Back to Journals » Nature and Science of Sleep » Volume 17
Restorative Effects of Daytime Naps on Inhibitory Control: A Neuroimaging Study Following Sleep Deprivation
Authors Li L, Li Y, Yu S, Xu Z , Wang C, Guo F, Chang Y, Zhang R, Fang P, Zhu Y
Received 8 October 2024
Accepted for publication 4 February 2025
Published 18 March 2025 Volume 2025:17 Pages 475—487
DOI https://doi.org/10.2147/NSS.S499702
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sarah L Appleton
Leilei Li,1,* Ya Li,1,* Sihang Yu,2,* Ziliang Xu,1 Chen Wang,1 Fan Guo,1 Yingjuan Chang,1 Ran Zhang,3 Peng Fang,3– 5 Yuanqiang Zhu1
1Department of Radiology, Xijing Hospital, Air Force Medical University, Xi’an, Shaanxi, People’s Republic of China; 2School of Computer Science and Engineering, Xi’an Technological University, Xi’an, Shaanxi, People’s Republic of China; 3Department of Military Medical Psychology, Air Force Medical University, Xi’an, Shaanxi, People’s Republic of China; 4Shaanxi Provincial Key Laboratory of Bioelectromagnetic Detection and Intelligent Perception, Xi’an, Shaanxi, People’s Republic of China; 5Military Medical Innovation Center, Fourth Military Medical University, Xi’an, Shaanxi, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yuanqiang Zhu, Department of Radiology, Xijing Hospital, Air Force Medical University, No. 127 West Changle Road, Xi’an, 710032, People’s Republic of China, Tel /Fax +86-29-84775415, Email [email protected] Peng Fang, Department of Military Medical Psychology, Air Force Medical University, Xi’an, Shaanxi, 710032, People’s Republic of China, Email [email protected]
Background: Sleep deprivation is known to impair cognitive performance, particularly inhibitory control, which is crucial for goal-directed behavior. While extended recovery sleep is the ideal solution, the fast-paced demands of modern life often make this impractical. Brief daytime naps have emerged as a potential countermeasure, but the neural mechanisms underlying their restorative effects remain underexplored.
Objective: This study aimed to investigate the effects of a 30-minute daytime nap on brain activation patterns and cognitive performance following sleep deprivation. We used task-based functional magnetic resonance imaging (fMRI) to examine how naps modulate brain regions involved in inhibitory control.
Methods: Forty-five participants completed a dual-choice Oddball task under three conditions: Resting Wakefulness (RW), Sleep Deprivation (SD), and Post-Nap (Nap). Reaction times (RT), accuracy, and brain activation patterns were measured and analyzed across these states. Task-related brain activation was examined using fMRI, focusing on regions involved in the frontoparietal and default mode networks (DMN).
Results: Sleep deprivation significantly impaired inhibitory control, as reflected by slower RTs and reduced accuracy. A 30-minute nap partially restored cognitive performance, with RTs and accuracy showing intermediate improvement between RW and SD. Neuroimaging data revealed that the nap restored positive activation in the prefrontal cortex, occipital lobes, and middle frontal regions, which had been significantly reduced during SD. Furthermore, the nap enhanced negative activation in the middle temporal gyrus and cingulate gyrus, regions associated with the DMN, reducing cognitive interference from irrelevant stimuli.
Conclusion: Daytime naps significantly mitigate the cognitive deficits induced by SD through two primary mechanisms: (1) enhancing positive activation in task-relevant brain regions and (2) increasing negative activation in areas involved in the DMN. These findings provide novel insights into the neural basis of nap-induced cognitive recovery, underscoring the value of naps as an effective intervention to restore inhibitory control following SD.
Keywords: sleep deprivation, inhibitory control, daytime nap, functional MRI, fMRI
Introduction
Insufficient sleep has become a growing concern in modern society, impacting not only specialized sectors such as military operations but also various areas of public life.1,2 Studies have shown that a significant proportion of the global population consistently fails to meet the recommended seven to nine hours of sleep per night, leading to detrimental effects on health, cognition, and daily performance.3,4 Sleep deprivation (SD) has been extensively demonstrated to impair cognitive functions across various domains, including reduced attention, impaired working memory, and weakened inhibitory control.5–7 These deficits are especially concerning for individuals in high-stakes professions that require sustained attention, such as healthcare, transportation, and emergency services, where even brief lapses in cognitive performance can have serious consequences.
