Back to Journals » International Journal of Nanomedicine » Volume 20
Research Progress of Bone Grafting: A Comprehensive Review
Authors Zhang J, Zhang W, Yue W, Qin W, Zhao Y, Xu G
Received 5 December 2024
Accepted for publication 8 April 2025
Published 15 April 2025 Volume 2025:20 Pages 4729—4757
DOI https://doi.org/10.2147/IJN.S510524
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Lijie Grace Zhang
Jing Zhang,1,2 Wanhao Zhang,1,2 Wenjie Yue,1,2 Wenhe Qin,1,2 Yantao Zhao,3,4 Gang Xu1,2
1Department of Orthopaedics, First Affiliated Hospital of Dalian Medical University, Dalian, 116011, People’s Republic of China; 2Key Laboratory of Molecular Mechanism for Repair and Remodeling of Orthopaedic Diseases, Dalian, Liaoning Province, 116011, People’s Republic of China; 3Senior Department of Orthopedics, the Fourth Medical Center of PLA General Hospital, Beijing, 100048, People’s Republic of China; 4Beijing Engineering Research Center of Orthopaedic Implants, Beijing, 100048, People’s Republic of China
Correspondence: Yantao Zhao; Gang Xu, Email [email protected]; [email protected]
Abstract: Bone tissue, the second most transplanted tissue after blood, is utilized in over 2.2 million bone grafts annually to address various bone-related conditions including fractures, tumors, bone infections, scoliosis, congenital defects, osteoporosis, osteoarthritis, and osteogenesis imperfecta. According to incomplete statistics, $4.3 billion was spent on bone graft materials in 2015 alone, with projections suggesting this figure may reach $66 billion by 2026. The limited availability of autogenous bone graft considered the gold standard due to their three critical biological properties: osteoconduction, osteoinduction, and osteogenesis-alongside the increasing global aging population, may be contributing to this rising expenditure. Furthermore, advancements in biomaterials and engineering technologies have created opportunities for the exploration of new bone graft substitutes. In this review, we will examine the fundamental structure of natural bone and the characteristics of ideal bone graft, highlighting common bone graft materials currently available, such as true bone ceramics, decalcified bone matrix, freeze-dried bone and demineralized freeze-dried bone, bioactive glasses, bone marrow aspirate concentrate, polymer nanocomposites, which have different characteristics in osteogenic, osteoconductivity, osteoinductivity, biocompatibility, mechanical properties, and resorption. How to utilize its advantages to maximize the osteogenic effect will be the focus of this review, and some of the current challenges in the field of bone grafting will be identified, outlining potential directions for future development. In conclusion, the choice of bone graft is critical to bone repair and regeneration, and a comprehensive understanding of the advantages and disadvantages of bone graft materials can improve the effectiveness of related surgical interventions.
Keywords: bone graft, true bone ceramics, decalcified bone matrix, freeze-dried bone, bone regeneration
Introduction
Over a decade ago, the National Center for Health Statistics reported that 4,392,000 orthopedic-related surgeries were performed in 2010 alone. Among these, approximately 1 million involved surgical interventions on cranial bones, extremities, ribs and sternum affected by trauma, postoperative deformities, oncological conditions, and inflammatory diseases. Additionally, 139,400 surgeries were lower extremity joint replacements, with at least 20–25% of these cases requiring bone graft material, the majority of which utilized bone substitute materials. Consequently, the total number of surgeries involving bone graft material is estimated to be between 1.3 and 1.5 million.1 Nowadays, the aging global population, increased life expectancy, enhanced access to advanced health care services and a heightened incidence of sports injuries among youth contribute to an annual rise in these figures. Bone defects resulting from trauma, disease, surgery or congenital malformations represent a significant health challenge worldwide, necessitating improved methods for the repair and regeneration of bone tissue.2,3 Furthermore, bone is the second most transplanted tissue after blood, with over 2.2 million bone grafts performed each year to address various bone-related diseases, including fractures, tumors, bone infections, scoliosis, congenital defects, osteoporosis, osteoarthritis and osteogenesis imperfecta.4,5 This substantial demand for bone replacement materials has spurred the growth of the orthopedic implant market, which was valued at $4.3 billion in 2015 and is projected to reach $66.0 billion by 2026.6,7 Bone grafting is frequently employed in the fields of traumatology, orthopedics and maxillofacial surgery. The treatment approach is primarily influenced by the degree and nature of the pathological condition that results in the bone defect. Bone graft materials are predominantly utilized in traumatology and orthopedics for applications involving the spine, significant bone defects, and degenerative diseases affecting major joints. Additionally, these materials are employed in dentistry and maxillofacial surgery to address atrophy in both the upper and lower alveolar ridges.8 Among the various types of bone grafts, autologous bone grafts, which are obtained in the form of bone fragments or pellets, are considered the gold standard due to their three critical biological properties: osteoconduction, osteoinduction and osteogenesis.9,10 However, autografts have notable limitations, primarily due to the restricted availability of donor sites, which are mainly the ilium, tibia, and fibula.11 Furthermore, complications related to bone collection occur in approximately 20.6% of cases and may include issues such as limited availability, the necessity for additional surgeries, bleeding at the donor site, deformities, scarring, infection, inflammation, chronic pain, and increased costs. Moreover, autografts may not be suitable for larger bone defects.12–14 While inert non-bioactive metal implants have been employed to address large bone defects. However, issues related to the integration and compatibility of these grafts with surrounding tissues and natural bone have hindered their widespread clinical application.15 In contrast, allograft bone exhibits similar properties, offering excellent osteogenic and osteoconductive characteristics without the complications and issues associated with donor sites, thereby serving as an effective alternative to autologous bone. However, allograft bone also has its own limitations, which includes risks of disease transmission, antigenicity, osteochondrosis, limited availability, lack of uniformity, graft resorption, and high costs.16–18 The development of biomaterials and advancements in engineering technology have opened new avenues for modern bone tissue engineering (BTE). Both natural and synthetic biomaterials have been utilized for tissue repair, with various porous structures enhancing cell adhesion, differentiation, and proliferation, thus promoting better integration and improving the physical properties of implants. However, how do common xenografts compare to the ideal bone graft? This review aims to provide a detailed examination of the advantages and limitations of prevalent xenografts, highlight the challenges currently faced in the field of bone grafts, and outline potential directions for future development.
Common Xenograft Bones
Bone is a highly metabolically active, multifunctional, and complex organ characterized by unique regenerative and repair properties. In addition to its weight-bearing and auxiliary functions, bone plays a vital role in various physiological processes, including hematopoiesis, the protection of essential organs (such as the brain and heart), and the storage of minerals and various growth factors.19 Furthermore, bone is a dynamic and highly vascularized tissue with a nanocomposite structure, exhibiting approximately 80–90% porosity and accounting for about 15% of the total body weight.20,21 It comprises a diverse array of cells, including osteoprogenitor cells, osteoblasts, osteoclasts, and osteocytes, along with collagen, hydroxyapatite, and water. The extracellular matrix (ECM) serving as a scaffold for bone deposition, enhances the strength of bone tissue, and accommodates signaling factors critical for bone formation, growth, remodeling, and resorption (Figure 1).22,23 The process of bone repair following a fracture is intricate, necessitating the mobilization of a continuous stream of cells and molecules regulated by both systemic and local factors.24 Although bone tissue possesses the ability to self-repair, it can only regenerate and reshape minor injuries (less than 8 mm).25 When a bone defect surpasses the critical size threshold (approximately greater than 2 cm) or when more than 50% of the bone circumference is compromised, it can lead to inadequate fusion, abnormal fusion, or pathological fractures.26 To address large bone defects, surgical intervention and the use of bone substitutes are essential. The selection of appropriate bone substitutes is a critical step in resolving this challenge. Xenograft bones are emerging as an effective alternative to autologous bone grafts due to their availability, cost-effectiveness, and reduced morbidity at the donor site (Figure 2).27,28 Moreover, xenografts are structurally and morphologically similar to properties to human bone, providing another viable option (Table 1).29 In North America, the proportional use of bone graft materials reveals that allografts account for just over 50% of cases, while autografts constitute approximately 15%, xenografts 22%, synthetic materials 5%, and recombinant human bone morphogenetic protein (BMP)-2 also 5%.30 Bone graft hold significant potential for the healing and regeneration of bone defects. Under the influence of BTE, bone graft materials have been vigorously developed, with an increasing emphasis on biomaterials that fulfill three primary characteristics (Table 2).31–37 In addition to the aforementioned characteristics of replicating bone, the ideal bone replacement material should possess the following attributes:38–40 ① a three-dimensional (3D) structure resembling real bone, with similar porosity and good biocompatibility, is suitable for cell and vascular implantation, while being cost-effective; ② the ability to maintain in vivo mechanical stability and withstand physiological loads at the defect site, be radiopaque, and facilitate the use of non-invasive methods (such as X-ray or micro-computed tomography) for implant monitoring; ③ the capacity to degrade at a controlled rate that aligns with the rate of new bone formation, and be easy to handle and sterilize.
