Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Relationship Between Timing of PCSK9 Inhibitor Monoclonal Antibody Initiation and Clinical Outcomes in Patients with Prior Cardiovascular Events
Authors Sidelnikov E, Kalich BA, Miglins ML, Multani JK, Tuly R, Hawkins K, Baber U
Received 17 December 2024
Accepted for publication 29 April 2025
Published 21 May 2025 Volume 2025:21 Pages 727—736
DOI https://doi.org/10.2147/TCRM.S512708
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Eduard Sidelnikov,1 Bethany A Kalich,2 Margot Lisa Miglins,3 Jasjit K Multani,4 Rifat Tuly,5 Kevin Hawkins,5 Usman Baber6
1Global Value & Access, Amgen (Europe) GmbH, Rotkreuz, Switzerland; 2Medical Affairs, Amgen Inc., Thousand Oaks, CA, USA; 3Medical Value & Access Communications, Amgen, Thousand Oaks, CA, USA; 4Health Economics and Outcomes Research, IQVIA, Falls Church, VA, USA; 5Health Economics and Outcomes Research, IQVIA, Wayne, PA, USA; 6The Zena and Michael A. Weiner Cardiovascular Institute, The Mount Sinai Medical Center, New York, NY, USA
Correspondence: Eduard Sidelnikov, Global Value & Access, Amgen (Europe) GmbH, Suurstoffi 22, Rotkreuz, CH-6343, Switzerland, Tel +41 (0) 41 369 04 42, Email [email protected]
Purpose: Timing of initiation of proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) monoclonal antibody (mAb) therapy and its impact on cardiovascular outcomes is unknown. The aim was to identify any association between timing of PCSK9i mAb initiation after a major adverse cardiovascular event (MACE) and the rate of subsequent MACE.
Patient and Methods: A retrospective cohort study of adult patients in the United States with a MACE (myocardial infarction, stroke, unstable angina, or coronary revascularization) from January 1, 2017 to February 28, 2022 was conducted using administrative claims databases (index date = first observed MACE during this period). Patients were required to have ≥ 360 days of data visibility prior to (baseline period) and for ≥ 30 days after the index date (minimum, variable follow up period), and ≥ 1 prescription claim for PCSK9i mAb therapy on or after the index date. Subsequent MACE rates, time from index MACE to PCSK9i mAb initiation, and time to subsequent MACE were reported.
Results: A total of 58,997 patients with ≥ 1 MACE were identified (mean age = 64 years; 58% male; median follow up=1,241 days). Over half of the patients did not initiate a PCSK9i mAb in the first year after the index MACE. Overall, 35% (n = 20,465) had ≥ 1 subsequent MACE. Compared to the period between index MACE and prior to PCSK9i mAb initiation, rates of subsequent MACE after PCSK9i mAb initiation were reduced in a time-dependent manner by 70% among patients who initiated PCSK9i mAb therapy within 30 days, 78% (31– 90 days), 76% (91– 180 days), 65% (181– 360 days), and 42% (> 360 days) after the index MACE. Those who initiated PCSK9i mAb within 30 days of the index MACE had longer median time to the first subsequent MACE (111 days) compared to patients who initiated at later times.
Conclusion: This study provides evidence that earlier initiation of PCSK9i mAb therapy after a MACE appeared to be associated with longer time to a subsequent MACE. Patients without timely treatment are left at an unnecessarily elevated risk of further MACE.
