Back to Journals » International Journal of Nanomedicine » Volume 19
Recent Progress of Exosomes in Hematological Malignancies: Pathogenesis, Diagnosis, and Therapeutic Strategies
Authors Zhang H , Xia J, Wang X , Wang Y , Chen J , He L, Dai J
Received 24 May 2024
Accepted for publication 15 October 2024
Published 9 November 2024 Volume 2024:19 Pages 11611—11631
DOI https://doi.org/10.2147/IJN.S479697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Hu Zhang,1,* Jingyi Xia,2,* Xueqing Wang,1 Yifan Wang,2 Jie Chen,3 Lin He,1 Jingying Dai2
1Department of Pharmacy, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China; 2Department of Hematology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China; 3Central Laboratory, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, 610072, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jingying Dai, Department of Hematology, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, No. 32, West Section 2, First Ring Road, Qingyang District, Chengdu, 610072, People’s Republic of China, Tel +86-15756317270, Email [email protected] Lin He, Department of Pharmacy, Sichuan Academy of Medical Sciences & Sichuan Provincial People’s Hospital, School of Medicine, University of Electronic Science and Technology of China, No. 32, West Section 2, First Ring Road, Qingyang District, Chengdu, 610072, People’s Republic of China, Tel +86-17708130632, Email [email protected]
Abstract: Hematological malignancies originate from the hematopoietic system, including lymphoma, multiple myeloma, leukaemia, etc. They are highly malignant with a high incidence, a poor prognosis and a high mortality. Although the novel therapeutic strategies have partly improved the clinical efficacy of hematological malignancies, patients still face up with drug resistance, refractory disease and disease relapse. Many studies have shown that exosomes play an important role in hematological malignancies. Exosomes are nanoscale vesicles secreted by cells with a size ranging from 40 to 160 nm. They contain various intracellular components such as membrane proteins, lipids, and nucleic acids. These nanoscale vesicles transmit information between cells with the cargos. Thus, they participate in a variety of pathological processes such as angiogenesis, proliferation, metastasis, immunomodulation and drug resistance, which results in important role in the pathogenesis and progression of hematological malignancies. Furthermore, exosomes and the components carried in them can be used as potential biomarkers for the diagnosis, therapeutic sensitivity and prognosis in hematological malignancies. In the therapy of hematologic malignancies, certain exosome are potential to be used as therapeutic targets, meanwhile, exosomes are suitable drug carriers with lipid bilayer membrane and the nanostructure. Moreover, the tumor-derived exosomes of patients with hematologic malignancies can be developed into anti-tumor vaccines. The research and application of exosomes in hematological malignancies are summarized and discussed in this review.
Keywords: hematological malignancies, exosomes, biomarkers, targets, drug carriers, vaccines
Introduction
Hematological malignancies are malignancies originating from the hematopoietic system, mainly including lymphoma, multiple myeloma, and leukemia.1–3 Lymphoma is a clonal malignancy of lymphocyte cells with the top 1 incidence in hematological malignancies. It can be classified based on the morphology and immunohistochemistry as Hodgkin’s lymphoma and non-Hodgkin’s lymphoma.4 Multiple myeloma (MM) is characterized by abnormal proliferation of clonal plasma cells in the bone marrow with the top 2 incidence in hematological malignancies. MM remain incurable.5 Leukemia is a hematological malignancies originate from uncontrolled growth and differentiation of leukemic cells. Leukemia is generally divided into 2 types - acute leukemia and chronic leukemia. Acute leukemia can be subdivided into subtypes including acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). Chronic leukemia can be subdivided into subtypes including chronic lymphocytic leukemia (CLL), chronic myelogenous leukemia (CML).6,7 AML is the most common type of acute leukemias while CLL is the most common type of chronic leukemias.8,9 Although there have been significant improvement in the therapy of hematological malignancies, part of the patients after conventional therapy still face up with relapse, refractory disease and poor prognosis.
The researches on the pathogenesis and progression of hematological malignancies are ongoing in order to search for novel strategies for the control of these malignancies. Exosomes are nanoscale vesicles with the size ranging from 40 to 160 nm. They contain various components including proteins, lipids, nucleic acids, etc. These nanoscale vesicles transmit information between cells with the cargos.10–12 Exosomes and the components carried by them involve in interaction between hematological malignant cells and tumor microenvironment. In the recent ten years, scientists started to reveal that exosomes play an important role in the occurrence, progression, and metastasis of various hematological malignancies.13 For instance, exosomes regulate on angiogenesis with exosomal miR-let-7c/miR-135b/miR-340 in MM14–16 and exosomal miR-155 in lymphoma.17 Exosomes carry TNF-Related Apoptosis-Inducing Ligand (TRAIL), miR-15a, miR-1305, S100A4, etc to regulate on the proliferation, metastasis and transformation of hematologic malignant cells.18–21 Exosomes can also reflect the drug resistance in hematological malignancies such as exosomal RPL13/RPL14 for imatinib resistance in CML,22 exosomal Transforming Growth Factor Beta 1(TGFB1)/miR-155/miR-375 for TKI resistance in AML,23 exosomal HSP70 for bortezomib resistance in MM, etc.24
Furthermore, exosomes can be used as potential biomarkers for the diagnosis, therapeutic sensitivity and prognosis of hematological malignancies. Exosomes contain various specific proteins, nucleic acids, metabolites and lipids, which can reflect the properties of the origin cells of exosomes.25,26 Thus, exosomes can be valuable biomarkers for early disease detection and disease monitoring such as exosomal miR-99a-5P, miR125b-5P, miR-200C, miR141, etc in lymphomas.27,28 Moreover, exosomes can be associated with prognosis such as exosomal miR-532 in AML,29 exosomal miR-99a-5P/miR-125b-5P in DLBCL, etc.27 As exosomes involve in pathogenesis and development of hematological malignancies, some exosomes are potential to be used as therapeutic targets for hematological malignancies including exosomal miR-107 for DLBCL,30 exosomal Hsa_circ_0058493 for CML,31 exosomal miR-23b-5P for AML, etc.32 In the therapeutic strategies of hematological malignancies, exosomes are suitable to be used as drug carriers with lipid bilayer membrane and the nanostructure.33,34 For instance, exosomes are used to deliver BCL6 siRNA for the treatment of DLBCL in mice,35 deliver Imatinib or BCR-ABL siRNA for the treatment of CML in mice,33,36 deliver TRAIL for the treatment of MM in mice, etc18 Tumor-derived exosomes and dendritic cell derived exosomes carry tumor antigens. Thus, these exosomes are potential to be used as anti-tumor vaccines for the stimulation of anti-tumor immune efficacy.37,38 The research and application of exosomes in hematological malignancies are summarized and discussed in the following parts.
Exosome
Overview of Exosomes
Exosomes were initially discovered by Trams et al during the 1980.39 In 1987, Johnstone et al made the discovery that exosomes contain a significant quantity of bioactive components derived from reticulocytes. These exosomes participate in the maturation of reticulocytes into red blood cells.40 In 1991, it was revealed that exosomes serve a dual purpose – not only promoting the growth of reticulocytes, but also providing the primary route for eliminating membrane proteins during the process of membrane remodeling.41 In recent years, exosomes have been a hot topic in the medical and biological research fields. The key milestones in exosome research over the years have been depicted in Figure 1.42–49
 |
Figure 1 Timeline of exosome research milestones. |
During the initial phase of exosome studies, exosomes were commonly described as extracellular vesicles ranging in size from 30 to 150 nm.50 Currently, exosomes are specifically referred to as discoid vesicles with diameters ranging from 40 to 160 nm. Initially, exosomes were believed to be a byproduct of cellular secretion.51 However, recent research has shown that exosomes possess a lipid bilayer structure, allowing them to directly fuse with recipient cell membranes through endocytosis. This mechanism enables exosomes to facilitate the transfer of substances from one cell to another. Exosomes contain various bioactive components such as lipids, proteins, mRNA, lncRNA, miRNA, etc.52 In addition, different cells can employ the release of exosomes containing unique components as a means of achieving intercellular communication. The receiving cells capture and internalize these exosomes, allowing for interaction and communication through the exchange or release of their contents.
Biogenesis of Exosomes
Exosomes can originate from a variety of sources, including humans, animals, plants, and bacteria. Almost all cells can secrete exosomes. Exosomes are widely distributed and can be found in peripheral blood, cerebrospinal fluid, saliva, urine, as well as breast milk.53–55 The biogenesis of exosomes is a complex process that encompasses three distinct stages (Figure 2): (a) In the first stage, the endocytosis of the plasma membrane leads to the formation of endocytic vesicles. These vesicles subsequently fuse to form early endosomes within the cell. Early endosomes further mature into late endosomes. (b) In the second stage, there is inward budding of the late endosome membrane, leading to the formation of intraluminal vesicles (ILVs). Many ILVs compose the multivesicular bodies (MVBs). (c) In the final stage, the MVBs further fuse with the cell surface membrane and release their ILVs, termed as exosome.56–58
Composition of Exosomes
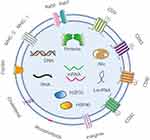
Exosomes are nanoscale vesicles secreted by cells, containing various cellular components such as membrane proteins, lipids, and nucleic acids from the cell (Figure 3).59 The membrane proteins are primarily categorized into two different groups. One group includes proteins found in nearly all exosomes. These proteins serve as distinguishing factors that can effectively differentiate exosomes and other vesicles, such as tetraspanins (CD9, CD63, CD81, CD82), cell adhesion molecules (integrins, CD11b, and CD54), and major histocompatibility complex (MHC) molecules (MHC-I and MHC-II). These membrane proteins play crucial roles in the identification and specific binding of exosomes.60 Another group includes proteins found in exosomes derived from specific cell types rather than all cell types. Various proteins of this group have been identified in exosomes. These proteins include fusion and trafficking proteins such as Rab2, Rab7, flotillin and annexins; heat shock proteins such as Hsc70 and Hsc90; cytoskeletal proteins such as actin, myosin and tubulin; as well as proteins that can facilitate the formation of MVBs, such as Alix.61–63 Exosomes possess numerous proteins that serve diverse functions such as signal transmission, cellular binding and immune regulation. Lipids are fundamental elements present in all cell types. The lipids found in exosomes originate from the cell membrane. The main components of lipids consist of phospholipids, including phosphatidylcholine and phosphatidylinositol. In addition, sphingomyelin, ganglioside GM3 and cholesterol are common lipids found in the cell membrane.64 Nucleic acids within exosomes encompass DNA and RNA. RNA is the main functional component in target cells. The RNA in exosomes primarily includes messenger RNAs (mRNAs), microRNAs (miRNAs), and long non-coding RNAs (LncRNAs). MiRNAs are functional RNA that can potentially regulate the levels of RNA and proteins within cells, thereby affecting both the morphology and function of the cell. These nucleic acids can be transmitted between different cells via exosomes. It has been reported that in specific diseases, the nucleic acids of exosomes may also play important biological roles, including the regulation of tumor development.65,66
In general, exosomes are vesicles with a complex composition, which encompass a variety of biomacromolecules. The interaction and collaboration of these elements exhibit the biological functions of exosomes. Moreover, with the continuous advancement of research related to exosomes, the composition and function of exosomes are being further clarified.
