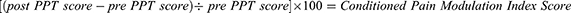Back to Journals » Journal of Pain Research » Volume 18
Revealing the Progression of Pain Pathways and Identifying Chronification of Pain Predictors After an Isolated Lateral Ankle Sprain: Project RECOIL
Authors Kosik KB , Hoch MC, Patlan I, Slone S , Torp DM, Van Wyngaarden JJ, Roach MH
Received 24 July 2024
Accepted for publication 10 February 2025
Published 26 February 2025 Volume 2025:18 Pages 931—945
DOI https://doi.org/10.2147/JPR.S488420
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alaa Abd-Elsayed
Kyle B Kosik,1 Matthew C Hoch,1 Ilana Patlan,1 Stacey Slone,2 Danielle M Torp,1 Joshua J Van Wyngaarden,3 Megan H Roach4– 6
1Department of Athletic Training & Clinical Nutrition – Sports Medicine Research Institute, University of Kentucky, Lexington, KY, 40536, USA; 2Dr Bing Zhang Department of Statistics, University of Kentucky, Lexington, KY, 40536, USA; 3Army-Baylor University, Doctoral Program of Physical Therapy, Baylor University, San Antonio, TX, USA; 4Extremity Trauma and Amputation Center of Excellence, Defense Health Agency, Falls Church, VA, 22042, USA; 5Department of Clinical Investigations, Womack Army Medical Center, Fort Bragg, NC, 28310, USA; 6Department of Surgery, Uniformed Services University of the Health Sciences, Bethesda, MD, 20814, USA
Correspondence: Kyle B Kosik, Department of Athletic Training & Clinical Nutrition – Sports Medicine Research Institute, University of Kentucky, Lexington, KY, 40536, USA, Tel +1-859-323-9850, Email [email protected]
Abstract: Persistent pain is a common complaint among civilians and military personnel after a lateral ankle sprain (LAS). Most individuals who experience pain after an LAS self-report a moderate pain intensity level that interferes with activity. This pain experience is mostly described through study designs and outcomes that limit the understanding of the acute to chronic pain transition after an LAS. The purpose of this prospective study is to quantify the prevalence rate of chronic ankle pain at 6-months post-injury and identify susceptibility and resiliency factors that contribute to pain chronification after an LAS. The objective of this study will be accomplished through a two-site prospective cohort study design with data collected at four timepoints (< 7 days post-LAS, 3-, 6-, and 12-months post-LAS). A target sample size of 200 men or women (100 per site) between 18 and 45 years of age who sustain an acute LAS within the previous 7-days will be enrolled. Participants will complete a series of standardized electronic surveys at each timepoint to self-report the presence of chronic ankle pain, healthcare utilization patterns, subsequent musculoskeletal injury, and new co-morbid conditions. Additionally, participants will complete validated patient-reported outcomes (PROs) electronically to characterize the pain burden and undergo quantitative sensory testing to assess mechanical pain sensitivity via pressure pain thresholds, pain facilitation via temporal summation, and pain inhibition via a conditioned pain modulation response at all timepoints. Lastly, clinician-based outcomes will be completed at 3-, 6-, and 12-months post-LAS to examine dynamic postural control, functional performance, and walking mechanics. We hypothesize that 30% of participants will self-report chronic ankle pain at 6-months post-injury. In addition, chronic pain at 6-months will be predicted by a combination of healthcare utilization patterns, prolonged levels of peripheral sensitization and pain facilitation, and worse functional performance and PROs.
Keywords: comorbidity, epidemiology, dynamic balance, healthcare utilization, patient-reported outcomes, prospective, observational, quantitative sensory techniques, walking
Introduction
More than 2 million people in the United States are treated annually for a lateral ankle sprain (LAS).1,2 An LAS commonly occurs because of excessive inversion and internal rotation of the rearfoot on the tibia, which may or may not occur with an increased plantarflexion angle.3–5 This biomechanical mechanism typically results in the anterior talofibular ligament and calcaneofibular ligament to be injured.5 As a result, individuals with an acute LAS experience immediate pain, swelling, and loss of function that can cause substantial time loss from work (~7 days) and physical activity (~14 days).6,7 An LAS is also the number one reason for lost duty days within the United States Military, with the average lost number of duty days ranging from 45 to 55 days.8 Therefore, an LAS is a common joint injury that interferes with a person’s ability to perform activities of daily living, participate in physical activity, and complete required training or occupational duties among Service members and civilians.
Unfortunately, many individuals who sustain an LAS continue to experience symptoms long after returning to work, physical activity, or duty. Prolonged ankle pain is one of the primary symptoms that most individuals report.9,10 Up to 40% of active-duty Service members report ankle pain 6-months post-LAS.11 Similar prevalence rates (50–79%) have been documented among civilian populations, with the longest follow-up occurring 6-years post-LAS.12 Individuals who develop persistent ankle pain report moderate intensity levels that interfere with walking, running, and vigorous activities.12 Those with persistent pain display worse fear of injury, physical health, and emotional well-being than those without pain.13 Persistent pain is also the chief complaint that individuals report when seeking long-term follow-up care.14,15 Lastly, prior authors have suggested persistent pain associated with a history of multiple ankle sprains is a contributing symptom of early separation from military service.16 These data collectively demonstrate that persistent pain post-LAS is a debilitating symptom for both civilians and active-duty Service members that warrants further attention.
Authors investigating persistent pain post-LAS have used various definitions for long-term pain (ie, chronic),12 varying endpoints to assess for the presence of pain or outcomes,12 and only evaluated the effect pain has on physical function or quality of life.13,17–20 This limited scope of research is also based on secondary analyses or cross-sectional and retrospective study designs that limit the understanding of the complex transition from acute to chronic pain.12,13,17–20 As a result, significant gaps in knowledge exist for the basic epidemiology of chronic ankle pain, the natural evolution of pain-generating pathways, or how changes in clinician and patient outcomes interact with co-morbidities and contribute to the chronification of ankle pain. Prospectively evaluating such outcomes can provide valuable insight for identifying risk or protective factors in patients susceptible to pain chronification – a vital component to developing personalized therapies at critical windows of opportunity.
To generate a substantial step towards this goal, the current study will implement a prospective study design that will follow the Heuristic Model of Pain to assess the longitudinal course of pain mechanisms and pain burden.21 For example, Quantitative Sensory Testing (QST) techniques can provide an avenue to evaluate prolonged peripheral sensitization after an LAS through pressure algometry.21–23 At the same time, other QST techniques can be used to evaluate central sensitization by assessing the balance between levels of pain facilitation (ie, temporal summation) and inhibition (ie, condition pain modulation).21,24 Secondly, the Heuristic Model of Pain outlines the importance of characterizing the sensory and affective qualities of pain through patient-reported outcomes (PROs) because chronic pain is a deeply personal experience.21 A direct benefit of this comprehensive model for pain assessment is that other biopsychosocial outcomes can be incorporated to identify risk or protective factors for individuals susceptible to chronification. Therefore, this study will accomplish the following specific aims:
- Specific Aim 1: Quantify the prevalence rate of chronic ankle pain and its relationship to healthcare utilization patterns, episodes of ankle joint “giving way”, and the new musculoskeletal injuries or co-morbid conditions throughout the first 12-months post-LAS.
- Specific Aim 2: Compare mechanical pain sensitivity levels, pain facilitation and inhibition levels between participants who do and do not develop chronic pain 6- and 12-months post-LAS.