While extended recovery sleep is ideal for mitigating the effects of SD,8 modern lifestyles often make it difficult for individuals to get sufficient rest.9 Consequently, short daytime naps have emerged as a practical countermeasure to alleviate some of the cognitive deficits caused by SD.10,11 Nap duration is known to influence cognitive recovery, with 30-minute naps showing particular promise. Research suggests that 30-minute naps are effective in reducing fatigue and enhancing alertness, without the grogginess often associated with longer naps.12 This makes 30 minutes an ideal duration for studying the effects of naps on cognitive functions, as it provides a feasible and efficient intervention without inducing sleep inertia.13,14
Despite the known benefits of naps, the specific mechanisms through which naps restore cognitive function—particularly inhibitory control—remain underexplored.5,15 Inhibitory control is supported by complex neural mechanisms involving both positive and negative activation pathways.16–18 Positive activation refers to the engagement of task-relevant brain regions, such as the prefrontal cortex,17 while negative activation involves the suppression of the default mode network (DMN), which is active during rest.18,19 Most studies have focused on positive activation in task-related brain areas, while the role of negative activation, especially in the context of post-nap recovery, has received less attention. This gap in understanding limits our ability to fully comprehend how naps restore cognitive function.
Although previous research has explored the general cognitive benefits of naps, few studies have specifically examined the recovery of inhibitory control. Moreover, the neural mechanisms underlying nap-induced recovery of inhibitory control have not been fully elucidated, particularly with the use of functional magnetic resonance imaging (fMRI). In this study, we aim to address these gaps by investigating how daytime naps influence inhibitory control and the corresponding neural mechanisms following 24 hours of sleep deprivation. Using fMRI and a dual-choice Oddball task, we will examine brain activation patterns across three conditions: Resting Wakefulness, Sleep Deprivation, and Post-Nap. We hypothesize that naps will not only enhance positive activation in brain regions associated with cognitive control, such as the prefrontal cortex, but also restore negative activation in the DMN, including regions like the middle temporal gyrus and cingulate gyrus.
Methods
The study was carried out in line with the Declaration of Helsinki and received approval from the Ethics Committee of Xijing Hospital. Fifty participants gave written informed consent before the study. The sample consisted of cadets from the Air Force Military Medical University. The inclusion criteria required participants to be (1) right-handed and (2) aged between 18 and 30. Exclusion criteria included (1) a history or presence of any medical condition, (2) a history or presence of sleep disorders, (3) a history or presence of psychiatric conditions, (4) shift work, (5) any history of substance abuse or dependency, and (6) contraindications to MRI scanning. In addition, all participants completed the Pittsburgh Sleep Quality Index (PSQI) to assess their sleep quality. Only participants with PSQI scores of 5 or lower, indicating normal sleep quality, were included in the study. This helped ensure that none of the participants had significant sleep disturbances or disorders that could affect the study results.
Study Procedure
This study utilized a within-subjects design, where each participant experienced all three experimental conditions: Resting Wakefulness (RW), Sleep Deprivation (SD), and Post-Nap (Nap). The within-subjects design allowed for comparisons of brain activation patterns and cognitive performance within the same individual across these conditions, minimizing between-subject variability. Each participant visited the laboratory three times. During the first visit, participants were briefed on the study procedures and given a wrist-mounted Actiwatch (Philips Respironics, Mini Mitter) to monitor their sleep patterns for at least one week.20 This monitoring allowed us to collect data on their sleep durations before work days and sleep durations before free days, providing baseline information on their typical sleep-wake patterns prior to the experimental sessions. They also signed an informed consent form at this time. For the second and third visits, participants either underwent a 24-hour sleep deprivation (SD) period followed by MRI scanning or completed the protocol after a normal night of sleep. The order of these last two sessions was pseudo-randomized to control for order effects, and a minimum interval of one week was maintained between the visits to prevent any lingering effects from the SD condition.
The SD phase began at 8:00 a.m. and continued until 8:00 a.m. the following day. During this period, participants were allowed to engage in passive activities such as reading, watching television, or using the internet, but strenuous physical activities were prohibited, and participants were instructed to avoid caffeine. The environment was maintained at a stable temperature (approximately 23°C) with standard lighting conditions (340 lux). Two researchers remained with each participant to ensure they did not fall asleep during the sleep deprivation period.
For the RW and SD conditions, MRI scans were scheduled between 8:00 a.m. and 9:00 a.m. Upon completing the MRI scans following the SD session, participants were allowed to take a 30-minute nap, during which polysomnography (PSG) was recorded using six electroencephalography (EEG) channels (F3, F4, C3, C4, O1, O2), two electrooculography (EOG) channels, and bipolar electromyography (EMG) from the submental region. EEG impedance levels were kept below 5 kΩ, while EOG and EMG impedances were kept under 10 kΩ. After the nap, participants were awakened for a final MRI scan. To reduce the effects of sleep inertia,21 participants were given a 20-minute rest period before the final scan. This detailed experimental procedure is illustrated in Figure 1.
 |
Figure 1 Overview of the experimental procedure. |
The Dual-Choice Oddball Task
To assess inhibitory control, participants completed a dual-choice Oddball task during each scanning session. The dual-choice Oddball task is a well-established paradigm in cognitive neuroscience for probing inhibitory control, especially in the context of attentional shifts and the need to suppress habitual responses.22 This task involved the presentation of two types of visual stimuli: a frequent standard stimulus (the letter “W”), which appeared in 85% of trials, and an infrequent deviant stimulus (the letter “M”), presented in 15% of the trials. Participants were instructed to respond to each stimulus by pressing a specific key: pressing the left button in response to the frequent “W” and the right button when the infrequent “M” appeared.
