Back to Journals » International Journal of Nanomedicine » Volume 20
Reprogramming of Glucose Metabolism by Nanocarriers to Improve Cancer Immunotherapy: Recent Advances and Applications
Authors Jiang K, Liu H, Chen X, Wang Z, Wang X, Gu X, Tong Y, Ba X, He Y, Wu J , Deng W, Wang Q , Tang K
Received 19 December 2024
Accepted for publication 20 March 2025
Published 5 April 2025 Volume 2025:20 Pages 4201—4234
DOI https://doi.org/10.2147/IJN.S513207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Yan Shen
Kehua Jiang,1,* Hongming Liu,2,* Xiaolong Chen,1 Zhen Wang,1 Xiaodong Wang,1 Xiaoya Gu,3 Yonghua Tong,4 Xiaozhuo Ba,4 Yu He,4 Jian Wu,4 Wen Deng,4 Qing Wang,1 Kun Tang4,5
1Department of Urology, Guizhou Provincial People’s Hospital, Guiyang, Guizhou, People’s Republic of China; 2Department of Urology, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, People’s Republic of China; 3Guizhou University of Traditional Chinese Medicine, Guiyang, Guizhou, People’s Republic of China; 4Department of Urology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, People’s Republic of China; 5Shenzhen Huazhong University of Science and Technology Research Institute, Shenzhen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kun Tang, Email [email protected] Qing Wang, Email [email protected]
Abstract: Although immunotherapy has made significant progress in cancer treatment, its limited responsiveness has greatly hindered widespread clinical application. The Warburg effect in tumor cells creates a tumor microenvironment (TME) characterized by hypoxia, low glucose levels, and high lactate levels, which severely inhibits the antitumor immune response. Consequently, targeting glucose metabolism to reprogram the TME is considered an effective strategy for reversing immunosuppression and immune evasion. Numerous studies have been conducted on enhancing cancer immunotherapy efficacy through the delivery of glucose metabolism modulators via nanocarriers. This review provides a comprehensive overview of the glucose metabolic characteristics of tumors and their impacts on the immune system, as well as nanodelivery strategies targeting glucose metabolism to enhance immunotherapy. These strategies include inhibiting key glycolytic enzymes, blocking glucose and lactate transporters, and utilizing glucose oxidase and lactate oxidase. Furthermore, this article reviews recent advancements in synergistic antitumor therapy involving glucose metabolism-targeted therapy combined with other treatments, such as chemotherapy, radiotherapy (RT), phototherapy, and immunotherapy. Finally, we discuss the limitations and future prospects of nanotechnology targeting glucose metabolism therapy, hoping to provide new directions and ideas to improve cancer immunotherapy.
Keywords: nanoparticles, glycolysis, lactate metabolism, cancer immunotherapy, tumor microenvironment
Graphical Abstract:

Introduction
Immunotherapy has shown significant potential in cancer treatment and has revolutionized the landscape of cancer therapy. Among these, immune checkpoint blockade (ICB) therapies targeting co-inhibitory receptors on T cells have been the focus of extensive research, particularly those involving programmed death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte antigen 4 (CTLA-4).1–3 However, only a minority of patients exhibit a favorable objective response, possibly due to weak antigen specificity and insufficient T-lymphocyte infiltration.4,5 Additionally, cancer cells display high levels of heterogeneity and plasticity, enabling them to develop multiple mechanisms for immune escape, ultimately leading to unsatisfactory clinical outcomes.6 Tumors can also induce the transition from an antitumor microenvironment to a protumor microenvironment by regulating metabolism. For example, hepatocellular carcinoma cells can upregulate hypoxia-inducing factor 1α (HIF1α) to induce the overexpression of hexokinase 2 (HK2), resulting in enhanced glycolytic metabolism and promoting the formation of a tumor immunosuppressive microenvironment (TIME).7
The specific glucose metabolism in tumors plays a pivotal role in shaping the TIME. Even under aerobic conditions, cancer cells rely primarily on glycolysis rather than oxidative phosphorylation (OXPHOS) to obtain energy, a phenomenon known as the Warburg effect.8 Tumor competition for glucose limits the supply of glucose to T cells, hindering the antitumor immune response. Glycolysis creates a low-glucose, hypoxic, and acidic TME by converting glucose into lactate. The byproducts of glycolysis, such as lactate, impair the function of antitumor immune cells and facilitate the recruitment of immunosuppressive cells such as M2 macrophages and regulatory T cells (Tregs).9 Alterations within the TME result in immunosuppression and facilitate immune evasion, thereby limiting the efficacy of immunotherapy and promoting tumor progression and metastasis. Consequently, an increasing number of studies have considered targeting glucose metabolism as a potential approach for enhancing immunotherapy. Reducing the glucose source of tumor cells and inhibiting key enzymes involved in glycolysis are effective strategies for reshaping the TME. Directly reducing lactate levels in the TME is also an important method for reversing the TIME.
In addition, the development of targeted delivery systems represents a revolutionary advancement in cancer therapy. A diverse array of nanocarriers, including polymer nanoparticles (NPs),10 lipid-based materials,11 metallic NPs,12 and extracellular vesicles (EVs)13 etc., have been utilized in anti-tumor therapy. One of the most prevalent applications involves the delivery of drugs through these nanocarriers, which leverage their specific targeting and responsive delivery capabilities to enhance the efficacy of anti-tumor treatments while minimizing adverse effects. Furthermore, more advanced functional nanocarriers have been developed, including nanovaccines designed to activate immune responses and improve the effectiveness of immunotherapy,14 as well as nanozymes that regulate the TME by catalyzing H2O2.15 Additionally, bionic nanocarriers have been engineered to overcome biological barriers,16 and photothermal conversion nanomaterials facilitate precise ablation of deep tumors under near-infrared (NIR) light.17 More importantly, there have been significant advancements in various targeted metabolic nanomedicines that enhance immunotherapy by inhibiting the Warburg effect.
Therefore, we conducted a comprehensive literature search and review utilizing various databases, including PubMed, ScienceDirect, Google Scholar, and Medline etc. The key terms employed in the search encompassed nanoparticles, glycolysis, lactate metabolism, tumor microenvironment, cancer, and immunotherapy etc. This review summarizes the characteristics of tumor glycolytic metabolism and its role in immune regulation and tumor progression. Furthermore, this paper presents a comprehensive overview of various emerging strategies aimed at targeting glycolytic metabolic pathways to enhance immunotherapy, as well as recent advances in combining these strategies with other therapeutic approaches. Finally, the challenges and future possibilities of targeted therapies for glucose metabolism are also discussed.
Tumor Glucose Metabolism-Mediated Immunosuppression and Therapy Resistance
Characteristics of Glucose Metabolism in Cancer
Cancer cells often overexpress glucose transporters (GLUTs) to improve the efficiency of transporting glucose into the cell and obtaining sufficient energy.18 Glucose is subsequently catalyzed to glucose 6-phosphate (G-6-P) via HK. G-6-P is converted to pyruvate by a range of enzymes, such as phosphofructokinase (PFK) and pyruvate kinase (PK). Under aerobic conditions, the anaerobic oxidation pathway of normal cells is inhibited, and pyruvate generates acetyl-CoA via pyruvate dehydrogenase (PDH) that finally enters the tricarboxylic acid (TCA) cycle to completely oxidize and produce abundant ATP, known as OXPHOS. However, in tumor cells, pyruvate preferentially enters the glycolysis pathway and is reduced to produce lactate by lactate dehydrogenase (LDH) (Figure 1).
Although aerobic glycolysis is the primary pathway in tumor cells, these cells can adjust their metabolic mode and adapt to the TME in response to different stresses. Hypoxia-induced tumor cells located far from blood vessels mainly undergo glycolysis, while oxygen-rich tumor cells located around blood vessels can obtain energy through OXPHOS.19 Tumor cells can selectively express monocarboxylic acid transporter (MCT) 4 or MCT1, depending on different conditions. Oxygen-depleted tumor cells export lactate extracellularly in an MCT1-dependent manner, thereby mitigating lactate feedback inhibition and enhancing glycolysis.20 Oxygen-rich breast cancer cells transport lactate into cells through MCT4 and serve as an energy source, allowing cancer cells to survive long-term glucose starvation and resist anti-PI3K/ mammalian target of rapamycin (mTOR) inhibitor therapy.21
In addition, Warburg reported that cancer cells consumed more glucose and other nutrients than did normal cells. Cancer cells possess higher enzymatic activity and glycolytic efficiency to obtain sufficient energy.22,23 This idea was further validated by the discovery that highly glycolytic tumors were more aggressive.24 Although a single glycolysis produces much less ATP than OXPHOS, the rate of ATP production is approximately 10–100 times that of OXPHOS.25 This efficient and rapid ATP generation pathway and abundant intermediate products provide sufficient energy for tumor progression and migration. Uncontrollable proliferation of cancer cells leads to the accumulation of toxic metabolites, such as lactate, adenosine, and reactive oxygen species (ROS), resulting in a TME with high lactate levels, hypoxia and acidosis.26 Excess lactate stabilizes HIF1α in the TME and further induces the production of proangiogenic cytokines. In addition, increased lactate levels promote endothelial cell production of CXCL8, which contributes to tumor progression and metastasis.27 Metabolic alterations not only are extremely important for maintaining tumor growth and survival but also may trigger carcinogenic signals. For instance, neutrophils in the TME transmit the SPI1 mRNA through EVs, leading to increased expression of SPI1 in tumor cells, and SPI1 further drives the expression of glycolysis genes, promoting the progression of colon cancer.28 Therefore, reprogramming glucose metabolism to reverse TME may be an effective anti-tumor approach.
Tumor Glucose Metabolism Mediates Immunosuppression and Treatment Resistance
The TME encompasses various immune cells, such as T cells, dendritic cells (DCs), natural killer (NK) cells, and macrophages. Tumor cells reprogram the TME to allow immune cells to develop in a direction that supports tumor progression. Mature T cells differentiate into CD4+ and CD8+ T cell subtypes, the latter of which can be further divided into Tregs and helper T cells. Due to the competition for glucose by cancer cells, the proliferation and effector function of T cells are inhibited.29,30 The glucose content in the TME of degenerative tumors is higher than that in progressive tumors with immune evasion; as a consequence, degenerative tumors can exert an immune function by promoting CD8+ T cell production of IFN-γ and cancer cell death31 (Figure 2). Hypoxia also hinders the function of T cells. Although CD8+ T cells can still differentiate into functional effector cells under continuous stimulation or hypoxia, the combination of both conditions leads to elevated levels of ROS and impaired mitochondrial function, thereby inhibiting T cells proliferation and antitumor activity.32,33 A high lactate level is an important marker of the TME and inhibits the nuclear factor of activated T cells and mitogen activated protein kinase pathways.34,35 A low pH activates V-domain immunoglobulin suppressor of T cells activation signaling.36 Lactate can inhibit the survival, proliferation and function of CD8+ T cells through the above mechanism. Additionally, T cells exhaustion exacerbates immunosuppression, specifically due to the continuous stimulation of T cells by antigens in the TME, resulting in a gradual loss of effector function and immune memory in T cells.37 T cells exhaustion is characterized by reduced production of antitumor cytokines such as IL-2, TNF-α, and IFN-γ and increased the expression of inhibitory receptors such as PD-1, CTLA4, and TIM-3.38
Tregs possess immunosuppressive properties, enabling them to inhibit the activation and proliferation of effector T cells (Teffs), suppress antitumor and anti-inflammatory effects, and mediate immune evasion and therapy resistance. Moreover, Tregs may show superior adaptability to metabolic alterations and nutrient limitations compared to other T cells subsets. Teffs rely on OXPHOS for energy production, whereas Tregs exhibit decreased GLUT1 levels, reduced glucose uptake, and increased reliance on fatty acid (FAO) oxidation.39 Additionally, Tregs can tolerate a TME with high lactate levels and a low pH through metabolic remodeling mediated by the Treg transcription factor Foxp3.40 In short, Tregs can utilize FAO metabolism and the lactate pathway to maintain their proliferation and function. Furthermore, hypoxia promotes the recruitment of Tregs, and indoleamine 2,3-dioxygenase (IDO) and adenosine in the TME can facilitate Tregs proliferation and immunosuppressive functions.41
DCs are the most important antigen-presenting cells, which can trigger a specific cytotoxic T lymphocyte (CTL) antitumor immune response. Following tumor cell immunogenic cell death (ICD), the release of tumor-associated antigens (TAAs) and damage-associated molecular patterns (DAMPs) activate the immune response by promoting DCs maturation. Activation of DCs leads to an increased demand for glucose; however, the nutrient-restricted TME limits their antigen-presenting capacity.42 Moreover, elevated levels of lactate, low pH, hypoxia and ROS resulting from tumor metabolism impair the antitumor effects mediated by DCs. On the one hand, lactate inhibits major histocompatibility complex (MHC) II presentation on the surface of DCs by activating G protein-coupled receptor 81.43 On the other hand, lactate hampers cross-presentation by DCs through the inhibition of Toll-like receptor 3 and downstream IFN-I and stimulator of interferon genes signaling to accelerate antigen degradation.43,44 An acidic TME can inhibit mTOR activity while promoting monocyte differentiation into DCs in a cytokine-dependent manner.45 Hypoxia affects the differentiation process, as well as the antigen uptake and migration abilities of DCs.46 The maturation of DCs is induced by high mobility group protein B1 (HMGB1), whose function is inhibited by the oxidation of ROS accumulated in the TME.32
Innate immunity is an important antitumor mechanism in which macrophages play a pivotal role. Tumor-associated macrophages (TAMs) can be categorized into two phenotypes: M1 and M2. The M1-like TAMs exhibit anti-inflammatory and antitumor effects, while the M2-like phenotype promotes angiogenesis and facilitates tumor progression and metastasis.47 Tumor metabolites not only inhibit phagocytic activity but also induce the transformation of M1 macrophages to M2 macrophages, significantly compromising the antitumor potential of TAMs.9 Hypoxia triggers A2A and A2B receptor activation, where adenosine binding to adenosine A2A receptors reduces M1-like polarization, and activating adenosine A2B receptors induces M2-like polarization.48,49 Lactate stabilizes HIF-1α and induces the polarization of M2 TAMs through G protein-coupled receptor 132.50,51 HIF-1α also modulates CD47 and CD24 signaling in cancer cells, enabling the evasion of phagocytosis by liver cells.47 Decreasing lactate levels or eliminating A2A expression can promote the polarization of M1 TAMs.52
MHCI molecules on target cells bind to NK cells suppressor receptors to avoid the self-destruction of NK cells.53 Therefore, NK cells play an important role in clearing cancer cells by downregulating MHC molecules. As NK cells are activated, trophic receptors such as GLUT1, CD98, and CD71 are upregulated, resulting in increased glucose uptake and glycolysis efficiency.54 However, the exhaustion of multiple nutrients in the TME inhibits the function of NK cells. Studies have shown that low glucose levels in the TME can interfere with IFN-γ and granzyme B expression by inhibiting mTORC1, and hypoxia can inhibit NK cells activated receptors.55,56 Oxidative stress associated with lipid peroxidation can restrict glucose metabolism and cause NK cells dysfunction.57 Furthermore, Tregs, M2 TAMs and myeloid-derived suppressor cells (MDSCs) secrete immunosuppressive factors such as IL-10 and TGF-β that hinder the activation and function of NK cells.
Tumor-associated MDSCs show enhanced FAO, hypoxia can induce MDSCs to express PD-L1 and recruit MDSCs to metastatic sites and induce MDSCs to differentiate into TAMs.58–60 The upregulation of CD39 by hypoxia can induce AMP production, inhibit MDSCs differentiation to DCs, and promote MDSCs aggregation.61 Alterations in the metabolism of tumors upregulate MDSCs, and MDSCs show good adaptability to energy depletion, hypoxia, and a high lactate level in the TME, which promotes cancer progression and immunosuppression.