 |
Table 1 Summary of Representative Commercial Xenograft and DBM-Based Bone Graft |
 |
Table 2 Summary of the Characteristics of Common Bone Graft |
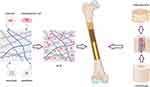 |
Figure 1 Schematic diagram of the anatomy and main components of natural bone. Abbreviation: ECM, extracellular matrix. |
True Bone Ceramics
True bone ceramics (TBC) are organic crystals of bone minerals derived from fresh bovine cancellous bone, which are calcined at high secondary temperatures (Figure 3).41,42 Their crystalline properties closely resemble those of artificial hydroxyapatite and other biomaterials, establishing TBC as a bone substitute material characterized by excellent biocompatibility and biological activity.43 Specifically, TBC is completely deproteinized bone that retains the microskeletal structure of native bone, akin to the micropore structure of cancellous bone, and exhibits bone conduction properties.44,45 Additionally, TBC possesses high porosity, which enhances the surface area, thereby promoting cytokine release to adjacent cells, facilitating the growth of new bone, and accelerating osteoblast proliferation.46,47 The degradation of TBC releases calcium and phosphate ions, which are essential for new bone formation.43 Bovine-derived xenografts are regarded as more biocompatible with human organisms. One study indicated that the use of bovine-derived grafts resulted in dense, mineralized bone encapsulated with bovine graft particles, alongside the presence of capillaries and neoplastic cells colonizing Haversian canals.44,48 Furthermore, Tamaki et al41 reported the findings from a study on TBC, demonstrating that TBC implanted in bone marrow exhibited good biocompatibility with surrounding bone, thus suggesting that TBC, with their natural bone structure, promoted blood vessel growth within the material and created an optimal environment for bone formation. Qiao et al49 prepared calcined xenogeneic bone using a high-temperature calcination method and subsequently co-cultured the extract of this calcined xenogeneic bone with L929 cells in vitro. Cytotoxicity experiments indicated that the cytotoxicity of the calcined xenogeneic bone extract ranged from 0 to 1, while cytocompatibility tests demonstrated that L929 cells adhered well to the surface of the calcined xenogeneic bone and proliferated within its pores. In vivo experiments revealed varying degrees of new bone formation at 4 and 26 weeks. The advantages of TBC include its lack of antigenicity, absence of cytotoxicity, and higher alkaline phosphatase activity in osteoblasts cultured with TBC compared to those cultured with hydroxyapatite (HA) materials.50 However, TBC alone is not an ideal bone substitute due to its poor surface activity, insufficient cell adhesion, and low osteogenic induction capacity.51,52 With the rapid development of BTE, the surface modification of TBC have been implemented to retain its beneficial properties, enhance the biological activity of the TBC surface and improve the adhesion between seed cells and the scaffold. Consequently, the incorporation of bioactive molecules or bone-promoting metal ions, such as BMP-2-related peptide, rhBMP-2 and Sr, and (DSS)6-liposome/Casein kinase 2-interacting protein (CKIP)-1 siRNA, has been shown to possess osteoinductive properties that facilitate more effective bone defect repair (Table 3). 53–63
 |
Table 3 Studies Related to the Modification of TBCs by Adding Bioactive Molecules or Metal Ions |
Decalcified Bone Matrix
The use of decalcified bone as a substitute for treating bone defects can be traced back to Senn,64 while Urist elaborated on the preparation of decalcified bone matrix (DBM) in 1965, highlighting the role of BMP in facilitating bone formation.65 With the rapid advancement in bone graft technologies, DBM is now regarded as a highly processed allogeneic bone derivative, produced through standardized procedures that include acidification to remove at least 40% of the mineral content from the matrix (Figure 3). This matrix primarily consists of collagen (predominantly type I, along with types IV and X, which together account for over 93% of its total composition), proteins (including BMPs), various growth factors such as transforming growth factor-beta (TGF-β), and residual minerals (1–6%).66,67 The surface area and porosity of decalcified bone meal are significantly greater than those of non-decalcified bone meal to enhance its capacity for bone conduction. Following decalcification,68 BMP and vascular endothelial growth factor (VEGF) released from surrounding mineral components synergistically promote osteoinductive potential.69 Additionally, the residual collagen in DBM imparts essential physical and biological properties to the matrix, facilitating a 3D configuration that supports the growth of host capillaries, perivascular tissues, and bone progenitor cells within the graft, thereby enabling bone formation.70 Pan et al71 obtained a composite bone graft with anti-inflammatory, prevascularization and endogenous stem cell homing by subcutaneous implantation of DBM, which was confirmed that it significantly promoted the regeneration of skull defects in rats. Liu et al72 used fresh halibut bone as raw material to make fish DBM (FDBM), and the results showed that FDBM not only had a good repair effect on bone defects, but also had good physicochemical properties, biosecurity, cell adhesion and lower economic cost. Furthermore, Mahyudin et al73 constructed a rabbit femoral defect model and implanted allogeneic lyophilized bovine cortical bone, allogeneic lyophilized New Zealand white rabbit cortical bone, xenogeneic hydroxyapatite bovine bone, and xenogeneic decalcified bone matrix bovine bone. Their results revealed that the decalcified bone matrix group exhibited the most favorable bone healing outcomes. The raw materials of DBM derived from xenogeneic sources are more abundant, which greatly improve the utilization rate of resources and provide a promising biomaterial for the treatment of bone defects.74 A study conducted at the New York Special Surgery Hospital from 2002 to 2004 found that 10% of all used bone substitutes were allogeneic bone, while 82% were DBM products.66 Consequently, DBM has been demonstrated as a viable alternative for bone conduction and osteoinduction. The use of DBM in bone reconstruction presents several advantages:70 ① it is independent of the number of grafts; ② it minimizes complications associated with the acquisition of autologous bone grafts at the donor site; ③ it reduces both surgery and recovery time. DBM is extensively utilized in clinical practice, particularly for rotator cuff repair and anterior cruciate ligament reconstruction, as it promotes tendon-bone healing.75 The efficacy of DBM in cervical and lumbar fusion procedures has been corroborated by multiple studies.76–78 In a level I prospective multicenter randomized clinical trial, Kang et al79 reported on the effectiveness of Grafton DBM™ compared to iliac crest autograft for single-segment posterior lumbar fusion. The final follow-up results indicated a fusion rate of 86% in the DBM™ group and 92% in the autograft group. Commercially available DBM is primarily derived from human allogeneic bone and is available in various forms, including powders, granules, gels, putty, and bars. While there have been no documented cases of infectious disease transmission associated with commercial DBM products, the processing methods can not eliminate the risk of prion contamination.66 Furthermore, the osteoinductive properties of DBM are influenced by exposure time and HCl concentration, with significant variability in osteoinductive potential observed across different donor characteristics.80–82 Despite the development of various DBM products, limitations remain:70,83 ① no DBM product currently satisfies all the ideal conditions for bone grafting materials; ② there are inconsistencies in the osteogenic activity of DBM products processed under different materials, methods, and reagents, even when produced under identical conditions; ③ the required mechanical properties to withstand tension and load continue to pose challenges. In light of these issues, BTE seeks to leverage the characteristics of DBM and enhance its osteogenic activity (Table 4). 84–102
 |
Table 4 Studies of Composite Materials for Repair of Bone Defects Based on DBM |
Freeze-Dried Bone and Demineralized Freeze-Dried Bone
Freeze-dried bone (FDB) is derived from various sources, predominantly of human origin. The processing of FDB encompasses several steps, beginning with donor screening, followed by soft tissue stripping, size reduction, decontamination, antimicrobial treatment, freeze-drying, dehydration, secondary size reduction, and final sterilization (Figure 3).103 This meticulous process primarily aims to significantly reduce the water content in the graft to less than 5%, enabling a storage duration of up to five years at room temperature. Additionally, the removal of water serves to disrupt the lipid envelope, thereby inactivating envelope viruses and further decreasing the risk of disease transmission.104,105 Although the treatment of FDB preserves the structural characteristics of the natural donor bone tissue and minimizes the impacts on osteoconductivity and biocompatibility, it can result in the destruction of osteoblasts and a reduction in the expression of major histocompatibility complex class I antigens in these cells.106,107 Meanwhile, FDB is recognized as an inert, rapidly fixable, and degradable bone graft.108 Its low failure rate for bulk grafts and high implant survival rate, particularly in complex defects with significant bone loss, address the limitations of granular bone grafts, with the solid form of FDB compensating for this shortcoming.109 FDB is extensively utilized in clinical practices such as spine surgery, trauma orthopedics, dentistry, and maxillofacial surgery, yielding promising clinical outcomes.110–113 A comprehensive 10-year review of augmentation rhinoplasty indicated that FDB grafts represented a safe and equivalent alternative.114 The osteoplasticity of FDB was believed to be comparable to that of autologous bone grafts.115–117 Kreuz et al118 demonstrated that FDB and autologous bone integrated similarly in canine models, as evidenced by the qualitative similarity in the extent of new bone formation and subsequent bone incorporation. Novell et al105 reconstructed the atrophied maxilla using FDB and conducted a follow-up over five years, finding that the outcomes were comparable to those achieved with autologous bone. Notably, the degree of FDB resorption was lower than that observed with autologous bone. Iasella et al119 assessed osteogenesis in FDB and reported approximately 65% bone presence after six months, which included 28% vital bone and 37% non-significant graft remnants. Furthermore, clinical evaluations of FDB indicated predictable outcomes comparable to those of autologous bone when guided bone regeneration surgery was performed.120 There was no significant difference in the effects of bone regeneration effect with or without the application of a layer of autologous bone for augmentation, suggesting that FDB can effectively stimulate bone regeneration and may serve as a viable alternative to autologous bone.121 FDB demonstrates superiority over allogeneic materials regarding dimensional stability, new bone formation, and cost-efficiency.122,123 Additionally, specific bone remodeling genes can be upregulated during two-stage maxillary sinus augmentation with FDB, with gene expression results aligned with osteopontin (OPN) immunoreactivity findings. The expression patterns of FDB are similar to those of natural bone, and bone formation-related genes are more highly expressed, indicating its potential clinical superiority over deproteinized bovine bone (DBB).124 Concurrently, in vitro studies have demonstrated that FDB particles possess greater potential than HA/β-tricalcium phosphate (β-TCP) particles in supporting the attachment and proliferation of human dental pulp stem cells (DPSCs), as well as in inducing their alkaline phosphatase (ALP) activity.125 Compared to fresh frozen bone, FDB is easier to maintain and has lower isoimmunogenicity, as well as a reduced risk of infection. However, it is important to note that freeze-drying significantly decreases the Young’s modulus of FDB by 15%, leading to a reduction in its mechanical properties.126 Te Stroet et al127 reported 10-year survival rates for any cause and aseptic loosening of acetabular prostheses at 87% and 97%, respectively, when fresh frozen allografts were used. In contrast, Villatte et al128 reported clinical and radiographic 10-year survival rates of 96.2% and 84.5% for acetabular components using irradiated FDB allografts. Unlike FDB, DFDB is processed with varying concentrations of hydrochloric acid for different durations. Although Heiple et al129 compared the osteogenic properties of various types of bone grafts in dogs, they found that histologically, FDB ranked second only to autologous implants and was superior to frozen, decalcified, frozen irradiated, and fresh deproteinized allografts. The demineralization process of DFDB enhances the proximity and release of various growth factors, including BMP-2, 4 and 7, which facilitate rapid revascularization and hard tissue growth at bone defects.130,131 Consequently, this promotes regeneration, making DFDB more osteoinducible than FDB. However, due to the demineralization process, DFDB is not visible on X-rays, and the ultimate strength of the bone is significantly reduced (by 93%),126 which complicates its application in high-stress limb bone defects.132 FDB can be utilized in specific cases of early bone formation, including immediate implantation and maxillary sinus lift surgery, to achieve functional rehabilitation.131 Consequently, there are notable differences between FDB and DFDB (Table 5). Histological analysis by Wood et al133 demonstrated that, following the implantation of DFDB in humans, after 19 weeks, there was significantly greater bone formation and reduced graft material compared to FDB. In vitro studies examining the osteoinductiveness of both FDB and DFDB revealed that both possessed osteoinducibility and could promote the osteogenic differentiation of osteoblast-like cells (Saos-2 and MG-63), with the DFDB group exhibiting a superior capacity for osteogenic differentiation, characterized by a calcium/phosphorus ratio approaching that of native bone (1.67).134 However, certain studies comparing treatments for chronic periodontitis noted that, through the evaluation of clinical and imaging parameters preoperatively, as well as at three and six months postoperatively, DFDB could not demonstrate any improvement in the clinical and imaging parameters of intraosseous defects relative to FDB.135 A study investigating surgical and clinical complications following maxillary sinus floor lift and dental implant survival, comparing DFDB to bovine-derived xenografts, concluded that extensive rehabilitation of the atrophic maxilla using DFDB was a reliable treatment option, with a success rate comparable to that of xenografts for maxillary sinus augmentation.136 The rate of bone formation in DFDB varies significantly based on factors such as the age of the donor, medical condition, preparation protocol and sterilization procedure.137,138 It is also important to recognize that the amount of growth factor released by DFDB is just one of several factors influencing the success of the graft, alongside the accuracy of the procedure, cleanliness and the condition of the recipient.139
 |
Table 5 Differences Between DFDB and FDB |
Others
Bioactive Glasses
