Q, Li X, et al. Biocompatibility of magnetic Fe₃O₄ nanoparticles and their cytotoxic effect on MCF-7 cells. Int J Nanomed. 2012;7:4973–4982. doi:10.2147/ijn.S35140
82. Hersi AF, Eriksson S, Ramos J, Abdsaleh S, Wärnberg F, Karakatsanis A. A combined, totally magnetic technique with a magnetic marker for non-palpable tumour localization and superparamagnetic iron oxide nanoparticles for sentinel lymph node detection in breast cancer surgery. Eur J Surg Oncol. 2019;45(4):544–549. doi:10.1016/j.ejso.2018.10.064
83. Jha A, Nikam AN, Kulkarni S, et al. Biomimetic nanoarchitecturing: a disguised attack on cancer cells. J Control Release. 2021;329:413–433. doi:10.1016/j.jconrel.2020.12.005
84. Zhao M, Li H, Bu X, Lei C, Fang Q, Hu Z. Quantitative Proteomic Analysis of Cellular Resistance to the Nanoparticle Abraxane. ACS Nano. 2015;9(10):10099–10112. doi:10.1021/acsnano.5b03677
85. Park J, Sun B, Yeo Y. Albumin-coated nanocrystals for carrier-free delivery of paclitaxel. J Control Release. 2017;263:90–101. doi:10.1016/j.jconrel.2016.12.040
86. Tan S, Wu T, Zhang D, Zhang Z. Cell or cell membrane-based drug delivery systems. Theranostics. 2015;5(8):863–881. doi:10.7150/thno.11852
87. Hamidi M, Zarrin A, Foroozesh M, Mohammadi-Samani S. Applications of carrier erythrocytes in delivery of biopharmaceuticals. J Control Release. 2007;118(2):145–160. doi:10.1016/j.jconrel.2006.06.032
88. Khatoon N, Zhang Z, Zhou C, Chu M. Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater Sci. 2022;10(5):1193–1208. doi:10.1039/d1bm01664d
89. Luk BT, Zhang L. Cell membrane-camouflaged nanoparticles for drug delivery. J Control Release. 2015;220(Pt B):600–607. doi:10.1016/j.jconrel.2015.07.019
90. Wu CY, Du SL, Zhang J, Liang AL, Liu YJ. Exosomes and breast cancer: a comprehensive review of novel therapeutic strategies from diagnosis to treatment. Cancer Gene Ther. 2017;24(1):6–12. doi:10.1038/cgt.2016.69
91. Hannafon BN, Trigoso YD, Calloway CL, et al. Plasma exosome microRNAs are indicative of breast cancer. Breast Cancer Res. 2016;18(1):90. doi:10.1186/s13058-016-0753-x
92. Li QY, Lin B, Li YZ, Lu N. Erythrocyte-Camouflaged Mesoporous Titanium Dioxide Nanoplatform for an Ultrasound-Mediated Sequential Therapies of Breast Cancer. Int j Nanomed. 2021;16:3875–3887. doi:10.2147/ijn.S301855
93. Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001;220(1):13–30. doi:10.1148/radiology.220.1.r01jl3113
94. Yelland A, Graham MD, Trott PA, et al. Diagnosing breast carcinoma in young women. BMJ. 1991;302(6777):618–620. doi:10.1136/bmj.302.6777.618
95. White RR, Halperin TJ, Olson Jr JA, Soo MS, Bentley RC, Seigler HF. Impact of core-needle breast biopsy on the surgical management of mammographic abnormalities. Ann Surg. 2001;233(6):769–777. doi:10.1097/00000658-200106000-00006
96. Łukasiewicz E, Ziemiecka A, Jakubowski W, Vojinovic J, Bogucevska M, Dobruch-Sobczak K. Fine-needle versus core-needle biopsy - which one to choose in preoperative assessment of focal lesions in the breasts? Literature review. J Ultrason. 2017;17(71):267–274. doi:10.15557/JoU.2017.0039
97. Jiang Y, Jiang Z, Wang M, Ma L. Current understandings and clinical translation of nanomedicines for breast cancer therapy. Adv Drug Deliv Rev. 2022;180:114034. doi:10.1016/j.addr.2021.114034
98. Chintamaneni PK, Nagasen D, Babu KC, et al. Engineered upconversion nanocarriers for synergistic breast cancer imaging and therapy: current state of art. J Control Release. 2022;352:652–672. doi:10.1016/j.jconrel.2022.10.056
