Back to Journals » Cancer Management and Research » Volume 17
Recurrence Prediction by Multi-Omics in the Patient with Colorectal Peritoneal Metastases After Cytoreductive Surgery: A Prospective Biomarker Study
Authors Chen C , Gong Z, Zhang Z, Gu H, Zhu A, Peng D, Li B, Wang J, Hu Y, Wang D, Sun L
Received 31 January 2025
Accepted for publication 22 April 2025
Published 29 April 2025 Volume 2025:17 Pages 893—904
DOI https://doi.org/10.2147/CMAR.S519094
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Chao Chen,1– 3,* Zhiyuan Gong,1– 3,* Zhiwei Zhang,1– 3 Haochen Gu,1– 3 Akao Zhu,1– 3 Di Peng,4 Bing Li,4 Jian Wang,1 Yeting Hu,1 Da Wang,1 Lifeng Sun1
1Department of Colorectal Surgery and Oncology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China; 2Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education, Beijing, Zhejiang Province, People’s Republic of China; 3Zhejiang University Cancer Center, Hangzhou, Zhejiang Province, People’s Republic of China; 4Guangzhou Ranshi Medical Laboratory Co., Ltd, Guangzhou, Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Da Wang, Department of Colorectal Surgery and Oncology, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang Province, People’s Republic of China, Tel +86-571-87784720, Email [email protected] Lifeng Sun, The Second Affiliated Hospital, Zhejiang University School of Medicine, 88 Jiefang Road, Hangzhou, Zhejiang, 310009, People’s Republic of China, Tel +86-571-87783586, Email [email protected]
Background: Colorectal cancer with peritoneal metastases (CRCPM) exhibits high recurrence post-cytoreductive surgery (CRS). This study evaluated tumor tissue biomarkers and combined circulating tumor DNA (ctDNA) and methylation analysis via ultra-deep next-generation sequencing (NGS) for recurrence prediction.
Methods: CRCPM patients undergoing surgery were enrolled (n=21). Blood samples was collected at preoperative and postoperative, and tumor and adjacent tissues were collected. NGS assessed ctDNA and methylation in blood samples, while tumor mutations and methylation were analyzed in tumor. Recurrence was determined via imaging. Outcomes included progression-free survival (PFS) and overall survival (OS).
Results: Of 17 patients with paired pre-/postoperative ctDNA testing, preoperative ctDNA levels were higher in those with extraperitoneal metastases versus peritoneal-only disease (0.1064 vs 0.0037). Postoperative ctDNA positivity correlated with 100% peritoneal recurrence. ctDNA-positive subgroups showed shorter PFS (HR=2.5; 95% CI:1.6– 6.6). Patients persistently ctDNA-negative pre-/postoperatively had improved PFS versus those with positivity (HR=5.07; 95% CI:0.53– 48.38). ctDNA methylation positivity was observed in all extraperitoneal metastasis cases and 70% of peritoneal-only cases. Baseline methylation positivity predicted worse OS overall and in peritoneal-only subgroups. Postoperative dual negativity for ctDNA and methylation correlated with better OS. Tumor mutations in EPHB1 (P = 0.012), ARFRP1 (P = 0.048), and ATR (P = 0.048) were significantly associated with PFS.
Conclusion: Dynamic ctDNA and methylation monitoring, and tumor mutation profiling, may serve as sensitive biomarkers for early recurrence detection in CRCPM underwent CRS. These tools could enhance recurrence prediction and guide clinical management.
Keywords: circulating tumor DNA, colorectal cancer, peritoneal metastasis, ctDNA methylation, timing of recurrence, mutations
Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide and the third leading cause of death of all cancers.1 The peritoneum is the second most common metastatic site for colorectal cancer (after liver). Approximately 17–40% of patients will experience synchronous peritoneal metastases and 44–50% of metachronous peritoneal metastases, contributing to worse overall survival compared with patients without peritoneal involvement.2 Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) remain the gold standard treatment that can be curative for CRC patients with peritoneal metastases (CRCPM).3,4 Most CRCPM patients develop recurrent disease (it can occur within the peritoneal cavity, systemically, or both) after CRS and HIPEC within 1 year.5,6 Unfortunately, conventional clinical assessment and current imaging tools are not sufficient for detecting recurrence.7,8
Due to the rapid development in techniques, circulating tumor DNA (ctDNA) detected in patient blood samples could be an effective prognostic marker for tumorigenesis to recurrence.9,10 There are many researches have explored the effectiveness of ctDNA in minimal residual disease (MRD), and the clinical utility in managing treatment, and surveillance following cytoreductive surgery.11,12 In recent years, ctDNA methylation has emerged as an alternative biomarker for CRC.13,14 Research on ctDNA methylation has been accumulating to investigate its feasibility in detecting early relapse in patients with CRC.15,16 However, limited studies to date have established the combined analysis of circulating tumor DNA (ctDNA) and methylation based on ultra-deep next-generation sequencing (NGS) to predict patient recurrence in CRCPM patients.
