Back to Journals » Cancer Management and Research » Volume 17
Retrospective Analysis of the Timing of Radiotherapy Intervention After Induction Chemoimmunotherapy in Unresectable Locally Advanced Lung Squamous Cell Carcinoma
Authors Zeng L, Zhang Y, Zeng A, Ma D
Received 13 February 2025
Accepted for publication 25 June 2025
Published 3 July 2025 Volume 2025:17 Pages 1301—1311
DOI https://doi.org/10.2147/CMAR.S517837
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Kattesh Katti
Li Zeng, Yu Zhang, Aiju Zeng, Daiyuan Ma
Department of Oncology, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, People’s Republic of China
Correspondence: Daiyuan Ma, Email [email protected]
Background: The optimal combination of immune checkpoint inhibitors (ICIs), radiotherapy, and chemotherapy for unresectable locally advanced lung squamous cell carcinoma (LA-LUSC) remains undefined. This study evaluated induction chemoimmunotherapy followed by radiotherapy ± consolidation ICI in unresectable LA-LUSC, specifically exploring radiotherapy timing impact.
Methods: We retrospectively analyzed 54 unresectable LA-LUSC patients receiving induction chemoimmunotherapy followed by radiotherapy. Patients were grouped by radiotherapy timing: Early (after 2– 3 induction cycles, n = 18) and Late (after 4– 6 cycles, n = 36). Survival analysis (Kaplan–Meier, Log-rank) compared progression-free survival (PFS), local PFS (LPFS), distant metastasis-free survival (DMFS), overall survival (OS), and safety. Prognostic factors for PFS/OS were explored.
Results: Median follow-up was 30.7 months. Median PFS for all patients was 21.9 months. Early radiotherapy improved PFS (HR = 0.43, p = 0.024) and LPFS (HR = 0.36, p = 0.038). Radiotherapy after 2– 3 induction cycles was an independent predictor of improved PFS (p = 0.040). Overall treatment tolerance was good; grade ≥ 3 pneumonitis incidence was 5.56%. After propensity score matching, OS was significantly longer in patients receiving induction plus consolidation ICI versus induction ICI alone (HR = 0.51, p = 0.038).
Conclusion: Induction chemoimmunotherapy followed by radiotherapy demonstrates promising efficacy and manageable toxicity in unresectable LA-LUSC. Initiating radiotherapy earlier (after 2– 3 induction cycles) improves PFS and LPFS and is an independent favorable prognostic factor. Consolidation ICI after combined chemoimmunotherapy and radiotherapy further extends OS compared to induction ICI alone.
Keywords: locally advanced non-small cell lung cancer, immune checkpoint inhibitors, radiotherapy, lung cancer
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide. Data shows that approximately 1.8 million people die from lung cancer each year.1 Non-small cell lung cancer (NSCLC) is the most common type, accounting for about 85% of all lung cancers.2 Clinically, over one-third of NSCLC patients are diagnosed as locally advanced at first visit, with the majority being unresectable.3 Definitive chemoradiotherapy (CRT) is the standard treatment for unresectable locally advanced NSCLC (LA-NSCLC), but the prognosis remains poor, with a 5-year survival rate of only 15%.3 The emergence of immune checkpoint inhibitors (ICIs) has significantly improved the survival rates of unresectable LA-NSCLC patients.4 However, the 5-year progression-free survival (PFS) rate for consolidation ICI is only 33.1%,5 indicating that nearly 70% of unresectable LA-NSCLC cases remain uncontrolled. According to the criteria of the PACIFIC study, almost half of the unresectable LA-NSCLC patients who received CRT were not eligible for consolidation ICI.6,7 The MOOREA8 study is a prospective, non-intervention study designed to evaluate real-world treatment patterns and outcomes in Chinese patients with stage III NSCLC. Mid-term analysis showed that in the unresectable III phase NSCLC cohort, only 26.8% of patients received consolidation ICI after CRT. Therefore, the new permutation of ICIs and CRT may extend the benefits of ICIs to all patients suitable for CRT, thus bringing further benefits to patients with unresectable LA-NSCLC.