- Specific Aim 3: Identify co-morbid conditions, clinician-based outcomes, and patient-reported outcomes that are predictive of chronic ankle pain at 6-months and 12-months post-LAS.
Material and Methods
Study Design
A multi-site prospective cohort study design will be used to enroll civilians and active-duty Service members from the University of Kentucky (UK) and Womack Army Medical Center (WAMC) at Fort Bragg (Figure 1).
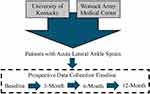 |
Figure 1 Study sites, target population, study design, and data collection timepoints. |
Ethical Approval
The Revised Common Rule’s Cooperative Research Provision (45 CFR 46.114) established in the United States for institutions engaged in multi-site research was followed to obtain ethical approval. Ethical approval was first obtained by the UK Institutional Review Board (IRB# 87032, IRB of Record) in accordance with the Declaration of Helsinki, with a reliance agreement and ethical approval granted by the WAMC Human Research Protection Program Office. Additionally, the protocol was reviewed and approved by the United States Army Medical Research and Development Command (USAMRDC) Office of Human Research Oversight.
Participants
A target sample size of 200 hundred men and women will be enrolled between UK (n = 100) and WAMC (n = 100). Participants will be recruited from the campus surrounding community associated with UK. Meanwhile, active-duty Service members and dependents will be recruited through WAMC physical therapy and orthopedic clinics that provide care to diverse populations at Fort Bragg.
Individuals between 18 and 45 years of age who sustain an acute LAS within the previous 7-days will be included. An LAS will be defined as an incident in which the rearfoot was inverted and resulted in a combination of swelling, pain, and time lost from activity for at least 1 day.25
Individuals will be excluded if they: 1) are diagnosed with a concomitant injury (eg, fracture), 2) sustain an LAS more than 8-days prior to enrollment, 3) report a history of any lower extremity injury within 6-months prior to enrollment, 4) report a history of lower extremity surgery, 5) diagnosed with chronic low back pain, neck pain, or other joint pain besides at the ankle, 6) report any neurological disease, vestibular or visual disturbance, or any other condition that would affect balance and gait, 7) are pregnant, or 8) do not speak English.
Enrollment and Informed Consent Procedures
All individuals interested in participating will be pre-screened over the phone by a member of the study team using an inclusion/exclusion criteria checklist. Individuals who are eligible to participate based on the pre-screen will be sent a copy of the informed consent document to review and scheduled to meet in-person with a member of the study team who is trained to obtain informed consent. During the in-person meeting, the trained study team member will further discuss the details of the study, confirm inclusion/exclusion criteria, and provide ample opportunity for potential participants to ask questions. All study team members discussing consent will be in civilian clothing and identify themselves by their professional title and not their military rank (if relevant) to minimize coercion or undue influences. Written informed consent will be obtained from all participants prior to formal engagement in any research activities.
Study Procedures
Participants who are deemed eligible and provided written informed consent will complete a standardized baseline assessment at the time of enrollment (Figure 1). Participants will then be scheduled for an in-person standardized assessment at 3-, 6- and 12-months post-LAS (Figure 1).
An overview of the outcomes to be collected at each timepoint is provided in Table 1. The overall order in which outcomes are collected will be the same at both sites and consistent across all timepoints. Specifically, participants will first undergo the QST described below. In between these assessments, participants will be asked to complete all self-reported questionnaires electronically through REDCap (Research Electronic Data Capture). Participants will then finish the testing session by completing the Star Excursion Balance Test, Hop-to-Stabilization, and walking mechanics assessment. A more complete description of how each outcome measure will be performed is described below. To reduce attrition, participants will complete the self-reported questionnaires electronically if they are unable to attend a follow-up assessment in-person.
 |
Table 1 Outcome Measures to Be Collected at Each Timepoint |
Primary Outcome Measure
Chronic Ankle Pain
The presence of chronic ankle pain will be documented 6- and 12-months post-LAS through a self-reported questionnaire. Chronic pain will be defined as “pain that occurs on at least half of the days for 6-months or more”.26 This is aligned with the Fiscal Year 19 Chronic Pain Management Research Program congressional appropriation definition of chronic pain caused by combat- and training related physical trauma including musculoskeletal joint injuries. Therefore, participants will be asked to answer the question (yes or no) “Have you experienced ankle pain on at least half of the days for the past 6 months?”.
Secondary Outcome Measures
Healthcare Utilization Patterns
Healthcare utilization patterns 3-, 6-, and 12-months post-LAS will be collected through a self-reported questionnaire. Specific healthcare utilization patterns captured will include the use of opioid or non-opioid prescriptions related to the index injury and outcomes associated with physical rehabilitation.
Opioid and Non-Opioid Prescriptions
Participants will be asked to self-report any opioid or non-opioid medications prescribed by their treating physician at the time of injury. Additionally, participants will be asked to self-report any opioid or non-opioid prescription refills for their injury at 3-, 6-, and 12-months post-LAS. The prescription dosage (ie, strength, number of pills) will be documented for each opioid or non-opioid medication prescribed at all timepoints.
Physical Rehabilitation
Participants will be asked to self-report if they were referred to physical rehabilitation at the time of injury (ie, baseline). At the subsequent follow-up timepoints, participants will be asked: 1) the date of their first rehabilitation visit, and 2) the overall number of physical rehabilitation visits attended.
Episodes of Ankle Joint “Giving Way”
Participants will be asked to self-report if they have experienced an episode of ankle joint “giving way” at 3-, 6- and 12-months post-LAS. An episode of “giving way” will be defined as “a temporary uncontrollable sensation of instability or rolling over one’s ankle”.25,27 Data collected will include the number of “giving way” episodes for each limb and time since most recent episode.
New Musculoskeletal Injury
Participants will be asked to self-report any new musculoskeletal injury occurring in the upper extremity, back, or lower extremity at 3-, 6-, and 12-months post-LAS. A musculoskeletal injury will be defined as 1) an injury that required the participant to seek medical attention from a healthcare provider or 2) an injury that required the participant to not participate in normal physical activity for one or more consecutive days. Data collected for each musculoskeletal injury will include: 1) body site, 2) time since injury, 3) if the injury is recurrent, 4) pain intensity at its worst following the injury, and 5) if medical treatment was provided. Specific emphasis will be placed on quantifying the number of lower extremity musculoskeletal injuries at 6- and 12-months post-LAS.
New Physician Diagnosed Co-Morbidities
Participants will be asked to complete a self-reported questionnaire to identify if they were diagnosed by a physician with a new co-morbidity since their LAS. Co-morbid conditions of interest will include cardiometabolic syndromes, sleep disorders, mental health conditions, chronic pain, concussion/traumatic brain injury, alcohol substance abuse, numbness, phobia, or psychosocial and behavioral disorders.14
Quantitative Sensory Techniques
Mechanical Pain Sensitivity
Peripheral mechanical pain sensitivity will be assessed via pressure pain thresholds (PPTs). A digital, handheld, clinical-grade pressure algometer (Model FPX 50, Wagner Instruments, Greenwich, CT) equipped with a 1cm2 rubber disc attachment will be used to introduce mechanical pain and quantify PPTs.
