Back to Journals » International Journal of Nanomedicine » Volume 19
Research Progress of Disulfide Bond Based Tumor Microenvironment Targeted Drug Delivery System
Authors Ma W , Wang X , Zhang D, Mu X
Received 1 April 2024
Accepted for publication 17 July 2024
Published 24 July 2024 Volume 2024:19 Pages 7547—7566
DOI https://doi.org/10.2147/IJN.S471734
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Kamakhya Misra
Weiran Ma,1,2 Xiaoying Wang,2 Dongqi Zhang,3 Xupeng Mu1
1Scientific Research Center, China-Japan Union Hospital of Jilin University, Changchun, 130033, People’s Republic of China; 2Jilin University School of Pharmaceutical Sciences, Changchun, 130021, People’s Republic of China; 3Department of Urology, The First Hospital of Jilin University, Changchun, 130021, People’s Republic of China
Correspondence: Xupeng Mu, Email [email protected]
Abstract: Cancer poses a significant threat to human life and health. Chemotherapy is currently one of the effective cancer treatments, but many chemotherapy drugs have cell toxicity, low solubility, poor stability, a narrow therapeutic window, and unfavorable pharmacokinetic properties. To solve the above problems, target drug delivery to tumor cells, and reduce the side effects of drugs, an anti-tumor drug delivery system based on tumor microenvironment has become a focus of research in recent years. The construction of a reduction-sensitive nanomedicine delivery system based on disulfide bonds has attracted much attention. Disulfide bonds have good reductive responsiveness and can effectively target the high glutathione (GSH) levels in the tumor environment, enabling precise drug delivery. To further enhance targeting and accelerate drug release, disulfide bonds are often combined with pH-responsive nanocarriers and highly expressed ligands in tumor cells to construct drug delivery systems. Disulfide bonds can connect drug molecules and polymer molecules in the drug delivery system, as well as between different drug molecules and carrier molecules. This article summarized the drug delivery systems (DDS) that researchers have constructed in recent years based on disulfide bond drug delivery systems targeting the tumor microenvironment, disulfide bond cleavage-triggering conditions, various drug loading strategies, and carrier design. In this review, we also discuss the controlled release mechanisms and effects of these DDS and further discuss the clinical applicability of delivery systems based on disulfide bonds and the challenges faced in clinical translation.
Keywords: disulfide bond, drug delivery systems, tumor microenvironment, GSH/ROS
Introduction
Cancer incidence is widespread in all countries and regions, showing an increasing trend annually, and imposes a significant economic burden, making it a major public health concern affecting people’s lives and health. Cancer has become the second leading cause of death in the United States.1 Currently, traditional methods for treating cancer mainly include surgery, radiotherapy, and chemotherapy.2–5 Chemotherapy has been widely used in tumor treatment, but its clinical use is limited due to its severe toxic side effects as it can distribute widely in normal tissues and organs such as the heart, liver, spleen, kidneys, and lungs.6–8 At the same time, tumor cells are usually embedded in the dense extracellular matrix composed of collagen, proteoglycan, protein, and glycoprotein,9 and tumor blood vessels are highly disorganized and the blood viscosity within the tumor is high,10 which hinders the entry of anti-tumor drugs and affects the curative effect.11 Therefore, an increasing number of scholars are focusing on constructing targeted drug delivery systems to achieve precise drug release, reduce systemic reactions, decrease dosage, improve efficacy, and enhance water solubility.
 |
Table 1 Summary of Drug Delivery Systems Based on Disulfide Bonds |
In the past few decades, drug delivery systems with different mechanisms of drug release have been widely proposed and researched. The selective release of nanomedicine at the target site is crucial for the effectiveness and safety of the drug.12,13 Due to the rapid proliferation and growth of tumors, it has a series of special microenvironments such as low oxygen, slightly acidic, high oxidative stress, high concentration of GSH, and overexpression of some enzymes.14 In recent years, based on these special characteristics of the tumor microenvironment, different stimulus-responsive nanomedicine have been developed and applied in cancer therapy.15–18
With deepening research into the tumor microenvironment, many studies have indicated that disulfide bonds are crucial units for constructing targeted conjugates.19,20 Drug delivery systems targeting the tumor microenvironment based on disulfide bonds demonstrate broad application prospects. Disulfide bonds can serve as connections between drugs and polymer molecules, between drugs themselves, and between drug carrier molecules in drug delivery systems (Table 1). Although the positions of disulfide bonds in the system may vary, they essentially function through thiol-disulfide exchange reactions, consuming GSH and subsequently breaking the disulfide bonds to release drugs and exert therapeutic effects.21 Due to the stability of disulfide bonds at physiological pH in plasma, prodrugs constructed based on disulfide bonds can remain stable in normal tissues, effectively avoiding severe systemic toxicity while exhibiting good targeting towards reactive oxygen species (ROS) and GSH, demonstrating unique advantages and importance in the design of anti-tumor drugs.22,23
Starting from a drug delivery system targeting tumor microenvironment based on disulfide bonds, this paper introduced the response mechanism of disulfide bonds, the conditions for targeting tumor microenvironment, carrier types, and multifunctional carriers, especially from the perspective of the connection position of disulfide bonds in the carrier. The purpose and application of disulfide bonds between drug and drug, between drug and carrier molecule, and between carrier molecule and carrier molecule were discussed to show the new and more comprehensive application and prospect of disulfide bonds in the field of drug delivery.
Basis of Action of Disulfide Bond
Disulfide bonds are chemical bonds that widely exist in proteins within the human body, connecting different peptide chains or the same peptide chain via cysteine residues’ thiol groups.24,25 In eukaryotic cells, nearly one-third of protein structures contain at least one disulfide bond.26 As an important component of structure, the formation of disulfide bonds ensures that proteins or protein complexes have the correct conformation and maintain the stability of peptides or proteins.27 In addition to their structural role, disulfide bonds also play a major role in signal transduction, thiol protection, and regulation of cellular redox homeostasis.28 Reducing agents such as GSH, dithiothreitol (DTT), and β-mercaptoethanol can react with disulfide bonds, converting them into thiol groups.29,30 The thiol group on cysteine in GSH serves as its active center. Through redox reactions and nucleophilic substitution reactions, disulfide bonds are broken down into thiol groups by GSH, leading to the rapid degradation of the carrier and facilitating drug release for therapeutic effects.31 Furthermore, disulfide bonds can undergo oxidative reactions with reactive oxygen species (ROS) in environments with high ROS levels.32 The reaction mechanism is similar to that of thioether bonds, where sulfur is oxidized into hydrophilic sulfone or sulfoxide, increasing the hydrophilicity of the drug delivery system. This also facilitates hydrolytic reactions of the carrier, promoting the disintegration of nanoparticles and drug release.32 Tumor tissues have high levels of GSH and ROS, which facilitate the degradation of drug delivery systems constructed via disulfide bonds to exert therapeutic effects. The specific drug release mechanism is illustrated in the diagram (Figure 1). Before entering tumor cells, disulfide bonds exhibit higher stability in the cellular microenvironment under normal physiological conditions, preventing premature drug release. Based on the aforementioned research foundation, an increasing number of researchers are focusing on designing various responsive and multifunctional composite intelligent nanomedicine delivery systems using disulfide bonds.
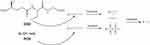 |
Figure 1 REDOX response mechanism of disulfide bond. |
Targeting Conditions of Tumor Microenvironment
The tumor microenvironment refers to the intrinsic environment generated and maintained by tumor cells, which, in addition to tumor cells, comprises various types of cells such as endothelial cells, fibroblasts, immune cells, and extracellular components including cytokines, growth factors, hormones, and extracellular matrix components.33–35 Due to the ability of tumor cells to interact with the surrounding environment through blood and lymphatic circulation, the tumor microenvironment plays a significant regulatory role in aspects such as tumor initiation, growth, migration, invasion, and the efficacy of drug therapy.36 Targeting the tumor microenvironment offers significant therapeutic advantages compared to directly targeting cancer cells because cancer cells are prone to developing resistance due to the instability of their genome, while non-tumor cells in the tumor microenvironment are more genetically stable and susceptible to damage.37 This unique local environment, distinct from normal tissue, not only provides new insights for cancer therapy but also offers novel strategies for targeted delivery of anti-cancer drugs. The common tumor microenvironment response mechanism of disulfide bond delivery systems is shown in Figure 2.
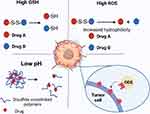 |
Figure 2 Tumor microenvironment response mechanism of disulfide bond-based drug delivery system. Created with BioRender.com. |
GSH/ROS Targeting
Glutathione (GSH) is the most common non-protein thiol in eukaryotic cells.38 It is a common antioxidant in the body and exists in two forms: reduced GSH and oxidized glutathione disulfide (GSSG).39 It plays important roles in scavenging ROS, reducing oxidative stress, maintaining thiol status, and regulating cell growth and differentiation.40–42 Compared to normal tissues, various tumor cells often have higher levels of GSH. In normal tissue cells and plasma, GSH levels range from 0.5 to 10 mmol/L, while GSH concentration in tumor tissue is more than four times that of normal cells.43,44 This concentration difference is an important prerequisite for targeting tumors through disulfide bonds. Studies have shown that GSH can counteract the anti-tumor effects of chemotherapy drugs by promoting tumor cell proliferation, binding to or responding to drugs, interacting with reactive oxygen species, alleviating damage to proteins or DNA, or participating in DNA repair processes.43,45,46 At the same time, some drugs, such as platinum-based chemotherapy drugs, can also induce the production of GSH, further weakening its anti-tumor ability.47 In conclusion, the high level of GSH is a disadvantage for anti-tumor, and its consumption by disulfide bonds is more conducive to tumor inhibition.