Here, we analyzed the change of ctDNA and ctDNA methylation in CRCPM patient plasma samples obtained preoperatively and postoperatively throughout the surveillance and the mutations of tumor samples. Specifically, the combined analysis of circulating tumor DNA (ctDNA) and methylation can be used as a promising biomarker in postoperative surveillance and be an adjuvant tool for guiding further treatment. This study aimed to provide valuable insights into the utility of liquid biopsy for identifying disease recurrence of CRCPM patients underwent CRS and HIPEC, and it will help guide future surveillance pathways for these patients.
Methods and Materials
Study Design
Between July 2020 and December 2022, the study enrolled 21 patients with CRCPM from the Second Affiliated Hospital of Zhejiang University School of Medicine who underwent CRS. The inclusion criteria specified patients who had resectable CRCPM before CRS as determined by a multidisciplinary team (MDT). Patients with other simultaneous malignancies were excluded from the study. Preoperative staging and evaluation of PM were performed by computed tomography (CT) scan or MRI of the abdomen and chest for each patient according to standard clinical protocols. Peripheral blood samples were collected at the start of surgery and 4 weeks after postoperatively. All the patients had samples collected after radical resection for PM.
All enrolled patients underwent surgical resection of primary tumor or conduct CRS procedure. CRC and PM were diagnosed by pathology. This study was conducted with the approval of the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine, and according to the Declaration of Helsinki. All patients in our center provided signed informed consent.
All patients were followed up in the outpatient unit at approximately two weeks after CRS/HIPEC, and at least every 3 months for 2 years, then every six months progression free survival (PFS) and overall survival (OS) were used as the primary outcome, assessed with standard radiologic criteria, and calculated from the date of surgery to the date of verified radiologic recurrence.
Capture‑Based Targeted DNA Sequencing
Genomic DNA was isolated from formalin-fixed, paraffin-embedded (FFPE) tumor tissue samples using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany), while cell-free DNA (cfDNA) was extracted from 4–5 mL of plasma using the QIAamp Circulating Nucleic Acid Kit (Qiagen) following the manufacturer’s standard protocol. The DNA from both sources was quantified using the Qubit 2.0 fluorometer with the double-stranded DNA HS assay kit (Life Technologies, CA, USA). For library preparation for next-generation sequencing (NGS), 20–80 ng of either tissue DNA or purified cfDNA was utilized.Tissue DNA was fragmented using the M220 ultrasonicator (Covaris, MA, USA). Subsequent steps, including end repair, addition of a single dA overhang, and adaptor ligation, were performed. For cfDNA, adaptors were ligated using the Burning Rock UHS library preparation kit with unique molecular identifiers (UMIs). Hybridization with probe baits, magnetic beads selection, and PCR amplification were carried out afterward. Tumor DNA samples were captured using a commercial panel consisting of 520 cancer-related genes, spanning 1.64 Mb of the human genome (OncoScreen Plus, Burning Rock Biotech).17 Plasma DNA samples were sequenced using a panel consisting of 168 cancer-related genes spanning 237 Kb of the human genome.18 The quality and fragment size of the DNA libraries were evaluated using Bioanalyzer 2100 (Agilent Technologies, CA, USA). The indexed samples were sequenced on a NovaSeq 6000 platform (Illumina, Inc., CA, USA) with 150-base paired-end reads. Target sequencing depths were set at 1000 × for tissue samples and 30,000 × for plasma samples.