The stimuli were presented in a randomized sequence to ensure that participants could not predict the appearance of the deviant stimulus. The task was designed to probe inhibitory control by measuring participants’ reaction times (RT) and accuracy. In particular, the task assesses how participants suppress automatic responses to the frequent standard stimulus and instead respond to the less frequent deviant stimulus. RT and accuracy were recorded for each stimulus type. The task specifically measured participants’ ability to inhibit prepotent responses to the frequent standard stimulus and correctly respond to the less frequent deviant stimulus. Performance on the task is expected to decline under conditions of cognitive fatigue, such as sleep deprivation.
Task-Based fMRI Data Acquisition and Preprocessing
Functional MRI data were collected during the dual-choice Oddball task using a Philips 3-Tesla MRI scanner with a 32-channel head coil. Blood-oxygen-level-dependent (BOLD) signals were acquired using an echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 220 mm × 220 mm, slice thickness = 3 mm, and 45 axial slices covering the entire brain. Each scanning session corresponded to one of the three experimental conditions: Resting Wakefulness (RW), Sleep Deprivation (SD), and Post-Nap (Nap).
The task-fMRI data preprocessing was conducted using SPM12 (Statistical Parametric Mapping), beginning with the discarding of the first 10 time points to allow for signal stabilization. Subsequent steps included slice timing correction to account for temporal differences in slice acquisition, realignment to correct for head motion, and normalization to the Montreal Neurological Institute (MNI) template to standardize brain images. Finally, spatial smoothing was applied using a Gaussian kernel of 6 mm full width at half maximum (FWHM) to enhance the signal-to-noise ratio.23
Task-Based fMRI Data Analysis
The analysis of fMRI data followed a two-stage mixed-effects model approach to investigate neural activity across the different experimental conditions. In the first stage, an event-related design was implemented to precisely isolate the neural correlates of task performance. For each condition (RW, SD, Nap), a general linear model (GLM) was applied to the stimulus sequence, convolved with the hemodynamic response function (HRF). This model identified brain regions exhibiting significantly higher or lower activity in response to the deviant stimulus (“M“) compared to the standard stimulus (“W”). To control for head motion artifacts, six motion parameters from the preprocessing stage were included in the model.
In the second stage, brain activation patterns were examined using a one-sample t-test for each condition during the dual-choice Oddball task. Specifically, we calculated the difference in brain activation between the two stimulus conditions (Deviant vs Standard) for each participant, and a one-way ANOVA was then conducted to compare activation differences across the three experimental conditions. Post-hoc pairwise comparisons were employed to explore differences between RW vs SD, RW vs Nap, and SD vs Nap. Finally, brain activation values were extracted from regions showing significant differences, and visual representations of these findings were generated to facilitate interpretation.
Statistical Analysis
In the dual-choice Oddball task, behavioral performance was assessed using two primary measures: reaction times (RT) and accuracy for both standard stimuli (“W”) and deviant stimuli (“M”). Reaction times and accuracy were calculated separately for standard and deviant stimuli under each of the three conditions: Resting Wakefulness (RW), Sleep Deprivation (SD), and Post-Nap (Nap). Reaction times were reported as the average time taken to respond to each stimulus type, while accuracy was expressed as the percentage of correct responses for both standard and deviant stimuli.
The differences in performance between deviant and standard stimuli were calculated for both reaction times and accuracy. For reaction times, the difference was computed by subtracting the mean RT for standard stimuli from the mean RT for deviant stimuli (RT: deviant - standard). Similarly, accuracy differences were calculated by subtracting the accuracy for standard stimuli from that of deviant stimuli (Accuracy: deviant - standard). These difference scores provided insights into participants’ inhibitory control, specifically how their performance changed when responding to infrequent, unexpected stimuli compared to frequent, expected stimuli.