In recent years, preoperative neoadjuvant chemoimmunotherapy has achieved surprising results. In the CheckMate-816 trial,9 preoperative neoadjuvant chemoimmunotherapy brought greater survival benefits to patients with IIIA stage NSCLC than patients with stage IB-II disease, which may be due to the fact that neoadjuvant chemoimmunotherapy can achieve the goal of decreasing stage by reducing tumor volume and contribute to the efficacy of subsequent radical treatment. Therefore, it is reasonable that induction chemoimmunotherapy before radical CRT can benefit patients with unresectable LA-NSCLC, especially in the GEMSTONE-301 trial,10 when the proportion of patients with stage IIIB/IIIC NSCLC (70%) is larger, showing an unsatisfactory treatment response and survival outcome to initial radical CRT and consolidation ICI.
At present, prospective studies on induction chemoimmunotherapy in unresectable LA-NSCLC are under way. According to the reported data,11,12 induction chemoimmunotherapy followed by radiotherapy seems to provide some survival benefits for patients with unresectable LA-NSCLC. Its safety is controllable and the short-term effect is good. However, it is not clear when radiotherapy intervention will be carried out after induction chemoimmunotherapy, and whether it is still necessary to take consolidation ICI. Prior to the Pacific trial, two cycles of induction chemotherapy prior to CRT were common for unresectable LA-NSCLC,13 likely because the two-cycle time window not only reduced tumor size but also provided timely free radical therapy to prevent tumor progression. For patients with LA-NSCLC who were unresectable after receiving induction chemoimmunotherapy, Yang et al14 showed that a trend in PFS benefit was observed compared to other induction cycles in less than 3 cycles of induction therapy. Wu et al15 also found that 2–3 cycles of induced immunochemotherapy may be best, as longer induction may delay CRT and worsen the prognosis. However, the above studies focused more on reporting the efficacy and safety of induction chemoimmunotherapy, and did not study the timing of radiotherapy interventions in more detail. Lung squamous cell carcinoma has a higher tumor mutation load, and the positive rate of driving gene is lower than that of non-squamous cell carcinoma.16,17 The subgroup analysis of the GESTONE-301 study also showed that the HR of patients with squamous cell carcinoma was 0.57, while the HR of non-squamous cell carcinoma was 0.77.10 From the second line to the first line of immunotherapy, the immune benefit of patients with squamous cell carcinoma seems to be better than that of non-squamous cell carcinoma. Therefore, the purpose of this study was to evaluate the efficacy and safety of induction chemoimmunotherapy followed by radiotherapy with or without consolidation ICI in unresectable locally advanced lung squamous cell carcinoma (LA-LUSC) and to explore the effect of intervention timing of radiotherapy on the efficacy.
Materials and Methods
Patient Selection
Patients with unresectable LA-LUSC who received induction chemoimmunotherapy followed by radiotherapy in the affiliated Hospital of North Sichuan Medical College from December 2019 to November 2023 were collected retrospectively. Inclusion criteria: 1. Confirmed by pathology as LUSC; 2. Patients with unresectable LA-LUSC assessed by multidisciplinary evaluation; 3. First-line receiving induction chemoimmunotherapy for 2 or more cycles; 4. There was no disease progression before radiotherapy.; 5. All patients completed the local thoracic radiotherapy during the period of receiving 4–6 cycles of chemotherapy and immunotherapy combined system therapy. Exclusion criteria: 1. Incomplete local thoracic radiotherapy 2. Did not complete the total course of treatment of 4–6 cycles of chemotherapy and immunotherapy combined with system treatment; 3. The case data are incomplete.
Radiotherapy after 2–3 cycles of induction chemoimmunotherapy was defined as early radiotherapy group, and radiotherapy after 4–6 cycles of induction chemotherapy was defined as late radiotherapy group. This study complies with the provisions of the Helsinki Declaration (revised in 2013). The review committee of the affiliated hospital of North Sichuan Medical College gave ethical approval to this study.
Treatment Strategy
All patients received first-line induction chemoimmunotherapy followed by radiotherapy with or without consolidation ICI. ICIs are anti-PD-1 antibodies delivered at the same time as or after chemotherapy. The ICIs used in the induction regimen included Carellizumab, Nivolumab, Sintilimab and Tirelizumab. Based on the good efficacy of NSCLC patients, these four ICIs drugs have been approved for the treatment of NSCLC. Conventional fractionated intensity radiotherapy was used for radiotherapy. The typical dose of radiotherapy is 60Gy, which is divided into 30 times and is used continuously for 5 days a week. Consolidation ICI is recommended for patients without treatment-related adverse events (TRAEs) at the end of first-line treatment.