Sequence Data Analysis and Mutation Calling
Paired-end sequencing reads were aligned to the human reference genome (hg19) using the Burrows-Wheeler Aligner (BWA) version 0.7.10.19 Local realignment, variant calling, and annotation were conducted with the Genome Analysis Toolkit (GATK) v.3.220 and VarScan v.2.4.3.21 Comparisons between tissue or plasma samples and matched white blood cells were performed to filter out variants related to clonal hematopoiesis. Variant calling required a minimum of eight supporting reads for single nucleotide variants (SNVs), and for insertions or deletions (Indels), at least five reads for tissue and two reads for plasma samples. Variants with population frequency greater than 0.1% in databases such as ExAC, 1000 Genomes, dbSNP, and ESP6500SI-V2 were classified as single nucleotide polymorphisms (SNPs) and excluded from subsequent analysis. The remaining variants were annotated with ANNOVAR (2016–02-01 release)22 and SnpEff (version 3.6).23 Structural variants (SVs), including large genomic rearrangements (LGRs), were detected using Factera (version 1.4.4).24 Copy number variations (CNVs) were determined based on capture interval depth, with thresholds of 1.5 for deletions and 2.75 for amplifications. Mutations identified in cfDNA that were also present in paired tumor tissue were classified as ctDNA-positive. The ctDNA fraction was determined as the maximum allele frequency (maxAF) detected in each plasma sample.
cfDNA Methylation Analysis
Library preparation for bisulfite-targeted sequencing was conducted in accordance with a previously established protocol for cfDNA methylation assays (ELSA-seq).25 Cancer-associated differentially methylated blocks (DMBs) were identified through systematic comparison of 195 matched tumor (≥30% purity) and tumor-adjacent normal tissue (<5% tumor cells) samples, using blood samples from 312 healthy donors as reference. DMBs selection required absolute methylation difference |Δβ| ≥ 0.2 between tumor and normal pairs and statistical significance (FDR-adjusted P < 0.05, Benjamini-Hochberg corrected paired t-test), yielding 3,159 tumor-specific DMBs. For individual patients, personalized DMBs subsets were selected based on methylation consistency (hyper/hypomethylation in ≥80% of tumor replicates) and dynamic range (top 20% of |Δβ| values), which were subsequently integrated into the timMRD model as features for beta-binomial regression to estimate tumor fraction (αi) through maximum likelihood estimation.26 To determine the presence of ctDNA, the Wald test was applied under the null hypothesis. A p-value of less than 0.001 was considered a methylation-positive result. Model accuracy was validated through: (1) spike-in experiments with lung cancer cell line DNA (H2209) diluted in normal DNA (GM24385), achieving 95% detection sensitivity at 0.02% tumor fraction; and (2) numerical simulations confirming that timMRD-scores accurately reflected simulated tumor fractions down to 0.01%, with minimal interference (<5%) from normal DNA at tumor fractions >0.1%.
Statistical Analysis
Fisher’s exact test was used to compare differences between the two groups. Cumulative survival was evaluated by Kaplan-Meier analysis. A two-sided P value < 0.05 was set significant. All analyses were performed in SPSS for windows (version 25.0) and R software (version 3.6.1).
Result
Clinicopathological Characteristics
The workflow of sample processing and sequencing experiments is presented briefly in Figure 1. Surgical tissues from all the patients were sequenced using the 520-gene panel. To assess differences in ctDNA and methylation at different blood collection times, peripheral blood samples from patients after a median of 4 postoperative weeks also were sequenced.
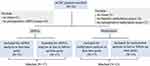 |
Figure 1 The workflow of sample processing and sequencing experiments. Abbreviations: mCRC, metastatic colorectal cancer; ctDNA, circulating tumor DNA. |
A total of 22 patients with CRCPM were recruited prospectively. 1 case was excluded for subsequent analysis, due to insufficient tissue and blood. 21 cases were finally enrolled into analysis in this study. NGS profiling of ctDNA in the baseline blood was available in 21 cases, and 17 cases were included for analyzing the impact of dynamic ctDNA changes on prognosis analysis. Methylation in blood was available in 17 cases at baseline, and in 12 cases were matched for analyzing the impact of dynamic changes on prognosis. DNA mutations in tumor tissue (TIS) were available in 21 cases (Figure 2). The demographic and clinicopathologic characteristics of the overall 21 cases in the cohort were summarized in Table 1. The median age was 53 years old, and 61.9% of the patients were male. Based on pathologic characteristics, 42.9% were synchronous for peritoneal metastasis timing. During the study period, 13 (61.9%) of the patients experienced recurrences: 7 patients had peritoneal-only recurrence and other patients had distant recurrence. The median follow-up period after radical resection was 6.6 months (range, 2.6–18.5).