Furthermore, tumor cells accumulate ROS due to high metabolism and mitochondrial dysfunction.48 Various human cancers exhibit high levels of ROS, DNA oxidative damage, increased lipid peroxidation, and a lack of antioxidant defense mechanisms.49 ROS are not only closely associated with DNA and protein damage and cell death but are also involved in inhibiting apoptosis and promoting cell survival.50 They play important roles in key physiological processes of tumor cells, such as tumor angiogenesis and epithelial-mesenchymal transition.51
Due to impairments in maintaining normal cell function, tumor cells generally have higher levels of intracellular oxidative stress compared to normal cells.52 Disulfide bonds exhibit good reduction responsiveness and can target GSH. Through GSH-mediated thiol-disulfide exchange reactions, disulfide bonds can be rapidly cleaved while GSH is consumed, thus reducing the GSH level in tumor cells. Additionally, ROS can oxidize disulfide bonds into sulfones or sulfoxides, increasing hydrophilicity and promoting drug release.36 In addition to their targeting capabilities, disulfide bonds deplete excessive GSH or ROS within tumor cells. Malignant tumor cells are more vulnerable to oxidative stress than healthy cells. An intratumoral drug delivery system based on disulfide bonds can not only achieve targeted drug release within tumor tissues but also alter the oxidative-reductive state in the tumor microenvironment, leading to an imbalance in redox status and inducing programmed cell death of tumor cells.
pH Targeting
In addition to redox states, the pH of tumor tissue also differs from normal tissue. Due to significantly higher rates of aerobic and anaerobic glycolysis in tumor tissue compared to normal cells, acidic interstitial fluid accumulates. The pH of most tumors ranges between 5.7 and 7.8, approximately 0.5 lower than normal tissue, providing another triggering condition for tumor drug delivery systems. Since GSH-triggered, thiol-disulfide exchange reaction-dependent redox drug delivery systems are kinetically slow, pH is often employed as another stimulus condition in conjunction with disulfide bonds to construct pH/redox dual-responsive drug delivery systems.53 Li et al designed a nano-carrier (RMOF) based on the “Trojan horse” concept, comprising a zeolitic imidazolate framework-8 (ZIF-8) core and a disulfide-linked amphiphilic polymer-drug conjugate steric shell. They encapsulated the highly reducible THPP within ZIF-8, followed by coverage with myristyl alcohol. The drug curcumin was combined with the disulfide bond-containing linker to form amphiphilic polymers mPEG-P(Lys-Cur) distributed on the periphery. Under acidic conditions, ZIF-8 ruptures and releases THPP, promoting disulfide bond cleavage and drug release. This design maintains the stability of the drug before reaching the target site and greatly accelerates the drug release rate, demonstrating unique advantages and significance (Figure 3).53
 |
Figure 3 Schematic illustration of redox-responsive metal organic framework (RMOF) nanocarrier for rapid cleavage of disulfide and enhanced delivery of a model drug, curcumin (Cur). The tailored nanocarrier consists of zeolitic imidazolate framework-8 (ZIF-8) as the core and amphiphilic copolymer, mPEG-P(Lys-Cur) as the steric shell. Upon tumor accumulation and endocytosis, the disassembly of RMOF liberates THPP that can significantly increase the disulfide cleavage kinetics and hence the intracellular drug concentration. Reproduced with permission from J Control Release, volume 342, Li Y, Feng S, Dai P, et al. Tailored Trojan horse nanocarriers for enhanced redox-responsive drug delivery. 201–209. Copyright © 2022, with permission from Elsevier B.V.53 |
Targeting of Highly Expressed Ligands on the Surface of Tumor Cells
Many tumor cells express ligands on their surface that are different from normal tissue cells, including antibodies, peptides, vitamins, hormones, etc.55 By actively targeting these ligands, therapeutic drugs can bind more accurately to cancer cells, enhancing drug accumulation at the tumor site and reducing systemic toxicity. For example, the CD44 receptor is often overexpressed in various tumors, including liver cancer.54 The folate receptor is frequently overexpressed in a range of solid tumors, including ovarian, lung, and breast cancers.56 Due to the high metabolic activity and rapid glucose uptake of tumor cells, the glucose transporter GLUT is overexpressed in many tumor cells.57 Utilizing disulfide bonds in combination with protein ligands that are highly expressed on tumor cells can construct drug delivery systems that achieve dual targeting of both GSH and proteins, further enhancing targeting functionality and reducing systemic side effects. Doxorubicin (DOX) is a broad-spectrum anticancer drug, but its severe systemic toxicity limits its clinical application. When DOX is covalently linked to folate via disulfide bonds, the cellular toxicity of DOX is reduced while maintaining dual targeting to the folate receptor and GSH.58 Additionally, Zhou et al used disulfide bonds to connect polyethylene glycol (PEG) and polylactic acid (PLA) to form an amphiphilic polymer PEG-SS-PLA, and then modified the polymer with aminoglucose (AG) targeting GLUT-1 to enable specific targeting delivery to tumor cells. AG-PEG-SS-PLA self-assembled into vesicles, encapsulating the highly hydrophobic anticancer drug paclitaxel (PTX) in the core, forming nano-micelles capable of tumor cell-specific recognition, uptake, and rapid intracellular release. The results showed that these dual-functional nano-micelles more effectively inhibited the proliferation of multidrug-resistant A549/ADR cells, demonstrating broad application prospects.57
Zeng et al synthesized amphiphilic glycolipid polymers by bridging chitosan and octadecylamine (ODA) via disulfide bonds. These polymers can self-assemble into micelles that can be used to encapsulate the chemotherapy drug DOX, LinTT1 peptide, and NO donor S-nitroso-n-acetylpenicillamine (SNAP), and link with CO to form TSCO-SS-ODA/DOX. The LinTT1 peptide targets the overexpressed p32/gC1q R/HABP1 protein in breast cancer cells. In endothelial cells, the micelles rapidly release NO in response, leading to vasodilation, increased infiltration of M1 macrophages and CD8+ T cells, and modulation of the tumor microenvironment. In tumor cells, DOX is released in response to GSH, exerting its therapeutic effect.59
Since hyaluronic acid (HA) can bind to CD44 on the surface of various tumor cells, Duan et al conjugated the anticancer drug geraniol (GER) with biocompatible HA via disulfide bonds, obtaining 110 nm diameter amphiphilic spherical nanoparticles (HSSG). After 96 hours of incubation at pH 5.5, the drug release rate of HSSG nanoparticles reached 74.7%, higher than the 69.9% achieved by nanoparticles (HCCG) constructed via a two-carbon atom linkage, demonstrating good responsiveness of disulfide bonds. Leveraging the high hyaluronidase content, reducing environment, and low pH in liver cancer tissue, HSSG NPs induced S-phase arrest in liver cancer HepG2 and Huh7 cells, significantly inhibiting cell proliferation.54 In another study by Yu et al, it was found that this nano-prodrug also exhibited good inhibitory effects on prostate cancer.60 Additionally, delivery systems based on multiple tumor overexpressed products such as carbonic anhydrase IX (CAIX), integrin αvβ3, and VIM have also been designed.61–63
Type of Drug Delivery Carrier Based on Disulfide Bond
A nanocarrier is a drug delivery device that is prepared by nanotechnology and has a tiny size, typically between 1 and 1000 nm, to facilitate better penetration through biological barriers such as cell membranes and the blood-brain barrier, enabling precise drug delivery.64 This system can regulate the release rate and targeting of drugs by altering the size, shape, surface properties, and drug encapsulation methods of the nanocarriers, thus achieving precise disease treatment.
Nanocarrier systems have broad prospects in the field of cancer therapy and can transport various types of anticancer drugs, including small molecules, genes, proteins, and other large molecules, to the target site. Targeted nanocarriers selectively accumulate at tumor sites, effectively improving the bioavailability and therapeutic effects of drugs, while reducing their toxic side effects on normal tissues. Additionally, they can enhance stability, solubility, blood circulation time, and cellular uptake.65–67 The most familiar and commonly used nanocarriers include polymeric nanoparticles, liposomes, magnetic nanoparticles, gold nanoparticles, mesoporous silica nanoparticles (MSNs), polymeric micelles, and dendritic polymers.55 Below, I will primarily discuss the types of nanocarriers commonly used for constructing disulfide bond-based systems.
Polymeric Nanoparticles
Polymer nanoparticles primarily consist of polymers, including drug/polymer conjugates, polymer micelles, etc., with sizes mostly ranging from 10 to 1000 nanometers. Due to their simple processing, good biocompatibility, structural diversity, and strong biomimicry, they are widely used in biomaterial research. Nanoparticles are divided into nanocapsules and nanospheres.68,69 Nanospheres uniformly disperse drugs within the matrix, while nanocapsules encapsulate drugs in cavities, surrounded by a polymeric shell. Common materials for constructing polymer nanoparticles include albumin, chitosan, gelatin, polylactides (PLA), poly(lactide-co-glycolides) (PLGA), polyglycolides (PGA), polyorthoesters (POE), polycyanoacrylates, polycaprolactone (PCL), poly(malic acid) (PMLA), polyglutamic acid (PGA), poly(methyl methacrylate) (PMMA), etc.69,70 Additionally, natural materials with excellent biocompatibility such as chitosan and its derivatives, HA and alginate are widely used in the construction of polymer nanoparticles.71 The carried drugs can be encapsulated within the particles, adsorbed onto the surface, or covalently linked to the particle surface.67 Moreover, the surface of polymer nanoparticles can be designed by using different polymer end groups or by coupling specific polymers to achieve targeting and prolonged circulation time. In the construction of polymer nanoparticles, disulfide bonds are often used as connections between different polymer molecules, or between polymer molecules and drugs. By using the unique function of the disulfide bond, the vector can be endowed with the ability of targeting and reduction response and has good biocompatibility and degradability. However, due to the nature of nanoparticles, the stability of this type of vector is poor, and further modification is often needed to improve its circulation and enhance its stability.
Zhang et al selected 2-octyl-1-dodecanol (OD), the most representative modification module to link mitoxantrone (MTO) via a redox-responsive disulfide bond, developing a tumor-selective MTO prodrug (MTO-SS-OD). They also utilized DSPE-PEG2K as a surface functionalized module to develop MTO prodrug nanocomposites. To determine the impact of the surface functionalized module DSPE-PEG2K on the stability and circulation time of nanoparticles, the authors constructed nano-prodrugs with different PEG ratios. The results showed that nanoparticles without any surfactant significantly increased in size when exposed to physiological conditions (pH=7.4 PBS containing 10% FBS), indicating instability in blood circulation. In contrast, 40% of NPs exhibited excellent assembly stability and cellular uptake efficiency, providing a new approach for the rational design of MTO prodrug nanocomposites (Figure 4).72
 |
Figure 4 Schematic diagram of the impact of surface functionalized module on the performance of MTO prodrug nanocomposites for efficient cancer therapy. Reproduced with permission from Zhang B, Li L, Huang M, et al. Probing the impact of surface functionalization module on the performance of mitoxantrone prodrug nanoassemblies: improving the effectiveness and safety. Nano Lett. 2024;24(12):3759–3767. Copyright©2024 American Chemical Society.72 |
Polymeric micelles (PMs) are a common type of nanoscale structure, typically ranging in size from 10 to 200 nm, formed through the self-assembly of polymer molecules in an aqueous solution.74 These polymeric micelles consist of a hydrophobic core and a hydrophilic or amphiphilic shell composed of structural units. In this structure, drugs are encapsulated within the core through hydrophobic interactions, hydrogen bonding, and pi-pi interactions, which shield them from the aqueous environment.73,75 The shell, on the other hand, stabilizes the drugs in the body and controls their release rate.