Methods of Exosome Isolation
The small size of exosomes has posed a significant challenge in their isolation. To further introduce exosomes into clinical applications, it is necessary to effectively extract exosomes from various cellular components. In the past, ultracentrifugation was predominantly employed to isolate exosomes, segregating them based on different centrifugal speeds. A progressively accelerated centrifugation process is commonly used, resulting in the precipitation of exosomes.67 This method yields exosomes of relatively high purity. However, high-speed centrifugation may cause mechanical or thermal damage to the exosomes, which could ultimately alter their structure or compromise their biological activity.68 Therefore, novel separation methods have been developed based on membrane filtration and density gradient centrifugation (Table 1). In recent years, there has been a significant advancement in research techniques for exosome separation, including the utilization of immunomagnetic beads, microfluidic chips, aerodynamic sorting and electrophoretic separation methods. These methods contain advantages, such as minimizing the required sample volume, reagent consumption and separation time.69 As different exosome separation methods possess their respective advantages and disadvantages, an appropriate method should be selected according to the specific experimental requirements and sample properties. However, at the same time, to enhance the purity and efficiency of exosome separation, a combination of multiple methods can be used.
 |
Table 1 Techniques of Exosome Isolation |
Methods for Exosome Identification
Exosomes are cup-shaped vesicular structures with diameters ranging from 40 to 160nm, which are not visible to the naked eye or an optical microscope. To solve this problem, transmission electron microscopy (TEM) has become a popular method for examining the morphology of exosomes, as it allows for the observation of their distinctive cup-shaped structure.83 The characterization methods of exosomes mainly fall into two distinct categories: external (physical) methods focus on their morphology and particle size, while characterization (composition/biochemical) methods examine their membrane protein and lipid content (Table 2).84 The selection of these methods depends on the purpose of the study and the available resources, as there are both advantages and disadvantages associated with each approach. For instance, Nanoparticle Tracking Analysis (NTA) or immunological methods can be used for preliminary screening of exosomes. However, for a more comprehensive investigation of the biological characteristics and composition, more refined methods such as Mass Spectrometry (MS) can be used. At present, there is no single method available that can directly determine whether a vesicle is an exosome. For example, according to the requirements of the vesicle association, it is necessary to utilize at least two different methods for accurately characterizing a single vesicle for mutual complementarity in identification (Scanning Electron Microscopy (SEM)/ TEM + NAT, etc). Thus, the combination of these identification methods can more comprehensively determine the presence of exosomes and their characteristics, including quantity, size and component composition. This serves as a crucial groundwork for future investigation into the functions of exosomes.
 |
Table 2 Technology of Exosome Identification |
The Roles of Exosomes in the Pathogenesis and Progression of Hematological Malignancies
Angiogenesis
Although hematological malignancies do not rely on vascular networks in the same direct manner as solid tumors, they still depend on vascular supply to some extent for acquiring nutrients as well as oxygen, and also require blood vessels for the metastasis of malignant cells. Exosomes released by hematologic malignant cells can alter the bone marrow microenvironment and impact angiogenesis. The vascular system plays a crucial role in supplying oxygen, nutrients, hormones, growth factors, and eliminating metabolic wastes during the repair and regeneration of damaged tissues.91 In the field of lymphoma research, exosomes derived from human EBV-positive (EBV+) Burkitt lymphoma cells (Raji) were found to promote angiogenesis in lymphoma retinal pigment epithelial cells (ARPE-19) by delivering overexpression of miR-155.17
Many studies have suggested that exosomes can effectively regulate the process of angiogenesis in the bone marrow microenvironment, which is an integral part of the multiple myeloma (MM) microenvironment. For example, it was reported in a study that exosome miR-let-7c secreted by mesenchymal stem cells could increase the polarization of M2 macrophages and promote the ability of angiogenesis in the bone marrow microenvironment.14 In another study reported by Umezu et al, investigators successfully established a hypoxia-resistant MM [HR-MM] cell model in vitro. The study found that HR-MM cells could significantly increase exosome secretion under both normoxic as well as acute hypoxic conditions and the miR-135b expression level in these exosomes was also increased. This study further revealed that miR-135b in MM exosomes can significantly promote angiogenesis by inhibiting hypoxia-inducible factor 1 (FIH-1).15 In addition, another study found that exosomes from bone marrow mesenchymal stem cells can potentially influence the process of angiogenesis. The expression of exosomal microRNAs (miRNAs) differed in bone marrow stromal cells derived from younger and older populations. The researchers observed that miR-340 was preferentially expressed in exosomes originating from young bone marrow mesenchymal stem cells (BMSCs) by direct transfection of miR-340. It effectively inhibited angiogenesis through the hepatocyte growth factor/c-MET (HGF/c-MET) signaling pathway. These findings may provide new directions for investigating and treating MM patients.16
In CLL, tumor cell-derived exosomes play an important role in creating a favorable environment that supports tumor cell growth. They enhance the formation of isolated neo-arterial microvessels, promote endothelial tube formation, and induce the production of vascular endothelial growth factor (VEGF) thereby increasing angiogenesis.92 In another elegant study, Taverna et al93 demonstrated that adding CML cell-derived exosomes to Human Umbilical Vein Endothelial Cells (HUVECs) could significantly increase Interleukin 8(IL8) and vascular cell adhesion molecule-1 (VCAM1) concentrations, but exosomes encapsulating curcumin reversed these effects and attenuated angiogenesis. Experimental studies have validated this antiangiogenic property under both in vitro and in vivo settings.
In conclusion, Malignant cells in hematologic malignancies can promote or inhibit angiogenesis by releasing exosomes and altering the bone marrow microenvironment to facilitate their growth and spread. This provides new perspectives for understanding and treating hematological tumors and the possibility of developing new therapies for the management of hematological malignancies.
Proliferation and Metastasis
Exosomes play a key role in the proliferation and spread of hematologic malignant cells, as they transport key signaling molecules, such as growth factors, kinases, and miRNAs, which are required for tumor cell growth and survival. These molecules can stimulate cell division as well as proliferation and inhibit apoptosis, thereby promoting the growth of hematologic malignant cells.94,95 In addition, certain molecules in exosomes, such as Matrix metalloproteinases (MMPs) and miRNAs, can effectively alter cell adhesion and migration. This can aid in dissemination of the tumor cells from their primary site and facilitate their migration to the bone marrow or other tissues. A study by Rivoltini et al18 reported that K562 cell-derived exosomes carrying TRAIL resulted in a decreased growth of lymphoma cells and an increase in programmed cell death. Injection of TRAIL exosomes into tumors further reduced tumor growth in Stanford University-Diffuse Histiocytic Lymphoma-4 (SUDHL4) mice, thereby leading to decreased lymphoma proliferation in SUDHL4 mice.
Wang et al96 analyzed the potential effect of bone marrow stromal cells(BMSCs)-derived exosomes on the viability, proliferation, and spreading of MM cells using a mouse 5T33MM model and human MM samples. Interestingly, it was found that In myeloma patients, exosomes derived from BMSCs induced tumor growth in vivo and promoted tumor cell spread in myeloma transformation models. A study using an in vivo myeloma transformation model found that BMSC-derived exosomes obtained from myeloma patients could significantly promote tumor cell growth and metastasis in vivo. The study delved into the down-regulation of the tumor suppressor gene regulator miRNA-miR-15a by BMSC-derived exosomes. The results showed that miR-15a produced by BMSC-derived exosomes could inhibit both tumour cell growth and metastasis.19 Furthermore, another study investigated the increased secretion of exosomes by MM cells under hypoxic conditions. Interestingly, decrease of cytoplasmic miR-1305 and the increase in miR-1305 target gene expression due to increased exosomal miR-1305 as well as the promoting effects of mouse double sensitive 2 homolog (MDM2), insulin-like growth factor 1 (IGF1), and fibroblast growth factor 2 (FGF2) on MM oncogenic activity were observed. This ultimately resulted in exosomal miR-1305-induced transfer of MM cells to the macrophages.20
A recent study found that Bone marrow mesenchymal stem cells-derived exosomes (BM-MSC-exos) could significantly increase the number of leukemia stem cells (LSCs) and the metastatic potential of leukemia cells. Mechanistically, this phenomenon can be explained by the up-regulation of S100A4 into the leukemia cells by BM-MSC-exos. S100A4 is a member of the S100 family of calcium-binding proteins and can reported to modulate different biological processes, such as cellular accretion, cell spreading, and apoptosis. The proliferation and spreading of leukemia cells were significantly enhanced due to the increase of S100A4 after BM-MSC-exo treatment.21
Thus, the release of exosomes by blood tumor cells is significant in altering both the cells themselves and their neighboring environment. This process plays a critical role in the growth, spread, and treatment resistance, making it a current hotspot in tumor research and therapy. Hence, targeting exosomes or key molecules within them may provide a novel therapeutic strategy for hematological malignancies.
Immunomodulation
Exosomes play an important role in the immunomodulation of hematologic malignant cells. The hematological tumor cells can release exosomes containing a variety of immunosuppressive factors, including PD-L1 and tumor growth factor (TGF)-β, which can effectively inhibit the activity of effector T cells and NK cells while enhancing the function of immunosuppressive cells such as regulatory T cells and myeloid-derived suppressor cells.97,98 This mechanism has the potential to partly mitigate the attack of the immune system on tumors. In recent studies, it has been revealed that the primary cause for the generation of anti-tumor immunosuppression in the body is due to exosome mediation of tumor cell origin. For instance, Ferguson Bennit et al99 found that lymphoma cell-derived exosomes contain the inhibitor of apoptosis (IAP) protein survivin, which can act on the immune function of NK cells. NK cells were extracted from the peripheral blood of the healthy donors and processed using pure survivin proteins or exosomes from two distinct lymphoma cell lines, namely Diffuse Large B-cell lymphoma 2(DLCL2) and Follicular Small Cleaved Cell Lymphoma (FSCCL). Moreover, research has shown that although exosome treatment does not significantly alter NK cell function, it can have an effect on the immune function of NK cells by decreasing natural killer group 2D receptor (NKG2D) levels and intracellular protein levels of perforin, granzyme B, TNF-alpha and IFN-γ.