Data Collection and Follow-Up
Baseline demographic and therapeutic data were extracted from electronic medical records. The disease staging is based on the American Joint Committee on Cancer and the eighth edition of the International Union against Cancer Lung Cancer staging classification TNM. Routine follow-up includes vital signs, physical status, and other hematological tests, as well as any adverse events. According to the solid tumor response evaluation criteria version 1.1, CT or positron emission tomography was used to evaluate the tumor response. Patients were followed up every three months in the first two years, every six months in the following two years, and once a year after five years. Clinical examinations include routine blood tests, chest enhanced CT, and neck/abdominal CT or ultrasound and indicative PET/CT. The recurrence was confirmed by radiological study or tissue cell biopsy.
Outcome and Statistical Analysis
PFS was calculated from the date of induction therapy to the date of progression, death of any cause, or the last follow-up. Local progression-free survival (LPFS) was calculated from the date of induction therapy to the date of local progression or death for any reason. Survival without distant metastasis (DMFS) is defined as the time from induction therapy to distant metastasis or death from any cause. Overall survival (OS) is defined as the time from the start of induction therapy to the date of death of any cause or the date of the last follow-up. Common terminology standards for adverse events (CTCAE version 5.0) were used to assess TRAEs. All statistical analyses were performed using SPSS version 25.0 (SPSS, Chicago, USA) comparing baselines using Pearson’s chi-square test or Fisher’s exact test, and describing count data in terms of number of cases and percentages. The Kaplan–Meier method was used to evaluate PFS, LPFS, DMFS and OS, and the Log rank test was used to compare the survival differences between the groups, and the survival curve was plotted by GraphPadPrism 9.0. Cox regression analysis was used to determine the prognostic factors affecting PFS and OS, and the variables of p < 0.3 in the univariate Cox regression analysis were included in the multivariate Cox regression analysis. The prognostic factors affecting PFS and OS were subjected to a propensity matching score (PSM) and then subgroup survival analysis. In this paper, when the p < 0.05 (two-tailed), it was declared statistically significant. Power calculation assumed a hazard ratio (HR) of 0.5, α = 0.05, and 80% power, with a 1:2 allocation ratio. Using the Schoenfeld formula, the required sample size was 54 patients, which aligns with our cohort. Post hoc analysis revealed that the observed HR = 0.43 (stronger effect size) and 35 progression events provided 92% power (α = 0.05), further supporting the robustness of our findings.
Results
Baseline Characteristics
A total of 54 eligible patients were included in the study: 18 cases (33.3%) in the early radiotherapy group and 36 cases (66.7%) in the late radiotherapy group. The median age of the whole group was 61 years old (IQR, 46–79 years). Most of the patients were males with previous smoking history. In terms of tumor stage, there were 38 cases (70.4%) in IIIB/IIIC stage. The typical dose of whole group radiotherapy is 60Gy. The median number of cycles of induction chemoimmunotherapy was 4 (IQR, 2–6). Nineteen patients (35.2%) were treated with consolidation ICI after first-line treatment, including early radiotherapy group (27.8%) and late radiotherapy group (36.8%). Patients who did not take consolidation ICI included economic reasons (60.0%), intolerable toxicity (14.3%), disease progression (20.0%) and death (5.7%). The two sets of baseline data are well balanced (Table 1).
 |
Table 1 The Baseline Demographic and Clinical Characteristics of Patients |
Survival Outcomes
PFS and OS in the Total Population
The study was followed up to December 2024, with a median follow-up period of 30.7 months (10.5–51.8 months) in the entire cohort. The median PFS of the whole group was 21.9 months. One- and 2-year PFS rates were 83.4% and 48.1%, respectively (Figure 1A). As of the date, the median OS data was immature. One- and 2-year OS rates of 92.5% and 79.2%, respectively (Figure 1B). Thirty-five patients (64.8%) developed disease progression, including 8 cases (22.9%) in the early radiotherapy group and 27 cases (77.1%) in the late radiotherapy group. Among them, there were only 16 cases of local recurrence, 14 cases of distant metastasis and 5 cases of simultaneous local recurrence and distant metastasis. A total of 12 patients died at the time of analysis.
