Back to Journals » Drug Design, Development and Therapy » Volume 19
Research Trends and Developments in Nanomaterials for Rheumatoid Arthritis: A Comprehensive Bibliometric Analysis
Authors Jia X, He L, Chang Y, Li J, Wang J, Zhang X, Guo J
Received 30 December 2024
Accepted for publication 4 May 2025
Published 27 May 2025 Volume 2025:19 Pages 4355—4371
DOI https://doi.org/10.2147/DDDT.S514898
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qiongyu Guo
Xuezhao Jia,1,* Lei He,1,* Yonglong Chang,2 Jiajie Li,1 Jing Wang,3 Xiaojun Zhang,1 Jinchen Guo1,4
1Anhui University of Chinese Medicine, Hefei, 230012, People’s Republic of China; 2Department of Integrated Traditional Chinese and Western Medicine, The Second Xiangya Hospital, Central South University, Changsha, 410011, People’s Republic of China; 3Institute of Basic Theory of Traditional Chinese Medicine, Anhui Academy of Traditional Chinese Medicine, Hefei, 230012, People’s Republic of China; 4Key Laboratory of Xin’an Medical Education, Anhui University of Traditional Chinese Medicine, Hefei, 230012, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaojun Zhang, Email [email protected] Jinchen Guo, Email [email protected]
Background: Rheumatoid arthritis (RA) is one of the common diseases associated with job loss and disability. However, the existing diagnosis and treatment methods are limited by factors such as misdiagnosis, missed diagnosis, and toxic side effects. In recent years, remarkable progress has been made in applying nanomedicine for RA treatment. However, previous studies lack a systematic and comprehensive analysis of the development trajectory of nanomaterials in the diagnosis and treatment of RA, the contributions of key researchers, and the evolution of research focuses. This study aims to fill this gap by providing a detailed bibliometric analysis of the global research landscape on nanomaterial applications in RA diagnosis and treatment, highlighting the significance of this field in advancing therapeutic interventions and identifying future research directions.
Methods: Relevant literature on the application of nanomaterials in RA treatment was searched in the Web of Science Core Collection (WoSCC) database from January 1, 1985 to December 31, 2023. VOSviewer, CiteSpace, “bibliometrix” R-package, and Microsoft Office Excel 2021 were used for bibliometric analysis to evaluate the number of publications, research hotspots, main researchers, and institutions.
Results: A total of 524 articles were included, involving 33 countries or regions, 784 institutions, and 2751 authors, covering 19 disciplines, including biochemistry and molecular biology, chemistry, engineering, immunology, and materials science. Countries with the highest publication output were China, India, and the United States, with China and the United States having the closest cooperation. The University of California at San Diego and CHEN X were the most influential institutions and authors. Journal of Controlled Release had the highest publication output and emerged as the most influential journal in the field. In recent years, research hotspots of nanomaterials in RA included “dexamethasone”, “micelle”, and “diagnosis”.
Conclusion: This study is the first comprehensive bibliometric analysis of nanotechnology in RA application. It highlights the importance of nanomaterials in advancing RA diagnosis and treatment and provides a valuable reference for future research. By identifying key research trends, influential contributors, and emerging hotspots, this analysis offers actionable insights for researchers to build upon, ultimately driving innovation and improving therapeutic outcomes in the field of nanomedicine for RA.
Keywords: bibliometric analysis, rheumatoid arthritis, nanomaterial, research trend
Introduction
Rheumatoid arthritis (RA) is one of the most common chronic inflammatory diseases. Without timely treatment, it can lead to bone damage, joint deformity, and loss of function.1 RA is one of the main diseases leading to job loss and disability. The global incidence of RA is about 0.4–1.3%, posing a huge economic burden on patients and society.2 However, due to the lack of early diagnostic methods with high specificity and sensitivity, some RA patients are easily misdiagnosed and miss the best opportunity for treatment.3 Although non-steroidal anti-inflammatory drugs, antirheumatic drugs, glucocorticoids, and other drugs can improve the symptoms of RA to a certain extent, they are limited by drug resistance and serious side effects, and their long-term efficacy remains uncertain.4,5 These limitations highlight the urgent need for innovative approaches that can address the shortcomings of conventional treatments, such as improving drug delivery efficiency, reducing side effects, and enhancing diagnostic accuracy.
Nanotechnology, which focuses on the properties and applications of nanomaterials with sizes ranging from 1 nm to 100 nm, including polymers, metals, macromolecules, lipids, semiconductors, and chemicals,6 has emerged as a promising solution to overcome these challenges. Compared to traditional drug delivery methods, nanotechnology offers significant advantages in therapy. It enables targeted drug delivery, enhances local therapeutic efficacy, and significantly reduces systemic toxicity.7,8 Furthermore, nanotechnology also demonstrates great potential in the diagnostic field. For example, nanosensors can detect RA-related biomarkers with high sensitivity and specificity, while nanoprobe applications in magnetic resonance imaging (MRI) or other imaging techniques enable effective visualization of joint tissue structure and lesions, thus aiding in the early diagnosis of RA.9 In addition, when compared to other emerging treatment strategies such as biologics and gene therapy, nanotechnology offers a more versatile platform for both diagnostic and therapeutic applications, with the potential to integrate multiple functionalities into a single system. This unique advantage positions nanotechnology as a powerful alternative for addressing the multifaceted challenges of RA management.
Despite the growing interest in nanotechnology for RA diagnosis and treatment, there remains a significant research gap in systematically evaluating the current state of this field. While numerous studies have demonstrated the potential of nanotechnology in various aspects of RA management, a comprehensive understanding of the trends, challenges, and future directions is still lacking. Bibliometric analysis, which combines mathematical, statistical, and data visualization methods to quantitatively and qualitatively analyze the quantity, quality, impact, and structure of academic literature, offers an ideal tool to fill this gap.10 By assessing the current state of research in a field and emerging trends, bibliometric analysis can provide valuable insights into the development of RA nanotechnology and guide future investigations.
The present study presents the first systematic analysis of the literature on RA nanotechnology using bibliometric methods, providing an objective overview of the application of nanotechnology in diagnosing and treating RA. This research aims to address the existing research gap by identifying key trends, challenges, and future opportunities in this rapidly evolving field, thereby offering valuable insights for future investigations.