In summary, the glucose metabolic reprogramming TME by tumor cells leads to the downregulation and impairment of the function of antitumor immune cells, including T cells, NK cells, and DCs. Simultaneously, more immunosuppressive cells, such as Tregs, M2 macrophages, and MDSCs, are recruited. The TIME allows tumor cells to evade immune surveillance and develop treatment resistance. Consequently, reconfiguring the TIME can markedly diminish therapeutic resistance and enhance the effectiveness of immunotherapy; furthermore, inhibiting tumor glycolysis represents a viable strategy for reprogramming TIME.
Strategies of Nanoparticles Targeting Glucose Metabolism for Cancer Immunotherapy
Given the critical role of glucose metabolism in tumor progression and immune evasion, numerous studies have concentrated on targeting glucose metabolism to enhance the efficacy of anti-tumor immunotherapy. With the ongoing development and application of nanomaterials, nanotechnology have also made remarkable progress in the delivery of glycolysis regulators. In comparison to conventional drugs, nanomaterials have garnered considerable attention due to their remarkable advantages: firstly, they substantially improve therapeutic efficacy while effectively minimizing side effects through precise targeting of tumor cells.62 Secondly, nanomaterials facilitate the integration and assembly of multiple therapeutics, enabling coordinated approaches against tumors that involve various drugs or treatment modalities.63 Furthermore, distinct nanomaterials exhibit unique characteristics: some can penetrate deeply into tumor tissues, others can release drugs under specific conditions, and certain types possess photothermal conversion capabilities or demonstrate enzyme-like activity—allowing researchers to leverage these properties to enhance the anti-tumor effects of NPs.64–67 To inhibit glycolysis effectively, nano-systems can first target key glycolytic enzymes such as HK2, PFK, M2-type PK (PKM2), pyruvate dehydrogenase kinase (PDK), and LDH; additionally, they may obstruct glucose entry into cells and disrupt lactate shuttling by inhibiting transporters. Moreover, strategies that involve blocking the glucose supply for tumor cells via glucose oxidase (GOx) and reducing lactate accumulation by glucose oxidase (LOx) are also recognized as effective anti-tumor approaches (Figure 1, Table 1).
 |
Table 1 Strategies for Enhancing Cancer Immunotherapy by Targeting Glucose Metabolism with Nanoparticles |
Nanoparticles Targeting Glycolysis-Related Enzymes for Cancer Immunotherapy
Nanoparticles Targeting Hexokinase (HK2)
HK2 functions as a crucial regulator of glycolysis, with its upregulation frequently observed in tumor cells.107 The glucose analogue 2-deoxyglucose (2DG), recognized as the first developed inhibitor of HK2, undergoes phosphorylation by HK to yield 2-DG-6-phosphate.108 2-DG-6-phosphate subsequently inhibits both HK and G-6-P isomerase, ultimately leading to energy depletion and apoptosis in tumor cells.109 Liu et al engineered targeted bionic nanovesicles (NVs) derived from genetically modified non-small cell lung cancer cell lines that overexpress PD-1. These P-NVs were loaded with 2DG and (DOX), referred to as PDG-NVs.69 The homologous targeting capability of P-NVs facilitates effective binding between PD-1 on the P-NVs and PD-L1 on cancer cells. Notably, PDG-NVs significantly downregulated the expression of PD-L1 by interfering with its glycosylation via 2DG; concurrently, the presence of PD-1 protein on NVs disrupted the PD-1/PD-L1 axis within cancer cells, effectively enhancing anti-tumor immune responses. Luo et al developed PLGA microspheres co-loaded with hollow gold nanoshells (HAuNS) and metformin, termed HAuNS-Met@MS.68 This nanosystem enables sustained pulsed release of metformin for over three weeks, contributing to enhanced anti-tumor effects.110 When combined with 2DG, HAuNS-Met@M can inhibit glycolysis while reducing heat stress responses in tumor cells; it also enhances photothermal therapy (PTT) induced ICD, reverses heat tolerance in tumor cells, and improves their sensitivity to photothermal treatment.
Lonidamine (LND), an indazole derivative, effectively suppresses aerobic glycolysis by modifying mitochondrial ultrastructure and inhibiting HK2 activity.71 It has entered clinical trials for various cancers, including breast cancer.111 Jiang et al developed a high-fidelity spatio-temporal delivery strategy utilizing apatinib-loaded nanomedicine in conjunction with LND-loaded formulation.70 Specifically, apatinib-loaded nanodurg initially targets tumor endothelial cells to selectively block the vascular endothelial growth factor receptor, facilitating vascular normalization therapy that promotes T cells infiltration. Subsequently, LND-loaded nanodurgexhibits delayed accumulation at tumors while reprogramming glucose metabolism to activate T cells against malignancies. Zhao et al designed a straightforward self-assembled nanoparticle named TerBio without additional carriers, synthesized through intermolecular interactions among LND, chlorine e6 (Ce6), and SB50512473 (Figure 3). SB505124 effectively inhibits TGF-β signaling pathways while synergizing with metabolic modulation from LND to counteract immunosuppression.106 Furthermore, both liposomes loaded with mannose and polymeric NPs containing JQ1 significantly inhibited HK2 activity, leading to successful suppression of glycolysis.74,102,103 In summary, the targeted delivery of HK2 inhibitors via NPs effectively reprogrammed the TME and mitigated immunosuppression, demonstrating promising anti-tumor potential.
Lonidamine (LND), an indazole derivative, effectively suppresses aerobic glycolysis by modifying mitochondrial ultrastructure and inhibiting HK2 activity.71 It has entered clinical trials for various cancers, including breast cancer.111 Jiang et al developed a high-fidelity spatio-temporal delivery strategy utilizing apatinib-loaded nanomedicine in conjunction with LND-loaded formulation.70 Specifically, apatinib-loaded nanodurg initially targets tumor endothelial cells to selectively block the vascular endothelial growth factor receptor, facilitating vascular normalization therapy that promotes T cells infiltration. Subsequently, LND-loaded nanodurgexhibits delayed accumulation at tumors while reprogramming glucose metabolism to activate T cells against malignancies. Zhao et al designed a straightforward self-assembled nanoparticle named TerBio without additional carriers, synthesized through intermolecular interactions among LND, chlorine e6 (Ce6), and SB50512473 (Figure 3). SB505124 effectively inhibits TGF-β signaling pathways while synergizing with metabolic modulation from LND to counteract immunosuppression.106 Furthermore, both liposomes loaded with mannose and polymeric NPs containing JQ1 significantly inhibited HK2 activity, leading to successful suppression of glycolysis.74,102,102 In summary, the targeted delivery of HK2 inhibitors via NPs effectively reprogrammed the TME and mitigated immunosuppression, demonstrating promising anti-tumor potential.
 |
Figure 3 Nanosystem targets HK2 to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the self-assembly of TerBio nanosystem and (B) its antitumor mechanism. (C) True real-time and in vitro fluorescence images of major organs and tumor tissues after intravenous Ce6 or TerBio administration, He, Li, Sp, Lu, Ki, and Tu stand for heart, liver, spleen, lung, kidney, and tumor respectively. (D) Tumor tissue and (E) average tumor weight in the presence or absence of light exposure. Flow cytometry (FCM) quantitative analysis of (F) CD49b+ NK cells, (G) CD11c+CD86+ DCs, (H) CD3+CD8+ T cells and (I) CD3+ CD4+ Foxp3+ T regulatory cells in tumor tissues after different treatments. *p < 0.05, **p < 0.01, and ***p < 0.001 were tested via a Student’s t test. Adapted with permission from Zhao L-P, Zheng R-R, Kong R-J, et al. Self-delivery ternary bioregulators for photodynamic amplified immunotherapy by tumor microenvironment reprogramming. ACS Nano. 2022;16(1):1182–1197. Copyright © 2022 American Chemical Society.73 |
Nanoparticles Targeting Phosphofructokinase (PFK)
PFK, an enzyme crucial for regulating the rate of glycolysis, catalyzes the conversion of fructose-6-phosphate (F-6-P) to fructose-1,6-diphosphate. Furthermore, F-6-P can be converted into fructose-2,6-bisphosphate by fructose-2-kinase 6-phosphate/fructose-2,6-diphosphatase 3 (PFKFB3), recognized as the most potent allosteric activator of PFK. The overexpression of PFKFB3 has been observed to meet the metabolic needs of aggressive tumors, such as prostate cancer, colon cancer, and breast cancer.112 3-pyridine-1-(4-pyridine-2-propenyl-1-1) (3PO) and its derivative PFK15 are selective inhibitors of PFKFB3, effectively inhibiting glycolysis and consequently reducing lactic acid production.113,114
To more effectively counteract acidic TME, Gao et al encapsulated both 3PO and LOx within hollow manganese dioxide (HMnO2) NPs115 (Figure 4). The compound 3PO successfully inhibits lactic acid and ATP production by obstructing glycolysis; concurrently, LOx oxidizes lactic acid to generate H2O2. This hydrogen peroxide is subsequently catalyzed by the catalytic-like activity of PMLR NPs to produce O2—thereby accelerating lactic acid consumption and enhancing sensitivity to 3PO. This strategy not only targets lactic acid production but also promotes its depletion effectively reversing TIME dynamics while significantly improving ICB therapy efficacy.
 |
Figure 4 Nanosystem targets PFK to reprogram TME and enhance immunotherapy. (A) Schematic of PMLR nanosystem construction and (B) lactate exhaustion process via 3PO-mediated inhibition of production and LOx-mediated oxidation. (C) ATP inhibition and (D) cytotoxicity of PMLR NPs in B16F10 cells under normoxia or hypoxia. Lactate consumption effect of PMLR NPs under (E) normoxia or (F) hypoxia. Quantitative analyses of the (G) CD3+CD8+ T cells, (H) CD3+CD8+GranzymeB+ T cells, (I) CD3+CD8+IFN-γ+ T cells and (J) M2 macrophages according to the corresponding FCM data. *p < 0.05, **p < 0.01, ***p < 0.001. Adapted from Gao F, Tang Y, Liu W, et al. Intra/extracellular lactic acid exhaustion for synergistic metabolic therapy and immunotherapy of tumors. Adv Mater. 2019;31(51):1904639. © 2019 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.115 |
TME is a complex, network-like system in which multiple pathways targeting the same objective are essential. However, directing attention to different targets within the TME also represents an innovative strategy. Cancer-associated fibroblasts (CAFs), as the predominant stromal cells in tumors, provide metabolites to tumor cells through glycolysis and collaboratively shape the TIME alongside these cells.116 Zang et al encapsulated liposomes within hybrid cell membranes derived from 4T1 and CAF-like NIH3T3 cells, thereby facilitating homologous targeting of both tumor and CAF cells.75 The solid lipid NPs were loaded with the chemotherapeutic agent paclitaxel (PTX) and PFK15, achieving a synergistic integration of chemotherapy and immunotherapy. These findings suggest that inhibiting PFK, a pivotal enzyme in glycolysis, constitutes an effective approach to enhance immunotherapy; furthermore, simultaneous targeting of multiple entities or pathways may yield more pronounced anti-tumor effects.
Nanoparticles Targeting Pyruvate Kinases (PKM2)
Fructose-1,6-diphosphate is catalyzed by a series of enzymes to produce phosphoenolpyruvate, which produces pyruvate via PK. PK is the third rate-limiting enzyme of glycolysis, and (PKM2 is often overexpressed in tumors. Shikonin (SK) is the primary active component isolated from the roots of the traditional Chinese herb Zicao. SK can not only downregulate the expression of PKM2 and inhibit glycolysis and lactate production but also promote the production of ROS, induce necroptosis and ICD, and inhibit NF-κB regulated gene products, thus inhibiting tumor progression.117
Li et al utilized NPs to co-deliver SK and PD-L1 knockdown siRNAs (SK/SIR-NPs) to explore the roles of SK in regulating the TME and enhancing the efficacy of immunotherapy76 (Figure 5). SK/SIR-NPs have been shown to diminish the production of lactic acid and ATP by down-regulating PKM2 expression, while simultaneously enhancing anti-tumor immunotherapy when combined with PD-1 knockdown. Naturally derived components tend to have more potential side effects. To improve the safety and therapeutic efficacy of SK, Li’s team modified liposomes using M1 macrophage EVs and RS 17 peptide.77 The innate homing capability of M1 EVs, along with the selective binding affinity of RS17 peptide to CD47 on tumor cells, allows these NPs to accurately target tumor cells.118 Furthermore, the NPs are also equipped with photosensitizer IR 820 to exert PTT effect and augment anti-tumor immunotherapy in conjunction with SK.
 |
Figure 5 Nanosystem targets PKM to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the self-assembly of SK/siR-NPs nanosystem and (B) its antitumor mechanism. (C) Western blot analysis of the expression of PD-L1 and PKM2 after different treatments. (D) Tumor volume curve in different groups. (E) HE and TUNEL staining of tumor tissue after different treatments (scale bar, 10μm). (F) Qualitative analysis of CD8+ T cells infiltration in tumors by FCM. *p < 0.05, **p < 0.01, ***p < 0.001. Adapted with permission from Li J, Zhao M, Sun M, et al. Multifunctional nanoparticles boost cancer immunotherapy based on modulating the immunosuppressive tumor microenvironment. ACS Appl Mater Interfaces. 2020;12(45):50734–50747. Copyright © 2020 American Chemical Society.76 |
The initiation and progression of tumors are influenced by various components within the TIME, rendering single-target therapies less effective and prone to drug resistance. Given that mannose and lactoferrin can specifically bind to overexpressed mannose receptors and low-density lipoprotein receptor-associated proteins found in cancer cells as well as TAMs, Wang et al developed a mannose-lactoferrin nanoparticle (Man-LF NP) encapsulating both SK and JQ1 for simultaneous targeting of tumor cells and TAMs within TIME.119 JQ1 functions as a bromodomain inhibitor that targets terminal exo-motifs, effectively inhibiting HK-2, LDH, and PD-1 expression.120,121 SK and JQ1 synergistically inhibit multiple key steps of glycolysis and promote TAMs repolarization towards M1 phenotype, thereby reversing TIME.
Nevertheless, the limited specificity and low cross-expression of antigens, coupled with the TIME, pose significant challenges to the efficacy of immunotherapy. Enhancing in situ ICD to generate autologous tumor cell lysates for antigen release and presentation can effectively trigger a cascade immune response and reverse the TIME. Consequently, Shi et al simultaneously induced necrotic apoptosis and ferroptosis by loading Fe²+ and SK onto alum NPs to elicit robust ICD.78 Ferroptosis is not influenced by common tumor-associated mutations in apoptotic proteins, while necrotic apoptosis represents a more potent form of ICD compared to traditional apoptosis, significantly enhancing immunogenicity.122 Furthermore, immunoadjuvant cytosine guanine dinucleotide (CpG) oligodeoxynucleotides (ODNs) and inorganic aluminum salts further enhance the antitumor efficacy of the glycolysis inhibitor SK. This treatment strategy activates an immune response through multiple dimensions and steps, demonstrating remarkable efficacy in inhibiting tumor growth, recurrence, and metastasis. These PKM2-targeted therapeutic strategies improve the delivery of NPs via EVs, lactoferrin, peptides, and various modifications, while also enhancing ICD and amplifying anti-tumor immune responses through the use of immune adjuvants, PTT, and ferroptosis.
Nanoparticles Targeting Lactate Dehydrogenase (LDHA)
LDHA plays a pivotal role as an enzyme in glycolysis, facilitating the conversion of pyruvate to lactic acid. The overexpression of LDHA in tumor cells can result in the infiltration of TAMs, thereby promoting immune evasion and therapeutic resistance.123 GSK2837808A (GSK) has established a TME characterized by elevated glucose levels and reduced lactate concentrations through the inhibition of LDHA and restriction of glucose consumption. Yan et al demonstrated significant anti-tumor effects by combining GSK, an LDHA inhibitor, with sonodynamic therapy (SDT).79 The HPP-Ca@GSK nanoplatform was concurrently integrated with PEG-IR 780, CaCl2, and GSK, effectively reversing the TME while addressing calcium overload and ultrasound-mediated ROS-induced mitochondrial damage. By integrating these components, a robust ICD is induced that enhances the antitumor immune efficacy of anti-CTLA-4 therapy. Although small molecule inhibitors often exhibit poor stability, nanomaterials can alleviate this limitation. Two-dimensional tin selenide nanosheets (SnSe NSs) also demonstrate commendable LDH-like enzymatic activity under challenging conditions; they are capable of converting lactic acid back to pyruvate while effectively inhibiting glycolysis.83,124,125 Moreover, SnSe NSs can successfully reverse acidic TME by delivering carbonic anhydrase inhibitors.126–128 Furthermore, SnSe NSs possess remarkable photothermal properties that induce excessive ROS production upon NIRlight irradiation leading to apoptosis in tumor cells.