 |
Figure 1 Kaplan–Meier curves of progression-free survival (A) and overall survival (B) for the whole group. |
Grouping PFS, LPFS, DMFS and OS
The median PFS of early radiotherapy group and late radiotherapy group was 25.9 and 20.1 months, respectively, the 1-year PFS rate was 93.5% vs.77.6%, 2-year PFS rate was 69.5% vs 36.8% (HR = 0.43, p = 0.024, Figure 2A). The median LPFS of the early radiotherapy group was NR, and that of the late radiotherapy group was 19.9 months. The 1-year LPFS rate was 100% and 82.8%, respectively, and the 2-year LPs rate was 73.4% and 45.4%, respectively (HR = 0.36, p = 0.038, Figure 2B). The median DMFS is NR vs 26.9 months (HR = 0.41, p = 0.084, Figure 2C). The median OS is not yet mature, with 1-year OS rates of 94.5% and 91.7%, and 2-year OS rates of 86.7% and 75.4%, respectively. (HR = 0.43, p = 0.262, Figure 2D).
 |
Figure 2 Kaplan–Meier curves of progression-free survival (A) local progression-free survival (B) distant metastasis-free survival (C) and overall survival (D) between two groups. |
Treatment-Related Adverse Events
Forty-four patients had any grade of TRAEs. Grade 1–2 TRAEs occurred in 39 patients (72.22%). Hematological toxicity was the most common, including anemia (51.85%), leukopenia (27.78%), neutropenia (25.93%) and thrombocytopenia (14.81%), followed by pneumonia (38.89%) and thyroid dysfunction (35.19%). Five patients (9.26%) developed ≥grade 3 TRAES. The incidence of ≥grade 3 pneumonia was 5.56%, and one case died of dyspnea caused by pneumonia radiation (Table 2). Through timely symptomatic intervention, most of the above TRAEs did not affect the overall treatment of patients, and the safety of patients can be controlled during the whole treatment period.
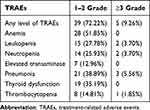 |
Table 2 Treatment-Related Adverse Events |
Analysis of Prognostic Factors Affecting Survival Outcomes
Univariate Cox regression analysis was performed on the baseline data collected, such as gender, age, smoking history, clinical stage, radiotherapy dose, timing of radiotherapy intervention, and whether immunotherapy was consolidated, and the results showed that the timing of radiotherapy intervention (p = 0.034) was associated with PFS prognosis. The variables of p < 0.3 in the univariate were included in the multivariate analysis, and early interventional radiotherapy after 2–3 induction cycles was a better independent predictor of PFS (p = 0.040) (Table 3). No statistically significant predictors of OS were found in the collected data (Table 4).
 |
Table 3 Univariate and Multivariate Cox Regression Analysis of PFS in Patients in Early Radiotherapy Group and Late Radiotherapy Group |
 |
Table 4 Univariate and Multivariate Cox Regression Analysis of OS in Patients in Early Radiotherapy Group and Late Radiotherapy Group |
Subgroup Analysis
A total of 19 patients received further consolidation ICI. The median PFS of patients receiving consolidation ICI and patients without consolidation ICI was 20.8 months and 21.9 months, respectively, and the 2-year PFS rate was 34.6% vs.50.0% (HR = 1.16, p = 0.670, Figure 3A). The median OS of both groups was NR. The 2-year OS rate of patients who received consolidation ICI was 87.0%, and that of patients without consolidation ICI was 77.4% (HR = 0.32, p = 0.120, Figure 3B). After using PSM, the median PFS of patients receiving consolidation ICI was 20.9 months, the median PFS of patients without consolidation ICI was 18.0 months, and the 2-year PFS rate was 34.8% vs.47.3% (HR = 0.84, p = 0.636, Figure 3C). The median OS of the group receiving consolidation ICI was NR, while that of the group without consolidation ICI was 34.8 months. The 2-year OS rate of patients who received consolidation ICI was 83.1%, and that of patients without consolidation ICI was 67.3% (HR = 0.51, p = 0.038, Figure 3D).