Keywords: MACE, cardiovascular risk, lipid-lowering therapy, claims analysis
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality, resulting in more than 940,000 deaths in the United States (US) in 2022.1 Atherosclerotic cardiovascular disease (ASCVD) is a severe form of CVD that frequently leads to major adverse cardiovascular events (MACE).1 MACE refers to a composite clinical endpoint that is often used for outcome evaluations in clinical trials for cardiovascular (CV) research. MACE is frequently defined as a composite of non-fatal stroke, non-fatal MI or cardiovascular CV death but can also include heart failure, coronary revascularization, and unstable angina.2–4 The American College of Cardiology (ACC) categorizes patients as having very high risk of future ASCVD events by the occurrence of past major ASCVD events and by patient risk factors, such as elevated lipid risk factors.5
Elevated low-density lipoprotein cholesterol (LDL-C) levels are a well-established causal risk factor for ASCVD. However, LDL-C levels can be effectively modified using highly efficacious lipid lowering therapies (LLT).6,7 The ACC recommends the addition of a non-statin therapy to maximally tolerated statin therapy for ASCVD patients with an LDL-C of ≥55 mg/dL and at very high risk (VHR) of future ASCVD events.5 Non-statin therapies include ezetimibe or monoclonal antibody (mAb) therapies, such as proprotein convertase subtilisin/kexin type 9 inhibitor (PCSK9i) mAbs. PCSK9i mAbs have been shown to substantially reduce LDL-C levels in patients who cannot achieve and maintain an optimal LDL-C level on statins alone.5,7–10 Despite the ACC recommendations, studies have shown undertreatment with LLT in patients with high CV risk. A real-world assessment in 2019 found that just over half (58.8%) of patients with LDL-C >130 mg/dL received an LLT, with only 1% of patients utilizing PCSK9i mAbs.9
Prompt reduction of LDL-C in ASCVD patients is critical. As available clinical evidence shows, aggressive reduction of LDL-C increases the magnitude of risk reduction.9 There is evidence that early reduction of LDL-C after an MI is associated with lower risk of major adverse cardiac events (MACE) and CV mortality.11,12 Further, longer duration of treatment has been shown to correlate with lower LDL-C levels.6,11 As evidence also suggests a cumulative effect of long-term LLT for continued LDL-C reduction, robust attempts to lower LDL-C rapidly in eligible patients are imperative.6 However, information on the association between timing of PCSK9i mAb initiation and risk of subsequent CV outcomes is insufficient to determine optimal timing of treatment administration.
This descriptive study aimed to evaluate the association between timing of PCSK9i mAb initiation in patients with history of MACE and risk of a subsequent MACE a real-world setting. The occurrence and rate of subsequent MACE outcomes were assessed in US administrative claims data. These occurrences were used to characterize optimal timing of PCSK9i mAb medication initiation and its association with subsequent MACE.
Materials and Methods
Study Design
This retrospective cohort study utilized the IQVIA PharMetrics® Plus adjudicated health plan claims supplemented with the IQVIA open-source medical (Dx) and longitudinal pharmacy (LRx) claims databases, with a subset linked to Prognos and Quest LDL-C data. IQVIA PharMetrics® Plus is a health plan claims database comprised of fully adjudicated medical and pharmacy claims for more than 190 million unique enrollees since 2006. Data contributors are largely commercial health plans, and the database is representative of the commercially insured US national population for patients under 65 years of age with respect to age and sex. IQVIA LRx captures information on adjudicated dispensed prescriptions sourced from retail, mail, long-term care, and specialty pharmacies. IQVIA Dx contains unadjudicated medical claims from office-based physicians, ambulatory facilities, and hospital-based physicians, and is sourced from clearing houses involved in the claims processing. The IQVIA databases linked to the Prognos and Quest LDL-C databases were accessible to the authors of this study, and a primary study dataset was constructed using linked patient data from the databases. All records in these databases are deidentified.
Patients with ≥1 MACE from January 1, 2017 to November 30, 2021, for PharMetrics Plus and from January 1, 2017 to February 28, 2022, for Dx/LRx were included in the study, with the total study period extending from January 1, 2016 to March 31, 2022. The date of the first documented MACE (defined as an ICD-10-CM code for myocardial infarction [MI], stroke, or unstable angina in the primary position on an inpatient claim, an ICD-10-CM for MI or stroke in any position on an emergency department claim, or a procedure code for coronary revascularization in any position on an inpatient or outpatient claim) was designated as the index date. CV death was not included in the MACE composite because mortality data was not available. For patients identified both in PharMetrics Plus and Dx/LRx, the earlier date was used as the index date. For patients identified with the same date both in PharMetrics Plus and Dx/LRx, data visibility was checked using both the PharMetrics Plus and the Dx/LRx criteria; patients were kept in the sample provided that the criteria were met in either of the data sources. A study design schematic is depicted in Supplementary Figure 1.
This study complied with all applicable laws regarding subject privacy, including the Health Insurance Portability and Accountability Act of 1996 (HIPAA). All data analyzed in this study were secondary and anonymized; no direct subject contact or primary collection of individual human subject data occurred. Therefore, this study did not require informed consent, ethics committee, or Institutional Review Board review.