Bioactive glasses (BGs) are a category of synthetic, silicate-based ceramics originally composed of various inorganic compounds. These compounds were subsequently modified to form more stable materials through the incorporation of potassium oxide (K2O), magnesium oxide (MgO), and boron oxide (B2O3), with silicate constituting 45–52% of the total weight (Figure 3).140 BGs are preferred for bone regeneration due to their effective bone conduction and osteoinduction properties.141,142 They rapidly form a HA layer via ion dissolution, facilitating the binding of proteins, collagen, fibrin and growth factors. This layer is crucial for promoting the migration and adhesion of bone-forming cells, thereby aiding the bone remodeling process.143 Over time, during long-term implantation, this HA layer is partially replaced by bone through the creep replacement process.144 Additionally, the release of ions from BGs interacts with surrounding cells, enhancing their affinity for bone,145 and contributing to the expression of bone markers such as ALP, collagen type 1, and osteocalcin. These makers increase osteoinductive properties and promote bone healing.146 The biological activity and absorptive capacity of BGs vary according to their chemical composition, and in vivo studies indicate that they promote new bone growth on their surfaces, demonstrating a balance between intramedullary bone formation and material resorption, thus exhibiting effective bone conduction.147 BGs exhibit remarkable biocompatibility, demonstrating minimal inflammatory response, foreign body reaction, or fibrous encapsulation148 when implanted in human or animal models. Moreover, BGs have been shown to upregulate essential genes for new bone formation, such as insulin-like growth factor (IGF-II) and VEGF, which facilitate osteoblast proliferation.149 However, similar to other ceramics, BGs possess brittle ness, slow absorption rates, a theoretical risk of fracture, and limited clinical data regarding their application in trauma orthopedics.150 Additionally, local pH changes may induce cytotoxicity in vitro, although no significant clinical reports have documented this concern.151 When combined with growth factors (GF), BGs can be utilized for the reconstruction of facial defects and can also serve as carriers for drugs and biologics.152,153 To address various clinical needs, BGs have been developed with antibacterial properties against microorganisms.154 Additionally, they promote osteogenesis and angiogenesis by incorporating various of functional elements such as strontium and zinc.155 In a prospective comparative study of periodontal defects treated with autografts and BGs, Sumer et al 156 concluded that both grafts led to significant improvements in clinical and radiographic parameters at six months post-surgery, although these outcomes could be influenced by the morphology or location of the bone defects. Katuri et al 157 compared BGs with DFDB as treatments for periodontal defects and found significant differences after twelve months. Specifically, sites treated with DFDB exhibited greater reductions in periodontal probing depth (PPD), increased clinical attachment levels and a higher percentage of bone filling compared to those treated with BGs. Given their osteoconductive and osteoinductive properties, as well as promising in vitro and in vivo results, BGs continue to be a focal point of research as composite materials for bone substitutes.158
Bone Marrow Aspirate Concentrate
Bone marrow aspirate concentrate (BMAC) is a cellular graft characterized by its osteogenic and osteoinductive properties. It is composed of pluripotent stem cells, including mesenchymal stem cells, hematopoietic stem cells, and endothelial progenitor cells, as well as heterogeneous aggregates of various monocyte types, such as macrophages, lymphocytes, mast cells, and other cells. Additionally, BMAC contains cytokines and GFs,159–161 with CD11b+ macrophages constituting approximately 70% of the total number of cell population, T cells accounting for 15%, and it also includes 2–5 colony-forming units (CFUs)/106 cells.162 BMAC is primarily harvested from the posterior region of the iliac bone, with a maximum volume of up to 150 mL obtainable.163 Muschler et al164 noted that 85% of the pluripotent stem cells are found in the initial 4 mL of BMAC; however, significant variability exists in the stem cell counts among different patients. This diverse cell mixture from BMAC contributes to the establishment of a stable microenvironment conducive to osteogenesis, with each cell type potentially playing a distinct role in tissue regeneration. Some studies indicated that a mixed population of bone marrow-derived cells could demonstrate superior potential for bone regeneration compared to populations enriched with specific cell types.165 BMAC serves as a rich source of GFs, including TGF-β, platelet-derived growth factor (PDGF), BMP-2, BMP-7, and fibroblast growth factor-2 (FGF-2), which are believed to exhibit anti-inflammatory, angiogenic, trophic, and immunomodulatory properties,166,167 potentially facilitating tissue repair through paracrine and autocrine mechanisms.168 Clusters of monocytes derived from fresh bone marrow are equipped with angiogenic factors supplied by CD34+ endothelial precursor cells and CD34− cells, which may assist not only in revascularization but also in the differentiation of osteoblasts and endothelial progenitor cells into bone and endothelial cells, ultimately promoting angiogenesis and supporting bone regeneration.169 Du et al170 compared the efficacy of concentrated fresh bone marrow mononuclear cells and cultured bone marrow mesenchymal stem cells (BMSCs) in Beagle dogs. They found that the fresh group promoted bone regeneration more effectively than the cultured group. Specifically, the grafts in the fresh group exhibited superior mineralization and demonstrated collagen arrangement and biomechanical properties akin to those of the natural tibia. This suggests that concentrated fresh bone marrow mononuclear cells may be more effective than in vitro-expanded stem cells in repairing segmental bone defects. In a comparative study of BMAC versus platelet-rich fibrin (PRF), Koyanagi et al171 reported that BMAC clots were uniformly distributed and contained a higher density of hematoxylin-stained cells, including leukocytes, adipocytes, and bone marrow-derived stem cells, indicating greater bone regeneration potential. BMAC was found to release higher GF than PRF (whether arterial or venous-derived) and exhibited enhanced capabilities for cell migration, angiogenesis, collagen synthesis, and osteoblast differentiation compared to the control PRF group. This positions BMAC as a promising option for promoting wound healing and bone regeneration. Lim et al172 utilized BMAC alongside an autologous bone graft to address a 14 mm segmental defect in a rabbit ulna model. They concluded that both treatment strategies yielded comparable results, suggesting that BMAC could serve as an alternative to autologous bone therapy for long bone healing. The potential advantages of BMAC include its straightforward harvesting technique, the absence of risk for allogeneic disease transmission,173 and its applicability in treating cartilage lesions, bone defects, tendon injuries, and maxillofacial diseases.174–177 However, its poor mechanical properties limit its suitability for load-bearing applications. The combination of BMAC with osteoconductive biomaterials to create composite grafts possessing osteoinductive, osteoconductive, and osteogenic properties can stimulate bone formation, thereby introducing new biological functionalities.178 Consequently, the efficacy of BMAC is significantly enhanced when utilized in conjunction with autologous bone graft, PRF and other scaffolding materials.176 Kanakaraj et al179 demonstrated adequate bone formation was found in the mandible using a combination of BMAC and autogenous cortical cancellous bone for treating odontogenic keratocysts. Furthermore, Saad et al180 developed a rabbit model featuring a 10×15 mm bone defect in the mandibular region, filled the defect site with bone marrow-derived undifferentiated mesenchymal stem cells (BM-MSCs)/β-TCP, as well as BM-MSCs without scaffolds. Their findings indicated that the combination of BM-MSCs with the β-TCP scaffold exhibited superior and accelerated bone regeneration potential. Overall, the integration of BMAC with graft materials can possess bone conduction properties and maximize its benefits demonstrating significant promise for bone regeneration.
Polymer Nanocomposites
Inadequate integration with host tissues, inflammatory responses, and infections may limit the performance of bone implants. The surface characteristics of these bone biomaterials significantly influence the biological activity of immune cells and osteoblasts.181 With the rapid development of BTE and modern nanotechnology, nanocomposites have gradually gained prominence. These materials combine polymer and biodegradable biomatrix structures with biologically active and easily absorbable nanofillers (Figure 3).182 The objective is to endow biomaterials with critical physical and chemical properties, such as increased surface area, enhanced mechanical strength and stability, and improved cell adhesion, proliferation, and differentiation (Table 6).183–194 Furthermore, various characterization tests (eg, electron microscopy, spectroscopy and mechanical stress testing) evaluate osteoblast adhesion, viability, and mineralization, thereby creating nanostructured surfaces that can influence osteogenesis and immune cell activity while adjusting physicochemical properties.195,196 Polymers are favored for their capacity to rapidly absorb and stimulate autologous bone repair in vivo; Thus, composites combined with nanofillers may facilitate bone tissue repair.197,198 The application of bone tissue regeneration necessitates specific modifications to the polymer structure to fabricate composites that are flexible, rigid, and bioactive.199,200 Common polymers utilized in BTE include polycaprolactone (PCL), polylactic acid (PLA), and poly (lactic-co-glycolic) acid (PLGA). PCL is an aliphatic and semi-crystalline polymer known for its excellent toughness, adjustable mechanical properties, high crystallinity, non-toxicity, and adequate biocompatibility.201 However, it is limited by its slow degradation rate.202 Karimipour-Fard et al203 prepared PCL/Nano-HA (nHA) /Chitin-Nano-Whisker (CNW) nanocomposites using PCL/nHA and PCL/CNW as raw materials. They found that the inclusion of nHA and CNW nanofillers enhanced the biodegradation rate of PCL, and resulting nanocomposites significantly improved the biological and mechanical properties of 3D printed bone tissue scaffold. PLA, derived from the polyesterification of lactic acid, exhibits essential properties for bone regeneration, including non-toxicity, biocompatibility, thermal stability, and biodegradability. However, it lacks the mechanical strength required for effective bone tissue regeneration systems,204 which can be addressed through the incorporation of various nanofillers create nanocomposite fibers.205,206 Canales et al207 developed a PLA-based composite nanomaterial utilizing bioglass (n-BG) and zinc oxide (n-ZnO) as fillers, demonstrating that this material possesses bioactive and bactericidal properties suitable for BTE applications. PLGA is a linear copolymer composed of PLA and Poly (Glycolic Acid) (PGA), characterized by its biocompatibility,208 biodegradability, controllable degradation rate, and ease of processing. However, PLGA is constrained by its inadequate mechanical properties, limited osteoinduction, and poor cell adhesion. Li et al209 prepared MgO2/PLGA nanocomposite scaffolds with good mechanical properties and activity by low-temperature 3D printing, and the results showed that this material was proved to promote bone repair by enhancing the differentiation of BMSCs to osteoblasts and the formation of a pro-osteoporotic immune microenvironment through macrophage M2. The unique effects arising from the interaction between polymers and organic or inorganic nanomaterials suggest that the functionalization of polymer nanocomposites presents a significant advantage,210 with substantial potential in applications such as bone tissue engineering, drug delivery, biosensors wound healing, and magnetic hyperthermia. This advancement could significantly transform the landscape of nanomedicine, particularly for individuals suffering from bone diseases today.211,212
 |
Table 6 Summary of Representative Studies, Advantages and Limitations of Different Types of Nanocomposites for Bone Regeneration |
Challenges and Prospects
The scale of xenograft bone research has steadily increased over the decade from 2013 to 2023.213 While the desirable properties of bone graft have been extensively documented for decades, no biomaterials currently available on the market encompass all of these properties. Presently, there appears to be a trend towards simulating the natural bone structure as closely as possible, often incorporating one or two active ingredients that promote bone repair and regeneration. This approach has led to the development of new materials for bone defect repair. Although it is encouraging to see the emergence of numerous reparative materials in tissue engineering, it is crucial to remember the ultimate goal of these new bone repair materials, namely the application in clinical practice to effectively address patients’ bone defects. The apparent disconnect between research teams and clinical practitioners seems to contribute to this issue. Strengthening communication and collaboration among researchers from various disciplines is a fundamental and essential step towards improving the ideal bone graft. From the perspective of tissue engineering researchers, the ideal bone graft should not only focus on the aforementioned characteristics but also emphasize extending the retention period, enhancing vascularization, and eliminating size limitations, while paying closer attention to the intricate details of the graft material itself. Conversely, clinicians prioritize the effectiveness of bone defect repair and economic viability, ensuring safety as a prerequisite. Only by integrating the perspectives of both researchers and clinicians can we advance more effectively and consistently towards the development of ideal bone graft. As advancements in characterization methods continue to reveal new insights into the structural arrangement and crystalline phases of bone, an additional challenge arises from the potential overemphasis on the surface microstructure of active tissues like bone. Currently, regardless of the source of bone tissue repair materials, most approaches have exhausted various methods to enhance synthesis, primarily aiming to better replicate the microstructure of natural bone. This includes aspects such as mechanical properties, interconnected voids, surface structure, and pore morphology. However, these efforts often overlook the critical role of the bone microenvironment as a metabolic tissue essential for survival, as well as the integration with surrounding tissues and vascular nerves. Fortunately, an increasing number of researchers are now focusing on promoting bone repair and regeneration within the extracellular matrix, highlighting the significance of the bone microenvironment. The application of 3D printing rapid prototyping technology to create complex scaffold materials that mimic the properties of natural bone, along with utilizing these scaffolds as carriers for load various cytokines and active substances with osteogenic potential, may offer promising solutions to overcome the limitations in treating bone-related diseases in the future. Another challenge lies in the fact that current in vivo experiments on bone graft are predominantly conducted in small animal models, with a notable lack of high-quality animal studies and randomized controlled trials to validate the feasibility of the target materials. While xenografts still face certain limitations, including immune rejection, biocompatibility issues, risk of infectious diseases, poor functional recovery, and uncertainty regarding long-term effects, they nonetheless present a viable treatment option for patients and an avenue for clinical practice. Thanks to the rapid advancements in tissue engineering technology, the future xenotransplantation may increasingly emphasize immunomodulatory techniques, biomaterials engineering, stem cell and gene therapy, personalized therapy, bioprinting technology and regenerative medicine, thereby enhancing the development of xenotransplantation in a safer, more effective, and personalized manner.