To assess the effects of the different conditions (RW, SD, Nap) on both the raw scores and the differences (deviant - standard) for reaction times and accuracy, a one-way repeated measures ANOVA was conducted. This analysis examined whether sleep deprivation or post-nap recovery influenced participants’ ability to respond to deviant versus standard stimuli. Post-hoc Tukey’s HSD tests were applied to explore significant pairwise differences between conditions for both reaction time and accuracy measures, including the calculated differences. The significance level was set at p < 0.05, and multiple comparisons were corrected using the false discovery rate (FDR). Additionally, for neuroimaging data, FDR corrections were applied to both F-tests comparing activation across conditions and post-hoc comparisons, with the threshold set at pFDR < 0.05. Correlation analysis was conducted to examine the relationship between changes in brain activation and improvements in cognitive performance (accuracy and reaction time) specifically between the Sleep Deprivation (SD) and Post-Nap (Nap) conditions. Pearson’s correlation coefficient was used to calculate the strength and direction of these associations. To control for multiple comparisons, all correlation results were corrected using the FDR method at a threshold of p < 0.05.
Results
Due to the head motion, data from five participants were excluded from the analysis, Therefore, 45 participants were included in the final analysis, and each participant completed the dual-choice Oddball task under all three conditions (Resting Wakefulness, Sleep Deprivation, and Post-Nap). The demographic details of the remaining participants are provided in Table 1.
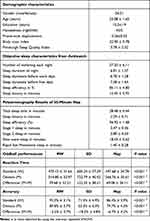 |
Table 1 Demographic Characteristics, Objective Sleep Measures and Oddball Performances |
Dual-Choice Oddball Behavioral Performance
Participants’ performance in the dual-choice Oddball task was evaluated through reaction times (RT) and accuracy for both standard and deviant stimuli across three experimental conditions In terms of reaction times, participants in the RW condition showed the fastest responses, with an average RT of 475.12 ± 31.64 ms for standard stimuli and 514.80 ± 32.97 ms for deviant stimuli, resulting in the smallest RT difference of 39.68 ± 33.21 ms. In contrast, participants in the SD condition had the slowest reaction times, with 600.24 ± 37.29 ms for standard stimuli and 722.79 ± 40.42 ms for deviant stimuli, leading to the largest RT difference of 122.55 ± 38.21 ms. Participants in the Nap condition demonstrated intermediate RTs, averaging 497.68 ± 34.78 ms for standard stimuli and 566.76 ± 35.61 ms for deviant stimuli, with an RT difference of 69.08 ± 36.11 ms. A one-way ANOVA revealed a significant variation in RT differences across the three conditions (F(2, 132) = 286.53, p < 0.0001). Post-hoc Tukey’s HSD tests indicated significant differences between all pairs of conditions: SD vs RW (Mean difference = 82.87 ms, p < 0.0001), SD vs Nap (Mean difference = 53.47 ms, p < 0.0001), and Nap vs RW (Mean difference = 29.39 ms, p < 0.0001).
In terms of accuracy, participants in the RW condition achieved an average accuracy of 92.3% ± 3.1% for standard stimuli and 89.8% ± 3.7% for deviant stimuli, resulting in the smallest accuracy difference (−0.025 ± 0.037). In contrast, participants in the SD condition had much lower accuracy, with 71.5% ± 4.9% for standard stimuli and 53.3% ± 5.3% for deviant stimuli, producing the largest accuracy discrepancy (−0.182 ± 0.048). Participants in the Nap condition performed better than the SD group but worse than the RW group, with an accuracy of 86.4% ± 3.9% for standard stimuli and 79.7% ± 4.3% for deviant stimuli, resulting in an accuracy difference of −0.067 ± 0.042. A one-way ANOVA confirmed a highly significant effect of condition on accuracy differences (F(2, 132) = 477.06, p < 0.0001). Post-hoc Tukey’s HSD tests again showed significant differences between all groups: SD vs RW (Mean difference = −0.157, p < 0.0001), SD vs Nap (Mean difference = −0.115, p < 0.0001), and Nap vs RW (Mean difference = −0.042, p < 0.0001). The results are summarized in Table 1 and illustrated in Figure 2.
Task-Based fMRI Activation Patterns
Figure 3A–C presents the results of the one-sample t-test, illustrating the brain activation patterns in response to deviant stimuli compared to standard stimuli. The activation patterns were consistent across all three conditions, with key regions of activation including the right and left middle frontal gyri, right and left supplementary motor areas, right and left inferior frontal gyri, right insula, left cingulate gyrus, and the right and left inferior parietal gyri.
 |
Figure 3 (A–C) One-sample t test result for the three conditions (RW, SD and Nap). (D) One-way repeated ANOVA results across the three conditions. |
Figure 3D and Table 2 summarize the ANOVA results, which reveal significant differences in brain activation across the three conditions. These differences were observed in regions such as the bilateral occipital lobes, middle temporal gyrus, middle cingulate gyrus, medial frontal lobes, and dorsolateral prefrontal cortex. The distinct activation patterns among the three groups suggest that task performance was influenced by the specific condition participants were in.