Methods
Search Strategy
On April 7, 2024, the relevant literature on the application of nanomaterials in RA treatment was searched in the Web of Science core collection (WoSCC) database (https://webofscience.clarivate.cn/wos/woscc/basic-search) from January 1, 1985 to December 31, 2023, using the search notation TS = (nano*) AND TS = (rheumatoid arthritis). Only English-language publications were included. Eligible study types encompassed original research, reviews, experimental studies, and clinical trials, while conference abstracts, reviews, and non-peer-reviewed articles were excluded. The initial search yielded a large number of results, which were subsequently screened by two authors who independently reviewed the titles and abstracts to exclude publications unrelated to the research topic. Duplicate records were identified and removed using the EndNote reference management software, followed by a manual review to ensure that no duplicates were overlooked. Any discrepancies between the authors regarding the inclusion or exclusion of articles were resolved through consensus discussions with a third author. A total of 524 articles were included in the final analysis. The study workflow is depicted in Figure 1.
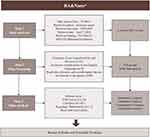 |
Figure 1 Flowchart of the literature screening process. |
Data Analysis
VOSviewer (1.6.18), CiteSpace (6.3 R1), “bibliometrix” R-package (4.4.1), and Microsoft Office Excel 2021 were used for visualization and analysis. VOSviewer was used to analyze countries and regions, institutions, author cooperation, and keyword clustering. CiteSpace was used to analyze citation bursts, keywords, and cited references. The “bibliometrix” R-package was used for quantitative evaluation of basic information such as author, institution, country, and keywords. Microsoft Office Excel 2021 and tidyverse (ggplot2) R-package were used for quantitative analysis and visual drawing. VOSviewer was applied to visualize the cooperation among countries, regions, institutions, and authors (cooperation map) and keyword clustering (co-occurrence map). In the generated plot, each dot represents an element, and the size of the dot indicates the number of publications related to the country, the institution, or the frequency of keywords. The lines connecting these points indicate the number of cooperative relationships or keyword co-occurrences, and the colors indicate different clusters or years. In addition, CiteSpace was employed to further analyze the citation outbreak, keyword time series, and citation bursts, and conduct time series analysis on literature data to show the evolution trend of citations and keywords, which helps understand the development trends in this field.
Result
Descriptive Bibliometric Analysis
A total of 524 articles on nanomaterials and RA were authored by 2751 researchers affiliated with 784 institutions across 33 countries or regions, and published in 199 journals (Table 1). The first study entitled “Suppression of collagen-induced arthritis by single administration of poly (lactic-co-glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance” was published by a Korean scholar Kim WU in Arthritis Rheum in 2002, who developed a nanodrug delivery system named “poly(lactic-co-glycolic acid) nanoparticles encapsulating type II collagen”, which was successfully employed for the treatment of collagen-induced arthritis (CIA) mice.11 In the early stage, there was a low publication rate in this field. However, with the rapid advancements in nanotechnology, the number of published papers has significantly surged since 2015, reaching 95 by 2023, with an average annual growth rate of 27.08%. The polynomial curve analysis demonstrated a positive correlation between the yearly publication count and the publication year (R2 = 0.9415), indicating an upward trend in article numbers (Figure 2). Numerous scholars continue to explore the application of nanotechnology in RA diagnosis and treatment.
 |
Table 1 Main Information Identified by the Bibliometric Analysis |
 |
Figure 2 Global publication growth trends from 2002 to 2023. |
Research Countries and Institutions
The 524 studies were contributed by authors from 33 countries/regions The top five countries with the highest publication output were China (239), India (69), the United States (36), South Korea (35), and Pakistan (21) (Table 2). Since 2017, China has witnessed exponential growth in the number of publications, establishing itself as the leading country in terms of annual publication count (Figure 3A). Figure 3C represents the publication counts across different countries. Simultaneously, we conducted a comprehensive analysis by tallying the total number of citations received by published papers in each country and calculating their average citation count, as shown in the Figure 3B. The top 10 most cited countries were China (5646), followed by the United States (2231), South Korea (2147), India (1315), Iran (560), Pakistan (457), Portugal (420), Denmark (373), Japan (316), and Germany (234). However, the top 10 countries with the highest average citation frequency were the United States (61.97), South Korea (61.34), Denmark (53.29), Portugal (46.67), Iran (35.00), Germany (33.43), Japan (31.60), China (23.62), Pakistan (21.76), and India (19.06). Figure 3D depicts the cooperation around the world. The United States had the highest level of international research collaborations (63). The most research collaborations were between China and the United States(30).
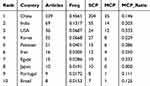 |
Table 2 Top 10 Countries with the Most Publications |
A total of 784 institutions worldwide were engaged in research related to nanotechnology and RA, of which the institution with the largest output is Jilin University (8), but the total number of citations is only 238 times, failing to enter the top 10 most influential institutions. It is worth noting that although the University of California, San Diego and Stanford University in the United States only published one paper, the total citations were as high as 543 and 361 times, respectively, indicating that American institutions conducted high-quality research. China, South Korea, India, scholars number is more, total cited frequency is limited, but the quality remains to be improved (Table 3). In addition, the intensity of cooperation between institutions is not high at present, and most institutions conduct related research independently.
 |
Table 3 Top 10 Most Influential Institutions |
Analysis of Journals and Co-Cited Journals
The 524 articles were published in 199 academic journals. The Journal of Controlled Release published the highest number of articles and had the highest impact factor. In addition, the International Journal of Pharmaceutics, Biomaterials, International Journal of Nanomedicine, and ACS Nano ranked in the top 10 in terms of the number of publications and co-citations, providing important references in the field of nanometer materials and RA-related research. According to the 2024 journal citation reports (JCR) data, the other cited journals were distributed in the Q1 region, except for Arthritis Research & Therapy and International Journal of Nanomedicine, which were distributed in the Q2 region. Particularly, Advanced Drug Delivery Review, with an impact factor of 98.7, ranked the highest among the most cited journals, and thus research on this topic has a significant influence (Table 4).
 |
Table 4 Top 10 Journals and Co-Cited Journals |
Analysis of Authors and Collaboration Networks
The H-index is an important indicator for evaluating the influence of an author based on the quality and quantity of their published papers.12 Table 5 presents the top 10 authors with the highest H-index, of whom PARK JH and PARK JS were from South Korea, while the remaining authors were from China. It is noteworthy that PARK JS remains the pioneering figure in nanotechnology research in the field of RA, which has greatly contributed to his high H-index and significant academic influence. Despite their relatively late entry into this domain, six Chinese authors, including CHEN X, SUN FY, and TENG LS, managed to publish numerous articles within a short period gaining recognition within the academic community. They represent crucial emerging forces in this particular field. Furthermore, although ZHANG Y had a substantial publication count, her citation numbers were comparatively lower, which resulted in a lower H-index and her absence from Table 5. Figure 4 illustrates author collaborations wherein nodes are sparsely distributed, indicating insufficient closeness in terms of collaborative efforts. This may be attributed to factors such as disciplinary boundaries, geographical concentration, and fragmented research directions. Representative South Korean scholars, such as PARK JH, tend to lead independent research efforts within single institutions. While emerging Chinese authors are highly productive, their collaborations are largely confined to domestic institutions.