Khalife et al100 reported that MM cells release miR-16 in large quantities through EVs, which can correlate with intracellular expression associated with chromosome 13 deletion (Del13). Indeed, miR-16 can directly target the Inhibitor of KB kinase α/β (IKKα/β) complex of the NF-ĸB-typical pathway, which can lead to activation of M2-type Tumor-Associated Macrophages (M2TAMs). In addition, overexpressed miR-16 can significantly enhance anti-MM immunoreactivity through proteasome inhibitors in the presence of MM-resident bone marrow TAMs. A recently reported study demonstrated that treatment of MM cells with eicosapentaenoic acid or docosahexaenoic acid reduced the immunosuppressive effects of MM cells, while still maintaining their immunostimulatory response.101 The activation or inhibition of the immune response is contingent on the cell of origin of exosomes.
Exosomes have garnered significant attention as a novel anti-tumor immunotherapeutic approach. For instance, in a study involving AML patients, it was found that serum exosomes served as a valuable source of dendritic cell (DC) antigens. These exosomes affected the cytotoxicity against tumors by evaluating the percentage of specific lysis of K562 leukemia cells in co-culture. The results of the study revealed that the co-culture of exosomes with DCs significantly reduced the lysis rate of K562 cells. This implies that exosomes may influence the immunomodulatory effects of DCs.102 In another study, Hu et al103 encoded two B7 co-stimulatory molecules (CD80 and CD86) using lentiviral vectors and transduced them into L1210 leukemia cells, and successfully obtained leukemia cell exosomes (LEXs) with high expression of CD80 and CD86. LEXs modified with the B7 gene could effectively induce antigen-specific anti-leukemia cytotoxic T lymphocyte (CTL) responses. Subsequent studies in animals revealed that immunomodulation by B7-modified LEX, especially LEX-CD8086, significantly delayed leukemia tumor growth compared to controls. This development could boost the immune response against leukemia and offer a promising approach for immunotherapy in treating the disease.
In summary, exosomes play a key role in the immunomodulation of hematological tumors. They can affect the interaction of the tumor cells with the immune system, inhibit anti-tumor immune responses and potentially modulate the efficacy of immunotherapy. Therefore, a deeper understanding of the specific mechanisms of exosomes in immunomodulation, particularly in terms of immunosuppression and antigen presentation, may contribute to developing new immunotherapeutic strategies. For example, it may be possible to enhance the effect of immunotherapy if the action of immunosuppressive factors (such as PD-L1 or TGF-β) in exosomes can be inhibited, or if the response of antigen-presenting cells to exosomes can be enhanced.
Drug Resistance
Exosomes can also carry anti-apoptotic factors and signals that induce autophagy, thereby enhancing the resistance of the tumor cells against apoptosis caused by drugs. In addition, exosomes can promote the development of drug resistance by altering the tumor microenvironment.104 For example, exosomes can stimulate surrounding fibroblasts or immune cells, triggering the production of signals that promote growth and resistance to drugs.105 Exosomes released from cancer-associated fibroblasts (CAF) in the lymphoma microenvironment have been implicated in lymphoma cell survival and antipyrimidine drug resistance. Moreover, RNA sequencing studies of miRNAs in exosomes conducted by Kunou et al revealed that miR-4717-5p, one of the most abundant miRNAs in exosomes, not only can inhibit ENT2 expression under both in vivo and in vitro settings but also induce antipyrimidine resistance.106
Jones et al24 further demonstrated that bortezomib-resistant MM cells could transfer resistance to sensitive cells via exosomal HSP70 delivery. In another recent study, exosomes from MM cell lines treated with melphalan or bortezomib showed an elevated expression of acid sphingomyelinase (ASM). The exosomes high in ASM were able to transfer drug-resistant phenotypes to chemosensitive cells, thereby indicating the potential protective impact of ASM on tumors.107
In a groundbreaking study, Viola et al23 revealed that the release of exosomes enriched in Transforming Growth Factor Beta 1(TGFB1), miR-155 and miR-375 from stromal cells in AML may mediate resistance to tyrosine kinase inhibitors during chemotherapy. In addition, LI et al extracted exosomes from mixed plasma samples of 9 patients with imatinib (IM)-resistant CML and 9 patients with IM-sensitive CML, followed by protein characterization, and found that RPL13 and RPL14 in the exosomes showed abnormal up-regulation in patients with imatinib-resistant CML, which induced IM resistance.22
Hence, the crucial role of exosomes in the development of drug resistance in suggests that targeting or modifying these mechanisms may be an effective strategy for overcoming drug resistance in hematological tumors. For example, drugs can be designed to target exosomes to block their formation, release, or action, thereby facilitating the prevention of drug resistance. The development of drug resistance can also be predicted and monitored by analyzing the contents of exosomes.
The Role of Exosomes in the Diagnosis and Therapy of Hematological Malignancies
Biomarkers
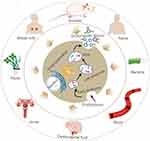
Exosomes due to their stability in body fluids such as blood, are being considered a promising source of biomarkers that can reflect the properties of cells of origin. For example, exosomes produced by tumor cells may contain specific proteins, nucleic acids, metabolites, and lipids, which can serve as potential biomarkers for the tumors (Table 3).108–110 In addition, some studies have indicated that the tumor cells may produce more exosomes compared to the normal cells, so blood levels of exosomes could also be useful for tumor diagnosis.111 In various types of malignant hematological neoplasms, including AML, ALL, CLL, CML and MM, researchers discovered that the number of extracellular vesicles in the peripheral blood was significantly increased. These vesicles derived from the tumor cells, especially exosomes, offers a valuable noninvasive approach for detecting and monitoring tumor development.95,112–115 Exosomes play important roles in hematological malignancies (Figure 4). There is an increasing body of evidence suggesting that miRNAs have the potential to serve as valuable biomarkers for early disease detection and monitoring patients following chemotherapy treatments.116,117 In a study targeting exosome-derived miRNAs as biomarkers for DLBCL, researchers identified 37 significantly increased and 17 significantly decreased miRNAs. Among the four miRNAs identified to be upregulated, the elevation of miR-99a-5p and miR-125b-5p was particularly significant. Increased exosomal miR-99a-5p and miR-125b-5p in the serum samples obtained from DLBCL patients were strongly associated with progression-free survival time and effectively predicted response to chemotherapy. Therefore, exosomal miR-99a-5p and miR-125b-5p possess the potential to be employed as biomarkers for DLBCL.27 Mycosis fungoides (MF) is a T-cell-associated lymphoma, and Moyal et al’s study of microRNAs from exosomes derived from MF patients revealed that both miR-155 and miR-1246 microRNAs were delivered to target cells. Further studies revealed that miR-155 had a migratory effect on target cells and that plasma levels of miR-155 were up-regulated only in plaque/tumor MF. miR-1246 plasma levels were highest in combined plaque/tumor MF. The findings suggest that MF-derived exosome pairs grow cell motility. Based on miR-155 and miR-1246 plasma expression, they could serve as potential biomarkers for T-cell-associated lymphomas.118 Hu et al28 conducted a cerebrospinal fluid (CSF) exosomal microRNA (miRNA) study for the early diagnosis of primary central nervous system lymphoma (PCNSL) and monitoring of chemotherapy efficacy. They further verified the expression of miR-200c and miR-141 in cerebrospinal fluid exosomes using relative quantitative real-time polymerase chain reaction. Ultimately found that miR-200c and miR-141 were up-regulated in cerebrospinal fluid exosomes of PCNSL patients after chemotherapy, which suggests that they have the potential to be used as biomarkers for the diagnosis and monitoring of the efficacy of chemotherapy for PCNSL.
 |
Table 3 Exosomes as Potential Biomarkers and Targets for Hematologic Malignancies |
There is growing evidence that circRNAs in exosomes are associated with the invasion and metastasis of solid tumors. Their potential application as novel biomarkers for hematological malignancies is also highlighted.126 CircHNRNPU was identified as one of the most abundant and differentially expressed circRNAs in IgD MM. In addition, the growth of circHNRNPU was found to be associated with poor prognostic outcomes. Interestingly, MM cells produce and release circHNRNPU, which is responsible for encoding a specific protein called circHNRNPU_603aa. Moreover, overexpression of circHNRNPU_603aa resulted in enhanced MM cell growth under both in vitro and in vivo settings. However, targeted inhibition of circHNRNPU_603aa expression by siRNA technology was able to significantly eliminate this promoting effect. Notably, MM cells release circHNRNPU through exosomes, which can affect multiple cells in the BM microenvironment. Thus, circHNRNPU_603aa can be potentially considered a promising diagnostic and therapeutic marker in MM cells as well as the BM microenvironment.124 The growing interest in studying circulating microRNAs has led to extensive research on their potential use as biomarkers in malignant tumors. Zhang et al120 isolated serum exosomal microRNAs and serum circulating microRNAs from sera of patients with SMM or MM and healthy individuals. They then quantified the expression levels of the selected microRNAs by real-time polymerase chain reaction (PCR). The differences in the levels of miR-20a-5p, miR-103a-3p, and miR-4505 in serum exosomes of MM patients, SMM patients, and healthy subjects were found to be statistically significant. Interestingly, significant differences in serum levels of let-7c-5p, miR-185-5p, and miR-4741 were observed in MM patients compared to SMM patients or healthy controls. Consequently, serum exosomal microRNAs possess the capacity to act as independent and novel MM biomarkers. In another elegant study, Gu et al121 used microarray technology to screen the differently expressed miRNAs from bone marrow stromal stem cells (BMSCs) from patients with multiple myeloma (MM-MSCs) or benign diseases (BD-MSCs). Thereafter, by conducting in-depth experimental observation, it was found that miR-483-5p expression was significantly increased in MM-MSCs. In addition, MM-MSCs could effectively transport miR-483-5p to MM cells through exosomes, thereby increasing miR-483-5p expression in these cells. Compelling evidence suggests that the release of exosomes by MM-MSCs and their regulation of the miR-483-5p/TIMP2 axis play a pivotal role in promoting the malignant progression of MM. These important findings reveal the central role of BMSC-derived exosomal miRNAs in MM and emphasize their potential as significant biomarkers for the diagnosis and treatment of MM. Zhang et al found that serum exosomal miR-451 expression was significantly lower in MM patients compared to healthy controls. At the same time, the ROC curve showed that the area under the curve of miR-451 was reduced, suggesting that miR-451 may be a biomarker for the diagnosis of MM.122
The discovery and identification of new miRNAs may hold promising opportunities for the creation of innovative therapeutic strategies for leukemia patients127,128 For instance, in a study of AML patients, Lin et al29 found an interesting phenomenon that an increase in plasma exosome-produced miR-532 was associated with better overall survival (OS). Thus, the detection of exosome-derived miR-532 could potentially serve as a new biomarker with an important reference value for treating AML. A recent study comparing exosomes isolated from plasma samples of patients with ALL and B-ALL cell lines found significantly elevated levels of miR-326 in the exosomes of the patients. This suggests that miR-326 in exosomes may have potential as a biomarker for ALL diagnosis.119
In conclusion, exosomal miRNAs play an important potential in tumour genesis, progression, clinical typing and early diagnosis. They hold great clinical importance and show potential as a reliable tumor biomarker. Although the application of exosomes in the diagnosis and treatment of hematological malignancies is still being studied, the potential of exosomes makes this research area remarkably interesting and promising.
