Back to Journals » International Journal of Nanomedicine » Volume 19
Revolutionizing Intervertebral Disc Regeneration: Advances and Future Directions in Three-Dimensional Bioprinting of Hydrogel Scaffolds
Authors Zhang X, Gao X, Zhang X, Yao X , Kang X
Received 17 March 2024
Accepted for publication 10 August 2024
Published 21 October 2024 Volume 2024:19 Pages 10661—10684
DOI https://doi.org/10.2147/IJN.S469302
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Anderson Oliveira Lobo
Xiaobo Zhang,1,* Xidan Gao,1,* Xuefang Zhang,1 Xin Yao,1 Xin Kang2
1Department of Spine Surgery, Honghui Hospital, Xi’an Jiaotong University, Xi’An, Shaanxi, P.R. China; 2Department of Sports Medicine, Honghui Hospital, Xi’an Jiao Tong University, Xi’An, Shaanxi, P.R. China
*These authors contributed equally to this work
Correspondence: Xin Kang, Email [email protected]
Abstract: Hydrogels are multifunctional platforms. Through reasonable structure and function design, they use material engineering to adjust their physical and chemical properties, such as pore size, microstructure, degradability, stimulus-response characteristics, etc. and have a variety of biomedical applications. Hydrogel three-dimensional (3D) printing has emerged as a promising technique for the precise deposition of cell-laden biomaterials, enabling the fabrication of intricate 3D structures such as artificial vertebrae and intervertebral discs (IVDs). Despite being in the early stages, 3D printing techniques have shown great potential in the field of regenerative medicine for the fabrication of various transplantable tissues within the human body. Currently, the utilization of engineered hydrogels as carriers or scaffolds for treating intervertebral disc degeneration (IVDD) presents numerous challenges. However, it remains an indispensable multifunctional manufacturing technology that is imperative in addressing the escalating issue of IVDD. Moreover, it holds the potential to serve as a micron-scale platform for a diverse range of applications. This review primarily concentrates on emerging treatment strategies for IVDD, providing an in-depth analysis of their merits and drawbacks, as well as the challenges that need to be addressed. Furthermore, it extensively explores the biological properties of hydrogels and various nanoscale biomaterial inks, compares different prevalent manufacturing processes utilized in 3D printing, and thoroughly examines the potential clinical applications and prospects of integrating 3D printing technology with hydrogels.
Keywords: intervertebral disc degeneration, 3D bioprinting, hydrogel, novel therapy, challenges
Introduction
Lower back pain is a prevalent condition that impacts approximately 80% of the global population. Intervertebral disc (IVD) degeneration (IVDD) is recognized as the most common cause of lower back pain. Over the years, the incidence of IVDD has significantly risen, particularly with advancing age, leading to a substantial increase in disability rates. Consequently, IVDD poses a considerable economic burden on society.1,2 IVDD is a natural consequence of the aging process, characterized by a gradual decline in IVD cell viability, dehydration of the extracellular matrix (ECM), and structural abnormalities as the disease advances. The etiology of IVDD is multifactorial and encompasses various factors, such as mechanical trauma, genetic predisposition, sedentary behavior lifestyle, and certain metabolic disorders, but the exact pathogenesis is unknown. Despite decades of persistent efforts by scholars, clinical treatment is still focused on alleviating patients’ pain and reducing the incidence of disability, including physiotherapy, closed therapy, and surgery,3,4 which not only fail to cure the disease at the etiological level but also may lead to complications associated with drugs and surgery. Therefore, it is necessary to develop various novel therapies to meet the goals of tissue regeneration and targeted minimally invasive treatment of diseases.
Current Novel Therapies
Recently, many novel therapies for IVDD have been developed, including stem cell regenerative therapies, gene-targeted therapies, and regenerative alternative therapies combined with tissue-engineered hydrogels, which cover the current mainstream of novel therapies in IVDD, all of which have various advantages and disadvantages. Significant advancements in medical technology have paved the way for gene-targeted therapy in treating IVDD. This breakthrough represents a promising and thrilling opportunity for IVDD.5 It is achieved at the genetic level by foreign gene expression of key products such as ECM or inhibition of disease-causing gene expression to delay disease progression, and commonly used vectors include viral vectors, nonviral vectors or gene editing to transport specific bioactive substances. Viral vectors can efficiently replicate and proliferate in cells, and the technology is now mature, mainly including retroviruses, lentiviruses, adenoviruses, and baculoviruses, with certain safety concerns.6,7 Nearly all nonviral vectors have high safety but low transfection efficiency, including inorganic nanoparticles, natural polymeric nanoparticles, and RNA interference.8 DNA nuclease gene editing techniques have demonstrated the capability to achieve precise and efficient gene editing in normal eukaryotic cells. These techniques offer a high degree of accuracy and effectiveness in modifying specific genes within the cellular DNA of eukaryotes.9,10 However, since IVDD is an aging disease, its causative factors are multifactorial, and its pathogenesis is poorly studied. It is currently believed that IVDD is associated with inflammation, the immune response, and genetics. Indeed, the widespread adoption of gene therapy tools has been hindered by several factors. These include concerns regarding the long-term safety of gene modifications, challenges in the mass production of gene drugs, difficulties in controlling gene expression in vivo, limitations in achieving stable and long-term expression of foreign genes in vivo, low efficiency of targeted gene transfer, alterations in the biological properties of target cells, and ethical considerations. These factors collectively contribute to the limited popularity and utilization of gene therapy tools, as shown in Table 1.
 |
Table 1 Summary of Novel Therapies for IVDD |
IVD cell loss is a significant pathological feature of IVDD. Restoring the lost IVD cells through various approaches, such as inhibiting apoptotic cell death16 or cell transplantation,11 has been recognized as a direct and effective strategy for IVDD treatment. This approach involves introducing autologous or allogeneic adult cells or stem cells from the patient into the degenerated IVD in a targeted manner. The goal is to replenish the lost IVD cells, enhance the content of proteoglycans and collagen, and ultimately restore the tissue structure and biomechanical function of the IVD.12 Commonly used cell types for transplantation in IVDD treatment include bone marrow mesenchymal stem cells (BMSCs), adipose mesenchymal stem cells, nucleus pulposus (NP) mesenchymal stem cells, umbilical cord mesenchymal stem cells, and induced pluripotent stem cells.13 Transplanted cells in the IVD face numerous challenges due to the harsh microenvironment. Factors such as high osmotic pressure, mechanical load, nutritional deficiency, low oxygen levels, and acidic conditions can significantly impact the viability, proliferation, and differentiation of these transplanted cells.17,18 Moreover, the natural aging process of cells and the possibility of undirected differentiation of stem cells lead to low transplantation efficiency, limiting cell-based IVDD therapy (Table 1).
Exosomes, or extracellular vesicles (EVs), are small vesicles (40–100 nm) that play a role in cell regeneration and apoptosis. They carry genetic and functional information from the parent cell and can act as carriers of biomolecules with specific functions, making them potential therapeutic vehicles for targeted drug delivery and regenerative medicine.19,20 Exosomes replicate the genetic and functional information of the parent cell and act as carriers of small molecules, playing a therapeutic role in delivering biomolecules with specific functions. In short, EVs are stable, nonimmunogenic, more convenient to store and transport and have obvious advantages.14,15 However, the extraction, administration method, dose, source, growth state, and culture conditions of EVs affect their biological functions,13 and the currently extracted and stored EVs are not yet ready for commercialization, with cumbersome extraction steps, high costs, and storage difficulties. The biological mechanisms of exosomes are not fully understood, and their safety has yet to be verified (Table 1).
Tissue engineering, which was first proposed in 1987 at the Washington Science Foundation and officially confirmed in 1988, applies engineering principles to develop biological substitutes for improving and restoring the function of injured IVDs.21 Tissue engineering has become a bridge for the abovementioned new therapies due to its unique advantages, demonstrating greater feasibility and application prospects. In recent years, there has been rapid development in hydrogel technology for tissue engineering. Hydrogels are hydrophilic network polymers that contain a significant amount of water and can be used in environments that require large deformations. Due to its adjustable physical properties, it has been widely used in clinical practice. The commonly used techniques for manufacturing hydrogel products are molding and casting, but these techniques have difficulty producing hydrogel products with complex geometries. Material scientists have been exploring various bioprinting processes for hydrogels. 3D printing, based on the principle of additive manufacturing, utilizes digital model files and adhesive materials to construct objects layer by layer. It has emerged as a promising solution for addressing the global organ shortage by printing artificial organs. Additionally, 3D printing is an effective method for preparing gel-like drug formulations of different shapes and colors, enhancing medication compliance for pediatric patients in clinical settings.22 In recent years, 3D printing technology has made significant advancements in the field of spinal surgery. The use of 3D printing-assisted manufacturing enables the construction of IVD structures with complex 3D microchannels, meeting the specialized requirements for the rapid production of multifunctional artificial IVD bodies. Specifically, the printing of vertebral body bone models, including lesion models, has proven to be a simple, cost-effective, and practical approach. These models have become valuable tools for clinical education, auxiliary diagnosis, doctor-patient communication, surgical planning, and more precise surgeries. Furthermore, the design and production of navigation templates based on the printing of vertebral body bone models have shown significant clinical benefits in guiding internal fixation screws during surgery.23 This article aims to showcase the recent advancements in hydrogels through 3D printing technology in the treatment of IVDD, to provide valuable insights and references for IVDD management and to offer guidance for the optimal utilization of 3D printing technology in various clinical domains.