 |
Table 1 Demographic Characteristics of the Patients |
Baseline ctDNA Levels Across Disease Sites
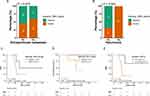
The ctDNA fraction was lower in patients with peritoneal-only disease compared with those with other metastases (Figure 3A). We analyzed the correlation between the level of ctDNA and the different metastatic sites at baseline. Baseline ctDNA was tested for association with patients’ clinical features (site of metastases, peritoneal metastasis timing, the number of extra-peritoneal metastasis); however, no significant associations were found (Figure S1).
Association of ctDNA Levels and Clinical Outcome
At 4 weeks after surgery, ctDNA was positive in 5 of 5 patients with peritoneal-only recurrence (100%), while 2 of 6 patients with distant recurrence showed positive ctDNA results (33.3%) (P = 0.045) (Figure 3B). In patients with peritoneal-only metastasis before surgery, the sensitivity and specificity of follow-up blood ctDNA in detection of recurrence were 80%, 66.67%, respectively. The PPV and NPV were 80% and 66.67%, respectively (Table S1).
Follow-up ctDNA was found to be associated with longer RFS and OS in patients with peritoneal-only metastasis before surgery, however, no significant associations due to the small sample size. The PFS was 12.7 months (95% CI, 6.8 to 18.5) and 4.4 months (95% CI, 2.56 to 12.7) for patients with negative (4 patients) and positive (4 patients) follow-up ctDNA, respectively (log-rank P = 0.309; HR negative vs positive, 2.5; 95% CI, 1.6 to 6.6, Figure 3C). The OS was 8.73 months for patients with positive follow-up ctDNA and not reached for patients with negative follow-up ctDNA (log-rank P =0.157, Figure 3D).
The 17 patients were divided into two subgroups by ctDNA (-/-) or ctDNA (+) according to the status of ctDNA at baseline and follow-up. The Kaplan-Meier survival analysis showed that the PFS in patients with persistently negative ctDNA (-/-) exhibited longer PFS compared to those who had positive ctDNA (including converted to ctDNA positive (-/+) or had a positive baseline ctDNA result (+/+ or ±) (p = 0.124; ctDNA (-/-) vs ctDNA (+); HR= 5.07 [0.53–48.38]; Figure 3E).
Ability to Predict Recurrence and Survival Using the ctDNA and Methylation
The ctDNA methylation was positive in all CRC patients with extraperitoneal metastases and 70% was positive in patients with only PM (Figure S2). The baseline ctDNA methylation positive was related to worse OS both in all CRC patients (p = 0.505) and patient only with PM (P = 0.335) (Figure 4A and B). Only one patient showed negative postoperative ctDNA methylation and did not experienced recurrences until now. To clarify the prognostic value of combined analysis of ctDNA and methylation, the 17 patients were divided into two subgroups by the state of ctDNA and methylation (all positive (ctDNA/methylation (+/+)) and not all positive ((±), (-/+), (-/-)). The Kaplan-Meier survival analysis revealed a clinically observable trend toward reduced overall survival in patients demonstrating concurrent positivity for circulating tumor DNA and methylation biomarkers, though this difference lacked statistical significance (P=0.121, Figure 4C).
Ability to Predict Recurrence and Survival Using the 520 Gene Panel
In the 21 tumor tissue samples sequenced by the 520-gene panel, the mutation AFs ranged from 4.8 to 81%. The mutation profile of tissue samples is shown in Figure 5. The 520-gene panel detected 117 mutations in 21 samples, for a mean of 5.57 mutations per sample, and the mean mutation AF was 9.2% (range, 4.8–81%). The frequently mutated genes were TP53, KRAS and APC in the operative samples. The Kaplan-Meier survival analysis showed that EPHB1 (p = 0.012; HR, 7.21 [1.18–43.94]; Figure 6A), ATR (p = 0.048; HR, 4.57 [0.88–23.84]; Figure 6B), and ARFRP1 (p = 0.048; HR, 4.57 [0.88–23.84]; Figure 6C) are associated with the PFS. We also identified 3 genes that were significantly associated with OS. These included: AXIN2 (p = 0.008; HR, 13.77 [1.33–142.18]; Figure 6D), BARD1 (p = 0.019; HR, 13.44 [0.81–233.94]; Figure 6E), LRP1B (p = 0.025; HR, 9.44 [0.85–104.63]; Figure 6F).