Discussion
Although CRT followed consolidation ICI is currently the standard treatment for unresectable LA-LUSC, the benefit population is limited. Real-world studies have shown that about 22% of 30% of patients can fail to complete the full course of treatment during CRT due to disease progression or intolerable toxicity,18,19 resulting in the loss of opportunities to consolidation ICI. Preclinical studies have shown that induction immunotherapy can promote the release of new tumor specific antigens, stimulating effect and memory T cell proliferation. In addition, after induction therapy, tumor-specific CD8+T cells can persist in the blood for a long time.20 Clinical studies have observed that the number of CD8+ T cells in tumor infiltrating lymphocytes in patients with gastric cancer who received immunotherapy before radiotherapy was higher than that in patients without immunotherapy.21 According to research reports, the increase in the number of CD8+T cells can independently predict the better response of cancer patients to radiotherapy.22,23 Therefore, induction immunotherapy may achieve radiosensitization by directly or indirectly increasing the number of CD8+T cells. In addition, preclinical studies have shown that the use of induction immunotherapy before radiotherapy may deplete Tregs24,25 and normalize blood vessels to achieve radiotherapy sensitization,26,27 and can also play a more powerful anti-tumor function through contact with the healthy immune system.28 Clinical studies have also shown that immunotherapy before radiotherapy may further benefit patients with unresectable LA-NSCLC.11,29 To the best of our knowledge, this is the first real-world study to explore the timing of radiotherapy on the prognosis of patients with unresectable LA-LUSC receiving induction chemoimmunotherapy followed radiotherapy.
The 1- and 2-year PFS rates of induction chemoimmunotherapy were 83.4% and 48.1%, respectively, which were longer than those reported in the PACIFIC trial (55.7% and 45.0%, respectively). The median PFS (21.9 months) was also better than that of the PACIFIC trial (16.9 months).4 It is worth noting that in the PACIFIC trial, IIIB/IIIC accounted for 47.1% of patients, while most of the patients in this study were IIIB/ IIIC (70.4%). Induction chemoimmunotherapy followed by radiotherapy may benefit patients with unresectable LA-LUSC, which is similar to the results of Wang et al30 (median PFS is 30.6 months, the 1-year and 2-year PFS rates were 85.8% and 64.2%, respectively). In the 2023 WCLC, a similar prospective study11 was reported, with a median PFS of 22.0 months, 1-year PFS rates were 84.7%. Another retrospective study31 also showed that unresectable LA-NSCLC could benefit from induction chemoimmunotherapy before CRT, with a median PFS of 20.4 months and a 1-and 2-year PFS rate of 70.4% and 41.9%, respectively. On OS, the 1-year and 2-year OS rates of induction chemoimmunotherapy in this study were 92.5% and 79.2%, respectively. This is similar to what Wang et al30 reported, with OS rates of 91.5% and 75.1% for 1 and 2 years, respectively. A similar study11 of WCLC in 2023 reported a 1-year OS rate of 91.8%. In the SPRINT study,32 a 1-year OS rate of 95% and a 2-year OS rate of 73.7% in the AFT-16 study.33 Based on the above studies, induction chemoimmunotherapy followed radiotherapy has a promising effect in patients with unresectable LA-NSCLC. More related prospective studies are under way, and we look forward to reporting the follow-up results.
Our study showed that patients in the early radiotherapy group who received 2–3 cycles of induction chemoimmunotherapy had an improvement in median PFS compared with the late radiotherapy group (NR vs.18.0 months, p = 0.036). In terms of local recurrence control, the early radiotherapy group was also better than the late radiotherapy group (NR vs.19.9 months, p = 0.049). Although there was no significant statistical difference in distant metastasis control between the two groups, the trend was better in the early radiotherapy group. Before the PACIFIC trial, two cycles of induction chemotherapy before CRT were common for unresectable LA-LUSC,13 probably because the time window of two cycles can not only reduce the size of the tumor but also provide timely radical treatment to prevent tumor progression. For unresectable LA-LUSC patients with CRT after receiving induction chemoimmunotherapy, Yang et al14 showed that the benefit trend of PFS could be observed in less than 3 cycles of induction chemoimmunotherapy than other induction cycles. Wang et al30 found that the disease control rate after 2 cycles of induction chemoimmunotherapy was significantly higher than that after more cycles of induction chemoimmunotherapy. The follow-up simulated radiotherapy plan showed that all target volume and dose parameters decreased significantly after 2 cycles of induction therapy. Wu et al15 also found that 2–3 cycles of induced immunochemotherapy may be the best, because longer induction may delay CRT and worsen the prognosis. Overall, for patients with unresectable LA-LUSC, early interventional radiotherapy after 2–3 cycles of induction chemoimmunotherapy seems to bring more survival benefits. However, this study is a retrospective study, and further prospective studies are needed to determine whether the specific cycles of induction chemoimmunotherapy followed by interventional radiotherapy are the best.