 |
Table 2 Significant Differences in Brain Responses Among the Three Groups |
Post-hoc analyses, as depicted in Figure 4A, identified specific group differences in the medial frontal cortex, prefrontal cortex, and bilateral occipital lobes. The SD group exhibited significantly reduced activity in these regions compared to the RW group (medial frontal cortex: t = −7.00, p < 0.001, FDR corrected; prefrontal cortex: t = −7.70, p < 0.001, FDR corrected; left occipital lobe: t = −12.67, p < 0.001, FDR corrected; right occipital lobe: t = −7.70, p < 0.001, FDR corrected) whereas the Nap group showed a recovery in activation compared to the SD group (medial frontal cortex: t = 6.86, p < 0.001, FDR corrected; prefrontal cortex: t = 6.82, p < 0.001, FDR corrected; left occipital lobe: t = 6.40, p < 0.001, FDR corrected; right occipital lobe: t = 5.86, p < 0.001, FDR corrected), approaching the levels seen in the RW group (Nap vs RW, all p > 0.05, FDR corrected). These results suggest that a nap may help restore normal brain activity in regions associated with cognitive control and visual attention.
A different pattern was observed in the middle temporal gyrus and cingulate gyrus, as shown in Figure 4B. Under normal conditions (RW), these areas typically display negative activation during the task. However, the SD condition showed reduced negative activation in these regions compared to RW (middle temporal gyrus: t = 3.00, p < 0.01, FDR corrected; cingulate gyrus: t = 3.00, p < 0.01, FDR corrected). The Nap condition, on the other hand, restored the negative activation in these regions compared to the SD condition (middle temporal gyrus: t = −3.50, p < 0.01, FDR corrected; cingulate gyrus: t = −3.50, p < 0.01, FDR corrected). However, the SD condition showed reduced negative activation in these regions compared to RW (middle temporal gyrus: t = 3.00, p < 0.01, FDR corrected; cingulate gyrus: t = 3.00, p < 0.01, FDR corrected). The Nap condition, on the other hand, restored the negative activation in these regions compared to the SD condition (middle temporal gyrus: t = −3.50, p < 0.01, FDR corrected; cingulate gyrus: t = −3.50, p < 0.01, FDR corrected).
Correlation Analysis
A significant positive correlation was found between increased activation in the prefrontal cortex and improvements in accuracy in the Nap condition (r = 0.38, p < 0.01, Figure 4C). Additionally, a positive correlation was observed between increased deactivation in the middle temporal gyrus and improvements in reaction time in the Nap condition (r = 0.32, p < 0.05, Figure 4C). These findings suggest that naps may promote both enhanced task performance and more effective neural deactivation, contributing to improved cognitive control.
Discussion
This study provides preliminary evidence supporting the effectiveness of brief daytime naps in improving inhibitory control performance following sleep deprivation. By analyzing brain activity dynamics during a dual-choice Oddball task under three conditions (RW, SD, and Nap). We observed that a brief nap enhanced positive activation in the prefrontal and occipital regions and increased negative activation in the middle temporal gyrus and cingulate cortex. These changes contributed to improved conflict control performance, offering insights into how sleep deprivation impairs cognitive function and highlighting the potential of naps as a recovery strategy.
Our results from one-sample t-tests revealed that task-related brain activation involved multiple key networks, including the default mode network (DMN), the frontoparietal network, and the salience network. Consistent with previous studies,24,25 the middle frontal gyrus, inferior frontal gyrus, supplementary motor areas, insula, and cingulate cortex were activated during task performance. As illustrated in Figure 3A–C, positive activation was most prominent during resting wakefulness, decreased significantly during sleep deprivation, and was partially restored after the post-nap condition. The ANOVA results identified significant differences in brain activation across the three conditions, particularly in the occipital lobes, middle temporal gyrus, cingulate cortex, medial frontal lobes, and dorsolateral prefrontal cortex. Post-hoc analyses revealed that the improvements in conflict control following a nap were driven by two key neuroimaging mechanisms.
Firstly, there was a partial restoration of positive activation in regions such as the dorsolateral prefrontal cortex, occipital lobes, and medial frontal lobes. These areas showed a significant reduction in activation during sleep deprivation compared to resting wakefulness, but this activation was partially recovered after the nap, suggesting that naps help to restore functions critical for cognitive control and visual processing, reducing the deficits caused by sleep deprivation.9 Secondly, naps contributed to the restoration of negative activation, particularly in the middle temporal gyrus and cingulate cortex, which are part of the default mode network (DMN). Sleep deprivation was associated with reduced deactivation in these regions, indicating difficulties in disengaging from task-irrelevant processes and a decline in cognitive efficiency. After a nap, however, negative activation was restored, surpassing even the levels observed during resting wakefulness. This suggests that naps enhance the brain’s ability to allocate cognitive resources more efficiently by modulating DMN activity, helping to filter out irrelevant stimuli, which is similar with our previous studies.26,27 Overall, these findings suggest that naps improve inhibitory control through a dual mechanism: enhancing positive activation in task-relevant regions and increasing negative activation in the DMN, both of which play a crucial role in restoring efficient cognitive processing after sleep deprivation.
