Back to Journals » Clinical Ophthalmology » Volume 19
Retrospective Study of 2-year Clinical Outcomes of Combination Ab-Interno Canaloplasty and a Microstent Compared to Ab-Interno Canaloplasty in Cataract Surgery Patients
Authors Trubnik V, Huang L, Hall B
Received 28 January 2025
Accepted for publication 10 June 2025
Published 25 June 2025 Volume 2025:19 Pages 1991—1997
DOI https://doi.org/10.2147/OPTH.S519826
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Valerie Trubnik,1 Lucy Huang,1 Brad Hall2
1Ophthalmic Consultants of Long Island, Manhasset, NY, USA; 2Sengi, Penniac, NB, Canada
Correspondence: Valerie Trubnik, Ophthalmic Consultants of Long Island, 1355 Northern Boulevard, Suite 300, Manhasset, NY, 11030, USA, Tel +1 516-627-3232, Email [email protected]
Purpose: To evaluate the postoperative outcomes of patients undergoing cataract surgery, OMNI canaloplasty, and Hydrus Microstent implantation compared to patients undergoing cataract surgery with OMNI canaloplasty alone.
Methods: This was a retrospective, single surgeon study. Eligible charts were from adults that had a diagnosis of mild to moderate primary open angle glaucoma and preoperative mild to moderate visual field loss. Outcome measures included mean number of ocular hypotensive medication prescribed, intraocular pressure (IOP), number of glaucoma secondary surgical interventions (SSI), and visual field mean deviation and pattern standard deviation. Data were collected up to 24 months postoperatively.
Results: The chart review has identified 45 eyes in the Hydrus/OMNI Group and 35 eyes in the OMNI Group. The mean number of glaucoma medications improved from 2.9 preoperatively to 2.1 at 24 months postoperatively in the Hydrus/OMNI Group compared to 1.9 preoperatively to 1.6 at 24 months postoperatively in the OMNI Group. Mean postoperative IOP was stable in both groups.
Conclusion: The results of this study suggest that after both combination cataract, Hydrus Microstent/OMNI surgery and combination cataract and OMNI surgery, the number of glaucoma medications was reduced for 2 years postoperatively, but the reduction was not significantly different between groups.
Plain Language Summary: The progression of glaucoma is strongly linked to intraocular pressure (IOP). As such, treatments tend to focus on lowering IOP, such as microinvasive glaucoma surgery (MIGS). The Hydrus Microstent and the OMNI canaloplasty procedures are both MIGS procedures that are considered less invasive that traditional glaucoma surgeries, with faster visual recovery, and can result in less serious complications. Combining these two MIGS procedures may also be useful in avoiding future potentially riskier surgeries. However, there are minimal data on the effectiveness of these combined MIGS procedures in patients undergoing cataract surgery. The purpose of this study was to evaluate the postoperative outcomes of patients undergoing cataract surgery, OMNI canaloplasty, and Hydrus implantation compared to patients undergoing cataract surgery with OMNI canaloplasty alone. The results of this study suggest that after both combination cataract, Hydrus Microstent/OMNI surgery and combination cataract and OMNI surgery, the number of glaucoma medications is reduced for 2 years postoperatively, but the reduction was not significantly different between groups. Results also suggest that IOP was stable in both groups over the same period.
Keywords: Hydrus microstent, MIGS, glaucoma, OMNI
Introduction
Glaucoma affects an estimated 60.5 million people worldwide and 5.7 million people in the United States.1 The progression of this disease is strongly linked to intraocular pressure (IOP).2,3 As such, treatments tend to focus on lowering IOP through medications, laser therapy, traditional surgery, and microinvasive glaucoma surgery (MIGS). The use of MIGS compared to traditional glaucoma surgery may lead to fewer complications,4,5 but may also have reduced efficacy.6
The OMNI surgical system (Sight Sciences, Inc., Menlo Park, CA, USA) is a MIGS procedure that facilitates ab-interno canaloplasty followed by trabeculotomy using a single device.7 It was approved by the US Food and Drug Administration in 2017 for cases of mild to moderate primary open angle glaucoma (POAG). The OMNI Surgical System is a manually operated device used to thread Schlemm’s canal circumferentially (180 or 360 degrees) using a microcatheter.8 With this device, the surgeon is able to perform an ab interno canaloplasty and address outflow obstruction by delivering viscoelastic to dilate Schlemm’s canal and the distal outflow channels. The OMNI system does not provide a bypass of the resistance at the trabecular meshwork and the dilatory effect on the canal may be temporary. In the GEMINI study, outcomes of phacoemulsification used in combination with the OMNI surgical system were evaluated over 36 months.9,10 The results suggest long-term effectiveness of IOP lowering and reduction in the number of glaucoma medications.