 |
Figure 5 The mutational profiling of ctDNA in TIS of PM. Abbreviations: peri_metastasis, peritoneal metastasis; extraperi_metastas, extraperitoneal metastasis. |
Discussion
A considerable proportion of patients with CRCPM can experience recurrence after CRS and HIPEC.27–30 Early detection of recurrence is a difficult clinical issue due to the low sensitivity and specificity of CEA and the limitation of detecting occult micro metastatic nodules by imaging examination.31 In this study, we evaluating the robust sensitivity and specificity of the analysis of circulating tumor DNA (ctDNA) and methylation in blood, and tumor mutations and methylation for detecting recurrence and its association with PFS and OS.
An increasing number of studies in recent years have proposed that the concentrations of ctDNA are up-regulated in most CRC patients with metastases, however, the ctDNA burden was different in different organ metastases. Consistent with reports from other groups, the ctDNA fraction was lower in patients with peritoneal-only metastasis compared with those with other sites of metastases.32–35
An increasing number of studies in recent years have indicated the clinical utility of ctDNA and for MRD assessment, monitoring recurrence, and postoperative management in the clinical cancer course.36–38 In our study, we showed that the patients who tested ctDNA at postoperative could predict recurrence and the patients with ctDNA-positive had a worse PFS than who with ctDNA-negative, indicating the reliability and effectiveness of ctDNA in detecting molecular residual disease and recurrence. It is worth noting that the uncertainties persist regarding optimal detection timing of ctDNA. Recent studies showed the ctDNA positivity typically emerged over three months postoperatively in patients with isolated peritoneal involvement.9 However, our observational cohort utilizing a standardized 4-week postoperative surveillance protocol, the detection of circulating tumor DNA (ctDNA) demonstrated a 100% positive predictive value for subsequent peritoneal carcinomatosis (P = 0.045). The heterogeneous postoperative sampling windows further obscure the clinical utility of ctDNA in this context. Further large-scale clinical trials are warranted to validate the optimal postoperative timing for ctDNA detection. Moreover, we assessed the persistent status of ctDNA in patients’ outcomes, in which ctDNA-positive patients at preoperative with retained residual ctDNA at postoperative more likely to have shorter PFS than persistent ctDNA-negative patients.
Nowadays, most researches about ctDNA pay attention to genomic variations, which are special to every patient. Furthermore, the application of fixed panel would loss the cancer signals, the ctDNA methylation was applied to the fullest extent.16 According to other research, ctDNA methylation was a significant prognostic factor associated with recurrence in multivariable analysis.14 Here we combined the status of ctDNA and ctDNA methylation at postoperative to assess their potential to improve detection of relapse. For patients who tested all positive ctDNA and ctDNA methylation at postoperative was showed worse PFS than patients with negative status. The clinical utility of the combination of ctDNA and ctDNA methylation as a valuable biomarker was confirmed for recurrence warning throughout the disease course in CRCPM patients underwent CRS. This pragmatic test can largely simplify and facilitate the postoperative monitoring in standard patient care.