Hydrogel Scaffolds: Composition, Structure, and Customization in Tissue Engineering
The development of hydrogel tissue engineering has emerged as a promising and effective approach for treating IVDD. Hydrogels are three-dimensional (3D) polymer networks that can absorb and retain a significant amount of water. They possess adjustable physicochemical properties, making them suitable for various conditions and applications. Hydrogels have found extensive use in the biomedical field, including studies on physiological and pathological mechanisms, tissue regeneration, and disease treatment.24–26 The characteristics of hydrogel scaffolds are influenced by several factors, including the material composition and concentration, crosslinking method and density, and manufacturing techniques. The choice of materials such as collagen, gelatin, or polyethylene glycol (PEG)-based hydrogels can result in different scaffold structures. For example, collagen-based hydrogels often exhibit fibrous structures, while gelatin-based hydrogels may have macroporous structures. On the other hand, PEG-based hydrogels can possess nanoporous structures. These structural variations allow for the customization of hydrogel scaffolds to meet specific requirements in tissue engineering applications.27 The mechanical properties of hydrogels can be modified through physical or chemical crosslinking methods. Physical crosslinking, which involves hydrophobic interactions, hydrogen bonding, and polymer entanglement, typically leads to lower mechanical strength. On the other hand, covalent crosslinking, achieved through processes such as free radical polymerization or enzyme-induced crosslinking, can impart higher mechanical performance to hydrogels.28,29 Similarly, a higher crosslinking density contributes to denser structures and enhanced stiffness. It is important to note that manufacturing techniques such as micropatterning30 and 3D bioprinting31 also influence the characteristics of hydrogels and determine their applications. The interaction between cells and hydrogels is a complex and dynamic process that plays a crucial role in tissue physiology, including cell proliferation32 and migration,33 as well as in pathological processes such as cell apoptosis.34 Hydrogels have a variety of physical and chemical advantages, such as high strength, self-healing, elastic conductivity, synergistic antibacterial activity, and stimulus responsiveness. Therefore, it is essential to have a comprehensive and in-depth understanding of these cell-hydrogel interactions. Hydrogels have been extensively studied and utilized in various biomedical applications due to their diverse physicochemical, biological, and structural properties. They have been used in aesthetic medicine35 as 3D models for different diseases, such as corneal disease models,36 neurodegenerative disease models,37 and inflammatory bowel disease.38 In addition to their use in tissue engineering, hydrogels have been utilized as carriers for enzymes, enabling biocatalytic monitoring and regulation of enzymatic bioactivity39 (Figure 1). This application has facilitated research on disease mechanisms and targeted drug screening, providing a valuable tool for studying and understanding various biological processes.
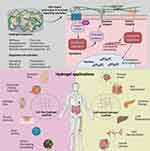 |
Figure 1 Interactions between hydrogels and extracellular matrix and their clinical implications are depicted in the schematic diagram. Hydrogels influence cellular biology through signal cascade reactions triggered by their physicochemical properties, and they have extensive applications in various clinical fields. Reproduced from Cao H, Duan L, Zhang Y, et al. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6(1):426. http://creativecommons.org/licenses/by/4.0/.40 |
Hydrogels provide a favorable microenvironment for cell survival and attachment of small molecules. This microenvironment facilitates enhanced intercellular signaling, promoting cell proliferation and maintaining cell lineage commitment.41 Under specific light sources, hydrogels exhibit tunable mechanical properties. Hydrogels can be processed using various techniques, including 3D printing, bioprinting, self-assembly, and microfluidics. These techniques allow for the fabrication of hydrogel constructs with controlled structures, providing a 3D microenvironment that supports cell proliferation and tissue regeneration. This enables the creation of complex and customized tissue constructs that closely mimic the native tissue architecture, promoting better integration and functionality in tissue engineering applications. Hydrogels possess excellent biocompatibility and biological activity, along with higher solubility and lower antigenicity. Importantly, the mechanical properties, pH, and photolysis time can be adjusted to meet the requirements of transplantation therapy for different diseases.42 Hydrogels can also induce MSCs to produce more ECM, proteoglycans, collagen type II, and glycosaminoglycans, ensuring the necessary nutrient supply for cell growth and proliferation, thus facilitating the repair of IVD tissue. Additionally, hydrogels can serve as carriers for stem cells, osteogenic proteins, or transforming growth factors, maintaining the stability of the transplanted molecules and maximizing their potential in inducing osteogenic differentiation and reducing inflammatory reactions. This, to some extent, addresses the limitations of previous transplantation therapies.43 By adjusting the fiber spacing or porosity, the mechanical properties of hydrogel scaffolds can be optimized to achieve a balance between compression and tension, as well as dynamic modulus. This optimization allows for the generation of scaffolds with tailored mechanical properties that fall within the physiological range of spine motion segments. These findings provide a solid theoretical foundation for the use of hydrogels in transplantation therapy for IVDD (Figure 2). Hydrogels offer promising potential in this field, as they can provide a suitable microenvironment for tissue regeneration and contribute to the restoration of disc function.
As an Ideal Printing Material
Microhydrogel
At present, IVDD therapies that involve the local injection of various biologics are showing promise. However, their application is hindered by limitations such as the harsh external environment and inefficient carriers. Hydrogels, with their adjustable biological properties and rapid in situ curing ability, are well suited for IVD regeneration. Microgels, in particular, exhibit distinct properties and functions compared to macroscopic materials.44 Additionally, different particle sizes of microgels can have varying modular properties,45 which can be advantageous for tailoring the mechanical and biological characteristics of hydrogel-based therapies for IVDD. Microgels are utilized to construct aggregates in the form of microscaffolds, which facilitate cellular infiltration. They can also be embedded within bulk hydrogels to create multilevel structural materials.46 The biological, mechanical, and structural properties of microgels can be tailored through factors such as polymer composition, crosslink density, and fabrication methods. This versatility allows for the customization of microgels to meet specific requirements in terms of their intended application, enabling the development of hydrogel-based materials with desired properties for various biomedical applications. In addition to their tunable properties, microgels can also be programmed to undergo dynamic processes triggered by internal stimuli (such as temperature) or external stimuli (such as light).44 This programmability enables precise control over the release of biomolecules and drugs, making microgels valuable carriers for therapeutic delivery. While hydrogel-based delivery systems alone may not fully replicate all the properties of IVD tissues, the integration of 3D printing technology allows for the customization of IVD structures. This combination provides a temporal and spatial guarantee for the delivery of therapeutic agents, enhancing the potential of hydrogel-based systems for IVD regeneration and treatment.
Microspheres
Microspheres are particulate dispersion systems formed when a drug is dispersed or adsorbed in a polymer or polymeric matrix. Compared to microgels, microspheres have the advantage of slow long-term drug release (Figure 3). Microspheres are produced by various methods, including double emulsion solvent evaporation, electrospray, microfluidics and supercritical fluids.
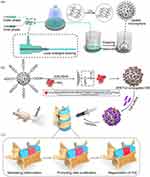 |
Figure 3 GelMA microsphere fabrication process, including (A) crosslinking of microspheres, (B) modification of microspheres with loading of biologics, and (C) use for IVDD treatment in rats. Reprinted with permission from Bian J, Cai F, Chen H, et al. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21(6):2690–2698. Copyright 2021 American Chemical Society.47. |
The double emulsification solvent evaporation method is a commonly used technique for manufacturing hydrophilic polymer microspheres. It is favored for its ease of handling, low cost, rapidity, and reproducibility. This method involves forming a primary water-in-oil emulsion, followed by a secondary oil-in-water emulsion. The solvent is then evaporated, resulting in solid microspheres. This versatile technique allows for the encapsulation of various substances within the microspheres, making it suitable for drug delivery and other biomedical applications.44 The controlled release of drugs from microspheres depends on the rate of degradation and is regulated by the internal monomer ratio or molecular weight.45 In addition, varying the solvent phase viscosity, homogenization rate and emulsifier concentration allows for the customization of microspheres with different particle sizes.46 Polyester amide (PEA) may be an effective delivery material, and it has been found that the in vivo degradation products of PEA (α-amino acids and diols and adipic acid) are minimally harmful to cells and tissues and have good in vitro cytotoxicity and in vivo biocompatibility.48,49 Long degradation and release profile of PEA microspheres with immunoprivilege against IVDs.50,51 In addition, it is able to drive acquired and innate immune cells to the site of the lesion for immunization and can be safely and sustainably released in the body after loading.52,53 PEA microspheres have been found to be effective carriers for the prolonged release of small molecule drugs, including corticosteroids. In vitro studies have demonstrated that these microspheres can sustain the release of celecoxib for up to 80 days.54
Electrospraying (ES) is liquid atomization under high pressure by a formulation consisting of a polymer and a drug. The formation of Taylor cones is driven by the electrostatic force, and when the cohesive forces of these surface tensions are surpassed, it leads to the generation of uniform microsphere particles with particle sizes ranging from 10 to 100 μm.55,56 ES requires less solvent and allows for finer control of microsphere particle size and higher encapsulation efficiency, and it leaves less residue. In addition, by designing and customizing the core features of microspheres that can be prepared to meet the needs of the particles, compared with the traditional method, ES is prone to produce opposite properties. Rapid synthesis of injectable microspheres with controllable and homogeneous particle sizes for cellular and drug carriers is achieved by the ES method. However, equipment factors (eg, applied voltage) and solution factors (eg, polymer concentration) affect the properties of microspheres,55 and precise and controlled condition factors need to be developed.