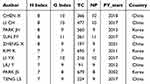 |
Table 5 Top 10 Most Influential Authors Ranked by the H Index |
 |
Figure 4 Visualization of author collaboration. |
WoSCC Research Areas
To facilitate classification, Clarivate Analytics classifies research papers in different research areas in the WoSCC database. Generally, each paper can be classified into at least one research area in the WoSCC database. The statistics on research areas can help grasp the changing focus of research in a particular field.13 We analyzed the research fields of nanotechnology applied to RA and found that the number of fields covered increased from 1 in 2002 to 19 in 2020, with an overall fluctuating growth trend (Figure 5A). There were 491 papers in the top 10 most active research areas, covering 93.70% of the literature in this field, mainly involving biochemistry and molecular biology, biotechnology and applied microbiology, chemistry, engineering, immunology, materials science, pharmacology and pharmacy, physics, and research and experimental medicine. The annual changes in the top 10 most productive fields by line plots are displayed in Figure 5B, among which pharmacology had the highest number of publications and remained the research focus in this field.
 |
Figure 5 (A) Annual changes in the number of WOS research areas; (B) Annual Publication Trends in the Top Ten WOS research areas. |
Analysis of the Most Influential Publications and Citation Bursts
The top ten most cited publications are shown in Table 6. Among them, “Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis”14 was published in Nature Nanotechnology in 2018 had the highest total number of citations, up to 543, with an average of 77.57 citations per year, indicating its high quality. Another study published in the journal Nano Letters of “Gold nanoparticles: a revival in precious metal administration to patients”15 was cited 361 times, ranking second, but it was cited 25.79 times per year on average. The corresponding authors of both articles are from the United States, and three of the top ten most cited publications are from China, indicating that both the United States and China provide a certain number of high-quality publications in this field.
 |
Table 6 Top ten Papers According Total Citation |
The term “citation burst” refers to the time frame in which an academic paper or research result receives a large number of citations, underscoring the academic impact and importance of a publication.16 Figure 6 depicts the top 15 citation bursts in the literature ranked by citation burst intensity. The earliest citation burst occurred in 2013, the latest occurred in 2021, and the longest burst occurred across four years. The most recent burst was from LI, a scholar at Fudan University in China, who published a study entitled “Route to Rheumatoid Arthritis by Macrophage-Derived Microvesicle-Coated Nanoparticles” in Nano Letters in 2019. This study developed a Macrophage-Derived Microvesicle (MMV)-Coated Nanoparticle (MNP) and the targeting effect of MNP in the CIA mice was significantly augmented. The targeting mechanism was revealed by proteomic analysis, and Mac-1 and CD44 were considered to be responsible for the significant targeting effect of MNP. The study also included the model drug tacrolimus, which was encapsulated in MNP (T-RNP) packages and significantly suppressed the progression of RA in mice. This literature is still in the citation explosion stage.17
 |
Figure 6 Top 15 references with stronger citation burst. |
Analysis of Keyword
The core and essence of a paper are encapsulated in keywords. Keyword co-occurrence analysis not only enables the identification of research hotspots within a scientific field but also provides an effective means to comprehend the research direction of a specific topic. The “Most Frequent Words” analysis using the “bibliometrix” R-package revealed that in addition to rheumatoid arthritis and nanoparticles, the most frequent keywords were delivery (97), drug-delivery (77), collagen-induced arthritis (69), inflammation (59), therapy (58), in-vitro (54), methotrexate (54), and cells (40) (Figure 7A).
 |
Figure 7 (A)Top 10 keywords with the highest frequency; (B)Clustering analysis of keywords; (C) Keyword co-occurrence clustering map; (D)Top 12 keywords with the strongest citation burst. |
To present the research hotspots more intuitively, VOSviewer was utilized to create a network visualization of the 524 articles based on the co-occurrence of keywords. The links between articles were established based on the strength of keyword co-occurrence. Forty-one keywords that appeared at least 20 times were selected for visualization purposes. These terms were further divided into four distinct network clusters (Figure 7B), with green and yellow clusters being the largest ones, comprising 19 and 10 terms respectively. The themes within the green cluster primarily revolved around RA, angiogenesis, cells, collagen-induced arthritis, cytokines, delivery, etc., while topics within the yellow cluster mainly focused on drug delivery aspects such as nanomedicine and drug delivery systems both in vitro and in vivo.
CiteSpace was employed to generate a keyword timeline (Figure 7C). Ten keywords, including “chitosan”, “nanomedicine”, “inflammation”, “nanomaterials”, “cytotoxicity”, “gold nanoparticles”, “collagen-induced arthritis”, “anti-inflammatory effect”, “teriflunomide” and “microneedles”, were clustered. The “chitosan” cluster appeared the earliest and lasted the longest, highlighting its research significance in this field. All other clusters were a hot topic in 2023, except for “teriflunomide”.
The 12 keywords with the strongest citation burst are shown in Figure 7D. Among them, “efficacy” was the keyword with the strongest citation burst, indicating that this field paid more attention to the clinical efficacy of nanomaterials in the treatment of RA. In addition, the longest-lasting keyword was “antigen-induced arthritis” (AIA), which refers to animal models in which researchers mimic the pathogenesis and pathophysiology of RA by inducing joint inflammation in mice or rats by injection of specific antigens (such as collagen). This indicates that the application of nanotechnology in RA is still mainly focused on basic research. Additionally, keywords such as “dexamethasone”, “micelle”, and “diagnosis” continued to attract attention in 2023, implying that these are still hot research topics.
Discussion
Nanotechnology and RA research have developed rapidly in recent years. However, there is still a dearth of bibliometric studies in this field. The present study provides a systematic review of the current research status and potential research hotspots of RA nanotechnology. To the best of our knowledge, this is the first bibliometric analysis of literature in this field between 1985 and 2023. Research trends and changes in this field are summarized as follows.