All test sites were selected based on published data evaluating PPTs after an acute LAS.22 Specifically, PPTs will be assessed across the second metacarpal on the ipsilateral side of the injured limb and bilaterally over the 1) anterior talofibular ligament (ATFL); 2) deltoid ligament; 3) lateral malleolus; and 4) tibialis anterior muscle belly. Table 2 provides a description detailing how each test site will be identified and participant positioning. The limb tested first will alternate with every consecutive participant enrolled at each site, with half the participants being tested on the injured limb first and the other half tested on the un-injured limb first.
 |
Table 2 Body Site Description and Participant Position for Each Anatomical Site Tested for All Pressure Pain Thresholds |
The test sites will be marked using the anatomical landmark descriptions listed in Table 2 and each participant will be instructed on how the PPT test is performed. All PPTs will be performed by placing the rubber disc attachment over the skin and applying a constant rate of 40 KPa/sec pressure perpendicular to the anatomical landmark. Participants will verbally acknowledge the moment they perceive the mechanical pressure to become painful. The algometer will then be immediately removed, and the PPT will be recorded in Newtons.
Two practice trials will be performed over the participant shoulder at a non-painful site. Two test trials will be performed at each body site with a 30-second rest between trials. The average PPT at each body site will be used for statistical analysis.
Temporal Summation
Pain facilitation is commonly assessed through temporal summation of second pain. Temporal summation of second pain is the increased perception of pain response to repetitive noxious stimuli and is viewed as an indirect method for evaluating the hyperexcitability of the central nervous system.28–30 Previously published methodology for performing a temporal summation assessment through mechanical stimuli will be followed.31,32 Mechanical stimuli will be applied using a von Frey filament (Touch-Test Sensory Evaluator; North Coast Medical, Gilroy, CA) calibrated to bend at 180g of force.
Temporal summation will be tested at: 1) the ATFL of the injured ankle and 2) between the 2nd and 3rd metacarpal (not over a vein or bone) and approximately half the distance from the metacarpophalangeal joint and wrist.
Participants will first be positioned in a chair with their feet flat on the ground. Both test sites will be marked, and each participant will be instructed on how the temporal summation test is performed. First, a single mechanical stimulus will be applied with the filament at the tested body site. Participants will then be asked to rate their level of perceived pain intensity using a “0” (no pain at all) to “10” (worst pain imaginable) Numerical Rating Scale (NRS). Next, a series of 10 mechanical stimuli will be applied with the filament to the tested body site at a rate of 1 tap per second. Participants will then be asked to immediately rate the greatest level of pain intensity during the 10 stimuli using the same “0” to “10” NRS.
One trial at each body site will be performed. Temporal summation will be calculated as the difference between the single stimulus pain rating and the pain rating after the 10 stimuli. Scores at each tested body site will then be used for statistical analysis.
Condition Pain Modulation
A conditioned pain modulation (CPM) protocol is commonly used to evaluate centrally derived pain inhibition pathways. This is accomplished by quantifying the reduction of pain experienced at one body site in response to the introduction of a second noxious conditioning stimuli applied to a remote region of the body (eg, “pain inhibits pain”). Therefore, recommendations for experimentally performing a CPM protocol will be followed.33
The most commonly applied conditioning stimulus utilized in pain studies is cold water immersion.34 Therefore, the conditioning stimulus will be introduced by requiring participants to submerge the contralateral hand of the injured ankle (eg, right ankle, left hand) into a cold-water bath cooled to 2–5° Celsius. Participants will be instructed to submerge their hand until the water covers the wrist and refrain from closing their hand. While their hand is submerged, participants will be asked to verbally report their cold-water pain intensity every 30 seconds using a “0” (no pain at all) to “10” (worst pain imaginable) NRS. Participants will be allowed to voluntarily remove their hand from the cold-water bath at any time or verbally instructed to remove their hand once they report a 7/10 pain using the NRS or after 3-minutes of immersion. The time mark that participants remove their hand from the cold-water bath will be recorded.
Two mechanical test stimuli will be applied across the injured ATFL and ipsilateral tibialis anterior muscle belly immediately after the conditioning stimulus (Table 2). Each mechanical test stimuli will be administered consistent with the methods described above for obtaining a PPT.
A CPM index score will be calculated for the ATFL and the ipsilateral tibialis anterior muscle belly. This index score will be calculated using the average PPT values obtained during the battery of mechanical pain sensitivity testing and those captured immediately after the cold-water immersion. Therefore, the following equation will be used:
A positive percent change score would represent an increase in PPT after the conditioning stimulus and suggest the presence of pain inhibition.
Patient-Reported Outcomes
Table 3 provides a complete description of the measurement scale, number of items, scoring, and interpretation of each PRO to be collected. Therefore, a brief description of the PROs is listed below.
 |
Table 3 Description of the Design, Scoring and Interpretation for Each Patient-Reported Outcome to Be Collected |
Pain Catastrophizing Scale (PCS)
The PCS is a dimension-specific PRO that assesses catastrophic thinking related to pain.35 In addition to calculating the total score, items can be divided to assess feelings of rumination, magnification, and helplessness. The PCS has demonstrated good test–retest reliability (Spearman ρ = 0.88) and high internal consistency (Cronbach’s α = 0.92).36
Defense & Veterans Pain Rating Scale (DVPRS)
The DVPRS was developed by the Army Pain Management Task Force over concerns associated with the inconsistent implementation of a standard NRS across military settings and its clinical value.37 The DVPRS has demonstrated acceptable psychometric properties.37,38
Patient-Reported Outcomes Measurement Information System (PROMIS) Pain Intensity Scale & Interference Short-Form
Surveys from the PROMIS were selected because of the methodology around the validation process for multiple clinical pathologies, and psychometric properties of health domains, including pain intensity and pain-interference.39
Fear-Avoidance Beliefs Questionnaire (FABQ)
The FABQ is a dimension-specific PRO designed to assess fear of pain and avoidance beliefs.40 The FABQ has demonstrated high test–retest reliability (intraclass correlation coefficient [ICC] = 0.90)41 and acceptable internal consistency (Cronbach’s α = 0.662 to 0.704).42
Short-Form McGill Pain Questionnaire-2 (SF-MPQ-2)
The SF-MPQ-2 is an updated PRO that assesses the sensory and affective symptoms commonly associated with neuropathic and non-neuropathic pain.43 The SF-MPQ-2 is suggested to be a useful instrument for studies examining the epidemiology, natural progression, mechanisms, and treatment responses for neuropathic or non-neuropathic pain.43 The SF-MPQ-2 has demonstrated high internal consistency (Cronbach’s α = 0.91 to 0.95).43
Pain Self-Efficacy Questionnaire (PSEQ)
The PSEQ is a dimension-specific PRO that examines a persons’ confidence performing activities despite their pain.44 The PSEQ has demonstrated acceptable test–retest reliability (r = 0.73) and high internal consistency (Cronbach’s α = 0.92).44
Perceived Stress Scale (PSS-10)
The PSS-10 is a dimension-specific PRO that measures the personal stress an individual is currently experiencing.45 The PSS has demonstrated good test–retest reliability (r = 0.85) and internal consistency (Cronbach’s α = 0.84).45
Modified Disablement in the Physically Active Scale (mDPA)
The mDPA is a 16-item generic PRO designed to evaluate activity limitations, participation restrictions, and emotional well-being.46 The mDPA has demonstrated high test–retest reliability (ICC = 0.943)46 with the summary components demonstrating adequate internal consistency (Cronbach’s α = 0.941 to 0.878).47
Foot and Ankle Disability Index (FADI)
The FADI is a 34-item region-specific PRO created to assess functional limitations during activities of daily living and physical activity related to foot and ankle conditions.48 The FADI has demonstrated good test–retest reliability (ICC = 0.84 to 0.89) and is sensitive to detecting change.48
Clinician-Based Outcomes
Star Excursion Balance Test (SEBT)
The modified SEBT is a clinical measure of dynamic balance that includes only the anterior, posteromedial, and posterolateral reach directions. The SEBT requires participants to balance on a single-limb (ie, involved limb) and performs a maximal reach with the non-stance limb (ie, un-involved limb).49
Participants will perform each direction of the SEBT barefoot for the involved limb. Participants will be positioned facing a tape measure secured to the floor and toes at zero for the anterior reach direction.49 In contrast, participants will be positioned with their heel at zero and the tape measure at a 135° angle for the posteromedial and posterolateral reach directions.49
Participants will first be instructed to place their hands on their hips and then transition from a double-limb to a single-limb stance on the involved limb. Once stable, participants will perform a maximal reach with their non-injured limb for each direction and gently touch the tape measure with the most distal part of the foot while maintaining a single-limb stance. Participants will then return to a double-limb stance without losing balance, and the distance reached is recorded. Participants will be provided with four practice trials, followed by three test trials in each reach direction.50 A trial will be discarded and repeated if 1) the participant loses balance, 2) hands are removed from the hips, 3) weight is transferred to the non-stance limb; or 4) the heel of the stance limb raises off the floor.
