Back to Journals » International Journal of Nanomedicine » Volume 20
Recent Advances in Spatiotemporal Manipulation of Engineered Bacteria for Precision Cancer Therapy
Authors Chang X, Liu X, Wang X, Ma L, Liang J , Li Y
Received 10 January 2025
Accepted for publication 25 April 2025
Published 7 May 2025 Volume 2025:20 Pages 5859—5872
DOI https://doi.org/10.2147/IJN.S516523
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jie Huang
Xueke Chang,1,* Xiaolin Liu,1,* Xiumei Wang,2,* Lin Ma,1 Jing Liang,1 Yan Li1
1Department of Oncology, The First Affiliated Hospital of Shandong First Medical University & Shandong Provincial Qianfoshan Hospital, Shandong Lung Cancer Institute, Jinan, 250000, People’s Republic of China; 2Department of Oncology, Yuncheng Chengxin Hospital, Heze, Shandong, 274000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jing Liang, Email [email protected] Yan Li, Email [email protected]
Abstract: Solid tumours possess a hypoxic and immunosuppressive microenvironment, presenting a significant challenge to anticancer treatments. Certain anaerobic microorganisms thrive in this setting, rendering them promising candidates for targeted antitumour therapy delivery. In contrast to traditional nanodrug delivery systems, bacterial-based drug delivery systems can be engineered to produce and secrete therapeutics without the need for intricate post-purification or protective delivery methods. Nevertheless, bacteria can potentially migrate beyond their intended niche, causing off-target drug release and substantial toxicity to healthy tissues. Consequently, to enhance the effectiveness of cancer treatments while minimizing side effects, it is essential to precisely manipulate bacteria for accurate and controlled drug delivery directly to the tumour site. This can be achieved by employing inducible or repressible systems that allow for precise regulation of gene expression at specific times and locations. Ideally, engineering bacteria capable of rapidly and precisely transitioning between “on” and “off” states as required will enable them to recognize and react to targeted stimuli. While various techniques such as optical, magnetic, acoustic, and hyperbaric oxygen micromanipulation have been developed for the manipulation of particles or cells, each technique boasts its unique set of pros and cons. This review article provides an updated overview of the recent progress in the spatiotemporal control of engineered bacteria via these methods and discusses the benefits and constraints of each approach.
Keywords: engineered bacteria, spatiotemporal manipulation, cancer, light, magnetic, ultrasound, hyperbaric oxygen
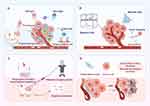
Graphical Abstract:

Introduction
Solid tumours are known to have a hypoxic and immunosuppressive microenvironment, which is a major barrier to anticancer treatment.1 Specific obligatory and facultative anaerobic microorganisms preferentially grow in this environment,2,3 which makes them suitable candidates for the delivery of antitumour therapies.4,5 Unlike traditional nanoscale drug delivery systems, bacterial-based drug carriers can be engineered to produce and release medications, thus eliminating the requirement for complex postpurification or delivery protection measures.6 However, bacteria can occasionally proliferate beyond their usual habitats, which results in off-target therapeutic release and may cause significant toxicity to normal tissues.7,8 Thus, to boost the effectiveness of cancer treatments while reducing adverse reactions, it is essential to fine-tune bacteria for accurate and on-demand medication delivery directly to the tumour location. Depending on the requirements, exact control over gene expression can be achieved through the activation of inducible or repressible systems at precise times and locations. Ideally, designing bacteria that rapidly and precisely switch between “ON” and “OFF” modes, as needed, would allow them to identify and respond to physiological or pathological conditions in response to targeted triggers.9 Bacteria are administered endogenous chemical inducers, which allow them to react to the acidic or hypoxic milieu of solid tumours to control bacterial activity spatially. However, these systems still cannot control bacterial activity temporally.4,8 Exogenous chemical inducers can closely regulate the corresponding inducible promoters to manage bacterial colonization or gene expression for temporal control, but they are unable to provide spatial control of bacterial activity.4,8 Chemical inducers cannot easily accumulate to the proper concentration in the tumour microenvironment due to the high interstitial fluid pressure, and increasing their dosages may result in unintended consequences.10–12 Certain biological techniques, such population sensing systems for bacteria, can modify gene expression based on population size such that therapeutic gene expression only occurs when the population density reaches a certain threshold.13,14 Nevertheless, accurately mimicking gene expression in a spatiotemporally controlled manner using biological approaches can be challenging, particularly in living animals. Compared to the two ways mentioned above, physical methods are more controllable in terms of both space and time.