The Hydrus Microstent (Alcon Vision LLC, Fort Worth, TX, USA) is a MIGS device that is designed to be implanted with the inlet in the anterior chamber and the rest of the device inserted into Schlemm’s canal. The aim of this device is to bypass the trabecular meshwork and permanently stent open Schlemm’s canal.11 One potential drawback is that it only spans 90° of the canal’s circumference. In 2018, Hydrus was approved by the US Food and Drug Administration for cases of mild to moderate POAG in conjunction with cataract surgery. The HORIZON pivotal trial, compared the outcomes of cataract surgery alone to cataract surgery combined with Hydrus Microstent, up to 5 years postoperatively.12 Reports suggest a greater reduction in the number of glaucoma medications in the combined group compared with cataract surgery alone.12,13
The Hydrus Microstent and the OMNI canaloplasty procedures are both ab interno and are considered less invasive raditional glaucoma surgeries. They can have a faster visual recovery, and result in less serious complications compared to traditional glaucoma surgeries, all while sparing the conjunctiva for future interventions.7 The addition of Hydrus Microstent to OMNI Canaloplasty may be beneficial in maintaining the long term scaffolding of Schlemm’s canal after the initial dilation and by allowing aqueous to bypass the resistance of the trabecular meshwork. The addition of OMNI Canaloplasty may be beneficial in providing up to 360° of treatment compared to 90° with the Hydrus alone. Thus, these two MIGS procedures may be synergistic as a first surgical intervention to avoid potentially riskier filtering surgeries. However, there are minimal data on the postoperative medication reduction of combined MIGS procedures in patients undergoing cataract surgery. The purpose of this study is to evaluate the postoperative outcomes of patients undergoing cataract surgery, OMNI canaloplasty, and Hydrus implantation compared to patients undergoing cataract surgery with OMNI canaloplasty alone.
Methods
This was a retrospective chart review of 2-year clinical outcomes with OMNI canaloplasty compared to combination OMNI canaloplasty and Hydrus Microstent in cataract surgery patients with mild to moderate glaucoma. An independent institutional review board approved the study (Salus IRB, approval VT-23-01). A waiver of informed consent was granted as this was a non-interventional chart review of anonymized data. Confidentiality was maintained for all data. As a retrospective study, there was no need to register in a clinical trials database. The tenets of the Declaration of Helsinki, International Harmonization (ICH) guidelines, and good clinical practice were adhered to.
Consecutive charts were reviewed for patients that had surgery between November 2019 and October 2023. Charts were included from patients with mild-to-moderate POAG who were on topical glaucoma medications and had undergone cataract extraction with OMNI canaloplasty plus successful Hydrus implantation or cataract extraction with OMNI canaloplasty alone, and who had pre-operative mild-to-moderate visual field (VF) loss as defined by Hodapp-Anderson-Parrish criteria.14 Charts were excluded for patients with angle closure glaucoma or secondary forms of glaucoma due to neovascularization of the angle, uveitic glaucoma, congenital glaucoma, or glaucoma due to congenital anomalies, abnormal anterior segment examination other than cataract. Patients with pigment dispersion and pseudoexfoliation, or refractory cystoid macular edema (CME) or CME persisting 3 months or more postoperatively were also excluded.
From eligible charts, data were collected preoperatively, and at 1 week, 1, 3, 6, 12, and 24 months postoperatively. Data included number of glaucoma medications, IOP (measured by Goldmann applanation tonometry), visual field mean deviation and pattern standard deviation (measured with the Humphrey Field Analyzer (Carl Zeiss Meditec) using SITA standard 24–2, retinal nerve fiber layer (measured with the Spectralis optical coherence topographer (Heidelberg Engineering) at the central retina), and secondary surgical interventions. The primary outcomes measure was the change in number of glaucoma medications. Other outcomes measures were IOP, visual field mean deviation and pattern standard deviation, retinal nerve fiber layer, visual acuity, and secondary surgical interventions.
A single experienced surgeon performed all procedures. The primary reason for surgery was cataract extraction. Patients were controlled on their current IOP regimen but had a desire to reduce topical glaucoma mediation burden. Choice of MIGS procedure was at the discretion of the surgeon and based on patient individual characteristics. The ophthalmic viscosurgical device used was Healon (Johnson & Johnson). In the Hydrus/OMNI group, 1 hydrus Microstent was implanted per eye.