In addition to utilizing nanocarriers loaded with LDHA inhibitors, silencing or editing the LDHA gene represents another viable strategy. Zhang et al restructured the TME by silencing the LDHA gene utilizing cationic lipid-assisted NPs delivery methods.82 Ju’s team employed lipid NPs for delivering CRISPR/Cas9 plasmid DNA targeting LDHA, which effectively edited tumor cell genomes and inhibited LDHA expression.81 She et al achieved dual inhibition of both glycolysis and TCA cycle activity by combining CRISPR/Cas9 technology with CPI-Z2 treatment80 (Figure 6). CPI-Z2 downregulates mitochondrial TCA cycling activity while inducing ROS production via inhibition of pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase complex pathways. This ROS catalyzes L-arginine into nitric oxide, subsequently reducing drug resistance by inhibiting P-glycoprotein.129–131 Furthermore, AMANC@M NPs were coated with erythrocyte-4 T1 hybrid membranes to enhance their targeting ability towards tumors while improving blood circulation stability. Collectively, these various nanodelivery strategies underscore that inhibiting LDHA constitutes an effective target for augmenting the efficacy of immunotherapy.
 |
Figure 6 Nanosystem targets LDH to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the synthesis of AMANC@M nanosystem. (B) AMANC@M blocks the energy supply of tumor cells. (i) Glycolytic inhibition by knocking down LDHA using CRISPR/Cas9 technology. (ii) TCA inhibition induced by CPI-Z2. (C) Schematic diagram of inhibition of P-gp to alleviate chemotherapy resistance via increasing NO mediated by CPI-Z2. (D) Tumor picture of mouse after different treatments. (E) immunofluorescence staining images of CD4+ T cells (red), CD8+ T cells (red), and granzyme B (green) in the tumor tissues (scale bar, 50μm). Notes: (1) control (PBS), (2) AM, (3) AMA, (4) AMAN, (5) AMANC, (6) AMANC@M. Adapted from J Control Release, volume 366, She W, Li H, Wang Z, et al. Site-specific controlled-release nanoparticles for immune reprogramming via dual metabolic inhibition against triple-negative breast cancer. 204–220. Copyright 2024, with permission from Elsevier.80 |
JQ1 down-regulates the expression of oncogenes c-Myc and PD-L1 by inhibiting bromodomain and extraterminal protein 4.121 The reduction in c-Myc levels subsequently leads to decreased expression of key glycolytic enzymes, including HK2 and LDHA.120,132 Furthermore, polymeric NPs encapsulating JQ1 effectively mitigate the lactate-driven TIME, elicit a robust immune response, and overcome the immune resistance associated with chemotherapy and photodynamic therapy (PDT).102,103
Nanoparticles Targeting Pyruvate Dehydrogenase Kinase (PDK)
In addition to obstructing the glycolytic metabolic pathway, transitioning tumor cells from glycolysis to OXPHOS presents a novel strategy for metabolic therapy. PDK phosphorylates and inactivates PDH, thereby inhibiting the conversion of pyruvate into acetyl-CoA and preventing its entry into the TCA. Dichloroacetic acid (DCA), a small molecule inhibitor of PDK, facilitates this transition from glycolysis to OXPHOS; however, its bioavailability remains suboptimal.133
Consequently, Dai et al utilized MnFe2O4 NPs for targeted delivery of DCA to cancer cells, effectively reducing levels of immunosuppressive lactate and adenosine, thus reversing the TIME84 (Figure 7). Furthermore, ultra-small MnFe2O4 NPs (6 nm) can efficiently penetrate mitochondria to degrade Glutathione (GSH) and H2O2, releasing oxygen and hydroxyl radicals (∙OH). This process enhances DCA efficacy and promotes apoptosis in tumor cells. In conclusion, the inhibition of key glycolytic enzymes to reverse aberrant glycolytic metabolism can reinvigorate the immune response and enhance the effectiveness of various therapeutic approaches.
 |
Figure 7 Nanosystem targets PDK to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the preparation of SMDNs nanosystem. (B) TEM image, (C) dark field image and corresponding area-elemental mappings of SMDNs (scale bar, 10nm). (D) SMDNs reverses immunosuppression by converting glycolysis to OXPHOS and inhibiting ADO production. SMDNs activated the immune response by regulating (E) ATP, (F) ADO, and (G) lactate levels. The proportion of (H) mature DCs, (I) CD8+T cells, and (J) effector memory T cells with different treatments via FCM analysis. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. Adapted from Biomaterials, volume 284; Dai Z, Wang Q, Tang J, et al. A Sub-6 Nm MnFe2O4-dichloroacetic acid nanocomposite modulates tumor metabolism and catabolism for reversing tumor immunosuppressive microenvironment and boosting immunotherapy, page number: 121533. Copyright 2022, with permission from Elsevier.84 |
Nanoparticles Targeting Glucose and Lactate Transporters for Cancer Immunotherapy
Nanoparticles Targeting Glucose Transporters
The efficiency of glucose uptake and metabolism improves significantly to ensure a supply of nutrients and energy for the infinite proliferation of cancer cells. Blocking the intracellular glycolysis pathway may results in the adaptive upregulation of GLUT expression, particularly that of GLUT1, in tumors.134 Consequently, targeting GLUT1-mediated glucose transport is considered an attractive antitumor therapeutic strategy. This strategy, which causes cancer cells death by blocking the supply of energy and nutrients, is called starvation therapy. Wu et al successfully constructed a “nanoenabled energy interrupter” (HZ@GD) by embedding a GLUT1 mRNA-cleaving DNAzyme into the nanocarrier zeolitic imidazolate framework-8 (ZIF-8) and then tethered it to hyaluronic acid to improve the effectiveness of starvation therapy.135 The CD44-mediated active targeting mechanism promotes the uptake of HZ@GD by tumor cells, and the presence of hyaluronidase and an acidic pH allow Zn2+ and the DNAzyme to be released into the tumor cytoplasm. Zn2+ inhibits glycolysis by triggering a decrease in NAD+ levels and the inactivation of GAPDH, while the Zn2+-activated DNAzyme reduces GLUT1 expression and decreases the glucose supply within tumor cells. Although HZ@GD has shown excellent tumor-suppressing effects, its impact on the immune system has not been assessed.
The effectiveness of ICB is often limited by increasing numbers of immunosuppressive cells and inhibitory cytokines in the TME and negative immune regulation mediated by CTLA-4. Ren et al addressed this challenge by introducing self-assembled aptamer polymers for targeted delivery of specific GLUT1 inhibitor BAY-876116. The supramolecular nanostructure (DNA-PAE@BAY-876) was designed by attaching BAY-876 to a nanocore DNA- polyβ-amino ester (PAE), which was formed by modifying PD-L1 and CTLA-4-antagonizing aptamers (aptPD-L1 and aptCTLA-4) with PAE (Figure 8). AptPD-L1 and aptCTLA-4 bind PD-L1 and CTLA-4 with high affinity and specificity to enhance immunotherapy. BAY-876 reduced the glycosylation of PD-L1 and enhanced the efficacy of aptPD-L1 by reducing lactate levels and negatively regulating the Glu-F6-P-UDP-GlcNAc axis. Furthermore, DNA-PAE@BAY-876 has the potential to enhance the immune response and induce systemic immune memory, thereby preventing TNBC metastasis and recurrence.
 |
Figure 8 Nanosystem targets GLUT to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the preparation of DNA-PAE@BAY-876 nanosystem and (B) the mechanism of reprogramming TIME. (C) ELISA analysis of the level of lactic acid following different treatments, I NC, (II) PAE, (III) aptCTLA-4, (IV) aptPD-L1, (V) BAY-876, (VI) DNA-PAE, (VII) DNA-PAE@BAY-876. (D)The comparative of mice tumors among the distinct treatment. FCM analysis of the (E) total immune cell population (CD45+) and (F) IFN-γ+CD8+ T cells within the tumor tissues. Adapted from Ren X, Cheng Z, He J, et al. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat Commun. 2023;14(1):7021. Creative Commons.85 |
The metabolic nanomodulator D/B/CQ@ZIF-8@CS was successfully constructed by encapsulating 2DG, BAY-876 and chloroquine within ZIF-8 NPs, followed by coating with chondroitin sulfate to improve the targeting of NPs.104 2DG and BAY-876 maximally block the supply of energy and nutrients to tumor cells, and indirectly increases the glucose supply to immune cells. As anticipated, the NPs activated an immune response characterized by the upregulation of T cells and the cytokines IFN-γ and TNF-α.
Nanoparticles Targeting Lactate Transporters
As mentioned earlier, rapid glycolysis in tumor cells leads to the production of plenty of lactate, which is transported out of the cell by MCT to form an acidic TME. MCT1 and MCT4 are the most important MCTs in cancer, and their overexpression is associated with a poor prognosis for various malignant tumors.136 The efflux of lactate mediated by MCT4 helps to prevent intracellular acidification and alleviate the feedback inhibition of glycolysis caused by lactate accumulation in hypoxic cancer cells, while the influx of lactate mediated by MCT1 provides fuel for the respiration of oxygen-rich cancer cells.86 In addition to inhibiting lactate production, inhibiting lactate transport by targeting MCT is also a promising antitumor therapy.
Blocking MCT1 inhibited lactate-fueled OXPHOS in oxygen-rich tumor cells and converted metabolism to glycolysis, which inhibited the energy metabolism of hypoxic tumor cells through competition for glucose. Additionally, blocking MCT1 inhibited glycolysis in hypoxic cancer cells by reducing the production of glycolytic intermediates, ultimately leading to the inhibited growth and proliferation of cancer cells. AZD-ultra-pH-sensitive (UPS) NPs are designed to selectively load the MCT1 inhibitor AZD3965 inside an UPS micelle via a microfluidic method.86 These NPs are stable at pH 7.4 and can be quickly released in acidic environments. Additionally, AZD-UPS NPs combined with anti-PD-1 therapy significantly improved the immune response of CD8+ T cells. However, MCT1 is more highly expressed in oxygen-rich tumor cells located near blood vessels, suggesting that drugs targeting MCT1 may penetrate more easily into tumors due to their closer proximity to blood vessels. However, Fischer et al reported that MCT1 is also expressed on normal cells, such as infiltrating CD8+ T lymphocytes.137 Consequently, MCT1-blocking therapy may affect the immune activity of CD8+ T lymphocytes. Therefore, enhancing nanoparticle targeting to cancer cells can potentially enhance the antitumor effects of MCT1 while minimizing adverse reactions.
Inhibition of MCT4 expression can lead to intracellular acidosis and the inhibition of glycolysis. Li et al loaded siMCT-4 and the chemotherapy drug hydroxycamptothecin (HCPT) with hollow mesoporous organosilica NPs modified with PEG-CDM and coated them with bovine serum albumin (Figure 9) 121. The NPs successfully silenced the expression of MCT-4, leading to intracellular lactate accumulation and promoting the necrosis and apoptosis of tumor cells. Tian et al proposed that a reprogrammed TME enhances immunotherapy efficacy by synergistically inhibiting lactate production and blocking lactate shuttling.138 Syrosingopine, which inhibits MCT-4 expression, and the HK2 inhibitor LND were simultaneously encapsulated in PEGylated liposomes to form immune and metabolically regulated nanosheets (L@S/L). LND and syrosingopine synergically reduced extracellular lactate levels, increased the numbers of NK cells and M1-like TAMs, and upregulated IFN-γ and TNF-α, showing great potential for antitumor therapy. In summary, these nanoplatforms reversed the TIME, significantly inhibiting tumor growth and progression. However, MCT4 is expressed in hypoxic tumor cells far from blood vessels, making it more difficult for MCT4-blocking drugs to reach therapeutic targets. Therefore, improving the penetration of NPs may further improve their antitumor effects.
 |
Figure 9 Nanosystem targets MCT to reprogram TME and enhance immunotherapy. (A) Schematic illustration of the preparation HMONs@HCPT-BSA-PEI-CDM-PEG@siMCT-4 nanosystem and the mechanism of reverse TIME. (B) SEM and (C) TEM images of HMONs. (D) MCT-4 expression in B16F10 tumor tissues by Western blotting. (E) Standardized lactate levels in TME, FCM analysis of (F) M2-TAMs (CD206+) and (G) M1-TAMs (CD86+) in tumor tissues. (a: Saline, b: HMONs-BSA-PEI-CDM-PEG, c: HCPT, d: HMONs@HCPT-BSA-PEI-CDM-PEG@siNC, e: HMONs@HCPT-BSA-PEI-CDM-PEG@siMCT-4+forskolin, f: HMONs@HCPT-BSA-PEI-CDM-PEG@siMCT-4). **p < 0.01. Adapted from Li K, Lin C, He Y, et al. Engineering of cascade-responsive nanoplatform to inhibit lactate efflux for enhanced tumor chemo-immunotherapy. ACS Nano. 2020;14(10):14164–14180. Copyright © 2020 American Chemical Society.87 |
Nanoparticles Targeting Glucose Oxidase (GOx) for Cancer Immunotherapy
Tumor cells need to consume more glucose to maintain growth, and thus starvation therapy has been proposed to block the energy supply to tumor cells. GOx is an endogenous oxidoreductase that blocks glycolysis and inhibiting the production of lactate and ATP via converting glucose into gluconic acid.92,139 However, its efficacy has been severely limited by poor bioavailability and rapid inactivation when encountering biological barriers.140 Additionally, the adverse effects associated with GOx, such as hypoglycemia and organ damage, have hindered its clinical application.141,142 Nevertheless, the advent of nanomaterials effectively addresses the limitations associated with GOx. Therefore, mesoporous silica NPs (MSNs) have been utilized to deliver GOx to construct the CMSN-Gox NPs, which can effectively target tumor due to the cancer cell membrane enveloping the CMSN-GOx.92 CMSN-GOx effectively induced an immune response and inhibited tumor growth. LMGC, an acidic TME-responsive spherical nanoparticle, was prepared by immobilizing GOx onto the surface of liquid metal (LM) nanocapsules, followed by modification with mineralized CaCO3 and the copolymer PEG-PAsp.143 Gox-induced glycolytic inhibition and Ca2+-mediated mitochondrial dysfunction result in a significant reduction in ATP production. Upon NIR light irradiation, LM nanocapsules can effectively kill tumor cells via PTT.91 Surprisingly, LMGC markedly reduces the expression of heat shock protein (HSP), enhancing the sensitivity of PTT and reversing tumor cell heat resistance.