Based on the biological characteristics of the target site, polymeric micelles can be designed for active targeting and responsive release by incorporating ligand conjugation or pH- and GSH-sensitive moieties. Hydrophobic polymers that are commonly used for encapsulating the hydrophobic core include PLA, PLGA, polypeptides, and lipids.69,76 Hydrophilic polymers used to form the shell include PEG, chitosan, dextran, and HA.77,78 This system finds extensive applications in cancer therapy, inflammation treatment, and infectious disease treatment, providing strong support for precision medicine and personalized therapy. Disulfide bonds are often used as linking molecules in polymer micelles, giving them reductive responsiveness. At the same time, this combination not only improves the hydrophilicity of the drug but also can trigger the reducing state and release at a specific location. To address the shortcomings of PTX, including poor aqueous solubility and serious side effects, Ding et al coupled poly(ethylene glycol) methyl ether methacrylate (PEGMEA) to PTX prodrug through a disulfide bond.79 And Li et al conjugated high-water-soluble hydroxyethyl starch (HES) to PTX via a disulfide bond and prepared HES-SS-PTX.80 In another case, Yang et al coupled D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) with PTX and DOX, respectively.81
Liposomes
Liposomes are colloidal spheres formed by self-assembly of amphiphilic lipid molecules in solution,82 originally discovered by Dr. Alec D. Bangham in the 1960s.83 These liposomes consist of hydrophilic heads and hydrophobic tails, forming a bilayer structure akin to cell membranes. For drug delivery applications, the ideal size of liposomes ranges between 50 and 200 nanometers.84 Different water-soluble drugs can be loaded in various ways: hydrophilic molecules may reside within the aqueous core, hydrophobic molecules can penetrate the lipid bilayer, and amphiphilic molecules may be located at the water/lipid interface. Compared with other carriers, the bilayer membrane structure of liposomes has better biocompatibility and membrane permeability and has unique advantages in tumor treatment. The disulfide bond is often used as a bridge between lipid molecules and other functional molecules, and the vector is endowed with targeting and response-ability through the disulfide bond. Enhanced vector stability, improved circulation, or enhanced targeting by other functional molecules.
To improve liposome transportation in vivo, avoid uptake by the mononuclear phagocyte system, and prolong circulation time, hydrophilic polymers such as PEG are commonly used to modify the liposome surface, achieving a “stealth” effect.85 Due to their excellent performance, liposomes are frequently employed as delivery vehicles for targeted cancer therapy.82,86 For instance, Wang et al engineered a drug delivery platform for multiple drugs and various tumor treatments by connecting cholesterol and sphingomyelin (SM) lipid bilayers through disulfide bonds.87 He et al combined camptothecin (CPT) with glycerophosphoryl choline couples through disulfide bonding agents to form CPT-phosphorylcholine coupling (CPT-SS-GPC), which can be assembled into liposomes for transport and function in the body.88 Similarly, some scholars have constructed Gemcitabine and ONC201 liposomal dual drug delivery systems through a disulfide bond, which also shows unique advantages in anti-tumor and has a broad application prospect for the treatment of pancreatic ductal adenocarcinoma.89 However, research indicates significant issues with the current manufacturing processes of liposomes and the low efficiency of intracellular delivery, limiting their widespread clinical application.90
Mesoporous Silica Nanoparticles
Nanocarriers can be divided into organic and inorganic. Organic nanoparticles, such as the polymeric nanoparticles and liposomes described above, have high biocompatibility and degradability, but with low drug-carrying capacity and poor stability. In contrast, MSNs and other inorganic carriers have better stability and more modification sites, but poor biocompatibility and trace toxicity. By modifying the disulfide bond, the surface sites are covered, giving the vector new functions and reducing side effects.
MSNs are nanomaterials with a mesoporous structure, typically composed of silicon oxide. Compared to other drug delivery systems, MSNs possess unique mesoporous structures and high surface area ratios.91,92 Due to their highly controllable pore sizes and surface areas, they have broad potential applications in drug delivery, biosensing, catalysis, and energy storage. In the field of drug delivery, MSNs can be used as drug carriers to efficiently load and control the release of drugs by controlling pore size and surface properties. This enhances the bioavailability and targeting of drugs while reducing their toxic side effects. However, MSNs are generally considered to have low toxicity.91 The silanol groups on their surface can interact with biomolecules such as cell membrane lipids and proteins, leading to the disruption of their structures. Therefore, surface modification of MSNs plays a crucial role. PEG is the most common surface modification material, and studies have shown that when MSNs are modified with PEG, the surface silanol groups are covered, resulting in a significant reduction in cytotoxicity and hemolysis.93,94 However, PEG modification may also trigger specific hypersensitivity reactions and accelerate drug clearance from the bloodstream, limiting the widespread biomedical applications of MSNs.95 Apart from surface modification, the size of MSNs is also considered a key factor influencing their deposition site, biocompatibility, cellular uptake, and toxicity.96–100 In the study by Zhao et al, MSNs were used for co-delivery of siRNA and DOX to achieve redox-controlled release. The mesoporous silica core was loaded with DOX, while siRNA was connected to the core surface through disulfide bonds, acting as a gatekeeper. The disulfide bonds were also utilized for targeting intracellular GSH, whose cleavage triggered the release of DOX and siRNA. The nanoparticles showed satisfactory therapeutic effects in inhibiting tumor growth in vivo.101 In another study by Zhang et al, HA was conjugated to the surface of MSNs via disulfide bonds, and DOX was physically encapsulated in HA-modified MSNs as a model drug (MSNs/SS/HA@DOX). In Hela cells, MSNs/SS/HA@DOX was internalized into cells through the enhanced permeability and retention (EPR) effect and receptor-mediated endocytosis. The release of the drug was induced by the response to GSH, leading to cell death.102 Some researchers also use MSNs as the carrier core, which is loaded with fluorescent dye (ferrubin O) or chemotherapy agent (DOX), and functionalized with PEG chains containing disulfide bonds on the surface. To achieve the effect of continuous drug release in the local tumor, the nanoparticles are dispersed with HA and chitosan to make a gel state. The results show that payload delivery is triggered in the presence of reducing agent GSH. This design provides a new idea for tumor-targeted controlled release, which can realize the local transfer of drugs into the tumor (Figure 5).103
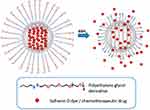 |
Figure 5 Schematic representation of the gated material (S2 and S3) capped with PEG chains via disulfide linkage, and its activation with intracellular GSH. Reproduced with permission from de la Torre C, Coll C, Ultimo A, et al. In situ-forming gels loaded with stimuli-responsive gated mesoporous silica nanoparticles for local sustained drug delivery. Pharmaceutics. 2023; 15(4): 1071. Creative Commons.103 |
Different drug delivery systems based on disulfide bonds have their advantages and disadvantages. In general, polymeric nanoparticles and liposomes have better biocompatibility and degradability with higher safety. MSNs are more stable and more convenient to modify, but less secure. Modification of disulfide bonds gives them the ability to target and reduce response, which plays a role in improving transport, improving stability, or reducing toxicity. Due to the wide range of materials and good biocompatibility, polymer nanoparticles, and polymer micelles are the most widely used carrier types in current research. In addition, other types of nanocarriers, such as magnetic nanoparticles, gold nanoparticles, dendritic polymers, etc., have also been used to construct disulfide bond drug delivery carriers.
Connection Mode of Disulfide Bond in Drug Delivery System
Disulfide bonds are often located in different positions in the drug delivery system, connecting different components, such as it can connect drugs to polymer molecules, drugs to drugs, and polymer molecules to polymer molecules. Through different connection modes, the functions of coupled drug delivery, controlled release or targeting can be achieved. At present, most reviews of the same type mainly start from the perspective of carrier types, but this paper innovatively discusses the connection location of disulfide bonds, and more comprehensively and carefully demonstrates the application and development direction of drug delivery systems based on disulfide bonds.