A shift in response strategy following the nap could also explain the changes observed in performance. Specifically, while reaction time (RT) in the Nap condition was slower than during resting wakefulness, accuracy (ACC) was significantly improved. This pattern suggests that participants may have adjusted their responding strategy after the nap, prioritizing accuracy over speed. Such a shift could be viewed as a compensatory mechanism, where participants took more time to process information carefully, resulting in more accurate responses but slower reaction times. This strategy shift is consistent with findings from previous studies indicating that sleep, particularly naps, can enhance cognitive control, such as response inhibition and attention regulation.11,12,28 The increase in accuracy after the nap may reflect enhanced decision-making, possibly supported by greater activation in the prefrontal cortex, a region critical for cognitive control. Meanwhile, the slower reaction time could be related to increased inhibition of impulsive responses, potentially reflecting activity in the anterior cingulate cortex, which is involved in conflict monitoring and response control. Overall, the observed slower RT but higher ACC after the nap may indicate that participants adopted a more cautious and deliberate approach to the task, likely aided by improvements in cognitive control and neural efficiency following the nap. These findings suggest that naps may promote both more accurate decision-making and better control of cognitive interference, enhancing performance after sleep deprivation.
In recent years, studies using functional magnetic resonance imaging (fMRI) have highlighted the central role of the prefrontal cortex and anterior cingulate cortex in inhibitory control.29,30 The prefrontal cortex plays a crucial role in cognitive regulation through top-down control, and its connectivity with other cortical and subcortical areas is positively correlated with inhibitory performance. When individuals experience sleep deprivation, activity in the prefrontal cortex is significantly reduced, undermining its regulatory functions across a range of cognitive processes. Similarly, the anterior cingulate cortex, which monitors cognitive conflicts and integrates information from motor and limbic circuits,31 shows reduced activation under SD, limiting its ability to detect and resolve conflicts in task performance.
Previous research has demonstrated the high sensitivity of inhibitory control to sleep deprivation. For example, Drummond et al (2006) found that after 55 hours of sleep deprivation, participants exhibited reduced accuracy and increased reaction times in a Go/No-Go task, indicating a diminished ability to inhibit inappropriate responses.32 Similarly, Slama et al (2018) reported that a single night of sleep deprivation impaired response inhibition and prolonged reaction times during a No-Go task.33 While most research has focused on interventions such as transcranial stimulation to mitigate these effects, few studies have explored the role of naps. Our study, which used task-based fMRI, demonstrated that daytime naps partially restore inhibitory control performance by both enhancing positive activation in the prefrontal cortex and occipital lobes and increasing negative activation in DMN-related regions. These findings offer new insights into the role of naps in cognitive function, particularly in restoring inhibitory control after sleep deprivation.
The primary limitation of this study is the homogeneity of the participants, as all were cadets from the Air Force Military Medical University. This restricts the generalizability of the findings to non-military populations. The challenging nature of the sleep deprivation task necessitated the use of this specific group, but future studies should aim to include a more diverse participant pool to confirm the findings across different demographics. While the sample size of 45 participants is standard for studies involving complex methodologies such as sleep deprivation and fMRI scanning, a larger and more diverse sample could help increase the external validity of the findings. Although our findings suggest that a nap after SD improved cognitive function, as indicated by changes in oddball task performance, this conclusion needs validation using other cognitive tasks. Another key aspect of the study involves the role of naps, and future research should explore whether shorter naps (less than 30 minutes) have a reduced effect on cognitive recovery, as well as how factors like dreaming before waking impact cognitive function.
In conclusion, this study demonstrates that sleep deprivation and daytime naps primarily affect brain regions involved in the frontoparietal network, default mode network, and occipital lobes. These results provide a foundation for future investigations into the neuroimaging mechanisms underlying the cognitive effects of sleep deprivation and naps. While task-based fMRI effectively captures brain region activity during specific tasks, it has limitations in characterizing the broader impact of sleep and naps on cognitive functions. Future research utilizing resting-state fMRI will help uncover how sleep deprivation and naps affect brain network connectivity, providing a more comprehensive understanding of their role in cognitive processes.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This investigation was performed in accordance with the Declaration of Helsinki and was approved by the Ethical Committee of the Sports Institute of Xijing Hospital.
Consent for Publication
Consent for publication has been obtained.