However, glucose oxidation requires the participation of O2, and a severely hypoxic TME limits this process.144 A nanocarrier that can relieve the hypoxic TME must be designed to improve the catalytic efficiency and sustainability of GOx. Therefore, various nanocarriers with dual GOx and catalase activities have been developed to catalyze H2O2 to produce O2, which improved the oxidation efficiency of GOx. For example, Zhang et al hybridized GOx with MnCl2·4H2O using a biomineralization method to synthesize GOx-Mn/HANPs, creating a virtuous cycle with dual enzyme activity.90 Furthermore, cationic gold nanoclusters (CAuNCs) adsorbed GOx and catalase through electrostatic interactions, followed the NPs were further condensed with HK2 siRNA to create nanomotors NM-Si.105 The CAT-catalyzed cascade of enzymatic reactions continued to generate oxygen bubbles, prompting nanomotors using H2O2 as fuel to move autonomously to tumor tissues with higher H2O2 concentrations.105 NM-si significantly reduces tumor cell migration and invasion by downregulating the expression of HIF1α and HK2.145
GOx can also enhance the Fenton reaction by increasing the production of H2O2, which is catalyzed by Fe2+ to generate ∙OH and enhance chemodynamic therapy (CDT).88,146 GOx and ∙OH work synergistically to promote ICD induction, facilitate DCs maturation, and enhance the antitumor immune response. Ren et al introduced PTX into FeSGOx through hydrophobic interactions to construct FGP NPs.94 The photothermal effect of FGP can promote the activity of GOx and increase the production of ∙OH. FGP serves as a cascade bioreactor that combines starvation therapy, CDT, PTT, and chemotherapy to amplify ICD induction and generate a potent antitumor immune response. Similarly, another biomimetic nanosystem, mEHGZ, was developed by loading epirubicin, GOx and hemin into porous ZIF-8 NPs.147 The mEHGZ displayed outstanding tumor-targeting capabilities via the coating of tumor cell membranes with overexpressed CRT.148 The Fe2+ in hemin reacts with H2O2 to produce abundant ∙OH and Fe3+, while Fe3+ oxidizes intracellular GSH. A decrease in GSH levels disrupts the redox balance, inhibits the clearance of ∙OH, accelerates ROS production and enhances endoplasmic reticulum stress.149–151 A metal–organic framework (MOF) nanoreactor was equipped with GOx and the IDO inhibitor 1-methyltryptophan (1-MT) to construct a nanoplatform93 (Figure 10). Another nanoparticle was fabricated by loading DOX and aspirin onto PEG-modified manganese-doped hollow mesoporous silica-coated gold NPs.95 The two NPs exhibited the enzymatic properties of GOx and catalase and increased ·OH levels through a Fenton-like reaction mediated by Mn2+. These agents synergized with the immunomodifiers 1-MT and aspirin to counteract immunosuppression, and enhance the antitumor efficacy of the NPs.
 |
Figure 10 Nanosystem targets GOx to reprogram TME and enhance immunotherapy. (A) Schematic illustration of constructing PCP-Mn-DTA@GOx@1-MT nanosystem and its antitumor mechanism. Quantitative analysis of (B) migration and (C) invasion assays of B16F10 cells treated with this nanosystem. (D) FCM and Quantitative analysis on the populations of CD8+ CTLs, Treg cells, NK cells, matured DCs, and B cells with different treatments. Adapted from Dai L, Yao M, Fu Z, et al. Multifunctional metal-organic framework-based nanoreactor for starvation/oxidation improved indoleamine 2,3-dioxygenase-blockade tumor immunotherapy. Nat Commun. 2022;13(1):2688. Creative Commons. **p < 0.01.93 |
Nanoparticles Targeting Lactate Oxidase (LOx) for Cancer Immunotherapy
The above studies have focused mainly on reducing lactate production, but strategies for direct lactate depletion have been quite compelling. LOx converts lactate to pyruvate and H2O2 to improve the TIME. Microbial-derived recombinant LOx was encapsulated in a thin polymer shell using a single enzyme encapsulation platform to construct n(LOx) NPs.97 The n(LOx) NPs significantly reduced the level of lactate while promoting the continuous release of H2O2. H2O2 successfully activates nuclear factor κB signaling and promotes the secretion of antitumor cytokines, thereby activating T cells and guiding their migration into tumors.99,152,153 Wang et al developed the biomimetic nanosystem PMOL, which encapsulates LOx and the chemotherapy drug oxaliplatin within MOFs.99 The coating of a platelet membrane onto NPs enables specific tumor targeting. Oxaliplatin induces the apoptosis of tumor cells and triggers an ICD-mediated immune response while working in conjunction with LOx to distinctly suppress tumor growth.
Although the antitumor effects of Lox have been verified, its function is limited due to its instability and inhibition by the anoxic TME.154 Hence, hollow Prussian blue (HPB) carriers were introduced to solve these problems. HPB has CAT-like activity and can catalyze H2O2 to provide oxygen for lactate consumption.155 Furthermore, HPB can prevent the leakage of LOx and protect its activity. LOx was coated with HPB NPs and PD-L1 siRNA (siPD-L1) was loaded into the NPs by electrostatic adsorption to further explore the combination therapy of LOx and PD-1100 (Figure 11). High GSH levels in tumor cells promote the cleavage of disulfide bonds, effectively releasing LOx and siPD-L1. The NPs mitigated hypoxia, enhanced lactate consumption and ROS production, and downregulated PD-L1 expression in cancer cells. MCuLP NPs were constructed by integrating LOx and Cu2+ onto pegylated mesoporous polydopamine (mPDA) NPs.96 Upon the chelation of Cu2+, mPDA exhibits catalase-like properties and forms a closed loop with the catalytic activity of LOx,156 leading to a significant reduction in the lactate concentration without increasing pyruvate levels, and thereby significantly enhancing immunosuppression. The mCuLP nanosystem also showed excellent photothermal conversion and stability,157,158 with a limited impact on the activity of LOx. Furthermore, the PTT-induced apoptosis of tumor cells promotes the exposure of DAMPs (such as CRT and HMGB1), induces DCs maturation, and enhances T-cell-mediated killing of cancer cells. Xie et al designed PNDPL NPs by coating PtBi nanozymes and NLG919 with an amphiphilic polymer and linking LOx to the surface of nanomicelles through amide bonds.98 Upon NIR light irradiation, PtBi nanozymes demonstrated efficient photothermal conversion and stability. An increase in temperature facilitated the release of PtBi nanozymes and NLG919 while also enhancing the Cat-like enzyme activity of PtBi nanozymes.159,160 Furthermore, oxygen provided by PtBi nanozymes promotes lactate oxidation catalyzed by LOx, resulting in a significant reduction in lactate levels. In combination with metabolic immunoregulation and PTT, PNDPL enhances antitumor immunogenicity, induces powerful ICD, and achieves a robust immune response. Additionally, Chen et al reported that HMnO2 NPs loaded with LOx overcame hypoxia and immunosuppression after the local thermal ablation of hepatocellular carcinoma.101
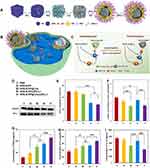 |
Figure 11 Nanosystem targets LOx to reprogram TME and enhance immunotherapy. (A) Schematic illustration of constructing HPB-S-PP@LOx/siPD-L1 nanosystem. (B)Lox-mediated downregulation of lactate to (C) enhance the effect of PD-1/PDL-1 blocking therapy by up-regulation of CD8+ T cells and down-regulation of Tregs. (D) Western blot analysis of PDL-1 expression in resected tumors after different treatments. Quantitative analysis of (E) the expression of PDL-1, (F) the levels of lactate, (G) TNF-α, (H) IL-2 and (I) IL-10 after different treatments. *p < 0.05, **p < 0.01, ***p < 0.001. Adapted from Biomater Adv, volume 150, Tang Y, Chang Q, Chen G, et al. Tumor immunosuppression relief via acidity modulation combined PD-L1 siRNA for enhanced immunotherapy. 213425. Copyright 2023, with permission from Elservier.100 |
GOx/LOx facilitates the production of H2O2, making the incorporation of nanomaterials with catalase-like activity advantageous for alleviating hypoxia and enhancing glucose and lactic acid oxidation. Furthermore, employing nanoenzyme capable of catalyzing the Fenton reaction in conjunction with GOx provides a dual benefit. Gox/LOx regulates lactate metabolism while supplying substrates for the Fenton reaction, whereas nano-enzyme contributes to tumor suppression through its catalytic role in CDT.
Combination Therapy with Nanoparticles Reprogramming Glucose Metabolism
With ongoing research into cancer therapies yielding promising results, more effective approaches are being developed for combating tumors effectively, including chemotherapy, PTT/PDT, ICB, RT, SDT, CDT and more. Unfortunately, tumor resistance to treatment is a significant issue that cannot be ignored, as it seriously hinders the effectiveness of tumor treatment. The glucose metabolism of tumor cells and the reprogrammed immune microenvironment are important factors in tumor resistance. Glucose metabolism-targeted therapy has the potential to enhance the immune response, reverse drug resistance, and improve antitumor therapeutic effects. Nanosystem represent a powerful strategy for the synergistic integration of multiple therapeutic modalities. On one hand, nanomaterials can encapsulate various drugs with distinct properties; on the other hand, they often exhibit inherent therapeutic effects themselves. Moreover, glycolytic metabolism therapy has been demonstrated to enhance the efficacy of diverse anti-tumor treatments, thereby achieving a synergistic effect characterized by “1+1>2” (Figure 12).
Combination with Chemotherapy
Chemotherapy is a systemic antitumor therapy that has been widely applied in clinical practice. However, patients often have to discontinue treatment due to drug resistance and unacceptable side effects. Drug resistance is a main adaptive regulatory mechanism in aggressive tumors, but its specific mechanism is not fully understood. Currently, the evidence suggests that the TME affects the accumulation/retention of chemotherapy drugs within tumor cells, which is considered a contributing factor to clinical chemotherapy failure. First, an acidic pH reduces the uptake of drugs by cancer cells; for example, the uptake of DOX by MCF-7 cells at pH 6.8 is significantly lower than that at pH 7.4.161 Second, the low pH and hypoxia of the TME enhance P-gp activity, which promotes drug transport out of cells, reducing its ability to kill cancer cells.162 The aforementioned nanomaterials targeting key nodes of glucose metabolism can reprogram the TME, improve hypoxia and acidity in the TME, and effectively alleviate chemotherapeutic resistance.
The combination of chemotherapeutic drugs with metabolic regulators synergistically enhances antitumor efficacy. The increases in CRT and HMGB-1 indicate that DOX@HA NPs has the ability to induce ICD during chemotherapy.135 However, DOX@HA NPs loaded with the HK2 inhibitor JQ1 induced stronger ICD and amplified the antigen-presenting effect of DCs. Moreover, DOX can attract more CD8+ T cells and activate more inflammatory cytokines in tumors.163 Moreover, studies have revealed that 2DG promotes the activation of CD8+T cells by downregulating the level of PD-L1.164 Therefore, PDG-NV NPs carried DOX and 2DG enhance the potential of chemical immunotherapy.69 PTX/PFK15-SLN@[4T1-3T3] NPs target both tumor cells and CAFs, inhibit glycolysis and decrease the supply of glycolytic metabolites derived from CAFs to cancer cells. This process significantly increases the sensitivity of cancer cells to PTX and enhances its antitumor effect. This finding was further verified by calculating the CI for the PTX and PFK15 combination.75,165 The CI values at the IC50 in 4T1 cells (0.30±0.01) and 3T3 cells (0.48±0.03) were significantly less than 1, indicating a synergistic effect between these two drugs.75 Similarly, other nanoplatforms co-equipped with chemotherapy drugs and regulators of glucose metabolism have also achieved superior antitumor effects.87,94,99
In addition, targeted drug delivery through nanomaterials can significantly reduce the concentration and frequency of administration. The starvation effect of 2DG can also decrease the toxicity of DOX toward normal cells and chemotherapy-induced side effects.166 Furthermore, some NPs can be selectively decomposed and released under specific conditions; for example, GSH-responsive nanomaterials are released in weakly acidic TME and tumor cells with high levels of GSH, while the release of PTX is almost undetectable in normal cells with low GSH levels, thus preventing damage to normal cells to the greatest extent.87 Although metabolic nanomedicine holds great potential for synergistic immunotherapy and chemotherapy, further exploration is necessary to understand the mechanism of synergistic therapy and the binding effect between different metabolic targets and various chemotherapeutic drugs.
Combination with Radiotherapy
RT is an important method for treating tumors. However, studies have indicated that RT can cause an increase in intracellular PD-L1 expression, leading to enhanced tumor resistance to DNA damage and significantly limiting the effectiveness.167,168 LND has been demonstrated to reduce PD-L1 expression and reverse hypoxia by interfering with mitochondrial OXPHOS. Hence, Wang et al combined LND with triphenylphosphine cations (TPP+) to construct TPP-LND@Lip NPs.72 These NPs effectively targeted the mitochondria through TPP+ and enhanced RT sensitivity and immune activity. The combination of TPP-LND@Lip and RT exhibited superior efficacy compared to PD-1 therapy. Furthermore, increased EVs secretion from cancer cells after RT can promote the polarization of M2-like TAMs and inhibit the immune response.169 Shen et al developed the M/LM-Lipo NPs by utilizing mannose to interfere with glucose metabolism and reduce PD-L1 expression.74 Additionally, levamisole hydrochloride not only facilitates the glycolysis of mannose but also induces mitochondrial degradation and autophagy in M2 macrophages. The combination of M/LM-Lipo and RT induces a robust immune response, alleviates RT resistance, and significantly inhibits local tumors, distant tumors, and metastatic tumors. However, research on the combination of RT with metabolic immunotherapy targeting glycolysis is limited, and further efforts are needed to fill these gaps in the field.
Combination with Immunotherapy
Immunotherapy exerts a crucial function in inhibiting tumor growth and metastasis by reactivating and sustaining the immune cycle and restoring the body’s innate immune response. Treatments targeting the PD-1/PD-L1 pathway and anti-CTLA-4 therapy are the most extensively researched ICB therapies, with widespread clinical use and remarkable results. However, tumors have developed various defense mechanisms against the immune system. Intervening in glucose metabolism and reducing negative immune metabolites are important strategies for reversing immunosuppression and improving immune escape. Previous studies have shown that various NPs targeting glucose metabolism can alleviate immunosuppression and enhance the efficacy of anti-PD-1 therapy.82,97 The combination of PMLR NPs equipped with LOx and 3PO, with α-PDL-1 induced the largest area of damage to tumor tissue and the highest survival rate in mice.101 In addition, T cells and M1 TAMs were upregulated, and the secretion of the immune activators IFN-γ and IL-6 was significantly increased. Nanomaterials targeting PD-1/PD-L1 have been developed to further validate the synergistic effect of ICB and glucose metabolism regulation therapy.
F-6-P is responsible for synthesizing UDP-GlcNAc through the action of glutamine fructose-6-phosphate amidotransferase, which serves as a crucial substrate in the glycosylation of numerous proteins and lipids.170 The functionality of PD-1/PDL-1 relies on glycosylation; therefore, the enzymes that inhibit F-6-P production, including GLUTs, HK2, and GOx, can potentially diminish the immunosuppressive effect of PD-1/PDL-1. For instance, Li et al developed biomimetic NVs (PDG-NVs) derived from cancer cell membranes overexpressing PD-1.69 PDG-NVs affect the glycosylation of PD-L1 via 2DG to inhibit glucose metabolism. Additionally, the binding of PD-1 on P-NVs to PDL-1 on cancer cells blocks the PD-1/PD-L1 axis. A nanosystem equipped with LOx/siPD-1 has been developed.100 LOx reduces lactate levels and reprograms the TIME into an immunostimulatory state. It collaborates with siPD-1 to block the binding of PD-1 and PDL-1, thus enhancing the effect of immunotherapy. Ren et al combined the GLUT1 inhibitor BAY-876 with aptCTLA-4 and aptPD-L1 in a nanoplatform to establish an effective antitumor therapy system.85 BAY-876 improves immunosuppression by inhibiting glucose competition and glycolysis in cancer cells. Additionally, it promotes the establishment of a strong and stable immune response through aptPD-L1 binding to PD-L1 and aptCTLA-4-mediated CTLA-4 blockade during therapy. In combination with a CTLA-4 checkpoint blocker, FGP NPs loaded with GOx and PTX promoted the upregulation of CD8+ T cells and the downregulation of Tregs and enhanced the curative effect on local and distant tumors in mice.94
Additionally, IDO, a heme enzyme highly expressed in tumor cells, inhibits the proliferation of Teffs and induces the expansion of Tregs by catalyzing the conversion of tryptophan (Trp) to kynuridine (Kyn).171,172 The IDO inhibitor 1-MT can improve immune tolerance, while starvation therapy with LOx can initiate and enhance the immune response. Nanocarriers can synergistically promote tumor cells apoptosis and inhibit tumor growth by combining GOx and 1-MT.93 Similarly, targeted tumor therapy combining lactate depletion of LOx with the IDO inhibitor NLG919 has shown promising antitumor effects.98 NLG919 blocks the Trp/Kyn metabolic pathway and mitigates the immunosuppressive effects of Kyn.173 Furthermore, combination therapies involving other immunotherapy agents with glycolysis-targeted therapy have also been shown to enhance antitumor effects. For example, blocking the CD47-SIRPα signaling pathway, which is used in combination with the PKM2 inhibitor SK, promotes macrophage phagocytosis, and the TGF-β receptor inhibitor SB505124 is used in combination with the glycolysis regulator LND.73,77 CpG ODN activates Toll-like receptor 9, which promotes DC maturation and MHC-II expression, thereby inducing both innate and adaptive immunity.174 Additionally, the presence of inorganic aluminum salts in NPs allows the effective adsorption of antigens, and NPs act as an “antigen pool” that continuously releases antigens to initiate and prolong the adaptive immune response.175 Immunoadjuvant CpG ODN and inorganic aluminum salts further enhance the antitumor efficacy of the glycolysis inhibitor SK. The effect of immunotherapy was enhanced through the integration of immune adjuvant CpG ODN, inorganic aluminum salt and SK on the same nanoparticle.78
Nevertheless, maintaining the long-term effectiveness of immunotherapy and addressing adverse reactions caused by prolonged use remain unresolved issues that require attention. Overall, significant advances in immunotherapy will be achieved through a greater understanding of these immune mechanisms and targeted interventions.