Microfluidics enables the preparation of microspheres with high monodispersity, precise tunability of structure, and excellent encapsulation efficiency.57 Droplets are formed in microfluidic devices with micrometer-sized channels (eg, T-shaped channels) that combine immiscible continuous phases at high shear, causing microspheres to be formed at the intersection of the channels.58 Among them, T-shaped channels produce highly monodisperse microspheres.59 It was shown that Mg@PLGA microspheres functionalized with magnesium metal, prepared by microfluidic coemulsification, possessed a higher Mg encapsulation efficiency (87%) than that of ultrasonically homogenized microspheres (50%),60 and the photothermal effect of microspheres fabricated by this method was determined by the Mg content, independent of size and structural features.61 However, the droplet generation and gelation steps of this method are relatively long, making the cell viability of microsphere-encapsulated cells relatively low and preventing large-scale production. Microfluidics greatly reduces the complexity of operations and reagent consumption in single-cell analysis while enabling the integration of multiple functionalities.62 Human brain organoids can be generated based on microfluidics technology.63 Additionally, the flexibility and selectivity of microfluidics technology, combined with the hydrogel degradation method using alginate and methacrylated gelatin, have resulted in the formation of multivalve structures of multimolecular microparticles with excellent adhesive capabilities. When encapsulating dexamethasone, microparticle delivery systems exhibit sustained drug release properties and anti-inflammatory effects.64
Supercritical fluid is the formation of microspheres after passing a polymer solution through a nozzle into a low-pressure environment for rapid depressurization.65 Currently, the key process parameters for microsphere preparation include the pressure at the nozzle, nozzle diameter, solution concentration, and temperature. The resulting aerogel microspheres prepared using supercritical fluid exhibit uniform particle size and morphology, and their porous structure imparts suitable aerodynamic properties.66 Recently, supercritical carbon dioxide (scCO2) has attracted much attention due to its lower critical temperature (31.1°C) and critical pressure (73.8 bar) to solubilize some specific polymers, and the interactions of scCO2 with the active ingredient, polymeric materials and solvents should be considered in microsphere fabrication.67 In a one-pot approach using scCO2 solution, polyethylene oxide and poly (heptafluorodecyl ester) with photosensitive groups were utilized to produce drug-carrying microspheres of HEMA (hydroxyethyl methacrylate) through free radical dispersion polymerization. These microspheres exhibited a spherical morphology with a particle size ranging from 250 to 350 nm. Under UV conditions, the stabilizers present on the surface of the microspheres were destroyed, causing the microspheres to swell up to 2.1–3.6 µm. This swelling allowed for the continuous release of the drug for approximately 10 days.68
Liposome Derivatives
In addition to microspheres, other nanosized hydrogel-based particles (eg, liposomes) can serve as drug delivery systems for IVDD (Figure 4). Liposomes are spherical lipid bilayers with a diameter of 50–1000 nm that can be made of the same material as cell membranes and contain at least one lipid bilayer around a hydrophilic core, which can be used as a delivery vehicle for bioactive compounds69 (Figure 5). HA microgels were incorporated into lecithin-based microemulsions to create microgel liposomes (M-i-L) with excellent loading efficiency. These M-i-L structures comprise a core of HA microgel surrounded by a lipid membrane shell, resembling the structure of skin keratinocytes. The lecithin-based microemulsion effectively stabilizes the dispersion of HA microgel particles, preventing phase separation or aggregation. Furthermore, the high concentration of natural moisturizing factor in M-i-L helps maintain the water content of the hydrogel, keeping it virtually unchanged.70 It is shown that this system plays a crucial role in preventing hydrogel water loss, which is very helpful in maintaining the highly hydrated structure of IVDs. Liposomes have the remarkable ability to release encapsulated drugs into cells through various mechanisms, such as adsorption, fusion, endocytosis, or lipid transfer. The release kinetics of liposomes can be controlled by factors including liposome composition, pH, osmolarity, temperature, and other parameters11,71 (Figure 4). On the other hand, the multicompartmental drug delivery platform ensures the corelease of several drugs within the same carrier for more effective and safer therapeutic outcomes. Novel multicompartmental carriers were developed by assembling liposomes with a protective poly (dopamine) shell and a PEG layer, along with temperature-sensitive poly (N-isopropylacrylamide-co-acrylic acid) microgels. This unique combination of components results in a multifunctional carrier system with multiple compartments. PEG on the carrier surface prevented protein adsorption regardless of the presence or absence of shear stress (Figure 4). In addition, the multicompartmental carrier did not exhibit inherent toxicity.72 This multicompartmental carrier delivery mode offers a therapeutic strategy with great potential.
 |
Figure 4 Crosslinking process for the fabrication of liposomes. (A) and (B) PDA-HAMA-coated modified liposomes; (C) local injection of adhesion and release biologics for treating disease. Reproduced from Lin F, Wang Z, Xiang L, Deng L, Cui W. Charge-Guided Micro/Nano-Hydrogel Microsphere for Penetrating Cartilage Matrix. Adv. Funct. Mater. 2021, 31, 2107678. © 2021 Wiley-VCH GmbH.73 |
Dendritic Polymers
Dendritic polymers are novel drug delivery systems in which drugs can be physically encapsulated or covalently coupled to dendritic macromolecules through noncovalent interactions, and the encapsulation and release efficiency of the drug can be modulated by changing the surface and structure of the dendritic macromolecule.74 Polyamidoamine dendritic polymers are ideal building blocks for the preparation of hydrogels due to their highly functionalized surfaces and well-defined, highly branched structures,75 which has led to their increasing popularity in the fields of drug delivery, gene therapy, etc. In the study of Wang et al,76 micrometer-sized dendrimer hydrogels (μDHs) were prepared using the water-in-oil inverse microemulsion method combined with highly efficient aza-Michael addition. These μDHs demonstrated pH-dependent degradation behavior. The unique structure of μDHs offers a programmable platform for advanced drug delivery and release, allowing for precise control over the release kinetics and targeting of drugs. On the other hand, polyamidoamine dendritic polymers also promote cytokine and chemokine release and inhibit proinflammatory factor secretion,77,78 a property that is expected to be used for the treatment of inflammatory responses in the IVDD microenvironment (Figure 5).
Multiwalled Carbon Nanotubes
Multiwalled carbon nanotubes (CNTs) are widely utilized in biomedicine due to their distinctive atomic structure, high surface area-to-volume ratio, and exceptional electronic, mechanical, and thermal properties.79 These CNTs serve as innovative nanocarriers for drug delivery in the biomedical field. Additionally, they can reinforce hydrogels and enhance the mechanical strength of the system.80 For example, enhanced CNT-GelMA mixtures were used to create 3D structures loaded with cells. The number of CNTs can modulate the mechanical properties of hybridized materials, making them suitable for different types of tissue engineering applications. Notably, CNTs do not decrease the GelMA hydrogel porosity. MSCs easily diffuse and proliferate in CNT-GelMA hybrid microgels,81 which is very meaningful for hydrogel regeneration for repairing IVDs. The highest electrosensitivity of hybridized hydrogels was conferred when the CNT mass fraction was 35%.82 Notably, cytotoxicity and aggregation are key factors affecting the application of CNTs.83 Conductive double cross-linked microgel-carbon nanotube composites (DX-MG/CNT) were synthesized by combining vinyl-functionalized pH-responsive microgel particles and CNTs through a free radical reaction. The microgel particles served as dispersants for CNTs and acted as macroscopic crosslinkers. Increasing the concentration of CNTs resulted in improved ductility and modulus of the system. Additionally, DX-MG/CNT exhibited high cellular activity, with MSCs reaching 99% viability and sustained metabolic activity for at least 7 days.84 This injectable and biocompatible DX-MG/CNT system may have IVD tissue repair potential (Figure 5).
In summary, we have listed several common microhydrogel delivery systems currently available, including their fabrication methods, their advantages and disadvantages, and the obvious advantages of microspheres for slow long-term drug release compared to microgels. Previous research has demonstrated that drugs encapsulated within or attached to the surface of nanoparticles are released through processes such as dissolution and diffusion. Nanoscale biomaterials are easily adhered to and absorbed by cells.85 In addition, nanocarriers are widely used in various biomedical applications due to their in situ degradability and their ability to not induce inflammatory and immune responses.86 We believe that the fabrication of microgels or microspheres through a special process and then 3D printing to create structurally specific material scaffolds for IVDD treatment is a very promising strategy.
3D Bioprinting Technology
3D printing has emerged as a prominent manufacturing technology in healthcare and medicine, finding applications in various fields, such as dentistry, regenerative medicine, tissue engineering, medical devices, anatomical models, and drug formulations.87 It offers commercial medical products and platforms for emerging research areas such as tissue and organ printing. Hydrogels, known for their biocompatibility, play a crucial role in tissue engineering and are widely used in this field.
Although hydrogels can be adjusted to create artificial IVD scaffolds with desired properties, there are challenges in achieving perfect in vivo defect filling and long-term stability. Additionally, the presence of micro- or nanosized hydrogel particles can hinder oxygen and nutrient supply, as well as impede cell proliferation and differentiation. Bio3D printing offers unique advantages, such as enhanced properties of individual components, high-resolution cell deposition, scalability, and cost-effectiveness. This makes it an ideal approach for creating implants that conform to the human body’s shape. Furthermore, the interconnected pores and high surface area ratios of 3D-printed delivery systems facilitate cell adhesion, proliferation, intercellular communication, and gas and nutrient exchange.88 Therefore, bio3D printing technology is well suited for constructing multifunctional artificial IVD structures.