Drug and Polymer Molecular Connection
Currently, most polymers used in drug delivery system design are biocompatible, non-toxic, and biodegradable materials that do not produce adverse effects on drug efficacy while altering the water solubility of drugs. Some anticancer drugs exhibit high lipid solubility, resulting in poor solubility in plasma. To improve their solubility, reduce the dosage, increase stability, and prolong blood circulation time, drug-polymer conjugates are often prepared by linking the drug to hydrophilic polymers via disulfide bonds. These amphiphilic drug delivery carriers can self-assemble in aqueous solutions into spherical nanoparticles, with the hydrophobic drug as the core and the hydrophilic polymer as the shell, thereby improving the transport of the drug in the bloodstream. Compared to other nanoscale drug delivery systems, self-assembly can reduce the use of excipients, enhance drug loading capacity, lessen the financial burden on patients, and improve safety.105
Temozolomide (TMZ) is a moderately lipophilic alkylating agent and is currently the only chemotherapy drug available for treating glioblastoma multiforme (GBM). Due to its short half-life and hydrophobicity, TMZ is typically used at high doses, resulting in significant side effects. Researchers have coupled TMZ to β-glucan using a disulfide bond-containing linker (2,2’-dithioethanol) to prepare a prodrug self-assembled nanoparticle. The hydrophilic β-glucan wraps around TMZ, improving its water solubility. Compared with free TMZ, macrophages (Mφ) cells treated with prodrug nanoparticles maintained higher viability, indicating that Mφ could be used to transport TMZ. In the tumor microenvironment, the disulfide bond is cleaved in the presence of GSH, releasing TMZ to exert its therapeutic effect. In vivo experiments, compared with free TMZ, mice treated with prodrug nanoparticles had less weight loss, higher survival rate, more tumor volume and weight reduction, and showed significantly higher anti-tumor efficacy.106 Temozolomide (TMZ) is a prodrug of 5-(3-methyltriazene-1- yl)imidazole-4-carboxamide (MTIC). Du et al designed a diblock copolymer-based MTIC prodrug installed with a disulfide linkage and achieved self-assembly of polymer micelles under physiological conditions without the fear of MTIC leakage. Through thiol/sulfide exchange, polymer micelles could be induced to decompose in response to GSH, which facilitated the release of MTIC. The in vitro results showed that GSH-responsive polymeric micelles could inhibit the TMZ-sensitive and TMZ-resistant GBM cells.107 Ding et al synthesized a PTX prodrug via esterification reaction using 3,3’-dithiodipropionic acid (DTPA), 2-hydroxyethyl methacrylate (HEMA) and PTX. They then copolymerized it with poly(ethylene glycol) methyl ether methacrylate (PEGMEA) to self-assemble into spherical micelles in aqueous solution, with PTX as the core and hydrophilic PEG as the shell. The PTX loading capacity was 23%, and in vitro experiments confirmed its inhibitory effect on tumor cells.79 Li et al prepared a novel and simple GSH-responsive theranostic nanoparticle for tumor therapy. The theranostic nanoparticle, DHP, was composed of HES and PTX conjugate (HES-SS-PTX) connected by disulfide bonds and near-infrared (NIR) cyanine fluorophore DiR, which was prepared by a simple one-step dialysis method. Furthermore, DHP could also be used as an in vivo probe for fluorescence and photoacoustic imaging, while obtaining effective anti-tumor effects through chemical-photothermal combination therapy.80
In addition to coupling with hydrophilic polymers, orally administered antitumor drugs can be coupled with lipophilic polymers to enhance their lipid solubility. Lei et al respectively used two commonly used disulfide bond linkers disulfanyl acetate (ACSS) and disulfanyl-ethyl carbonate (ETCSS) to connect CPT and oleic acid (OA) to construct nano-prodrugs and studied their release. They found that the cleavage of ETCSS directly coupled with the release of CPT, while the cleavage of ACSS led to the generation of CPT-SH intermediates, and their chemical stability determined the release of CPT. Additionally, the presence of disulfide bonds significantly reduced the chemical stability of CPT-OA, leading to a significant increase in drug release in the culture medium.104
Due to the propensity of single chemotherapy drugs to induce tumor resistance, leading to tumor recurrence, multiple chemotherapy drugs are typically used in the clinical treatment of malignant tumors. Variations in drug solubility, transport, and utilization make it difficult to control the actual utilization ratio, which may result in different clinical responses, affecting treatment outcomes.108 Both DOX and PTX are frontline drugs for treating breast cancer, and their combined use can enhance efficacy, albeit with more pronounced side effects. To improve targeting, reduce toxicity, and enhance efficacy, Yang et al utilized D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) as a carrier and employed the disulfide-containing small molecule 3, 3′-dithio-dipropionic acid (DTDP) as a linker to connect the drugs with TPGS, synthesizing amphiphilic TPGS-SS-DOX and TPGS-SS-PTX prodrugs. These two prodrugs are self-assembled into mixed micelles loaded with DOX and PTX in an ideal ratio. Compared to formulations without DTT, the addition of DTT increased the release of PTX and DOX, indicating that the highly reducing tumor environment promotes prodrug release.81
Drug and Drug Connection
In addition to linking to polymer molecules, drugs can also be linked to the same or different drugs through disulfide bonds, thereby improving the drug loading rate or exerting the synergistic effect of two drugs. Zou et al synthesized a redox-sensitive nanosystem based on the amphiphilic PTX-ss-TMP conjugate, in which PTX and tetramethylpyrazine (TMP) are linked via a disulfide bond. The PTX-ss-TMP nanoparticles rapidly disintegrated in tumor tissue in response to a high intracellular GSH concentration. Furthermore, tumor progression may be effectively suppressed by the combined effects of PTX and TMP on cancer cell proliferation arrest and angiogenesis inhibition, respectively.109 Sun et al used carbon chains containing disulfide bonds of different lengths to connect PTX and citronellol (CIT), resulting in the synthesis of three prodrugs: α-PTX-SS-CIT, β-PTX-SS-CIT, and γ-PTX-SS-CIT. These prodrugs were self-assembled into uniform nanostructures once dispersed in water. The use of DSPE-PEG2K improved the colloidal stability of the drug precursor nanocomplexes. The results showed a drug loading rate of over 55% for the nano-prodrugs. Further studies demonstrated that β-PTX-SS-CIT NPs and γ-PTX-SS-CIT NPs exhibited delayed release and weaker cytotoxicity. On the other hand, α-PTX-SS-CIT NPs displayed superior dual-reactive drug release at the tumor site, leading to a stronger chemotherapy effect.32
In addition, Tang et al synthesized a drug dimer connected by disulfide bonds and encapsulated with F127. This dimer links LND and NLG919 via disulfide bonds, which can be cleaved by excessive GSH in the tumor microenvironment to release these two drugs. Released LND can reduce the expression of hexokinase II, disrupt mitochondria, and inhibit glycolysis to block energy supply. NLG919, on the other hand, can reduce the accumulation of kynurenine and regulate the number of regulatory T cells, thereby alleviating the immunosuppressive microenvironment.110 Li et al constructed a novel prodrug, CPT-SS-NLG919, using disulfide bonds. This drug can induce immunogenic cell death (ICD) while inhibiting indoleamine 2,3-dioxygenase (IDO) to promote dendritic cell (DC) maturation, reduce Treg cells, alleviate tryptophan consumption, and decrease the levels of IL-6, IL-13, and TGF-β. Consequently, it transforms the ecological niche of cancer stem cells (CSCs) into an environment unfavorable for CSC survival and effectively eliminates CSCs.111 In a study by Jong Seung Kim’s research group, a prodrug composed of triphenylphosphonium, PP2A inhibitor demethylcantharidin (DMC), and floxuridine (FUDR) was designed and synthesized via disulfide bond linkage. The triphenylphosphonium group targets mitochondria, delivering the prodrug M1 to the mitochondria of tumor cells. The disulfide bond can be selectively degraded by GSH in mitochondria, releasing DMC and FUDR, which cause mitochondrial DNA damage and subsequently induce mitochondria-mediated cell apoptosis.112
Polymer Molecular and Polymer Molecular Connection
In the construction of drug carriers, sometimes disulfide bonds are not directly connected to anti-tumor drugs but serve as linkers to connect polymer systems, forming nanogels for drug transport. The drug molecules are encapsulated within the nanogels, and when they reach the tumor environment, the disulfide bonds are cleaved by excessive levels of GSH, leading to the degradation of the nanogels and the release of the drugs to exert their therapeutic effects. This connection method is connected between polymer molecules, avoiding the direct transformation of the drug, and effectively preventing the change of drug effect caused by the change of drug molecules. More than any other connection, this is like weaving a pocket containing a drug using disulfide bonds. When transported to a specific location, the disulfide bonds break, causing the pocket to disintegrate, and the drug to be released. Pan et al prepared redox/pH dual stimuli-responsive poly(methacrylic acid) (PMAA)-based nanohydrogels from methacrylic acid and N,N-bis(acryloyl)cystamine crosslinker via distillation-precipitation polymerization. The anticancer drug DOX was loaded into PMAA, achieving a maximum drug loading efficiency of up to 95.7%. Water-soluble reducing agents like DTT and GSH can degrade the nanohydrogels into short polymer chains, and different pH values also influence the release of DOX. Results demonstrated that the nanohydrogels exhibited higher release efficiency and greater cytotoxicity in glioma cells compared to normal cells, confirming their targeting ability.22 Zhou et al successfully synthesized AG-PEG-SS-PLA, a PEG-PLA polymer conjugate linked by disulfide bonds and coupled with glucosamine (AG). PTX was solubilized in dimethyl sulfoxide and added to AG-PEG-SS-PLA, resulting in PTX encapsulation within the nano-sized micelles. After internalization, the disulfide bonds in AG-PEG-SS-PLA were cleaved by the highly reducing environment inside cells, leading to the decomposition of the nanomicelles and rapid release of the encapsulated PTX.57
In addition to large polymer systems, Cao et al successfully constructed a tunable gel using the abundant intramolecular disulfide bonds in keratin. The disulfide bonds within the keratin molecules can be cleaved by reducing agents such as cysteine, releasing free thiol groups. Subsequently, the thiol groups on different keratin molecules can form intermolecular disulfide bonds through thiol oxidation, resulting in the cross-linking of keratin to form a gel as a drug transport carrier. The researchers studied the drug release behavior of the drug-loaded keratin hydrogel using ciprofloxacin and DOX as examples. The results showed that all the hydrogels exhibited near zero-order degradation rate curves in both PBS and GSH culture media. The drug release rate was positively correlated with the gel degradation rate, indicating that the crosslinking degree of the gel and the drug release rate can be regulated by adjusting the cysteine level. Besides, the study also found a positive correlation between gel degradation rate and GSH level.113
In addition to the traditional encapsulation approach, Zhou et al designed a double-layered vesicle structure resembling a sandwich biscuit. It links triethoxysilyl head and hydrophobic alkyl double chain through a cleavable disulfide bond, forming amphiphilic molecules termed CFL. These CFL molecules were then further assembled into lipid bilayer vesicles using a sol-gel method in an aqueous solution. PTX was loaded between the two CFL layers, forming a highly stable crosslinked siloxane network structure. Compared to other drug delivery systems, the crosslinked siloxane surface exhibited excellent stability, effectively avoiding premature drug leakage and associated side effects.114 Yang et al using the strong host-guest interaction between cyclodextrins (CDs) and drug molecules, employed a thiol-initiated ring-opening polymerization reaction to synthesize a main-chain product consisting of the chemotherapeutic drug DM1 and poly(thioctic acid) (PTA). β-cyclodextrins (β-CDs) were attached to the main chain, and DOX and CPT were loaded separately onto the β-CDs. Due to the microtubule-inhibitory activity of DM1, it can exert a synergistic effect with DOX or PTA. This nanoparticle exhibited dual responsiveness to pH and GSH, providing a new strategy for combination therapy.115
In summary, disulfide bonds are connected in the drug delivery vehicle in the three ways described above. To improve drug transport and increase water solubility or fat solubility, drugs can be linked to polymer molecules with different hydrophilicity. To improve drug loading or play a synergistic role, drugs can be connected with the same or different drugs; To target drug transport without altering the structure of the drug itself, the drug can be loaded with polymer molecules linked by disulfide bonds. With the deepening of more and more research, sometimes the carrier does not use only one connection mode, but a combination of two or more connection modes. In the design of the carrier, the characteristics of the drug itself should be fully considered to select the most suitable disulfide bond connection mode.