Potential Targets
Exosomes play a crucial role in facilitating intercellular communication, ultimately creating a conducive environment within the bone marrow for tumor development. Therefore, an increasing number of research studies are directing their attention towards investigating the secretion mechanism of exosomes in order to explore their potential as novel therapeutic targets (Table 3).129,130 Many studies have suggested that various miRNAs can act as tumor suppressors and oncogenes and can be potential therapeutic targets for hematologic malignancies.131 In a study of cancer-associated fibroblasts (CAF) and their derived exosomes in the lymphoma microenvironment, miR-4717-5p was found to be one of the most abundant miRNAs in exosomes by RNA sequencing analysis of miRNAs in exosomes, and further studies revealed that exosomes secreted by CAF including miR-4717-5p play a key role, suggesting that they are a promising potential target for the treatment of lymphoma.106 By exploring the role of exosomal miR-107 in lymphomagenesis, Liu et al30 detected a significant reduction of exosomal miR-107 in the plasma of DLBCL patients and further found that miR-107 significantly eliminated cell proliferation, induced apoptosis, and inhibited cell invasion in vitro, and inhibited tumor growth in vivo. Thus highlighting the potential of miR-107 as a new therapeutic target for DLBCL treatment. Shao et al found elevated levels of Enolase 2 (ENO2) in serum and DLBCL cell exosomes from DLBCL patients. In addition, DLBCL-derived exosomes were assimilated by macrophages, which then regulated macrophage polarisation. DLBCL-derived exosome ENO2 promoted DLBCL proliferation and migration by affecting glucose degradation through the GSK3β/β-catenin/c-Myc signalling pathway, which ultimately transformed macrophages into an M2-like phenotype. The results suggest that exosomal ENO2 may be a promising therapeutic target for DLBCL.125
In the tumor microenvironment, the transformation of normal fibroblasts (NFs) into cancer-associated fibroblasts (CAFs) promotes angiogenesis and influences the course of tumorigenesis. MicroRNA-21 (miR-21) is highly expressed in MM. CAF-derived exosomes have been found to contain large amounts of miR-21, and thus miR-21 is expected to be a potential therapeutic target for MM.123 The important role of circular RNAs (circRNAs) in tumor progression and exosome-mediated intracellular communication are considered to be key factors in the pathogenesis of MM. However, Zhang et al132 initially explored the role of exosome-derived circulating RNAs (exo-circRNAs) in MM and MM-induced peripheral neuropathy (PN) by investigating the role of exosomal circulating RNAs in MM-associated PN. Abnormal expression of serum exo-circular RNAs was found to be associated with MM-associated PN, suggesting that exo-circular RNAs may be a novel therapeutic target for MM-associated PN. Barrera et al133 performed miRNA sequencing against exosomes isolated from bone marrow-derived MSC from AML patients and healthy controls. The study discovered that the targets of miRNA in exosomes derived from AML-infected MSCs were distinct from those in control samples. Interestingly, two molecules whose gene expression was predicted to be down-regulated in AML by miRNA analysis (EZH3 and GSK2β) showed up-regulation in AML-derived cells. Therefore, by analyzing miRNAs in exosomes of AML-derived MSCs, potential miRNAs related to AML can be detected, thus providing new insights into leukemogenesis and new possibilities for therapeutic targets. In addition, Zhong et al31 demonstrated the presence of a specific molecule called Hsa-circ-0058493 in PBMC. Hsa_circ_0058493 was found to be significantly increased in imatinib-resistant cell-derived exosomes, suggesting its potential as a promising prognostic biomarker and providing a potential therapeutic target for CML treatment. A recent study conducted by Cheng and other researchers32 discovered TRIM14’s role in stimulating the proliferation of AML cells through the activation of the PI3K/AKT pathway. On the contrary, exosomes from human bone marrow mesenchymal stem cells (HMSC) were able to reverse the state of AML cells by delivering miR-23b-5p. These results imply that miR-23b-5p and TRIM14 have potential roles as therapeutic targets for AML.
However, despite the enormous potential of exosomes, there are several challenges that need to be addressed before exosomes can be used as therapeutic targets. For example, it is imperative to gain deeper insights into the distribution and metabolism of exosomes in the body as well as to develop novel methodologies to precisely target exosomes to specific cells or tissues.
Drug Carriers
Exosomes are ideal drug delivery vehicles due to their various natural properties such as good biocompatibility, low toxicity, and low immunogenicity. They have great potential for effectively delivering small molecules of nucleic acids and small molecules of chemical drugs to specific locations.134–136 They can transport a variety of biomolecules, including proteins, lipids, and nucleic acids, between the source and target cells. Exosomes possess the unique ability to cross various biological barriers due to their nanoscale dimensions and ability to carry cell surface molecules.137–140 The surface of exosomes can be genetically engineered or chemically modified to carry specific markers for targeting the tumor cells and to further improve the precision of drug delivery.141 Secondly, exosomes play a vital role in protecting the drug enclosed within them from enzymatic degradation in the body, resulting in enhanced drug bioavailability. Nucleic acid drugs have shown potential as novel therapeutic agents in treating various diseases. However, the absence of efficient cell-specific delivery mechanisms poses significant obstacles to the systemic administration of these drugs, particularly for precise targeting of cancer cells, notably hematologic malignant cells. There are two major challenges encountered in siRNA therapy’s clinical use, namely the immunogenicity of siRNA and the effectiveness of delivering siRNA to the target cells. However, recent studies have revealed that exosomes can effectively transport siRNA to tumor tissues in mice, offering fresh perspectives on the subject. To reduce the immunogenicity of siRNAs, the investigators opted to employ immature DCs to produce exosomes. The researchers then modified these DCs to express Lysosomal-associated membrane protein 2b (Lamp2b). This protein was fused with an AV integrin-specific iRGD peptide to produce a tumor-targeting exosome (iRGD-Exo). The resulting exosome, known as iRGD-Exo, was then loaded with BCL6 siRNA using electroporation to effectively target tumor cells. Subsequently, these iRGD-Exo were delivered in vivo by intravenous injection and successfully delivered BCL6 siRNA to the tumor tissues, leading to significant inhibition of tumor growth in DLBCL. Furthermore, the study discovered that exosomes containing BCL6 siRNA did not trigger significant toxic responses in mice. This finding holds great significance for the future implementation of siRNA therapy in a clinical setting. Thus, exosomes have the possibility and potential to be tools for siRNA delivery.35 This discovery can not only provide novel ideas to solve the problems of siRNA therapy in clinical application but may also provide innovative possibilities and avenues for treating diseases such as DLBCL.
The TRAIL has the ability to selectively induce apoptosis in tumor cells, but the clinical application of artificial recombinant TRAIL is limited due to its short half-life in vivo. In another study, Rivoltini et al18 found that exosomes expressing TRAIL induced apoptosis and inhibited tumor progression in vitro. Moreover, results of in vivo experiments revealed that exosomes loaded with TRAIL could significantly reduce the size of the tumors in the MM mouse model and exhibited good anti-tumor effects.
BCR-ABL fusion proteins play a driving role in the pathogenesis of CML. Imatinib (IM) is a selective inhibitor approved against BCR-ABL with significant therapeutic effects. In an elegant study, Bellavia et al designed HEK293T cells to express the exosome protein Lamp2b and further attached the protein to interleukin 3 (IL3) fragments. It was observed that IL3L exosomes loaded with IM or BCR-ABL siRNA could precisely target CML cells. Thus, it was able to effectively inhibit the growth of tumor cells and reduce drug resistance as well as adverse reactions during the treatment.36 In addition, curcumin also plays an important role in the treatment of CML. It can inhibit BCR-ABL expression in CML cells through exosome-borne miR-21 and thereby prevent tumor cell growth. This mechanism suggests that the incorporation of miR-21 in exosomes may enhance curcumin’s efficacy against leukemia in CML treatment, offering a novel approach for therapeutic intervention in medical practice.141
Although there is considerable potential in using exosomes as drug carriers, there are various challenges that need to be effectively addressed. Examples include methods for producing exosomes in large quantities, enhancing their stability, improving exosome drug loading efficiency, and ensuring their proper distribution and clearance in the body. The treatment of hematological malignancies will be a step closer to utilizing exosomes as drug carriers once these issues are resolved.
Vaccines
The application of exosomes as potential vaccines in hematological malignancies is an emerging area of research. Cancer cells can generate tumor-specific antigens and present them to the immune system through exosomes due to their distinct characteristics, such as the ability to move freely around the body and carry antigens associated with the exosome cell of origin. This allows the immune system to recognize and attack tumor cells with the same antigen.142 In addition, exosomes can directly engage with immune cells such as DCs and T cells, playing a role in triggering and regulating immune reactions. Furthermore, DCs play a key role in the tumor microenvironment in the activation and modulation of innate and adaptive immune mechanisms. Exosomes produced by the tumor cells can be effectively captured by DCs, which in turn can elicit an immune response to the tumor.143 Chaperone-rich cell lysates (CRCLs) have gained significant attention in antitumor vaccine development as tumor-derived CRCL can trigger an effective anti-tumor response by activating DCs. In particular, after CRCL derived from GL261 glioma cells was loaded into DCs, the DEXs generated from these DCs were found to exhibit important biological functions. This method was employed to treat DCs to produce DEX in Casitas B-cell spectrum lymphoma. It was found that (Cbl)-b and c-Cbl signaling pathways were negatively regulated. Furthermore, phosphatidylinositol 261-kinase (PI3K)/Akt and extracellular signal-regulated kinase (ERK) signaling pathways in T cells were activated, which were reported to augment anti-tumor activity. These findings provide innovative and promising insights for developing anti-lymphoma vaccines.142 Chen and other researchers143 offered valuable information on exosomes derived from DLBCL cell lines, which were demonstrated to contain malignancy molecules such as c-Myc, Bcl-2, Mcl-1, CD19, and CD20. The researchers found that exosomes from DLBCL cell lines are readily captured by DCs as well as lymphoma cells. These exosomes acted primarily as immunosuppressive mediators by inducing apoptosis in T cells and up-regulating programmed cell death protein 1 (PD-1). In vivo, these exosomes were able to enhance cell growth and migration as well as promote angiogenesis, thereby facilitating tumor development. However, exosomes from DLBCL cell lines did not induce apoptosis in DCs. These exosomes can interact with DCs to stimulate the clonal expansion of T cells and increase the secretion of interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFα). At the same time, they can cause significant inhibition of the immunosuppressive cytokines interleukin 4 (IL-4) and interleukin 10 (IL-10). This discovery opens up new possibilities for immunovaccine therapy for lymphoma.