Patient Population
Patients were required to be ≥18 years on the index date, with ≥360 days of data visibility prior to the index date and for ≥30 days after, as well as having ≥1 prescription claim for PCSK9i mAb therapy on or after the index date. The 360-day period prior to the index date was considered the baseline period, used to assess patient baseline characteristics. Outcomes were assessed during the 30-day minimum, variable follow-up period (including the index date). Patients with evidence of PCSK9i mAb therapy during the baseline period were excluded. Patients were followed until the end of data visibility or end of study period (December 31, 2021, in PharMetrics Plus or March 31, 2022 in open-source claims), whichever occurred earliest. Patients with missing or invalid data for year of birth or gender were excluded.
Outcomes
The primary outcomes were the time from index MACE to PCSK9i mAb initiation and the occurrence of subsequent MACE during the follow-up period. After the index MACE, all subsequent MACE of the same type were counted as the same event if they occurred within 30 days of the discharge date of the previous event. Coronary revascularization occurring within 30 days of discharge from a prior MI/stoke/unstable angina event, or a prior revascularization, was not considered a distinct event. Time to first subsequent MACE was captured, both from index MACE and from PCSK9i mAb initiation. The secondary objective of this study was to evaluate the relationship between timing of PCSK9 mAb initiation and rates of total subsequent MACE. Use of LLTs in patients was captured over the follow-up period, as separate therapies, using NDC codes. Additionally, duration of PCSK9i mAb therapy was captured. LDL-C levels were captured among patients with available laboratory data, and relative change in value was calculated.
Statistical Analysis
Baseline demographic and clinical characteristics are summarized using descriptive statistics: mean (standard deviation [SD]) and median (interquartile range [IQR]) for continuous variables, frequencies and percentages for categorical ones. Rates of subsequent MACE were expressed per 100 person-years with 95% confidence interval (CI), for first subsequent MACE documented and for all MACE documented during the follow-up period. Time to first subsequent MACE and time to PCSK9i mAb initiation from index MACE were estimated. Patients without a subsequent MACE were censored at the end of their follow up. The timing of PCSK9i mAb initiation after index MACE was assessed and categorized into the following groups: 0–30 days, 31–90 days, 91–180 days, 181–360 days, and >360 days from index date. Qualifying patients were stratified by the subgroups of timing of PCSK9i mAb initiation as stated above. Patients with ≥1 LDL-C laboratory value during the 360-day pre-index period and the variable follow-up period, were evaluated for LDL-C outcomes. Somers’ D test was used to test the proportional difference in MACE rate per 100-person-years before and after initiation of PCSK9i mAb among the timing initiation groups; p-value <0.05 was considered statistically significant. All analyses were performed using SAS® version 9.4.
Results
Patient Characteristics
A total of 58,997 patients with at least one prior MACE were included in the analysis. The mean age of the study participants was 64.1 (SD: 10.8) years, 58.5% were male and the majority (72.5%) were commercially/self-insured (Table 1). Median (IQR) follow up was 1241 (830, 1,538) days. Of these, 41.7% (N = 24,618) had a coronary revascularization, 31.2% (N = 18,301) had an MI, 15.4% (N = 9,078) had unstable angina, and 11.9% (N = 7,000) had a stroke. During the baseline period, more than half (55.8%) of patients had evidence of hypertension and nearly 40% had evidence of diabetes. Approximately 41.1% (n = 24,244) of patients did not receive an LLT during the baseline period. Of those patients utilizing an LLT (n = 34,753), 44.1% utilized only statins, with 21.3% of patients on a high-intensity statin only. Among the 15,088 (25.6%) patients with LDL-C data available at baseline, the mean LDL-C was 127.1 mg/dL (SD: 49.3 mg/dL) and 90% had LDL-C levels above 70 mg/dL.
 |
Table 1 Patient Demographic and Clinical Characteristics |
Time to PCSK9i mAb Therapy
Due to the nature of the inclusion criteria, all patients received a PCSK9i mAb. In this analysis, we found that more than half of all patients (55.2%) initiated PCSK9i mAb therapy >360 days following the index MACE (Figure 1). The median time to PCSK9i mAb initiation was 427 days (IQR: 145–838 days). Fewer than 20% of patients initiated PCSK9i mAb in the first 90 days after index MACE, and only 7.1% initiated the therapy within the first 30 days after the index MACE.