In this study, we also investigated the genetic mutations in tumor tissues, and three genes (EPHB1, ATR, ARFRP1) which related to PFS are identified in this study. EPHB1 belonged to Erythropoietin-producing hepatoma (Eph) receptor tyrosine kinases, which regulate the positioning of cell types along the crypt-villus axis in the intestinal epithelium.39 EPHB1 is the most most frequently mutated Eph receptor in metastatic CRC. The migration of CRC cells was unable to suppress due to the mutation of EPHB1.40 ATR (Ataxia Telangiectasia and Rad3-related) is the member of the PIKK family, which is associated with DNA damage response. ATR inhibitor has been reported its potentiating effect on cancer cells when combined with DNA damaging agents.41,42 Egger’s research showed that the mutation of ATR sensitizes the cells to several DNA damaging drugs, and exacerbates their synergistic effects, which could open new doors in the management of ATR-mutated cancers. The special mutation bottlenecks the replication checkpoint leading to extensive DNA damage. ATR frameshift mutations found in patients may also represent important prognostic factors.43 ARFRP1 (ADP-ribosylation factor-related protein 1) is the commonly altered small GTPasess, which is involved in trans-Golgi network through regulating ARL1-mediated Golgi recruitment and involved in lipidation and assembly of lipoproteins. However, there are not many studies about its role in cancer.44 Furthermore, ARFRP1 could regulate radiation sensitivity in breast cancer cells, ARFRP1 could be a novel protein biomarker for selecting radiotherapy for patients, as well as a new target for overcoming resistance in cancer radiotherapy.45
Limitations
The combination of ctDNA and ctDNA methylation and genetic mutations were analyzed for identifying disease recurrence of CRCPM patients underwent CRS and HIPEC, however, our study had some limitations. First, the variable plasma collection restricted our sample size, which resulted in non-significant p-values for all survival analysis while there was difference. Second, the single-institution nature and predominance of patients who underwent CRS for PM may limit the generalizability of our results. These limitations could be ameliorated by recruitment of more patients for inclusion in a future prospective study. Studies including more samples should be carried out to assess effectiveness of treatments and explore accurately stratify postoperative recurrence risk for CRC patients with PM.
Conclusion
In summary, our data show that peritoneal-only disease was associated with lower ctDNA levels compared to distant disease. The status of ctDNA at postoperative is a potential sensitive and reliable biomarker for detecting recurrence. The combination of ctDNA and ctDNA methylation was more sensitive than ctDNA-only in predicting PFS performance and detecting of recurrence in CRCPM patients underwent operation. The mutation of EPHB1, ATR, and ARFRP1 are related to PFS. Therefore, these findings support the potential incorporation of the combination of ctDNA, ctDNA methylation and and genomic variations assessments in clinical practice, and it is also expected to translate into an improved recurrence indicator for patients with CRCPM.
Ethics Declaration
This study was conducted with the approval of the Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine (2022-0632), and according to the Declaration of Helsinki. All patients in our center provided signed informed consent.
Patient Consent Statement
The study protocol was reviewed and approved by the Ethics Committee of the Second Affiliated Hospital of Zhejiang University School of Medicine (SAHZU).
Acknowledgments
Thanks to all authors for their efforts on this study. Our special acknowledgments to Leqi Wei for helping us with collecting samples.
Funding
The study was funded by the National Natural Science Foundation of China (81472819,81672342, 82072621, 82303951); Zhejiang Provincial Key R&D Program of China (2019C03018), and the Zhejiang Provincial Natural Science Foundation of China (LY20H160038).
Disclosure
The authors report no potential conflicts of interest for this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. Ca Cancer J Clin. 2021;71(1):7–33. doi:10.3322/caac.21654
2. Razenberg LG, van Gestel YR, Creemers GJ, Verwaal VJ, Lemmens VE, de Hingh IH. Trends in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of synchronous peritoneal carcinomatosis of colorectal origin in the Netherlands. Eur J Surg Oncol. 2015;41(4):466–471. doi:10.1016/j.ejso.2015.01.018