To manipulate particles or cells, a number of technologies, such as optical,15,16 magnetic,17,18 acoustic19,20 tweezers, and hyperbaric oxygen (HBO)21 have been developed to date. Through photon momentum transfer, optical tweezers use intensely concentrated laser beams for noncontact capture and manipulation of small objects. Optogenetics has recently advanced swiftly as a noninvasive method that is characterized by excellent spatiotemporal precision and reversibility.22,23 However, optical tweezers can be used only in clear media, which limits their use in vivo. Moreover, biological specimens may sustain photodamage from direct exposure to high-intensity lasers, which can lead to photothermal or photochemical inactivation of bioactive medications. Magnetic fields enable the in vivo manipulation of cells through the use of magnetic tweezers, which have the ability to penetrate opaque media and boast a high level of biosafety.24 The targets subjected to the applied magnetic field must be magnetized or associated with magnetized particles.25,26 Owing to the unfocused nature of magnetic fields, magnetic tweezers have difficulty accurately identifying the target position in vivo. The viability and bioactivity of biological samples may be permanently impacted by magnetic labelling. Therefore, acoustic tweezers have recently become popular tools for working with biological particles because they cause low damage and have high tissue penetration, significant micron-scale spatial precision, and adaptability to a variety of environments.27,28 Despite the numerous benefits of ultrasonic therapy, its implementation has inherent hurdles, and its efficacy in inducing the expression of genes is limited by the tumour depth and dissemination. Consequently, hyperbaric oxygen therapy techniques are employed to efficiently deplete the extracellular matrix (ECM), facilitate the buildup of anticancer agents within the tumour and enhance the drug penetration depth and delivery efficacy.29 Nonetheless, the precise mechanism of hyperbaric oxygen therapy needs further elucidation. The effectiveness and safety of its clinical implementation need additional validation. Each of the above particle manipulation methods has advantages and disadvantages. This review encapsulates the latest progresses in the spatiotemporal manipulation of engineered bacteria, utilizing the aforementioned techniques, and it further discusses their respective strengths and limitations. (Figure 1)
Spatiotemporal Manipulation by Light
Light is noninvasive and harmless and can be delivered or retracted at any moment. Photosensitive bacteria that react to visible light have been extensively utilized in dynamic industrial manufacturing and the modulation of signalling pathways.30,31 (Table 1) With an aggregation-induced emission (AIE) photosensitizer MA, metabolic engineering was used to create a live therapeutic system based on attenuated Salmonella. The modified bacteria may settle in tumour tissues and cause the colonization and expression of VEGFR2 from outside sources. The protein encoded by the VEGFR2 gene, which can reduce angiogenesis caused by a T-cell-mediated autoimmune response and prevent tumour development, was released and expressed in tumour tissues upon exposure to light. The administered MA-engineered Salmonella harbouring VEGFR2 plasmids were located in the tumour tissues, and the bacteria continued to colonize and express exogenous genes since the MA labelling approach had no adverse effects on Salmonella reproduction. Using an AIE photosensitizer and metabolic engineering, we created a live therapeutic system based on attenuated Salmonella to achieve light-controlled gene release for breast cancer treatment. Notably, the excitation wavelength of MA was in the visible spectrum, which restricted its ability to penetrate light in in vivo applications.32 Future research will concentrate on enhancing the metabolic engineering technique with NIR or two-photon photosensitizers in order to increase the range of theranostic applications. Existing optogenetic methods are effective under certain conditions; however, they often lead to poor tissue penetration. Therefore, optogenetic manipulations frequently use intrusive fibre optic insertion.33–35 The upconversion materials transform deep tissue-penetrating NIR light into localized visible light, thereby facilitating subsequent photoresponsive manipulations within cells. Upconversion optogenetics has been extensively used in tumour therapy and neuroscience, providing a greater spatiotemporal manipulation accuracy than conventional regulatory methods do.36–39 To minimize trauma while penetrating deep into tissues, an optogenetic system has been developed that is responsive to NIR light. This system employs lanthanide-doped upconversion nanoparticles to transform NIR light into blue light, which indirectly stimulates the expression of optogenetic genes in bacteria that have been genetically modified to combat tumours.40–42 This optogenetic system that is activated by NIR light effectively inhibits tumour growth but is unable to completely eradicate tumours. We engineered α-haemolysin from Escherichia coli Nissle 1917 (EcN) to be expressed upon exposure to blue light, which is emitted by lanthanide-doped upconversion nanoparticles, to overcome this limitation. As an in situ photothermal agent in tumours, the αHL not only kills tumour cells but, more significantly, damages endothelium to cause thrombosis and facilitate a cascade of photogenetic and photothermal therapeutic effects, which can result in the complete eradication of surface tumours. Thrombi with strong NIR absorbance like this allow precise photothermal therapy to totally stop tumour growth with very little off-target toxicity, which is uncommon in purely genetically engineered bacterial systems. Furthermore, αHL secretion triggers an immune response and inhibits 4T1 tumour metastasis. Therefore, the in situ PTT generated by bacteria-based optogenetic systems has a lot of potential for precise cancer treatment.43
 |
Table 1 Spatiotemporal Manipulation by Light or Magnetic Field |
Another photocontrolled engineered bacterium exerts an antitumor effect by synthesizing antitumour agents and metabolizing compounds that facilitate tumour proliferation. People have engineered the gram-negative E. coli strain MG1655 and genetically programmed the outer membrane of the bacterial cells with therapeutic TRAIL using the YiaT surface display system. The therapeutic protein might be supplied straight to the intended tumour areas using this tactic after bacterial targeting of tumors. Additionally, the surface-programmed bacteria act as light-driven nitric oxide generators that reduce nitrate to nitric oxide, which is released locally in the tumour region, increases treatment efficiency, and polarizes tumour-associated macrophages towards the M1 phenotype. The simultaneous production of reactive oxygen species causes the death of immune cells, enhancing the anticancer effect. This study highlights the use of microbial metabolism and programmable bacteria with functional materials to create living, multipurpose anticancer systems.44 In addition, a study used the transdermal therapeutic cryomicroneedle patch to deliver bacteria to the tumour site. Following laser irradiation at a set time, Rhodospirillum rubrum (R.r-Au) efficiently produced hydrogen, depleted lactate, and enhance the activation of anticancer immunity. This study shows the potential of cryomicroneedle delivery of nanogold-engineered Rhodospirillum rubrum as a minimally invasive in situ delivery method for living bacterial drugs and provides new opportunities for nanoengineering bacteria to convert tumour metabolites into useful compounds for tumour optical biotherapy.45
Another study developed a photogenetic clearance approach for engineered bacteria. Initially, a photogenetic elimination gene circuit that utilizes the blue light-responsive system EL222, which incorporates the dark-induced antitoxic protein ccdA and the blue light-induced toxin ccdB, was developed. The engineered bacteria perished upon exposure to 488-nm laser irradiation, thereby enabling the engineered bacteria to function for the desired period of time.46