99. Miripour ZS, Aghaee P, Abbasvandi F, et al. Electrically guided interventional radiology, in-vivo electrochemical tracing of suspicious lesions to breast cancer prior to core needle biopsy. Biosens Bioelectron. 2020;161:112209. doi:10.1016/j.bios.2020.112209
100. Yang X, Zhao M, Wu Z, et al. Nano-ultrasonic Contrast Agent for Chemoimmunotherapy of Breast Cancer by Immune Metabolism Reprogramming and Tumor Autophagy. ACS Nano. 2022;16(2):3417–3431. doi:10.1021/acsnano.2c00462
101. Ramesh K, Truong A, Wang Y, Rusckowski M, Gkikas M. Ligand-Specific Nano-Contrast Agents Promote Enhanced Breast Cancer CT Detection at 0.5 mg Au. Int J Mol Sci. 2022;23:17. doi:10.3390/ijms23179926
102. Schaffner F, Ray AM, Dontenwill M. Integrin α5β1, the Fibronectin Receptor, as a Pertinent Therapeutic Target in Solid Tumors. Cancers. 2013;5(1):27–47. doi:10.3390/cancers5010027
103. D’Souza AA, Shegokar R. Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert Opin Drug Deliv. 2016;13(9):1257–1275. doi:10.1080/17425247.2016.1182485
104. Farzin A, Etesami SA, Quint J, Memic A, Tamayol A. Magnetic Nanoparticles in Cancer Therapy and Diagnosis. Adv Healthc Mater. 2020;9(9):e1901058. doi:10.1002/adhm.201901058
105. Zhuang D, Zhang H, Hu G, Guo B. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J Nanobiotechnol. 2022;20(1):284. doi:10.1186/s12951-022-01479-6
106. Jain P, Patel K, Jangid AK, et al. Biotinylated Mn(3)O(4) nanocuboids for targeted delivery of gemcitabine hydrochloride to breast cancer and MRI applications. Int J Pharm. 2021;606:120895. doi:10.1016/j.ijpharm.2021.120895
107. Zhang C, Li L, Han FY, et al. Integrating Fluorinated Polymer and Manganese-Layered Double Hydroxide Nanoparticles as pH-activated (19) F MRI Agents for Specific and Sensitive Detection of Breast Cancer. Small. 2019;15(36):e1902309. doi:10.1002/smll.201902309
108. Ding J, Zhou Y, Li J, Jiang L, He Z, Zhu JJ. Screening of HER2 Overexpressed Breast Cancer Subtype In Vivo by the Validation of High-Performance, Long-Term, and Noninvasive Fluorescence Tracer. Anal Chem. 2015;87(24):12290–12297. doi:10.1021/acs.analchem.5b03580
109. Wei T, Xing H, Wang H, et al. Bovine serum albumin encapsulation of near infrared fluorescent nano-probe with low nonspecificity and cytotoxicity for imaging of HER2-positive breast cancer cells. Talanta. 2020;210:120625. doi:10.1016/j.talanta.2019.120625
110. Liang N, Liu L, Li P, et al. Efficient isolation and quantification of circulating tumor cells in non-small cell lung cancer patients using peptide-functionalized magnetic nanoparticles. J Thorac Dis. 2020;12(8):4262–4273. doi:10.21037/jtd-20-1026A
111. Tan B, Huang L, Wu Y, Liao J. Advances and trends of hydrogel therapy platform in localized tumor treatment: a review. J Biomed Mater Res A. 2021;109(4):404–425. doi:10.1002/jbm.a.37062
112. Wang Z, Zhang Y, Yin Y, et al. High-Strength and Injectable Supramolecular Hydrogel Self-Assembled by Monomeric Nucleoside for Tooth-Extraction Wound Healing. Adv Mater. 2022;34(13):e2108300. doi:10.1002/adma.202108300
113. Fang YL, Huang SS, Hu QY, et al. Injectable Zwitterionic Physical Hydrogel with Enhanced Chemodynamic Therapy and Tumor Microenvironment Remodeling Properties for Synergistic Anticancer Therapy. Acs Nano. 2023;17(24):24883–24900. doi:10.1021/acsnano.3c05898
114. Lee D, Huntoon K, Wang Y, Jiang W, Kim BYS. Harnessing Innate Immunity Using Biomaterials for Cancer Immunotherapy. Adv Mater. 2021;33(27):e2007576. doi:10.1002/adma.202007576