The average reach distance for all three directions will be calculated, normalized to the length of each participants stance leg, and represented as a percentage of leg length. Leg length will be measured as the linear distance between the anterior superior iliac spine and the medial malleolus. Greater normalized reach distances represent better dynamic balance.
Hop-to-Stabilization
Functional performance will be assessed using the forward hop-to-stabilization task. To accomplish this task, participants will begin standing at a distance equal to 40% of their height from a landing zone outlined on the floor (40 × 60 cm box). Participants will initiate this task by performing a double-limb forward jump over a 30 cm hurdle placed halfway between the starting line and landing zone. Participants will then land on their involved limb within the landing zone, obtain their balance, place their hands on the hips, and remain as still as possible for five seconds. Trials will be discarded and repeated if they do not land completely in the target area, touch down with the other foot, or twist or hop on the stance-leg after landing.
Prior to beginning the task, an inertial measurement unit (IMU) will be secured to the low back (L4/L5). Tri-axial data from the IMU will be sampled at 120Hz. Data synchronization, sensor settings, and trial identifications will be controlled using the IMU research Application (for iOS developed by Xsens Technologies) on a tablet. Acceleration and gyroscopic data for each test trial will be processed using a custom MATLAB code (the MathWorks, Natick, MA, USA). The stabilization phase will be assessed using the dynamic postural stability index (DPSI) values calculated from the root-mean-square of accelerometer and rotational velocity magnitudes.
Participants will be provided with three practice trials and 10 attempts to successfully complete 5 test trials to minimize fatigue. The DPSI from five successful trials will be averaged and used for statistical analysis. Greater DPSI values will indicate greater postural disturbance and worse task performance.
Overground Walking Mechanics
All participants will be fitted with wireless force insoles (Loadsol, Novel Electronics, St. Paul, MN, USA) that are placed in their normal footwear. The insoles are designed to cover the entire foot and are comprised of 3-sensors dividing the foot into posterior, medial, and lateral region. Forces in each region will be sampled at 100 hz and recorded via Bluetooth through the Loadapp iOS application (Novel Electronics, St. Paul, MN, USA).
The insoles will be calibrated prior to data collection using recommended procedures described in thorough detail elsewhere.51 Briefly, participants will be weighed prior to data collection and their is weight calculated in Newtons (N). Calibration of each insole will be confirmed and accepted if the insole is within 5% of the participants bodyweight when fully loaded during a single-limb stance. If the calibration is not acceptable, the manufacturer’s guidelines will be followed to re-calibrate the insole.
Participants will walk across a 9.11m walkway at a self-selected pace to assess walking mechanics. Participants will start and stop each trial 1.52m on either side of the walkway to prevent any acceleration or deceleration effect (total walkway 12.15m).52,53 Participants' self-selected walking speed will first be determined by having participants complete five practice trials and calculating the average walking speed using time gates at each end of the walkway. Five test trials within 5% of each participants average walking speed will then be performed and used for statistical analysis.
Raw force data for each test trial will be exported from the Loadapp application to a.csv file for processing. The middle 5 steps from each trial (n = 25 steps total) will be identified and averaged for statistical analysis. A publicly available MATLAB code will be used to calculate the average loading rate (Δ Force (N)/Δ Time (seconds)) for each limb.51,54
Power Analysis
The primary outcome is the development of chronic ankle pain at 6-months. We anticipate that 30% of participants will develop chronic ankle pain at 6-months based on a prospective study.11 Thus, 167 participants will allow for estimating the proportion of patients who develop chronic ankle pain with a 7% margin of error and 95% confidence intervals. Additionally, a sample size of 167 participants will allow for 80% power to detect moderate effect sizes for the quantitative sensory testing techniques between those who do and do not develop chronic ankle pain. Furthermore, a sample size of 167 participants will allow for identifying predictors of chronification using up to 5 covariates (co-morbidity, clinical outcomes, PROs) assuming p-values less than 0.05 are statistically significant. Lastly, to account for 15% attrition during the study, a target of 200 participants will be enrolled (ie, 100 participants per site).
Statistical Plan and Data Analysis
All statistical analysis will be completed with Statistical Analysis System (SAS) v9.4 (SAS Institute, Cary, NC, USA). Summary statistics will be calculated for demographic variables, prevalence rate of chronic ankle pain at 6- and 12-months, healthcare utilization measures, subsequent musculoskeletal injury, and new co-morbidities. Normality and constant various assumptions will be assessed. Continuous data will be assessed by two-sample t-tests and categorical variables will be assessed with chi-square tests to look for differences between the participants who do and do not develop chronic ankle pain. Binary logistic regressions will be used to examine the relationship between chronic pain at 6- and 12-months with healthcare utilization measures, subsequent musculoskeletal injury, and new co-morbidities. Separate analysis of covariance models controlling for unbalanced demographic data (age, sex, race/ethnicity) and injury severity will be performed if needed. The asymptotic level of significance will be set at p ≤0.05 for all analyses.
Pain assessments will be measured at baseline, 3-months, and 6-months post-LAS. Normality and constant various assumptions will be assessed. A linear mixed model will be fit to assess differences over time between groups (chronic pain vs no pain) for each dependent variable. Non-parametric analyses, transformations, or separate analyses of covariance models controlling for unbalance demographic data (age, sex, race/ethnicity) and injury severity will be performed if needed. In addition, various correlation structures will be considered to estimate the within-subject correlation. The patterns of missing endpoint data will be assessed and, if necessary, techniques to handle missing data will be considered including, but not limited to, multiple imputation and last value carried forward and their sensitivity evaluated. P-values obtained for the multiple comparisons will be adjusted using appropriate methods.
The dichotomous outcome (chronic pain vs no pain) will be fit with a generalized linear mixed model to identify previously diagnosed co-morbid conditions, clinical outcomes, and PROs that predict chronic pain at 6- and 12-months post-LAS. These statistical models will allow for comparisons and examination of complex relationships among outcomes and co-morbid conditions and outcomes. Models which include demographic variables and injury severity will also be considered. Models with statistical interaction effects will be used to check for any combinations of variables under study that are related to each quantitative outcome. The asymptotic level of significance will be set at p ≤0.05 for all analyses. Planned exploratory analysis for all aims will be performed to examine differences in military and civilian populations.