Multifunctional Delivery Carrier Based on Disulfide Bond
Photodynamic therapy (PDT) has gradually emerged as an alternative treatment for superficial tumors in recent years, relying on its safety, minimally invasive nature, and fast response. It primarily utilizes photosensitizers and the combined action of light and oxygen to locally generate ROS and induce tumor cell death.116,117 However, due to the low water solubility and cytotoxicity of photosensitizers, precise delivery to the tumor site is challenging, leading to reduced effectiveness and potential adverse effects on normal tissues. The delivery of photosensitizers in conjunction with disulfide linkages has become a major focus in the field of tumor drug delivery. Under the action of photosensitizers, a large amount of ROS is generated locally, causing tumor cell rupture and the release of a series of immunogenic substances, thereby activating the immune system to eliminate the tumor. Some researchers have utilized disulfide-bridged hollow mesoporous organosilica nanoparticles (-SS-HMONs) as carriers, with the photosensitizer MB loaded inside and an outer layer of the MnO2 membrane. Both manganese dioxide and disulfide linkages possess GSH-responsive properties, allowing them to rapidly consume excessive GSH and alleviate the inhibitory effect of GSH on ROS generation during photodynamic therapy. The encapsulation of photosensitizers with manganese dioxide protects them from direct contact with human tissue cells before reaching the tumor site, thereby reducing their toxic effects on normal tissues and effectively preventing the deactivation of MB. These advancements contribute to improving the therapeutic effect of PDT.118 In another study by Jiao et al, a photosensitizer (protoporphyrin IX) was connected to a quencher (disperse blue 3) using disulfide linkages. The LND-1 fragment was conjugated with a mitochondrial targeting peptide sequence Phe-Arg-Phe-Lys, a tissue protease B (CTSB) cleavable sequence Gly-Phe-Leu-Gly, and a hydrophilic PEG tail. The two components were mixed and assembled into nanoparticles. Due to the higher GSH levels in cancer cells, the disulfide linkages break, activating the photosensitizer and promoting cancer-specific fluorescence imaging and precise PDT. Simultaneously, under the guidance of the LND-1 mitochondrial targeting sequence, precise targeting of mitochondria is achieved (Figure 6).119
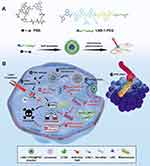 |
Figure 6 Schematic illustration of redox and enzyme dual-responsive nanoplatform for co-delivery of lonidamine (LND) and activable photosensitizer (PSD) with enhanced selectivity and efficacy toward cancer cells. (A) Nanoparticle formation through self-assembly of LND-1-PEG and PSD. (B) The cellular mechanism of LND-1-PEG@PSD-induced cell death through the synergy of PDT and chemotherapy. Reproduced with permission from Jiao Q, Zheng Y, Xie Q, et al. A dual-responsive morphologically-adaptable nanoplatform for targeted delivery of activatable photosensitizers in precision photodynamic therapy. Small. 2023; e2309054. Copyright © 2023 Wiley‐VCH GmbH.119 |
Yu et al used chondroitin sulfate A (CSA), a highly hydrophilic and biodegradable natural polysaccharide with CD44 targeting function, as a carrier material, connected the photosensitizer chlorin e6 (Ce6) to CAS via a disulfide bond, and then incorporated DOX into it to make a CSA delivery platform with CD44 targeting and reduction sensitivity, CSSC-D. In vivo experiments showed that after 12 days of treatment, compared with CSSC treated with drugs alone or without DOX, CSSC-D had a better tumor inhibition effect, and no weight loss was observed in mice, suggesting that it has good therapeutic effect and safety.121 In another study, Yu et al constructed a monoclonal antibody-modified dual drug delivery system using two macromolecular prodrugs. Firstly, DOX prodrug (GMA-ss-DOX) was synthesized by disulfide bonding, and maleimide pegyl-Ce6 (Mal-PEG-Ce6) was prepared by acylation. The two polymers were self-assembled to form DOX and Ce6-co-loaded mixed micelles (DCMMs). The maleimide group (Mal-) on its surface was further linked to the anti-EGFR monoclonal antibody nistuzumab, and the mixed micelle modified with Nimotuzumab (NTTZ-DCMMS) was obtained. The experimental results showed that the cumulative release of DOX in the buffer solution with 10 mM GSH was as high as 63.4%, which was much higher than the 7.2% without GSH, demonstrating its good reduction responsiveness. On the other hand, compared with free DOX, the toxicity of prodrug to H22 cells was significantly reduced, and the surface carrier had the function of reducing side effects. At the same time, compared with the single drug delivery system Mal-PEG-Ce6 and GMA-ss-DOX, NTZ-DCMMs have a better cell-killing effect, indicating that DOX and Ce6 can play a synergistic role in inhibiting H22 cells.122
Cyclodextrin (CD) is a cyclic oligosaccharide connected by 1,4-glycosidic bonds, which is produced by the enzymatic hydrolysis of starch.123 It possesses a hydrophobic central cavity and a hydrophilic exterior. The hydrophobic cavity consists of interconnected glucose units. Low water-soluble drugs of appropriate size can be captured in this cavity through host-guest interactions between the guest molecules and the hydrophobic host cavity, thereby increasing the drug’s solubility and expanding its application range. The encapsulated drug can be continuously released from the hydrophobic cavity, resulting in sustained therapeutic effects.120 Additionally, as a natural biomaterial, cyclodextrin exhibits characteristics such as renewable, biodegradable, non-carcinogenic, non-antigenic, and antibacterial properties.124 It provides a good structural basis for targeted drug delivery systems and is an advantageous choice for drug delivery carriers. To improve the water solubility of the photosensitizer Ce6, researchers have utilized succinylated β-CD to form a connection with Ce6 through a disulfide bond, enhancing transport and endowing it with reduction-responsive capability. He et al designed a acid-sensitive β-carboxylic acid amide bond to connect the immune modulator demethylcantharidin (DMC) with the carrier β-CD to form DMC-CD. Additionally, the hydrophobic photosensitizer Ce6 was connected with the hydrophobic terminal peptide FFVLGGGC through a disulfide bond, forming Ad-ss-pep-Ce6. These two components self-assembled through host-guest recognition to form DMC-CD/Ad-ss-pep-Ce6 nanoparticles (DACss). The results showed its excellent dual responsiveness to GSH and low PH. By combining the photosensitizer and immune modulator, the simultaneous delivery of both was achieved, reducing the toxicity of the photosensitizer and compensating for the immune suppression in the tumor microenvironment during PDT. Ultimately, this approach achieved outstanding anti-tumor and anti-metastasis effects in situ lung metastasis.125
The treatment of tumors is a complex and lengthy process. With the development of increasingly advanced technologies and the interdisciplinary application of multiple disciplines, multifunctional mixed drug delivery systems that integrate therapy and diagnosis, combine physics and chemistry, or have real-time imaging capabilities will become more common. Scholars have used a combination of fluorescent probes with high fluorescence quantum yields such as BODIPY and the anticancer drug PTX to monitor the real-time release of drugs in cells.126 When the drug enters the tumor tissue, the high concentration of GSH cleaves the disulfide bond, leading to the release of PTX and fluorescence quenching, thereby achieving drug release monitoring.
Although existing technologies such as magnetic resonance imaging (MRI), computed tomography scans, endoscopic examinations, and other imaging techniques can aid in cancer detection, early diagnosis is very challenging due to their difficulty in distinguishing between cancer cells and healthy cells.127 Dicyanomethylene-4H-pyran (DCM) is a hydrophobic near-infrared dye whose fluorescence is quenched when occupied at the amino end and restored when free. Therefore, by connecting DCM with DOX through disulfide bonds, an intelligent nanocarrier integrating diagnosis and treatment can be constructed, enabling immediate treatment after diagnosis without significant time delays, thereby more effectively treating tumors. When the disulfide bond is broken due to the high concentration of GSH in tumor tissue, DCM is released and activated, and DOX is released to exert its anti-tumor function.128 Mo et al utilized molybdenum disulfide nanosheets modified with strong near-infrared absorption and excellent photothermal performance, connecting them with DOX via disulfide bonds. The molybdenum disulfide nanosheets were photothermally induced for tumor ablation, and disulfide bonds delivered antitumor drugs to tumor cells.129 Sun et al combined photothermal therapy with chemodynamic therapy (CDT) by assembling Cu2+-tannic acid (TA) coordination shells on a zeolitic imidazolate framework-8 (ZIF-8) skeleton filled with phycocyanin (PC), and then preparing HA polymer via disulfide bonds. This achieved pH, GSH responsiveness, and tumor targeting, with HA imparting targeting functionality through CD44. Under NIR-II irradiation, PC can promote the generation of endogenous photothermal nanoagents Cu, thereby activating photothermal therapy. At the same time, Cu2+ acts as a good Fenton-like drug, consuming GSH, enhancing CDT, and synergistically acting with photothermal therapy in cancer treatment.130
In addition to coupling with conventional carriers, magnetic nanoparticles capable of precise targeting via electromagnetic driving can also be combined with disulfide bonds for drug delivery.131 While exerting the targeting effect of disulfide bonds, they can be further precisely delivered to the lesion site using external electromagnetic fields, demonstrating good efficacy.
In general, single delivery of chemotherapy drugs may not meet the needs of future diagnosis and treatment, and multi-functional carriers based on disulfide bond design are gradually becoming the focus of research. Cyclodextrin vectors are increasingly used in the construction of multifunctional vectors because of their unique “host-guest interaction”. At the same time, photodynamic therapy, chemodynamic therapy, diagnosis, imaging, and other functions are increasingly combined with chemotherapy. The functions of carriers are gradually enriched, and the disulfide bond plays an important role as a response element.
Conclusion
With the deepening of research on the mechanism of tumor development, the drug delivery system targeting tumor microenvironment mediated by disulfide bonds has shown obvious advantages. Due to abnormalities in maintaining their environment and function, tumor tissues are often in a microenvironment different from normal tissues, including high reoxidation-reduction levels and low pH. Meanwhile, tumors are more susceptible to environmental interference. Changing the tumor microenvironment can effectively inhibit tumor growth. Disulfide bond has a good ability to target tumor microenvironment, while targeting, it can consume excessive GSH and ROS, and destroy the environment conducive to tumor growth. The drug delivery system constructed by disulfide bonds can not only assist drug delivery but also interfere with the tumor microenvironment to play a synergistic anti-tumor effect and reduce multi-drug resistance. At the same time, when the drug does not reach the site of action, the drug is not active due to the presence of disulfide bonds, which can reduce the damage to normal tissues. Disulfide bonds have been widely used in the construction of various anti-tumor nanomaterials. However, small differences in the disulfide bond connection locations can significantly affect the assembly performance, reactivity, and release rate of nanomedical drugs.132 The safety of structures with high reactivity cannot be guaranteed, while structures with low reactivity cannot achieve rapid and massive release of drugs in tumor tissues. Therefore, appropriate reactivity can be designed. A connection structure that guarantees both security and effectiveness is essential. In addition, the carrier materials used to construct the drug delivery system also significantly affect the circulation, metabolism, biodegradability, and safety of drugs, and there are still many challenges in treatment. In the future, drug delivery systems targeting tumor microenvironments based on disulfide bonds will be more and more used in the research of nano-agents, playing a unique and important value and significance in the drug therapy of tumors.
The drug delivery system based on disulfide bonds can effectively improve the therapeutic effect and safety of anti-tumor drugs and has application value in the treatment of a variety of tumors, especially those with high local reduction states. In addition, it can also play a variety of targeting and response functions through the connection with various functional groups, to meet the needs of different tumor therapy. Through precise targeted release, the side effects of drugs can be reduced, the quality of life of patients can be improved, and problems such as multi-drug resistance can be overcome, providing a new solution for the treatment of refractory tumors. At the same time, such systems also face many challenges, such as the accuracy of response release, the safety of polymer materials, and the cycling stability of drug delivery systems.