The software R was used for all statistical analyses (version 4.4.0, The R Foundation for Statistical Computing, Vienna, Austria). An unfortunate drawback with a retrospective study is that not all data may be available for all subjects at each timepoint. Thus, the analysis for this study focused on the mean difference from the same eyes preoperative to postoperative rather than summarizing the means of all data collected at each timepoint or summarizing the difference in means, where possible. Data for mean number of medication and IOP were compared using linear mixed effect models, which accounted for bias from multiple measures with the same eye. All other data are presented with descriptive statistics only. A p ≤0.05 was considered significant. We estimated that the study would require a total sample size of 51 eyes to detect a 0.5 difference in the change in number of medications between groups, assuming a pooled standard deviation of 0.6, power 80%, and alpha 0.05.
Results
A total of 45 eyes (of 35 patients) and 35 eyes (of 27 patients) eligible charts were identified in the Hydrus/OMNI and OMNI groups respectively. Preoperative and patient demographics are summarized in Table 1.
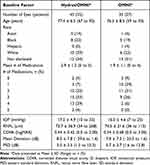 |
Table 1 Preoperative and Demographic Data |
Changes in number of medications compared to baseline are summarized in Table 2. Reductions in number of medications compared to baseline were sustained in both groups for up to 24 months. At 24 months postoperatively, the mean number of medications were 0.6 less (23%) in the Hydrus/OMNI group and 0.7 less (30%) in the OMNI group compared to baseline, respectively. There were no significant differences between groups at any timepoint. However, it should be noted that subjects in the Hydrus/OMNI group were on an average of 2.9 medications at baseline compared to 1.9 in the OMNI group.
 |
Table 2 Number of Medications |
Changes in IOP compared to baseline are summarized in Table 3. Mean postoperative IOP were stable in both groups up to 24 months, compared to baseline. At 24 months postoperatively, mean IOP was 17.2 mmHg in the Hydrus/OMNI group (17.1 mmHg at baseline for the same subjects) and 14.8 mmHg in the OMNI group (15.6 mmHg at baseline for the same subjects), respectively. In addition, at 24 months postoperatively 75% of eyes achieved medicated IOP 18 mmHg or less in the Hydrus/OMNI group compared to 70% in the OMNI group. There were no significant differences between groups at any timepoint.
 |
Table 3 Intraocular Pressure |
Table 4 summarizes mean deviation from baseline to 24 months postoperatively. Average mean deviation at 24 months postoperatively was −13.3 dB in the Hydrus/OMNI group (−6.9 dB at baseline for the same subjects) and −13.0 dB in the OMNI group (−7.8 dB at baseline for the same subjects), respectively. Likewise, mean postoperative PSD were stable in both groups, compared to baseline. At 24 months postoperatively, mean PSD was 6.7 dB in the Hydrus/OMNI group (5.6 dB at baseline for the same subjects) and 7.3 dB in the OMNI group (6.0 dB at baseline for the same subjects), respectively.
 |
Table 4 Mean Deviation |
Changes in RNFL thickness overtime are summarized in Table 5. Mean postoperative RNFL thicknesses were stable in both groups, compared to baseline. At 24 months postoperatively, mean RNFL thickness was 72.4 µm in the Hydrus/OMNI group (74.3 µm at baseline for the same subjects) and 74.0 µm in the OMNI group (77.0 µm at baseline for the same subjects), respectively.
 |
Table 5 Retinal Nerve Fiber Layer Thickness |
There were 3/44 subjects that required secondary surgical interventions (SSI) in the Hydrus/OMNI group. These were 1) Xen gel stent (Allergan) and intraoperative mitomycin C, selective laser trabeculoplasty, and bleb needling with 5FU, 2) Xen gel stent and intraoperative mitomycin C, selective laser trabeculoplasty, and Iridex micropulse laser, and 3) Xen gel stent and intraoperative mitomycin C. There were no SSI in the OMNI group.