General Information
Relevant studies on nanotechnology and RA were thoroughly searched in the WoSCC database from 1985 to 2023. A total of 2751 authors from 784 institutions in 33 countries or regions published 524 studies on nanotechnology and RA in 199 journals. Although the annual number of papers related to nanotechnology and RA showed an overall upward trend, the growth trend was not obvious due to the small number of papers published in the early stage. However, the number of published papers increased rapidly since 2015. The annual number of publications indicates that this is an emerging field and predicts future research prospects. The most influential author was CHEN X from China. Meanwhile, China also emerged as the most productive and cited country. However, the average number of citations of Chinese publications was low because of the low-quality papers. China and the United States had the most research collaborations in this field. Many countries independently conduct research in this field, which suggests that academic barriers—such as competition, intellectual property concerns, discrepancies in global research standards, and limited funding—impede cross-border collaboration among researchers. This underscores the necessity for future collaborative efforts. At present, the related field of nanotechnology for the treatment of RA involves 19 disciplines such as biochemistry and molecular biology, chemistry, engineering, immunology, and materials science, which indicates that the research in this field is extensive. The journal impact factor of the top 10 Q1 journals in JCR partitions was above 5 points, suggesting that high-quality research is being carried out in this field. Moreover, the Journal of Controlled Release was the most prolific and cited journal. The article “Nanotherapeutics alleviate rheumatoid arthritis” garnered 84 citations, serving as a pivotal reference for comprehending the intersection of nanotechnology and RA.18 Notably, in this journal, almost all RA nano therapy-related publications between 2019 and 2023 were from China, suggesting that the Chinese government is paying close attention to quality issues, thereby gradually improving the quality of research.
Knowledge Base
By consulting highly cited papers, a comprehensive understanding of related research on nanotechnology and RA can be obtained. Gold nanoparticles (AuNPs) are one of the highlights in agro-scientific research in nanotechnology and can more easily penetrate the joint tissue owing to their excellent chemical stability and biocompatibility, as well as their smaller size, inhibiting the inflammatory response to alleviate the symptoms of RA, to relieve pain and swelling of the joints.19,20 Tsai et al observed the binding of 13 nm AuNPs to vascular endothelial growth factor in human RA synovial fluid, and its effect on RA synovial fluid induced endothelial cell proliferation and migration, demonstrating for the first time that intra-articular administration of AuNPs can improve the clinical course of RA by inhibiting angiogenic factors and reducing macrophage infiltration and inflammation.21 AuNPs can absorb and convert light energy to generate heat, increasing the local temperature, and thereby causing cell apoptosis, necrosis, or the release of heat-sensitive drugs to achieve therapeutic effects.22,23 However, AuNP photothermal therapies have mainly been used to treat cancer. Lee et al attempted to explore this property of AuNPs for the treatment of RA. Given that synovial hyperplasia of RA is similar to solid tumors in terms of capillary leakage and other aspects, they developed arginine (R)-glycine (G)-aspartic acid (D) (RGD)-attached gold (gold) half-shell nanoparticles coated with methotrexate (MTX). Under near-infrared irradiation, the gold half-shell generates heat and the drug is rapidly released from the poly (lactic-co-glycolic acid) (PLGA) nanoparticles, thereby achieving photothermally controlled drug delivery. It was found that MTX nanoparticles containing small doses, when used in combination with near-infrared irradiation, showed greater therapeutic effects than conventional MTX solutions in CIA mice.24 However, the cost of preparation of AuNPs is relatively high, and the long-term safety of treatment remains elusive. Studies have shown that the particle size, surface modification, and particle shape of AuNPs can affect their toxicity in vitro, as well as their blood circulation, biodistribution and accumulation, and the immune system in vivo.25,26
Natural biomimetic nanomaterials, such as liposomes, albumin, extracellular vesicles, etc., are also often used in this field. These nanomaterials are usually derived from organisms and have natural biocompatibility and biodegradability and diverse structures and functions, which can simulate complex biological processes in organisms, such as molecular recognition and cell signaling.27–29 Compared with AuNPs, these nanomaterials are safer and environmentally friendly and have better tissue permeability and targeting, which improves the therapeutic effect.30 Cell-coated nanoparticles are promising natural biomimetic nanoparticles that use biological methods to wrap cell membranes on the surface of nanoparticles, thereby endowing nanoparticles with characteristics and functions similar to the original cells. This technique combines the advantages of biology and nanotechnology, to overcome challenges posed by the traditional application of nanoparticles, such as immune rejection, biological incompatibility, lack of targeting, etc.31–33 By fusing neutrophil membranes to polymer nuclei, Zhang et al developed neutrophil membrane-coated nanoparticles that inherit the antigenic appearance and associated membrane functions of the source cells, making them ideal baits for neutrophil targeting biomolecules to bind immunomodulatory molecules. These nanoparticles have been shown to neutralize proinflammatory cytokines, inhibit synovial inflammation, penetrate deep into the cartilage matrix, and provide strong cartilage protection against joint damage. Neutrophil membrane-coated nanoparticles showed significant therapeutic effects in a CIA mouse model and a human transgenic mouse model of arthritis by improving joint damage and inhibiting the overall severity of arthritis.14 Li et al developed macrophage-derived microvesicle (MMV)-coated nanoparticles (MNPs) and evaluated the inflammation-mediated targeting ability of MNPs in vitro and in vivo. The results showed that MNPs exhibited stronger binding to inflammatory human umbilical vein endothelial cells (HUVECs) than erythrocyte membrane-coated nanoparticles (RNPs) in vitro and significantly enhanced targeting in vivo in the CIA mouse model. The targeting mechanism was subsequently revealed by proteomic analysis, which showed that Mac-1 and CD44 played a significant targeting role of MNPs. Meanwhile, MNPs encapsulated tacrolimus significantly inhibited the progression of RA in mice, indicating that MNPs may have certain clinical value.17
The pathogenesis of RA involves intricate cellular and molecular interactions. Although its mechanism remains unclear, it is closely associated with autoimmune disorders and inflammation.34,35 The infiltration of synovial tissue by various inflammatory cells, especially macrophages, is one of the important factors in the pathogenesis of RA. Activation of M1 macrophages produces a series of inflammatory cytokines, such as TNF-α/β and IL-6, IL-1, to maintain and increase joint inflammation. Therefore, targeting macrophages and inflammatory states is considered an important therapeutic target for relieving RA symptoms.36,37 To alleviate synovial inflammation, Yang et al developed folate-modified silver nanoparticles (FA-AgNPs) to eliminate M1 macrophages or convert them to an anti-inflammatory M2 phenotype. Research indicates that M1 macrophages can be specifically targeted by enhancing the expression of folate receptors on their surface. After entering cells, FA-AgNPs dissolve and release Ag+ to cope with intracellular glutathione, thereby promoting the apoptosis of M1 macrophages and scavenging reactive oxygen species (ROS) to promote the polarization of M2 macrophages to achieve therapeutic effects. FA-AgNPs were gradually cleared from the body mainly through feces after treatment, without tissue accumulation and with no obvious long-term toxicity.38 Jain et al successfully encapsulated the plasmid DNA encoding IL-10 anti-inflammatory cytokine onto alginate nanoparticles and targeted the tuftsin peptide on the surface of the nanoparticle to achieve macrophage activity. 