In the future, drug delivery systems based on disulfide bonds will be developed more and more and will be further improved in terms of response accuracy, multi-targeting, versatility, sustainability and safety, biocompatibility and safety, etc. In particular, multi-functional drug carriers are becoming the focus of research by scholars. These carriers can realize multi-function integration and regulation through the reasonably designed disulfide bond structure, so that they are not only the delivery vehicles of drugs, but also the functions of diagnosis, image navigation, or treatment monitoring. In general, the tumor microenvironment-responsive drug delivery system based on disulfide bonds represents an important advance in the field of drug delivery technology and has broad clinical application potential, which is expected to bring breakthroughs and improvements in tumor therapy.
Acknowledgments
This study was supported by grants from the Science and Technology Development Plan Projects of Jilin Province (YDZJ202401466ZYTS).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
2. Koti S, Demyan L, Deutsch G, et al. Surgery for oligometastatic pancreatic cancer: defining biologic resectability. Ann Surg Oncol. 2024;31(6):4031–4041. doi:10.1245/s10434-024-15129-8
3. Shah C, Bauer-Nilsen K, McNulty RH, et al. Novel radiation therapy approaches for breast cancer treatment. Semin Oncol. 2020;47(4):209–216. doi:10.1053/j.seminoncol
4. Sampath VS. Four-drug therapy for multiple myeloma. N Engl J Med. 2024;390(15):1440. doi:10.1056/NEJMc2402133
5. Katsikis PD, Ishii KJ, Schliehe C. Challenges in developing personalized neoantigen cancer vaccines. Nat Rev Immunol. 2024;24(3):213–227. doi:10.1038/s41577-023-00937-y
6. Zong JF, Lin PJ, Tsou HH, et al. Comparison the acute toxicity of two different induction chemotherapy schedules with cisplatin and fluorouracil in nasopharyngeal carcinoma patients. Radiother Oncol. 2023;184:109699. doi:10.1016/j.radonc.2023.109699
7. Zhang J, Ye ZW, Tew KD, et al. Cisplatin chemotherapy and renal function. Adv Cancer Res. 2021;152:305–327. doi:10.1016/bs.acr.2021.03.008
8. Songbo M, Lang H, Xinyong C, et al. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol Lett. 2019;307:41–48. doi:10.1016/j.toxlet.2019.02.013
9. Tan Q, Xu L, Zhang J, et al. Breast cancer cells interact with tumor-derived extracellular matrix in a molecular subtype-specific manner. Biomater Adv. 2023;146:213301. doi:10.1016/j.bioadv.2023.213301
10. Andreucci E, Peppicelli S, Ruzzolini J, et al. Physicochemical aspects of the tumour microenvironment as drivers of vasculogenic mimicry. Cancer Metastasis Rev. 2022;41(4):935–951. doi:10.1007/s10555-022-10067-x
11. Lee PJ, Ho CC, Ho H, et al. Tumor microenvironment-based screening repurposes drugs targeting cancer stem cells and cancer-associated fibroblasts. Theranostics. 2021;11(19):9667–9686. doi:10.7150/thno.62676
12. Bargakshatriya R, Pramanik SK. Stimuli-responsive prodrug chemistries for cancer therapy. Chembiochem. 2023;24(18):e202300155. doi:10.1002/cbic.202300155
13. El-Sawy HS, Al-Abd AM, Ahmed TA, et al. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano. 2018;12(11):10636–10664. doi:10.1021/acsnano.8b06104
14. Alavi SE, Alharthi S, Alavi SZ, et al. Bioresponsive drug delivery systems. Drug Discov Today. 2024;29(1):103849. doi:10.1016/j.drudis.2023.103849
15. Chen K, Li Y, Li Y, et al. Stimuli-responsive electrospun nanofibers for drug delivery, cancer therapy, wound dressing, and tissue engineering. J Nanobiotechnology. 2023;21(1):237. doi:10.1186/s12951-023-01987-z
16. Zhang H, Wang J, Wu R, et al. Self-supplied reactive oxygen species-responsive mitoxantrone polyprodrug for chemosensitization-enhanced chemotherapy under moderate hyperthermia. Adv Healthc Mater. 2024;13(12):e2303631. doi:10.1002/adhm.202303631
17. Ding Y, Yu W, Shen R, et al. Hypoxia-responsive tetrameric supramolecular polypeptide nanoprodrugs for combination therapy. Adv Healthc Mater. 2024;13(6):e2303308. doi:10.1002/adhm.202303308
18. Song X, Cai H, Shi Z, et al. Enzyme-responsive branched glycopolymer-based nanoassembly for co-delivery of paclitaxel and Akt inhibitor toward synergistic therapy of gastric cancer. Adv Sci. 2024;11(2):e2306230. doi:10.1002/advs.202306230
19. Gao J, Hou B, Zhu Q, et al. Engineered bioorthogonal POLY-PROTAC nanoparticles for tumour-specific protein degradation and precise cancer therapy. Nat Commun. 2022;13(1):4318. doi:10.1038/s41467-022-32050-4
20. Yan K, Feng Y, Gao K, et al. Fabrication of hyaluronic acid-based micelles with glutathione-responsiveness for targeted anticancer drug delivery. J Colloid Interface Sci. 2022;606(Pt 2):1586–1596. doi:10.1016/j.jcis.2021.08.129
21. Lee MH, Yang Z, Lim CW, et al. Disulfide-cleavage-triggered chemosensors and their biological applications. Chem Rev. 2013;113(7):5071–5109. doi:10.1021/cr300358b
22. Pan YJ, Chen YY, Wang DR, et al. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials. 2012;33(27):6570–6579. doi:10.1016/j.biomaterials.2012.05.062
23. Wei J, Qian Y, Bao L, et al. Disulfide bonds as a molecular switch of enzyme-activatable anticancer drug precise release for fluorescence imaging and enhancing tumor therapy. Talanta. 2024;278:126394. doi:10.1016/j.talanta.2024.126394
24. Okumura M, Noi K, Inaba K. Visualization of structural dynamics of protein disulfide isomerase enzymes in catalysis of oxidative folding and reductive unfolding. Curr Opin Struct Biol. 2021;66:49–57. doi:10.1016/j.sbi.2020.10.004
25. Kajihara D, Hon CC, Abdullah AN, et al. Analysis of splice variants of the human protein disulfide isomerase (P4HB) gene. BMC Genomics. 2020;21(1):766. doi:10.1186/s12864-020-07164-y
26. Kober FX, Koelmel W, Kuper J, et al. The crystal structure of the protein-disulfide isomerase family member ERp27 provides insights into its substrate binding capabilities. J Biol Chem. 2013;288(3):2029–2039. doi:10.1074/jbc.M112.410522
27. Bhopatkar AA, Uversky VN, Rangachari V. Disorder and cysteines in proteins: a design for orchestration of conformational see-saw and modulatory functions. Prog Mol Biol Transl Sci. 2020;174:331–373. doi:10.1016/bs.pmbts.2020.06.001
28. Messens J, Collet JF. Thiol-disulfide exchange in signaling: disulfide bonds as a switch. Antioxid Redox Signal. 2013;18(13):1594–1596. doi:10.1089/ars.2012.5156
29. Zlotnikov ID, Savchenko IV, Kudryashova EV. Specific FRET probes sensitive to chitosan-based polymeric micelles formation, drug-loading, and fine structural features. Polymers. 2024;16(6):739. doi:10.3390/polym16060739
30. Klinger GE, Zhou Y, Hao P, et al. Biomimetic reductive cleavage of keto aryl ether bonds by small-molecule thiols. ChemSusChem. 2019;12(21):4775–4779. doi:10.1002/cssc.201901742
31. Guo X, Cheng Y, Zhao X, et al. Advances in redox-responsive drug delivery systems of tumor microenvironment. J Nanobiotechnology. 2018;16(1):74. doi:10.1186/s12951-018-0398-2
32. Sun B, Luo C, Yu H, et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018;18(6):3643–3650. doi:10.1021/acs.nanolett.8b00737
33. Chen X, Song E. The theory of tumor ecosystem. Cancer Commun. 2022;42(7):587–608. doi:10.1002/cac2.12316
34. Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the Tumor Microenvironment. Cancer Discov. 2021;11(4):933–959. doi:10.1158/2159-8290.CD-20-1808
35. Pitt JM, Marabelle A, Eggermont A, et al. Targeting the tumor microenvironment: removing obstruction to anticancer immune responses and immunotherapy. Ann Oncol. 2016;27(8):1482–1492. doi:10.1093/annonc/mdw168
36. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–1437. doi:10.1038/nm.3394
37. Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. doi:10.1016/j.pharmthera.2020.107753
38. Lu SC. Glutathione synthesis. Biochim Biophys Acta. 2013;1830(5):3143–3153. doi:10.1016/j.bbagen.2012.09.008
39. Averill-Bates DA. The antioxidant glutathione. Vitam Horm. 2023;121:109–141. doi:10.1016/bs.vh.2022.09.002
40. Chen T, Chen J, Zeng T, et al. WZ35 inhibits gastric cancer cell metastasis by depleting glutathione to promote cellular metabolic remodeling. Cancer Lett. 2023;555:216044. doi:10.1016/j.canlet.2022.216044
41. Ebner J, Schmoellerl J, Piontek M, et al. ABCC1 and glutathione metabolism limit the efficacy of BCL-2 inhibitors in acute myeloid leukemia. Nat Commun. 2023;14(1):5709. doi:10.1038/s41467-023-41229-2
42. Liu T, Sun L, Zhang Y, Wang Y, Zheng J. Imbalanced GSH/ROS and sequential cell death. J Biochem Mol Toxicol. 2022;36(1):e22942. doi:10.1002/jbt.22942
43. Roy N, Paira P. Glutathione depletion and stalwart anticancer activity of metallotherapeutics inducing programmed cell death: opening a new window for cancer therapy. ACS Omega. 2024;9(19):20670–20701. doi:10.1021/acsomega.3c08890
44. Wu H, Gao X, Luo Y, et al. Targeted delivery of chemo-sonodynamic therapy via brain targeting, glutathione-consumable polymeric nanoparticles for effective brain cancer treatment. Adv Sci. 2022;9(28):e2203894. doi:10.1002/advs.202203894
45. Xiong Y, Xiao C, Li Z, Yang X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem Soc Rev. 2021;50(10):6013–6041. doi:10.1039/d0cs00718h