Conclusion
A brief procedure for the preparation of bone grafts is summarized (Figure 3). The selection of bone graft is crucial for effective bone repair and regeneration, as each option presents distinct advantages and disadvantages (Table 7).43,52,66,70,105,107,110–112,126,131,145,150,152,159,178,182,214–217 In summary, the decision regarding bone graft is influenced by specific clinical requirements, including the specific characteristics of the bone defect, the patient’s overall health, and the expected treatment outcome. A thorough evaluation of the advantages and disadvantages of each material is essential to optimize patient outcomes. A comprehensive understanding of these factors can enhance the efficacy of surgical interventions related to bone repair and regeneration.
 |
Table 7 Advantages, Disadvantages and Potential Clinical Applications of the Materials Described in the Review |
Data Sharing Statement
The data are available from the corresponding author Gang Xu on reasonable request.
Funding
This research was funded by National Natural Science Foundation of China (82204820).
Disclosure
The authors do not have any conflict on interest in this paper.
References
1. Centers for Disease Control and Prevention. Number of All-Listed Procedures for Discharges From Short-Stay Hospitals, by Procedure Category and Age: United States. 2010.
2. Wang C, Yue H, Huang W, et al. Cryogenic 3D printing of heterogeneous scaffolds with gradient mechanical strengths and spatial delivery of osteogenic peptide/TGF-β1 for osteochondral tissue regeneration. Biofabrication. 2020;12(2):025030. doi:10.1088/1758-5090/ab7ab5
3. Haugen HJ, Lyngstadaas SP, Rossi F, Perale G. Bone grafts: which is the ideal biomaterial? J Clin Periodontol. 2019;46(21):92–102. doi:10.1111/jcpe.13058
4. Volgas DA, Stannard JP, Alonso JE. Nonunions of the humerus. Clin Orthop Relat Res. 2004;(419):46–50. doi:10.1097/00003086-200402000-00008
5. Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev. 2015;94:53–62. doi:10.1016/j.addr.2015.03.013
6. Grandviewresearch. Orthopedic Implants Market Analysis, by Application (Spinal Fusion, Long Bone, Foot & Ankle, Craniomaxillofacial, Joint Replacement, Dental), and Segment Forecasts to 2024, 2016. https://www.grandviewresearch.com/industry-analysis/orthopedic-implants-market.
7. Hasan A, Byambaa B, Morshed M, et al. Advances in osteobiologic materials for bone substitutes. J Tissue Eng Regen M. 2018;12(6):1448–1468. doi:10.1002/term.2677
8. Deev RV, Drobyshev AY, Bozo IY, Isaev AA. Ordinary and Activated Bone Grafts: applied Classification and the Main Features. Biomed Res Int. 2015;2015:365050. doi:10.1155/2015/365050
9. Sivakumar PM, Yetisgin AA, Sahin SB, Demir E, Cetinel S. Bone tissue engineering: anionic polysaccharides as promising scaffolds. Carbohydr Polym. 2022;283:119142. doi:10.1016/j.carbpol.2022.119142
10. Roddy E, DeBaun MR, Daoud-Gray A, Yang YP, Gardner MJ. Treatment of critical-sized bone defects: clinical and tissue engineering perspectives. Eur J Orthop Surg Traumatol. 2018;28(3):351–362. doi:10.1007/s00590-017-2063-0
11. Guo L, Liang Z, Yang L, et al. The role of natural polymers in bone tissue engineering. J Control Release. 2021;338:571–582. doi:10.1016/j.jconrel.2021.08.055
12. Allison DC, McIntyre JA, Ferro A, Brien E, Menendez LR. Bone grafting alternatives for cavitary defects in children. Current Orthopaedic Practice. 2013;24(3). doi:10.1097/BCO.0b013e3182910f94
13. Sohn HS, Oh JK. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater Res. 2019;23(1):9. doi:10.1186/s40824-019-0157-y
14. Kashirina A, Yao Y, Liu Y, Leng J. Biopolymers as bone substitutes: a review. Biomater Sci. 2019;7(10):3961–3983. doi:10.1039/c9bm00664h
15. Fan L, Chen S, Yang M, Liu Y, Liu J. Metallic Materials for Bone Repair. Adv Healthc Mater. 2024;13(3):e2302132. doi:10.1002/adhm.202302132
16. Myeroff C, Archdeacon M. Autogenous bone graft: donor sites and techniques. J Bone Joint Surg Am. 2011;93(23):2227–2236. doi:10.2106/JBJS.J.01513
17. Gleeson JP, Plunkett NA, O’Brien FJ. Addition of hydroxyapatite improves stiffness, interconnectivity and osteogenic potential of a highly porous collagen-based scaffold for bone tissue regeneration. Eur Cell Mater. 2010;20:218–230. doi:10.22203/ecm.v020a18
18. Allison DC, McIntyre JA, Ferro A, Brien E, Menendez LR. Bone grafting alternatives for cavitary defects in children. Current Orthopaedic Practice. 2013;24(3):267–279. doi:10.1097/BCO.0b013e3182910f94
19. Filippi M, Born G, Chaaban M, Scherberich A. Natural Polymeric Scaffolds in Bone Regeneration. Front Bioeng Biotechnol. 2020;8:474. doi:10.3389/fbioe.2020.00474
20. Presbítero G, Gutiérrez D, Lemus-Martínez WR, Vilchez JF, García P, Arizmendi-Morquecho A. Assessment of Quality in Osteoporotic Human Trabecular Bone and Its Relationship to Mechanical Properties. Appl Sci. 2021;11(12):5479. doi:10.3390/app11125479
21. Schindeler A, McDonald MM, Bokko P, Little DG. Bone remodeling during fracture repair: the cellular picture. Semin Cell Dev Biol. 2008;19(5):459–466. doi:10.1016/j.semcdb.2008.07.004
22. Pei B, Wang W, Dunne N, Li X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: superiority, Concerns, Current Advancements, and Prospects. Nanomaterials. 2019;9(10):1501. doi:10.3390/nano9101501
23. Bi Y, Stuelten CH, Kilts T, et al. Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem. 2005;280(34):30481–30489. doi:10.1074/jbc.M500573200
24. Arvidson K, Abdallah BM, Applegate LA, et al. Bone regeneration and stem cells. J Cell mol Med. 2011;15(4):718–746. doi:10.1111/j.1582-4934.2010.01224.x
25. Fratzl P, Weinkamer R. Hierarchical structure and repair of bone: deformation, remodelling, healing. In: van der Zwaag S editor. Self Healing Materials: An Alternative Approach to 20 Centuries of Materials Science. Springer Netherlands; 2007:323–335. doi:10.1007/978-1-4020-6250-6_15.
26. Bezstarosti H, Metsemakers WJ, Van Lieshout EMM, et al. Management of critical-sized bone defects in the treatment of fracture-related infection: a systematic review and pooled analysis. Arch Orthop Trauma Surg. 2021;141(7):1215–1230. doi:10.1007/s00402-020-03525-0
27. Canullo L, Del Fabbro M, Khijmatgar S, et al. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: a systematic review and network meta-analysis. Clin Oral Investig. 2022;26(1):141–158. doi:10.1007/s00784-021-04248-1
28. Costa KLD, Abreu LF, Tolomei CB, et al. Use of Local Melatonin with Xenogeneic Bone Graft to Treat Critical-Size Bone Defects in Rats with Osteoporosis: a Randomized Study. J Funct Biomater. 2024;15(5):124. doi:10.3390/jfb15050124
29. Long B, Dan L, Jian L, Yunyu H, Shu H, Zhi Y. Evaluation of a novel reconstituted bone xenograft using processed bovine cancellous bone in combination with purified bovine bone morphogenetic protein. Ann Ny Acad Sci. 2012;19(2):122–132. doi:10.1111/j.1399-3089.2012.00694.x
30. Miron RJ. Optimized bone grafting. Periodontol. 2000;12517. doi:10.1111/prd.12517
31. Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114–124. doi:10.4161/org.23306
32. Polo-Corrales L, Latorre-Esteves M, Ramirez-Vick JE. Scaffold design for bone regeneration. J Nanosci Nanotechnol. 2014;14(1):15–56. doi:10.1166/jnn.2014.9127
33. Inchingolo F, Hazballa D, Inchingolo AD, et al. Innovative Concepts and Recent Breakthrough for Engineered Graft and Constructs for Bone Regeneration: a Literature Systematic Review. Materials. 2022;15(3):1120. doi:10.3390/ma15031120
34. Khan SN, Cammisa FP, Sandhu HS, Diwan AD, Girardi FP, Lane JM. The biology of bone grafting. J Am Acad Orthop Surg. 2005;13(1):77–86.
35. Greenwald AS, Boden SD, Goldberg VM.
36. Shepard NA, Rush AJ, Scarborough NL, Carter AJ, Phillips FM. Demineralized Bone Matrix in Spine Surgery: a Review of Current Applications and Future Trends. Int J Spine Surg. 2021;15(s1):113–119. doi:10.14444/8059
37. Baldwin P, Li DJ, Auston DA, Mir HS, Yoon RS, Kova KJ. Autograft, Allograft, and Bone Graft Substitutes: clinical Evidence and Indications for Use in the Setting of Orthopaedic Trauma Surgery. J Orthop Trauma. 2019;33(4):203–213. doi:10.1097/BOT.0000000000001420
38. Bow A, Anderson DE, Dhar M. Commercially available bone graft substitutes: the impact of origin and processing on graft functionality. Drug Metab Rev. 2019;51(4):533–544. doi:10.1080/03602532.2019.1671860
39. de Grado FG, Keller L, Idoux-Gillet Y, et al. Bone substitutes: a review of their characteristics, clinical use, and perspectives for large bone defects management. J Tissue Eng. 2018;9:2041731418776819. doi:10.1177/2041731418776819
40. Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4(8):743–765. doi:10.1002/mabi.200400026
41. Tamaki T, Sakurai K, Kasamatsu N. Results of basic and clinical studies of true bone ceramics. In: Urist MR, O’Connor BT, Burwell RG, editors. Bone Grafts: Derivatives and Substitutes. Oxford: Butterworth-Heinemann; 1994:235–244.
42. Herliansyah MK, Hamdi M, Ide-Ektessabi A, Wildan MW, Toque JA. The influence of sintering temperature on the properties of compacted bovine hydroxyapatite. Mater Sci Eng C. 2009;29(5):1674–1680. doi:10.1016/j.msec.2009.01.007
43. Xu G, Guo R, Han L, et al. Comparison of osteogenesis of bovine bone xenografts between true bone ceramics and decalcified bone matrix. J Mater Sci Mater Med. 2022;33(10):75. doi:10.1007/s10856-022-06696-x
44. Orsini G, Traini T, Scarano A, et al. Maxillary sinus augmentation with Bio-Oss particles: a light, scanning, and transmission electron microscopy study in man. J Biomed Mater Res B Appl Biomater. 2005;74(1):448–457. doi:10.1002/jbm.b.30196
45. Gasperini FM, Fernandes GVO, Mitri FF, et al. Histomorphometric evaluation, SEM, and synchrotron analysis of the biological response of biodegradable and ceramic hydroxyapatite-based grafts: from the synthesis to the bed application. Biomed Mater. 2023;18(6):065023. doi:10.1088/1748-605X/ad0397
46. Ueno Y, Shima Y, Ueyoshi A, et al. Experimental studies of sintered bone implantation. Bessatsu Seikeigeka. 1985;8:85–88.