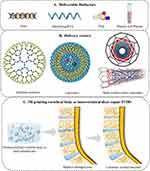
Currently, no single bioprinting technology can effectively produce synthetic tissues of all sizes and complexities. Inkjet printing (IJP), extrusion printing (EXP), laser-assisted printing (LAP), and stereolithography (SLA) each have advantages, disadvantages, and limitations. However, the overall manufacturing process involves preprocessing, processing, and postprocessing steps. Initially, natural IVD tissues are imaged and reconstructed as 3D models using computed tomography, magnetic resonance imaging, or ultrasound. These models are then converted to the STL file format compatible with printers.89 Bioinks are subsequently used to fabricate artificial IVDs using bio3D printers, and postprocessing involves artificial IVD structure maturation supported by the bioprinter. For a concise comparison of these bioprinting methods, see Table 2.
 |
Table 2 Comparison of Several 3D Printing Technologies Used to Build Artificial IVDs |
IJP
IJP is a noncontact bio3D printing technology that offers several advantages, including low cost, fast printing speed, and high cell survival rates ranging from 80% to 90%,119 and the most common type of printing is based on piezoelectric and thermal technology. Before printing, a hydrogel prepolymer solution loaded with cells acts as a bioink, which is loaded into a cartridge and connected to the printer head. The ideal bioinks consist of the patient’s cells and biomaterials that closely mimic the properties of the target tissue.120 During printing, the head is compressed and deformed by an encoded actuator to produce droplets of controlled size, and this deformation capacity is generally small.92 Gao et al22 found that poly ethylene glycol diacrylate (PEGDA) cartilage-based tissue-engineered scaffolds printed with thermal IJP achieved precise distribution and alignment of hMSCs and that the scaffolds showed natural strips of tissue structure and desirable mechanical properties. The resolution of the IJP is wide (1–500 μm), depending on the bioink properties and nozzle size.97 Bioinks must meet strict fluidic property requirements, such as viscosity and surface tension, to ensure successful cell deposition. Higher cell density in bioinks can increase viscosity, and the heat generated during printing can cause cell precipitation. These factors can potentially lead to outlet clogging, thereby limiting the application of bioinks.103
To overcome this limitation, Daisuke et al121 prevented cell settling at the nozzle by piezoelectric film vibration and mixing motion jointly driving the formed droplets, and this method stabilized cell viability over time. On the other hand, microreactor IJP polymerizes poly (3,4-ethylene dioxythiophene), poly (styrene sulfonate), and ionic liquid in air to form an aerogel, which can show homogeneous gel properties and potential for free patterning on a glass substrate and effectively prevent outlet clogging. In addition, aerogels can be transformed into specific 3D structures without molding, and the structures are highly conductive and biocompatible,122 which contributes to the large-scale scalable patterning of conductive films and hydrogels. The volume size of dried particles is linearly correlated with the concentration of the jet solution, and Shintaroh et al123 combined the drying process with IJP to create uniform and size-controlled ALG particles by varying the solution concentration. However, conventional drop-on-demand printers cannot store enough bioink to print large tissues or organs. To improve these limitations while expanding the cartridge capacity and increasing its sensitivity, printer heads with multiple nozzles have been developed to increase the printing speed and structural dimensions, but the poor resolution and slow speed limit their fidelity and clinical applications. Ferris et al124 utilized GG microgel produced in a standard cell culture medium as a bioink for cell deposition. They employed a microvalve deposition system and a multinozzle piezoelectric IJP, which met the strict fluidic requirements for multinozzle printing. This approach allowed for precise control of live cell positioning and introduced innovative design concepts for fabricating multicellular structures and functional tissues.
Ideal IVD scaffolds should match all the properties of natural tissue components, structure, mechanical stress, and allowable cell behavior. Cellulose nanofibers (CNFs) enhance 3D structural mechanical properties and can be applied to IVD tissue regeneration with stringent mechanical property requirements by cross-linking modification. Bioinks that combine CNF with saponified GelMA/ALG and fibrinogen composite hydrogels can be effectively utilized with IJP technology. This allows for the rapid and reliable use of these bioinks. By employing IJPs, it is possible to create high-fidelity and stiffness-controlled hydrogel scaffolds loaded with cells. This approach ensures excellent printing performance and structural resolution without compromising the bioactivity and proliferation of the cells being loaded.125 Consequently, it becomes feasible to fabricate high-resolution multiscale delivery systems at high speeds using IJP. In addition, Dufour et al115 combined melt electrowriting with IJP to generate hyaline hybrid cartilage scaffolds, a system that closely resembles immature synovial joint tissue and can be successfully loaded with a large number of cell aggregates. Importantly, melt electrowriting was able to orient the growth of spontaneously generated cell aggregates within the scaffolds, which could provide tensile enhancement, and after a period of incubation, the scaffolds had a greatly increased compressive stiffness and displayed a tension-compression nonlinear curve similar to that of natural tissues. This IJP-modified strategy provided a possible construction strategy for ideal artificial IVD scaffolds (Figure 6).
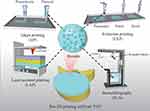 |
Figure 6 Several printing methods are used to print artificial IVDs, including IJP, EXP, LAP, and SLA. Reproduced from Gao XD, Zhang XB, Zhang RH, et al Aggressive strategies for regenerating intervertebral discs: stimulus-responsive composite hydrogels from single to multiscale delivery systems.J Mat Chem B. 2022;10(30):5696–5722, with permission from the Royal Society of Chemistry.126 |
EXP
EXP, an advanced modification of inkjet printing, is a highly popular bio3D printing technology. It enables higher cell density in printed structures, although it typically offers lower resolution (greater than 100 μm) and slower print speeds compared to other techniques,127 which allows for less fidelity in cell patterning and tissue structure. EXP typically dispenses bioink via a pneumatic pump or a mechanical screw plunger, which when applying continuous force can print uninterrupted fine cylindrical thin lines of controlled size.93 In addition, the wide range of biomaterials that can be used for EXP makes it possible to print hydrogel prepolymers of different viscosities and aggregates with high cell densities. However, strong mechanical pressure can damage the cells, decreasing cell viability.61 Li et al128 used a cyclic heating and cooling treatment to prepare semi-IPN bioinks with excellent shear-thinning properties, which dramatically improved cell activity while ensuring the high resolution of the scaffolds. Sahai et al129 developed CS-Gel-ALG composite hydrogels that exhibited excellent characteristics for biomedical applications, including tissue engineering and drug delivery. These hydrogels were highly porous, possessing optimal porosity and suitable mechanical strength. Furthermore, they demonstrated the ability to support chondrocyte differentiation, further highlighting their potential in the field.
EXP has demonstrated good compatibility with stimuli-responsive hydrogels of varying viscosities. Several researchers have proposed innovative design concepts for IVD tissue engineering scaffolds. Photocrosslinkable hyaluronate hydrogels have encountered challenges in terms of their formation and mechanical strength. These limitations arise from the insufficient substitution of photoactive groups on the hyaluronate backbone and the inhibitory effect of oxygen. Zhang et al130 developed mediated hyaluronic acid/thiol-terminated poly (ethylene glycol) hydrogel scaffolds using EXP and photopolymerization reactions. These scaffolds address the oxygen inhibition effect, are noncytotoxic, and promote cell adhesion. However, it is crucial to ensure that the bioink possesses shear-thinning properties to ensure the continuous extrusion of a cylindrical filament. Additionally, high shear stress on the nozzle tip wall and prolonged printing time can potentially impact cell viability. Lin et al131 introduced a novel approach for hydrogel 3D printing called multidimensional microvibration-assisted printing. This method utilizes a piezoelectric ceramic-driven compliant mechanism to generate multidimensional microvibrations. These microvibrations effectively reduce the shear stress and viscosity of hydrogels, promoting cell adhesion. This technique improves printing resolution and speed, making it a valuable reference for the rapid printing of high-viscosity bioinks.
Currently, there is a lack of research reports on the use of EXP in constructing artificial IVD structures. However, the extensive application of EXP in constructing drug delivery systems, as well as in addressing cartilage and bone defects, provides valuable insights for IVD regeneration. For example, Moien et al132 combined a composite hydrogel formed by cellulose nanocrystals and cellulose nanocrystal suspension/methacrylamide with small molecule antibiotics, silver nanoparticles and proteins to produce a multifunctional filamentous dressing by EXP technology, where the number of EXP filaments determines the release profile of the agent. At the same time, the application of dressings improves granulation tissue formation and different levels of vascular density, contributing to personalized wound healing. Customized porous structures in scaffolds by 3D printing technology can overcome the limitations of hydrogel particles in multiscale delivery systems. Li et al133 developed a macroporous hydrogel scaffold using extrusion-based low-temperature 3D printing. This scaffold was created through horseradish peroxidase (HRP)-mediated crosslinking of silk fibroin (SF) and tyramine-substituted gelatin (GT). The scaffold exhibited remarkable structural stability, mechanical properties, and a controlled degradation rate. It facilitated the differentiation of MSCs into cartilage-like structures and promoted articular cartilage regeneration in a rabbit cartilage defect model. This research provides a promising approach to meeting the structural requirements of an ideal artificial IVD. Ragab et al134 utilized extrusion-based 3D printing (EXP) to produce cellulose nanofiber/polyvinyl alcohol 3D scaffolds through a freeze-drying technique. These scaffolds demonstrated a significant increase in Young’s modulus when mineralized in situ with calcium superphosphate. The presence of functional groups, strong hydrogen bonding, and effective cross-linking between cellulose nanofiber/polyvinyl alcohol molecules contributed to this enhancement. By controlling the polymer concentration, these nanofibrous scaffolds achieved a biocompatible material suitable for skin repair. This research highlights the potential of EXP in fabricating biocompatible scaffolds for skin regeneration. Tamo et al116 filled CNF into a CS gel precursor solution, and the functional hydrogel scaffolds developed by optimizing 3D printing parameters exhibited excellent mechanical properties, anisotropic microstructure and supported high cell viability, providing a new perspective for refining the IVD regenerative multiscale delivery system (Figure 6).