Light-induced control elements provide high spatiotemporal precision49–51and show great potential in basic research and clinical applications, but they still have some significant limitations in practice. First, because optical tweezers are only used in clear media, their in vivo usefulness is limited. Furthermore, biological samples may be photodamaged and biologically active medications may be inactivated by photothermal or photochemical methods when the target is directly exposed to high-intensity laser radiation.52 Therefore, in clinical applications, these treatments may damage healthy tissues in the irradiation pathway or affect the effect of biologically active drugs.53 Therefore, reducing the intensity or duration of light required and reducing damage to cells and tissues may be possible in the future by developing genetic light-responsive tools that are sensitive to a low light intensity.54,55 The introduction of antioxidants or photothermal protectants during light exposure may attenuate the damage to cells and active drugs caused by light exposure.56 Another significant limitation lies in the restricted penetration depth of light,57 which consequently constrains its accessibility to deep-seated tumour tissues.58 Implantable luminescent technology may represent a potentially viable solution.59,60
Spatiotemporal Manipulation by Magnetic Field
Alternating magnetic fields serve as ideal signals for the manipulation of microorganisms because of their nearly unrestricted tissue penetration capacity and superior biosafety.61–63 (Table 1) Recently, Escherichia coli cells were effectively bound to magnetic nanoparticles and nanoliposomes, which were loaded with photothermolysin and chemotherapeutic medicines. In order to locate the bacterial biohybrids in tumour tissues, magnetic fields align and direct them across three-dimensional porous microenvironments. Activated by pH and NIR light, photothermal and pH-sensitive liposomal carriers on biohybrids provide a flexible and on-demand delivery platform that enables the spatiotemporal release of chemotherapeutic molecules at the target. A multifunctional microrobotic platform has been developed for directed navigation inside three-dimensional biological networks and for delivering stimulus-responsive medicines for diverse medical applications.47 Afterward, Ma X et al created a prototype of a bacterium-based micro-biorobot called AMF-manipulated tumour-homing bacteria (AMF-Bac), which has five modules: active navigation, signal decoding, signal feedback, signal processing, and signal output. The active navigation module gives AMF-Bac active tumor-targeting to orthotopic colon cancers when administered colon-specifically. Once orthotopic colon tumours are targeted, the Fe3O4 nanoparticles in the engineered bacteria allow AMF-Bac to receive and transform magnetic signals into heat signals. This process triggers the expression of lytic proteins in the bacteria, which leads to bacterial lysis and the release of the antitumor protein CD47nb, which was previously stored and pre-expressed in the AMF-Bac. The AMF-Bac also permitted continual magnetic field-controlled mobility, which improved tumour targeting and dramatically increased the therapeutic efficacy. These findings not only represent a technique for the noninvasive, spatiotemporal, real-time AMF manipulation of tumour-homing bacterial gene expression but they also integrate the idea of modular design into the development of bacterial systems.48
Magnetic field-based spatiotemporal manipulation shows great potential for clinical applications. Unfortunately, magnetic tweezers cannot easily or accurately locate target sites in vivo due to the unfocused nature of the magnetic field, but the use of imaging techniques, such as magnetic resonance imaging or magnetic particle imaging, to precisely locate deep tumours may be possible in the future.64 Magnetic labelling can have irreversible effects on the viability and biological activity of biological samples. Engineered bacteria are difficult to modify and require the integration of specific magnetic materials or receptor genes into the bacterial genome. This process is complex and costly, and the success rate is not always high. In addition, a potential risk of an immune response to the introduced exogenous material may exist. Prolonged exposure to magnetic fields may also have an impact on the physiological functions and metabolism of the bacteria themselves.65 Therefore, much more work is needed to achieve clinical translation. Continuous efforts are needed to improve the related technologies, develop more efficient and safe magnetic materials and methods, increase the precision and convenience of gene editing tools, and explore innovative ways of multidisciplinary cross-fertilization in order to further improve the safety and anti-tumour efficacy.
Spatiotemporal Manipulation by Acoustic Waves
Recently, acoustic tweezers have become useful tools for working with biological particles because of their high tissue penetration, low damage, significant micrometre spatial accuracy, and adaptability to a variety of media.27 (Table 2) Recently, two US-inducible transgenic expression methods for cancer therapy have been developed in bacteria using the thermosensitive bacteriophage λ repressor TcI.66,67.(Figure 2) The pR-pL tandem promoter was activated to boost the expression of therapeutic outputs for triggering the immune system after the tumours were exposed to targeted ultrasonic irradiation to reach a temperature of 42°C to 45°C after bacterial colonization. The integrase-based state switch is uncontrollable once it is activated. Thus, immune checkpoint inhibitor synthesis over an extended period of time may be harmful to the immune system.66 Another bacterium that responds to ultrasound (US) was created by incorporating a US-activated therapeutic circuit into the E. coli MG1655 laboratory strain.66,67 In conclusion, by combining URB with FUS, we were able to induce highly spatiotemporally controllable gene expression in deep-seated tumours. Healthy tissue can be subjected to thermal damage in the range of temperatures from 42°C to 45°C. Therefore, it is essential to create US-activated transgenic expression systems that are more sensitive and manageable. These systems should be activated at temperatures of 39°C to 40°C and should be constructed by using safer, nonpathogenic, or weakened bacterial strains that are suitable for therapeutic purposes. Thus, the team developed an US-controlled activatable gene circuit, termed SINGER, which employs the heat-sensitive transcriptional regulator TlpA39. (Figure 2) The modified bacteria containing this system can be activated by US and locally heated to 39°C to precisely trigger efficient expression of designated genes. This study successfully achieved US -triggered protein expression and secretion in specifically engineered bacteria with minimal disruption and increased induction rates through promoter modification and the tuning of a ribosome-binding site.68 Designing therapeutic bacteria to be controlled by FUS, a form of energy that can be applied noninvasively to specific anatomical sites (eg, solid tumours), may address limitations such as the release of the payload into healthy tissue outside of the tumour resulting from systemic drug delivery and the transplantation of small numbers of bacteria. This control is provided by temperature-driven genetic state switches that respond to brief bursts of FUS-induced hyperthermia to generate a sustained therapeutic effect that successfully initiates and induces significant inhibition of tumour growth in situ. This technique provides a key tool for the spatiotemporal targeting of potent bacterial therapies in a variety of biological and clinical scenarios.