46. Zhong ZX, Li XZ, Liu JT, et al. Disulfide bond-based SN38 prodrug nanoassemblies with high drug loading and reduction-triggered drug release for pancreatic cancer therapy. Int J Nanomedicine. 2023;18:1281–1298. doi:10.2147/IJN.S404848
47. Fronik P, Gutmann M, Vician P, et al. A platinum(IV) prodrug strategy to overcome glutathione-based oxaliplatin resistance. Commun Chem. 2022;5(1):46. doi:10.1038/s42004-022-00661-z
48. Sarmiento-Salinas FL, Perez-Gonzalez A, Acosta-Casique A, et al. Reactive oxygen species: role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021;284:119942. doi:10.1016/j.lfs.2021.119942
49. Hyun DH. Insights into the new cancer therapy through redox homeostasis and metabolic shifts. Cancers. 2020;12(7):1822. doi:10.3390/cancers12071822
50. Cheung EC, Vousden KH. The role of ROS in tumor development and progression. Nat Rev Cancer. 2022;22(5):280–297. doi:10.1038/s41568-021-00435-0
51. Dai X, Wang D, Zhang J. Programmed cell death, redox imbalance, and cancer therapeutics. Apoptosis. 2021;26(7–8):385–414. doi:10.1007/s10495-021-01682-0
52. Zhang M, Guo X, Wang M, et al. Tumor microenvironment-induced structure changing drug/gene delivery system for overcoming delivery-associated challenges. J Control Release. 2020;323:203–224. doi:10.1016/j.jconrel.2020.04.026
53. Li Y, Feng S, Dai P, et al. Tailored Trojan horse nanocarriers for enhanced redox-responsive drug delivery. J Control Release. 2022;342:201–209. doi:10.1016/j.jconrel.2022.01.006
54. Duan S, Xia Y, Tian X, et al. A multi-bioresponsive self-assembled nano drug delivery system based on hyaluronic acid and geraniol against liver cancer. Carbohydr Polym. 2023;310:120695. doi:10.1016/j.carbpol.2023.120695
55. Kumar A, Lunawat AK, Kumar A, et al. Recent trends in nanocarrier-based drug delivery system for prostate cancer. AAPS PharmSciTech. 2024;25(3):55. doi:10.1208/s12249-024-02765-2
56. Scaranti M, Cojocaru E, Banerjee S, et al. Exploiting the folate receptor α in oncology. Nat Rev Clin Oncol. 2020;17(6):349–359. doi:10.1038/s41571-020-0339-5
57. Zhou Y, Wen H, Gu L, et al. Aminoglucose-functionalized, redox-responsive polymer nanomicelles for overcoming chemoresistance in lung cancer cells. J Nanobiotechnology. 2017;15(1):87. doi:10.1186/s12951-017-0316-z
58. Santra S, Kaittanis C, Santiesteban OJ, et al. Cell-specific, activatable, and theranostic prodrug for dual-targeted cancer imaging and therapy. J Am Chem Soc. 2011;133(41):16680–16688. doi:10.1021/ja207463b
59. Zeng Y, Song G, Zhang S, et al. GSH-responsive polymeric micelles for remodeling the tumor microenvironment to improve chemotherapy and inhibit metastasis in breast cancer. Biomacromolecules. 2023;24(11):4731–4742. doi:10.1021/acs.biomac.3c00523
60. Yu H, Ning N, He F, et al. Targeted delivery of geraniol via hyaluronic acid-conjugation enhances its anti-tumor activity against prostate cancer. Int J Nanomedicine. 2024;19:155–169. doi:10.2147/IJN.S444815
61. Zhou J, Li Y, Wang L, et al. Bifunctional drug delivery system with carbonic anhydrase IX targeting and glutathione-responsivity driven by host-guest amphiphiles for effective tumor therapy. Carbohydr Polym. 2024;326:121577. doi:10.1016/j.carbpol.2023.121577
62. Wang W, Zhang Y, Jian Y, et al. Sensitizing chemotherapy for glioma with fisetin mediated by a microenvironment-responsive nano-drug delivery system. Nanoscale. 2023;16(1):97–109. doi:10.1039/d3nr05195a
63. Li S, Zhang T, Xu W, et al. Sarcoma-targeting peptide-decorated polypeptide nanogel intracellularly delivers shikonin for upregulated osteosarcoma necroptosis and diminished pulmonary metastasis. Theranostics. 2018;8(5):1361–1375. doi:10.7150/thno.18299
64. Qiu Z, Yu Z, Xu T, et al. Novel nano-drug delivery system for brain tumor treatment. Cells. 2022;11(23):3761. doi:10.3390/cells11233761
65. Prakash S. Nano-based drug delivery system for therapeutics: a comprehensive review. Biomed Phys Eng Express. 2023;9(5). doi:10.1088/2057-1976/acedb2
66. Chen M, Huang Z, Xia M, et al. Glutathione-responsive copper-disulfiram nanoparticles for enhanced tumor chemotherapy. J Control Release. 2022;341:351–363. doi:10.1016/j.jconrel.2021.11.041
67. Ma W, Zhao Q, Zhu S, et al. Construction of glutathione-responsive paclitaxel prodrug nanoparticles for image-guided targeted delivery and breast cancer therapy. RSC Adv. 2024;14(18):12796–12806. doi:10.1039/d4ra00610k
68. Beach MA, Nayanathara U, Gao Y, et al. Polymeric nanoparticles for drug delivery. Chem Rev. 2024;124(9):5505–5616. doi:10.1021/acs.chemrev.3c00705
69. Sunazuka Y, Ueda K, Higashi K, et al. Mechanistic analysis of temperature-dependent curcumin release from poly(lactic-co-glycolic acid)/poly(lactic acid) polymer nanoparticles. Mol Pharm. 2024;21(3):1424–1435. doi:10.1021/acs.molpharmaceut.3c01066
70. Behnke M, Holick CT, Vollrath A, et al. Knowledge-based design of multifunctional polymeric nanoparticles. Handb Exp Pharmacol. 2024;284:3–26. doi:10.1007/164_2023_649
71. Anfray C, Varela CF, Ummarino A, et al. Polymeric nanocapsules loaded with poly(I:C) and resiquimod to reprogram tumor-associated macrophages for the treatment of solid tumors. Front Immunol. 2024;14:1334800. doi:10.3389/fimmu.2023.1334800
72. Zhang B, Li L, Huang M, et al. Probing the impact of surface functionalization module on the performance of mitoxantrone prodrug nanoassemblies: improving the effectiveness and safety. Nano Lett. 2024;24(12):3759–3767. doi:10.1021/acs.nanolett.4c00300
73. Hwang D, Ramsey JD, Kabanov AV. Polymeric micelles for the delivery of poorly soluble drugs: from nanoformulation to clinical approval. Adv Drug Deliv Rev. 2020;156:80–118. doi:10.1016/j.addr.2020.09.009
74. Deshmukh AS, Chauhan PN, Noolvi MN, et al. Polymeric micelles: basic research to clinical practice. Int J Pharm. 2017;532(1):249–268.
75. Shi Y, Lammers T, Storm G, et al. Physico-chemical strategies to enhance stability and drug retention of polymeric micelles for tumor-targeted drug delivery. Macromol Biosci. 2017;17(1). doi:10.1002/mabi.201600160
76. Abdallah M, Lin L, Styles IK, et al. Functionalisation of brush polyethylene glycol polymers with specific lipids extends their elimination half-life through association with natural lipid trafficking pathways. Acta Biomater. 2024;174:191–205. doi:10.1016/j.actbio.2023.12.002
77. Varela-Aramburu S, Su L, Mosquera J, et al. Introducing hyaluronic acid into supramolecular polymers and hydrogels. Biomacromolecules. 2021;22(11):4633–4641. doi:10.1021/acs.biomac.1c00927
78. Tang X, Wen Y, Zhang Z, et al. Rationally designed multifunctional nanoparticles as GSH-responsive anticancer drug delivery systems based on host-guest polymers derived from dextran and β-cyclodextrin. Carbohydr Polym. 2023;320:121207. doi:10.1016/j.carbpol.2023.121207
79. Ding Y, Chen W, Hu J, et al. Polymerizable disulfide paclitaxel prodrug for controlled drug delivery. Mater Sci Eng C Mater Biol Appl. 2014;44:386–390. doi:10.1016/j.msec.2014.08.046
80. Li Y, Wu Y, Chen J, et al. A simple glutathione-responsive turn-on theranostic nanoparticle for dual-modal imaging and chemo-photothermal combination therapy. Nano Lett. 2019;19(8):5806–5817. doi:10.1021/acs.nanolett.9b02769
81. Yang M, Ding H, Zhu Y, et al. Co-delivery of paclitaxel and doxorubicin using mixed micelles based on the redox sensitive prodrugs. Colloids Surf B Biointerfaces. 2019;175:126–135. doi:10.1016/j.colsurfb.2018.11.086
82. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
83. Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8:660–668. doi:10.1016/s0022-2836(64)80115-7
84. Elsana H, Olusanya TOB, Carr-Wilkinson J, et al. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci Rep. 2019;9(1):15120. doi:10.1038/s41598-019-51065-4
85. Shimizu T, Abu Lila AS, Fujita R, et al. A hydroxyl PEG version of PEGylated liposomes and its impact on anti-PEG IgM induction and on the accelerated clearance of PEGylated liposomes. Eur J Pharm Biopharm. 2018;127:142–149. doi:10.1016/j.ejpb.2018.02.019
86. Kim Y, Choi J, Kim EH, et al. Design of PD-L1-targeted lipid nanoparticles to turn on PTEN for efficient cancer therapy. Adv Sci. 2024;11(22):e2309917. doi:10.1002/advs.202309917
87. Wang Z, Li W, Jiang Y, et al. Cholesterol-modified sphingomyelin chimeric lipid bilayer for improved therapeutic delivery. Nat Commun. 2024;15(1):2073. doi:10.1038/s41467-024-46331-7
88. He W, Du Y, Zhou W, et al. Redox-sensitive dimeric camptothecin phosphatidylcholines-based liposomes for improved anticancer efficacy. Nanomedicine. 2019;14(23):3057–3074. doi:10.2217/nnm-2019-0261
89. Shrestha P, Ghanwatkar Y, Mahto S, et al. Gemcitabine-lipid conjugate and ONC201 combination therapy effectively treats orthotopic pancreatic tumor-bearing mice. ACS Appl Mater Interfaces. 2024;16(23):29686–29698. doi:10.1021/acsami.4c02626
90. Shah S, Dhawan V, Holm R, et al. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Deliv Rev. 2020;154–155:102–122. doi:10.1016/j.addr.2020.07.002
91. Chen ZA, Wu CH, Wu SH, et al. Receptor ligand-free mesoporous silica nanoparticles: a streamlined strategy for targeted drug delivery across the blood-brain barrier. ACS Nano. 2024;18(20):12716–12736. doi:10.1021/acsnano.3c08993