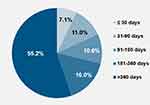 |
Figure 1 Distribution of timing of PCSK9i mAb initiation cohorts after index MACE. |
Subsequent MACE
Among all patients, 34.7% (N = 20,465) had ≥1 subsequent MACE during the study period. A total of 33,954 MACE events were documented over the variable follow-up period, translating into a rate of 17.5 per 100 person-years (95% CI: 17.3–17.7; Supplementary Table 1); 60.6% (20,593/33,954; Supplementary Tables 2 and 3, respectively) of subsequent MACE events occurred prior to PCSK9i mAb initiation. Among patients who initiated PCSK9i mAb within 30, 31–90, 91–180, 181–360, and >360 days after index MACE, 25%, 27%, 30%, 33%, and 39% had a subsequent MACE, respectively.
Among patients who experienced a subsequent event, those who initiated PCSK9i mAb within 30 days after the index event (N = 4205) had longer median time to first subsequent MACE (111 days) compared to patients who initiated at later times (Figure 2). Further, eligible patients who did not receive PCSK9i mAb soon after a MACE event remained at increased risk of further events: more than 30% of those who received PCSK9i mAb >360 days after the index event experienced ≥1 additional event and 10.5% experienced ≥2 additional events (Figure 3).
 |
Figure 2 Median time (days) between PCSK9i mAb initiation and first subsequent MACE.1 aEvaluated among patients with a subsequent MACE in the post-index period. A positive value indicates that the subsequent MACE event occurred after PCSK9i mAb therapy; a negative value indicates that the subsequent MACE event occurred before the patient received PCSK9i mAb therapy. Note: Bars represent the interquartile range from index MACE to PCSK9i mAb initiation. |
 |
Figure 3 Proportion of patients with subsequent MACE and number of MACE prior to PCSK9i mAb initiation by timing of PCSK9i mAb initiation cohort. |
When rates of subsequent MACE that occurred prior to PCSK9i mAb initiation were evaluated against rates of subsequent MACE that occurred after PCSK9i mAb initiation (Supplementary Tables 2 and 3, respectively), the rates were reduced by 70%, 78%, 76%, 65%, and 42% for patients who initiated within 30, 31–90, 91–180, 181–360, and >360 days after index MACE, respectively (p < 0.0001; Figure 4).
LDL-C Levels
The mean (SD) LDL-C of patients from index date to the date of PCSK9i mAb initiation was 111.5 (48.3) mg/dL, and the absolute change in LDL-C from the last value in the baseline period to the closest value to the date of initiation of PCSK9i mAb therapy in the post-index period was −13.3 mg/dL (relative change: −1.6%). The mean (SD) LDL-C of patients from date of PCSK9i mAb initiation to the end of follow-up was 77.7 (48.5) mg/dL, and the absolute change in LDL-C was −49.9 mg/dL (relative change: −35.9%).
Discussion
The findings from this study underscore the impact of delay in treatment with advanced LLT in patients at high risk for ASCVD events. The majority of patients initiated PCSK9i mAb therapy more than a year after their index MACE event. Fewer than 20% of eligible patients initiated PCSK9i mAb therapy within the first 90 days of the event. The substantial delay in PCSK9i mAb therapy observed in this study is relatively consistent with findings from prior research which identify access barriers and cost.8,13 Despite the price reductions for PCSK9i mAbs that were instituted in the US beginning in 2018, older patients and those on Medicare continue to experience barriers in accessing PCSK9i mAb therapy.13 The delay in PCSK9i mAb therapy as demonstrated by real world studies is contrary to current ACC guidelines, which recommend adding further LLT to statins for patients with VHR ASCVD with a prior MACE.14
A recent study of US administrative claims data by McKinley et al assessed time to PCSK9i mAb initiation after an acute MI occurring between July 2015 and December 2018 and found that PCSK9i mAb was initiated by 42% of patients within 90 days after hospital discharge, and by 25% of patients 90–179 days after discharge; our estimates are lower for the same time periods among patients with MI as the index MACE (19% and 11%, respectively).15 This may be due to the difference in the study periods, as the current study used more recently available data and access to PCSK9i mAb therapy has improved over time. McKinley et al also found evidence of additional events prior to treatment initiation in 13% of those initiating PCSK9i mAb >365 days.15 This was echoed in our study, with one-third of patients who initiated PCSK9i mAb >360 days after the index event experiencing a subsequent MACE prior to that initiation.