 |
Table 2 Spatiotemporal Manipulation by Acoustic Waves or HBO |
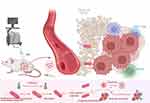 |
Figure 2 Spatiotemporal manipulation based on temperature-sensitive transcriptional regulators. Ultrasound stimulation-induced temperature increase activates thermosensitive transcriptional repressors, which in turn prompt the release of anti-tumor payloads from engineered bacteria. This action can lead to tumor cell apoptosis or stimulate an anti-tumor immune response.bacteria by employing temperature-sensitive transcriptional regulators. (Created in BioRender. Chang, x. (2025) https://BioRender.com/a36h404). Abbreviation: US, ultrasound. |
Nevertheless, manipulating normal cells directly is challenging because of their minute sizes and a close acoustic impedance match with the adjacent medium. Heterologous gene cluster expression has enabled the creation of bacteria that are capable of producing a multitude of submicron gas vesicles (GVs) within their cytoplasm. The incorporation of GVs significantly reduces the average bacterial density and enhances compressibility, which in turn increases acoustic responsiveness, making bacterial cells controllable via US. (Figure 3) By employing phased-array acoustic tweezers, it is possible to trap and manoeuvre clusters of these engineered bacteria, both inside and outside living organisms, by manipulating acoustic beams electronically. This method allowed for controlled, reverse, or on-demand circulation of bacteria within the vascular network of mice, thus facilitating targeted tumour therapy in preclinical models. This work also shows that this method can be used to increase the aggregation efficiency of engineered bacteria in malignancies. This work offers a framework for manipulating living cells in vivo, which will advance cell-based biomedical applications.69 Recently, an acoustic reporter gene (ARG1) has been engineered to produce gas vesicles in bacteria, which facilitates real-time imaging guidance for FUS. The operator would receive instant feedback when the tissue was exposed to hHIFU irradiation since the contrast signals would vanish as a result of GVs collapsing under the strong acoustic pressure. This advancement allows for the precise localization of the US focal point to the engineered bacteria within the tumour, prompting the bacteria to express and secrete IFN-γ locally within the tumour. The simultaneous application of IFN-γ and DOX on engineered bacteria stimulates tumour-specific T-cell responses, yielding a synergistic effect that significantly amplifies the antitumour response. Notably, the IFN-γ gene could be induced and therapeutic effects produced with just 25 minutes of high-intensity FUS irradiation at 42°C to 45°C. This process significantly increases safety for potential clinical applications.64
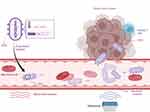 |
Figure 3 Spatiotemporal manipulation based on the combination of biosynthetic GVs and acoustic tweezers. Ultrasound enabled the controlled, reversible, or on-demand circulation of bacteria within the vascular network of mice; it also stimulated the release of IFN-γ and DOX from engineered bacteria, which in turn promotes tumour-specific T-cell responses. (Created in BioRender. Chang, x. (2025) https://BioRender.com/j04y108). Abbreviations: GVs, gas vesicles; DOX, doxorubicin. |
Sonodynamic therapy (SDT) utilizes US to activate sonosensitizers, which generate reactive oxygen species (ROS) to kill cancer cells. This method is emerging as a promising cancer treatment strategy because of its desired tissue penetration capacity, noninvasiveness, and low toxicity.71–74 A novel in vivo biotherapeutic drug (E. coli-pE@PCN) was developed by electrostatic adsorption of a nanoacoustic sensitizer onto engineered bacteria to be used with SDT. It was found that E. coli-pE@PCN could effectively accumulate in and penetrate the entire tumour owing to the instinctive bacterial targeting ability and active mobility. Engineered bacteria not only sustainably produce catalase to relieve tumour hypoxia but also facilitate the enriched and expanded distribution of the carried sonosensitizer at tumour sites. By utilizing the potency of sonodynamic therapy in promoting the release of tumour-associated antigens and bacterial pathogen-associated molecular patterns, E. coli-pE@PCN-based SDT could stimulate strong anti-tumour immune responses against cancer progression, metastasis, and recurrence. Thus, the biohybrids based on programmable bacteria created here open the door to developing next-generation sonodynamic immunotherapeutics to eradicate cancer and stop its spread and recurrence.70
While many benefits associated with the use of US for antitumor therapy have been noted, it also has inherent difficulties, especially when dealing with deep-seated or metastatic malignancies. Its efficacy in modulating gene expression is impeded by the tumour spread and depth, which necessitate more accurate heat transfer techniques. Potential remedies for more specialized treatment include external beam radiation and high-intensity FUS. Additionally, additional toxicological investigations are necessary to thoroughly assess the harmful effects of bacteria that have been created. The anticancer efficacy of engineered bacteria in patient-derived xenograft models requires further research in order to yield more insightful findings for clinical use.
Spatiotemporal Manipulation by HBO
It has been found in patients that HBO, a noninvasive treatment method, can effectively reduce the compact extracellular matrix, thereby promoting bacterial buildup inside tumours. (Table 2) Inspired by this finding, Xu et al modified EcN by incorporating cypate molecules to create EcN-cypate, a PTT tool that can initiate immunogenic cell death (ICD). HBO significantly boosts the accumulation of EcN-cypate within tumours and encourages the penetration of immune cells. When EcN–cypate is exposed to near-infrared (NIR) laser radiation, it achieves PTT and stimulates immunogenic cell death (ICD), which in turn triggers systemic immunological responses, including dendritic cell (DC) maturation, to destroy malignancies. HBO treatment dramatically increases the intratumoural accumulation of EcN–cypate and promotes the intratumoural infiltration of immune cells to achieve the desired tumour elimination. This work provides a straightforward and noninvasive way to boost the intratumoural transport efficiency of natural or engineered bacteria, which may be useful for the clinical application of bacteria-mediated synergistic cancer therapy.21
However, the therapeutic use of hyperbaric oxygen treatment is more complicated and expensive due to the need for specialist equipment and strict operation. Moreover, long-term efficacy may be limited by the stability and activity of engineered bacteria under hyperbaric oxygen. Therefore, we must use genetic engineering to improve the stability and targeting of modified bacteria in a hyperbaric oxygen environment. And the effectiveness and safety of its clinical application should be further validated. In addition, to further understand the mechanism of action of hyperbaric oxygen, we need to do further basic research.
Challenges and Outlook
Engineered bacteria have been combined with optical, magnetic, and acoustic tweezers and hyperbaric oxygen to precisely control multiple key steps in the process of bacterial treatment of tumours and to spatiotemporally control the release of therapeutic drugs, which can greatly improve treatment effectiveness and reduce costs for future cancer patients. In particular, focused ultrasound (FUS)-actuated bacterial medicines provide a route to eventual clinical implementation thanks to the expanding corpus of research on bacteria-based therapies75–77 and the growing clinical acceptance of FUS.78,79 More effort will be required to optimize the timing, dosage, and molecular identity of FUS-activated treatment release for every application. Combining FUS-activated bacterial treatments with other molecular or cellular therapies may improve therapeutic success. For instance, tumor entrance and engraftment patterns of immune cells and engineered microbes are different and frequently complementary. An environment that is more accessible to modified T cells may be created by creating bacteria that can enter immunosuppressed tumor areas and release cytokines or checkpoint inhibitors. In this manner, the bacteria and T cells can work together to fulfill their respective therapeutic roles.80 Despite notable advancements in achieving the spatiotemporal manipulation of engineered bacteria, critical challenges must be overcome to enable their successful clinical translation.