Combination with Phototherapy (PDT/PTT)
Phototherapy, which mainly includes PTT and PDT, has been applied to treat various tumors and has achieved certain curative effects. With active targeting and enhanced permeability and retention effects, nano photothermal transduction agents can selectively accumulate in tumors to reduce adverse reactions.80,96,176 PTT converts NIR light energy into heat, causing a rapid increase in temperature within the target tissue, which can selectively kill local tumor cells.177,178
PTT can induce an ICD cascade reaction, which induces DCs maturation by releasing recognizable TAAs and DAMPs and further activates the immune response. In addition, PTT can improve the immunogenicity of tumor cells through photothermal effects; thus, PTT has become an important method for inducing in situ vaccines.179 Mild PTT can also increase the permeability of T cells, effectively treat primary tumors, and inhibit tumor metastasis.180 However, tumor cells develop thermal resistance by activating cytoprotective pathways during treatment.181 Damaged tumor cells can dramatically increase glycolysis efficiency to produce sufficient ATP that promotes HSP synthesis, expression, and function.182 HSPs can promote the repair of damaged proteins, thus compromising the efficacy of PTT.182,183 Glycolysis inhibitors overcome the thermal tolerance of PTT by blocking the energy supply of tumor cells. LM nanomaterials have a good photothermal conversion efficiency and low nonspecific toxicity. LM-loaded GOx and Ca2+ inhibit glycolysis and mitochondrial respiration, resulting in the substantial inhibition of ATP production and HSP expression and enhancing the tumor-killing effect of PTT.143 Luo et al combined 2DG with Hauns-Met@M to treat tumors, 2DG blocked the energy supply by inhibiting glycolysis, significantly improved the sensitivity of tumor cells to PTT, and reversed heat tolerance.68 At the same time, 2DG induced endoplasmic reticulum stress by inhibiting protein glycosylation, synergistically enhancing PTT-induced ICD, activating the immune response and inducing and enhancing long-term immune memory, which are conducive to the complete elimination of primary tumors. In addition, the heat generated through PTT can not only promote drug release from nanosystem but also improve enzyme activity and enhance metabolic immunotherapy.98
PDT promotes apoptosis via generating a large amount of cytotoxic ROS, thereby initiating the ICD cascade to enhance tumor immunogenicity and activate the immune response. However, the effectiveness of PDT is limited by immune resistance. Glycolytic regulators can reprogram the TIME and induce or promote ICD to activate the immune response. Therefore, researchers are highly interested in exploring the combined effects of these two approaches. For instance, pyropheophorbide-a can produce ROS and induce ICD under NIR irradiation. The nanoplatform HCJSP combined with JQ1 and pyropheophorbide-a improved the acidic and hypoxic TME by inhibiting HK2 and LDHA expression, and alleviated PDT-induced immune resistance.103 Importantly, an appropriate light source, adequate photosensitizer and adequate O2 are crucial factors for effective PDT.184,185 However, the hypoxic nature of the TME significantly limits PDT efficacy. Liu et al constructed an automatic oxygen regulation nanomedicine (IR-LND@Alb) that could overcome this problem.71 The photosensitizer IR-68 can be transported to mitochondria through organic anion transport peptides, and cooperate with LND to reduce the efficiency of OXPHOS by inhibiting mitochondrial complexes, thereby reducing endogenous oxygen consumption and enhancing the antitumor effect of PDT.186 IR-LND@Alb NPs successfully inhibit HIF-a expression, reverse hypoxia, amplify ROS production, and promote tumor cell apoptosis and immune system activation.71 In addition to reducing oxygen consumption, strategies to increase oxygen production have also been developed. Zhang et al designed self-oxygenated nanomaterials using Mn,Cu-doped CDs as a photosensitizer for PDT and promoted the endocytosis and retention of the NPs through the action of γ-PGA.89 GOx oxidizes glucose to produce H2O2, which is catalyzed by Mn and Cu-CD to produce O2. GOx and Mn,Cu-CD synergistically increased O2 production and the singlet oxygen (1O2) content in tumors, greatly improving the efficiency of PDT. In conclusion, PTT/PDT has overcome some shortcomings in the presence of nanotechnology and glycolysis regulators, showing broad application prospects. However, active explorations of more effective binding of NPs to PTT/PDT and glycolysis regulators are needed, including screening for photosensitizers, glycolysis targets and the design of nanosystem.
Combination with Other Therapies (SDT, CDT)
CDT can produce highly cytotoxic ∙OH through Fenton or Fenton-like reactions in the presence of H2O2, independent of external stimuli or local O2 levels.187 Various nanomaterials that can catalyze Fenton reactions, such as FeS-GOx nanodots and gold NPs (Au@HMnMSNs), have been developed for targeted tumor therapy.94,95 The Fenton reaction is more efficient under acidic conditions (pH 3.0–5.0), while a weakly acidic TME (pH 6.5–6.9) cannot fully increase the efficiency of the Fenton reaction.188,189 Therefore, combination therapy with GOx and CDT can solve this problem effectively.94,95,147,190 GOx catalyzes glucose to produce gluconic acid and H2O2, which not only provides a substrate for the Fenton reaction but also reduces the pH of the TME. This self-reinforcing Fenton reaction is sustainable and leads to robust ROS production, triggering an effective immune response via ICD. GOx starvation therapy combined with GOx-enhanced CDT is a highly effective antitumor strategy. LOx accelerates the Fenton reaction by oxidizing lactate to produce H2O2 and collaborates with CDT to promote tumor cell apoptosis.191 Wang et al enhanced the efficacy of CDT by targeting lactate transport.192 SiMCT-4 inhibited MCT4-mediated lactate efflux from cells through gene silencing, leading to a decrease in the intracellular pH and amplifying the tumor damage caused by CDT.
Nowadays, SDT has garnered significant attention due to the high tissue permeability and safety of ultrasound.193 It activates and produces ROS via external ultrasound to induce tumor cell apoptosis.194 Zhang et al designed a nanoplatform, PALF, that enhances SDT by regulating metabolism.195 Under the action of the sonosensitizer PEG-Ce6 polyphenol, PALF significantly increased 1O2 and cytotoxic ROS during ultrasound exposure, leading to therapeutic cell death. LOx oxidizes lactate to reverse the immunosuppressive state and enhance SDT, while atovaquone improves the hypoxic TME by interfering with mitochondrial respiration to promote the effects of LOx and SDT. PALF activates an effective immune response, thereby inhibiting tumor proliferation and metastasis. Furthermore, the multifunctional metal–phenol nanomedicine HPP-Ca@GSK exerted synergistic antitumor effects on glucose metabolism and SDT.79 GSK successfully reduced lactate levels by inhibiting LDHA, while ultrasound drove the sonosensitizer GIR780 to produce a large amount of ROS, which cooperated with calcium overload to induce mitochondrial damage. HPP-Ca@GSK increases the recruitment of effective immune cells and the secretion of proinflammatory cytokines and has good therapeutic effects on tumors. However, current research seems to focus more on how to generate excessive ROS to maximize its tumor-suppressive effect but less on the sustainability, drug resistance, and toxicity of ROS in normal cells.
Conclusions and Future Perspectives
Overall, the reprogramming of glucose metabolism in tumor cells plays a significant role in immunosuppression and immune evasion, thereby enabling these cells to better endure treatment-induced stress. Targeting glucose metabolism has the potential to reverse the TIME, enhance the efficacy of immunotherapy, and mitigate treatment resistance. In comparison to conventional pharmaceuticals, nanomaterials present several advantages: superior targeting capabilities, precise drug release mechanisms, enhanced permeability, and minimal adverse effects. The modulation of glucose metabolism through nanomaterials elicits a more pronounced anti-tumor immune response, positioning this approach as a promising strategy for cancer therapy. Furthermore, nanocarriers can simultaneously deliver drugs with diverse properties and therapeutic targets, facilitating synergistic and complementary effects across multiple therapeutic modalities while further augmenting anti-tumor efficacy.
Nevertheless, nanomaterials designed to target glucose metabolism for the enhancement of immunotherapy continue to face significant challenges. (1) the mechanisms underlying tumor development are highly complex; the metabolic network is intricately interconnected and exhibits considerable plasticity and flexibility, enabling tumor cells to potentially evade treatment through alternative metabolic bypasses. Furthermore, the administration of these drugs in humans may activate metabolic regulatory switches that disrupt normal cellular metabolic pathways, potentially leading to uncontrolled outcomes. This issue is particularly pronounced when drug targeting is suboptimal or when off-target effects result in unpredictable consequences. (2) While NPs can be sufficiently small to traverse physiological barriers for optimal tumor treatment, they also pose potential risks.66 Studies have indicated that free radicals generated by NPs can inflict damage on cell membranes, organelles, and DNA.196,197 Although current research suggests that nanomaterials do not exhibit greater toxicity than conventional drugs, their long-term biosafety and potential impacts remain inadequately assessed. Thus, further investigation into their transport mechanisms, metabolic processes, and transformation pathways is warranted. (3) Due to the inherent complexity of tumors, an increasing number of studies are opting for a combination of metabolic therapy with other treatment modalities that demonstrate superior outcomes compared to monotherapies.76,145 However, whether such combinatorial approaches can achieve a synergistic effect remains questionable. Moreover, when integrating various targeted drugs into nanoparticle formulations, it is crucial to consider their properties and interactions in order to avert potential inefficiencies or adverse effects.
In summary, nanosystem target glucose metabolism to reprogram TIME, thereby reconfiguring the TIME and considerably enhancing the efficacy of immunotherapy. However, developing an optimal nanosystem presents a considerable challenge. (1) Primarily, it is crucial to select an appropriate nanocarrier that demonstrates high drug loading capacity, exceptional stability, and robust biocompatibility. Enhancing the drug loading capacity facilitates a reduction in the quantity of nanomaterials required, necessitating the selection of nanomaterials with high surface area-to-volume ratios while considering their physical and chemical properties. The modification of small molecules or the construction of biomimetic nanocapsules can avoid immune system clearance and enzyme degradation to improve their stability within circulation.198,199 (2) Furthermore, strategies must be devised for the precise delivery of therapeutic agents to the tumor site; this may involve selecting targeted nanocarriers or modifying nanosystem with small molecules (hyaluronic acid, folic acid etc)., targeting peptides, antibodies, aptamers and cell membranes derived from various cell types.76,85,92,200,201 Additionally, engineering nanosystem capable of disintegration under specific conditions—such as in the presence of high GSH or at acidic PH—can facilitate complete drug release within tumor cells for effective action.135,202 (3) Leveraging the unique properties of nanomaterials can significantly enhance therapeutic efficacy while minimizing required drug dosages. For instance, certain nanomaterials possess NIR or ultrasound stimuli responsive capabilities that allow for concurrent application of PTT, PDT or SDT.79,98 Moreover, some nanomaterials exhibit catalytic functions—such as CAT and Fe2+—which can convert O2 to alleviate hypoxic conditions within tumors and initiate Fenton or Fenton-like reactions for improved anti-tumor effects through CDT.93,94 (4) It is essential to select glycolysis inhibitors that have well-established efficacy and safety profiles. Initially, it is crucial to identify an appropriate glycolytic target, followed by developing a drug that demonstrates strong specificity towards this target. However, given that drugs may have potential adverse effects, innovative strategies could also involve silencing or editing genes associated with critical glycolytic enzymes.81,82 (5) Ultimately, prioritizing the maximization of immune cascade activation and enhancement of immune responses is essential. This can be achieved by amplifying both the quantity and functionality of normal TME cells as well as immune cells through targeted glycolysis interventions. Furthermore, exploring various methods to induce ferroptosis, PDT, CDT, etc., can effectively trigger robust ICD and facilitate DAMPs release to promote antigen presentation.78,95,103 Additionally, synergistic enhancement of immune responses and prolongation of anti-tumor immunity through adjunctive use of immune checkpoint inhibitors, antibodies and immune adjuvants represent viable strategies.76,89,98 Nevertheless, in our pursuit of efficacy, it is essential to recognize that nanomaterials demonstrating enhanced superior properties and ease of synthesis are more likely to be integrated into clinical practice.
In summary, nanotechnology targeting glucose metabolism offers a revolutionary strategy for reprogramming TME and enhancing cancer immunotherapy. As research progresses, a deeper understanding of the role of glucose metabolism in tumor biology is expected to drive further innovation. We believe that continued research efforts will ultimately optimize nanosystem targeting glucose metabolism, thereby improving clinical anti-tumor therapy and benefiting patients.
Abbreviations
OH, Hydroxyl radicals; 1-MT, 1-methyltryptophan; 1O2, Singlet oxygen; 2DG, 2-deoxyD-glucose; 3PO, 3-pyridine-1-(4-pyridine-2-propenyl-1-1); APA, Apatinib; CAFs, Cancer-associated fibroblasts; CDT, Chemodynamic therapy; Ce6, Chlorine e6; CpG ODN, Cytosine guanine dinucleotide oligodeoxynucleotide; CRT, Calreticulin; CTL, Cytotoxic T lymphocyte; CTLA-4, Cytotoxic T lymphocyte-associated antigen-4; DAMPs, Damage-associated molecular patterns; DCA, Dichloroacetate; DCs, Dendritic cells; DOX, Doxorubicin; EVs, Extracellular vesicles; F-6-P, Fructose 6-phosphate; FAO, Fatty acid oxidation; FCM, Flow cytometry; G-6-P, Glucose 6-phosphate; GLUTs, Glucose transporters; GOx, Glucose oxidase; GSH, Glutathione; GSK, GSK2837808A; HCPT, Hydroxycamptothecin; HIF1α, Hypoxia-inducing factor 1α; HK2, Hexokinase 2; HMGB1, High mobility group protein B1; HMnO2, hollow manganese dioxide; HPB, Hollow Prussian blue; HSP, Heat shock protein; ICB, Immune checkpoint blocking; ICD, Immunogenic cell death; IDO, Indoleamine 2,3-dioxygenase; Kyn, kynuridine; LDH, Lactate dehydrogenase; LM, Liquid metal; LND, Lonidamine; LOx, Lactate oxidase; MCT, Monocarboxylic acid transporter; MDSCs, Myeloid-derived suppressor cells; MHC, major histocompatibility complex; MOF, Metal–organic framework; mTOR, Mammalian target of rapamycin; mPDA, Pegylated mesoporous polydopamine; MSNs, Mesoporous silica nanoparticles; NIR, Near-infrared; NK, Natural killer; NPs, nanoparticles; NVs, Nanovesicles; OXPHOS, Oxidative phosphorylation; PAE, Polyβ-amino ester; PD-1, Programmed death-1; PD-L1, Programmed cell death 1 ligand 1; PDH, pyruvate dehydrogenase; PDK, Pyruvate dehydrogenase kinase; PDT, Photodynamic therapy; PEI, Poly (ether imide); PFK, Phosphofructokinase; PFKFB3, Fructose-2-kinase 6-phosphate/fructose-2, diphosphatase 3; P-gp, P-glycoprotein; PK, Pyruvate kinase; PKM2, Pyruvate kinase-M2; PTT, Photothermal therapy; PTX, Paclitaxel; ROS, Reactive oxygen species; RT, Radiotherapy; SDT, Sonodynamic therapy; SK, Shikonin; TAAs, Tumor-associated antigens; TAMs, Tumor-associated macrophages; TCA, Tricarboxylic acid; Teffs, Effector T cells; TIME, Tumor immunosuppressive microenvironment; TME, Tumor microenvironment; Tregs, Regulatory T cells; Trp, Tryptophan; TPP+, triphenylphosphine cations; UPS, Ultra-pH-sensitive; ZIF-8, Zeolitic imidazolate framework-8.