6 days after intraperitoneal injection, 66% of macrophages in the synovium of arthritic rats were in the M2 state. The reduction of proinflammatory cytokines in the whole body and joint tissues indicated that this method could repolarize macrophages from M1 to M2 functional subtype and prevent the progression of inflammation and joint damage.39 Kim et al developed manganferrite and cerium nanoparticle anchored mesoporous silica nanoparticles (MFC-MNS) that synergistically scavenged ROS and produced O2 to reduce M1 macrophage levels and induce M2 macrophages for RA therapy.40 Small interfering RNA (siRNA) is a type of short double-stranded RNA molecule, usually composed of about 20–25 bases, with a specific sequence, which can mediate gene silencing by targeting RNA interference pathway. It is one of the natural gene regulatory mechanisms in cells and has certain advantages in the treatment of RA.41,42 However, it has the characteristics of large volume, strong hydrophilicity, and anionic charge, limiting its ability to enter cells, and thus requires different drug delivery carriers to achieve delivery.43 Howard et al developed chitosan/siRNA nanoparticles and administered them intraperitoneally to CIA mice and found that chitosan/siRNA nanoparticles reduced the expression of TNF-α in the whole-body macrophages and improved the inflammatory state in CIA mice.44
Emerging Topics
Keywords are usually the core concepts of research topics. The study of core keywords in bibliometrics helps to explore important and emerging topics and provides valuable insights for the development of the respective field. By identifying the most significant citation bursts associated with specific keywords through CiteSpace, “dexamethasone” and “micelle” were identified as the main areas of future research.
Dexamethasone (DEX) is a type of long-acting and potent glucocorticoid. Its mechanism of action is that the glucocorticoid receptor binds to the glucocorticoid, and then reaches the nucleus to reduce the activity of nuclear factor-kappa B, thereby reducing the production of inflammatory cytokines to alleviate inflammation. However, due to the widespread distribution of glucocorticoid receptors in the body, the use of DEX may lead to cardiovascular disease, muscle atrophy, peptic ulcer, osteoporosis, and other toxic side effects.45–47 The precise delivery of drugs to inflammatory sites by nanotechnology is an important strategy to reduce toxic side effects.48 The delivery and release mechanisms of DEX have been optimized through nanotechnology. Dextran sulfate (DS)-modified DEX-loaded flexible liposome hydrogel (DS-FLS/DEX hydrogel),47 N-(2-hydroxypropyl) methacrylamide copolymer nanocellulators,49,50 Acetone-Based Ketal-Linked Nanomedicine by Dexamethasone Prodrugs (AKP-dexs),51 18 amine functional nano diamond (ND-oda),52 beta CD/PAA nano gel (nanodexa),53 liposomes (L-Dex), nuclear crosslinked micelles (M-Dex) and slow-release polymer prodrug (P-Dex-missile) and quick release polymer prodrug (P-Dex-fast)54 have been developed. These carriers can increase the concentration of DEX in the inflammatory area of arthritis, improve the bioavailability of the drug, and reduce systemic exposure, thereby reducing the impact on other organs. Despite these encouraging findings, the application of nanotechnology in the field of DEX drug delivery is still at a relatively nascent stage. Future studies are warranted to address the question of how to achieve the manufacturing, stability, cost-effectiveness, and regulatory approval of these systems in a clinical setting. With more clinical trials and technological development, it is anticipated that nanotechnology will play an important role in improving the efficacy of DEX in the treatment of RA.
Micelle is a nanoscale structure formed spontaneously by amphiphilic molecules in aqueous solution. The formation process involves the outward interaction of the hydrophilic part with water molecules, while the hydrophobic part is oriented inward to form a hydrophobic core. This structure enables the micelle to carry and transport hydrophobic drug molecules within its interior.55 Micellar drug delivery systems in rheumatoid arthritis offer several advantages. Primarily, micelles enhance the solubility, stability, and bioavailability of drugs. Moreover, they can target drug delivery to inflamed areas, reducing potential side effects throughout the body. For example, Li et al synthesized the microenvironment targeting micelles (PVGLIG-MTX-Que-Ms) by thin film hydration method, and tested the drug-loaded micelles in vitro for their sustained release performance, low cytotoxicity, strong targeting and anti-inflammatory properties. In vivo, the drug-loaded micelles can accumulate in joints for a long time to improve drug availability.56 Zhou et al, synthesized a micelle named HA@RH-CeO2 that utilizes HA as a biocompatible carrier and incorporates a cerium oxide nanoenzyme responsive to ROS. The micelle showed precise delivery of the nanoenzyme and RH to M1 macrophages into the inflamed synovial tissue. This targeted delivery system effectively reduced local inflammation in RA. Although micelles can effectively deliver rheumatoid arthritis drugs, their design, cost, biocompatibility, and patient specific conditions need to be optimized for clinical application.57 Each nanomaterial has advantages and disadvantages, which explains why different nanomaterials have been developed and evaluated, and the appropriate nanomaterials should be selected according to the specific clinical situation.58
Traditional diagnostic methods for RA, such as autoantibody detection and imaging, provide valuable information, but their limitations in sensitivity and specificity can lead to missed diagnoses, particularly in atypical cases. This has spurred significant research interest in the potential of nanotechnology for RA diagnosis. The recent advancements in the field of nanotechnology have led to the creation of some new diagnostic methods that combine with traditional RA diagnostic. For example, Veigas et al first reported the target-induced aggregation gold nanoprobe quantitative colorimetric detection of autoantibodies. They found that the detection speed was faster than that of the enzyme-linked immunosorbent assay, with a higher sensitivity, facilitating rapid and reliable RA screening and diagnosis.59 Chen et al was synthesized with the leukocyte differentiation antigen (cluster of differentiation, CD) 3 monoclonal antibody combining with carboxyl of the polyethylene glycol (peg) - superparamagnetic iron oxide nanoparticles – IOPC - CD3, MRI specific targeted contrast agent, as a marker of T cell markers and tracking the T cells in the body. This approach demonstrates high target specificity in the CIA rat model, suggesting significant potential for applications in immune molecular imaging.60 Gawne et al encapsulated GC into long-circulating liposomes, synthesized PEG-liposome GC nanoparticles, and conjugated with radioactive zirconia. In an experimental mouse model of inflammatory arthritis, the nanoparticles exhibited high uptake into inflamed joints, but also in the joints with hidden inflammation. Studies in mice have demonstrated that these visible nanomaterials not only accumulate in the target area but also alleviate inflammation within the treatment group. This promising combination of properties suggests broad applicability of these materials in the field of PET imaging.61
Looking ahead, several underexplored areas and technological challenges in RA nanomedicine warrant greater attention. The long-term biosafety and biocompatibility of nanomaterials within the body remain unclear, necessitating extensive preclinical and clinical studies to ensure patient safety. Achieving efficient and precise targeting of nanomedicines to RA-affected joints while avoiding unwanted accumulation in other tissues is another critical challenge that requires innovative solutions. The complex pathophysiological environment of RA, characterized by chronic inflammation and tissue remodeling, poses obstacles to the effective delivery and sustained action of nanomedicines, calling for the development of smarter nanosystems that can adapt to these conditions. Moreover, the translation of nanomedicine research into clinical practice is impeded by regulatory hurdles, the need for standardized manufacturing protocols, and the establishment of robust quality control measures. Addressing these issues will require collaborative efforts across academia, industry, and regulatory bodies to bridge the gap between laboratory research and real-world clinical application.