115. Overgaard J. Effect of hyperthermia on malignant cells in vivo. A review and a hypothesis. Cancer. 1977;39(6):2637–2646. doi:10.1002/1097-0142(197706)39:6<2637::aid-cncr2820390650>3.0.co;2-s
116. Yuan A, Wu J, Tang X, Zhao L, Xu F, Hu Y. Application of near-infrared dyes for tumor imaging, photothermal, and photodynamic therapies. J Pharm Sci. 2013;102(1):6–28. doi:10.1002/jps.23356
117. Chen Q, Wen J, Li H, Xu Y, Liu F, Sun S. Recent advances in different modal imaging-guided photothermal therapy. Biomaterials. 2016;106:144–166. doi:10.1016/j.biomaterials.2016.08.022
118. Chen G, Roy I, Yang C, Prasad PN. Nanochemistry and Nanomedicine for Nanoparticle-based Diagnostics and Therapy. Chem Rev. 2016;116(5):2826–2885. doi:10.1021/acs.chemrev.5b00148
119. Zhang X, Lai Y, Zhang L, et al. Chitosan-modified molybdenum selenide mediated efficient killing of Helicobacter pylori and treatment of gastric cancer. Int J Biol Macromol. 2024;275(Pt 1):133599. doi:10.1016/j.ijbiomac.2024.133599
120. Wang S, Ma X, Hong X, et al. Adjuvant Photothermal Therapy Inhibits Local Recurrences after Breast-Conserving Surgery with Little Skin Damage. ACS Nano. 2018;12(1):662–670. doi:10.1021/acsnano.7b07757
121. Mu J, Xiao M, Shi Y, et al. The Chemistry of Organic Contrast Agents in the NIR-II Window. Angew Chem Int Ed Engl. 2022;61(14):e202114722. doi:10.1002/anie.202114722
122. Qu R, He D, Wu M, et al. Afterglow/Photothermal Bifunctional Polymeric Nanoparticles for Precise Postbreast-Conserving Surgery Adjuvant Therapy and Early Recurrence Theranostic. Nano Lett. 2023;23(10):4216–4225. doi:10.1021/acs.nanolett.3c00191
123. Wen Y, Truong VX, Li M. Real-Time Intraoperative Surface-Enhanced Raman Spectroscopy-Guided Thermosurgical Eradication of Residual Microtumors in Orthotopic Breast Cancer. Nano Lett. 2021;21(7):3066–3074. doi:10.1021/acs.nanolett.1c00204
124. Deroose CM, De A, Loening AM, et al. Multimodality imaging of tumor xenografts and metastases in mice with combined small-animal PET, small-animal CT, and bioluminescence imaging. J Nucl Med. 2007;48(2):295–303.
125. Branca RT, Cleveland ZI, Fubara B, et al. Molecular MRI for sensitive and specific detection of lung metastases. Proc Natl Acad Sci U S A. 2010;107(8):3693–3697. doi:10.1073/pnas.1000386107
126. Sobral-Filho RG, DeVorkin L, Macpherson S, Jirasek A, Lum JJ, Brolo AG. Ex Vivo Detection of Circulating Tumor Cells from Whole Blood by Direct Nanoparticle Visualization. ACS Nano. 2018;12(2):1902–1909. doi:10.1021/acsnano.7b08813
127. Ghaderinia M, Khayamian MA, Abadijoo H, et al. Capture-free deactivation of CTCs in the bloodstream; a metastasis suppression method by electrostatic stimulation of the peripheral blood. Biosens Bioelectron. 2021;183:113194. doi:10.1016/j.bios.2021.113194
128. Wang Z, Xu W, Yang Y, et al. Impact of changing treatment strategy based on circulating tumor cells on postoperative survival of breast cancer. Front Oncol. 2022;12:1006909. doi:10.3389/fonc.2022.1006909
129. Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–264. doi:10.1200/jco.2014.55.7827
130. Kuemmel S, Heil J, Bruzas S, et al. Safety of Targeted Axillary Dissection After Neoadjuvant Therapy in Patients With Node-Positive Breast Cancer. JAMA Surg. 2023;158(8):807–815. doi:10.1001/jamasurg.2023.1772
131. Kapoor MM, Patel MM, Scoggins ME. The Wire and Beyond: recent Advances in Breast Imaging Preoperative Needle Localization. Radiographics. 2019;39(7):1886–1906. doi:10.1148/rg.2019190041