Discussion
The best available data indicate that between 50% and 79% of civilians and 40% of active-duty Service members report persistent pain within 6-months post-LAS.11,12 Individuals who experience persistent ankle pain have worse functional outcomes and display diminished physical and mental health-related quality of life.13,17,18 Despite this empirical evidence, there is a significant gap in the current knowledge regarding the basic prevalence rate of chronic pain after an LAS and factors that contribute to the progression from acute to chronic pain. To our knowledge, this will be the first prospective study to quantify the prevalence of chronic ankle pain post-LAS and examine its relationship with healthcare utilization patterns, subsequent musculoskeletal injury, and the development of new co-comorbidities. Additionally, this study will be the first to identify the susceptibility and resiliency factors underlying the transition from acute to chronic pain by prospectively assessing pain-generating pathways, clinician-based outcomes, and PROs post-LAS. We hypothesize that individuals who report chronic pain at 6-months post-LAS will receive delayed/fewer healthcare services, prolonged levels of peripheral sensitization and pain facilitation, and worse functional performance and PROs will be predictive of chronic pain.
Several clinical practice guidelines recommend that an LAS be managed conservatively with timely physical rehabilitation, non-steroidal anti-inflammatory drugs or non-opioid analgesics, and rest, ice, compression, and elevation.55–58 Additionally, systematic reviews have supported the use of a prophylactic ankle brace or other devices to reduce the risk of injury during activity after an LAS.59,60 Unfortunately, research has shown that many patients (~30%) are prescribed opioids to minimize their pain, and very few (~20%) are referred for physical rehabilitation.14,61–64 Published data also indicate that the odds of a recurrent LAS, proximal joint injury, and functional limitations can increase if a patient fails to receive timely physical rehabilitation.14,15,65 These data provide support to our hypothesis that healthcare utilization patterns post-LAS contribute to the development of chronic pain. Furthermore, once chronic pain develops, prior studies have documented that the pain intensity and severity of functional deficits are amplified by an increase in ongoing co-morbidities.66,67 We believe that identifying the relationship between time to specialized care, co-morbidities, and the development of chronic ankle pain may elucidate strategies to minimize the downstream effects currently documented post-LAS in both civilians and military populations.
Traumatic joint injuries activate peripheral nociceptors that relay signals to the central nervous system (CNS) that are processed with the cortex as acute pain.68,69 Ramiro-González et al22 demonstrated reduced sensitivity to mechanical pain over the ligamentous stabilizers within the first 2-days of being diagnosed with an acute LAS. Reduced sensitivity to mechanical pain provides evidence that an acute LAS is associated with the presence of localized peripheral sensitization.22 Although these findings were cross-sectional, prolonged activation of peripheral nociceptors can extend the time pain is experienced and amplify its intensity. This is supported by prior studies that have shown reduced levels of mechanosensitivity over various lower extremity neuromuscular structures among individuals with chronic ankle instability, regardless of whether they experience persistent pain or not.70 Therefore, these published data provide evidence to support our hypothesis that peripheral nociceptors around the ankle may continue to be activated long after an acute LAS and may contribute to the progression of chronic ankle pain.
Noxious stimuli relayed to the CNS from peripheral nociceptors are under constant modulation by cortical pathways. This constant modulation of noxious stimuli can disrupt pain processing pathways within cortical structures, causing more widespread pain. Changes in central pain processing are referred to as nociplastic pain and can occur independently of nociceptive pain or in combination.71 While empirical data indicating that the presence of nociplastic pain occurs post-LAS is sparse, there is indirect evidence that suggests this pain pattern may develop. Wang et al72 found evidence of neuroplastic alterations within the functional connectivity of pain processing (ie, insula) and motor control areas (ie, cingulate motor areas) among patients with ankle pain. Individuals with chronic ankle instability exhibit central sensorimotor alterations such as lower levels of corticomotor excitability,73–75 increased cortical inhibition,76 and less microstructural integrity of cortical tracts responsible for governing movement.77 As a result, we cannot rule out the possibility that our prospective study may also find that individuals who develop chronic ankle pain present with an imbalanced pain modulation profile.24 In other words, individuals who develop chronic pain may experience increased pain facilitation and diminished pain inhibition levels throughout the first 12-months post-LAS.
Regardless of the exact pain pattern found in this prospective study, there will be direct benefits of examining both peripheral and central pain mechanisms post-LAS. For instance, the QST data collected will allow for investigation into how different pain generating pathways evolve naturally and contribute to the chronification of pain or change in response to rehabilitation or recurrent injury. Secondly, characterizing the pattern of pain will help inform future clinical trials on selecting novel mechanism-based therapies to target either peripheral or central pathways. Lastly, the QST data collected during this prospective study can serve as normative data for future authors or clinicians to reference. Therefore, we believe that the QST data collected will have multiple short-term and long-term benefits.
While the descriptive healthcare utilization patterns and comparison of pain pathways will advance our current knowledge of the chronification of pain, the Federal Pain Research Strategy lists identifying susceptibility and resilience factors that underline the transition from acute to chronic pain as a top priority.78 In response to that objective, our prospective study aims to examine a variety of biopsychosocial outcomes post-LAS that are supported by the existing literature. For example, a recent systematic review found individuals with chronic ankle instability who experience pain report moderate intensity levels that interfere with walking, running, and vigorous activities.12 This pain-related interference is supported by empirical data demonstrating individuals with painful chronic ankle instability have worse dynamic postural control and more episodes of giving way.17 Pain is also identified as a known barrier to human performance and job-related duties among military personnel.79 However, pain does not only impact physical function as evidence suggests that psychosocial outcomes account for as much or more of the variation in symptoms than accounted by an LAS itself. Notably, lower self-efficacy levels within the first 3-weeks after injury were found to be better predictors of pain intensity than injury severity.80 Similar relationships between levels of pain catastrophizing, injury-related fear, pain, and physical function have been documented among individuals with chronic ankle instability.81 A secondary analysis of published data has also shown that individuals with painful chronic ankle instability report worse fear of injury, physical health, and emotional well-being than those without pain.13 Therefore, we believe the outcomes selected for this objective will allow our research team to engage with patients by capturing their positive experiences with recovery post-LAS (ie, resilience factors) and ineffective coping strategies (ie, susceptibility factors). Prospectively capturing these data will allow for future research to examine the effectiveness of multi-modal therapies that target both physical and psychosocial side effects of an LAS.
The nature of our study design will have an impact on both short- and long-term patient care post-LAS. For example, prospectively evaluating the course of pain from an acute episode to chronic is likely to reveal vital information for developing new therapeutic targets to prevent chronic pain or reverse its course including the underlying mechanisms to address, key timepoints to intervene, endpoints and outcomes to assess treatment effectiveness. The translation of this information into clinical practice will be driven by knowledge products in the form of peer-reviewed journal submissions, clinician training for pain assessments, and patient education materials that will be available for open access.