Innovations and Limitations
This study pioneers a systematic bibliometric analysis of the field of nanomaterials in relation to RA. By employing quantitative methods, we provide a comprehensive overview of publication and collaboration patterns, research trends, and hotspots across various levels – countries, regions, institutions, and authors. This analysis sheds light on the evolving landscape of this field, ultimately aiding researchers in navigating and understanding its current development. However, this study has some limitations that need to be acknowledged. First, we only searched publications in English in the WoSCC database and ignored those published in other languages. Therefore, our findings may not be sufficiently comprehensive. Another limitation is the inherent bias in citation analysis based on publication age. Since older cited works have had more time to be referenced, they may appear more influential than recently published high-quality research. This time bias could lead to an underestimation of the impact of newer studies.
Conclusion
In this bibliometric analysis, we describe the application of nanomaterials in the diagnosis and treatment of RA. Current research in this field prioritizes developing nanocarrier systems to target joint inflammation and enhance therapeutic efficacy for rheumatoid arthritis. Notably, micelle-based formulations and dexamethasone delivery are prominent areas of investigation, alongside advancements in diagnostic applications utilizing nanotechnology. These areas show significant potential for development. However, the field is still at its early stages, with most research conducted through animal experiments and challenges in clinical translation, interdisciplinary collaboration, and regulatory approval.
To accelerate progress, we propose the following policy recommendations: 1) Establish interdisciplinary research funding mechanisms to bridge gaps between materials science, medicine, and chemistry, prioritizing grants for joint projects involving clinicians and engineers. 2) Develop standardized regulatory guidelines for RA nanomedicines, including toxicity evaluation criteria and fast-track approval pathways for targeted therapies, drawing from existing FDA nanotechnology guidance. 3) Foster international open science platforms to share preclinical data, animal models, and manufacturing protocols, reducing redundant research and IP disputes. 4) Incentivize industry-academia partnerships through tax breaks or subsidies, encouraging pharmaceutical companies to invest in scalable nanomaterial production. 5) Integrate nanotechnology into RA precision medicine initiatives by supporting biomarker-driven clinical trials.
These findings and policy recommendations significantly contribute to the development of precision medicine for RA, enabling more individualized diagnostic and therapeutic approaches while addressing systemic barriers through policy innovation.
Abbreviations
*, a wildcard symbol to represent variations of the root term “nano”, including nanotechnology, nanomaterials, nanoscience, etc; SCP, Single Country Publications; MCP, Multiple Country Publications; TC, Web of Science Core Collection times cited count; NP, number of scientific productions; PY_start, First year published.
Data Sharing Statement
The data analyzed in this study is included in the article. Further inquiries about the data should be directly to the corresponding author.
Acknowledgments
The authors would like to thank all the reviewers who participated in the review, as well as MJEditor (www.mjeditor.com) for providing English editing services during the preparation of this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Modern medical application foundation and development of traditional Chinese medicine research in Anhui province key laboratory open fund project (2021 AKLMCM001); Anhui University of Chinese Medicine High-level Talent Support Program (2022rcyb024).
Disclosure
The authors affirm that the study was carried out without any commercial or financial ties that could be perceived as a possible conflict of interest.
References
1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388:2023–2038. doi:10.1016/S0140-6736(16)30173-8
2. Lin Y-J, Anzaghe M, Schülke S. Update on the pathomechanism, diagnosis, and treatment options for rheumatoid arthritis. Cells. 2020;9:880. doi:10.3390/cells9040880
3. Cush JJ. Rheumatoid Arthritis. Med Clin North Am. 2021;105:355–365. doi:10.1016/j.mcna.2020.10.006
4. Köhler BM, Günther J, Kaudewitz D, Lorenz H-M. Current therapeutic options in the treatment of rheumatoid arthritis. J Clin Med. 2019;8:938. doi:10.3390/jcm8070938
5. Chatterjee A, Jayaprakasan M, Chakrabarty AK, et al. Comprehensive insights into rheumatoid arthritis: pathophysiology, current therapies and herbal alternatives for effective disease management. Phytother Res. 2024:
6. Khalid K, Tan X, Mohd Zaid HF, et al. Advanced in developmental organic and inorganic nanomaterial: a review. Bioengineered. 2020;11:328–355. doi:10.1080/21655979.2020.1736240