132. Mango VL, Wynn RT, Feldman S, et al. Beyond Wires and Seeds: reflector-guided Breast Lesion Localization and Excision. Radiology. 2017;284(2):365–371. doi:10.1148/radiol.2017161661
133. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(10):1674. doi:10.1093/annonc/mdz189
134. Yuan L, Qi X, Zhang Y, et al. Comparison of sentinel lymph node detection performances using blue dye in conjunction with indocyanine green or radioisotope in breast cancer patients: a prospective single-center randomized study. Cancer Biol Med. 2018;15(4):452–460. doi:10.20892/j.issn.2095-3941.2018.0270
135. Goel M, Mackeyev Y, Krishnan S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: principles and concepts. Cancer Nanotechnol. 2023;14(1):15. doi:10.1186/s12645-023-00165-y
136. Lauwerends LJ, van Driel P, Baatenburg de jong RJ, et al. Real-time fluorescence imaging in intraoperative decision making for cancer surgery. Lancet Oncol. 2021;22(5):e186–e195. doi:10.1016/s1470-2045(20)30600-8
137. Ekinci M, Alencar LMR, Lopes AM, Santos-Oliveira R, Ilem-özdemir D. Radiolabeled Human Serum Albumin Nanoparticles Co-Loaded with Methotrexate and Decorated with Trastuzumab for Breast Cancer Diagnosis. J Funct Biomater. 2023;14(9). doi:10.3390/jfb14090477
138. Ebrahimi A, Pirali Hamedani M, Mohammadzadeh P, et al. (99m)Tc-Anionic dendrimer targeted vascular endothelial growth factor as a novel nano-radiotracer for in-vivo breast cancer imaging. Bioorg Chem. 2022;128:106085. doi:10.1016/j.bioorg.2022.106085
139. Blau R, Epshtein Y, Pisarevsky E, et al. Image-guided surgery using near-infrared Turn-ON fluorescent nanoprobes for precise detection of tumor margins. Theranostics. 2018;8(13):3437–3460. doi:10.7150/thno.23853
140. Lan L, Xia Y, Li R, et al. A fiber optoacoustic guide with augmented reality for precision breast-conserving surgery. Light Sci Appl. 2018;7:2. doi:10.1038/s41377-018-0006-0
141. Maishman T, Cutress RI, Hernandez A, et al. Local Recurrence and Breast Oncological Surgery in Young Women With Breast Cancer: the POSH Observational Cohort Study. Ann Surg. 2017;266(1):165–172. doi:10.1097/sla.0000000000001930
142. Wei M, Bai J, Shen X, et al. Glutathione-Exhausting Nanoprobes for NIR-II Fluorescence Imaging-Guided Surgery and Boosting Radiation Therapy Efficacy via Ferroptosis in Breast Cancer. ACS Nano. 2023;17(12):11345–11361. doi:10.1021/acsnano.3c00350
143. Li C, Chen G, Zhang Y, Wu F, Wang Q. Advanced Fluorescence Imaging Technology in the Near-Infrared-II Window for Biomedical Applications. J Am Chem Soc. 2020;142(35):14789–14804. doi:10.1021/jacs.0c07022
144. Cheng Z, Jin Y, Li J, et al. Fibronectin-targeting and metalloproteinase-activatable smart imaging probe for fluorescence imaging and image-guided surgery of breast cancer. J Nanobiotechnol. 2023;21(1):112. doi:10.1186/s12951-023-01868-5
145. Dai H, Shen Q, Shao J, Wang W, Gao F, Dong X. Small Molecular NIR-II Fluorophores for Cancer Phototheranostics. Innovation. 2021;2(1):100082. doi:10.1016/j.xinn.2021.100082
146. Yang Y, Xie Y, Zhang F. Second near-infrared window fluorescence nanoprobes for deep-tissue in vivo multiplexed bioimaging. Adv Drug Deliv Rev. 2023;193:114697. doi:10.1016/j.addr.2023.114697
147. Malata CM, McIntosh SA, Purushotham AD. Immediate breast reconstruction after mastectomy for cancer. Br J Surg. 2000;87(11):1455–1472. doi:10.1046/j.1365-2168.2000.01593.x
148. Calobrace MB, Stevens WG, Capizzi PJ, Cohen R, Godinez T, Beckstrand M. Risk Factor Analysis for Capsular Contracture: a 10-Year Sientra Study Using Round, Smooth, and Textured Implants for Breast Augmentation. Plast Reconstr Surg. 2018;141:20s–28s. doi:10.1097/prs.0000000000004351