In terms of safety, the incidence of ≥grade 3 pneumonia was 5.56% in this study, which was similar to that of Wu et al (4.9%).34 Although the value is slightly higher than that of the PACIFIC trial (4.2%),4 it is lower than the combined use of induction and consolidation immunotherapy reported in the KEYNOTE-799 trial35 (phase 1, 10.0%, Phase 2, 8.0%).35 Overall, this study shows that the safety of induction chemoimmunotherapy before radiotherapy is relatively controllable for unresectable squamous LA-NSCLC.
Subgroup analysis showed that patients who received consolidation ICI had better OS values than those who did not receive consolidation ICI, but PFS did not have an advantage. Considering that the timing of radiotherapy intervention and clinical tumor stage may be the biggest prognostic factors in this study, PSM was adopted. After PSM, it was observed that the OS of the group receiving consolidation ICI was significantly better than that of the group without consolidation ICI (mOS NR vs 34.8 months, 2 years OS, 83.1% vs 67.3%, p = 0.038). Similar to the study of Wang et al,30 the OS value of patients who received consolidation ICI was better than that of patients who did not receive consolidation ICI (2 years OS, 85.5% vs 64.2%, p = 0.170). The study of Guan et al36 also suggested that compared with patients who did not receive consolidation ICI, patients receiving consolidation ICI had a trend of improvement in PFS (HR = 0.398, p = 0.274) and OS (HR = 0.018, p = 0.209). The AFT-16 trial37 and the KEYNOTE-799 trial35 suggest that immunotherapy before and after CRT shows prolonged survival, but both are single-group studies. In summary, consolidation ICI after induction immunotherapy seems indispensable for patients with unresectable LA-LUSC, but there is still a lack of prospective studies to further confirm it.
This study has several limitations. First of all, this study is a small sample, single-center retrospective study, which has the limitations of retrospective study and needs to be verified by prospective studies in more centers and populations. Second, there is little data on PD-L1 expression because of the patient’s economic or other reasons. In addition, different ICIs may also lead to biases in the results. Future studies need to use the same ICIs and stratify according to PD-L1 expression to reduce confounding factors. Finally, PFS may be overrated because real-world treatment evaluation is sometimes delayed. Despite these limitations, our analysis confirms the efficacy and safety of induction chemoimmunotherapy before radiotherapy. More importantly, our study also found that early interventional radiotherapy after induction therapy is conducive to the further survival and benefit of patients, which provides ideas for the design of related trials in the future.
Conclusion
For patients with unresectable LA-LUSC, induction chemoimmunotherapy followed by radiotherapy shows a good curative effect and its safety is controllable. Interventional radiotherapy as early as possible after 2–3 cycles of induction chemoimmunotherapy may prolong PFS and LPFS. Consolidation ICI after induction immunotherapy may still be indispensable.