Discussion
This study evaluated the postoperative outcomes of patients undergoing cataract surgery, OMNI canaloplasty, and Hydrus implantation compared to patients undergoing cataract surgery with OMNI canaloplasty alone. To the best of our knowledge, this is the first study to compare outcomes of Hydrus/OMNI to those of OMNI alone, in cataract surgery patients. The primary outcome measure was the change in number of medications compared to baseline. At 24 months postoperatively, the mean number of medications were 2.1 (21% reduction) and 1.6 (32% reduction) in the Hydrus/OMNI and OMNI groups respectively. The GEMINI trial reported a mean number of medications of 0.4 (75% reduction) for combined OMNI and cataract surgery at 24 months postoperatively,10 lower than our study. However, the baseline mean number of medications was 1.6, which was lower than baseline for both of the groups in our study. In the HORIZON trial, mean number of medications was 1.4 (18% reduction) at 24 months postoperatively, also lower than our study. As with the GEMINI study, the baseline number of medications (1.7) was lower than both groups in our study. We are aware of one report of combined Hydrus, OMNI, and cataract surgery. In a retrospective study, Dickinson et al15 compared combined Hydrus, OMNI, and cataract surgery to combined Hydrus and cataract surgery. The Hydrus/OMNI group was reported to have a mean number of medications of 0.2 (1.4 at baseline, 86% reduction), compared to 0.6 (1.7 at baseline, 65% reduction) in the Hydrus group, at 6 months postoperatively.
A secondary outcome measure was the change in IOP compared to baseline. At 24 months postoperatively, mean IOP were 17.2 mmHg (17.1 mmHg at baseline) and 14.9 mmHg (15.6 mmHg at baseline) in the Hydrus/OMNI and OMNI groups, respectively. The GEMINI trial reported a mean IOP of 16.7 mmHg (23.1 mmHg at baseline) for combined OMNI and cataract surgery at 24 months postoperatively.10 In the HORIZON trial, mean IOP was 16.6 mmHg (25.5 mmHg at baseline) at 24 months postoperatively. The IOP in our study was measured without washout, while those of the GEMINI and HORIZON trials were taken after washout, which may explain the differences in our study. Dickinson et al15 reported that the mean IOP in the Hydrus/OMNI group was 13.6 mmHg (16.4 mmHg at baseline) compared to 14.1 mmHg (16.2 mmHg at baseline) in the Hydrus group, at 6 months postoperatively.
We did not find any significant differences between the Hydrus/OMNI and OMNI groups. Though we cannot rule out that there is in fact no difference, statistical comparisons were hindered due to a few limitations with our study. First, the sample size was small and with the collection of real-world retrospective data, the sample size was smaller at the longer follow-up time periods. Patient retention was further challenged by the global pandemic occurring at the time. Second, patient-specific factors would have influenced surgeon selection for combined MIGS and cataract surgery, which can introduce bias. For example, patients in the Hydrus/OMNI group were on more medications, on average, than the OMNI group. This limitation in baseline medications could have been overcome using propensity score matching (PSM), however, given the small sample size and attrition overtime, PSM would have led to even fewer cases for meaningful analysis. Third, patient medication compliance could have also confounded our results as there was no medication washout in our retrospective study. Finally, it is also important to note that cataract surgery alone can have a significant impact on medication usage and IOP.9,10,12,13 Our study did not include a control group of cataract-only patients, and therefore we cannot discern the individual impact from each of the three procedures studied (phacoemulsification, Hydrus Microstent, and OMNI canaloplasty). Despite these limitations, our study provides real-world evidence of the efficacy and safety of using combined MIGS and cataract surgery in mild to moderate POAG patients. However, future studies with larger sample sizes are required to validate our findings.
In conclusion, the results of this study suggests that after combination cataract, Hydrus Microstent, and OMNI canaloplasty and combination cataract and OMNI surgery, the number of glaucoma medications were reduced in both groups for 2 years postoperatively, but the reduction was not significantly different between groups.
Acknowledgment
This paper was presented at the 2025 American Society of Cataract and Refractive Surgery (ASCRS) Annual Meeting as a conference poster.
Funding
This study was supported with an investigator-initiated study grant (88160413) from Alcon Vision, LLC, Fort Worth, TX, USA.
Disclosure
Valerie Trubnik reports an investigator initiated study grant from Alcon and consulting fees for Glaukos. The authors report no other conflict of interest for this work.
References
1. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi:10.1136/bjo.2005.081224
2. Maier PC, Funk J, Schwarzer G, Antes G, Falck-Ytter YT. Treatment of ocular hypertension and open angle glaucoma: meta-analysis of randomised controlled trials. BMJ. 2005;331(7509):134. doi:10.1136/bmj.38506.594977.E0
3. The AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130(4):429–440. doi:10.1016/S0002-9394(00)00538-9
4. Stead R, Azuara-Blanco A, King AJ. Attitudes of consultant ophthalmologists in the UK to initial management of glaucoma patients presenting with severe visual field loss: a national survey. Clin Exp Ophthalmol. 2011;39(9):858–864. doi:10.1111/j.1442-9071.2011.02574.x
5. SooHoo JR, Seibold LK, Radcliffe NM, Kahook MY. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol. 2014;49(6):528–533. doi:10.1016/j.jcjo.2014.09.002