The clinical translation of engineered bacteria necessitates addressing critical biosafety and stability challenges. These microorganisms pose inherent risks due to their pathogenic potential, immunogenic properties, and robust colonization capacity, which may precipitate adverse outcomes, including sepsis and a systemic inflammatory response. Furthermore, while engineered bacterial vectors demonstrate biocompatibility and immune evasion capabilities, these attributes may inadvertently drive uncontrolled proliferation, genetic instability, and chronic toxicity.81 These properties necessitate implementing fourfold safeguards: (1) the selection of virulence-attenuated strains; (2) the development of novel materials for engineered bacterial delivery;46 (3) real-time antibiotic therapeutic management;82 and (4) real-time infection monitoring systems. In the future, we may be able to improve the biosafety and stability of engineered bacteria by delivering them to tumor sites with degradable materials that can persist in the body for long periods and develop genetic circuits for physical control of engineered bacteria—enabling engineered bacteria to die after a corresponding physical stimulus (eg, laser irradiation) so that the engineered bacteria can function for a desired period and degrade in a timely manner.46 The use of materials that degrade at specific triggers to manage the breakdown of delivered materials after the death of these bacteria, as well as improved targeting of materials, are key to improving the reliability and efficiency of this approach.
Developing intelligent engineered bacterial systems has become imperative to address the evolving therapeutic demands and enhance treatment efficacy. Contemporary synthetic biology approaches enable the precise spatiotemporal control of therapeutic payload release through bacterial–environment interactions. Notably, recent advancements have established synergistic platforms combining engineered bacteria with multimodal anticancer strategies, including radiotherapy,83–85 chemotherapy,86 and immunotherapy,87,88 thereby expanding the therapeutic landscape. Future developments may integrate engineered bacteria with multimodal antitumor therapies and spatiotemporal manipulation techniques through advanced synthetic biology platforms. This paradigm could increase therapeutic precision while optimizing tumour targeting efficacy. In addition, the synergistic actions of numerous spatiotemporal manipulation techniques may transcend the limitations of each technique alone and provide fresh perspectives for the area.89 Furthermore, combinatorial approaches utilizing multiple engineered bacterial consortia may potentiate antitumor responses through interactions.90
To further improve the controllability and targeting of spatiotemporally manipulated engineered bacteria, it is crucial to further construct visual bacteria64 and to add termination switches to engineered synthetic cargoes.25 In addition, exploring new methods of spatiotemporal manipulation (eg electric current) may shed new light on further development.
The antitumor efficacy of the above strategies must be tested in future studies, which may provide valuable clinical translation lessons.
Abbreviations
HBO, hyperbaric oxygen; ECM, extracellular matrix; NIR, near-infrared; AIE, aggregation-induced emission; MA, MeTTPy-D-Ala; NIR, near-infrared; EcN, Escherichia coli Nissle 1917; PTT, photothermal therapy; AMF, alternating magnetic field; AMF-Bac, AMF-manipulated tumor-homing bacteria; GVs, gas vesicles; IFN-γ, interferon γ; DOX, doxorubicin; SDT, sonodynamic therapy; US, ultrasound; FUS, focused ultrasound; ROS, reactive oxygen species; ICD, immunogenic cell death.
Data Sharing Statement
No data were used for the research described in the article.
Acknowledgments
We would like to express our gratitude to the Figraw and Biorender platforms. All figures in the article have been drawn through www.figdraw.com and app.biorender.com (Graphical abstract is created in BioRender. Chang, x. (2025) https://BioRender.com/c43f361)
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Major Science and Technology Innovation Project of Shandong Province (No. 2018CXGC1220); Projects ZR2022MH066, ZR2022QH236, ZR202111300475 and ZR2023MH333 were supported by the Shandong Provincial Natural Science Foundation. 2021 Shandong Medical Association Clinical Research Fund - Qilu Special Project YXH2022ZX02119.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Li Y, Zhao L, Li XF. Hypoxia and the tumor microenvironment. Technol Cancer Res Treat. 2021;20:15330338211036304. doi:10.1177/15330338211036304
2. Fan JX, Niu MT, Qin YT, Sun YX, Zhang XZ. Progress of engineered bacteria for tumor therapy. Adv Drug Deliv Rev. 2022;185:114296. doi:10.1016/j.addr.2022.114296
3. Stritzker J, Weibel S, Hill PJ, Oelschlaeger TA, Goebel W, Szalay AA. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297(3):151–162. doi:10.1016/j.ijmm.2007.01.008
4. Zhou S, Gravekamp C, Bermudes D, Liu K. Tumour-targeting bacteria engineered to fight cancer. Nat Rev Cancer. 2018;18(12):727–743. doi:10.1038/s41568-018-0070-z
5. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003;4(9):548–556. doi:10.1016/s1470-2045(03)01194-x