Ethics Approval and Consent to Participate
The study protocol was approved by the University of Kentucky Institutional Review Board (IRB) in compliance with all applicable Federal regulations governing the protection of human subjects (IRB # 87032) and the Declaration of Helsinki. A reliance agreement and ethical approval have also been granted by Womack Army Medical Center’s Human Research Protection Program in compliance with the single IRB protocol. This protocol has also been reviewed and approved by the United States Army Medical Research and Development Command (USAMRDC) Office of Human Research Oversight. All methods will be carried out in accordance with relevant guidelines and regulations set forth by the approved IRB protocol. Informed consent will be obtained from all participants before volunteering.
Acknowledgments
We thank the Department of Defense Congressionally Directed Medical Research Programs for funding our research. We are also greatly appreciative of the countless support and regulatory staff at the University of Kentucky and Womack Army Medical Center for their assistance facilitating this work. This project will also be supported by REDCap services provided by the NIH Center for Advancing Translational Sciences through grant number UL1TR001998.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs and Defense Health Agency J9, Research and Development Directorate, or the US Army Medical Research Acquisition Activity at the US Army Medical Research and Development Command, in the amount of $1,066,469 through the Chronic Pain Management Research Program under Award No. HT94252311054. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily by the Department of Defense.
Disclosure
The views expressed in this manuscript are of those of the authors and do not necessarily reflect the views, opinions, or policies of the Uniformed Services University of the Health Sciences, the US Departments of Army/Navy/Air Force, Department of Defense, nor the US Government. The authors report no conflicts of interest in this work.
References
1. Shah S, Thomas AC, Noone JM, Blanchette CM, Wikstrom EA. Incidence and Cost of Ankle Sprains in United States Emergency Departments. Sports Health. 2016;8(6):547–552. doi:10.1177/1941738116659639
2. Waterman BR, Owens BD, Davey S, Zacchilli MA, Belmont Jr PJ. The epidemiology of ankle sprains in the United States. J Bone Joint Surg Am Vol. 2010;92(13):2279–2284. doi:10.2106/JBJS.I.01537
3. Mok KM, Fong DT, Krosshaug T, et al. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: 2 cases during the 2008 Beijing Olympics. Am J Sports Med. 2011;39(7):1548–1552. doi:10.1177/0363546511399384
4. Fong DT, Ha SC, Mok KM, Chan CW, Chan KM. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: five cases from televised tennis competitions. Am J Sports Med. 2012;40(11):2627–2632. doi:10.1177/0363546512458259
5. Hertel J. Functional Anatomy, Pathomechanics, and Pathophysiology of Lateral Ankle Instability. J Athletic Training. 2002;37(4):364–375.
6. Cross KM, Worrell TW, Leslie JE, Van Veld KR. The relationship between self-reported and clinical measures and the number of days to return to sport following acute lateral ankle sprains. J Orthopaedic Sports Phys Ther. 2002;32(1):16–23. doi:10.2519/jospt.2002.32.1.16
7. de Bie RA, de Vet HC, van den Wildenberg FA, Lenssen T, Knipschild PG. The prognosis of ankle sprains. Int J Sports Sci Med. 1997;18(4):285–289. doi:10.1055/s-2007-972635
8. Roy TC, Faller TN, Richardson MD, Taylor KM. Characterization of Limited Duty Neuromusculoskeletal Injuries and Return to Duty Times in the U.S. Army During 2017-2018. Mil Med. 2022;187(3–4):e368–e376. doi:10.1093/milmed/usaa392
9. Hiller CE, Nightingale EJ, Raymond J, et al. Prevalence and impact of chronic musculoskeletal ankle disorders in the community. Arch Phys Med Rehabil. 2012;93(10):1801–1807. doi:10.1016/j.apmr.2012.04.023
10. Mohrsen A, Sørensen T, Lund H, et al. ”I Feel Like I Have Lost Part Of My Identity” - A Qualitative Study Exploring The Impact Of Chronic Ankle Instability. Int J Sports Phys Ther. 2024;19(3):316–325. doi:10.26603/001c.92908
11. Gerber JP, Williams GN, Scoville CR, Arciero RA, Taylor DC. Persistent disability associated with ankle sprains: a prospective examination of an athletic population. Foot and Ankle Int. 1998;19(10):653–660. doi:10.1177/107110079801901002
12. Al Adal S, Pourkazemi F, Mackey M, Hiller CE. The Prevalence of Pain in People With Chronic Ankle Instability: a Systematic Review. J Athletic Training. 2019;54(6):662–670. doi:10.4085/1062-6050-531-17
13. Kosik KB, Hoch MC, Slone S, Bain KA, Gribble PA. Health-Related Quality of Life Among Patients With Painful Chronic Ankle Instability. Int J Athl Ther Train. 2022;2022:1–6. doi:10.1123/ijatt.2022-0077
14. Rhon DI, Fraser JJ, Sorensen J, Greenlee TA, Jain T, Cook CE. Delayed Rehabilitation Is Associated With Recurrence and Higher Medical Care Use After Ankle Sprain Injuries in the United States Military Health System. J Orthopaedic Sports Phys Ther. 2021;51(12):619–627. doi:10.2519/jospt.2021.10730
15. Rhon DI, Greenlee TA, Cook CE, Westrick RB, Umlauf JA, Fraser JJ. Fractures and Chronic Recurrence are Commonly Associated with Ankle Sprains: a 5-year Population-level Cohort of Patients Seen in the U.S. Military Health System. Int J Sports Phys Ther. 2021;16(5):1313–1322. doi:10.26603/001c.27912
16. Bulathsinhala L, Hill OT, Scofield DE, Haley TF, Kardouni JR. Epidemiology of Ankle Sprains and the Risk of Separation From Service in U.S. Army Soldiers. J Orthopaedic Sports Phys Ther. 2015;45(6):477–484. doi:10.2519/jospt.2015.5733
17. Chen Y, Cao S, Qian L, et al. The influence of local pain on balance control in patients with chronic ankle instability. BMC Musculoskelet Disord. 2022;23(1):699. doi:10.1186/s12891-022-05656-4
18. Wang L, Yu G, Zhang X, Y-z W, Y-p C. Relationship between ankle pain, range of motion, strength and balance in individuals with functional ankle instability: a cross-sectional study. BMC Musculoskeletal Disorders. 2023;24(1):955. doi:10.1186/s12891-023-07079-1
19. Koshino Y, Watanabe K, Akimoto M, et al. Factors associated with persistent pain in college athletes with a history of lateral ankle sprain. Phys Ther Sport. 2023;64:27–31. doi:10.1016/j.ptsp.2023.08.007
20. Adal SA, Mackey M, Pourkazemi F, Hiller CE. The relationship between pain and associated characteristics of chronic ankle instability: a retrospective study. J Sport Health Sci. 2020;9(1):96–101. doi:10.1016/j.jshs.2019.07.009
21. Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of Chronic Pain: domains, Methods, and Mechanisms. J Pain. 2016;17(9 Suppl):T10–20. doi:10.1016/j.jpain.2015.08.010
22. Ramiro-Gonzalez MD, Cano-de-la-Cuerda R, De-la-Llave-Rincon AI, Miangolarra-Page JC, Zarzoso-Sanchez R, Fernandez-de-Las-Penas C. Deep tissue hypersensitivity to pressure pain in individuals with unilateral acute inversion ankle sprain. Pain Med. 2012;13(3):361–367. doi:10.1111/j.1526-4637.2011.01302.x