47. Song J, Kim J, Woo HM, et al. Repair of rabbit radial bone defects using bone morphogenetic protein-2 combined with 3D porous silk fibroin/β-tricalcium phosphate hybrid scaffolds. J Biomater Sci Polym Ed. 2018;29(6):716–729. doi:10.1080/09205063.2018.1438126
48. Tapety FI, Amizuka N, Uoshima K, Nomura S, Maeda T. A histological evaluation of the involvement of Bio-Oss in osteoblastic differentiation and matrix synthesis. Clin Oral Implants Res. 2004;15(3):315–324. doi:10.1111/j.1600-0501.2004.01012.x
49. Qiao W, Ren X, Shi H, et al. Biocompatibility research of true bone ceramics. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2017;31(10):1250–1255. doi:10.7507/1002-1892.201705001
50. Matsumoto T, Kawakami M, Kuribayashi K, Takenaka T, Minamide A, Tamaki T. Effects of sintered bovine bone on cell proliferation, collagen synthesis, and osteoblastic expression in MC3T3-E1 osteoblast-like cells. J Orthop Res. 1999;17(4):586–592. doi:10.1002/jor.1100170419
51. Li J, Lin Z, Zheng Q, et al. Repair of rabbit radial bone defects using true bone ceramics combined with BMP-2-related peptide and type I collagen. Mater Sci Eng C. 2010;30(8):1272–1279. doi:10.1016/j.msec.2010.07.011
52. Li J, Zheng Q, Guo X, et al. Bone induction by surface-double-modified true bone ceramics in vitro and in vivo. Biomed Mater. 2013;8(3):035005. doi:10.1088/1748-6041/8/3/035005
53. Minamide A, Kawakami M, Hashizume H, Sakata R, Yoshida M, Tamaki T. Experimental study of carriers of bone morphogenetic protein used for spinal fusion. J Orthop Sci. 2004;9(2):142–151. doi:10.1007/s00776-003-0749-0
54. Zhang W, Li G, Deng R, Deng L, Qiu S. New bone formation in a true bone ceramic scaffold loaded with desferrioxamine in the treatment of segmental bone defect: a preliminary study. J Orthop Sci. 2012;17(3):289–298. doi:10.1007/s00776-012-0206-z
55. Lianfu Deng LD, Huarong Shao HS, Jin Shao JS, et al. OIC-A006-loaded true bone ceramic heals rabbit critical-sized segmental radial defect. Pharmazie. 2012;(3):247–252. doi:10.1691/ph.2012.1072
56. Li J, Yang L, Guo X, et al. Osteogenesis effects of strontium-substituted hydroxyapatite coatings on true bone ceramic surfaces in vitro and in vivo. Biomed Mater. 2017;13(1):015018. doi:10.1088/1748-605X/aa89af
57. Yang L, Huang J, Yang S, et al. Bone Regeneration Induced by Local Delivery of a Modified PTH-Derived Peptide from Nanohydroxyapatite/Chitosan Coated True Bone Ceramics. ACS Biomater Sci Eng. 2018;4(9):3246–3258. doi:10.1021/acsbiomaterials.7b00780
58. Cui W, Liu Q, Yang L, et al. Sustained Delivery of BMP-2-Related Peptide from the True Bone Ceramics/Hollow Mesoporous Silica Nanoparticles Scaffold for Bone Tissue Regeneration. ACS Biomater Sci Eng. 2018;4(1):211–221. doi:10.1021/acsbiomaterials.7b00506
59. Sun T, Yao S, Liu M, et al. Composite Scaffolds of Mineralized Natural Extracellular Matrix on True Bone Ceramic Induce Bone Regeneration Through Smad1/5/8 and ERK1/2 Pathways. Tissue Eng Part A. 2018;24(5–6):502–515. doi:10.1089/ten.TEA.2017.0179
60. Zhang C, Xu G, Han L, Hu X, Zhao Y, Li Z. Bone induction and defect repair by true bone ceramics incorporated with rhBMP-2 and Sr. J Mater Sci Mater Med. 2021;32(9):107. doi:10.1007/s10856-021-06587-7
61. Xu G, Hu X, Han L, Zhao Y, Li Z. The construction of a novel xenograft bovine bone scaffold, (DSS)6-liposome/CKIP-1 siRNA/calcine bone and its osteogenesis evaluation on skull defect in rats. J Orthop Transl. 2021;28:74–82. doi:10.1016/j.jot.2021.02.001
62. Hu Y, Wang Y, Feng Q, et al. Zn–Sr-sintered true bone ceramics enhance bone repair and regeneration. Biomater Sci. 2023;11(10):3486–3501. doi:10.1039/D3BM00030C
63. Jiang Y, Li T, Lou Y, et al. True-bone-ceramics / type I collagen scaffolds for repairing osteochondral defect. J Mater Sci Mater Med. 2024;36(1):1. doi:10.1007/s10856-024-06852-5
64. Senn N. On the healing of aseptic bone cavities by implantation of antiseptic decalcified bone. Am J Med Sci. 1889;98:219–243.
65. Urist MR. Bone: formation by autoinduction. Science. 1965;150(3698):893–899. doi:10.1126/science.150.3698.893
66. Brydone AS, Meek D, Maclaine S. Research of repairing rabbit knee joint cartilage defect by compound material of fibrin glue and decalcified bone matrix (DBM) and chondrocytes. China J Orthop Traumatol. 2010;224(12):1329–1343. doi:10.1016/j.addr.2012.06.008
67. Aly LAA, Hammouda NI. Evaluation of implant stability simultaneously placed with sinus lift augmented with putty versus powder form of demineralized bone matrix in atrophied posterior maxilla. Future Dent J. 2017;3(1):28–34. doi:10.1016/j.fdj.2016.12.001
68. Chen B, Lin H, Zhao Y, et al. Activation of demineralized bone matrix by genetically engineered human bone morphogenetic protein-2 with a collagen binding domain derived from von Willebrand factor propolypeptide. J Biomed Mater Res A. 2007;80(2):428–434. doi:10.1002/jbm.a.30900
69. Nauth A, Lane J, Watson JT, Giannoudis P. Bone Graft Substitution and Augmentation. J Orthop Trauma. 2015;29(Supplement 12):S34–S38. doi:10.1097/BOT.0000000000000464
70. Zhang H, Yang L, Yang X, et al. Demineralized Bone Matrix Carriers and their Clinical Applications: an Overview. Orthop Surg. 2019;11(5):725–737. doi:10.1111/os.12509
71. Pan Q, Zhang P, Xue F, et al. Subcutaneously Engineered Decalcified Bone Matrix Xenografts Promote Bone Repair by Regulating the Immune Microenvironment, Prevascularization, and Stem Cell Homing. ACS Biomater Sci Eng. 2024;10(1):515–524. doi:10.1021/acsbiomaterials.3c01331
72. Liu Z, Jiang X, Wang K, et al. Preparation of fish decalcified bone matrix and its bone repair effect in rats. Front Bioeng Biotechnol. 2023;11:1134992. doi:10.3389/fbioe.2023.1134992
73. Mahyudin F, Utomo DN, Suroto H, Martanto TW, Edward M, Gaol IL. Comparative Effectiveness of Bone Grafting Using Xenograft Freeze-Dried Cortical Bovine, Allograft Freeze-Dried Cortical New Zealand White Rabbit, Xenograft Hydroxyapatite Bovine, and Xenograft Demineralized Bone Matrix Bovine in Bone Defect of Femoral Diaphysis of White Rabbit: experimental Study In Vivo. Int J Biomater. 2017;2017:1–9. doi:10.1155/2017/7571523
74. Wu T, Han L, Zhu Y, et al. Application of decalcified bone matrix in Salmon bone for tibial defect repair in rat model. Int J Artif Organs. 2024;47(10):783–792. doi:10.1177/03913988241269498
75. Hexter AT, Pendegrass C, Haddad F, Blunn G. Demineralized Bone Matrix to Augment Tendon-Bone Healing: a Systematic Review. Orthop J Sports Med. 2017;5(10):232596711773451. doi:10.1177/2325967117734517
76. Fu TS, Wang IC, Lu ML, Hsieh MK, Chen LH, Chen WJ. The fusion rate of demineralized bone matrix compared with autogenous iliac bone graft for long multi-segment posterolateral spinal fusion. Bmc Musculoskel Dis. 2016;17(1):3. doi:10.1186/s12891-015-0861-2
77. Baumann F, Krutsch W, Pfeifer C, Neumann C, Nerlich M, Loibl M. Posterolateral fusion in acute traumatic thoracolumbar fractures: a comparison of demineralized bone matrix and autologous bone graft. Acta Chir Orthop Traumatol Cech. 2015;82(2):119–125.