LAP
LAP, or laser-induced forward transfer (LIFT), is a technology derived from laser direct writing and laser indirect-induced transfer methods. It offers several advantages over other techniques. First, LAP enables precise printing of microgels with different viscosities and accommodates a wider range of bioinks. This capability is crucial for constructing 3D hydrogel structures that closely resemble natural IVD tissue-loaded cells. Additionally, LAP allows for the printing of any desired number of cells onto the substrate, utilizing rapid pulse repetition rates to efficiently generate large cell structures. Moreover, the bioink is not in direct contact with the nozzle, eliminating issues related to shear pressure and clogged outlets.135–137 Furthermore, the noncontact nature of this printing method minimizes mechanical damage to cells. Compared to inkjet printing, LAP results in minor cell damage and higher cell viability. This is primarily due to the avoidance of thermal damage caused by nanosecond laser stimulation, which can lead to cell death.94 Previous studies have shown that the formation of cell-filled ALG microspheres from an aqueous solution of ALG gel precursor and CaCl2 using the LIFT technique and the formation of a thick gel film during droplet descent significantly increased fibroblast activity, but at the same time, this impeded the diffusion of nutrients and oxygen through the medium, resulting in a decrease in cell viability, and that cell viability decreased with increasing laser fluence or ALG concentration.104 In contrast, skin cell lines such as fibroblasts and keratinocytes, as well as hMSCs, have shown resistance to laser damage and exhibit high survival rates in LIFT. Moreover, LIFT does not induce the differentiation of hMSCs and effectively transfers them into the hydrogel with precision.138 Therefore, LIFT holds great promise as a tool for creating artificial 3D-printed scaffolds that facilitate MSC regeneration, offering potential benefits for the development of delivery systems for artificial IVDs. However, the high cost of laser diodes with high resolution and intensity, as well as the complex and cumbersome operation of the printing equipment, pose significant challenges to the widespread clinical application of LIFT (Figure 6). These factors make it difficult to control the production process.
SLA
SLA, or stereolithography, is a widely used 3D bioprinting technology that involves the deposition of continuous layers of easily polymerizable photosensitive material to construct complex scaffolds. It utilizes SLA printers, which employ a digital light projector to cure the bioink layer by layer. With a precreated pattern generated by a computer program, each layer is printed for a specific duration. By vertically moving the table, complex structures can be printed, significantly simplifying the control of the printing process.92 SLA can control hydrogel synthesis at ultrahigh resolution (27 μm), enabling hydrogel scaffolds to resemble natural tissues.139 In a recent study by Christina et al,106 they introduced innovative bioresins that are compatible with SLA technology. These resins were used to fabricate filamentary hydrogel scaffolds with an ultrahigh resolution of less than 8 μm in width. These scaffolds demonstrated the ability to support cell adhesion and proliferation. The ultrahigh resolution, similar to the size of cells, opens up possibilities for the precise distribution of individual MSCs and the targeted placement of biokines in regenerative IVD delivery systems. Furthermore, this technology allows for precise regulation of hydrogel porosity, which plays a crucial role in cell proliferation and differentiation.140 Recently, several novel SLA improvement techniques have been involved in hydrogel complex network construction. For instance, Johnson et al141 employed the continuous liquid interface production technique to create square pyramidal continuous liquid interface production microneedles. These microneedles, composed of trimethylolpropane triacrylate, polyacrylic acid, and photopolymerizable derivatives of polyethylene glycol and polycaprolactone, were capable of puncturing mouse skin and releasing the fluorescent drug rhodamine. This technique allows for precise control over parameters such as size, shape, aspect ratio, and spacing, enabling the rapid and efficient fabrication of ideally shaped delivery systems. In contrast, the encoded microstereolithography process utilizes parameters that control the phase transition temperature of thermosensitive pNIPAAM microgels142 This technique is fast, high-resolution, and scalable, but the fabrication process is relatively complex. Furthermore, Grigoryan et al143 recently developed an open-source SLA device that enables the construction of 3D tissue structures in a layer-by-layer process. This device utilizes a digitally tunable virtual photomask projection and a 405 nm blue light source. These novel fabrication processes significantly enhance SLA resolution and reduce printing time.
PEGDA has good mechanical properties and stability and cures rapidly at room temperature and in the presence of photoinitiators and UV light, making it the most commonly used material for SLA.144 PEGDA hydrogel scaffolds fabricated using SLA exhibit comparable thermal and chemical properties to UV-cured hydrogels. However, they possess superior compressive strength, tensile stiffness, and hydrophilic properties.139 In addition, in biocompatible PEGDA hydrogels printed by maskless SLA, the surface of the structure binds to adhesion proteins of fibronectin cells, potentially inoculating MSCs precisely into the substrate pattern.145 On this basis, SLA-made ascorbic acid-containing PEGDMA hydrogels in a structure-specific delivery system and the combination of honeycomb and annular tablet gels may exhibit higher release rates.146 There is a need for a future method to mass-produce biocompatible hydrogels, enabling the creation of solid hydrogel models of several centimeters in size within minutes. This would greatly minimize structural deformation and cellular damage caused by prolonged exposure to environmental stresses. The capability of hydrogel networks to sustain high cell viability and maintain cultured material input at depth in large models147 provides a basis for fabricating large tissue engineering models.
SLA, as the state-of-the-art technology for 3D printing, is being gradually improved in regenerative engineering research. Recently, Tang et al148 prepared a multiscale delivery system with a customizable regular macroporous network structure by SLA of a mixture of CNFs and PEGDA. It supports cell activity and proliferation properties and promotes cartilage-like differentiation of MSCs, which contributes to the design and construction of artificial IVDs. Interestingly, the amount of CNFs modulates the shape integrity, porosity and compression modulus of the scaffolds but does not affect the transparency, homogeneity and shear-thinning properties of the scaffolds. Experiments have shown that NIH 3T3 cells can spread tightly on scaffolds and undergo good proliferation and differentiation.149 In contrast, the incorporation of tendon ECM into PEGDA bioink can enhance the swelling rate and porosity of the hydrogel. This, in turn, significantly improves the proliferation and biomineralization of MSCs. Luo et al150 used SLA to make bone defect repair scaffolds with a porous structure and high mechanical strength by combining ECM with PEGDA, which resulted in the upregulation of the expression of RNAs that regulate osteogenic differentiation and better repair of bone defects. In conclusion, SLA technology holds promise as an effective production process for fabricating regenerative IVD stimulus-responsive hydrogel multiscale delivery systems (Figure 6).
Imitation Vertebrae and IVD Structures
Replicating IVD with Artificial Vertebrae
Bio3D printing technology has emerged as a valuable tool in the field of regenerative medicine, yielding promising outcomes. In a recent study by Serra et al,151 a lumbar fixator with the anatomical shape of an IVD was developed using 3D printing technology, enabling the fabrication of artificial IVDs. Successful IVD scaffolds have been created to mimic the macrostructure, microstructure, and physiological stresses of natural tissues. For instance, flexible polylactic acid (FPLA) scaffolds with adjustable viscoelasticity, tensile strength, and compressive modulus have been designed to match the properties of natural IVDs (Figure 5). These scaffolds can sustain the activity of NP cells (NPCs) and promote the deposition of ECM over an extended period.152 To enhance the functionality of IVD constructs, a multiscale delivery system has been developed by combining a hydrogel with in situ curing mechanisms and nanoparticle cross-linking. This system not only serves as an ideal bioink for 3D printing but also functions as a multifunctional scaffold that mimics natural IVD tissues. It enables long-term cellular proliferation and differentiation while facilitating the controlled release of drugs and biofactors. This breakthrough in regenerative therapy holds great potential for advancing the field.
Mimicking IVD Structure
The IVD is composed of a central NP surrounded by an AF and upper and lower hyaline cartilage endplates.153 The unique structural organization of the IVD is crucial for spinal motion and possesses mechanical properties such as high compressive and tensile strength.154,155 However, accurately replicating the IVD concentric structures using biomaterials and 3D printing presents a significant medical challenge. In a study by Zhu et al,156 GG/PEGDA composite hydrogels loaded with BMSCs were utilized to mimic the NP, while oriented porous poly (propylene glycol)/octa-armed polyhedral oligo-sesquiplane PLLA/POSS-PLLA8 fiber bundles were employed to mimic the AF. A composite stent for IVD was successfully fabricated by integrating 3D printing and electrostatic spinning techniques. This composite scaffold exhibited structural and mechanical properties that closely resembled natural IVDs. The AF-like fiber bundles were oriented concentrically and could withstand tension during NP deformation by precisely designing the 3D printing parameters. As a result, the BMSCs in the NP-like composite hydrogel network were highly energetic and uniformly distributed, and the scaffold could maintain the height of the rat IVD and generate new ECM. The composite scaffold demonstrated a compression modulus of approximately 10 MPa, which is similar to that of the natural IVD. This indicates that the scaffold can offer adequate mechanical support for tissue repair and regeneration. Additionally, the porosity and mechanical properties of the scaffolds can be tailored through 3D modeling techniques. The fiber ring structure of the scaffold consists of concentrically oriented fiber bundles, with each subsequent layer positioned at a 60° angle to the spine. This arrangement enables the scaffold to withstand the tension generated during the deformation of the NP.156 Engineered bionic IVD scaffolds show promise for personalized IVD repair and regeneration. Christiani et al157 developed 3D-printed scaffolds with PCL unidirectional notch-etched struts arranged in ±30° orientation to mimic the angular layer arrangement of natural AF. These scaffolds exhibited similar circumferential tensile modulus and axial compression properties to natural AF tissues, promoting AF attachment, proliferation, and expression of collagen I and glycosaminoglycans. PCL/PLLA composite hydrogels were also used to create electrostatically spun fiber scaffolds, simulating the AF bilayer structure and mechanical stiffness. This system facilitated AFC adhesion, influenced cellular alignment, and significantly improved tensile stiffness and strength. To better replicate AF morphology, a cell sheet rolling system process was employed, overcoming the limitations of electrostatic spinning in 2D translation. This allowed the creation of 3D multilayered tubular structures loaded with cells, accurately reproducing the AF structure. Furthermore, the system supported long-term cell survival, targeted distribution, and deposition of collagen I158 (Figure 5).