149. Berthet M, Gauthier Y, Lacroix C, Verrier B, Monge C. Nanoparticle-Based Dressing: the Future of Wound Treatment? Trends Biotechnol. 2017;35(8):770–784. doi:10.1016/j.tibtech.2017.05.005
150. Wang M, Huang X, Zheng H, et al. Nanomaterials applied in wound healing: mechanisms, limitations and perspectives. J Control Release. 2021;337:236–247. doi:10.1016/j.jconrel.2021.07.017
151. Wang W, Zhang Q, Zhang M, et al. A novel biodegradable injectable chitosan hydrogel for overcoming postoperative trauma and combating multiple tumors. Carbohydr Polym. 2021;265:118065. doi:10.1016/j.carbpol.2021.118065
152. Kahandal A, Chaudhary S, Methe S, Nagwade P, Sivaram A, Tagad CK. Galactomannan polysaccharide as a biotemplate for the synthesis of zinc oxide nanoparticles with photocatalytic, antimicrobial and anticancer applications. Int J Biol Macromol. 2023;253(Pt 3):126787. doi:10.1016/j.ijbiomac.2023.126787
153. Król A, Pomastowski P, Rafińska K, Railean-Plugaru V, Buszewski B. Zinc oxide nanoparticles: synthesis, antiseptic activity and toxicity mechanism. Adv Colloid Interface Sci. 2017;249:37–52. doi:10.1016/j.cis.2017.07.033
154. Liu Y, Qi Y, Chen C, et al. Platelet-mimetic nano-sensor for combating postoperative recurrence and wound infection of triple-negative breast cancer. J Control Release. 2023;362:396–408. doi:10.1016/j.jconrel.2023.08.057
155. Dehaini D, Wei X, Fang RH, et al. Erythrocyte-Platelet Hybrid Membrane Coating for Enhanced Nanoparticle Functionalization. Adv Mater. 2017;29(16). doi:10.1002/adma.201606209
156. Han X, Ju L, Saengow C, et al. Nano oxygen chamber by cascade reaction for hypoxia mitigation and reactive oxygen species scavenging in wound healing. Bioact Mater. 2024;35:67–81. doi:10.1016/j.bioactmat.2024.01.010
157. Ogawa R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int J Mol Sci. 2017;18:3. doi:10.3390/ijms18030606
158. Li Y, Fu R, Duan Z, Zhu C, Fan D. Artificial Nonenzymatic Antioxidant MXene Nanosheet-Anchored Injectable Hydrogel as a Mild Photothermal-Controlled Oxygen Release Platform for Diabetic Wound Healing. ACS Nano. 2022;16(5):7486–7502. doi:10.1021/acsnano.1c10575
159. Xiong Y, Chu X, Yu T, et al. Reactive Oxygen Species-Scavenging Nanosystems in the Treatment of Diabetic Wounds. Adv Healthc Mater. 2023;12(25):e2300779. doi:10.1002/adhm.202300779
160. Zhao Q, Ogino S, Lee S, et al. Development of new bioabsorbable implants with de novo adipogenesis. Regen Ther. 2023;24:311–317. doi:10.1016/j.reth.2023.07.008
161. Adelson D, Singolda R, Haran O, Madah E, Barsuk D, Barnea Y. Our Experience Using Round Nano-Surface Ergonomix Implants for Breast Reconstruction: a Single-Center Retrospective Study. Aesthet Surg J. 2023;43(2):Np102–np111. doi:10.1093/asj/sjac264
162. Hiller JG, Perry NJ, Poulogiannis G, Riedel B, Sloan EK. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15(4):205–218. doi:10.1038/nrclinonc.2017.194
163. Darby SC, Ewertz M, Hall P. Ischemic heart disease after breast cancer radiotherapy. N Engl J Med. 2013;368(26):2527. doi:10.1056/NEJMc1304601
164. Onstenk W, Gratama JW, Foekens JA, Sleijfer S. Towards a personalized breast cancer treatment approach guided by circulating tumor cell (CTC) characteristics. Cancer Treat Rev. 2013;39(7):691–700. doi:10.1016/j.ctrv.2013.04.001
165. Lin D, Shen L, Luo M, et al. Circulating tumor cells: biology and clinical significance. Signal Transduct Target Ther. 2021;6(1):404. doi:10.1038/s41392-021-00817-8
166. Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20(10):2553–2568. doi:10.1158/1078-0432.Ccr-13-2664
167. Zhou J, Wu J, Hao X, et al. An exploratory study on the checkout rate of circulating tumor cells and the prediction of efficacy of neoadjuvant therapy and prognosis in patients with HER-2-positive early breast cancer. Front Oncol. 2022;12:966624. doi:10.3389/fonc.2022.966624
168. Guo Z, Zou Y, He H, et al. Bifunctional Platinated Nanoparticles for Photoinduced Tumor Ablation. Adv Mater. 2016;28(46):10155–10164. doi:10.1002/adma.201602738
169. Chao B, Jiao J, Yang L, et al. Application of advanced biomaterials in photothermal therapy for malignant bone tumors. Biomater Res. 2023;27(1):116. doi:10.1186/s40824-023-00453-z
170. Wu H, Song L, Chen L, et al. Injectable magnetic supramolecular hydrogel with magnetocaloric liquid-conformal property prevents post-operative recurrence in a breast cancer model. Acta Biomater. 2018;74:302–311. doi:10.1016/j.actbio.2018.04.052
171. Zhao HJ, Song QL, Zheng CX, et al. Implantable Bioresponsive Nanoarray Enhances Postsurgical Immunotherapy by Activating Pyroptosis and Remodeling Tumor Microenvironment. Adv Funct Mater. 2020;30:51. doi:10.1002/adfm.202005747
172. Liu T, Si X, Liu L, et al. Injectable Nano-in-Gel Vaccine for Spatial and Temporal Control of Vaccine Kinetics and Breast Cancer Postsurgical Therapy. ACS Nano. 2024;18(4):3087–3100. doi:10.1021/acsnano.3c08376
173. Caudle AS, Yang WT, Krishnamurthy S, et al. Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: implementation of Targeted Axillary Dissection. J Clin Oncol. 2016;34(10):1072–1078. doi:10.1200/jco.2015.64.0094
174. de Boniface J, Filtenborg Tvedskov T, Rydén L, et al. Omitting Axillary Dissection in Breast Cancer with Sentinel-Node Metastases. N Engl J Med. 2024;390(13):1163–1175. doi:10.1056/NEJMoa2313487
175. Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001;93(21):1633–1637. doi:10.1093/jnci/93.21.1633
176. Sun J, Chu F, Pan J, et al. BRCA-CRisk: a Contralateral Breast Cancer Risk Prediction Model for BRCA Carriers. J Clin Oncol. 2023;41(5):991–999. doi:10.1200/jco.22.00833
177. de Lázaro I, Mooney DJ. Obstacles and opportunities in a forward vision for cancer nanomedicine. Nat Mater. 2021;20(11):1469–1479. doi:10.1038/s41563-021-01047-7
178. Rao VM, Levin DC, Parker L, Cavanaugh B, Frangos AJ, Sunshine JH. How widely is computer-aided detection used in screening and diagnostic mammography? J Am Coll Radiol. 2010;7(10):802–805. doi:10.1016/j.jacr.2010.05.019
179. Bera K, Braman N, Gupta A, Velcheti V, Madabhushi A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol. 2022;19(2):132–146. doi:10.1038/s41571-021-00560-7
180. Bi WL, Hosny A, Schabath MB, et al. Artificial intelligence in cancer imaging: clinical challenges and applications. CA Cancer J Clin. 2019;69(2):127–157. doi:10.3322/caac.21552
181. Liu J, Zhao H, Huang Y, et al. Genome-wide cell-free DNA methylation analyses improve accuracy of non-invasive diagnostic imaging for early-stage breast cancer. Mol Cancer. 2021;20(1):36. doi:10.1186/s12943-021-01330-w
182. Wang T, Li P, Qi Q, et al. A multiplex blood-based assay targeting DNA methylation in PBMCs enables early detection of breast cancer. Nat Commun. 2023;14(1):4724. doi:10.1038/s41467-023-40389-5
183. Huang Y, Zhu T, Zhang X, et al. Longitudinal MRI-based fusion novel model predicts pathological complete response in breast cancer treated with neoadjuvant chemotherapy: a multicenter, retrospective study. EClinicalMedicine. 2023;58:101899. doi:10.1016/j.eclinm.2023.101899