78. Han S, Park B, Lim JW, et al. Comparison of Fusion Rate between Demineralized Bone Matrix versus Autograft in Lumbar Fusion: meta-Analysis. J Korean Neurosurg S. 2020;63(6):673–680. doi:10.3340/jkns.2019.0185
79. Kang J, An H, Hilibrand A, Yoon ST, Kavanagh E, Boden S. Grafton and local bone have comparable outcomes to iliac crest bone in instrumented single-level lumbar fusions. Spine. 2012;37(12):1083–1091. doi:10.1097/BRS.0b013e31823ed817
80. Aaboe M, Pinholt EM, Schou S, Hjørting‐hansen E. Incomplete bone regeneration of rabbit calvarial defects using different membranes. Clin Oral Implants Res. 1998;9(5):313–320. doi:10.1034/j.1600-0501.1998.090504.x
81. Pietrzak WS, Ali SN, Chitturi D, Jacob M, Woodell-May JE. BMP depletion occurs during prolonged acid demineralization of bone: characterization and implications for graft preparation. Cell Tissue Bank. 2011;12(2):81–88. doi:10.1007/s10561-009-9168-6
82. Figueiredo M, Cunha S, Martins G, Freitas J, Judas F, Figueiredo H. Influence of hydrochloric acid concentration on the demineralization of cortical bone. Chem Eng Res Des. 2011;89(1):116–124. doi:10.1016/j.cherd.2010.04.013
83. Cho H, Bucciarelli A, Kim W, et al. Natural Sources and Applications of Demineralized Bone Matrix in the Field of Bone and Cartilage Tissue Engineering. Adv Exp Med Biol. 2020;1249:3–14. doi:10.1007/978-981-15-3258-0_1
84. Kirk JF, Ritter G, Waters C, Narisawa S, Millán JL, Talton JD. Osteoconductivity and osteoinductivity of NanoFUSE(®) DBM. Cell Tissue Bank. 2013;14(1):33–44. doi:10.1007/s10561-012-9297-1
85. Chen Y, Bai B, Zhang S, et al. Study of a novel three-dimensional scaffold to repair bone defect in rabbit. J Biomed Mater Res A. 2014;102(5):1294–1304. doi:10.1002/jbm.a.34788
86. Wang ZX, Chen C, Zhou Q, et al. The Treatment Efficacy of Bone Tissue Engineering Strategy for Repairing Segmental Bone Defects Under Osteoporotic Conditions. Tissue Eng Part A. 2015;21(17–18):2346–2355. doi:10.1089/ten.TEA.2015.0071
87. Horváthy DB, Vácz G, Szabó T, et al. Serum albumin coating of demineralized bone matrix results in stronger new bone formation. J Biomed Mater Res B Appl Biomater. 2016;104(1):126–132. doi:10.1002/jbm.b.33359
88. Man Z, Hu X, Liu Z, et al. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. doi:10.1016/j.biomaterials.2016.09.002
89. LoGuidice A, Houlihan A, Deans R. Multipotent adult progenitor cells on an allograft scaffold facilitate the bone repair process. J Tissue Eng. 2016;7:204173141665614. doi:10.1177/2041731416656148
90. Xie H, Wang Z, Zhang L, et al. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci Rep. 2017;7(1):45622. doi:10.1038/srep45622
91. Wu D, Wang Z, Zheng Z, et al. Effects of physiological aging factor on bone tissue engineering repair based on fetal BMSCs. J Transl Med. 2018;16(1):324. doi:10.1186/s12967-018-1686-1
92. Chang Z, Xing J, Yu X. Construction and evaluation of a novel tissue-engineered bone device. Exp Ther Med. 2021;22(4):1166. doi:10.3892/etm.2021.10600
93. Leng Q, Liang Z, Lv Y. Demineralized bone matrix scaffold modified with mRNA derived from osteogenically pre-differentiated MSCs improves bone repair. Mater Sci Eng C. 2021;119:111601. doi:10.1016/j.msec.2020.111601
94. Chen Z, Du W, Lv Y. Zonally Stratified Decalcified Bone Scaffold with Different Stiffness Modified by Fibrinogen for Osteochondral Regeneration of Knee Joint Defect. ACS Biomater Sci Eng. 2022;8(12):5257–5272. doi:10.1021/acsbiomaterials.2c00813
95. Chen Z, Cai D, Shi R, Ding W, Xu Y, Tan H. In vitro dynamic perfusion of prevascularized OECs-DBMs (outgrowth endothelial progenitor cell - demineralized bone matrix) complex fused to recipient vessels in an internal inosculation manner. Bioengineered. 2022;13(6):14270–14281. doi:10.1080/21655979.2022.2085560
96. Hao J, Bai B, Ci Z, et al. Large-sized bone defect repair by combining a decalcified bone matrix framework and bone regeneration units based on photo-crosslinkable osteogenic microgels. Bioact Mater. 2022;14:97–109. doi:10.1016/j.bioactmat.2021.12.013
97. He SK, Ning LJ, Hu RN, et al. Segmentally Demineralized Cortical Bone With Stem Cell-Derived Matrix Promotes Proliferation, Migration and Differentiation of Stem Cells in vitro. Front Cell Dev Biol. 2022;9:776884. doi:10.3389/fcell.2021.776884
98. Ye J, Liu N, Li Z, et al. Injectable, Hierarchically Degraded Bioactive Scaffold for Bone Regeneration. ACS Appl Mater Interfaces. 2023;15(9):11458–11473. doi:10.1021/acsami.2c18824
99. Yu M, Song D, Guo X, et al. Regeneration of Mechanically Enhanced Tissue-Engineered Cartilage Based on the Decalcified Bone Matrix Framework. ACS Biomater Sci Eng. 2023;9(8):4994–5005. doi:10.1021/acsbiomaterials.3c00488
100. Liu J, Chen F, Song D, et al. Construction of three-dimensional, homogeneous regenerative cartilage tissue based on the ECG-DBM complex. Front Bioeng Biotechnol. 2023;11:1252790. doi:10.3389/fbioe.2023.1252790
101. Wang X, Xiang C, Huang C, et al. The treatment efficacy of bone tissue engineering strategy for repairing segmental bone defects under diabetic condition. Front Bioeng Biotechnol. 2024;12:1379679. doi:10.3389/fbioe.2024.1379679
102. Chen Y, Luo Y, Hou X, et al. Natural Affinity Driven Modification by Silicene to Construct a “Thermal Switch” for Tumorous Bone Loss. Adv Sci. 2024;11(35):2404534. doi:10.1002/advs.202404534
103. Holtzclaw D, Toscano N, Eisenlohr L, Callan D. The safety of bone allografts used in dentistry: a review. J Am Dent Assoc. 2008;139(9):1192–1199. doi:10.14219/jada.archive.2008.0334
104. Salvucci JT. Bone tissue, lyophilized and stored at room temperature for 15 days or more, is not capable of transmitting HIV, HCV or HBV. Cell and Tissue Banking. 2011;12(2):99–104. doi:10.1007/s10561-010-9173-9
105. Novell J, Novell-Costa F, Ivorra C, Fariñas O, Munilla A, Martinez C. Five-year results of implants inserted into freeze-dried block allografts. Implant Dent. 2012;21(2):129–135. doi:10.1097/ID.0b013e31824bf99f
106. Green DP. Rockwood and Green’s Fractures in Adults. Lippincott Williams & Wilkins. 2010;Vol. 1.
107. Kolk A, Handschel J, Drescher W, et al. Current trends and future perspectives of bone substitute materials - from space holders to innovative biomaterials. J Craniomaxillofac Surg. 2012;40(8):706–718. doi:10.1016/j.jcms.2012.01.002
108. Jonck M, Ash AS, Raubenheimer E. Allogenic Bone Transplantation. 1981.
109. Motamedian SR, Khojaste M, Khojasteh A. Success rate of implants placed in autogenous bone blocks versus allogenic bone blocks: a systematic literature review. Ann Maxillofac Surg. 2016;6(1):78–90. doi:10.4103/2231-0746.186143
110. Ando K, Kobayashi K, Ito K, et al. Occipitocervical or C1-C2 fusion using allograft bone in pediatric patients with Down syndrome 8 years of age or younger. J Pediatr Orthop B. 2019;28(4):405–410. doi:10.1097/BPB.0000000000000622
111. Bianchini MA, Buttendorf AR, Benfatti CAM, Bez LV, Ferreira CF, de Andrade RF. The use of freeze-dried bone allograft as an alternative to autogenous bone graft in the atrophic maxilla: a 3-year clinical follow-up. Int J Periodontics Restorative Dent. 2009;29(6):643–647.
112. Ilyas I, Alrumaih H, Rabbani S. Freeze Dried Proximal Femoral Allografts in Revision of Femoral Stems. J Arthroplasty. 2017;32(1):171–176. doi:10.1016/j.arth.2016.06.016
113. Drosos GI, Kazakos KI, Kouzoumpasis P, Verettas DA. Safety and efficacy of commercially available demineralised bone matrix preparations: a critical review of clinical studies. Injury. 2007;38(4):S13–21. doi:10.1016/s0020-1383(08)70005-6
114. Clark RP, Pham PM, Ciminello FS, Hagge RJ, Drobny S, Wong GB. Nasal Dorsal Augmentation with Freeze-Dried Allograft Bone: 10-Year Comprehensive Review. Plast Reconstr Surg. 2019;143(1):49e–61e. doi:10.1097/PRS.0000000000005166
115. Starch-Jensen T, Deluiz D, Tinoco EMB. Horizontal Alveolar Ridge Augmentation with Allogeneic Bone Block Graft Compared with Autogenous Bone Block Graft: a Systematic Review. J Oral Maxillofac Res. 2020;11(1):e1. doi:10.5037/jomr.2020.11101
116. Tomislav C, Marko B, Luka M, Zoran K, Damir J. Regeneration of a twelve teeth wide horizontal defect in the completely edentulous maxilla using the shell technique with three allogenic bone plates, composite bone graft and only one intraoral donor site–Alternative to Hip bone grafting. Clinical Oral Implants Research. 2019;30:432. doi:10.1111/clr.388_13509
117. Motta SHG, Soares APR, Fernandes JCH, Fernandes GVO. Histological Assessment of Bone Regeneration in the Maxilla with Homologous Bone Graft: a Feasible Option for Maxillary Bone Reconstruction. J Renew Mater. 2024;12(1):131–148. doi:10.32604/jrm.2023.043940
118. Kreuz F, Hyatt G, Turner TC, Bassett AL. The preservation and clinical use of freeze-dried bone. JBJS. 1951;33(4):863–888. doi:10.2106/00004623-195133040-00005
119. Iasella JM, Greenwell H, Miller RL, et al. Ridge preservation with freeze-dried bone allograft and a collagen membrane compared to extraction alone for implant site development: a clinical and histologic study in humans. J Periodontol. 2003;74(7):990–999. doi:10.1902/jop.2003.74.7.990
120. Schlee M, Dehner JF, Baukloh K, Happe A, Seitz O, Sader R. Esthetic outcome of implant-based reconstructions in augmented bone: comparison of autologous and allogeneic bone block grafting with the pink esthetic score (PES). Head Face Med. 2014;10:21. doi:10.1186/1746-160X-10-21
121. Morato GO, Rocha AG, Chung DG, et al. Lyophilized and gamma-sterilized allogeneic bone implant used as a spacer for advancement of a modified tibial tuberosity in the treatment of cranial cruciate ligament disease in dogs. PLoS One. 2019;14(8):e0220291. doi:10.1371/journal.pone.0220291
122. Mau JL, Grodin E, Lin JJ, Chen MCJ, Ho CH, Cochran D. A comparative, randomized, prospective, two-center clinical study to evaluate the clinical and esthetic outcomes of two different bone grafting techniques in early implant placement. J Periodontol. 2019;90(3):247–255. doi:10.1002/JPER.17-0491
123. Avila-Ortiz G, Elangovan S, Kramer KWO, Blanchette D, Dawson DV. Effect of alveolar ridge preservation after tooth extraction: a systematic review and meta-analysis. J Dent Res. 2014;93(10):950–958. doi:10.1177/0022034514541127
124. Kungvarnchaikul I, Subbalekha K, Sindhavajiva PR, Suwanwela J. Deproteinized bovine bone and freeze‐dried bone allograft in sinus floor augmentation: a randomized controlled trial. Clin Implant Dent Rel Res. 2023;25(2):343–351. doi:10.1111/cid.13179
125. Kouhestani F, Dehabadi F, Hasan Shahriari M, Motamedian SR. Allogenic vs. synthetic granules for bone tissue engineering: an in vitro study. Prog Biomater. 2018;7(2):133–141. doi:10.1007/s40204-018-0092-3
126. Mansor A, Ariffin AF, Yusof N, et al. Effects of processing and gamma radiation on mechanical properties and organic composition of frozen, freeze-dried and demineralised human cortical bone allograft. Cell Tissue Bank. 2023;24(1):25–35. doi:10.1007/s10561-022-10013-9
127. Te Stroet MAJ, Rijnen WHC, Gardeniers JWM, Van Kampen A, Schreurs WB. Satisfying outcomes scores and survivorship achieved with impaction grafting for revision THA in young patients. Clin Orthop Relat Res. 2015;473(12):3867–3875. doi:10.1007/s11999-015-4293-y
128. Villatte G, Erivan R, Salles G, et al. Acetabular bone defects in THA revision: reconstruction using morsellised virus-inactivated bone allograft and reinforcement ring. Orthop Traumatol Surg Res. 2017;103(4):543–548. doi:10.1016/j.otsr.2017.03.008
129. Heiple KG, Chase SW, Herndon CH. A comparative study of the healing process following different types of bone transplantation. JBJS. 1963;45(8):1593–1616. doi:10.2106/00004623-196345080-00003
130. Shigeyama Y, D’Errico JA, Stone R, Somerman MJ. Commercially-prepared allograft material has biological activity in vitro. J Periodontol. 1995;66(6):478–487. doi:10.1902/jop.1995.66.6.478
131. Jaiswal Y, Kumar S, Mishra V, Bansal P, Anand KR, Singh S. Efficacy of decalcified freeze-dried bone allograft in the regeneration of small osseous defect: a comparative study. Natl J Maxillofac Surg. 2017;8(2):143–148. doi:10.4103/0975-5950.221714
132. Rodrigues L, Dos Reis LM, Denadai R, et al. Prefabricated bone flap: an experimental study comparing deep-frozen and lyophilized-demineralized allogenic bones and tissue expression of transforming growth factor β. J Craniofac Surg. 2013;24(6):1914–1921. doi:10.1097/SCS.0b013e3182a41be2
133. Wood RA, Mealey BL. Histologic comparison of healing after tooth extraction with ridge preservation using mineralized versus demineralized freeze-dried bone allograft. J Periodontol. 2012;83(3):329–336. doi:10.1902/jop.2011.110270
134. Lafzi A, Vahabi S, Ghods S, Torshabi M. In vitro effect of mineralized and demineralized bone allografts on proliferation and differentiation of MG-63 osteoblast-like cells. Cell Tissue Bank. 2016;17(1):91–104. doi:10.1007/s10561-015-9516-7
135. Gothi R, Bansal M, Kaushik M, Khattak BP, Sood N, Taneja V. A comparative evaluation of freeze dried bone allograft and decalcified freeze dried bone allograft in the treatment of intrabony defects: a clinical and radiographic study. J Indian Soc Periodontol. 2015;19(4):411–415. doi:10.4103/0972-124X.154169
136. Lisa DK, Flore D, Gaetan VDV, Yannick S, Constantinus P. Survival rate of implants following maxillary sinus floor augmentation using freeze-dried allografts vs bovine derived xenografts: a retrospective multicenter study. Journal of Stomatol Oral Maxillofacial Surg. 2023;124(6):101605. doi:10.1016/j.jormas.2023.101605
137. Borg TD, Mealey BL. Histologic healing following tooth extraction with ridge preservation using mineralized versus combined mineralized-demineralized freeze-dried bone allograft: a randomized controlled clinical trial. J Periodontol. 2015;86(3):348–355. doi:10.1902/jop.2014.140483
138. Wei L, Miron RJ, Shi B, Zhang Y. Osteoinductive and Osteopromotive Variability among Different Demineralized Bone Allografts. Clin Implant Dent Relat Res. 2015;17(3):533–542. doi:10.1111/cid.12118
139. McAllister BS, Haghighat K. Bone augmentation techniques. Journal of Periodontology. 2007;78(3):377–396. doi:10.1902/jop.2007.060048
140. Bhatt RA, Rozental TD. Bone graft substitutes. Hand Clin. 2012;28(4):457–468. doi:10.1016/j.hcl.2012.08.001
141. Rainer A, Giannitelli SM, Abbruzzese F, Traversa E, Licoccia S, Trombetta M. Fabrication of bioactive glass-ceramic foams mimicking human bone portions for regenerative medicine. Acta Biomater. 2008;4(2):362–369. doi:10.1016/j.actbio.2007.08.007
142. Xu M, Liu Y, Wang P, Hu Y. Research progress of bioactive glasses based bone repair materials. Chinese Journal of Orthopaedics. (Chin J Orthop. 2019;39(7):1.