MSC-Based Induced Differentiation: Harnessing the Potential of 3D-Printed MSCs for IVD Regeneration
Harnessing 3D-Printed MSCs for IVD Regeneration: Viscoelastic FPLA Scaffolds
Another approach is to utilize the differentiation potential of 3D-printed MSCs to proliferate and differentiate into IVD-like tissues. Marshall et al152 employed 3D printing of FPLA to create viscoelastic FPLA scaffolds with adjustable bionic mechanics. These scaffolds were biocompatible and supported the viability of NPCs in both 2D and FPLA+hydrogel composite cultures. The composite scaffolds, when cultured with NPCs, maintained their baseline physiomechanical properties and facilitated matrix deposition for up to 8 weeks. MSCs cultured on FPLA scaffolds adhered to the structure and exhibited fibrocartilage differentiation. This study demonstrates the potential of 3D-printed FPLA scaffolds, with viscoelastic mechanical properties matching those of native IVDs in tension and compression, as mechanically stable and biocompatible biomaterials for IVD replacement.
Multiscale Delivery Scaffold: Utilizing BMSC Differentiation for IVD Regenerative Therapy
In a recent study by Sun et al,159 BMSCs and polydopamine nanomaterials encapsulating connective tissue growth factor-tissue growth factor and transforming growth factor-β3 were used as bioinks to create a multiscale delivery scaffold through IJP. In vitro experiments demonstrated the controlled release of tissue growth factor-tissue growth factor and transforming growth factor-β3, leading to the differentiation of BMSCs into NPCs and AFCs. When implanted subcutaneously on the dorsal surface of nude mice, this scaffold exhibited IVD-like changes, with a core area predominantly expressing collagen II and glycosaminoglycans and a peripheral area showing significant deposition of collagen I. This multiscale delivery system, utilizing BMSC differentiation, holds potential for clinical applications in IVD regenerative therapy. Furthermore, Rosenzweig et al160 compared large-pore 3D-printed scaffolds made of acrylonitrile butadiene styrene and PLA. Chondrocytes and NPCs demonstrated inward cell growth, viability, and tissue production on both acrylonitrile butadiene styrene and PLA scaffolds, which were printed using a simple and cost-effective desktop 3D printer. Additionally, NPCs exhibited higher production of proteoglycans than chondrocytes, regardless of the thermoplastic material used. The authors suggest that future scaffold designs should incorporate larger pore sizes and better mimic natural tissue structures, along with more flexible or resorbable materials, to create implantable structures with appropriate structure, function, and cellular architecture for potential cartilage and IVD tissue repair in vivo.
Challenges and Considerations in 3D Printing for IVDD Treatment: Overcoming Limitations and Advancing Clinical Applications
While 3D printing has demonstrated significant potential in treating IVDD and replicating IVD tissues using hydrogels as printing ink, several challenges need to be addressed.
First, the high water content of hydrogels gives these materials the flexibility to mimic natural organization. However, flexibility also brings limitations since the lower the polymer concentration is, the higher the porosity, the larger the pore size, the higher the water absorption, and the lower the mechanical strength. Hydrogels are often not strong enough and are prone to deformation, making it difficult to support weight-bearing joints such as the knee, so finding the optimal polymer concentration to achieve optimal biomechanical loading is a prerequisite.
Second, the current printing technology is still immature, and the 3D printing parameters need to be continuously mapped out. Due to the low viscosity of hydrogels and the wide range of gelling temperatures, it is difficult to realize high-resolution hydrogel precision 3D printing. Moreover, hydrogel synthesis technology requires expensive, heavy equipment, and the operation flow is complex and cannot be easily mastered.
Again, previous studies have focused on animal and cellular experiments, and due to the complex pathogenetic mechanisms involved in IVDD, the metrics used by scholars may be incomplete. Currently, in vivo studies on 3D-printed bone structures have been limited, and there is a lack of long-term, large-sample follow-up results from clinical trials involving 3D-printed bone. Additionally, there is a lack of clinical reports on the relevance of hydrogel 3D-printed IVDs due to the unique and complex IVD structure. These limitations highlight the need for further research and clinical trials to assess the efficacy and long-term outcomes of 3D-printed bone and hydrogel-based IVD treatments.
Finally, hydrogels can also be used as carriers of bioactive therapeutic substances, but the hydrogel-specific ratio to drug concentration, drug dosage, time control, and other issues have not yet been solved. These factors greatly limit the widespread promotion of hydrogel 3D bioprinting in IVDD.
Summary and Prospects
In summary, tissue-engineered hydrogel 3D bioprinting technology holds great promise in treating IVDD. The adjustable properties of hydrogels allow for precise control over the bioink, enabling the fabrication of bionic IVDs through 3D bioprinting. Compared to other novel therapies, hydrogel-based 3D bioprinting shows greater feasibility and potential for IVDD treatment.
However, there are still challenges that need to be addressed. One major challenge is optimizing the mechanical properties of hydrogels to achieve optimal biomechanical loading and support weight-bearing joints. This requires finding the right balance between polymer concentration, porosity, and mechanical strength. Advancements in 3D printing technology are also necessary to improve the resolution and precision of hydrogel printing, making it more suitable for complex structures such as IVDs. Furthermore, comprehensive in vivo studies and long-term clinical trials are essential to evaluate the efficacy and long-term outcomes of 3D-printed hydrogel-based IVDs. These studies should consider the unique and complex structure of the IVD and include large sample sizes to ensure reliable results. Additionally, exploring the potential of hydrogels as carriers for bioactive therapeutic substances could enhance the effectiveness of IVDD treatment.
In conclusion, while there are challenges to overcome, hydrogel-based 3D bioprinting technology holds significant promise for IVDD treatment. With further research and advancements in technology, we can expect to see improvements in the mechanical properties of hydrogels, enhanced printing resolution, and a better understanding of the long-term outcomes of 3D-printed IVDs. This technology has the potential to revolutionize IVDD treatment and improve the quality of life for patients suffering from this condition.
Abbreviations
IVD, Intervertebral discs; IVDD, Intervertebral disc degeneration; ECM, Extracellular matrix; BMSCs, Bone marrow mesenchymal stem cells; NP, Nucleus pulposus; NPCs, Nucleus pulposus cells; EVs, Extracellular vesicles; 3D, Three-dimensional; PEG, polyethylene glycol; PEA, Polyester amide; ES, Electrospraying; M-i-L, Microgel liposomes; μDHs, micrometer-sized dendrimer hydrogels; CNTs, Carbon nanotubes; DX-MG/CNT, Double cross-linked microgel-carbon nanotube composites; IJP, Inkjet printing; EXP, Extrusion printing; LAP, Laser-assisted printing; SLA, Stereolithography; PEGDA, Poly ethylene glycol diacrylate; CNFs, Cellulose nanofibers; LIFT, Laser-induced forward transfer.
Acknowledgments
Thank you to AJE company for providing language polishing services.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas. All authors took part in drafting, revising or critically reviewing the article. All authors gave final approval of the version to be published. All authors have agreed on the journal to which the article has been submitted. All authors agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Paige NM, Miake-Lye IM, Booth MS, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain: systematic review and meta-analysis. JAMA. 2017;317(14):1451–1460. doi:10.1001/jama.2017.3086
2. Zhang Y, Hu Y, Wang W, et al. Current progress in the endogenous repair of intervertebral disk degeneration based on progenitor cells. Front Bioeng Biotechnol. 2020;8:629088. doi:10.3389/fbioe.2020.629088
3. Bron JL, Helder MN, Meisel HJ, et al. Repair, regenerative and supportive therapies of the annulus fibrosus: achievements and challenges. Eur Spine J. 2009;18(3):301–313. doi:10.1007/s00586-008-0856-x
4. Chen BL, Guo JB, Zhang HW, et al. Surgical versus non-operative treatment for lumbar disc herniation: a systematic review and meta-analysis. Clin rehabilitat. 2018;32(2):146–160. doi:10.1177/0269215517719952
5. Roh EJ, Darai A, Kyung JW, et al. Genetic therapy for intervertebral disc degeneration. Int J Mol Sci. 2021;22(4):1579. doi:10.3390/ijms22041579
6. Ono C, Okamoto T, Abe T, et al. Baculovirus as a tool for gene delivery and gene therapy. Viruses. 2018;10(9):510. doi:10.3390/v10090510
7. Lundstrom K. RNA viruses as tools in gene therapy and vaccine development. Genes. 2019;10(3):189. doi:10.3390/genes10030189
8. Rui Y, Wilson DR, Green JJ. Non-viral delivery to enable genome editing. Trends Biotechnol. 2019;37(3):281–293. doi:10.1016/j.tibtech.2018.08.010
9. Bao LW, Zhou YY, Zeng FY. Advances in gene therapy for β-thalassemia and hemophilia based on the CRISPR/Cas9 technology. Yi Chuan. 2020;42(10):949–964. doi:10.16288/j.yczz.20-110
10. Biagioni A, Laurenzana A, Margheri F, et al. Delivery systems of CRISPR/Cas9-based cancer gene therapy. J Biol Eng. 2018;12(1):33. doi:10.1186/s13036-018-0127-2
11. Henry N, Clouet J, Le Bideau J, et al. Innovative strategies for intervertebral disc regenerative medicine: from cell therapies to multiscale delivery systems. Biotechnol Adv. 2018;36(1):281–294. doi:10.1016/j.biotechadv.2017.11.009
12. Maidhof R, Rafiuddin A, Chowdhury F, et al. Timing of mesenchymal stem cell delivery impacts the fate and therapeutic potential in intervertebral disc repair. J Orthop Res. 2017;35(1):32–40. doi:10.1002/jor.23350
13. Zhang XB, Chen XY, Qi J, et al. New hope for intervertebral disc degeneration: bone marrow mesenchymal stem cells and exosomes derived from bone marrow mesenchymal stem cell transplantation. Curr Gene Ther. 2022;22(4):291–302. doi:10.2174/1566523221666211012092855
14. He GH, Zhang W, Ma YX, et al. Mesenchymal stem cells-derived exosomes ameliorate blue light stimulation in retinal pigment epithelium cells and retinal laser injury by VEGF-dependent mechanism. Int J Ophthalmol. 2018;11(4):559–566. doi:10.18240/ijo.2018.04.04
15. Vizoso FJ, Eiro N, Cid S, et al. Mesenchymal stem cell secretome: toward cell-free therapeutic strategies in regenerative medicine. Int J Mol Sci. 2017;18(9):1852. doi:10.3390/ijms18091852
16. Zhang XB, Hu YC, Cheng P, et al. Targeted therapy for intervertebral disc degeneration: inhibiting apoptosis is a promising treatment strategy. Int J Med Sci. 2021;18(13):2799–2813. doi:10.7150/ijms.59171
17. Binch ALA, Fitzgerald JC, Growney EA, et al. Cell-based strategies for IVD repair: clinical progress and translational obstacles. Nat Rev Rheumatol. 2021;17(3):158–175. doi:10.1038/s41584-020-00568-w
18. Ma K, Chen S, Li Z, et al. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27(1):41–48. doi:10.1016/j.joca.2018.08.021
19. Chen BY, Sung CW, Chen C, et al. Advances in exosomes technology. Int j clin Chem. 2019;493:14–19. doi:10.1016/j.cca.2019.02.021
20. Familtseva A, Jeremic N, Tyagi SC. Exosomes: cell-created drug delivery systems. Mol Cell Biochem. 2019;459(1–2):1–6. doi:10.1007/s11010-019-03545-4
21. Stergar J, Gradisnik L, Velnar T, et al. Intervertebral disc tissue engineering: a brief review. Bosn J Basic Med Sci. 2019;19(2):130–137. doi:10.17305/bjbms.2019.3778
22. Tagami T, Ito E, Kida R, et al. 3D printing of gummy drug formulations composed of gelatin and an HPMC-based hydrogel for pediatric use. Int J Pharm. 2021;594:120118. doi:10.1016/j.ijpharm.2020.120118