3. Parikh MS, Johnson P, Romanes JP, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal peritoneal metastases: a systematic review. Dis Colon Rectum. 2022;65(1):16–26. doi:10.1097/dcr.0000000000002315
4. Quénet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, Phase 3 trial. Lancet Oncol. 2021;22(2):256–266. doi:10.1016/s1470-2045(20)30599-4
5. Grange R, Rousset P, Williet N, et al. Metastatic colorectal cancer treated with combined liver resection, cytoreductive surgery, and hyperthermic intraperitoneal chemotherapy (HIPEC): predictive factors for early recurrence. Ann Surg Oncol. 2024;31(4):2378–2390. doi:10.1245/s10434-023-14840-2
6. Hassan S, Malcomson L, Soh YJ, et al. Patterns and timing of recurrence following CRS and HIPEC in colorectal cancer peritoneal metastasis. Eur J Surg Oncol. 2023;49(1):202–208. doi:10.1016/j.ejso.2022.07.019
7. Tseng J, Bryan DS, Poli E, Sharma M, Polite BN, Turaga KK. Under-representation of peritoneal metastases in published clinical trials of metastatic colorectal cancer. Lancet Oncol. 2017;18(6):711–712. doi:10.1016/s1470-2045(17)30336-4
8. Yuan Z, Xu T, Cai J, et al. Development and validation of an image-based deep learning algorithm for detection of synchronous peritoneal carcinomatosis in colorectal cancer. Ann Surg. 2022;275(4):e645–e651. doi:10.1097/sla.0000000000004229
9. Bansal VV, Belmont E, Godley F, et al. Utility of circulating tumor DNA assessment in characterizing recurrence sites after optimal resection for metastatic colorectal cancer. J Am Coll Surg. 2024;238(6):1013–1020. doi:10.1097/xcs.0000000000001028
10. Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11(10):726–734. doi:10.1038/nrc3130
11. Naidoo M, Gibbs P, Tie J. ctDNA and adjuvant therapy for colorectal cancer: time to re-invent our treatment paradigm. Cancers. 2021;13(2):346. doi:10.3390/cancers13020346
12. Liu W, Jin KM, Zhang MH, et al. Recurrence prediction by circulating tumor DNA in the patient with colorectal liver metastases after hepatectomy: a prospective biomarker study. Ann Surg Oncol. 2023;30(8):4916–4926. doi:10.1245/s10434-023-13362-1
13. Tham C, Chew M, Soong R, et al. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 2014;120(20):3131–3141. doi:10.1002/cncr.28802
14. Jin S, Zhu D, Shao F, et al. Efficient detection and post-surgical monitoring of colon cancer with a multi-marker DNA methylation liquid biopsy. Proc Natl Acad Sci USA. 2021;118(5). doi:10.1073/pnas.2017421118
15. Cai G, Cai M, Feng Z, et al. A multilocus blood-based assay targeting circulating tumor DNA methylation enables early detection and early relapse prediction of colorectal cancer. Gastroenterology. 2021;161(6):2053–2056.e2. doi:10.1053/j.gastro.2021.08.054
16. Mo S, Ye L, Wang D, et al. Early detection of molecular residual disease and risk stratification for stage I to III colorectal cancer via circulating tumor DNA methylation. JAMA Oncol. 2023;9(6):770–778. doi:10.1001/jamaoncol.2023.0425
17. Wang M, Chen X, Dai Y, et al. Concordance study of a 520-gene next-generation sequencing-based genomic profiling assay of tissue and plasma samples. Mol Diagn Ther. 2022;26(3):309–322. doi:10.1007/s40291-022-00579-1
18. Lin Z, Li Y, Tang S, Deng Q, Jiang J, Zhou C. Comparative analysis of genomic profiles between tissue-based and plasma-based next-generation sequencing in patients with non-small cell lung cancer. Lung Cancer. 2023;182:107282. doi:10.1016/j.lungcan.2023.107282
19. Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324
20. McKenna A, Hanna M, Banks E, et al. The genome analysis toolkit: a Mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi:10.1101/gr.107524.110
21. Koboldt DC, Zhang Q, Larson DE, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22(3):568–576. doi:10.1101/gr.129684.111
22. Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164. doi:10.1093/nar/gkq603
23. Cingolani P, Platts A, Wang le L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: sNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6(2):80–92. doi:10.4161/fly.19695
24. Newman AM, Bratman SV, Stehr H, et al. FACTERA: a practical method for the discovery of genomic rearrangements at breakpoint resolution. Bioinformatics. 2014;30(23):3390–3393. doi:10.1093/bioinformatics/btu549