6. Huang X, Pan J, Xu F, et al. Bacteria-Based Cancer Immunotherapy. Adv Sci. 2021;8(7):2003572. doi:10.1002/advs.202003572
7. Garcia-Alvarez R, Vallet-Regi M. Bacteria and cells as alternative nano-carriers for biomedical applications. Expert Opin Drug Deliv. 2022;19(1):103–118. doi:10.1080/17425247.2022.2029844
8. Forbes NS. Engineering the perfect (bacterial) cancer therapy. Nat Rev Cancer. 2010;10(11):785–794. doi:10.1038/nrc2934
9. Chien T, Harimoto T, Kepecs B, et al. Enhancing the tropism of bacteria via genetically programmed biosensors. Nat Biomed Eng. 2022;6(1):94–104. doi:10.1038/s41551-021-00772-3
10. Taylor ND, Garruss AS, Moretti R, et al. Engineering an allosteric transcription factor to respond to new ligands. Nat Methods. 2016;13(2):177–183. doi:10.1038/nmeth.3696
11. Lagator M, Igler C, Moreno AB, Guet CC, Bollback JP. Epistatic interactions in the Arabinose Cis-Regulatory element. Mol Biol Evol. 2016;33(3):761–769. doi:10.1093/molbev/msv269
12. Wen M, Zheng JH, Choi JM, et al. Genetically-engineered Salmonella typhimurium expressing TIMP-2 as a therapeutic intervention in an orthotopic glioma mouse model. Cancer Lett. 2018;433:140–146. doi:10.1016/j.canlet.2018.06.031
13. Din MO, Danino T, Prindle A, et al. Synchronized cycles of bacterial lysis for in vivo delivery. Nature. 2016;536(7614):81–85. doi:10.1038/nature18930
14. Wu MR, Jusiak B, Lu TK. Engineering advanced cancer therapies with synthetic biology. Nat Rev Cancer. 2019;19(4):187–195. doi:10.1038/s41568-019-0121-0
15. Diekmann R, Wolfson DL, Spahn C, Heilemann M, Schüttpelz M, Huser T. Nanoscopy of bacterial cells immobilized by holographic optical tweezers. Nat Commun. 2016;7(1):13711. doi:10.1038/ncomms13711
16. Zhang H, Liu KK. Optical tweezers for single cells. J R Soc Interface. 2008;5(24):671–690. doi:10.1098/rsif.2008.0052
17. Carlsen RW, Edwards MR, Zhuang J, Pacoret C, Sitti M. Magnetic steering control of multi-cellular bio-hybrid microswimmers. Lab Chip. 2014;14(19):3850–3859. doi:10.1039/c4lc00707g
18. de Lanauze D, Felfoul O, Turcot J-P, Mohammadi M, Martel S. Three-dimensional remote aggregation and steering of magnetotactic bacteria microrobots for drug delivery applications. The International Journal of Robotics. Research. 2013;33(3):359–374. doi:10.1177/0278364913500543
19. Hammarstrom B, Laurell T, Nilsson J. Seed particle-enabled acoustic trapping of bacteria and nanoparticles in continuous flow systems. Lab Chip. 2012;12(21):4296–4304. doi:10.1039/c2lc40697g
20. Gutierrez-Ramos S, Hoyos M, Ruiz-Suarez JC. Induced clustering of Escherichia coli by acoustic fields. Sci Rep. 2018;8(1):4668. doi:10.1038/s41598-018-22960-z
21. Xu KF, Wu SY, Wang Z, et al. Hyperbaric oxygen enhances tumor penetration and accumulation of engineered bacteria for synergistic photothermal immunotherapy. Nat Commun. 2024;15(1):5147. doi:10.1038/s41467-024-49156-6
22. Jayaraman P, Devarajan K, Chua TK, Zhang H, Gunawan E, Poh CL. Blue light-mediated transcriptional activation and repression of gene expression in bacteria. Nucleic Acids Res. 2016;44(14):6994–7005. doi:10.1093/nar/gkw548
23. Ong NT, Olson EJ, Tabor JJ. Engineering an E. coli near-infrared light sensor. ACS Synth Biol. 2018;7(1):240–248. doi:10.1021/acssynbio.7b00289
24. Buss MT, Ramesh P, English MA, Lee-Gosselin A, Shapiro MG. Spatial control of probiotic bacteria in the gastrointestinal tract assisted by magnetic particles. Adv Mater. 2021;33(17):e2007473. doi:10.1002/adma.202007473
25. Alapan Y, Yasa O, Schauer O, et al. Soft erythrocyte-based bacterial microswimmers for cargo delivery. Sci Rob. 2018;3(17). doi:10.1126/scirobotics.aar4423
26. Kolosnjaj-Tabi J, Wilhelm C, Clément O, Gazeau F. Cell labeling with magnetic nanoparticles: opportunity for magnetic cell imaging and cell manipulation. J Nanobiotechnology. 2013;1(Suppl 1):S7. doi:10.1186/1477-3155-11-s1-s7
27. Ozcelik A, Rufo J, Guo F, et al. Acoustic tweezers for the life sciences. Nat Methods. 2018;15(12):1021–1028. doi:10.1038/s41592-018-0222-9
28. Marzo A, Drinkwater BW. Holographic acoustic tweezers. Proc Natl Acad Sci U S A. 2019;116(1):84–89. doi:10.1073/pnas.1813047115
29. Wang X, Li S, Liu X, et al. Boosting nanomedicine efficacy with hyperbaric oxygen therapy. Adv Exp Med Biol. 2021;1295:77–95. doi:10.1007/978-3-030-58174-9_4
30. Zhao EM, Zhang Y, Mehl J, et al. Optogenetic regulation of engineered cellular metabolism for microbial chemical production. Nature. 2018;555(7698):683–687. doi:10.1038/nature26141
31. Tandar ST, Senoo S, Toya Y, Shimizu H. Optogenetic switch for controlling the central metabolic flux of Escherichia coli. Metab Eng. 2019;55:68–75. doi:10.1016/j.ymben.2019.06.002
32. Liu X, Wu M, Wang M, et al. Metabolically engineered bacteria as light-controlled living therapeutics for anti-angiogenesis tumor therapy. Mater Horiz. 2021;8(5):1454–1460. doi:10.1039/d0mh01582b
33. Chia N, Lee SY, Tong Y. Optogenetic tools for microbial synthetic biology. Biotechnol Adv. 2022;59:107953. doi:10.1016/j.biotechadv.2022.107953
34. Lindner F, Diepold A. Optogenetics in bacteria - applications and opportunities. FEMS Microbiol Rev. 2022;46(2). doi:10.1093/femsre/fuab055
35. Emiliani V, Entcheva E, Hedrich R, et al. Optogenetics for light control of biological systems. Nat Rev Meth Primers. 2022;2. doi:10.1038/s43586-022-00136-4