23. Cai H, Liu Z, Wei F, et al. 3D Printing in Spine Surgery. Adv Exp Med Biol. 2018;1093:345–359.
24. Zheng L, Liu S, Cheng X, et al. Intensified stiffness and photodynamic provocation in a collagen-based composite hydrogel drive chondrogenesis. Adv Sci 2019;6(16):1900099. doi:10.1002/advs.201900099
25. Lin W, Kluzek M, Iuster N, et al. Cartilage-inspired, lipid-based boundary-lubricated hydrogels. Science. 2020;370(6514):335–338. doi:10.1126/science.aay8276
26. Contessotto P, Orbanić D, Da Costa M, et al. Elastin-like recombinamers-based hydrogel modulates post-ischemic remodeling in a non-transmural myocardial infarction in sheep. Sci Transl Med. 2021;13(581). doi:10.1126/scitranslmed.aaz5380
27. Hogrebe NJ, Reinhardt JW, Gooch KJ. Biomaterial microarchitecture: a potent regulator of individual cell behavior and multicellular organization. J Biomed Mater Res Part A. 2017;105(2):640–661. doi:10.1002/jbm.a.35914
28. Sánchez-Fernández MJ, Rutjes J, Félix Lanao RP, et al. Bone-adhesive hydrogels based on dual crosslinked poly (2-oxazoline)s. Macromol biosci. 2021;21(12):e2100257. doi:10.1002/mabi.202100257
29. Kim S, Regitsky AU, Song J, et al. In situ mechanical reinforcement of polymer hydrogels via metal-coordinated crosslink mineralization. Nat Commun. 2021;12(1):667. doi:10.1038/s41467-021-20953-7
30. Huang F, Chen M, Zhou Z, et al. Spatiotemporal patterning of photoresponsive DNA-based hydrogels to tune local cell responses. Nat Commun. 2021;12(1):2364. doi:10.1038/s41467-021-22645-8
31. Daly AC, Davidson MD, Burdick JA. 3D bioprinting of high cell-density heterogeneous tissue models through spheroid fusion within self-healing hydrogels. Nat Commun. 2021;12(1):753. doi:10.1038/s41467-021-21029-2
32. Wei Q, Young J, Holle A, et al. Soft hydrogels for balancing cell proliferation and differentiation. ACS Biomate Sci Eng. 2020;6(8):4687–4701. doi:10.1021/acsbiomaterials.0c00854
33. Chen WW, Tjin MS, Chua AWC, et al. Probing the role of integrins in keratinocyte migration using bioengineered extracellular matrix mimics. ACS Appl Mater Interfaces. 2017;9(42):36483–36492. doi:10.1021/acsami.7b06959
34. Wang JK, Cheam NMJ, Irvine SA, et al. Interpenetrating network of alginate-human adipose extracellular matrix hydrogel for islet cells encapsulation. Macromol Rapid Commun. 2020;41(21):e2000275. doi:10.1002/marc.202000275
35. Mitura S, Sionkowska A, Jaiswal A. Biopolymers for hydrogels in cosmetics: review. J Mater Sci Mater Med. 2020;31(6):50. doi:10.1007/s10856-020-06390-w
36. Kong B, Chen Y, Liu R, et al. Fiber reinforced GelMA hydrogel to induce the regeneration of corneal stroma. Nat Commun. 2020;11(1):1435. doi:10.1038/s41467-020-14887-9
37. Hyung S, Lee SR, Kim J, et al. A 3D disease and regeneration model of peripheral nervous system-on-a-chip. Sci Adv. 2021;7(5). doi:10.1126/sciadv.abd9749
38. LeSavage BL, Heilshorn SC. Defined matrices bring IBD to 3D. Nature Mater. 2021;20(2):124–125. doi:10.1038/s41563-020-00904-1
39. Wang X, Wang Q. Enzyme-laden bioactive hydrogel for biocatalytic monitoring and regulation. Acc Chem Res. 2021;54(5):1274–1287. doi:10.1021/acs.accounts.0c00832
40. Cao H, Duan L, Zhang Y, et al. Current hydrogel advances in physicochemical and biological response-driven biomedical application diversity. Signal Transduct Target Ther. 2021;6(1):426. doi:10.1038/s41392-021-00830-x
41. Kharkar PM, Scott RA, Olney LP, et al. Controlling the release of small, bioactive proteins via dual mechanisms with therapeutic potential. Adv Healthcare Mater. 2017;6(24):1700713
42. Bettinger CJ, Bruggeman JP, Borenstein JT, et al. Amino alcohol-based degradable poly (ester amide) elastomers. Biomaterials. 2008;29(15):2315–2325. doi:10.1016/j.biomaterials.2008.01.029
43. Xin T, Gu Y, Cheng R, et al. Inorganic Strengthened Hydrogel Membrane as Regenerative Periosteum. ACS Appl Mater Interfaces. 2017;9(47):41168–41180. doi:10.1021/acsami.7b13167
44. Phromsopha T, Baimark Y. Preparation of starch/gelatin blend microparticles by a water-in-oil emulsion method for controlled release drug delivery. Int J Biomater. 2014;2014:829490. doi:10.1155/2014/829490
45. Keles H, Naylor A, Clegg F, et al. The application of non-linear curve fitting routines to the analysis of mid-infrared images obtained from single polymeric microparticles. Analyst. 2014;139(10):2355–2369. doi:10.1039/C3AN01879B
46. Mao S, Xu J, Cai C, et al. Effect of WOW process parameters on morphology and burst release of FITC-dextran loaded PLGA microspheres. Int J Pharm. 2007;334(1–2):137–148. doi:10.1016/j.ijpharm.2006.10.036
47. Bian J, Cai F, Chen H, et al. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21(6):2690–2698. doi:10.1021/acs.nanolett.0c04713
48. Willems N, Mihov G, Grinwis GC, et al. Safety of intradiscal injection and biocompatibility of polyester amide microspheres in a canine model predisposed to intervertebral disc degeneration. J Biomed Mater Res Part B. 2017;105(4):707–714. doi:10.1002/jbm.b.33579
49. Pang X, Chu CC. Synthesis, characterization and biodegradation of functionalized amino acid-based poly (ester amide)s. Biomaterials. 2010;31(14):3745–3754. doi:10.1016/j.biomaterials.2010.01.027
50. Sun Z, Zhang M, Zhao XH, et al. Immune cascades in human intervertebral disc: the pros and cons. Int J Clin Exp Pathol. 2013;6(6):1009–1014.