23. Lorenzo-Sanchez-Aguilera C, Rodriguez-Sanz D, Gallego-Izquierdo T, et al. Neuromuscular Mechanosensitivity in Subjects with Chronic Ankle Sprain: a Cross-Sectional Study. Pain Med. 2020;21(9):1991–1998. doi:10.1093/pm/pny299
24. Yarnitsky D, Granot M, Granovsky Y. Pain modulation profile and pain therapy: between pro- and antinociception. Pain. 2014;155(4):663–665. doi:10.1016/j.pain.2013.11.005
25. Gribble PA, Delahunt E, Bleakley C, et al. Selection criteria for patients with chronic ankle instability in controlled research: a position statement of the International Ankle Consortium. Br. J. Sports Med. 2014;48(13):1014–1018. doi:10.1136/bjsports-2013-093175
26. Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on research standards for chronic low back pain. J Pain. 2014;15(6):569–585. doi:10.1016/j.jpain.2014.03.005
27. Simon J, Donahue M, Docherty C. Development of the Identification of Functional Ankle Instability (IdFAI). Foot and Ankle Int. 2012;33(9):755–763. doi:10.3113/FAI.2012.0755
28. Price DD. Characteristics of second pain and flexion reflexes indicative of prolonged central summation. Exp Neurol. 1972;37(2):371–387. doi:10.1016/0014-4886(72)90081-7
29. Price DD, Dubner R. Mechanisms of first and second pain in the peripheral and central nervous systems. J Invest Dermatol. 1977;69(1):167–171. doi:10.1111/1523-1747.ep12497942
30. Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi:10.1016/j.pain.2010.09.030
31. Naugle KM, Corrona S, Smith JA, Nguyen T, Saxe J, White FA. Physical activity behavior in the first month after mild traumatic brain injury is associated with physiological and psychological risk factors for chronic pain. Pain Rep. 2021;6(4):e969. doi:10.1097/PR9.0000000000000969
32. Naugle KM, Ohlman T, Wind B, Miller L. Test-Retest Instability of Temporal Summation and Conditioned Pain Modulation Measures in Older Adults. Pain Med. 2020;21(11):2863–2876. doi:10.1093/pm/pnaa288
33. Yarnitsky D, Bouhassira D, Drewes AM, et al. Recommendations on practice of conditioned pain modulation (CPM) testing. Eur J Pain. 2015;19(6):805–806. doi:10.1002/ejp.605
34. Lewis GN, Rice DA, McNair PJ. Conditioned pain modulation in populations with chronic pain: a systematic review and meta-analysis. J Pain. 2012;13(10):936–944. doi:10.1016/j.jpain.2012.07.005
35. Sullivan MJ, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychological Assess. 1995;7(4):524. doi:10.1037/1040-3590.7.4.524
36. Wheeler CHB, Williams ACC, Morley SJ. Meta-analysis of the psychometric properties of the Pain Catastrophizing Scale and associations with participant characteristics. Pain. 2019;160(9):1946–1953. doi:10.1097/j.pain.0000000000001494
37. Buckenmaier III CC, Galloway KT, Polomano RC, McDuffie M, Kwon N, Gallagher RM. Preliminary validation of the Defense and Veterans Pain Rating Scale (DVPRS) in a military population. Pain Med. 2013;14(1):110–123. doi:10.1111/j.1526-4637.2012.01516.x
38. Nassif TH, Hull A, Holliday SB, Sullivan P, Sandbrink F. Concurrent Validity of the Defense and Veterans Pain Rating Scale in VA Outpatients. Pain Med. 2015;16(11):2152–2161. doi:10.1111/pme.12866
39. Amtmann D, Cook KF, Jensen MP, et al. Development of a PROMIS item bank to measure pain interference. Pain. 2010;150(1):173–182. doi:10.1016/j.pain.2010.04.025
40. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. 1993;52(2):157–168. doi:10.1016/0304-3959(93)90127-B
41. Jacob T, Baras M, Zeev A, Epstein L. Low back pain: reliability of a set of pain measurement tools. Arch Phys Med Rehabil. 2001;82(6):735–742. doi:10.1053/apmr.2001.22623
42. Powden CJ, Hoch MC, Jamali BE, Hoch JM. Response Shift After a 4-Week Multimodal Intervention for Chronic Ankle Instability. J Athletic Training. 2019;54(4):397–402. doi:10.4085/1062-6050-345-17
43. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144(1–2):35–42. doi:10.1016/j.pain.2009.02.007
44. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11(2):153–163. doi:10.1016/j.ejpain.2005.12.008
45. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi:10.2307/2136404
46. Vela LI, Denegar CR. The Disablement in the Physically Active Scale, part II: the psychometric properties of an outcomes scale for musculoskeletal injuries. J Athletic Training. 2010;45(6):630–641. doi:10.4085/1062-6050-45.6.630
47. Houston MN, Hoch JM, Van Lunen BL, Hoch MC. The development of summary components for the Disablement in the Physically Active scale in collegiate athletes. Qual Life Res. 2015;24(11):2657–2662. doi:10.1007/s11136-015-1007-6
48. Hale SA, Hertel J. Reliability and Sensitivity of the Foot and Ankle Disability Index in Subjects With Chronic Ankle Instability. J Athletic Training. 2005;40(1):35–40.
49. Gribble PA, Hertel J, Plisky P. Using the Star Excursion Balance Test to Assess Dynamic Postural-Control Deficits and Outcomes in Lower Extremity Injury: a Literature and Systematic Review. J Athletic Training. 2012;47(3):339–357. doi:10.4085/1062-6050-47.3.08
50. Robinson RH, Gribble PA. Support for a reduction in the number of trials needed for the star excursion balance test. Arch Phys Med Rehabil. 2008;89(2):364–370. doi:10.1016/j.apmr.2007.08.139
51. Peebles AT, Maguire LA, Renner KE, Queen RM. Validity and Repeatability of Single-Sensor Loadsol Insoles during Landing. Sensors. 2018;18(12):4082. doi:10.3390/s18124082
52. Kosik KB, Hoch M, Allison RL, Bain KA, Slone S, Gribble PA. Talar-Cartilage Deformation and Spatiotemporal Gait Patterns in Individuals With and Those Without Chronic Ankle Instability. J Athletic Training. 2022;57(6):564–570. doi:10.4085/1062-6050-733-20
53. Kosik KB, Terada M, McCann R, Thomas A, Johnson N, Gribble P. Differences in temporal gait mechanics are associated with decreased perceived ankle joint health in individuals with chronic ankle instability. Gait Posture. 2019;70:403–407. doi:10.1016/j.gaitpost.2019.03.032
54. Luftglass AR, Peebles AT, Miller TK, Queen RM. The impact of standardized footwear on load and load symmetry. Clin Biomech. 2021;88:105421. doi:10.1016/j.clinbiomech.2021.105421
55. Martin RL, Davenport TE, Fraser JJ, et al. Ankle Stability and Movement Coordination Impairments: lateral Ankle Ligament Sprains Revision 2021. J Orthopaedic Sports Physical Ther. 2021;51(4):CPG1–CPG80. doi:10.2519/jospt.2021.0302
56. Kaminski TW, Hertel J, Amendola N, et al. National Athletic Trainers’ Association position statement: conservative management and prevention of ankle sprains in athletes. J Athletic Training. 2013;48(4):528–545. doi:10.4085/1062-6050-48.4.02
57. Vuurberg G, Hoorntje A, Wink LM, et al. Diagnosis, treatment and prevention of ankle sprains: update of an evidence-based clinical guideline. Br. J. Sports Med. 2018;52(15):956. doi:10.1136/bjsports-2017-098106