Findings from the clinical studies FOURIER and FOURIER-OLE (NCT: 01764633, NCT: 03080935)16,17 indicate that early implementation of PCSK9i mAb therapy can have a beneficial preventative effect on CV events, particularly coronary artery disease.18 Our real-world study found that patients who did not initiate PCSK9i mAbs early (<90 days) remained at higher risk for further events. Of patients who waited for more than a year after the index event to initiate PCSK9i mAb therapy, 33% experienced an additional MACE, and 10% experienced 2 or more MACE prior to PCSK9i initiation. Finally, we found those who initiated PCSK9i mAbs earlier experienced greater risk reduction of subsequent MACE compared to those who initiated them later. This evidence is invaluable for physicians to prescribe LLT as early as possible when treating VHR patients.
This study provided important information about the impact of timing of PCSK9i mAb initiation on rates of subsequent MACE. Nevertheless, there are limitations in this study. The study sample from PharMetrics Plus was largely commercially insured or self-insured and may not be representative of uninsured or Medicare or Medicaid populations. This study relied on administrative data collected for billing, and not for clinical research, purposes. The open-source claims databases (LRx and Dx) do not contain patient enrolment information in pharmacy and medical claim benefits. Therefore, we applied proxy rules for data visibility/stability to ensure patients’ clinical history was adequately captured in the data. This analysis was descriptive and confounding factors were not controlled for in the results. Future studies warrant adjustment of confounders in the relationship between timing of PCSK9i mAb and subsequent MACE rates. Patients in this study had a variable length of follow-up. MACE are more likely to occur with longer follow-up time as is the proportion of patients with MACE prior to PCSK9i initiation since follow up time was truncated at the time of PCSK9i initiation. Immortal time bias may have affected the results, as these analyses are only able to evaluate patients who survived their index MACE until their subsequent MACE event (fatal or non-fatal), which could lead to underestimation of subsequent MACE risk. Data on CV-related deaths was not available, a common limitation of US-based administrative claims databases, for our composite MACE outcome. This is most likely to result in the underestimation of MACE rates during the follow up period. The pre- and post-PCSK9i mAb initiation MACE rates are driven by the duration of time prior to and after PCSK9i initiation, respectively. Hence, rates were reported per 100 patient-years to account for the variable observation periods. Change in LDL-C values during follow up was limited to one-quarter of the study sample with available LDL-C data. However, to date, this is the largest real-world data analysis of subsequent MACE rates among ASCVD patients with PCSK9i mAb use. This study offers valuable insight into the relationship between timing of PCSK9i mAb initiation and subsequent MACE.
Conclusions
Among patients who experienced a MACE, earlier initiation of PCSK9i mAb appeared to be associated with longer time to a subsequent MACE. However, we found that more than 50% of eligible patients did not initiate PCSK9i mAb therapy until more than a year after their index MACE, leaving them at an unnecessarily elevated risk of a subsequent MACE. Further study of the clinical consequences of delayed initiation of PCSK9i mAb therapy is warranted.
Ethical Statement
As a retrospective study using secondary data, no interventions were made to patients during this study. In compliance with the Health Insurance Portability and Accountability Act (HIPAA), patient data included in the analyses were de-identified; therefore, this study was not subject to Institutional Review Board (IRB) review.
Acknowledgments
The authors would like to thank Kate Lovett, MPH, of IQVIA, Inc. for assistance in manuscript preparation, and Laura Martinez, formerly of Amgen, Inc. for her assistance in study conceptualization.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Amgen, Inc.