A study by Xie et al144 showed that a vaccine developed using exosomes had a significant therapeutic effect against MM. This study used exosomes derived from MM cells to initiate an immune response against the tumor and establish prophylactic immunity. It was found that MM cell-derived exosomes with membrane-bound HSP70 were able to promote dendritic cell (DC) maturation by up-regulating the co-stimulatory molecules CD40/CD80, and the inflammatory cytokines IL-1/IL12/tumor necrosis factor (TNF)-alpha. These exosomes enhanced the anti-tumor immunity of CD8+ cytotoxic T lymphocytes (CTL). Therefore, these MM cell-derived exosomes with membrane-bound HSP70 as a vaccine are effective in inducing anti-MM immune effects. Moreover, the newly developed vaccine from multi-epitope exosomes based on MM-specific antigens could inhibit the progression of MM by inducing significant CTL responses. These findings provide new strategies and hope for individualized treatment of MM patients.
Nanoscale vesicles released by the cells have been demonstrated to induce anti-leukemic immune responses. LEXs, which are exosomes derived from leukemia cells, are potential components that can be used in anti-tumor vaccines for tumor immunotherapy. Since the LEX vaccine showed general potency in vivo, it may be influenced by immunosuppressive PD-L1 proteins in exosomes. However, a recent study was designed to potentially optimize the efficacy of a LEX-based vaccine by targeting the PD-L1 protein in exosomes. The investigators conducted a comparison between the effects of PD-L1-silenced acute lymphoblastic leukemia-derived leukemia cells (LEXPD-L1si) and unmodified exosomes in inducing antileukemic immunity. During the experiment, PD-L1 expression in parental leukemia cells and LEX was down-regulated by lentivirus-mediated PD-L1 shRNA. It was observed that in comparison to unmodified LEX, LEXPD-L1si induced better dendritic cell (DC) maturation and enhanced T cell activation. This approach allowed LEXPD-L1si to trigger more intense T cell expansion and Th1 cytokine secretion after immunization with LEX. Further examination revealed that LEXPD-L1si was more effective in triggering antigen-specific cytotoxic T-lymphocyte (CTL) responses. Furthermore, in preclinical experiments, the researchers vaccinated mice using an exosomal vaccine in which PD-L1 was silenced to investigate its ability to induce both protective and therapeutic anti-tumor CTL responses in vivo. The experimental results showed that vaccination with LEXPD-L1si substantially inhibited tumor growth and significantly prolonged the survival of immunized mice. Thus, this study demonstrated that the efficacy of LEX-based vaccines in generating more robust anti-leukemia immune reactions can be significantly improved by reducing the presence of PD-L1 protein in exosomes.145 In another study, Li et al146 optimized the LEX-based anti-leukemia vaccine. A novel strategy was adopted in this study to bind CD4+T cells to the genetically modified leukemia cell-derived exosomes. Lentiviral vectors carrying genes encoding CD80 and CD86 were thereafter successfully transfected with L1210 leukemia cells, which significantly elevated the expression of both CD80 and CD86 in leukemia cells. Exosomes derived from these genetically transduced leukemia cells (LEX expressing high levels of CD80 and CD86, or called LEX-CD8086) were found to efficiently promote the proliferation of CD4+T cells and the secretion of Th1 cytokines. This finding suggested that the efficacy of LEX-based vaccines can be remarkably improved by optimizing the expression of co-stimulatory molecules. LEX-CD8086 significantly increased CD4+T cells proliferation and Th1 cytokine release in comparison to the control LEX. Exosomes derived from leukemia cells, which have been genetically modified with CD80 and CD86 co-stimulatory molecules, allow better targeting of CD4+T cells and efficiently induce an anti-leukemic immune response. This optimized LEX-based vaccine may have promising potential for treating leukemia through immunotherapy.
Although exosomes have shown potential as anti-tumor vaccines in laboratory studies, many challenges need to be addressed before exosomes can be effectively utilized as clinical anti-tumor vaccines. For example, there is a need to find an efficient and scalable method for extracting exosomes from cancerous cells, alongside assessing the optimal means of transporting these exosomes to immune cells. In addition, further studies on the safety, efficacy, and immune memory functions of exosomal vaccines are needed. Although exosomes still face up with many challenges as tumor vaccines, their potential is enormous and could revolutionize future cancer therapies.
Conclusion and Prospects
Exosomes have become an important research field in both biology and medicine. Exosomes have been shown to carry and deliver a variety of biomolecules, including proteins, lipids, RNA, etc, which makes exosomes an important tool for intercellular communication. Exosomes play a vital role in hematological malignancies. Tumor cells of hematological malignancies effectively manipulate their microenvironment via exosomes, influencing the processes of angiogenesis, immune regulation, and tumor resistance, which can lead to the proliferation and spread of malignant cells. In hematologic malignancies, exosomes have been studied and recognized as potential biomarkers for diagnosis and therapy due to their abilities to reflect the physiological and pathological state of their cells of origin. Some aberrantly expressed components originating from exosomes of hematological malignancies, such as miRNAs, proteins, and sphingolipids, may become potential therapeutic targets. Exosomes have nanostructures and various natural properties, such as good biocompatibility, low toxicity, and low immunogenicity, which make them potentially excellent carriers for drug delivery in the treatment of hematologic malignancies. In addition, exosomes can efficiently deliver tumor antigens, thus they have a great potential to be developed into vaccines against hematologic malignancies.
Although the application of exosomes in the diagnosis and treatment of hematological malignancies is very promising, research on exosomes as a diagnostic and therapeutic tool is ongoing and still in the early stages. The development of innovative techniques for the accurate and effective detection of exosomes, the regulation of production and release of exosomes to influence processes of hematological malignancies, as well as the application of exosomes as drug carriers should be further studied and focused in future research. Moreover, the further understanding of differences in exosomes regarding individual differences, disease stages, and biological characteristics is important for the application of exosomes for the diagnosis and treatment of hematologic malignancies in the clinic.
Abbreviations
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ASM, acid sphingomyelinase; BM-MSC-exos, Bone marrow mesenchymal stem cells-derived exosomes; BMSCs, bone marrow mesenchymal stem cells; CAF, cancer-associated fibroblasts; CAF, cancer-associated fibroblasts; CLL, chronic lymphocytic leukemia; CML, chronic myelogenous leukemia; CRCLs, Chaperone-rich cell lysates; CSF, cerebrospinal fluid; CTL, cytotoxic T lymphocyte; DC, dendritic cell; Del13, chromosome 13 deletion; DLCL2, Diffuse Large B-cell lymphoma 2; ENO2, Enolase 2; ERK, extracellular signal-regulated kinase; exo-circRNAs, exosome-derived circRNAs; FCM, Flow Cytometry; FGF2, fibroblast growth factor 2; FIH-1, hypoxia-inducible factor 1; FSCCL, Follicular Small Cleaved Cell Lymphoma; HGF/c-MET, hepatocyte growth factor/c-MET; HM, Hematological malignancies; HMSC, human bone marrow mesenchymal stem cells; HSPs, heat shock proteins; HUVECs, Human Umbilical Vein Endothelial Cells; IAP, inhibitor of apoptosis; IGF1, insulin-like growth factor 1; IKKα/β, Inhibitor of KB kinase α/β; IL-10, interleukin 10; IL3, interleukin 3; IL-4, interleukin 4; IL-6, interleukin-6; IL8, Interleukin 8; ILVs, intraluminal vesicles; IM, imatinib; iRGD-Exo, iRGD peptide to produce a tumor-targeting exosome; JMML, juvenile myelomonocytic leukemia; Lamp2b, Lysosomal-associated membrane protein 2b; LEXPD-L1si, PD-L1-silenced acute lymphoblastic leukemia-derived leukemia cells; LEXs, leukemia cell exosomes; LncRNAs, long non-coding RNAs; LSCs, leukemia stem cells; M2TAMs, M2-type Tumor-Associated Macrophages; MDM2, mouse double sensitive 2 homolog; MF, Mycosis fungoides; MHC, major histocompatibility complex; miRNAs, microRNAs; miRNAs, microRNAs; MM, Multiple myeloma; MM-MSCs, multiple myeloma; MMPs, Matrix metalloproteinases; mRNAs, messenger RNAs; MVBs, multivesicular bodies; NFs, normal fibroblasts; NTA, Nanoparticle Tracking Analysis; NTA, Nanoparticle Tracking Analysis; OS, overall survival; PCNSL, primary central nervous system lymphoma; PCR, polymerase chain reaction; PD-1, programmed cell death protein 1; PI3K, phosphatidylinositol 261-kinase; PN, peripheral neuropathy; PS, Phosphatidylserine; qRT-PCR, real-time polymerase chain reaction; SEC, Size Exclusion Chromatography; SEM, Scanning Electron Microscopy; SUDHL4, Stanford University-Diffuse Histiocytic Lymphoma-4; TEM, transmission electron microscopy; TGF, -β tumor growth factor; TGFB1, Transforming Growth Factor Beta 1; TNFα, tumor necrosis factor alpha; TRAIL, TNF-Related Apoptosis-Inducing Ligand; TRAIL, tumor necrosis factor related apoptosis inducing ligand; VCAM1, vascular cell adhesion molecule-1; VEGF, vascular endothelial growth factor.