Acknowledgments
We thank Michael Irvine, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
Author Contributions
All authors contributed substantially to the research, including the conceptualization, design, implementation, data collection, analysis, and interpretation of the study. They were involved in drafting, revising, and critically reviewing the manuscript. Each author provided final approval for the version to be published, agreed on the journal for submission, and accepts responsibility for the article’s content. The individual contributions of the authors, based on the CRediT taxonomy, are as follows: Leilei Li: Conceptualization, Methodology, Data Curation, Writing, Original Draft Preparation. Ya Li: Methodology, Investigation, Writing, Review & Editing, Supervision. Sihang Yu: Data Collection, Formal Analysis, Visualization. Ziliang Xu: Software, Validation, Writing, Review & Editing. Chen Wang: Resources, Investigation, Writing, Review & Editing. Fan Guo: Data Curation, Investigation, Writing, Review & Editing. Yingjuan Chang: Supervision, Project Administration, Writing, Review & Editing. Ran Zhang: Data Collection, Resources, Writing, Review & Editing. Peng Fang: Formal Analysis, Visualization, Writing, Review & Editing. Yuanqiang Zhu: Conceptualization, Supervision, Funding Acquisition, Writing, Review & Editing.
Funding
This study has received funding by the Boost Program of Xijing Hospital (JSYXZ08 and XJZT24JC06), Rapid Response Project from Air Force Medical University (2023KXKT050), National Natural Science Foundation of China (No.32471081) and Shaanxi Provincial Natural Science Basic Research Program (2024JC-YBQN-0209).
Disclosure
The authors declare that they have no competing interests.
References
1. Zhao Y, Huang B, Yu Y. Exercise to prevent the negative effects of sleep deprivation: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2023;155:105433. doi:10.1016/j.neubiorev.2023.105433
2. Di H, Guo Y, Daghlas I, et al. Evaluation of Sleep Habits and Disturbances Among US Adults, 2017-2020. JAMA Netw Open. 2022;5(11):e2240788. doi:10.1001/jamanetworkopen.2022.40788
3. Chattu VK, Manzar MD, Kumary S, Burman D, Spence DW, Pandi-Perumal SR. The Global Problem of Insufficient Sleep and Its Serious Public Health Implications. Healthcare. 2018;7(1):1. doi:10.3390/healthcare7010001
4. Chan CMH, Siau CS, Wong JE, Wee LH, Jamil NA, Hoe VCW. Prevalence of Insufficient Sleep and Its Associated Factors Among Working Adults in Malaysia. Nat Science Sleep. 2021;13:1109–1116. doi:10.2147/NSS.S295537
5. Wüst LN, Capdevila NC, Lane LT, Reichert CF, Lasauskaite R. Impact of one night of sleep restriction on sleepiness and cognitive function: a systematic review and meta-analysis. Sleep Med Rev. 2024;76:101940. doi:10.1016/j.smrv.2024.101940
6. Fjell AM, Walhovd KB. Individual sleep need is flexible and dynamically related to cognitive function. Nat Human Behav. 2024;8(3):422–430. doi:10.1038/s41562-024-01827-6
7. Magnuson JR, Kang HJ, Dalton BH, McNeil CJ. Neural effects of sleep deprivation on inhibitory control and emotion processing. Behav Brain Res. 2022;426:113845. doi:10.1016/j.bbr.2022.113845
8. Kayser KC, Puig VA, Estepp JR. Predicting and mitigating fatigue effects due to sleep deprivation: a review. Front Neurosci. 2022;16:930280. doi:10.3389/fnins.2022.930280
9. Xu Z, Chang Y, Guo F, et al. The restoration ability of a short nap after sleep deprivation on the brain cognitive function: a dynamic functional connectivity analysis. CNS Neurosci Ther. 2024;30(2):e14413. doi:10.1111/cns.14413
10. Mesas AE, Núñez de Arenas-Arroyo S, Martinez-Vizcaino V, et al. Is daytime napping an effective strategy to improve sport-related cognitive and physical performance and reduce fatigue? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2023;57(7):417–426. doi:10.1136/bjsports-2022-106355
11. Yang Y-B, Zheng Y-B, Sun J. To nap or not? Evidence from a meta-analysis of cohort studies of habitual daytime napping and health outcomes. Sleep Med Rev. 2024;78:101989. doi:10.1016/j.smrv.2024.101989
12. Leong RLF, Lo JC, Chee MWL. Systematic review and meta-analyses on the effects of afternoon napping on cognition. Sleep Med Rev. 2022;65:101666. doi:10.1016/j.smrv.2022.101666