Acknowledgments
Kehua Jiang and Hongming Liu are co-first authors for this study. This manuscript has been edited and polished by AJE.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 82060462, No. 82270804), Science and Technology Plan project of Guizhou Province (Number: [2019]5405), Foundation of Health and Family Planning Commission of Guizhou Province (Number: gzwjkj2019-1-127), Doctoral Foundation of Guizhou Provincial People’s Hospital (GZSYBS[2018]02), the Natural Science Foundation of Hubei Province (No.2023AFB867) and Shenzhen Natural Science Foundation (No. JCYJ20230807143505011). The funding agencies and donors had no role in any aspect of this study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Agarwal S, Aznar MA, Rech AJ, et al. Deletion of the inhibitory co-receptor CTLA-4 enhances and invigorates chimeric antigen receptor T cells. Immunity. 2023;56(10):2388–2407.e9. doi:10.1016/j.immuni.2023.09.001
2. Wang F, Jin Y, Wang M, et al. Combined anti-PD-1, HDAC inhibitor and anti-VEGF for MSS/pMMR colorectal cancer: a randomized phase 2 trial. Nat Med. 2024;30(4):1035–1043. doi:10.1038/s41591-024-02813-1
3. Xue F, Ren X, Kong C, et al. Polymeric PD1/PDL1 bispecific antibody enhances immune checkpoint blockade therapy. Mater Today Bio. 2024;28:101239. doi:10.1016/j.mtbio.2024.101239
4. Xie P, Yu M, Zhang B, et al. CRKL dictates anti-PD-1 resistance by mediating tumor-associated neutrophil infiltration in hepatocellular carcinoma. J Hepatol. 2024;81(1):93–107. doi:10.1016/j.jhep.2024.02.009
5. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi:10.1056/NEJMoa1606774
6. Nam A, Jain S, Wu C, et al. Integrin Αvβ3 upregulation in response to nutrient stress promotes lung cancer cell metabolic plasticity. Cancer Res. 2024;84(10):1630–1642. doi:10.1158/0008-5472.CAN-23-2700
7. Gao R, Buechel D, Kalathur RKR, et al. USP29-mediated HIF1α stabilization is associated with sorafenib resistance of hepatocellular carcinoma cells by upregulating glycolysis. Oncogenesis. 2021;10(7):52. doi:10.1038/s41389-021-00338-7
8. Warburg O. On the Origin of Cancer Cells. Science. 1956;123(3191):309–314. doi:10.1126/science.123.3191.309
9. Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer. 2020;8(2):e001408. doi:10.1136/jitc-2020-001408
10. Xie M, Zhao J, Feng X, et al. Cell membrane-inspired chitosan nanoparticles for prolonged circulation and tumor-targeted drug delivery. Int J Biol Macromol. 2025;304:140934. doi:10.1016/j.ijbiomac.2025.140934
11. Chen S, Lan H, Liu M, et al. Less is more: biomimetic hybrid membrane nanocarriers for highly efficient tumor targeted drug delivery. Small. 2025;21(6):2407245. doi:10.1002/smll.202407245
12. Qu H, Hang L, Diao Y, et al. Mn-doped MOF nanoparticles mitigating hypoxia via in-situ substitution strategy for dual-imaging guided combination treatment of microwave dynamic therapy and chemotherapy. J Colloid Interface Sci. 2025;685:912–926. doi:10.1016/j.jcis.2025.01.202
13. Yang L-Y, Liang G-W, Cai B-J, et al. Enhanced tumor self-targeting of lemon-derived extracellular vesicles by embedding homotypic cancer cell membranes for efficient drug delivery. J Nanobiotechnol. 2025;23(1):74. doi:10.1186/s12951-025-03161-z
14. Liu H, Xie Z, Zheng M. Unprecedented chiral nanovaccines for significantly enhanced cancer immunotherapy. ACS Appl Mater Interfaces. 2022;14(35):39858–39865. doi:10.1021/acsami.2c11596
15. Zhang L-Y, Chen X-T, Li R-T, et al. Overcoming hypoxia-induced breast cancer drug resistance: a novel strategy using hollow gold-platinum bimetallic nanoshells. J Nanobiotechnol. 2025;23(1):85. doi:10.1186/s12951-025-03132-4
16. Yao Y, Zhang J, Huang K, et al. Engineered CAF-cancer cell hybrid membrane biomimetic dual-targeted integrated platform for multi-dimensional treatment of ovarian cancer. J Nanobiotechnol. 2025;23(1):83. doi:10.1186/s12951-025-03165-9
17. Kennedy BE, Noftall EB, Dean C, et al. Targeted intra-tumoral hyperthermia using uniquely biocompatible gold nanorods induces strong immunogenic cell death in two immunogenically “cold” tumor models. Front Immunol. 2025;15:1512543. doi:10.3389/fimmu.2024.1512543
18. Kim H, Choi S-Y, Heo T-Y, et al. Value of glucose transport protein 1 expression in detecting lymph node metastasis in patients with colorectal cancer. World J Clin Cases. 2024;12(5):931–941. doi:10.12998/wjcc.v12.i5.931
19. Singh M, Afonso J, Sharma D, et al. Targeting Monocarboxylate Transporters (MCTs) in Cancer: how Close Are we to the clinics? Semi Cancer Biol. 2023;90:1–14. doi:10.1016/j.semcancer.2023.01.007
20. Pisarsky L, Bill R, Fagiani E, et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 2016;15(6):1161–1174. doi:10.1016/j.celrep.2016.04.028
21. Park S, Chang C-Y, Safi R, et al. ERRα-regulated lactate metabolism contributes to resistance to targeted therapies in breast cancer. Cell Rep. 2016;15(2):323–335. doi:10.1016/j.celrep.2016.03.026
22. Yang Y, Ishak Gabra MB, Hanse EA, et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun. 2019;10(1):809. doi:10.1038/s41467-019-08759-0
23. Sousa CM, Kimmelman AC. The complex landscape of pancreatic cancer metabolism. Carcinogenesis. 2014;35(7):1441–1450. doi:10.1093/carcin/bgu097
24. Venturoli C, Piga I, Curtarello M, et al. Genetic perturbation of pyruvate dehydrogenase kinase 1 modulates growth, angiogenesis and metabolic pathways in ovarian cancer xenografts. Cells. 2021;10(2):325. doi:10.3390/cells10020325
25. Heydarzadeh S, Moshtaghie AA, Daneshpoor M, Hedayati M. Regulators of glucose uptake in thyroid cancer cell lines. Cell Commun Signal. 2020;18(1):83. doi:10.1186/s12964-020-00586-x
26. McNamee EN, Korns Johnson D, Homann D, Clambey ET. Hypoxia and hypoxia-inducible factors as regulators of T cell development, differentiation, and function. Immunol Res. 2013;55(1–3):58–70. doi:10.1007/s12026-012-8349-8
27. Ha H, Debnath B, Neamati N. Role of the CXCL8-CXCR1/2 axis in cancer and inflammatory diseases. Theranostics. 2017;7(6):1543–1588. doi:10.7150/thno.15625
28. Wang J, Wang X, Guo Y, et al. Therapeutic targeting of SPIB / SPI1 ‐facilitated interplay of cancer cells and neutrophils inhibits aerobic glycolysis and cancer progression. Clin Transl Med. 2021;11(11):e588. doi:10.1002/ctm2.588
29. Cham CM, Gajewski TF. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174(8):4670–4677. doi:10.4049/jimmunol.174.8.4670
30. Ren W, Liu G, Yin J, et al. Amino-acid transporters in T-cell activation and differentiation. Cell Death Dis. 2017;8(3):e2655–e2655. doi:10.1038/cddis.2016.222
31. Chang C-H, Qiu J, O’Sullivan D, et al. metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229–1241. doi:10.1016/j.cell.2015.08.016
32. Deng H, Yang W, Zhou Z, et al. Targeted scavenging of extracellular ROS relieves suppressive immunogenic cell death. Nat Commun. 2020;11(1):4951. doi:10.1038/s41467-020-18745-6
33. Scharping NE, Rivadeneira DB, Menk AV, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion. Nat Immunol. 2021;22(2):205–215. doi:10.1038/s41590-020-00834-9
34. Mendler AN, Hu B, Prinz PU, Kreutz M, Gottfried E, Noessner E. Tumor lactic acidosis suppresses CTL function by inhibition of P38 and JNK/c‐Jun activation. Int J Cancer. 2012;131(3):633–640. doi:10.1002/ijc.26410
35. Brand A, Singer K, Koehl GE, et al. LDHA-associated lactic acid production blunts tumor Immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–671. doi:10.1016/j.cmet.2016.08.011
36. Johnston RJ, Su LJ, Pinckney J, et al. Vista is an acidic pH-selective ligand for PSGL-1. Nature. 2019;574(7779):565–570. doi:10.1038/s41586-019-1674-5
37. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15(8):486–499. doi:10.1038/nri3862
38. Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. CD8+ T cell exhaustion in cancer. Front Immunol. 2021;12:715234. doi:10.3389/fimmu.2021.715234
39. Michalek RD, Gerriets VA, Jacobs SR, et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol. 2011;186(6):3299–3303. doi:10.4049/jimmunol.1003613
40. Angelin A, Gil-de-gómez L, Dahiya S, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. 2017;25(6):1282–1293.e7. doi:10.1016/j.cmet.2016.12.018
41. Facciabene A, Peng X, Hagemann IS, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg Cells. Nature. 2011;475(7355):226–230. doi:10.1038/nature10169
42. Leone RD, Powell JD. Metabolism of immune cells in cancer. Nat Rev Cancer. 2020;20(9):516–531. doi:10.1038/s41568-020-0273-y
43. Brown TP, Bhattacharjee P, Ramachandran S, et al. the lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. 2020;39(16):3292–3304. doi:10.1038/s41388-020-1216-5
44. Caronni N, Simoncello F, Stafetta F, et al. Downregulation of membrane trafficking proteins and lactate conditioning determine loss of dendritic cell function in lung cancer. Cancer Res. 2018;78(7):1685–1699. doi:10.1158/0008-5472.CAN-17-1307
45. Erra Díaz F, Ochoa V, Merlotti A, et al. Extracellular acidosis and mTOR inhibition drive the differentiation of human monocyte-derived dendritic cells. Cell Rep. 2020;31(5):107613. doi:10.1016/j.celrep.2020.107613
46. Peng X, He Y, Huang J, Tao Y, Liu S. Metabolism of dendritic cells in tumor microenvironment: for immunotherapy. Front Immunol. 2021;12:613492. doi:10.3389/fimmu.2021.613492
47. Yuen VW-H, Wong CC-L. Hypoxia-inducible factors and innate immunity in liver cancer. J Clin Investig. 2020;130(10):5052–5062. doi:10.1172/JCI137553
48. Haskó G, Pacher P. Regulation of macrophage function by adenosine. ATVB. 2012;32(4):865–869. doi:10.1161/ATVBAHA.111.226852
49. Ahmad A, Ahmad S, Glover L, et al. Adenosine A 2A receptor is a unique angiogenic target of HIF-2α in pulmonary endothelial cells. Proc Natl Acad Sci USA. 2009;106(26):10684–10689. doi:10.1073/pnas.0901326106
50. Chen P, Zuo H, Xiong H, et al. Gpr132 sensing of lactate mediates tumor–macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci USA. 2017;114(3):580–585. doi:10.1073/pnas.1614035114
51. Colegio OR, Chu N-Q, Szabo AL, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–563. doi:10.1038/nature13490
52. Cekic C, Day Y-J, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74(24):7250–7259. doi:10.1158/0008-5472.CAN-13-3583
53. Myers JA, Miller JS. Exploring the NK Cell Platform for Cancer Immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100. doi:10.1038/s41571-020-0426-7
54. Salzberger W, Martrus G, Bachmann K, et al. Tissue-resident NK cells differ in their expression profile of the nutrient transporters Glut1, CD98 and CD71. PLoS One. 2018;13(7):e0201170. doi:10.1371/journal.pone.0201170
55. Balsamo M, Manzini C, Pietra G, et al. Hypoxia downregulates the expression of activating receptors involved in
56. Donnelly RP, Loftus RM, Keating SE, et al. mTORC1-dependent metabolic reprogramming is a prerequisite for NK cell effector function. J Immunol. 2014;193(9):4477–4484. doi:10.4049/jimmunol.1401558
57. Poznanski SM, Singh K, Ritchie TM, et al. Metabolic flexibility determines human NK cell functional fate in the tumor microenvironment. Cell Metab. 2021;33(6):1205–1220.e5. doi:10.1016/j.cmet.2021.03.023
58. Wong CC-L, Gilkes DM, Zhang H, et al. Hypoxia-inducible factor 1 is a master regulator of breast cancer metastatic niche formation. Proc Natl Acad Sci USA. 2011;108(39):16369–16374. doi:10.1073/pnas.1113483108
59. Noman MZ, Desantis G, Janji B, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–790. doi:10.1084/jem.20131916
60. Hossain F, Al-Khami AA, Wyczechowska D, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. 2015;3(11):1236–1247. doi:10.1158/2326-6066.CIR-15-0036
61. Chiu DK-C, Tse AP-W, Xu IM-J, et al. Hypoxia inducible factor HIF-1 promotes myeloid-derived suppressor cells accumulation through ENTPD2/CD39L1 in hepatocellular carcinoma. Nat Commun. 2017;8(1):517. doi:10.1038/s41467-017-00530-7
62. Yang T, Mochida Y, Liu X, et al. Conjugation of glucosylated polymer chains to checkpoint blockade antibodies augments their efficacy and specificity for glioblastoma. Nat Biomed Eng. 2021;5(11):1274–1287. doi:10.1038/s41551-021-00803-z
63. Yang F, Teves SS, Kemp CJ, Henikoff SD. DNA torsion, and chromatin dynamics. BBA. 2014;1845(1):84–89. doi:10.1016/j.bbcan.2013.12.002
64. Liu M, Huang L, Zhang W, et al. A transistor-like pH-sensitive nanodetergent for selective cancer therapy. Nat Nanotechnol. 2022;17(5):541–551. doi:10.1038/s41565-022-01085-5
65. Zeng Z, Zhang C, Li J, Cui D, Jiang Y, Pu K. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv Mater. 2021;33(4):2007247. doi:10.1002/adma.202007247
66. Min HS, Kim HJ, Naito M, et al. Systemic brain delivery of antisense oligonucleotides across the blood–brain barrier with a glucose‐coated polymeric nanocarrier. Angew Chem Int Ed. 2020;59(21):8173–8180. doi:10.1002/anie.201914751
67. He H, Lin X, Guo J, Wang J, Xu B. Perimitochondrial enzymatic self-assembly for selective targeting the mitochondria of cancer cells. ACS Nano. 2020;14(6):6947–6955. doi:10.1021/acsnano.0c01388
68. Luo L, Qin B, Jiang M, et al. Regulating immune memory and reversing tumor thermotolerance through a step-by-step starving-photothermal therapy. J Nanobiotechnol. 2021;19(1):297. doi:10.1186/s12951-021-01011-2
69. Li B, Yang T, Liu J, et al. Genetically engineered PD-1 displaying nanovesicles for synergistic checkpoint blockades and chemo-metabolic therapy against non-small cell lung cancer. Acta Biomater. 2023;161:184–200. doi:10.1016/j.actbio.2023.03.002
70. Jiang Z, Xiong H, Yang S, et al. Jet‐lagged nanoparticles enhanced immunotherapy efficiency through synergistic reconstruction of tumor microenvironment and normalized tumor vasculature. Adv Healthcare Mater. 2020;9(12):2000075. doi:10.1002/adhm.202000075
71. Liu Y, Zhou Z, Hou J, et al. Tumor selective metabolic reprogramming as a prospective PD‐L1 depression strategy to reactivate immunotherapy. Adv Mater. 2022;34(41):2206121. doi:10.1002/adma.202206121
72. Wang S, Zhou Z, Hu R, et al. Metabolic intervention liposome boosted lung cancer radio‐immunotherapy via hypoxia amelioration and PD‐L1 restraint. Adv Sci. 2023;10(18):2207608. doi:10.1002/advs.202207608
73. Zhao L-P, Zheng R-R, Kong R-J, et al. Self-delivery ternary bioregulators for photodynamic amplified immunotherapy by tumor microenvironment reprogramming. ACS Nano. 2022;16(1):1182–1197. doi:10.1021/acsnano.1c08978
74. Shen W, Liu T, Pei P, et al. Metabolic homeostasis‐regulated nanoparticles for antibody‐independent cancer radio‐immunotherapy. Adv Mater. 2022;34(51):2207343. doi:10.1002/adma.202207343
75. Zang S, Huang K, Li J, et al. Metabolic reprogramming by dual-targeting biomimetic nanoparticles for enhanced tumor chemo-immunotherapy. Acta Biomater. 2022;148:181–193. doi:10.1016/j.actbio.2022.05.045
76. Li J, Zhao M, Sun M, et al. Multifunctional nanoparticles boost cancer immunotherapy based on modulating the immunosuppressive tumor microenvironment. ACS Appl Mater Interfaces. 2020;12(45):50734–50747. doi:10.1021/acsami.0c14909
77. Tang L, Yin Y, Cao Y, et al. Extracellular vesicles‐derived hybrid nanoplatforms for amplified CD47 blockade‐based cancer immunotherapy. Adv Mater. 2023;35(35):2303835. doi:10.1002/adma.202303835
78. Shi W, Feng W, Li S, et al. Ferroptosis and necroptosis produced autologous tumor cell lysates co-delivering with combined immnoadjuvants as personalized in situ nanovaccines for antitumor immunity. ACS Nano. 2023;17(15):14475–14493. doi:10.1021/acsnano.3c00901