36. Yang C, Cui M, Zhang Y, et al. Upconversion optogenetic micro-nanosystem optically controls the secretion of light-responsive bacteria for systemic immunity regulation. Commun Biol. 2020;3(1):561. doi:10.1038/s42003-020-01287-4
37. Pan H, Wang H, Yu J, et al. Near-infrared light remotely up-regulate autophagy with spatiotemporal precision via upconversion optogenetic nanosystem. Biomaterials. 2019;199:22–31. doi:10.1016/j.biomaterials.2019.01.042
38. Chen S, Weitemier AZ, Zeng X, et al. Near-infrared deep brain stimulation via upconversion nanoparticle–mediated optogenetics. Science. 2018;359(6376):679–684. doi:10.1126/science.aaq1144
39. Zheng B, Wang H, Pan H, et al. Near-infrared light triggered upconversion optogenetic nanosystem for cancer therapy. ACS Nano. 2017;11(12):11898–11907. doi:10.1021/acsnano.7b06395
40. Zhu X, Chen S, Hu X, et al. Near-infrared nano-optogenetic activation of cancer immunotherapy via engineered bacteria. Adv Mater. 2023;35(8):e2207198. doi:10.1002/adma.202207198
41. Zhang Y, Xue X, Fang M, et al. Upconversion optogenetic engineered bacteria system for time-resolved imaging diagnosis and light-controlled cancer therapy. ACS Appl Mater Interfaces. 2022;14(41):46351–46361. doi:10.1021/acsami.2c14633
42. Wu C, Cui M, Cai L, et al. NIR-responsive photodynamic nanosystem combined with antitumor immune optogenetics bacteria for precise synergetic therapy. ACS Appl Mater Interfaces. 2022;14(11):13094–13106. doi:10.1021/acsami.2c01138
43. Hu X, Chen J, Qiu Y, et al. Bacteria-based cascade in situ near-infrared nano-optogenetically induced photothermal tumor therapy. Theranostics. 2024;14(13):4933–4947. doi:10.7150/thno.98097
44. Chen B, Zhang X, Cheng L, et al. Surface programmed bacteria as photo-controlled NO generator for tumor immunological and gas therapy. J Control Release. 2023;353:889–902. doi:10.1016/j.jconrel.2022.12.030
45. Shi Q, Yin T, Zeng C, et al. Cryomicroneedle delivery of nanogold-engineered Rhodospirillum rubrum for photochemical transformation and tumor optical biotherapy. Bioact Mater. 2024;37:505–516. doi:10.1016/j.bioactmat.2024.03.032
46. Han C, Zhang X, Pang G, et al. Hydrogel microcapsules containing engineered bacteria for sustained production and release of protein drugs. Biomaterials. 2022;287:121619. doi:10.1016/j.biomaterials.2022.121619
47. Akolpoglu MB, Alapan Y, Dogan NO, et al. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci Adv. 2022;8(28):eabo6163. doi:10.1126/sciadv.abo6163
48. Ma X, Liang X, Li Y, et al. Modular-designed engineered bacteria for precision tumor immunotherapy via spatiotemporal manipulation by magnetic field. Nat Commun. 2023;14(1):1606. doi:10.1038/s41467-023-37225-1
49. Hartsough LA, Park M, Kotlajich MV, et al. Optogenetic control of gut bacterial metabolism to promote longevity. Elife. 2020;9. doi:10.7554/eLife.56849
50. Lalwani MA, Ip SS, Carrasco-López C, et al. Optogenetic control of the lac operon for bacterial chemical and protein production. Nat Chem Biol. 2021;17(1):71–79. doi:10.1038/s41589-020-0639-1
51. Liu Z, Zhang J, Jin J, Geng Z, Qi Q, Liang Q. Programming bacteria with light—sensors and applications in synthetic biology. Front Microbiol. 2018;9:2692. doi:10.3389/fmicb.2018.02692
52. Chai J, Zhao Y, Xu L, et al. A noncovalent photoswitch for photochemical regulation of enzymatic activity. Angew Chem Int Ed Engl. 2022;61(30):e202116073. doi:10.1002/anie.202116073
53. Nuyts S, Van Mellaert L, Theys J, Landuyt W, Lambin P, Anné J. The use of radiation-induced bacterial promoters in anaerobic conditions: a means to control gene expression in Clostridium -mediated therapy for cancer. Radiat Res. 2001;155(5):716–723. doi:10.1667/0033-7587(2001)155[0716:tuorib]2.0.co;2
54. Qiao L, Niu L, Wang M, et al. A sensitive red/far-red photoswitch for controllable gene therapy in mouse models of metabolic diseases. Nat Commun. 2024;15(1):10310. doi:10.1038/s41467-024-54781-2
55. Rost BR, Wietek J, Yizhar O, Schmitz D. Optogenetics at the presynapse. Nat Neurosci. 2022;25(8):984–998. doi:10.1038/s41593-022-01113-6
56. Zhang X, Pan W, Kuang J, Wang K, Li N, Tang B. A nitric oxide-boosting molecular agent to enhance mild-temperature photothermal therapy. Chem Commun. 2025;61(21):4212–4215. doi:10.1039/d5cc00219b
57. Ash C, Dubec M, Donne K, Bashford T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med Sci. 2017;32(8):1909–1918. doi:10.1007/s10103-017-2317-4
58. Fan J-X, Li Z-H, Liu X-H, Zheng D-W, Chen Y, Zhang X-Z. Bacteria-mediated tumor therapy utilizing photothermally-controlled TNF-α expression via oral administration. Nano Lett. 2018;18(4):2373–2380. doi:10.1021/acs.nanolett.7b05323
59. Zou P, Lin R, Fang Z, et al. Implanted, wireless, self-powered photodynamic therapeutic tablet synergizes with ferroptosis inducer for effective cancer treatment. Adv Sci. 2023;10(36):e2302731. doi:10.1002/advs.202302731
60. Tao L, Kong Y, Xiang Y, Cao Y, Ye X, Liu Z. Implantable optical fiber microelectrode with anti-biofouling ability for in vivo photoelectrochemical analysis. Chin Chem Lett. 2023;34(3):107481. doi:10.1016/j.cclet.2022.04.079
61. Sitti M, Wiersma DS. Pros and cons: magnetic versus optical microrobots. Adv Mater. 2020;32(20):e1906766. doi:10.1002/adma.201906766