Acknowledgments
This work was supported by grants from the Sichuan Academy of Medical Sciences and the Sichuan Provincial People’s Hospital Clinical Study and Translational Fund (No. 2020LZ01) and Sichuan Science and Technology Program (2023YFS0159).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
2. Kansara RR, Speziali C. Immunotherapy in hematologic malignancies. Current Oncol. 2020;27(Suppl 2):S124–s131. doi:10.3747/co.27.5117
3. Taylor J, Xiao W, Abdel-Wahab O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood. 2017;130(4):410–423. doi:10.1182/blood-2017-02-734541
4. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. 2018;68(6):394–424. doi:10.3322/caac.21492
5. Petrucci MT, Vozella F. The Anti-CD38 antibody therapy in multiple myeloma. Cells. 2019;8(12):1629. doi:10.3390/cells8121629
6. Chupradit S, Km Nasution M, Rahman HS, et al. Various types of electrochemical biosensors for leukemia detection and therapeutic approaches. Anal Biochem. 2022;654:114736. doi:10.1016/j.ab.2022.114736
7. Juliusson G, Hough R. Leukemia. Prog Tumor Res. 2016;43:87–100. doi:10.1159/000447076.2
8. Nitika WJ, Hui AM, Hui A-M. Role of Biomarkers in FLT3 AML. Cancers. 2022;14(5):1164. doi:10.3390/cancers14051164
9. Forbes Kaprive J, Samsel J, Loperfito A, Towe M, Garofola C. Beyond the surface: chronic lymphocytic leukemia diagnosis during mohs micrographic surgery. Cureus. 2024;16(8):e66771. doi:10.7759/cureus.66771
10. Wang H, You Y, Zhu X. The role of exosomes in the progression and therapeutic resistance of hematological malignancies. Front Oncol. 2022;12:887518. doi:10.3389/fonc.2022.887518
11. Allegra A, Petrarca C, Di Gioacchino M, et al. Exosome-mediated therapeutic strategies for management of solid and hematological malignancies. Cells. 2022;11(7):1128. doi:10.3390/cells11071128
12. Ghaffari K, Moradi-Hasanabad A, Sobhani-Nasab A, Javaheri J, Ghasemi A. Application of cell-derived exosomes in the hematological malignancies therapy. Front Pharmacol. 2023;14:1263834. doi:10.3389/fphar.2023.1263834
13. Litwińska Z, Łuczkowska K, Machaliński B. Extracellular vesicles in hematological malignancies. Leukemia Lymphoma. 2019;60(1):29–36. doi:10.1080/10428194.2018.1459606
14. Tian X, Sun M, Wu H, et al. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma by polarizing M2 macrophages in the bone marrow microenvironment. Leuk Res. 2021;105:106566. doi:10.1016/j.leukres.2021.106566
15. Umezu T, Tadokoro H, Azuma K, et al. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124(25):3748–3757. doi:10.1182/blood-2014-05-576116
16. Umezu T, Imanishi S, Azuma K, et al. Replenishing exosomes from older bone marrow stromal cells with miR-340 inhibits myeloma-related angiogenesis. Blood Adv. 2017;1(13):812–823. doi:10.1182/bloodadvances.2016003251
17. Yoon C, Kim J, Park G, et al. Delivery of miR-155 to retinal pigment epithelial cells mediated by Burkitt’s lymphoma exosomes. Tumor Biol. 2016;37(1):313–321. doi:10.1007/s13277-015-3769-4
18. Rivoltini L, Chiodoni C, Squarcina P, et al. TNF-related apoptosis-inducing ligand (TRAIL)-armed exosomes deliver proapoptotic signals to tumor site. Clin Cancer Res. 2016;22(14):3499–3512. doi:10.1158/1078-0432.CCR-15-2170
19. Krishnan SR, Bebawy M. Circulating biosignatures in multiple myeloma and their role in multidrug resistance. Mol Cancer. 2023;22(1):79. doi:10.1186/s12943-022-01683-w
20. Lee JY, Ryu D, Lim SW, et al. Exosomal miR-1305 in the oncogenic activity of hypoxic multiple myeloma cells: a biomarker for predicting prognosis. J Cancer. 2021;12(10):2825–2834. doi:10.7150/jca.55553
21. Lyu T, Wang Y, Li D, et al. Exosomes from BM-MSCs promote acute myeloid leukemia cell proliferation, invasion and chemoresistance via upregulation of S100A4. Exp Hematol Oncol. 2021;10(1):24. doi:10.1186/s40164-021-00220-7
22. Li MY, Zhao C, Chen L, et al. Quantitative proteomic analysis of plasma exosomes to identify the candidate biomarker of imatinib resistance in chronic myeloid leukemia patients. Front Oncol. 2021;11:779567. doi:10.3389/fonc.2021.779567
23. Viola S, Traer E, Huan J, et al. Alterations in acute myeloid leukaemia bone marrow stromal cell exosome content coincide with gains in tyrosine kinase inhibitor resistance. Br J Haematol. 2016;172(6):983–986. doi:10.1111/bjh.13551
24. Jones RJ, Singh RK, Shirazi F, et al. Intravenous immunoglobulin g suppresses heat shock protein (HSP)-70 expression and enhances the activity of HSP90 and proteasome inhibitors. Front Immunol. 2020;11:1816. doi:10.3389/fimmu.2020.01816
25. Bazzoni R, Tanasi I, Turazzi N, Krampera M. Update on the role and utility of extracellular vesicles in hematological malignancies. Stem Cells. 2022;40(7):619–629. doi:10.1093/stmcls/sxac032
26. Zhou Q, Xie D, Wang R, et al. The emerging landscape of exosomal CircRNAs in solid cancers and hematological malignancies. Biomarker Res. 2022;10(1):28. doi:10.1186/s40364-022-00375-3
27. Feng Y, Zhong M, Zeng S, et al. Exosome-derived miRNAs as predictive biomarkers for diffuse large B-cell lymphoma chemotherapy resistance. Epigenomics. 2019;11(1):35–51. doi:10.2217/epi-2018-0123
28. Hu Y, Zhang Q, Wu Z, et al. Exosomal miR-200c and miR-141 as cerebrospinal fluid biopsy biomarkers for the response to chemotherapy in primary central nervous system lymphoma. Discov. Oncol. 2023;14(1):205. doi:10.1007/s12672-023-00812-1
29. Lin X, Ling Q, Lv Y, et al. Plasma exosome-derived microRNA-532 as a novel predictor for acute myeloid leukemia. Cancer Biomarkers. 2020;28(2):151–158. doi:10.3233/CBM-191164
30. Liu J, Han Y, Hu S, et al. Circulating exosomal MiR-107 restrains tumorigenesis in diffuse large B-cell lymphoma by targeting 14-3-3η. Front Cell Dev Biol. 2021;9:667800. doi:10.3389/fcell.2021.667800
31. Zhong AN, Yin Y, Tang BJ, et al. CircRNA microarray profiling reveals hsa_circ_0058493 as a novel biomarker for imatinib-resistant CML. Front Pharmacol. 2021;12:728916. doi:10.3389/fphar.2021.728916
32. Cheng H, Ding J, Tang G, et al. Human mesenchymal stem cells derived exosomes inhibit the growth of acute myeloid leukemia cells via regulating miR-23b-5p/TRIM14 pathway. Mol Med. 2021;27(1):128. doi:10.1186/s10020-021-00393-1
33. Wang C, Fu W, Lei C, Hu S. Generation and functional characterization of CAR exosomes. Met Cell Biology. 2022;167:123–131. doi:10.1016/bs.mcb.2021.06.017
34. Zhang H, Wang S, Sun M, et al. Exosomes as smart drug delivery vehicles for cancer immunotherapy. Front Immunol. 2022;13:1093607. doi:10.3389/fimmu.2022.1093607
35. Liu Q, Dai G, Wu Y, et al. iRGD-modified exosomes-delivered BCL6 siRNA inhibit the progression of diffuse large B-cell lymphoma. Front Oncol. 2022;12:822805. doi:10.3389/fonc.2022.822805
36. Bellavia D, Raimondo S, Calabrese G, et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7(5):1333–1345. doi:10.7150/thno.17092
37. Habib A, Liang Y, Zhu N. Exosomes multifunctional roles in HIV-1: insight into the immune regulation, vaccine development and current progress in delivery system. Front Immunol. 2023;14:1249133. doi:10.3389/fimmu.2023.1249133
38. Yoo KH, Thapa N, Kim BJ, et al. Possibility of exosome‑based coronavirus disease 2019 vaccine (Review). Mol Med Rep. 2022;25(1). doi:10.3892/mmr.2021.12542
39. Trams EG, Lauter CJ, Salem N Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. BBA. 1981;645(1):63–70. doi:10.1016/0005-2736(81)90512-5
40. Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262(19):9412–9420. doi:10.1016/S0021-9258(18)48095-7
41. Johnstone RM, Mathew A, Mason AB, Teng K. Exosome formation during maturation of mammalian and avian reticulocytes: evidence that exosome release is a major route for externalization of obsolete membrane proteins. J Cell Physiol. 1991;147(1):27–36. doi:10.1002/jcp.1041470105
42. Raposo G, Nijman HW, Stoorvogel W, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183(3):1161–1172. doi:10.1084/jem.183.3.1161
43. Zitvogel L, Regnault A, Lozier A, et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med. 1998;4(5):594–600. doi:10.1038/nm0598-594
44. Wolfers J, Lozier A, Raposo G, et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nature Med. 2001;7(3):297–303. doi:10.1038/85438
45. Valadi H, Ekström K, Bossios A, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9(6):654–659. doi:10.1038/ncb1596
46. Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14(10):1036–1045. doi:10.1038/ncb2574
47. Hoshino A, Costa-Silva B, Shen TL, et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi:10.1038/nature15756
48. Morrissey SM, Zhang F, Ding C, et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33(10):2040–2058.e10. doi:10.1016/j.cmet.2021.09.002
49. Hu Z, Chen G, Zhao Y, et al. Exosome-derived circCCAR1 promotes CD8 + T-cell dysfunction and anti-PD1 resistance in hepatocellular carcinoma. Mol Cancer. 2023;22(1):55. doi:10.1186/s12943-023-01759-1
50. Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36(3):301–312. doi:10.1007/s10571-016-0366-z
51. Kalluri R. The biology and function of exosomes in cancer. J Clin Invest. 2016;126(4):1208–1215. doi:10.1172/JCI81135
52. Sheridan C. Exosome cancer diagnostic reaches market. Nat. Biotechnol. 2016;34(4):359–360. doi:10.1038/nbt0416-359
53. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9(1):19. doi:10.1186/s13578-019-0282-2
54. Chen L, Hou Y, Du D, et al. MiR-199a-3p in mouse bone marrow mesenchymal stem cell exosomes increases epithelial sodium channel expression in lung injury. Fundament Clinic Pharmacol. 2022;36(6):1011–1019. doi:10.1111/fcp.12807
55. Yi Q, Xu Z, Thakur A, et al. Current understanding of plant-derived exosome-like nanoparticles in regulating the inflammatory response and immune system microenvironment. Pharmacol Res. 2023;190:106733. doi:10.1016/j.phrs.2023.106733
56. Gurunathan S, Kang MH, Kim JH. A comprehensive review on factors influences biogenesis, functions, therapeutic and clinical implications of exosomes. Int j Nanomed. 2021;16:1281–1312. doi:10.2147/IJN.S291956
57. Ashrafizadeh M, Kumar AP, Aref AR, Zarrabi A, Mostafavi E. Exosomes as promising nanostructures in diabetes mellitus: from insulin sensitivity to ameliorating diabetic complications. Int j Nanomed. 2022;17:1229–1253. doi:10.2147/IJN.S350250
58. Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019;132(13). doi:10.1242/jcs.222406
59. Skouras P, Gargalionis AN, Piperi C. Exosomes as novel diagnostic biomarkers and therapeutic tools in gliomas. Int J Mol Sci. 2023;24(12):10162. doi:10.3390/ijms241210162
60. Cheng H, Yang Q, Wang R, et al. Emerging advances of detection strategies for tumor-derived exosomes. Int J Mol Sci. 2022;23(2):
61. Mashouri L, Yousefi H, Aref AR, et al. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi:10.1186/s12943-019-0991-5
62. Kugeratski FG, Hodge K, Lilla S, et al. Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol. 2021;23(6):631–641. doi:10.1038/s41556-021-00693-y
63. Dai J, Su Y, Zhong S, et al. Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020;5(1):145. doi:10.1038/s41392-020-00261-0
64. Donoso-Quezada J, Ayala-Mar S, González-Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic. 2021;22(7):204–220. doi:10.1111/tra.12803
65. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88(1):487–514. doi:10.1146/annurev-biochem-013118-111902
66. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478). doi:10.1126/science.aau6977
67. Livshits MA, Khomyakova E, Evtushenko EG, et al. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep. 2015;5(1):17319. doi:10.1038/srep17319
68. Chen J, Li P, Zhang T, et al. Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol. 2021;9:811971. doi:10.3389/fbioe.2021.811971
69. Wang X, He L, Huang X, et al. Recent progress of exosomes in multiple myeloma: pathogenesis, diagnosis, prognosis and therapeutic strategies. Cancers. 2021;13(7):
70. Coughlan C, Bruce KD, Burgy O, et al. Exosome isolation by ultracentrifugation and precipitation and techniques for downstream analyses. Curr Protoc Cell Biol. 2020;88(1):e110. doi:10.1002/cpcb.110
71. Guerreiro EM, Vestad B, Steffensen LA, et al. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS One. 2018;13(9):e0204276. doi:10.1371/journal.pone.0204276
72. Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in Exosome Isolation Techniques. Theranostics. 2017;7(3):789–804. doi:10.7150/thno.18133
73. Duong P, Chung A, Bouchareychas L, Raffai RL. Cushioned-density gradient ultracentrifugation (C-DGUC) improves the isolation efficiency of extracellular vesicles. PLoS One. 2019;14(4):e0215324. doi:10.1371/journal.pone.0215324
74. Konoshenko MY, Lekchnov EA, Vlassov AV, Laktionov PP. Isolation of extracellular vesicles: general methodologies and latest trends. Biomed Res Int. 2018;2018:8545347. doi:10.1155/2018/8545347
75. Patel GK, Khan MA, Zubair H, et al. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Scientific Rep. 2019;9(1):5335. doi:10.1038/s41598-019-41800-2
76. Cai S, Luo B, Jiang P, et al. Immuno-modified superparamagnetic nanoparticles via host-guest interactions for high-purity capture and mild release of exosomes. Nanoscale. 2018;10(29):14280–14289. doi:10.1039/C8NR02871K
77. Nakai W, Yoshida T, Diez D, et al. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci Rep. 2016;6(1):33935. doi:10.1038/srep33935
78. Echevarria J, Royo F, Pazos R, et al. Microarray-based identification of lectins for the purification of human urinary extracellular vesicles directly from urine samples. Chembiochem. 2014;15(11):1621–1626. doi:10.1002/cbic.201402058
79. Ghosh A, Davey M, Chute IC, et al. Rapid isolation of extracellular vesicles from cell culture and biological fluids using a synthetic peptide with specific affinity for heat shock proteins. PLoS One. 2014;9(10):e110443. doi:10.1371/journal.pone.0110443
80. Jia Y, Yu L, Ma T, et al. Small extracellular vesicles isolation and separation: current techniques, pending questions and clinical applications. Theranostics. 2022;12(15):6548–6575. doi:10.7150/thno.74305
81. Skryabin GO, Vinokurova SV, Elkina NV, et al. Comparison of methods for microRNA isolation from extracellular vesicles obtained from ascitic fluids. Biochem Biokhimiia. 2022;87(11):1354–1366. doi:10.1134/S0006297922110141
82. Sriram H, Khanka T, Kedia S, et al. Improved protocol for plasma microRNA extraction and comparison of commercial kits. Biochemia medica. 2021;31(3):030705. doi:10.11613/BM.2021.030705
83. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):
84. Yu D, Li Y, Wang M, et al. Exosomes as a new frontier of cancer liquid biopsy. Mol Cancer. 2022;21(1):56. doi:10.1186/s12943-022-01509-9
85. Zhang Y, Bi J, Huang J, et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int j Nanomed. 2020;15:6917–6934. doi:10.2147/IJN.S264498
86. Liu Q, Piao H, Wang Y, Zheng D, Wang W. Circulating exosomes in cardiovascular disease: novel carriers of biological information. Biomed Pharmacother. 2021;135:111148. doi:10.1016/j.biopha.2020.111148
87. Onozato M, Kobata K, Sakamoto T, Ichiba H, Fukushima T. LC-MS/MS analysis of thiol-containing amino acids in exosomal fraction of serum. J Chromatogr Sci. 2020;58(7):636–640. doi:10.1093/chromsci/bmaa028
88. Kowal EJK, Ter-Ovanesyan D, Regev A, Church GM. Extracellular Vesicle Isolation and Analysis by Western Blotting. Methods Mol Biol. 2017;1660:143–152.
89. Lai JJ, Chau ZL, Chen SY, et al. Exosome processing and characterization approaches for research and technology development. Adv. Sci. 2022;9(15):e2103222. doi:10.1002/advs.202103222
90. Guan P, Cui R, Wang Q, Sun Y. A 3D hydrogel loaded with exosomes derived from bone marrow stem cells promotes cartilage repair in rats by modulating immunological microenvironment. Nan fang yi ke da xue xue bao. 2022;42(4):528–537. doi:10.12122/j.issn.1673-4254.2022.04.08
91. Al-Ostoot FH, Salah S, Khamees HA, Khanum SA. Tumor angiogenesis: current challenges and therapeutic opportunities. Cancer Treat Res Commun. 2021;28:100422. doi:10.1016/j.ctarc.2021.100422
92. Paggetti J, Haderk F, Seiffert M, et al. Exosomes released by chronic lymphocytic leukemia cells induce the transition of stromal cells into cancer-associated fibroblasts. Blood. 2015;126(9):1106–1117. doi:10.1182/blood-2014-12-618025
93. Taverna S, Fontana S, Monteleone F, et al. Curcumin modulates chronic myelogenous leukemia exosomes composition and affects angiogenic phenotype via exosomal miR-21. Oncotarget. 2016;7(21):30420–30439. doi:10.18632/oncotarget.8483
94. Zhang L, Yu D. Exosomes in cancer development, metastasis, and immunity. Biochimica Et Biophysica Acta Rev Cancer. 2019;1871(2):455–468. doi:10.1016/j.bbcan.2019.04.004
95. Wang QM, Lian GY, Sheng SM, et al. Exosomal lncRNA NEAT1 inhibits NK-cell activity to promote multiple myeloma cell immune escape via an EZH2/PBX1 axis. Mol Cancer Res. 2024;22(2):125–136. doi:10.1158/1541-7786.MCR-23-0282
96. Wang J, Hendrix A, Hernot S, et al. Bone marrow stromal cell-derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124(4):555–566. doi:10.1182/blood-2014-03-562439
97. Rasihashemi SZ, Rezazadeh Gavgani E, Majidazar R, et al. Tumor-derived exosomal PD-L1 in progression of cancer and immunotherapy. J Cell Physiol. 2022;237(3):1648–1660. doi:10.1002/jcp.30645
98. Mazini L, Rochette L, Hamdan Y, Malka G. Skin immunomodulation during regeneration: emerging new targets. J Pers Med. 2021;11(2):85. doi:10.3390/jpm11020085
99. Ferguson Bennit HR, Gonda A, Kabagwira J, et al. Natural killer cell phenotype and functionality affected by exposure to extracellular survivin and lymphoma-derived exosomes. Int J Mol Sci. 2021;22(3):1255. doi:10.3390/ijms22031255
100. Khalife J, Ghose J, Martella M, et al. MiR-16 regulates crosstalk in NF-κB tolerogenic inflammatory signaling between myeloma cells and bone marrow macrophages. JCI Insight. 2019;4(21). doi:10.1172/jci.insight.129348
101. Moloudizargari M, Redegeld F, Asghari MH, Mosaffa N, Mortaz E. Long-chain polyunsaturated omega-3 fatty acids reduce multiple myeloma exosome-mediated suppression of NK cell cytotoxicity. Daru. 2020;28(2):647–659. doi:10.1007/s40199-020-00372-7
102. Benites BD, da Silva Santos Duarte A, Longhini ALF, et al. Exosomes in the serum of acute myeloid leukemia patients induce dendritic cell tolerance: implications for immunotherapy. Vaccine. 2019;37(11):1377–1383. doi:10.1016/j.vaccine.2019.01.079