79. Yan J, Li W, Tian H, et al. Metal-phenolic nanomedicines regulate T-cell antitumor function for sono-metabolic cancer therapy. ACS Nano. 2023;17(15):14667–14677. doi:10.1021/acsnano.3c02428
80. She W, Li H, Wang Z, et al. Site-specific controlled-release nanoparticles for immune reprogramming via dual metabolic inhibition against triple-negative breast cancer. J Control Release. 2024;366:204–220. doi:10.1016/j.jconrel.2023.12.022
81. Ju H, Kim D, Oh Y-K. Lipid nanoparticle-mediated CRISPR/Cas9 gene editing and metabolic engineering for anticancer immunotherapy. Asian J Pharm Sci. 2022;17(5):641–652. doi:10.1016/j.ajps.2022.07.005
82. Zhang Y-X, Zhao -Y-Y, Shen J, et al. Nanoenabled modulation of acidic tumor microenvironment reverses anergy of infiltrating T cells and potentiates anti-PD-1 therapy. Nano Lett. 2019;19(5):2774–2783. doi:10.1021/acs.nanolett.8b04296
83. Ling J, Chang Y, Yuan Z, Chen Q, He L, Chen T. Designing lactate dehydrogenase-mimicking SnSe nanosheets to reprogram tumor-associated macrophages for potentiation of photothermal immunotherapy. ACS Appl Mater Interfaces. 2022;14(24):27651–27665. doi:10.1021/acsami.2c05533
84. Dai Z, Wang Q, Tang J, et al. A Sub-6 Nm MnFe2O4-dichloroacetic acid nanocomposite modulates tumor metabolism and catabolism for reversing tumor immunosuppressive microenvironment and boosting immunotherapy. Biomaterials. 2022;284:121533. doi:10.1016/j.biomaterials.2022.121533
85. Ren X, Cheng Z, He J, et al. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat Commun. 2023;14(1):7021. doi:10.1038/s41467-023-42883-2
86. Payen VL, Mina E, Van Hée VF, Porporato PE, Sonveaux P. Monocarboxylate transporters in cancer. Mol Metabol. 2020;33:48–66. doi:10.1016/j.molmet.2019.07.006
87. Li K, Lin C, He Y, et al. Engineering of cascade-responsive nanoplatform to inhibit lactate efflux for enhanced tumor chemo-immunotherapy. ACS Nano. 2020;14(10):14164–14180. doi:10.1021/acsnano.0c07071
88. Hu -J-J, Liu M-D, Gao F, et al. Photo-controlled liquid metal nanoparticle-enzyme for starvation/photothermal therapy of tumor by Win-Win Cooperation. Biomaterials. 2019;217:119303. doi:10.1016/j.biomaterials.2019.119303
89. Zhang M, Wang W, Wu F, et al. Biodegradable Poly(γ-Glutamic Acid)@glucose Oxidase@carbon dot nanoparticles for simultaneous multimodal imaging and synergetic cancer therapy. Biomaterials. 2020;252:120106. doi:10.1016/j.biomaterials.2020.120106
90. Zhang S, Zhang Y, Feng Y, et al. Biomineralized two‐enzyme nanoparticles regulate tumor glycometabolism inducing tumor cell pyroptosis and robust antitumor immunotherapy. Adv Mater. 2022;34(50):2206851. doi:10.1002/adma.202206851
91. Chechetka SA, Yu Y, Zhen X, Pramanik M, Pu K, Miyako E. Light-driven liquid metal nanotransformers for biomedical theranostics. Nat Commun. 2017;8(1):15432. doi:10.1038/ncomms15432
92. Xie W, Deng -W-W, Zan M, et al. Cancer cell membrane camouflaged nanoparticles to realize starvation therapy together with checkpoint blockades for enhancing cancer therapy. ACS Nano. 2019;13(3):2849–2857. doi:10.1021/acsnano.8b03788
93. Dai L, Yao M, Fu Z, et al. Multifunctional metal-organic framework-based nanoreactor for starvation/oxidation improved indoleamine 2,3-dioxygenase-blockade tumor immunotherapy. Nat Commun. 2022;13(1):2688. doi:10.1038/s41467-022-30436-y
94. Ren H, Yong J, Yang Q, et al. Self-assembled FeS-based cascade bioreactor with enhanced tumor penetration and synergistic treatments to trigger robust cancer immunotherapy. Acta Pharmaceutica Sinica B. 2021;11(10):3244–3261. doi:10.1016/j.apsb.2021.05.005
95. Sun K, Hu J, Meng X, et al. Reinforcing the induction of immunogenic cell death via artificial engineered cascade bioreactor‐enhanced chemo‐immunotherapy for optimizing cancer immunotherapy. Small. 2021;17(37):2101897. doi:10.1002/smll.202101897
96. Zheng X, Liu Y, Liu Y, et al. Dual closed-loop of catalyzed lactate depletion and immune response to potentiate photothermal immunotherapy. ACS Appl Mater Interfaces. 2022;14(20):23260–23276. doi:10.1021/acsami.2c07254
97. Cao Z, Xu D, Harding J, et al. Lactate oxidase nanocapsules boost T cell immunity and efficacy of cancer immunotherapy. Sci Transl Med. 2023;15(717):eadd2712. doi:10.1126/scitranslmed.add2712
98. Xie Y, Wang M, Qiao L, et al. Photothermal‐enhanced dual inhibition of lactate/kynurenine metabolism for promoting tumor immunotherapy. Small Methods. 2024;8(3):2300945. doi:10.1002/smtd.202300945
99. Wang H, Wu C, Tong X, Chen S. A biomimetic metal-organic framework nanosystem modulates immunosuppressive tumor microenvironment metabolism to amplify immunotherapy. J Control Release. 2023;353:727–737. doi:10.1016/j.jconrel.2022.11.054
100. Tang Y, Chang Q, Chen G, et al. Tumor immunosuppression relief via acidity modulation combined PD-L1 siRNA for enhanced immunotherapy. Biomater Adv. 2023;150:213425. doi:10.1016/j.bioadv.2023.213425
101. Chen Y, Bei J, Chen M, et al. Intratumoral lactate depletion based on injectable nanoparticles−hydrogel composite system synergizes with immunotherapy against postablative hepatocellular carcinoma recurrence. Adv Healthcare Mater. 2024;13(6):2303031. doi:10.1002/adhm.202303031
102. Zhao H, Li Y, Shi H, et al. Prodrug nanoparticles potentiate tumor chemo-immunometabolic therapy by disturbing oxidative stress. J Control Release. 2022;352:909–919. doi:10.1016/j.jconrel.2022.11.011
103. Sun F, Zhu Q, Li T, et al. Regulating glucose metabolism with prodrug nanoparticles for promoting photoimmunotherapy of pancreatic cancer. Adv Sci. 2021;8(4):2002746. doi:10.1002/advs.202002746
104. Luo Y, Li Y, Huang Z, et al. A nanounit strategy disrupts energy metabolism and alleviates immunosuppression for cancer therapy. Nano Lett. 2022;22(15):6418–6427. doi:10.1021/acs.nanolett.2c02475
105. Kuang Y, Balakrishnan K, Gandhi V, Peng X. Hydrogen peroxide inducible DNA cross-linking agents: targeted anticancer prodrugs. J Am Chem Soc. 2011;133(48):19278–19281. doi:10.1021/ja2073824
106. DaCosta Byfield S, Major C, Laping NJ, Roberts AB. SB-505124 is a selective inhibitor of transforming growth factor-β type i receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2004;65(3):744–752. doi:10.1124/mol.65.3.744
107. Ma X, Chen J, Huang B, et al. ErbB2-upregulated HK1 and HK2 promote breast cancer cell proliferation, migration and invasion. Med Oncol. 2023;40(5):154. doi:10.1007/s12032-023-02008-7
108. Urakami K, Zangiacomi V, Yamaguchi K, Kusuhara M. Impact of 2-Deoxy-D-Glucose on the target metabolome profile of a human endometrial cancer cell line. Biomed Res. 2013;34(5):221–229. doi:10.2220/biomedres.34.221
109. Wang L, Yang Q, Peng S, Liu X. The combination of the glycolysis inhibitor 2-DG and sorafenib can be effective against sorafenib-tolerant persister cancer cells. OTT. 2019;12:5359–5373. doi:10.2147/OTT.S212465
110. Ma EH, Poffenberger MC, Wong AH-T, Jones RG. The role of AMPK in T cell metabolism and function. Curr Opin Immunol. 2017;46:45–52. doi:10.1016/j.coi.2017.04.004
111. Berruti A, Bitossi R, Gorzegno G, et al. Time to progression in metastatic breast cancer patients treated with epirubicin is not improved by the addition of either cisplatin or lonidamine: final results of a Phase III study with a factorial design. JCO. 2002;20(20):4150–4159. doi:10.1200/JCO.2002.08.012
112. Bartrons R, Rodríguez-García A, Simon-Molas H, Castaño E, Manzano A, Navarro-Sabaté À. The potential utility of PFKFB3 as a therapeutic target. Exp Opin Therap Targets. 2018;22(8):659–674. doi:10.1080/14728222.2018.1498082
113. Emini Veseli B, Perrotta P, Van Wielendaele P, et al. Small molecule 3PO inhibits glycolysis but does not bind to 6‐phosphofructo‐2‐kinase/Fructose‐2,6‐bisphosphatase‐3 (PFKFB3). FEBS Lett. 2020;594(18):3067–3075. doi:10.1002/1873-3468.13878
114. Li H-M, Yang J-G, Liu Z-J, et al. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2017;36(1):7. doi:10.1186/s13046-016-0481-1
115. Gao F, Tang Y, Liu W, et al. Intra/extracellular lactic acid exhaustion for synergistic metabolic therapy and immunotherapy of tumors. Adv Mater. 2019;31(51):1904639. doi:10.1002/adma.201904639
116. Melchionna R, Trono P, Di Carlo A, Di Modugno F, Nisticò P. Transcription factors in fibroblast plasticity and CAF heterogeneity. J Exp Clin Cancer Res. 2023;42(1):347. doi:10.1186/s13046-023-02934-4
117. Andújar I, Ríos J, Giner R, Recio M. Pharmacological properties of Shikonin – a review of literature since 2002. Planta Med. 2013;79(18):1685–1697. doi:10.1055/s-0033-1350934
118. Verweij FJ, Balaj L, Boulanger CM, et al. The power of imaging to understand extracellular vesicle biology in vivo. Nat Methods. 2021;18(9):1013–1026. doi:10.1038/s41592-021-01206-3
119. Wang H, Tang Y, Fang Y, et al. Reprogramming Tumor Immune Microenvironment (TIME) and metabolism via biomimetic targeting codelivery of Shikonin/JQ1. Nano Lett. 2019;19(5):2935–2944. doi:10.1021/acs.nanolett.9b00021
120. Sim DY, Lee H-J, Ahn C-H, et al. Negative regulation of CPSF6 suppresses the Warburg effect and angiogenesis leading to tumor progression Via C-Myc signaling network: potential therapeutic target for liver cancer therapy. Int J Biol Sci. 2024;20(9):3442–3460. doi:10.7150/ijbs.93462
121. Kim S-L, Choi HS, Lee D-S. BRD4/Nuclear PD-L1/RelB circuit is involved in the stemness of breast cancer cells. Cell Commun Signal. 2023;21(1):315. doi:10.1186/s12964-023-01319-6
122. Pasparakis M, Vandenabeele P. Necroptosis and Its Role in Inflammation. Nature. 2015;517(7534):311–320. doi:10.1038/nature14191
123. Wang S, Ma L, Wang Z, et al. Lactate dehydrogenase-A (LDH-A) preserves cancer stemness and recruitment of tumor-associated macrophages to promote breast cancer progression. Front Oncol. 2021;11:654452. doi:10.3389/fonc.2021.654452
124. Gao M, Wang Z, Zheng H, et al. Two‐dimensional Tin Selenide (SnSe) nanosheets capable of mimicking key dehydrogenases in cellular metabolism. Angew Chem Int Ed. 2020;59(9):3618–3623. doi:10.1002/anie.201913035
125. Jiang D, Ni D, Rosenkrans ZT, Huang P, Yan X, Cai W. Nanozyme: new horizons for responsive biomedical applications. Chem Soc Rev. 2019;48(14):3683–3704. doi:10.1039/C8CS00718G
126. Li J, Shi K, Sabet ZF, et al. New power of self-assembling carbonic anhydrase inhibitor: short peptide–constructed nanofibers inspire hypoxic cancer therapy. Sci Adv. 2019;5(9):eaax0937. doi:10.1126/sciadv.aax0937
127. Becker HM. Carbonic anhydrase IX and acid transport in cancer. Br J Cancer. 2020;122(2):157–167. doi:10.1038/s41416-019-0642-z
128. Lee S-H, Griffiths JR. How and why are cancers acidic? Carbonic Anhydrase IX and the homeostatic control of tumour extracellular pH. Cancers. 2020;12(6):1616. doi:10.3390/cancers12061616
129. Fan W, Lu N, Huang P, et al. Glucose‐responsive sequential generation of hydrogen peroxide and nitric oxide for synergistic cancer starving‐like/gas therapy. Angew Chem Int Ed. 2017;56(5):1229–1233. doi:10.1002/anie.201610682
130. Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22(47):7468–7485. doi:10.1038/sj.onc.1206948
131. Ji HB, Kim S-N, Kim CR, et al. Metal-organic framework for biomimetic nitric oxide generation and anticancer drug delivery. Biomater Adv. 2023;145:213268. doi:10.1016/j.bioadv.2022.213268
132. Gao L, Liu Y, Zhou Y, Qin X. Baicalein attenuates neuroinflammation in LPS-treated BV-2 cells by inhibiting glycolysis via STAT3/c-Myc pathway. Neurochem Res. 2023;48(11):3363–3377. doi:10.1007/s11064-023-03961-5
133. Stacpoole PW. Therapeutic targeting of the pyruvate dehydrogenase complex/Pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. JNCI. 2017;109:11. doi:10.1093/jnci/djx071
134. Marbaniang C, Kma L. Dysregulation of glucose metabolism by oncogenes and tumor suppressors in cancer cells. Asian Pac J Cancer Prev. 2018;19:9. doi:10.22034/APJCP.2018.19.9.2377
135. Wu S, Zhang K, Liang Y, et al. Nano‐enabled tumor systematic energy exhaustion via Zinc (II) interference mediated glycolysis inhibition and specific GLUT1 depletion. Adv Sci. 2022;9(7):2103534. doi:10.1002/advs.202103534
136. Sun X, Wang M, Wang M, et al. Role of proton-coupled monocarboxylate transporters in cancer: from metabolic crosstalk to therapeutic potential. Front Cell Dev Biol. 2020;8:651. doi:10.3389/fcell.2020.00651
137. Fischer K, Hoffmann P, Voelkl S, et al. Inhibitory effect of tumor cell–derived lactic acid on human T cells. Blood. 2007;109(9):3812–3819. doi:10.1182/blood-2006-07-035972
138. Tian L-R, Lin M-Z, Zhong -H-H, et al. Nanodrug regulates lactic acid metabolism to reprogram the immunosuppressive tumor microenvironment for enhanced cancer immunotherapy. Biomater Sci. 2022;10(14):3892–3900. doi:10.1039/D2BM00650B
139. Fu L, Qi C, Hu Y, Lin J, Huang P. Glucose oxidase‐instructed multimodal synergistic cancer therapy. Adv Mater. 2019;31(21):1808325. doi:10.1002/adma.201808325
140. Hao H, Sun M, Li P, Sun J, Liu X, Gao W. In situ growth of a cationic polymer from the N-terminus of glucose oxidase to regulate H 2 O 2 generation for cancer starvation and H 2 O 2 therapy. ACS Appl Mater Interfaces. 2019;11(10):9756–9762. doi:10.1021/acsami.8b20956
141. Zhang L, Wang Z, Zhang Y, et al. Erythrocyte membrane cloaked metal–organic framework nanoparticle as biomimetic nanoreactor for starvation-activated colon cancer therapy. ACS Nano. 2018;12(10):10201–10211. doi:10.1021/acsnano.8b05200
142. Zhou Z, Zhang Q, Yang R, et al. ATP-charged nanoclusters enable intracellular protein delivery and activity modulation for cancer theranostics. iScience. 2020;23(2):100872. doi:10.1016/j.isci.2020.100872
143. Ding X-L, Liu M-D, Cheng Q, et al. Multifunctional liquid metal-based nanoparticles with glycolysis and mitochondrial metabolism inhibition for tumor photothermal therapy. Biomaterials. 2022;281:121369. doi:10.1016/j.biomaterials.2022.121369
144. Zhang T, Zhang Z, Yue Y, et al. A general hypoxia‐responsive molecular container for tumor‐targeted therapy. Adv Mater. 2020;32(28):1908435. doi:10.1002/adma.201908435
145. Yu W, Lin R, He X, et al. Self-propelled nanomotor reconstructs tumor microenvironment through synergistic hypoxia alleviation and glycolysis inhibition for promoted anti-metastasis. Acta Pharmaceutica Sinica B. 2021;11(9):2924–2936. doi:10.1016/j.apsb.2021.04.006
146. Nam J, Son S, Ochyl LJ, Kuai R, Schwendeman A, Moon JJ. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074. doi:10.1038/s41467-018-03473-9
147. Li Z, Cai H, Li Z, et al. A tumor cell membrane-coated self-amplified nanosystem as a nanovaccine to boost the therapeutic effect of Anti-PD-L1 antibody. Bioact Mater. 2023;21:299–312. doi:10.1016/j.bioactmat.2022.08.028
148. Kroll AV, Fang RH, Jiang Y, et al. Nanoparticulate delivery of cancer cell membrane elicits multiantigenic antitumor immunity. Adv Mater. 2017;29(47):1703969. doi:10.1002/adma.201703969
149. Weinberg F, Ramnath N, Nagrath D. Reactive oxygen species in the tumor microenvironment: an overview. Cancers. 2019;11(8):1191. doi:10.3390/cancers11081191
150. Bansal A, Simon MC. Glutathione metabolism in cancer progression and treatment resistance. J Cell Biol. 2018;217(7):2291–2298. doi:10.1083/jcb.201804161
151. Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi:10.1016/j.cell.2010.02.034
152. Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. 2018;7:81–86. doi:10.1016/j.cotox.2017.11.002