13. Kharas N, Chelaru MI, Eagleman S, Parajuli A, Dragoi V. NREM sleep improves behavioral performance by desynchronizing cortical circuits. Science. 2024;386(6724):892–897. doi:10.1126/science.adr3339
14. Tassi P, Muzet A. Sleep inertia. Sleep Med Rev. 2000;4(4):341–353. doi:10.1053/smrv.2000.0098
15. Harrington MO, Cairney SA. Sleep Loss Gives Rise to Intrusive Thoughts. Trends Cognitive Sci. 2021;25(6):434–436. doi:10.1016/j.tics.2021.03.001
16. Mao T, Dinges D, Deng Y. Impaired Vigilant Attention Partly Accounts for Inhibition Control Deficits After Total Sleep Deprivation and Partial Sleep Restriction. Nat Science Sleep. 2021;13:1545–1560. doi:10.2147/NSS.S314769
17. Apšvalka D, Ferreira CS, Schmitz TW, Rowe JB, Anderson MC. Dynamic targeting enables domain-general inhibitory control over action and thought by the prefrontal cortex. Nat Commun. 2022;13(1):274. doi:10.1038/s41467-021-27926-w
18. Zilverstand A, Parvaz MA, Moeller SJ, et al. Whole-brain resting-state connectivity underlying impaired inhibitory control during early versus longer-term abstinence in cocaine addiction. mol Psychiatry. 2023;28(8):3355–3364. doi:10.1038/s41380-023-02199-5
19. Liddle EB, Hollis C, Batty MJ, et al. Task-related default mode network modulation and inhibitory control in ADHD: effects of motivation and methylphenidate. J Child Psychol Psychiat Allied Discipline. 2011;52(7):761–771. doi:10.1111/j.1469-7610.2010.02333.x
20. Xu Z, Chang Y, Wang C, et al. Cognitive impairment after sleep deprivation: the role of precuneus related connectivity on the intra-individual variability changes. NeuroImage. 2023;284:120462. doi:10.1016/j.neuroimage.2023.120462
21. Dawson D, Ferguson SA, Vincent GE. Safety implications of fatigue and sleep inertia for emergency services personnel. Sleep Med Rev. 2021;55:101386. doi:10.1016/j.smrv.2020.101386
22. Bi XY, Ma X, Abulaiti A, Yang J, Tao Y. The influence of pride emotion on executive function: evidence from ERP. Brain Behav. 2022;12(8):e2678. doi:10.1002/brb3.2678
23. Wang X, Li Y, Jiao F, et al. Functional Connectivity Alterations During Sleep Deprivation: investigating Key Brain Regions and Networks. J Integrat Neurosci. 2023;22(6):169. doi:10.31083/j.jin2206169
24. Lee TW, Almeida S, Tramontano G. Attention improvement to transcranial alternating current stimulation at gamma frequency over the right frontoparietal network: a preliminary report. Acta Neuropsychiatr. 2024;1–5. doi:10.1017/neu.2024.35
25. Nikolaidis A, He X, Pekar J, Rosch K, Mostofsky SH. Frontal corticostriatal functional connectivity reveals task positive and negative network dysregulation in relation to ADHD, sex, and inhibitory control. Develop Cognitive Neurosci. 2022;54:101101. doi:10.1016/j.dcn.2022.101101
26. Zhu Y, Xi Y, Sun J, et al. Neural correlates of dynamic changes in working memory performance during one night of sleep deprivation. Human Brain Mapp. 2019;40(11):3265–3278. doi:10.1002/hbm.24596
27. Zhu Y, Ren F, Zhu Y, et al. Gradually Increased Interhemispheric Functional Connectivity During One Night of Sleep Deprivation. Nat Science Sleep. 2020;12:1067–1074. doi:10.2147/NSS.S270009
28. Faraut B, Andrillon T, Vecchierini MF, Leger D. Napping: a public health issue. From epidemiological to laboratory studies. Sleep Med Rev. 2017;35:85–100. doi:10.1016/j.smrv.2016.09.002
29. Bartholdy S, O’Daly OG, Campbell IC. Neural Correlates of Failed Inhibitory Control as an Early Marker of Disordered Eating in Adolescents. Biol Psychiatry. 2019;85(11):956–965. doi:10.1016/j.biopsych.2019.01.027
30. Kim Y, Lee JH, Park JC, et al. Neuromodulation of inhibitory control using phase-lagged transcranial alternating current stimulation. J Neuroeng Rehabil. 2024;21(1):93. doi:10.1186/s12984-024-01385-y
31. Clairis N, Lopez-Persem A. Debates on the dorsomedial prefrontal/dorsal anterior cingulate cortex: insights for future research. Brain. 2023;146(12):4826–4844. doi:10.1093/brain/awad263
32. Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15(3):261–265. doi:10.1111/j.1365-2869.2006.00535.x
33. Slama H, Chylinski DO, Deliens G, Leproult R, Schmitz R, Peigneux P. Sleep Deprivation Triggers Cognitive Control Impairments in Task-Goal Switching. Sleep. 2018;41(2). doi:10.1093/sleep/zsx200
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