143. Abushahba F, Algahawi A, Areid N, Hupa L, Närhi TO. Bioactive Glasses in Periodontal Regeneration: a Systematic Review. Tissue Eng C: Methods. 2023;29(5):183–196. doi:10.1089/ten.tec.2023.0036
144. Neo M, Nakamura T, Ohtsuki C, Kasai R, Kokubo T, Yamamuro T. Ultrastructural study of the A-W GC-bone interface after long-term implantation in rat and human bone. J Biomed Mater Res. 1994;28(3):365–372. doi:10.1002/jbm.820280311
145. Matsumoto MA, Caviquioli G, Biguetti CC, et al. A novel bioactive vitroceramic presents similar biological responses as autogenous bone grafts. J Mater Sci Mater Med. 2012;23(6):1447–1456. doi:10.1007/s10856-012-4612-8
146. Varanasi VG, Saiz E, Loomer PM, et al. Enhanced osteocalcin expression by osteoblast-like cells (MC3T3-E1) exposed to bioactive coating glass (SiO2-CaO-P2O5-MgO-K2O-Na2O system) ions. Acta Biomater. 2009;5(9):3536–3547. doi:10.1016/j.actbio.2009.05.035
147. Schwartz Z, Doukarsky‐Marx T, Nasatzky E, et al. Differential effects of bone graft substitutes on regeneration of bone marrow. Clin Oral Implants Res. 2008;19(12):1233–1245. doi:10.1111/j.1600-0501.2008.01582.x
148. Scarano A, Degidi M, Iezzi G, et al. Maxillary sinus augmentation with different biomaterials: a comparative histologic and histomorphometric study in man. Implant Dent. 2006;15(2):197–207. doi:10.1097/01.id.0000220120.54308.f3
149. Xynos ID, Edgar AJ, Buttery LDK, Hench LL, Polak JM. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass 45S5 dissolution. J Biomed Mater Res. 2001;55(2):151–157. doi:10.1002/1097-4636(200105)55:2<151::aid-jbm1001>3.0.co;2-d
150. Kaur G, Kumar V, Baino F, et al. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: state-of-the-art review and future challenges. Mater Sci Eng C. 2019;104:109895. doi:10.1016/j.msec.2019.109895
151. Ferreira JM, Rebelo A. The key features expected from a perfect bioactive glass-how far we still are from an ideal composition. Biomed J Sci Tech Res. 2017;1:936–939.
152. Jia WT, Fu Q, Huang WH, Zhang CQ, Rahaman MN. Comparison of Borate Bioactive Glass and Calcium Sulfate as Implants for the Local Delivery of Teicoplanin in the Treatment of Methicillin-Resistant Staphylococcus aureus-Induced Osteomyelitis in a Rabbit Model. Antimicrob Agents Chemother. 2015;59(12):7571–7580. doi:10.1128/AAC.00196-15
153. Azenha MR, De Lacerda SA, Marão HF, Filho OP, Filho OM. Evaluation of Crystallized Biosilicate in the Reconstruction of Calvarial Defects. J Maxillofac Oral Surg. 2015;14(3):659–665. doi:10.1007/s12663-015-0755-8
154. Abushahba F, Gürsoy M, Hupa L, Närhi TO. Effect of bioactive glass air-abrasion on Fusobacterium nucleatum and Porphyromonas gingivalis biofilm formed on moderately rough titanium surface. Eur J Oral Sci. 2021;129(3):e12783. doi:10.1111/eos.12783
155. Abou Neel EA, Chrzanowski W, Pickup DM, et al. Structure and properties of strontium-doped phosphate-based glasses. J R Soc Interface. 2009;6(34):435–446. doi:10.1098/rsif.2008.0348
156. Sumer M, Keles GC, Cetinkaya BO, Balli U, Pamuk F, Uckan S. Autogenous cortical bone and bioactive glass grafting for treatment of intraosseous periodontal defects. Eur J Dent. 2013;7(1):6–14.
157. Katuri K, Kumar P, Swarna C, Swamy D, Arun K. Evaluation of bioactive glass and demineralized freeze dried bone allograft in the treatment of periodontal intraosseous defects: a comparative clinico-radiographic study. J Indian Soc Periodontol. 2013;17(3):367. doi:10.4103/0972-124X.115660
158. Ferraz MP. Bone Grafts in Dental Medicine: an Overview of Autografts, Allografts and Synthetic Materials. Materials. 2023;16(11):4117. doi:10.3390/ma16114117
159. Goodman SB, Maruyama M. Inflammation, Bone Healing and Osteonecrosis: from Bedside to Bench. J Inflamm Res. 2020;13:913–923. doi:10.2147/JIR.S281941
160. Eaves CJ. Hematopoietic stem cells: concepts, definitions, and the new reality. Blood. 2015;125(17):2605–2613. doi:10.1182/blood-2014-12-570200
161. Kfoury Y, Scadden DT. Mesenchymal cell contributions to the stem cell niche. Cell Stem Cell. 2015;16(3):239–253. doi:10.1016/j.stem.2015.02.019
162. Goodman SB, Zwingenberger S. Concentrated autologous bone marrow aspirate is not “stem cell” therapy in the repair of nonunions and bone defects. Biomater Biosyst. 2021;2:100017. doi:10.1016/j.bbiosy.2021.100017
163. Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87(7):1430–1437. doi:10.2106/JBJS.D.02215
164. Muschler GF, Boehm C, Easley K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: the influence of aspiration volume. J Bone Joint Surg Am. 1997;79(11):1699–1709. doi:10.2106/00004623-199711000-00012
165. Yin D, Wang Z, Gao Q, et al. Determination of the fate and contribution of ex vivo expanded human bone marrow stem and progenitor cells for bone formation by 2.3ColGFP. mol Ther. 2009;17(11):1967–1978. doi:10.1038/mt.2009.151
166. Chahla J, Dean CS, Moatshe G, Pascual-Garrido C, Serra Cruz R, LaPrade RF. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: a Systematic Review of Outcomes. Orthop J Sports Med. 2016;4(1):2325967115625481. doi:10.1177/2325967115625481
167. Sugaya H, Yoshioka T, Kato T, et al. Comparative Analysis of Cellular and Growth Factor Composition in Bone Marrow Aspirate Concentrate and Platelet-Rich Plasma. Bone Marrow Res. 2018;2018:1549826. doi:10.1155/2018/1549826
168. Koyanagi M, Fujioka-Kobayashi M, Inada R, Yoneyama Y, Satomi T. Skin and Bone Regeneration of Solid Bone Marrow Aspirate Concentrate Versus Platelet-Rich Fibrin. Tissue Eng Part A. 2023;29(5–6):141–149. doi:10.1089/ten.tea.2022.0175
169. Umemura T, Nishioka K, Igarashi A, et al. Autologous bone marrow mononuclear cell implantation induces angiogenesis and bone regeneration in a patient with compartment syndrome. Circ J. 2006;70(10):1362–1364. doi:10.1253/circj.70.1362
170. Du F, Wang Q, Ouyang L, et al. Comparison of concentrated fresh mononuclear cells and cultured mesenchymal stem cells from bone marrow for bone regeneration. Stem Cells Transl Med. 2021;10(4):598–609. doi:10.1002/sctm.20-0234
171. Koyanagi M, Fujioka-Kobayashi M, Yoneyama Y, Inada R, Satomi T. Regenerative Potential of Solid Bone Marrow Aspirate Concentrate Compared with Platelet-Rich Fibrin. Tissue Eng Part A. 2022;28(17–18):749–759. doi:10.1089/ten.tea.2021.0225
172. Lim ZXH, Rai B, Tan TC, et al. Autologous bone marrow clot as an alternative to autograft for bone defect healing. Bone Jt Res. 2019;8(3):107–117. doi:10.1302/2046-3758.83.BJR-2018-0096.R1
173. Chahla J, Mannava S, Cinque ME, Geeslin AG, Codina D, LaPrade RF. Bone Marrow Aspirate Concentrate Harvesting and Processing Technique. Arthrosc Tech. 2017;6(2):e441–e445. doi:10.1016/j.eats.2016.10.024
174. Betsch M, Schneppendahl J, Thuns S, et al. Bone marrow aspiration concentrate and platelet rich plasma for osteochondral repair in a porcine osteochondral defect model. PLoS One. 2013;8(8):e71602. doi:10.1371/journal.pone.0071602
175. Shi L, Tee BC, Sun Z. Effects of porcine bone marrow-derived platelet-rich plasma on bone marrow-derived mesenchymal stem cells and endothelial progenitor cells. Tissue Cell. 2021;71:101587. doi:10.1016/j.tice.2021.101587
176. Fontes Martins LC, Sousa Campos de Oliveira AL, Aloise AC, et al. Bone marrow aspirate concentrate and platelet-rich fibrin in fresh extraction sockets: a histomorphometric and immunohistochemical study in humans. J Craniomaxillofac Surg. 2021;49(2):104–109. doi:10.1016/j.jcms.2020.12.005
177. Koch M, Hammer S, Fuellerer J, et al. Bone Marrow Aspirate Concentrate for the Treatment of Avascular Meniscus Tears in a One-Step Procedure-Evaluation of an In Vivo Model. Int J mol Sci. 2019;20(5):1120. doi:10.3390/ijms20051120
178. Victorelli G, Aloise AC, Passador-Santos F, de Mello, Oliveira R, Pelegrine AA. Ectopic Implantation of Hydroxyapatite Xenograft Scaffold Loaded with Bone Marrow Aspirate Concentrate or Osteodifferentiated Bone Marrow Mesenchymal Stem Cells: a Pilot Study in Mice. Int J Oral Maxillofac Implants. 2016;31(2):e18–23. doi:10.11607/jomi.4509
179. Kanakaraj M, Manoharan S, Srinivas S, et al. Autologous bone marrow aspirate concentrate (BMAC) for treatment of keratocystic odontogenic tumour (KCOT)-a case report. Stem Cell Investig. 2021;8:16. doi:10.21037/sci-2020-059
180. Saad KAE, Abu-Shahba AGT, El-Drieny EAE, Khedr MS. Evaluation of the role of autogenous bone-marrow-derived mesenchymal stem cell transplantation for the repair of mandibular bone defects in rabbits. J Craniomaxillofac Surg. 2015;43(7):1151–1160. doi:10.1016/j.jcms.2015.04.013