Disclosure
ES is an employee of Amgen (Europe) GmbH; ES holds stock of Amgen Inc. BK is an employee of Amgen Inc. and holds stock of Amgen Inc. MLM was an employee of Amgen, Inc. at the time of study. JM and RT are employed by IQVIA, which was hired to perform this work. KH was an employee of IQVIA at the time of study. UB is an employee of the Mount Sinai Medical Center. He also reports personal fees from Amgen, AstraZeneca, Boston Scientific, and Abbott, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Martin SS, Aday AW, Allen NB, et al.; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Committee. 2025 heart Disease and Stroke Statistics: a Report of US and Global Data From the American Heart Association. Circulation. 2025;151(8):e41–e660. doi:10.1161/CIR.0000000000001303
2. Miao B, Hernandez AV, Alberts MJ, Mangiafico N, Roman YM, Coleman CI. Incidence and predictors of major adverse cardiovascular events in patients with established atherosclerotic disease or multiple risk factors. J Am Heart Assoc. 2020;9(2):e014402. doi:10.1161/JAHA.119.014402
3. Arnott C, Li Q, Kang A, et al. Sodium-glucose cotransporter 2 inhibition for the prevention of cardiovascular events in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9(3):e014908. doi:10.1161/JAHA.119.014908
4. Verma S, Bain SC, Buse JB, et al. Occurrence of first and recurrent major adverse cardiovascular events with liraglutide treatment among patients with type 2 diabetes and high risk of cardiovascular events: a post hoc analysis of a randomized clinical trial. JAMA Cardiol. 2019;4(12):1214–1220. doi:10.1001/jamacardio.2019.3080
5. Lloyd-Jones DM, Morris PB, Ballantyne CM, et al. 2022 ACC expert consensus decision pathway on the role of nonstatin therapies for LDL-cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2022;80(14):1366–1418. Erratum in: J Am Coll Cardiol. 2023 Jan 3;81(1):104. doi:10.1016/j.jacc.2022.07.006
6. Ference BA, Ginsberg HN, Graham I, et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2017;38(32):2459–2472. doi:10.1093/eurheartj/ehx144
7. Bardolia C, Amin NS, Turgeon J. Emerging non-statin treatment options for lowering low-density lipoprotein cholesterol. Front Cardiovasc Med. 2021;8:789931. doi:10.3389/fcvm.2021.789931
8. Myers KD, Farboodi N, Mwamburi M, et al. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12(8):e005404. doi:10.1161/CIRCOUTCOMES.118.005404
9. Chamberlain AM, Gong Y, Shaw KM, et al. PCSK9 inhibitor use in the real world: data from the national patient-centered research network. J Am Heart Assoc. 2019;8(9):e011246. doi:10.1161/JAHA.118.011246
10. Birtcher KK, Allen LA, Allen LA, et al. 2022 ACC expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2023;81(3):292–317. doi:10.1016/j.jacc.2022.08.754
11. Trialists CT. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170 000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi:10.1016/s0140-6736(10)61350-5
12. Schubert J, Lindahl B, Melhus H, et al. Low-density lipoprotein cholesterol reduction and statin intensity in myocardial infarction patients and major adverse outcomes: a Swedish nationwide cohort study. Eur Heart J. 2021;42(3):243–252. doi:10.1093/eurheartj/ehaa1011
13. Smith A, Johnson D, Banks J, Keith SW, Karalis DG. Trends in PCSK9 inhibitor prescriptions before and after the price reduction in patients with atherosclerotic cardiovascular disease. J Clin Med. 2021;10(17):3828. doi:10.3390/jcm10173828
14. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139(25). doi:10.1161/cir.0000000000000625
15. McKinley EC, Bittner VA, Brown TM, et al. Factors associated with time to initiation of a PCSK9 inhibitor after hospital discharge for acute myocardial infarction. J Clin Lipidol. 2022;16(1):75–82. doi:10.1016/j.jacl.2021.11.001
16. Fourier open-label extension study in subjects with clinically evident cardiovascular disease in selected European countries. Available from: https://clinicaltrials.gov/study/NCT03080935.
17. Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk (Fourier). Available from: https://clinicaltrials.gov/study/NCT03080935.
18. Chen H, Chen X. PCSK9 inhibitors for acute coronary syndrome: the era of early implementation. Front Cardiovasc Med. 2023;10:1138787. doi:10.3389/fcvm.2023.1138787
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Use of Contrave, Naltrexone with Bupropion, Bupropion, or Naltrexone and Major Adverse Cardiovascular Events: A Systematic Literature Review
Dahlberg S, Chang ET, Weiss SR, Dopart P, Gould E, Ritchey ME
Diabetes, Metabolic Syndrome and Obesity 2022, 15:3049-3067
Published Date: 29 September 2022