103. Hu W, Huang F, Ning L, et al. Enhanced immunogenicity of leukemia-derived exosomes via transfection with lentiviral vectors encoding costimulatory molecules. Cell Oncol. 2020;43(5):889–900. doi:10.1007/s13402-020-00535-3
104. Lv Y, Du X, Tang W, Yang Q, Gao F. Exosomes: the role in tumor tolerance and the potential strategy for tumor therapy. Pharmaceutics. 2023;15(2):462. doi:10.3390/pharmaceutics15020462
105. Cariello M, Squilla A, Piacente M, Venutolo G, Fasano A. Drug resistance: the role of exosomal miRNA in the microenvironment of hematopoietic tumors. Molecules. 2022;28(1):116. doi:10.3390/molecules28010116
106. Kunou S, Shimada K, Takai M, et al. Exosomes secreted from cancer-associated fibroblasts elicit anti-pyrimidine drug resistance through modulation of its transporter in malignant lymphoma. Oncogene. 2021;40(23):3989–4003. doi:10.1038/s41388-021-01829-y
107. Faict S, Oudaert I, D’Auria L, et al. The transfer of sphingomyelinase contributes to drug resistance in multiple myeloma. Cancers. 2019;11(12):1823. doi:10.3390/cancers11121823
108. Kalluri R, McAndrews KM. The role of extracellular vesicles in cancer. Cell. 2023;186(8):1610–1626. doi:10.1016/j.cell.2023.03.010
109. Van Morckhoven D, Dubois N, Bron D, et al. Extracellular vesicles in hematological malignancies: EV-dence for reshaping the tumoral microenvironment. Front Immunol. 2023;14:1265969. doi:10.3389/fimmu.2023.1265969
110. Salman DM, Mohammad TAM. Leukemia cancer cells and immune cells derived-exosomes: possible roles in leukemia progression and therapy. Cell Biochem Funct. 2024;42(2):e3960. doi:10.1002/cbf.3960
111. Thind A, Wilson C. Exosomal miRNAs as cancer biomarkers and therapeutic targets. J Extracell Vesicles. 2016;5(1):31292. doi:10.3402/jev.v5.31292
112. Chen L, Xie T, Wei B, Di DL. Tumour‑derived exosomes and their emerging roles in leukaemia (Review). Exp Ther Med. 2023;25(3):126. doi:10.3892/etm.2023.11825
113. Bang YH, Shim JH, Ryu KJ, et al. Clinical relevance of serum-derived exosomal messenger RNA sequencing in patients with non-Hodgkin lymphoma. J Cancer. 2022;13(5):1388–1397. doi:10.7150/jca.69639
114. Hernández-Walias FJ, Vázquez E, Pacheco Y, et al. Risk, diagnostic and predictor factors for classical Hodgkin lymphoma in HIV-1-infected individuals: role of plasma exosome-derived miR-20a and miR-21. J Clin Med. 2020;9(3):760. doi:10.3390/jcm9030760
115. Menu E, Vanderkerken K. Exosomes in multiple myeloma: from bench to bedside. Blood. 2022;140(23):2429–2442. doi:10.1182/blood.2021014749
116. Luo C, Xin H, Zhou Z, et al. Tumor-derived exosomes induce immunosuppressive macrophages to foster intrahepatic cholangiocarcinoma progression. Hepatology. 2022;76(4):982–999. doi:10.1002/hep.32387
117. Andre M, Caobi A, Miles JS, et al. Diagnostic potential of exosomal extracellular vesicles in oncology. BMC Cancer. 2024;24(1):322. doi:10.1186/s12885-024-11819-4
118. Moyal L, Arkin C, Gorovitz-Haris B, et al. Mycosis fungoides-derived exosomes promote cell motility and are enriched with microRNA-155 and microRNA-1246, and their plasma-cell-free expression may serve as a potential biomarker for disease burden. Br J Dermatol. 2021;185(5):999–1012. doi:10.1111/bjd.20519
119. Saffari N, Rahgozar S, Faraji E, Sahin F. Plasma-derived exosomal miR-326, a prognostic biomarker and novel candidate for treatment of drug resistant pediatric acute lymphoblastic leukemia. Sci Rep. 2024;14(1):691. doi:10.1038/s41598-023-50628-w
120. Zhang ZY, Li YC, Geng CY, et al. Serum exosomal microRNAs as novel biomarkers for multiple myeloma. Hematol. Oncol. 2019;37(4):409–417. doi:10.1002/hon.2639
121. Gu J, Wang M, Wang X, et al. Exosomal miR-483-5p in bone marrow mesenchymal stem cells promotes malignant progression of multiple myeloma by targeting TIMP2. Front Cell Dev Biol. 2022;10:862524. doi:10.3389/fcell.2022.862524
122. Zhang J, Luo C, Long H, et al. Circulating exosomal miRNA-451 as an effective diagnostic biomarker and prognostic indicator for multiple myeloma. Int J Biol Markers. 2024:3936155241283747. doi:10.1177/03936155241283747
123. Miaomiao S, Xiaoqian W, Yuwei S, et al. Cancer-associated fibroblast-derived exosome microRNA-21 promotes angiogenesis in multiple myeloma. Sci Rep. 2023;13(1):9671. doi:10.1038/s41598-023-36092-6
124. Tang X, Deng Z, Ding P, et al. A novel protein encoded by circHNRNPU promotes multiple myeloma progression by regulating the bone marrow microenvironment and alternative splicing. J Exp Clin Cancer Res. 2022;41(1):85. doi:10.1186/s13046-022-02276-7
125. Shao R, Liu C, Xue R, et al. Tumor-derived exosomal ENO2 modulates polarization of tumor-associated macrophages through reprogramming glycolysis to promote progression of diffuse large b-cell lymphoma. Int J Bio Sci. 2024;20(3):848–863. doi:10.7150/ijbs.91154
126. Hashemi M, Roshanzamir SM, Paskeh MDA, et al. Non-coding RNAs and exosomal ncRNAs in multiple myeloma: an emphasis on molecular pathways. Eur J Pharmacol. 2023;941:175380. doi:10.1016/j.ejphar.2022.175380
127. Jiang D, Wu X, Sun X, et al. Bone mesenchymal stem cell-derived exosomal microRNA-7-5p inhibits progression of acute myeloid leukemia by targeting OSBPL11. J Nanobiotechnology. 2022;20(1):29. doi:10.1186/s12951-021-01206-7
128. Yan W, Song L, Wang H, et al. Extracellular vesicles carrying miRNA-181b-5p affects the malignant progression of acute lymphoblastic leukemia. J Transl Med. 2021;19(1):511. doi:10.1186/s12967-021-03174-w
129. Zhou Y, Zhang Y, Gong H, Luo S, Cui Y. The role of exosomes and their applications in cancer. Int J Mol Sci. 2021;22(22):12204. doi:10.3390/ijms222212204
130. Han QF, Li WJ, Hu KS, et al. Exosome biogenesis: machinery, regulation, and therapeutic implications in cancer. Mol Cancer. 2022;21(1):207. doi:10.1186/s12943-022-01671-0
131. Hong Y, Liu KK, Wang NL, Xie ZW, Chu JH. [expression and clinical significance of exosome derived MiR-181b-5p in children with acute lymphoblastic leukemia]. Zhongguo shi yan xue ye xue za zhi. 2023;31(3):643–648. doi:10.19746/j.cnki.issn.1009-2137.2023.03.004
132. Zhang Y, Pisano M, Li N, et al. Exosomal circRNA as a novel potential therapeutic target for multiple myeloma-related peripheral neuropathy. Cell Signal. 2021;78:109872. doi:10.1016/j.cellsig.2020.109872
133. Barrera-Ramirez J, Lavoie JR, Maganti HB, et al. Micro-RNA profiling of exosomes from marrow-derived mesenchymal stromal cells in patients with acute myeloid leukemia: implications in leukemogenesis. Stem Cell Rev Rep. 2017;13(6):817–825. doi:10.1007/s12015-017-9762-0
134. Yang F, Wang M, Guan X. Exosomes and mimics as novel delivery platform for cancer therapy. Front Pharmacol. 2022;13:1001417. doi:10.3389/fphar.2022.1001417
135. Zhao X, Wu D, Ma X, et al. Exosomes as drug carriers for cancer therapy and challenges regarding exosome uptake. Biomed Pharmacother. 2020;128:110237. doi:10.1016/j.biopha.2020.110237
136. Kandasamy G, Maity D. Current advancements in self-assembling nanocarriers-based siRNA delivery for cancer therapy. Colloids Surf B. 2023;221:113002. doi:10.1016/j.colsurfb.2022.113002
137. Shao J, Zaro J, Shen Y. Advances in exosome-based drug delivery and tumor targeting: from tissue distribution to intracellular fate. Int j Nanomed. 2020;15:9355–9371. doi:10.2147/IJN.S281890
138. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183–3195. doi:10.7150/thno.52570
139. Jiang XC, Gao JQ. Exosomes as novel bio-carriers for gene and drug delivery. Int J Pharm. 2017;521(1–2):167–175. doi:10.1016/j.ijpharm.2017.02.038
140. Pandian SRK, Vijayakumar KK, Kunjiappan S, Babkiewicz E, Maszczyk P. Emerging role of exosomes in hematological malignancies. Clin Exp Med. 2023;23(4):1123–1136. doi:10.1007/s10238-022-00850-z
141. Huang L, Rong Y, Tang X, et al. Engineered exosomes as an in situ DC-primed vaccine to boost antitumor immunity in breast cancer. Mol Cancer. 2022;21(1):45. doi:10.1186/s12943-022-01515-x
142. Bu N, Wu H, Zhang G, et al. Exosomes from dendritic cells loaded with chaperone-rich cell lysates elicit a potent t cell immune response against intracranial glioma in mice. J Mol Neurosci. 2015;56(3):631–643. doi:10.1007/s12031-015-0506-9
143. Chen Z, You L, Wang L, et al. Dual effect of DLBCL-derived EXOs in lymphoma to improve DC vaccine efficacy in vitro while favor tumorgenesis in vivo. J Exp Clin Cancer Res. 2018;37(1):190. doi:10.1186/s13046-018-0863-7
144. Xie Y, Bai O, Zhang H, et al. Membrane-bound HSP70-engineered myeloma cell-derived exosomes stimulate more efficient CD8(+) CTL- and NK-mediated antitumour immunity than exosomes released from heat-shocked tumour cells expressing cytoplasmic HSP70. J Cell & Mol Med. 2010;14(11):2655–2666. doi:10.1111/j.1582-4934.2009.00851.x
145. Huang F, Li Z, Zhang W, Li J, Hao S. Enhancing the anti-leukemia immunity of acute lymphocytic leukemia-derived exosome-based vaccine by downregulation of PD-L1 expression. Cancer Immunol Immunother. 2022;71(9):2197–2212. doi:10.1007/s00262-021-03138-5
146. Li J, Huang F, Jiang Y, et al. A novel costimulatory molecule gene-modified leukemia cell-derived exosome-targeted CD4(+) T cell vaccine efficiently enhances anti-leukemia immunity. Front Immunol. 2022;13:1043484. doi:10.3389/fimmu.2022.1043484
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.