181. Wang Q, Huang Y, Qian Z. Nanostructured Surface Modification to Bone Implants for Bone Regeneration. J Biomed Nanotechnol. 2018;14(4):628–648. doi:10.1166/jbn.2018.2516
182. Venkata Prathyusha E, Gomte SS, Ahmed H, et al. Nanostructured polymer composites for bone and tissue regeneration. Int J Biol Macromol. 2025;284:137834. doi:10.1016/j.ijbiomac.2024.137834
183. Tavakoli M, Salehi H, Emadi R, et al. 3D printed polylactic acid-based nanocomposite scaffold stuffed with microporous simvastatin-loaded polyelectrolyte for craniofacial reconstruction. Int J Biol Macromol. 2024;258(Pt 1):128917. doi:10.1016/j.ijbiomac.2023.128917
184. Salehi S, Ghomi H, Hassanzadeh-Tabrizi SA, Koupaei N, Khodaei M. 3D printed polylactic acid/polyethylene glycol/bredigite nanocomposite scaffold enhances bone tissue regeneration via promoting osteogenesis and angiogenesis. Int J Biol Macromol. 2024;281(Pt 1):136160. doi:10.1016/j.ijbiomac.2024.136160
185. Kanniyappan H, Sundaram MK, Ravikumar A, et al. Enhancing bone repair through improved angiogenesis and osteogenesis using mesoporous silica nanoparticle-loaded Konjac glucomannan-based interpenetrating network scaffolds. Int J Biol Macromol. 2024;279(Pt 2):135182. doi:10.1016/j.ijbiomac.2024.135182
186. Chen S, Qiu Z, Zhao L, Huang X, Xiao X. Functionalized BP@(Zn+Ag)/EPLA Nanofibrous Scaffolds Fabricated by Cryogenic 3D Printing for Bone Tissue Engineering. Adv Healthc Mater. 2024;13(23):e2401038. doi:10.1002/adhm.202401038
187. Trzaskowska M, Vivcharenko V, Franus W, Goryczka T, Barylski A, Przekora A. Optimization of the Composition of Mesoporous Polymer-Ceramic Nanocomposite Granules for Bone Regeneration. Molecules. 2023;28(13):5238. doi:10.3390/molecules28135238
188. Vidane AS, Nunes FC, Ferreira JA, et al. Biocompatibility and interaction of porous alumina-zirconia scaffolds with adipose-derived mesenchymal stem cells for bone tissue regeneration. Heliyon. 2023;9(9):e20128. doi:10.1016/j.heliyon.2023.e20128
189. Tavakoli Z, Ansari M, Poursamar SA, et al. Synergetic effect of bioglass and nano montmorillonite on 3D printed nanocomposite of polycaprolactone/gelatin in the fabrication of bone scaffolds. Int J Biol Macromol. 2024;281(Pt 2):136384. doi:10.1016/j.ijbiomac.2024.136384
190. Avinashi SK, Mishra RK, Shweta, et al. 3D nanocomposites of β-TCP-H3BO3-Cu with improved mechanical and biological performances for bone regeneration applications. Sci Rep. 2025;15(1):3224. doi:10.1038/s41598-025-87988-4
191. Fu M, Li J, Liu M, et al. Sericin/Nano-Hydroxyapatite Hydrogels Based on Graphene Oxide for Effective Bone Regeneration via Immunomodulation and Osteoinduction. Int J Nanomed. 2023;18:1875–1895. doi:10.2147/IJN.S399487
192. Zhou S, Xiao C, Fan L, et al. Injectable ultrasound-powered bone-adhesive nanocomposite hydrogel for electrically accelerated irregular bone defect healing. J Nanobiotechnology. 2024;22(1):54. doi:10.1186/s12951-024-02320-y
193. Zha K, Tan M, Hu Y, et al. Regulation of metabolic microenvironment with a nanocomposite hydrogel for improved bone fracture healing. Bioact Mater. 2024;37:424–438. doi:10.1016/j.bioactmat.2024.03.025
194. Guo J, Yao H, Chang L, et al. Magnesium Nanocomposite Hydrogel Reverses the Pathologies to Enhance Mandible Regeneration. Adv Mater. 2025;37(2):e2312920. doi:10.1002/adma.202312920
195. Bharadwaz A, Jayasuriya AC. Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C Mater Biol Appl. 2020;110:110698. doi:10.1016/j.msec.2020.110698
196. Dulany K, Hepburn K, Goins A, Allen JB. In vitro and in vivo biocompatibility assessment of free radical scavenging nanocomposite scaffolds for bone tissue regeneration. J Biomed Mater Res A. 2020;108(2):301–315. doi:10.1002/jbm.a.36816
197. Abbas M, Alqahtani MS, Alhifzi R. Recent Developments in Polymer Nanocomposites for Bone Regeneration. Int J mol Sci. 2023;24(4):3312. doi:10.3390/ijms24043312
198. Geng Z, Yuan Q, Zhuo X, et al. Synthesis, Characterization, and Biological Evaluation of Nanostructured Hydroxyapatite with Different Dimensions. Nanomaterials. 2017;7(2):38. doi:10.3390/nano7020038
199. Felgueiras HP, Amorim MTP. Production of polymer–bioactive glass nanocomposites for bone repair and substitution. In: Materials for Biomedical Engineering. Elsevier; 2019:373–396.
200. Geng Z, Cheng Y, Ma L, et al. Nanosized strontium substituted hydroxyapatite prepared from egg shell for enhanced biological properties. J Biomater Appl. 2018;32(7):896–905. doi:10.1177/0885328217748124
201. Sharifi F, Atyabi SM, Norouzian D, Zandi M, Irani S, Bakhshi H. Polycaprolactone/carboxymethyl chitosan nanofibrous scaffolds for bone tissue engineering application. Int J Biol Macromol. 2018;115:243–248. doi:10.1016/j.ijbiomac.2018.04.045
202. Zhang Y, Wu D, Zhao X, et al. Stem Cell-Friendly Scaffold Biomaterials: applications for Bone Tissue Engineering and Regenerative Medicine. Front Bioeng Biotechnol. 2020;8:598607. doi:10.3389/fbioe.2020.598607
203. Karimipour-Fard P, Jeffrey MP, JonesTaggart H, Pop-Iliev R, Rizvi G. Development, processing and characterization of Polycaprolactone/Nano-Hydroxyapatite/Chitin-Nano-Whisker nanocomposite filaments for additive manufacturing of bone tissue scaffolds. J Mech Behav Biomed Mater. 2021;120:104583. doi:10.1016/j.jmbbm.2021.104583
204. Gregor A, Filová E, Novák M, et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng. 2017;11(1):31. doi:10.1186/s13036-017-0074-3
205. Li H, Qiao T, Song P, et al. Star-shaped PCL/PLLA blended fiber membrane via electrospinning. J Biomater Sci Polym Ed. 2015;26(7):420–432. doi:10.1080/09205063.2015.1015865
206. Qiao T, Song P, Guo H, Song X, Zhang B, Chen X. Reinforced electrospun PLLA fiber membrane via chemical crosslinking. Eur Polym J. 2016;74:101–108. doi:10.1016/j.eurpolymj.2015.11.012
207. Canales DA, Piñones N, Saavedra M, et al. Fabrication and assessment of bifunctional electrospun poly(l-lactic acid) scaffolds with bioglass and zinc oxide nanoparticles for bone tissue engineering. Int J Biol Macromol. 2023;228:78–88. doi:10.1016/j.ijbiomac.2022.12.195
208. Boukari Y, Qutachi O, Scurr DJ, Morris AP, Doughty SW, Billa N. A dual-application poly (dl-lactic-co-glycolic) acid (PLGA)-chitosan composite scaffold for potential use in bone tissue engineering. J Biomater Sci Polym Ed. 2017;28(16):1966–1983. doi:10.1080/09205063.2017.1364100
209. Li C, Zhang W, Nie Y, et al. Time-Sequential and Multi-Functional 3D Printed MgO2/PLGA Scaffold Developed as a Novel Biodegradable and Bioactive Bone Substitute for Challenging Postsurgical Osteosarcoma Treatment. Adv Mater. 2024;36(34):e2308875. doi:10.1002/adma.202308875
210. Qi W, Zhang X, Wang H. Self-assembled polymer nanocomposites for biomedical application. Curr Opin Colloid Interface Sci. 2018;35:36–41. doi:10.1016/j.cocis.2018.01.003
211. Obisesan OS, Ajiboye TO, Mhlanga SD, Mufhandu HT. Biomedical applications of biodegradable polycaprolactone-functionalized magnetic iron oxides nanoparticles and their polymer nanocomposites. Colloids Surf. B. 2023;227:113342. doi:10.1016/j.colsurfb.2023.113342
212. Luo W, Geng Z, Li Z, et al. Controlled and sustained drug release performance of calcium sulfate cement porous TiO2 microsphere composites. Int J Nanomed. 2018;13:7491–7501. doi:10.2147/IJN.S177784
213. Li J, Zhao Y, Chen S, Wang S, Zhong W, Zhang Q. Research Hotspots and Trends of Bone Xenograft in Clinical Procedures: a Bibliometric and Visual Analysis of the Past Decade. Bioengineering. 2023;10(8):929. doi:10.3390/bioengineering10080929
214. Nickoli MS, Hsu WK. Ceramic-based bone grafts as a bone grafts extender for lumbar spine arthrodesis: a systematic review. Global Spine J. 2014;4(3):211–216. doi:10.1055/s-0034-1378141
215. Pintore A, Notarfrancesco D, Zara A, et al. Intra-articular injection of bone marrow aspirate concentrate (BMAC) or adipose-derived stem cells (ADSCs) for knee osteoarthritis: a prospective comparative clinical trial. J Orthop Surg Res. 2023;18(1):350. doi:10.1186/s13018-023-03841-2
216. El-Kadiry AEH, Lumbao C, Rafei M, Shammaa R. Autologous BMAC Therapy Improves Spinal Degenerative Joint Disease in Lower Back Pain Patients. Front Med. 2021;8:622573. doi:10.3389/fmed.2021.622573
217. Manawar S, Myrick E, Awad P, et al. Use of allograft bone matrix in clinical orthopedics. Regener Med. 2024;19(5):247–256. doi:10.1080/17460751.2024.2353473
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.