62. Huang G, Qiu Y, Yang F, et al. Magnetothermally triggered free-radical generation for deep-seated tumor treatment. Nano Lett. 2021;21(7):2926–2931. doi:10.1021/acs.nanolett.1c00009
63. Ho D, Sun X, Sun S. Monodisperse magnetic nanoparticles for theranostic applications. Acc Chem Res. 2011;44(10):875–882. doi:10.1021/ar200090c
64. Yang Y, Wang Y, Zeng F, Chen Y, Chen Z, Yan F. Ultrasound-visible engineered bacteria for tumor chemo-immunotherapy. Cell Rep Med. 2024;5(5):101512. doi:10.1016/j.xcrm.2024.101512
65. Filipič J, Kraigher B, Tepuš B, Kokol V, Mandic-Mulec I. Effects of low-density static magnetic fields on the growth and activities of wastewater bacteria Escherichia coli and Pseudomonas putida. Bioresour Technol. 2012;120:225–232. doi:10.1016/j.biortech.2012.06.023
66. Abedi MH, Yao MS, Mittelstein DR, et al. Ultrasound-controllable engineered bacteria for cancer immunotherapy. Nat Commun. 2022;13(1):1585. doi:10.1038/s41467-022-29065-2
67. Chen Y, Du M, Yuan Z, Chen Z, Yan F. Spatiotemporal control of engineered bacteria to express interferon-γ by focused ultrasound for tumor immunotherapy. Nat Commun. 2022;13(1):4468. doi:10.1038/s41467-022-31932-x
68. Gao T, Niu L, Wu X, et al. Sonogenetics-controlled synthetic designer cells for cancer therapy in tumor mouse models. Cell Rep Med. 2024;5(5):101513. doi:10.1016/j.xcrm.2024.101513
69. Yang Y, Yang Y, Liu D, et al. In-vivo programmable acoustic manipulation of genetically engineered bacteria. Nat Commun. 2023;14(1):3297. doi:10.1038/s41467-023-38814-w
70. Wang C, Chen L, Zhu J, et al. Programmable bacteria‐based biohybrids as living biotherapeutics for enhanced cancer sonodynamic‐immunotherapy. Adv Funct Mater. 2024;34(30). doi:10.1002/adfm.202316092
71. Wan G-Y, Liu Y, Chen B-W, Liu -Y-Y, Wang Y-S, Zhang N. Recent advances of sonodynamic therapy in cancer treatment. Cancer Biol Med. 2016;13(3):325–338. doi:10.20892/j.issn.2095-3941.2016.0068
72. Qian X, Zheng Y, Chen Y. Micro/nanoparticle-augmented Sonodynamic Therapy (SDT): breaking the depth shallow of photoactivation. Adv Mater. 2016;28(37):8097–8129. doi:10.1002/adma.201602012
73. Son S, Kim JH, Wang X, et al. Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev. 2020;49(11):3244–3261. doi:10.1039/c9cs00648f
74. Pan X, Wang H, Wang S, et al. Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci. 2018;61(4):415–426. doi:10.1007/s11427-017-9262-x
75. Chien T, Doshi A, Danino T. Advances in bacterial cancer therapies using synthetic biology. Curr Opin Syst Biol. 2017;5:1–8. doi:10.1016/j.coisb.2017.05.009
76. Dou J, Bennett MR. Synthetic biology and the gut microbiome. Biotechnol J. 2018;13(5):e1700159. doi:10.1002/biot.201700159
77. Chang Z, Guo X, Li X, et al. Bacterial immunotherapy leveraging IL-10R hysteresis for both phagocytosis evasion and tumor immunity revitalization. Cell. 2025;188(7):1842–1857.e20. doi:10.1016/j.cell.2025.02.002
78. Haar &, Coussios C. High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia. 2007;23(2):89–104. doi:10.1080/02656730601186138
79. O’Reilly MA. Exploiting the mechanical effects of ultrasound for noninvasive therapy. Science. 2024;385(6714):eadp7206. doi:10.1126/science.adp7206
80. Vincent RL, Gurbatri CR, Li F, et al. Probiotic-guided CAR-T cells for solid tumor targeting. Science. 2023;382(6667):211–218. doi:10.1126/science.add7034
81. Cao Z, Cheng S, Wang X, Pang Y, Liu J. Camouflaging bacteria by wrapping with cell membranes. Nat Commun. 2019;10(1):3452. doi:10.1038/s41467-019-11390-8
82. Crull K, Weiss S. Antibiotic control of tumor-colonizing Salmonella enterica serovar Typhimurium. Exp Biol Med. 2011;236(11):1282–1290. doi:10.1258/ebm.2011.011111
83. Li H, Pei P, He Q, et al. Nanozyme-coated bacteria hitchhike on CD11b + immune cells to boost tumor radioimmunotherapy. Adv Mater. 2024;36(8):e2309332. doi:10.1002/adma.202309332
84. Zhang Y, Liu Y, Li T, et al. Engineered bacteria breach tumor physical barriers to enhance radio-immunotherapy. J Control Release. 2024;373:867–878. doi:10.1016/j.jconrel.2024.07.076
85. Wang Y, Dong A, Man J, et al. TREM2 scFv-engineering Escherichia coli displaying modulation of macrophages to boost cancer radio-immunotherapy. Adv Mater. 2025:e2417920. doi:10.1002/adma.202417920
86. Shen H, Zhang C, Li S, et al. Prodrug-conjugated tumor-seeking commensals for targeted cancer therapy. Nat Commun. 2024;15(1):4343. doi:10.1038/s41467-024-48661-y
87. Fu L, He Q, Lu X, Hu L, Qian H, Pei P. Surface engineering on bacteria for tumor immunotherapy: strategies and perspectives. Adv Funct Mater. 2024;34(42). doi:10.1002/adfm.202405304
88. Redenti A, Im J, Redenti B, et al. Probiotic neoantigen delivery vectors for precision cancer immunotherapy. Nature. 2024;635(8038):453–461. doi:10.1038/s41586-024-08033-4
89. Zhang X, Zang Z, Liang Z, et al. Nanobiohybrid oncolytic bacteria with optimized intratumoral distribution for combined sono–photodynamic/immunotherapy. ACS Nano. 2025;19(6):6437–6453. doi:10.1021/acsnano.4c16740
90. Wang Y, Fan Y, Zhang X, et al. In situ production and precise release of bioactive GM-CSF and siRNA by engineered bacteria for macrophage reprogramming in cancer immunotherapy. Biomaterials. 2025;317:123037. doi:10.1016/j.biomaterials.2024.123037
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.