92. Tan J, Ding B, Chen H, et al. Effects of skeleton structure of mesoporous silica nanoadjuvants on cancer immunotherapy. Small. 2024;20(3):e2305567. doi:10.1002/smll.202305567
93. Jiang L, Wang X, Raza F, et al. PEG-grafted arsenic trioxide-loaded mesoporous silica nanoparticles endowed with pH-triggered delivery for liver cancer therapy. Biomater Sci. 2023;11(15):5301–5319. doi:10.1039/d3bm00555k
94. Lee H, Choi M, Kim HE, et al. Mannosylated poly(acrylic acid)-coated mesoporous silica nanoparticles for anticancer therapy. J Control Release. 2022;349:241–253. doi:10.1016/j.jconrel.2022.06.064
95. Wen H, Huo G, Qin C, et al. Safety evaluation of PEGylated MNPs and p-PEGylated MNPs in SD rats. Sci Rep. 2023;13(1):21501. doi:10.1038/s41598-023-48742-w
96. García-Fernández A, Sancenón F, Martínez-Máñez R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv Drug Deliv Rev. 2021;177:113953. doi:10.1016/j.addr.2021.113953
97. Meka AK, Gopalakrishna A, Iriarte-Mesa C, et al. Influence of pore size and surface functionalization of mesoporous silica nanoparticles on the solubility and antioxidant activity of confined coenzyme Q10. Mol Pharm. 2023;20(6):2966–2977. doi:10.1021/acs.molpharmaceut.3c00017
98. Hong X, Zhong X, Du G, et al. The pore size of mesoporous silica nanoparticles regulates their antigen delivery efficiency. Sci Adv. 2020;6(25):eaaz4462. doi:10.1126/sciadv.aaz4462
99. Chen YP, Chou CM, Chang TY, et al. Bridging size and charge effects of mesoporous silica nanoparticles for crossing the blood-brain barrier. Front Chem. 2022;10:931584. doi:10.3389/fchem.2022.931584
100. Lérida-Viso A, Estepa-Fernández A, García-Fernández A, et al. Biosafety of mesoporous silica nanoparticles; towards clinical translation. Adv Drug Deliv Rev. 2023;201:115049. doi:10.1016/j.addr.2023.115049
101. Zhao S, Xu M, Cao C, et al. A redox-responsive strategy using mesoporous silica nanoparticles for co-delivery of siRNA and doxorubicin. J Mater Chem B. 2017;5(33):6908–6919. doi:10.1039/c7tb00613f
102. Zhang J, Sun Y, Tian B, et al. Multifunctional mesoporous silica nanoparticles modified with tumor-shedable hyaluronic acid as carriers for doxorubicin. Colloids Surf B Biointerfaces. 2016;144:293–302. doi:10.1016/j.colsurfb.2016.04.015
103. De la Torre C, Coll C, Ultimo A, et al. In situ-forming gels loaded with stimuli-responsive gated mesoporous silica nanoparticles for local sustained drug delivery. Pharmaceutics. 2023;15(4):1071. doi:10.3390/pharmaceutics15041071
104. Lei J, Zhang Q, Jin X, et al. Drug release from disulfide-linked prodrugs: role of thiol agents. Mol Pharm. 2021;18(7):2777–2785. doi:10.1021/acs.molpharmaceut.1c00326
105. Zhang Y, Wu X, Xu X, et al. Nanosized assemblies from amphiphilic solanesol derivatives for anticancer drug delivery. ACS Appl Bio Mater. 2023;6(9):3875–3888. doi:10.1021/acsabm.3c00508
106. Miao YB, Chen KH, Chen CT, et al. A noninvasive gut-to-brain oral drug delivery system for treating brain tumors. Adv Mater. 2021;33(34):e2100701. doi:10.1002/adma.202100701
107. Du K, Xia Q, Sun J, et al. Visible light and glutathione dually responsive delivery of a polymer-conjugated temozolomide intermediate for glioblastoma chemotherapy. ACS Appl Mater Interfaces. 2021;13(47):55851–55861. doi:10.1021/acsami.1c16962
108. Chen J, Zhang Y, Meng Z, et al. Supramolecular combination chemotherapy: a pH-responsive co-encapsulation drug delivery system. Chem Sci. 2020;11(24):6275–6282. doi:10.1039/d0sc01756f
109. Zou L, Liu X, Li J, et al. Redox-sensitive carrier-free nanoparticles self-assembled by disulfide-linked paclitaxel-tetramethylpyrazine conjugate for combination cancer chemotherapy. Theranostics. 2021;11(9):4171–4186. doi:10.7150/thno.42260
110. Liu X, Li Y, Wang K, et al. GSH-responsive nanoprodrug to inhibit glycolysis and alleviate immunosuppression for cancer therapy. Nano Lett. 2021;21(18):7862–7869. doi:10.1021/acs.nanolett.1c03089
111. Guan J, Wu Y, Liu X, et al. A novel prodrug and its nanoformulation suppress cancer stem cells by inducing immunogenic cell death and inhibiting indoleamine 2, 3-dioxygenase. Biomaterials. 2021;279:121180. doi:10.1016/j.biomaterials.2021.121180
112. Jangili P, Kong N, Kim JH, et al. DNA-damage-response-targeting mitochondria-activated multifunctional prodrug strategy for self-defensive tumor therapy. Angew Chem Int Ed Engl. 2022;61(16):e202117075. doi:10.1002/anie.202117075
113. Cao Y, Yao Y, Li Y, et al. Tunable keratin hydrogel based on disulfide shuffling strategy for drug delivery and tissue engineering. J Colloid Interface Sci. 2019;544:121–129. doi:10.1016/j.jcis.2019.02.049
114. Zhou G, Li L, Xing J, et al. Redox responsive liposomal nanohybrid cerasomes for intracellular drug delivery. Colloids Surf B Biointerfaces. 2016;148:518–525. doi:10.1016/j.colsurfb.2016.09.033
115. Yang K, Bai B, Huang F, et al. Drug-initiated poly(thiocitc acid) polymer incorporating host-guest interaction for cancer combination chemotherapy. iScience. 2024;27(3):109070. doi:10.1016/j.isci.2024.109070
116. Abdel Khalek MA, Abdelhameed AM, Abdel Gaber SA. The use of photoactive polymeric nanoparticles and nanofibers to generate a photodynamic-mediated antimicrobial effect, with a special emphasis on chronic wounds. Pharmaceutics. 2024;16(2):229. doi:10.3390/pharmaceutics16020229
117. Li Z, Li X, Lu Y, et al. Improved photodynamic therapy based on glutaminase blockage via tumor membrane coated CB-839/IR-780 nanoparticles. Small. 2024;20(10):e2305174. doi:10.1002/smll.202305174
118. Hao C, Shao Y, Tian J, et al. Dual-responsive hollow mesoporous organosilicon nanocarriers for photodynamic therapy. J Colloid Interface Sci. 2024;659:582–593. doi:10.1016/j.jcis.2024.01.034
119. Jiao Q, Zheng Y, Xie Q, et al. A dual-responsive morphologically-adaptable nanoplatform for targeted delivery of activatable photosensitizers in precision photodynamic therapy. Small. 2024;20(21):e2309054. doi:10.1002/smll.202309054
120. Abou Taleb S, Moatasim Y, GabAllah M, et al. Quercitrin loaded cyclodextrin based nanosponge as a promising approach for management of lung cancer and COVID-19. J Drug Deliv Sci Technol. 2022;77:103921. doi:10.1016/j.jddst.2022.103921
121. Yu J, Xu J, Jiang R, et al. Versatile chondroitin sulfate-based nanoplatform for chemo-photodynamic therapy against triple-negative breast cancer. Int J Biol Macromol. 2024;265(Pt 1):130709. doi:10.1016/j.ijbiomac.2024.130709
122. Yu L, Zhang M, He J, et al. A nanomedicine composed of polymer-ss-DOX and polymer-Ce6 prodrugs with monoclonal antibody targeting effect for anti-tumor chemo-photodynamic synergetic therapy. Acta Biomater. 2024;179:272–283. doi:10.1016/j.actbio.2024.02.048
123. Abruzzo A, Croatti V, Zuccheri G, et al. Drug-in-cyclodextrin-in-polymeric nanoparticles: a promising strategy for rifampicin administration. Eur J Pharm Biopharm. 2022;180:190–200. doi:10.1016/j.ejpb.2022.10.001
124. Kali G, Haddadzadegan S, Bernkop-Schnürch A. Cyclodextrins and derivatives in drug delivery: new developments, relevant clinical trials, and advanced products. Carbohydr Polym. 2024;324:121500. doi:10.1016/j.carbpol.2023.121500
125. He S, Wang L, Wu D, et al. Dual-responsive supramolecular photodynamic nanomedicine with activatable immunomodulation for enhanced antitumor therapy. Acta Pharm Sin B. 2024;14(2):765–780. doi:10.1016/j.apsb.2023.10.006
126. Nisar S, Starosta E, Elayyan M, et al. Photoinduced electron transfer-based glutathione-sensing theranostic nanoprodrug with self-tracking and real-time drug release monitoring for cancer treatment. ACS Appl Mater Interfaces. 2024;16(6):6859–6867. doi:10.1021/acsami.3c16585
127. Ansari MA, Thiruvengadam M, Venkidasamy B, et al. Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: current status and future perspectives. Semin Cancer Biol. 2022;86(Pt 2):678–696. doi:10.1016/j.semcancer.2022.04.005
128. Chi G, Lv Y, Chao S, et al. Glyconanoparticles with activatable near-infrared probes for tumor-cell imaging and targeted drug delivery. Int J Nanomedicine. 2022;17:1567–1575. doi:10.2147/IJN.S337082
129. Mo C, Wang Z, Yang J, et al. Rational assembly of RGD/MoS(2)/Doxorubicin nanodrug for targeted drug delivery, GSH-stimulus release and chemo-photothermal synergistic antitumor activity. J Photochem Photobiol B. 2022;233:112487. doi:10.1016/j.jphotobiol.2022.112487
130. Sun X, Liang X, Wang Y, et al. A tumor microenvironment-activatable nanoplatform with phycocyanin-assisted in-situ nanoagent generation for synergistic treatment of colorectal cancer. Biomaterials. 2023;301:122263. doi:10.1016/j.biomaterials.2023.122263
131. Lee H, Kim DI, Kwon SH, et al. Magnetically actuated drug delivery helical microrobot with magnetic nanoparticle retrieval ability. ACS Appl Mater Interfaces. 2021;13(17):19633–19647. doi:10.1021/acsami.1c01742
132. Sun Y, Wang S, Li Y, et al. Precise engineering of disulfide bond-bridged prodrug nanoassemblies to balance antitumor efficacy and safety. Acta Biomater. 2023;157:417–427. doi:10.1016/j.actbio.2022.12.005
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