Ethics Approval and Consent to Participate
The present study was approved by the Ethics Committee of the affiliated Hospital of North Sichuan Medical College (2024ER293-1). The requirement for informed consent was waived due to the retrospective nature of the research, which utilized pre-existing de-identified clinical data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7(12):1824–1832. doi:10.1001/jamaoncol.2021.4932
3. Detterbeck FC, Boffa DJ, Kim AW, Tanoue LT. The Eighth Edition Lung Cancer Stage Classification. Chest. 2017;151(1):193–203. doi:10.1016/j.chest.2016.10.010
4. Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. N Engl J Med. 2017;377(20):1919–1929. doi:10.1056/NEJMoa1709937
5. Spigel DR, Faivre-Finn C, Gray JE, et al. Five-year survival outcomes from the PACIFIC trial: durvalumab after chemoradiotherapy in stage III non-small-cell lung cancer. J Clin Oncol. 2022;40(12):
6. Cotarla I, Boron ML, Cullen SL, et al. Treatment decision drivers in stage III non-small-cell lung cancer: outcomes of a web-based survey of oncologists in the United States. JCO Oncol Pract. 2020;16(10):693. doi:10.1200/JOP.19.00781
7. Arunachalam A, Vasudevan A, Sura S, Murphy J, Goldschmidt J. Real world treatment patterns and outcomes among unresectable stage III non-small cell lung cancer patients initiating chemoradiotherapy. J Thorac Oncol. 2022;17(9):S264–5. doi:10.1016/j.jtho.2022.07.448
8. Xing L, Yu J, Zhao R, et al. Real-world treatment patterns in stage III NSCLC patients: interim results of a prospective, multicenter, non-interventional study (MOOREA). J Thorac Oncol. 2023;18(4):S111–2. doi:10.1016/S1556-0864(23)00380-5
9. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):
10. Zhou Q, Chen M, Jiang O, et al. Sugemalimab versus placebo after concurrent or sequential chemoradiotherapy in patients with locally advanced, unresectable, stage III non-small-cell lung cancer in China (GEMSTONE-301): interim results of a randomised, double-blind, multicentre, Phase 3 trial. Lancet Oncol. 2022;23(2):209–219.
11. Tang M, Li L, Zhang P, et al. The safety and efficacy of induction chemoimmunotherapy followed by radiotherapy and consolidation immunotherapy in locally advanced NSCLC. J Thorac Oncol. 2023;18(11):S297–S297. doi:10.1016/j.jtho.2023.09.510
12. Wang Y, Deng L, Wang J, et al. Induction PD-1 inhibitor toripalimab plus chemotherapy followed by concurrent chemoradiotherapy and consolidation toripalimab for bulky locally advanced non-small-cell lung cancer: protocol for a randomized Phase II trial (InTRist study). Front Immunol. 2023;14:1341584. doi:10.3389/fimmu.2023.1341584
13. Fournel P, Vergnenégre A, Robinet G, et al. Induction or consolidation chemotherapy for unresectable stage III non-small-cell lung cancer patients treated with concurrent chemoradiation: a randomised phase II trial GFPC - IFCT 02-01. Eur J Cancer. 2016;52:181–187. doi:10.1016/j.ejca.2015.10.072
14. Yang Y, Wang J, Zhang T, et al. Efficacy and safety of definitive chemoradiotherapy with or without induction immune checkpoint inhibitors in patients with stage III non-small cell lung cancer. Front Immunol. 2023;14:1281888. doi:10.3389/fimmu.2023.1281888
15. Wu L, Cheng B, Sun X, et al. Induction immunochemotherapy followed by definitive chemoradiotherapy for unresectable locally advanced non-small cell lung cancer: a multi-institutional retrospective cohort study. MedComm. 2024;5(3):e501. doi:10.1002/mco2.501
16. Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–550. doi:10.1038/nature13385
17. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi:10.1038/nature11404
18. Eichkorn T, Bozorgmehr F, Regnery S, et al. Consolidation immunotherapy after platinum-based chemoradiotherapy in patients with unresectable stage III non-small cell lung cancer-cross-sectional study of eligibility and administration rates. Front Oncol. 2020;10:586449. doi:10.3389/fonc.2020.586449
19. Sakaguchi T, Ito K, Furuhashi K, et al. Patients with unresectable stage III non-small cell lung cancer eligible to receive consolidation therapy with durvalumab in clinical practice based on PACIFIC study criteria. Respir Investig. 2019;57(5):466–471. doi:10.1016/j.resinv.2019.03.011