6. Balas M, Mathew DJ. Minimally invasive glaucoma surgery: a review of the literature. Vision. 2023;7:54.
7. Hirsch L, Cotliar J, Vold S, et al. Canaloplasty and trabeculotomy ab interno with the OMNI system combined with cataract surgery in open-angle glaucoma: 12-month outcomes from the ROMEO study. J Cataract Refract Surg. 2021;47(7):907–915. doi:10.1097/j.jcrs.0000000000000552
8. Vold SD, Williamson BK, Hirsch L, et al. Canaloplasty and trabeculotomy with the OMNI system in Pseudophakic patients with open-angle glaucoma: the ROMEO study. Ophthalmol Glaucoma. 2021;4(2):173–181. doi:10.1016/j.ogla.2020.10.001
9. Gallardo MJ, Pyfer MF, Vold SD, et al. Canaloplasty and trabeculotomy combined with phacoemulsification for glaucoma: 12-month results of the GEMINI study. Clin Ophthalmol. 2022;16:1225–1234. doi:10.2147/OPTH.S362932
10. Greenwood MD, Yadgarov A, Flowers BE, et al. 36-month outcomes from the prospective GEMINI study: canaloplasty and trabeculotomy combined with cataract surgery for patients with primary open-angle glaucoma. Clin Ophthalmol. 2023;17:3817–3824. doi:10.2147/OPTH.S446486
11. Samet S, Ong JA, Ahmed IIK. Hydrus microstent implantation for surgical management of glaucoma: a review of design, efficacy and safety. Eye Vis. 2019;6:32. doi:10.1186/s40662-019-0157-y
12. Ahmed IIK, De Francesco T, Rhee D, et al. Long-term outcomes from the HORIZON randomized trial for a schlemm’s canal microstent in combination cataract and glaucoma surgery. Ophthalmology. 2022;129(7):742–751. doi:10.1016/j.ophtha.2022.02.021
13. Samuelson TW, Chang DF, Marquis R, et al. A schlemm canal microstent for intraocular pressure reduction in primary open-angle glaucoma and cataract: the HORIZON study. Ophthalmology. 2019;126(1):29–37. doi:10.1016/j.ophtha.2018.05.012
14. Hodapp EA, Parrish RK, Anderson DR, Culbertson WW. Clinical decisions in glaucoma. Arch Ophthalmol 1993;111:394–395. doi:10.1001/archopht.1993.01090030114051
15. Dickinson A, Leidy L, Nusair O, et al. Short-term outcomes of hydrus microstent with and without additional canaloplasty during cataract surgery. J Glaucoma. 2023;32(9):769–776. doi:10.1097/IJG.0000000000002245
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Interim Analysis of STREAMLINE® Surgical System Clinical Outcomes in Eyes with Glaucoma
Lazcano-Gomez G, Garg SJ, Yeu E, Kahook MY
Clinical Ophthalmology 2022, 16:1313-1320
Published Date: 27 April 2022
Short-Term Efficacy of Combined ab Interno Canaloplasty and Trabeculotomy in Pseudophakic Eyes with Open-Angle Glaucoma
Bleeker AR, Litchfield WR, Ibach MJ, Greenwood MD, Ristvedt D, Berdahl JP, Terveen DC
Clinical Ophthalmology 2022, 16:2295-2303
Published Date: 21 July 2022
A Multicenter 12-Month Retrospective Evaluation of Canaloplasty and Trabeculotomy in Patients with Open-Angle Glaucoma: The ROMEO 2 Study
Murphy III JT, Terveen DC, Aminlari AE, Dhamdhere K, Dickerson Jr JE
Clinical Ophthalmology 2022, 16:3043-3052
Published Date: 14 September 2022
Canaloplasty and Trabeculotomy with the OMNI System in Patients with Open-Angle Glaucoma: Two-Year Results from the ROMEO Study
Williamson BK, Vold SD, Campbell A, Hirsch L, Selvadurai D, Aminlari AE, Cotliar J, Dickerson Jnr JE
Clinical Ophthalmology 2023, 17:1057-1066
Published Date: 6 April 2023
Comparison of Efficacy of Combined Phacoemulsification and iStent Inject versus Combined Phacoemulsification and Hydrus Microstent
Chee WK, Yip VCH, Tecson IO, Chua CH, Ang BCH, Kee AR, Hu JY, Kan TCJ, Yip LWL
Clinical Ophthalmology 2023, 17:1151-1159
Published Date: 14 April 2023