58. Tiemstra JD. Update on acute ankle sprains. Am Fam Phys. 2012;85(12):1170–1176.
59. Biz C, Nicoletti P, Tomasin M, Bragazzi NL, Di Rubbo G, Ruggieri P. Is Kinesio Taping Effective for Sport Performance and Ankle Function of Athletes with Chronic Ankle Instability (CAI)? A Systematic Review and Meta-Analysis. Medicina. 2022;58(5):620. doi:10.3390/medicina58050620
60. Kemler E, van de Port I, Backx F, van Dijk CN. A systematic review on the treatment of acute ankle sprain: brace versus other functional treatment types. Sports Med. 2011;41(3):185–197. doi:10.2165/11584370-000000000-00000
61. Bowers LC, Gribble PA, Hoch MC, Villasante Tezanos AG, Kosik KB. Physical therapy referral and medication for ankle sprain visits to physician offices: an analysis of the national ambulatory medical care survey. Phys Sportsmed. 2021;49(2):176–181. doi:10.1080/00913847.2020.1800369
62. Kosik KB, Bowers LC, Hoch MC, et al. Pain Medication Administered and Prescribed to Patients With an Ankle Sprain Treated in an Emergency Department: a Record-Based Cohort Study. J Emerg Nurs. 2021;47(4):609–620e3. doi:10.1016/j.jen.2020.12.011
63. Kosik KB, Hoch MC, Humphries RL, Villasante Tezanos AG, Gribble PA. Medications Used in U.S. Emergency Departments for an Ankle Sprain: an Analysis of the National Hospital Ambulatory Medical Care Survey. J Emerg Med. 2019;57(5):662–670. doi:10.1016/j.jemermed.2019.08.025
64. Feger MA, Herb CC, Fraser JJ, Glaviano N, Hertel J. Supervised rehabilitation versus home exercise in the treatment of acute ankle sprains: a systematic review. Clin Sports Med. 2015;34(2):329–346. doi:10.1016/j.csm.2014.12.001
65. Foster KS, Greenlee TA, Fraser JJ, Young JL, Rhon DI. The Influence of Therapeutic Exercise after Ankle Sprain on the Incidence of Subsequent Knee, Hip, and Lumbar Spine Injury. Med Sci Sports Exercise. 2022;55(2):177–185. doi:10.1249/MSS.0000000000003035
66. Saltzman CL, Zimmerman MB, O’Rourke M, Brown TD, Buckwalter JA, Johnston R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. J Bone Joint Surg Am Vol. 2006;88(11):2366–2372. doi:10.2106/JBJS.F.00295
67. Ahn BH, Cho BK. Persistent Pain After Operative Treatment for Chronic Lateral Ankle Instability. Orthop Res Rev. 2021;13:47–56. doi:10.2147/ORR.S299409
68. Takebayashi T, Yamashita T, Minaki Y, Ishii S. Mechanosensitive afferent units in the lateral ligament of the ankle. J Bone Joint Surg Br. 1997;79(3):490–493. doi:10.1302/0301-620X.79B3.0790490
69. Michelson JD, Hutchins C. Mechanoreceptors in human ankle ligaments. J Bone Joint Surg Br. 1995;77(2):219–224. doi:10.1302/0301-620X.77B2.7706334
70. Patlan I, Ohrnberger E, Kosik KB. Pain Mechanosensitivity in Individuals With and Without a History of Lateral Ankle Sprain: a Critically Appraised Topic. Int J Athl Ther Train. 2024;2024:1–6. doi:10.1123/ijatt.2023-0048
71. Chimenti RL, Frey-Law LA, Sluka KA. A Mechanism-Based Approach to Physical Therapist Management of Pain. Phys Ther. 2018;98(5):302–314. doi:10.1093/ptj/pzy030
72. Wang Y, Li Q, Xue X, et al. Neuroplasticity of pain processing and motor control in CAI patients: a UK Biobank study with clinical validation. Front Mol Neurosci. 2023;16:1096930. doi:10.3389/fnmol.2023.1096930
73. Kosik KB, Terada M, Drinkard CP, McCann RS, Gribble PA. Potential Corticomotor Plasticity in Those with and without Chronic Ankle Instability. Med Sci Sports Exercise. 2017;49(1):141–149. doi:10.1249/MSS.0000000000001066
74. Terada M, Kosik KB, McCann RS, Drinkard C, Gribble PA. Corticospinal activity during a single-leg stance in people with chronic ankle instability. J Sport Health Sci. 2022;11(1):58–66. doi:10.1016/j.jshs.2020.08.008
75. Pietrosimone BG, Gribble PA. Chronic Ankle Instability and Corticomotor Excitability of the Fibularis Longus Muscle. J Athletic Training. 2012;47(6):621–626. doi:10.4085/1062-6050-47.6.11
76. Terada M, Bowker S, Thomas AC, Pietrosimone B, Hiller CE, Gribble PA. Corticospinal Excitability and Inhibition of the Soleus in Individuals With Chronic Ankle Instability. PM R. 2016;8(11):1090–1096. doi:10.1016/j.pmrj.2016.04.006
77. Terada M, Johnson N, Kosik K, Gribble P. Quantifying Brain White Matter Microstructure of People With Lateral Ankle Sprain. Medicine and Science in Sports and Exercise. 2018;2018:1. doi:10.1249/MSS.0000000000001848
78. Gatchel RJ, Reuben DB, Dagenais S, et al. Research Agenda for the Prevention of Pain and Its Impact: report of the Work Group on the Prevention of Acute and Chronic Pain of the Federal Pain Research Strategy. J Pain. 2018;19(8):837–851. doi:10.1016/j.jpain.2018.02.015
79. Buckenmaier CC, Galloway KT, Polomano RC, Deuster PA. Pain as a Barrier to Human Performance: a Focus on Function for Self-Reporting Pain With the Defense Veterans Pain Rating Scale. J Spec Oper Med. 2016;16(2):82–87. doi:10.55460/S27H-8173
80. Briet JP, Houwert RM, Hageman M, Hietbrink F, Ring DC, Verleisdonk E. Factors associated with pain intensity and physical limitations after lateral ankle sprains. Injury. 2016;47(11):2565–2569. doi:10.1016/j.injury.2016.09.016
81. Suttmiller AMB, Cavallario JM, Baez SE, Martinez JC, McCann RS. Perceived Instability, Pain, and Psychological Factors for Prediction of Function and Disability in Individuals With Chronic Ankle Instability. J Athletic Training. 2022;57(11–12):1048–1054. doi:10.4085/1062-6050-0605.21
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Benralizumab in Severe Eosinophilic Asthma and Chronic Rhinosinusitis with Nasal Polyps: The Real-World, Multi-Country RANS Observational Study
Le TT, Emmanuel B, Katial R, Tran TN, Kwiatek JJ, Cohen DS, Daniel SR, Cao Y, Shih VH, Melcón MG, Devouassoux G, Pelaia G
Journal of Asthma and Allergy 2024, 17:313-324
Published Date: 5 April 2024
Interaction Between Multimorbidity and Hip Fracture Surgery Leads to Excess Risk of Infection: A Danish Registry-Based Cohort Study of 92,599 Patients With Hip Fracture
Hansen CM, Gadgaard NR, Vandenbroucke-Grauls C, Hailer NP, Pedersen AB
Clinical Epidemiology 2025, 17:167-176
Published Date: 24 February 2025