153. Oh H, Ghosh S.
154. Chen Q, Feng L, Liu J, et al. Intelligent Albumin‐MnO 2 nanoparticles as pH‐/H 2 O 2 ‐responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv Mater. 2018;30(8):1707414. doi:10.1002/adma.201707414
155. Komkova MA, Karyakina EE, Karyakin AA. Catalytically synthesized Prussian blue nanoparticles defeating natural enzyme peroxidase. J Am Chem Soc. 2018;140(36):11302–11307. doi:10.1021/jacs.8b05223
156. Hong S, Zhang Q-L, Zheng D-W, et al. Enzyme mimicking based on the natural melanin particles from human hair. iScience. 2020;23(1):100778. doi:10.1016/j.isci.2019.100778
157. Liu H, Yang Y, Liu Y, et al. Melanin‐like nanomaterials for advanced biomedical applications: a versatile platform with extraordinary promise. Adv Sci. 2020;7(7):1903129. doi:10.1002/advs.201903129
158. Wu D, Zhou J, Chen X, et al. Mesoporous polydopamine with built-in plasmonic core: traceable and NIR triggered delivery of functional proteins. Biomaterials. 2020;238:119847. doi:10.1016/j.biomaterials.2020.119847
159. Hao Y, Chen Y, He X, et al. Polymeric nanoparticles with ROS‐responsive prodrug and platinum nanozyme for enhanced chemophotodynamic therapy of colon cancer. Adv Sci. 2020;7(20):2001853. doi:10.1002/advs.202001853
160. Dong S, Dong Y, Jia T, et al. GSH‐depleted nanozymes with hyperthermia‐enhanced dual enzyme‐mimic activities for tumor nanocatalytic therapy. Adv Mater. 2020;32(42):2002439. doi:10.1002/adma.202002439
161. Wojtkowiak JW, Verduzco D, Schramm KJ, Gillies RJ. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol Pharm. 2011;8(6):2032–2038. doi:10.1021/mp200292c
162. Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O. Role of the tumor microenvironment in the activity and expression of the P-glycoprotein in human colon carcinoma cells. Oncol Rep. 2007;17(1):239–244.
163. Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. 2019;10:1654. doi:10.3389/fimmu.2019.01654
164. Hsu J-M, Li C-W, Lai Y-J, Hung M-C. Posttranslational modifications of PD-L1 and their applications in cancer therapy. Cancer Res. 2018;78(22):6349–6353. doi:10.1158/0008-5472.CAN-18-1892
165. Chou T-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi:10.1158/0008-5472.CAN-09-1947
166. Yang B, Chen Y, Shi J. Tumor‐specific chemotherapy by nanomedicine‐enabled differential stress sensitization. Angew Chem Int Ed. 2020;59(24):9693–9701. doi:10.1002/anie.202002306
167. Zhou Z, Liu Y, Jiang X, et al. Metformin modified chitosan as a multi-functional adjuvant to enhance cisplatin-based tumor chemotherapy efficacy. Int J Biol Macromol. 2023;224:797–809. doi:10.1016/j.ijbiomac.2022.10.167
168. Tu X, Qin B, Zhang Y, et al. PD-L1 (B7-H1) competes with the RNA exosome to regulate the DNA damage response and can be targeted to sensitize to radiation or chemotherapy. Molecular Cell. 2019;74(6):1215–1226.e4. doi:10.1016/j.molcel.2019.04.005
169. Zhang R, Yang Y, Dong W, et al. D-Mannose facilitates immunotherapy and radiotherapy of triple-negative breast cancer via degradation of PD-L1. Proc Natl Acad Sci USA. 2022;119(8):e2114851119. doi:10.1073/pnas.2114851119
170. Oikari S, Makkonen K, Deen AJ, et al. Hexosamine biosynthesis in keratinocytes: roles of GFAT and GNPDA enzymes in the maintenance of UDP-GlcNAc content and hyaluronan synthesis. Glycobiology. 2016;26(7):710–722. doi:10.1093/glycob/cww019
171. Yang P, Zhang J. Indoleamine 2,3-Dioxygenase (IDO) activity: a perspective biomarker for laboratory determination in tumor immunotherapy. Biomedicines. 2023;11(7):1988. doi:10.3390/biomedicines11071988
172. Labadie BW, Bao R, Luke JJ. Reimagining IDO pathway inhibition in cancer immunotherapy via downstream focus on the Tryptophan–Kynurenine–Aryl hydrocarbon axis. Clin Cancer Res. 2019;25(5):1462–1471. doi:10.1158/1078-0432.CCR-18-2882
173. Watson MJ, Delgoffe GM. Fighting in a wasteland: deleterious metabolites and antitumor immunity. J Clin Investig. 2022;132(2):e148549. doi:10.1172/JCI148549
174. Tan J, Ding B, Teng B, Ma P, Lin J. Understanding structure–function relationships of nanoadjuvants for enhanced cancer vaccine efficacy. Adv Funct Mater. 2022;32(16):2111670. doi:10.1002/adfm.202111670
175. Oleszycka E, McCluskey S, Sharp FA, et al. The vaccine adjuvant alum promotes IL‐10 production that suppresses Th1 responses. Eur J Immunol. 2018;48(4):705–715. doi:10.1002/eji.201747150
176. Hu T, Wang Z, Shen W, Liang R, Yan D, Wei M. Recent advances in innovative strategies for enhanced cancer photodynamic therapy. Theranostics. 2021;11(7):3278–3300. doi:10.7150/thno.54227
177. Zhou Z, Wang X, Zhang H, et al. Activating layered metal oxide nanomaterials via structural engineering as biodegradable nanoagents for photothermal cancer therapy. Small. 2021;17(12):2007486. doi:10.1002/smll.202007486
178. Zhao L-P, Zheng -R-R, Huang J-Q, et al. Self-delivery photo-immune stimulators for photodynamic sensitized tumor immunotherapy. ACS Nano. 2020;14(12):17100–17113. doi:10.1021/acsnano.0c06765
179. Patel RB, Ye M, Carlson PM, et al. Development of an in situ cancer vaccine via combinational radiation and bacterial‐membrane‐coated nanoparticles. Adv Mater. 2019;31(43):1902626. doi:10.1002/adma.201902626
180. Sweeney EE, Cano‐Mejia J, Fernandes R. Photothermal therapy generates a thermal window of immunogenic cell death in neuroblastoma. Small. 2018;14(20):1800678. doi:10.1002/smll.201800678
181. Nikfarjam M, Muralidharan V, Christophi C. Mechanisms of focal heat destruction of liver tumors. J Surg Res. 2005;127(2):208–223. doi:10.1016/j.jss.2005.02.009
182. Wu J, Liu T, Rios Z, Mei Q, Lin X, Cao S. Heat shock proteins and cancer. Trends Pharmacol Sci. 2017;38(3):226–256. doi:10.1016/j.tips.2016.11.009
183. Wang Z, Li S, Zhang M, et al. Laser‐triggered small interfering RNA releasing gold nanoshells against heat shock protein for sensitized photothermal therapy. Adv Sci. 2017;4(2):1600327. doi:10.1002/advs.201600327
184. Kruijt B, Van Der Snoek EM, Sterenborg HJCM, Amelink A, Robinson DJ. A dedicated applicator for light delivery and monitoring of PDT of intra-anal intraepithelial neoplasia. Photodiagn Photodyn Ther. 2010;7(1):3–9. doi:10.1016/j.pdpdt.2010.01.006
185. Huang RB, Mocherla S, Heslinga MJ, Charoenphol P, Eniola-Adefeso O. Dynamic and cellular interactions of nanoparticles in vascular-targeted drug delivery. Mol Membrane Biol. 2010;27(7):312–327. doi:10.3109/09687688.2010.522117
186. Huang Y, Zhou J, Luo S, et al. Identification of a fluorescent small-molecule enhancer for therapeutic autophagy in colorectal cancer by targeting mitochondrial protein translocase TIM44. Gut. 2018;67(2):307–319. doi:10.1136/gutjnl-2016-311909
187. Lin L-S, Huang T, Song J, et al. Synthesis of copper peroxide nanodots for H 2 O 2 self-supplying chemodynamic therapy. J Am Chem Soc. 2019;141(25):9937–9945. doi:10.1021/jacs.9b03457
188. Fu S, Yang R, Zhang L, et al. Biomimetic CoO@AuPt nanozyme responsive to multiple tumor microenvironmental clues for augmenting chemodynamic therapy. Biomaterials. 2020;257:120279. doi:10.1016/j.biomaterials.2020.120279
189. Ma B, Wang S, Liu F, et al. Self-assembled copper–amino acid nanoparticles for in situ glutathione “AND” H 2 O 2 sequentially triggered chemodynamic therapy. J Am Chem Soc. 2019;141(2):849–857. doi:10.1021/jacs.8b08714
190. Shi L, Wang Y, Zhang C, et al. An acidity‐unlocked magnetic nanoplatform enables self‐boosting ROS generation through upregulation of lactate for imaging‐guided highly specific chemodynamic therapy. Angew Chem Int Ed. 2021;60(17):9562–9572. doi:10.1002/anie.202014415
191. Tian Z, Yang K, Yao T, et al. Catalytically selective chemotherapy from tumor‐metabolic generated lactic acid. Small. 2019;15(46):1903746. doi:10.1002/smll.201903746
192. Wang X, Zhao Y, Shi L, et al. Tumor‐targeted disruption of lactate transport with reactivity‐reversible nanocatalysts to amplify oxidative damage. Small. 2021;17(20):2100130. doi:10.1002/smll.202100130
193. Tan X, Huang J, Wang Y, et al. Transformable nanosensitizer with tumor microenvironment‐activated sonodynamic process and calcium release for enhanced cancer immunotherapy. Angew Chem Int Ed. 2021;60(25):14051–14059. doi:10.1002/anie.202102703
194. Liang S, Xiao X, Bai L, et al. Conferring Ti‐Based MOFs with defects for enhanced sonodynamic cancer therapy. Adv Mater. 2021;33(18):2100333. doi:10.1002/adma.202100333
195. Zhang Z, Li B, Xie L, et al. Metal-phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy. ACS Nano. 2021;15(10):16934–16945. doi:10.1021/acsnano.1c08026
196. Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–1807. doi:10.1021/nl061025k
197. Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicol Sci. 2006;91(1):159–165. doi:10.1093/toxsci/kfj122
198. Dai Q, Wilhelm S, Ding D, et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano. 2018;12(8):8423–8435. doi:10.1021/acsnano.8b03900
199. Nienhaus K, Nienhaus GU. Mechanistic understanding of protein corona formation around nanoparticles: old puzzles and new insights. Small. 2023;19(28):2301663. doi:10.1002/smll.202301663
200. Tylawsky DE, Kiguchi H, Vaynshteyn J, et al. P-selectin-targeted nanocarriers induce active crossing of the blood–brain barrier via Caveolin-1-dependent transcytosis. Nat Mater. 2023;22(3):391–399. doi:10.1038/s41563-023-01481-9
201. Tietz O, Cortezon-Tamarit F, Chalk R, Able S, Vallis KA. Tricyclic cell-penetrating peptides for efficient delivery of functional antibodies into cancer cells. Nat Chem. 2022;14(3):284–293. doi:10.1038/s41557-021-00866-0
202. Liu X, Li M, Liu J, et al. In situ self-sorting peptide assemblies in living cells for simultaneous organelle targeting. J Am Chem Soc. 2022;144(21):9312–9323. doi:10.1021/jacs.2c01025
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