7. Patra JK, Das G, Fraceto LF, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16(71):1–33.
8. Rani R, Raina N, Sharma A, Kumar P, Tulli HS, Gupta M. Advancement in nanotechnology for treatment of rheumatoid arthritis: scope and potential applications. Naunyn Schmiedebergs Arch Pharmacol. 2023;396:2287–2310. doi:10.1007/s00210-023-02514-5
9. Radu A-F, Bungau SG. Nanomedical approaches in the realm of rheumatoid arthritis. Ageing Res Rev. 2023;87:101927. doi:10.1016/j.arr.2023.101927
10. Ninkov A, Frank JR, Maggio LA. Bibliometrics: methods for studying academic publishing. Perspect Med Educ. 2021;11:173–176. doi:10.1007/S40037-021-00695-4
11. Kim W, Lee W-K, Ryoo J-W, et al. Suppression of collagen‐induced arthritis by single administration of poly(lactic‐co‐glycolic acid) nanoparticles entrapping type II collagen: a novel treatment strategy for induction of oral tolerance. Arthritis Rheum. 2002;46:1109–1120. doi:10.1002/art.10198
12. Dinis-Oliveira RJ. The H-index in life and health sciences: advantages, drawbacks and challenging opportunities. Curr Drug Res Rev. 2019;11:82–84. doi:10.2174/258997751102191111141801
13. Sun G, Zhang Q, Dong Z, et al. Antibiotic resistant bacteria: a bibliometric review of literature. Front Public Health. 2022;10:1002015. doi:10.3389/fpubh.2022.1002015
14. Zhang Q, Dehaini D, Zhang Y, et al. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat Nanotechnol. 2018;13:1182–1190. doi:10.1038/s41565-018-0254-4
15. Thakor AS, Jokerst J, Zavaleta C, Massoud TF, Gambhir SS. Gold nanoparticles: a revival in precious metal administration to patients. Nano Lett. 2011;11:4029–4036. doi:10.1021/nl202559p
16. Chang Y, Ou Q, Zhou X, et al. Global research trends and focus on the link between rheumatoid arthritis and neutrophil extracellular traps: a bibliometric analysis from 1985 to 2023. Front Immunol. 2023;14:1205445. doi:10.3389/fimmu.2023.1205445
17. Li R, He Y, Zhu Y, et al. Route to rheumatoid arthritis by macrophage-derived microvesicle-coated nanoparticles. Nano Lett. 2019;19:124–134. doi:10.1021/acs.nanolett.8b03439
18. Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. J Controlled Release. 2017;252:108–124. doi:10.1016/j.jconrel.2017.02.032
19. Masse F, Desjardins P, Ouellette M, et al. Synthesis of ultrastable gold nanoparticles as a new drug delivery system. Molecules. 2019;24:2929. doi:10.3390/molecules24162929
20. Onaciu A, Braicu C, Zimta -A-A, et al. Gold nanorods: from anisotropy to opportunity. an evolution update. Nanomed. 2019;14:1203–1226. doi:10.2217/nnm-2018-0409
21. Tsai C, Shiau A-L, Chen S-Y, et al. Amelioration of collagen‐induced arthritis in rats by nanogold. Arthritis Rheum. 2007;56:544–554. doi:10.1002/art.22401
22. Abbas G, Maqbool S, Shahzad MK, et al. Analysis of gold nanospheres, nano ellipsoids, nanorods, and effect of core–shell structures for hyperthermia treatment. RSC Adv. 2022;12:9292–9298. doi:10.1039/D2RA00618A
23. Hussein E, Zagho M, Nasrallah G, Elzatahry A. Recent advances in functional nanostructures as cancer photothermal therapy. Int J Nanomedicine. 2018;13:2897–2906. doi:10.2147/IJN.S161031
24. Lee S-M, Kim HJ, Ha Y-J, et al. Targeted chemo-photothermal treatments of rheumatoid arthritis using gold half-shell multifunctional nanoparticles. ACS Nano. 2013;7:50–57. doi:10.1021/nn301215q
25. Jia Y-P, Ma B-Y, Wei X-W, Qian Z-Y. The in vitro and in vivo toxicity of gold nanoparticles. Chin Chem Lett. 2017;28:691–702. doi:10.1016/j.cclet.2017.01.021
26. Lopez-Chaves C, Soto-Alvaredo J, Montes-Bayon M, et al. Gold nanoparticles: distribution, bioaccumulation and toxicity. In vitro and in vivo studies. Nanomed Nanotechnol Biol Med. 2018;14:1–12. doi:10.1016/j.nano.2017.08.011
27. Tavasolian F, Moghaddam AS, Rohani F, et al. Exosomes: effectual players in rheumatoid arthritis. Autoimmun Rev. 2020;19:102511. doi:10.1016/j.autrev.2020.102511
28. Naskar A, Cho H, Lee S, Kim K. Biomimetic nanoparticles coated with bacterial outer membrane vesicles as a new-generation platform for biomedical applications. Pharmaceutics. 2021;13:1887. doi:10.3390/pharmaceutics13111887
29. Zhang Y, Long Y, Wan J, et al. Macrophage membrane biomimetic drug delivery system: for inflammation targeted therapy. J Drug Target. 2023;31:229–242. doi:10.1080/1061186X.2022.2071426
30. Narayanan KB, Sakthivel N. Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci. 2010;156:1–13. doi:10.1016/j.cis.2010.02.001
31. Hu C-MJ, Zhang L, Aryal S, et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc Natl Acad Sci. 2011;108:10980–10985. doi:10.1073/pnas.1106634108
32. Narain A, Asawa S, Chhabria V, Patil-Sen Y. Cell Membrane Coated Nanoparticles: next-generation Therapeutics. Nanomed. 2017;12:2677–2692. doi:10.2217/nnm-2017-0225
33. Vijayan V, Uthaman S, Park I-K. Cell membrane-camouflaged nanoparticles: a promising biomimetic strategy for cancer theragnostics. Polymers. 2018;10:983. doi:10.3390/polym10090983
34. Gravallese EM, Firestein GS. Rheumatoid arthritis — common origins, divergent mechanisms. N Engl J Med. 2023;388:529–542. doi:10.1056/NEJMra2103726
35. Mueller A-L, Payandeh Z, Mohammadkhani N, et al. Recent advances in understanding the pathogenesis of rheumatoid arthritis: new treatment strategies. Cells. 2021;10:3017. doi:10.3390/cells10113017
36. Alivernini S, MacDonald L, Elmesmari A, et al. Distinct synovial tissue macrophage subsets regulate inflammation and remission in rheumatoid arthritis. Nat Med. 2020;26:1295–1306. doi:10.1038/s41591-020-0939-8
37. Kondo N, Kuroda T, Kobayashi D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int J Mol Sci. 2021;22:10922. doi:10.3390/ijms222010922