20. Liu J, Blake SJ, Yong MCR, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov. 2016;6(12):1382–1399. doi:10.1158/2159-8290.CD-16-0577
21. Sasaki A, Nakamura Y, Togashi Y, et al. Enhanced tumor response to radiotherapy after PD-1 blockade in metastatic gastric cancer. Gastric Cancer. 2020;23(5):893–903. doi:10.1007/s10120-020-01058-4
22. Liu C, Hu Q, Hu K, et al. Increased CD8+CD28+T cells independently predict better early response to stereotactic ablative radiotherapy in patients with lung metastases from non-small cell lung cancer. J Transl Med. 2019;17:120. doi:10.1186/s12967-019-1872-9
23. Yang ZR, Zhao N, Meng J, et al. Peripheral lymphocyte subset variation predicts prostate cancer carbon ion radiotherapy outcomes. Oncotarget. 2016;7(18):26422–26435. doi:10.18632/oncotarget.8389
24. Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Rev Clin Oncol. 2019;16(6):356–371. doi:10.1038/s41571-019-0175-7
25. Oweida A, Darragh L, Bhatia S, et al. Regulatory T cells mediate resistance to radiotherapy in head and neck squamous cell carcinoma. J Clin Oncol. 2019;37(8):70. doi:10.1200/JCO.2019.37.8_suppl.70
26. Mittal A, Nenwani M, Sarangi I, Achreja A, Lawrence TS, Nagrath D. Radiotherapy-induced metabolic hallmarks in the tumor microenvironment. Trends Cancer. 2022;8(10):855–869. doi:10.1016/j.trecan.2022.05.005
27. Wang-Bishop L, Kimmel BR, Ngwa VM, et al. STING-activating nanoparticles normalize the vascular- immune interface to potentiate cancer immunotherapy. Sci Immunol. 2023;8(83):eadd1153. doi:10.1126/sciimmunol.add1153
28. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367(6477):525. doi:10.1126/science.aax0182
29. Liu H, Qiu B, Zhao Y, et al. A phase II randomized trial evaluating consolidative nivolumab in locally advanced non-small cell lung cancer post neoadjuvant chemotherapy plus nivolumab and concurrent chemoradiotherapy (GASTO-1091). 2024.
30. Wang Y, Zhang T, Wang J, et al. Induction immune checkpoint inhibitors and chemotherapy before definitive chemoradiation therapy for patients with bulky unresectable stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2023;116(3):590–600. doi:10.1016/j.ijrobp.2022.12.042
31. Zhao J, Miao D, Zhou J, et al. A retrospective comparison of induction chemoimmunotherapy versus chemotherapy followed by concurrent chemoradiotherapy and consolidation immunotherapy in stage III non-small cell lung cancer. Front Oncol. 2024;14:1432954. doi:10.3389/fonc.2024.1432954
32. Ohri N, Jolly S, Cooper BT, et al. The Selective Personalized Radio-immunotherapy for Locally Advanced NSCLC Trial (SPRINT): initial results. J Clin Oncol. 2022;40(16):8510. doi:10.1200/JCO.2022.40.16_suppl.8510
33. Ross HJ, Kozono D, Wang XF, et al. Atezolizumab before and after chemoradiation for unresectable stage III non-small cell lung cancer: a phase II nonrandomized controlled trial. JAMA Oncol. 2024;10(9):1212–1219. doi:10.1001/jamaoncol.2024.1897
34. Wu L, Yan Y, Xu Y. Induction immunochemotherapy followed by definitive chemoradiotherapy for unresectable locally advanced non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2023;117(2):E75–E75. doi:10.1016/j.ijrobp.2023.06.813
35. Jabbour SK, Lee KH, Frost N, et al. Pembrolizumab plus concurrent chemoradiation therapy in patients with unresectable, locally advanced, stage III non-small cell lung cancer: the phase 2 KEYNOTE-799 nonrandomized trial. JAMA Oncol. 2021;7(9):1–9. doi:10.1001/jamaoncol.2021.2301
36. Guan S, Zhang S, Ren K, Li X, Li X, Zhao L. Induction chemoimmunotherapy may improve outcomes of chemoradiotherapy in patients with unresectable stage III NSCLC. Front Immunol. 2023;14:1289207. doi:10.3389/fimmu.2023.1289207
37. Ross HJ, Kozono DE, Urbanic JJ, et al. AFT-16: phase II trial of neoadjuvant and adjuvant atezolizumab and chemoradiation (CRT) for stage III non-small cell lung cancer (NSCLC). JCO. 2021;39(15_suppl):8513. doi:10.1200/JCO.2021.39.15_suppl.8513
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