184. Lotter W, Diab AR, Haslam B, et al. Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat Med. 2021;27(2):244–249. doi:10.1038/s41591-020-01174-9
185. Yang Y, Liu Z, Huang J, et al. Histological diagnosis of unprocessed breast core-needle biopsy via stimulated Raman scattering microscopy and multi-instance learning. Theranostics. 2023;13(4):1342–1354. doi:10.7150/thno.81784
186. Jiang Y, Yang M, Wang S, Li X, Sun Y. Emerging role of deep learning-based artificial intelligence in tumor pathology. Cancer Commun. 2020;40(4):154–166. doi:10.1002/cac2.12012
187. Jing B, Zhang T, Wang Z, et al. A deep survival analysis method based on ranking. Artif Intell Med. 2019;98:1–9. doi:10.1016/j.artmed.2019.06.001
188. Lai YH, Chen WN, Hsu TC, Lin C, Tsao Y, Wu S. Overall survival prediction of non-small cell lung cancer by integrating microarray and clinical data with deep learning. Sci Rep. 2020;10(1):4679. doi:10.1038/s41598-020-61588-w
189. Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13(1):152. doi:10.1186/s13073-021-00968-x
190. Ferraz RO, Moreira-Filho DC. Survival analysis of women with breast cancer: competing risk models. Cien Saude Colet. 2017;22(11):3743–3754. doi:10.1590/1413-812320172211.05092016
191. Mu W, Jiang L, Zhang J, et al. Non-invasive decision support for NSCLC treatment using PET/CT radiomics. Nat Commun. 2020;11(1):5228. doi:10.1038/s41467-020-19116-x
192. Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S. Bioprinting for cancer research. Trends Biotechnol. 2015;33(9):504–513. doi:10.1016/j.tibtech.2015.06.007
193. Liu K, Li L, Chen J, et al. Bone ECM-like 3D Printing Scaffold with Liquid Crystalline and Viscoelastic Microenvironment for Bone Regeneration. ACS Nano. 2022;16(12):21020–21035. doi:10.1021/acsnano.2c08699
194. Liu H, He L, Kuzmanović M, et al. Advanced Nanomaterials in Medical 3D Printing. Small Methods. 2024;8(3):e2301121. doi:10.1002/smtd.202301121
195. Myung N, Kang HW. Local dose-dense chemotherapy for triple-negative breast cancer via minimally invasive implantation of 3D printed devices. Asian J Pharm Sci. 2024;19(1):100884. doi:10.1016/j.ajps.2024.100884
196. Liu S, Kumari S, He H, et al. Biosensors integrated 3D organoid/organ-on-a-chip system: a real-time biomechanical, biophysical, and biochemical monitoring and characterization. Biosens Bioelectron. 2023;231:115285. doi:10.1016/j.bios.2023.115285
197. Liu C, Nguyen RY, Pizzurro GA, et al. Self-assembly of mesoscale collagen architectures and applications in 3D cell migration. Acta Biomater. 2023;155:167–181. doi:10.1016/j.actbio.2022.11.011
198. Yang Y, Qiao X, Huang R, et al. E-jet 3D printed drug delivery implants to inhibit growth and metastasis of orthotopic breast cancer. Biomaterials. 2020;230:119618. doi:10.1016/j.biomaterials.2019.119618
199. Li W, Hu X, Yang S, et al. A novel tissue-engineered 3D tumor model for anti-cancer drug discovery. Biofabrication. 2018;11(1):015004. doi:10.1088/1758-5090/aae270
200. Su Y, Liu Y, Hu X, et al. Caffeic acid-grafted chitosan/sodium alginate/nanoclay-based multifunctional 3D-printed hybrid scaffolds for local drug release therapy after breast cancer surgery. Carbohydr Polym. 2024;324:121441. doi:10.1016/j.carbpol.2023.121441
201. Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials. 2020;226:119536. doi:10.1016/j.biomaterials.2019.119536
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