38. Yang Y, Guo L, Wang Z, et al. Targeted silver nanoparticles for rheumatoid arthritis therapy via macrophage apoptosis and Re-polarization. Biomaterials. 2021;264:120390. doi:10.1016/j.biomaterials.2020.120390
39. Jain S, Tran T-H, Amiji M. Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials. 2015;61:162–177. doi:10.1016/j.biomaterials.2015.05.028
40. Kim J, Kim HY, Song SY, et al. Synergistic oxygen generation and reactive oxygen species scavenging by manganese ferrite/ceria co-decorated nanoparticles for rheumatoid arthritis treatment. ACS Nano. 2019;13:3206–3217. doi:10.1021/acsnano.8b08785
41. Bumcrot D, Manoharan M, Koteliansky V, Sah DWY. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–719. doi:10.1038/nchembio839
42. Feng N, Guo F. Nanoparticle-siRNA: a potential strategy for rheumatoid arthritis therapy? J Controlled Release. 2020;325:380–393. doi:10.1016/j.jconrel.2020.07.006
43. Kanasty R, Dorkin JR, Vegas A, Anderson D. Delivery materials for siRNA therapeutics. Nat Mater. 2013;12:967–977. doi:10.1038/nmat3765
44. Howard KA, Paludan SR, Behlke MA, et al. Chitosan/siRNA nanoparticle–mediated TNF-α knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Mol Ther. 2009;17:162–168. doi:10.1038/mt.2008.220
45. Lorscheider M, Tsapis N, ur-Rehman M, et al. Dexamethasone palmitate nanoparticles: an efficient treatment for rheumatoid arthritis. J Controlled Release. 2019;296:179–189. doi:10.1016/j.jconrel.2019.01.015
46. Madamsetty VS, Mohammadinejad R, Uzieliene I, et al. Dexamethasone: insights into pharmacological aspects, therapeutic mechanisms, and delivery systems. ACS Biomater Sci Eng. 2022;8:1763–1790. doi:10.1021/acsbiomaterials.2c00026
47. Zhao Y-P, Han J-F, Zhang F-Y, et al. Flexible nano-liposomes-based transdermal hydrogel for targeted delivery of dexamethasone for rheumatoid arthritis therapy. Drug Deliv. 2022;29:2269–2282. doi:10.1080/10717544.2022.2096718
48. Pham CTN. Nanotherapeutic approaches for the treatment of rheumatoid arthritis. WIREs Nanomed Nanobiotechnol. 2011;3:607–619. doi:10.1002/wnan.157
49. Libánská A, Randárová E, Skoroplyas S, et al. Size-switchable polymer-based nanomedicines in the advanced therapy of rheumatoid arthritis. J Controlled Release. 2023;353:30–41. doi:10.1016/j.jconrel.2022.11.027
50. Rubanová D, Skoroplyas S, Libánská A, et al. Therapeutic activity and biodistribution of a nano-sized polymer-dexamethasone conjugate intended for the targeted treatment of rheumatoid arthritis. Nanomed Nanotechnol Biol Med. 2024;55:102716. doi:10.1016/j.nano.2023.102716
51. Xu Y, Mu J, Xu Z, et al. Modular acid-activatable acetone-based ketal-linked nanomedicine by dexamethasone prodrugs for enhanced anti-rheumatoid arthritis with low side effects. Nano Lett. 2020;20:2558–2568. doi:10.1021/acs.nanolett.9b05340
52. Pentecost AE, Witherel CE, Gogotsi Y, Spiller KL. Anti-inflammatory effects of octadecylamine-functionalized nanodiamond on primary human macrophages. Biomater Sci. 2017;5:2131–2143. doi:10.1039/C7BM00294G
53. Argenziano M, Dianzani C, Ferrara B, et al. Cyclodextrin-based nanohydrogels containing polyamidoamine units: a new dexamethasone delivery system for inflammatory diseases. Gels. 2017;3:22. doi:10.3390/gels3020022
54. Quan L, Zhang Y, Crielaard BJ, et al. Nanomedicines for inflammatory arthritis: head-to-head comparison of glucocorticoid-containing polymers, micelles, and liposomes. ACS Nano. 2014;8:458–466. doi:10.1021/nn4048205
55. Kore G, Kolate A, Nej A, Misra A. Polymeric Micelle as Multifunctional Pharmaceutical Carriers. J Nanosci Nanotechnol. 2014;14:288–307. doi:10.1166/jnn.2014.9021
56. Li X, Wang X, Qu X, et al. Microenvironmental enzyme-responsive methotrexate modified quercetin micelles for the treatment of rheumatoid arthritis. Int J Nanomedicine. 2024;19:3259–3273. doi:10.2147/IJN.S457004
57. Zhou F, Li M, Chen M, et al. Redox homeostasis strategy for inflammatory macrophage reprogramming in rheumatoid arthritis based on ceria oxide nanozyme-complexed biopolymeric micelles. ACS Nano. 2023;17:4358–4372. doi:10.1021/acsnano.2c09127
58. Yun L, Shang H, Gu H, Zhang N. Polymeric micelles for the treatment of rheumatoid arthritis. Crit Rev Ther Drug Carr Syst. 2019;36:219–238. doi:10.1615/CritRevTherDrugCarrierSyst.2018021833
59. Veigas B, Matias A, Calmeiro T, et al. Antibody modified gold nanoparticles for fast colorimetric screening of rheumatoid arthritis. The Analyst. 2019;144:3613–3619. doi:10.1039/C9AN00319C
60. Chen C-L, Siow TY, Chou C-H, et al. Targeted superparamagnetic iron oxide nanoparticles for in vivo magnetic resonance imaging of T-cells in rheumatoid arthritis. Mol Imaging Biol. 2017;19:233–244. doi:10.1007/s11307-016-1001-6
61. Gawne PJ, Clarke F, Turjeman K, et al. PET imaging of liposomal glucocorticoids using 89 zr-oxine: theranostic applications in inflammatory arthritis. Theranostics. 2020;10:3867–3879. doi:10.7150/thno.40403
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Scientific Knowledge of Rheumatoid Arthritis: A Bibliometric Analysis from 2011 to 2020
Xu J, Yu J, Jiao W, Chen G, Liu L, Zhang M, Wu D
Journal of Pain Research 2022, 15:2761-2772
Published Date: 8 September 2022
Global Trends in Orthopedic Biofilm Research: A Bibliometric Analysis of 1994-2022
Hu Z, Yin X, Fan G, Liao X
Journal of Multidisciplinary Healthcare 2024, 17:3057-3069
Published Date: 3 July 2024
Research Trends of Rheumatoid Arthritis and Depression from 2019 to 2023: A Bibliometric Analysis
Zhao Y, Chen GY, Fang M
Journal of Multidisciplinary Healthcare 2024, 17:4465-4474
Published Date: 17 September 2024