25. Liang N, Li B, Jia Z, et al. Ultrasensitive detection of circulating tumour DNA via deep methylation sequencing aided by machine learning. Nat Biomed Eng. 2021;5(6):586–599. doi:10.1038/s41551-021-00746-5
26. Chen K, Kang G, Zhang Z, et al. Individualized dynamic methylation-based analysis of cell-free DNA in postoperative monitoring of lung cancer. BMC Med. 2023;21(1):255. doi:10.1186/s12916-023-02954-z
27. Breuer E, Hebeisen M, Schneider MA, et al. Site of recurrence and survival after surgery for colorectal peritoneal metastasis. J National Cancer Inst. 2021;113(8):1027–1035. doi:10.1093/jnci/djab001
28. Portilla AG, Sugarbaker PH, Chang D. Second-look surgery after cytoreduction and intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal cancer: analysis of prognostic features. World J Surg. 1999;23(1):23–29. doi:10.1007/s002689900560
29. Frühling P, Moberg L, Ghanipour L, et al. Clinical significance of circulating tumor cells in epithelial appendiceal neoplasms with peritoneal metastases. Cancers. 2024;16(13):2441. doi:10.3390/cancers16132441
30. Frühling P, Moberg L, Ghanipour L, et al. Clinical significance of circulating tumor cells in colorectal cancer with peritoneal metastases: a prospective cohort study using a novel method for monitoring treatment response, and assessing minimal residual disease. Int J Surg. 2024;110(11):7187–7195. doi:10.1097/js9.0000000000001906
31. Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for Colorectal Cancer Screening. Gastroenterology. 2020;158(2):418–432. doi:10.1053/j.gastro.2019.06.043
32. Thomsen CB, Hansen TF, Andersen RF, Lindebjerg J, Jensen LH, Jakobsen A. Early identification of treatment benefit by methylated circulating tumor DNA in metastatic colorectal cancer. Ther Adv Med Oncol. 2020;12:1758835920918472. doi:10.1177/1758835920918472
33. Bando H, Kagawa Y, Kato T, et al. A multicentre, prospective study of plasma circulating tumour DNA test for detecting RAS mutation in patients with metastatic colorectal cancer. Br J Cancer. 2019;120(10):982–986. doi:10.1038/s41416-019-0457-y
34. Bando H, Nakamura Y, Taniguchi H, et al. Effects of metastatic sites on circulating tumor DNA in patients with metastatic colorectal cancer. JCO Precis Oncol. 2022:
35. Manca P, Corallo S, Lonardi S, et al. Variant allele frequency in baseline circulating tumour DNA to measure tumour burden and to stratify outcomes in patients with RAS wild-type metastatic colorectal cancer: a translational objective of the Valentino study. Br J Cancer. 2022;126(3):449–455. doi:10.1038/s41416-021-01591-8
36. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nature Med. 2008;14(9):985–990. doi:10.1038/nm.1789
37. Moding EJ, Nabet BY, Alizadeh AA, Diehn M. Detecting liquid remnants of solid tumors: circulating tumor DNA minimal residual disease. Cancer Disc. 2021;11(12):2968–2986. doi:10.1158/2159-8290.Cd-21-0634
38. Yu L, Lopez G, Rassa J, et al. Direct comparison of circulating tumor DNA sequencing assays with targeted large gene panels. PLoS One. 2022;17(4):e0266889. doi:10.1371/journal.pone.0266889
39. Kundu S, Nunes L, Adler J, Mathot L, Stoimenov I, Sjöblom T. Recurring EPHB1 mutations in human cancers alter receptor signalling and compartmentalisation of colorectal cancer cells. Cell Commun Signal. 2023;21(1):354. doi:10.1186/s12964-023-01378-9
40. Kim Y, Ahmed S, Miller WT. Colorectal cancer-associated mutations impair EphB1 kinase function. J Biol Chem. 2023;299(9):105115. doi:10.1016/j.jbc.2023.105115
41. Priya B, Ravi S, Kirubakaran S. Targeting ATM and ATR for cancer therapeutics: inhibitors in clinic. Drug Discov Today. 2023;28(8):103662. doi:10.1016/j.drudis.2023.103662
42. Suzuki T, Hirokawa T, Maeda A, et al. ATR inhibitor AZD6738 increases the sensitivity of colorectal cancer cells to 5‑fluorouracil by inhibiting repair of DNA damage. Oncol Rep. 2022;47(4). doi:10.3892/or.2022.8289
43. Egger T, Bordignon B, Coquelle A. A clinically relevant heterozygous ATR mutation sensitizes colorectal cancer cells to replication stress. Sci Rep. 2022;12(1):5422. doi:10.1038/s41598-022-09308-4
44. Jaschke A, Chung B, Hesse D, et al. The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium. Hum Mol Genet. 2012;21(14):3128–3142. doi:10.1093/hmg/dds140
45. Gao Z, Yang YY, Huang M, Qi TF, Wang H, Wang Y. Targeted proteomic analysis of small GTPases in radioresistant breast cancer cells. Anal Chem. 2022;94(43):14925–14930. doi:10.1021/acs.analchem.2c02389
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.