51. Rudnik-Jansen I, Colen S, Berard J, et al. Prolonged inhibition of inflammation in osteoarthritis by triamcinolone acetonide released from a polyester amide microsphere platform. J Control Release. 2017;253:64–72. doi:10.1016/j.jconrel.2017.03.014
52. Rudnik-Jansen I, Tellegen A, Beukers M, et al. Safety of intradiscal delivery of triamcinolone acetonide by a poly (esteramide) microsphere platform in a large animal model of intervertebral disc degeneration. Spine J. 2019;19(5):905–919. doi:10.1016/j.spinee.2018.10.014
53. Tellegen AR, Rudnik-Jansen I, Beukers M, et al. Intradiscal delivery of celecoxib-loaded microspheres restores intervertebral disc integrity in a preclinical canine model. J Control Release. 2018;286:439–450. doi:10.1016/j.jconrel.2018.08.019
54. Janssen M, Timur UT, Woike N, et al. Celecoxib-loaded PEA microspheres as an auto regulatory drug-delivery system after intra-articular injection. J Control Release. 2016;244(Pt A):30–40. doi:10.1016/j.jconrel.2016.11.003
55. Ali A, Zaman A, Sayed E, et al. Electrohydrodynamic atomisation driven design and engineering of opportunistic particulate systems for applications in drug delivery, therapeutics and pharmaceutics. Adv Drug Delivery Rev. 2021;176:113788. doi:10.1016/j.addr.2021.04.026
56. Plati F, Papi R, Paraskevopoulou A. Characterization of oregano essential oil (Origanum vulgare L. subsp. hirtum) particles produced by the novel nano spray drying technique. Foods. 2021;10(12):2923. doi:10.3390/foods10122923
57. Caballero Aguilar LM, Duchi S, Onofrillo C, et al. Formation of alginate microspheres prepared by optimized microfluidics parameters for high encapsulation of bioactive molecules. J Colloid Interface Sci. 2021;587:240–251. doi:10.1016/j.jcis.2020.12.026
58. He C, Zeng W, Su Y, et al. Microfluidic-based fabrication and characterization of drug-loaded PLGA magnetic microspheres with tunable shell thickness. Drug Delivery. 2021;28(1):692–699. doi:10.1080/10717544.2021.1905739
59. Li G, Yao L, Li J, et al. Preparation of poly (lactide-co-glycolide) microspheres and evaluation of pharmacokinetics and tissue distribution of BDMC-PLGA-MS in rats. Asian J Pharm Sci 2018;13(1):82–90. doi:10.1016/j.ajps.2017.09.002
60. Zhou W, Zhang Y, Meng S, et al. Micro-/nano-structures on biodegradable magnesium@plga and their cytotoxicity, photothermal, and anti-tumor effects. Small Methods. 2021;5(2):e2000920. doi:10.1002/smtd.202000920
61. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature Biotechnol. 2014;32(8):773–785. doi:10.1038/nbt.2958
62. Chen Y, Guo K, Jiang L, et al. Microfluidic deformability cytometry: a review. Talanta. 2023;251:123815. doi:10.1016/j.talanta.2022.123815
63. Zhu Y, Zhang X, Sun L, et al. Engineering human brain assembloids by microfluidics. Adv Mater 2023;35(14):e2210083. doi:10.1002/adma.202210083
64. Huang D, Wang J, Nie M, et al. Pollen-inspired adhesive multilobe microparticles from microfluidics for intestinal drug delivery. Adv Mater 2023;35(28):e2301192. doi:10.1002/adma.202301192
65. Duarte AR, Costa MS, Simplício AL, et al. Preparation of controlled release microspheres using supercritical fluid technology for delivery of anti-inflammatory drugs. Int J Pharm. 2006;308(1–2):168–174. doi:10.1016/j.ijpharm.2005.11.012
66. Obaidat RM, Tashtoush BM, Bayan MF, et al. Drying using supercritical fluid technology as a potential method for preparation of chitosan aerogel microparticles. AAPS Pharm Sci Tech. 2015;16(6):1235–1244. doi:10.1208/s12249-015-0312-2
67. Kawashima Y, York P. Drug delivery applications of supercritical fluid technology. Adv Drug Deliv Rev. 2008;60(3):297–298. doi:10.1016/j.addr.2007.10.011
68. Parilti R, Riva R, Howdle SM, et al. Sulindac encapsulation and release from functional poly (HEMA) microparticles prepared in supercritical carbon dioxide. Int J Pharm. 2018;549(1–2):161–168. doi:10.1016/j.ijpharm.2018.07.060
69. Daraee H, Etemadi A, Kouhi M, et al. Application of liposomes in medicine and drug delivery. Artif Cells Nanomed Biotechnol. 2016;44(1):381–391. doi:10.3109/21691401.2014.953633
70. An E, Jeong CB, Cha C, et al. Fabrication of microgel-in-liposome particles with improved water retention. Langmuir. 2012;28(9):4095–4101. doi:10.1021/la2046349
71. Dos Santos Giuberti C, de Oliveira Reis EC, Ribeiro Rocha TG, et al. Study of the pilot production process of long-circulating and pH-sensitive liposomes containing cisplatin. J Liposome Res. 2011;21(1):60–69. doi:10.3109/08982101003754377
72. York-Duran MJ, Ek PK, Godoy-Gallardo M, et al. Shear stress regulated uptake of liposome-decorated microgels coated with a poly (dopamine) shell. Colloids Surf, B. 2018;171:427–436. doi:10.1016/j.colsurfb.2018.07.031
73. Lin F, Wang Z, Xiang L, Deng L, Cui W Charge-Guided Micro/Nano-Hydrogel Microsphere for Penetrating Cartilage Matrix. Adv. Funct. Mater. 2021, 31, 2107678.
74. Chauhan AS. Dendrimers for Drug Delivery. Molecules. 2018;23(4):938. doi:10.3390/molecules23040938
75. Yang H, Kao WJ. Synthesis and characterization of nanoscale dendritic RGD clusters for potential applications in tissue engineering and drug delivery. Int j Nanomed. 2007;2(1):89–99. doi:10.2147/nano.2007.2.1.89
76. Wang J, Cooper RC, He H, et al. Polyamidoamine dendrimer microgels: hierarchical arrangement of dendrimers into micrometer domains with expanded structural features for programmable drug delivery and release. Macromolecules. 2018;51(15):6111–6118. doi:10.1021/acs.macromol.8b01006
77. Seelbach RJ, Fransen P, Pulido D, et al. Injectable hyaluronan hydrogels with peptide-binding dendrimers modulate the controlled release of BMP-2 and TGF-β1. Macromol biosci. 2015;15(8):1035–1044. doi:10.1002/mabi.201500082
78. Bosch X. Dendrimers to treat rheumatoid arthritis. ACS nano. 2011;5(9):6779–6785. doi:10.1021/nn203190x
79. Beg S, Rizwan M, Sheikh AM, et al. Advancement in carbon nanotubes: basics, biomedical applications and toxicity. J Pharm Pharmacol. 2011;63(2):141–163. doi:10.1111/j.2042-7158.2010.01167.x
80. Dong W, Huang C, Wang Y, et al. Superior mechanical properties of double-network hydrogels reinforced by carbon nanotubes without organic modification. Int J Mol Sci. 2013;14(11):22380–22394. doi:10.3390/ijms141122380
81. Shin SR, Bae H, Cha JM, et al. Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS nano. 2012;6(1):362–372. doi:10.1021/nn203711s
82. Spizzirri UG, Hampel S, Cirillo G, et al. Spherical gelatin/CNTs hybrid microgels as electro-responsive drug delivery systems. Int J Pharm. 2013;448(1):115–122. doi:10.1016/j.ijpharm.2013.03.013
83. Li D, Li S, Liu J, et al. Surface modification of carbon nanotube with gelatin via mussel inspired method. Mater Sci Eng C Mater Biol Appl. 2020;112:110887. doi:10.1016/j.msec.2020.110887
84. Cui Z, Zhou M, Greensmith PJ, et al. A study of conductive hydrogel composites of pH-responsive microgels and carbon nanotubes. Soft Matter. 2016;12(18):4142–4153. doi:10.1039/C6SM00223D
85. Wilczewska AZ, Niemirowicz K, Markiewicz KH, et al. Nanoparticles as drug delivery systems. Pharmacol Rep. 2012;64(5):1020–1037. doi:10.1016/S1734-1140(12)70901-5
86. Des Rieux A, Fievez V, Garinot M, et al. Nanoparticles as potential oral delivery systems of proteins and vaccines: a mechanistic approach. J Control Release. 2006;116(1):1–27. doi:10.1016/j.jconrel.2006.08.013
87. Liaw CY, Guvendiren M. Current and emerging applications of 3D printing in medicine. Biofabrication. 2017;9(2):024102. doi:10.1088/1758-5090/aa7279
88. Knowlton S, Anand S, Shah T, et al. Bioprinting for Neural Tissue Engineering. Trends Neurosci. 2018;41(1):31–46. doi:10.1016/j.tins.2017.11.001
89. Kamio T, Suzuki M, Asaumi R, et al. DICOM segmentation and STL creation for 3D printing: a process and software package comparison for osseous anatomy. 3D Print Med. 2020;6(1):17. doi:10.1186/s41205-020-00069-2
90. Pavan Kalyan BG, Kumar L. 3D printing: applications in tissue engineering, medical devices, and drug delivery. AAPS Pharm Sci Tech. 2022;23(4):92. doi:10.1208/s12249-022-02242-8
91. Ozbolat IT, Yu Y. Bioprinting toward organ fabrication: challenges and future trends. IEEE Trans Biomed Eng. 2013;60(3):691–699. doi:10.1109/TBME.2013.2243912
92. Mandrycky C, Wang Z, Kim K, et al. 3D bioprinting for engineering complex tissues. Biotechnol Adv. 2016;34(4):422–434. doi:10.1016/j.biotechadv.2015.12.011
93. Ozbolat IT, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi:10.1016/j.biomaterials.2015.10.076
94. Derakhshanfar S, Mbeleck R, Xu K, et al. 3D bioprinting for biomedical devices and tissue engineering: a review of recent trends and advances. Bioact Mater. 2018;3(2):144–156. doi:10.1016/j.bioactmat.2017.11.008
95. Xu T, Jin J, Gregory C, et al. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–99. doi:10.1016/j.biomaterials.2004.04.011
96. Hospodiuk M, Dey M, Sosnoski D, et al. The bioink: a comprehensive review on bioprintable materials. Biotechnol Adv. 2017;35(2):217–239. doi:10.1016/j.biotechadv.2016.12.006
97. Huang Y, Wu W, Liu H, et al. 3D printing of functional nerve guide conduits. Burns Trauma. 2021;9:tkab011.
98. Nahmias Y, Odde DJ. Micropatterning of living cells by laser-guided direct writing: application to fabrication of hepatic-endothelial sinusoid-like structures. Nat Protoc. 2006;1(5):2288–2296. doi:10.1038/nprot.2006.386
99. Martinez V, Forró C, Weydert S, et al. Controlled single-cell deposition and patterning by highly flexible hollow cantilevers. Lab on a Chip. 2016;16(9):1663–1674. doi:10.1039/C5LC01466B
100. Foresti D, Kroll KT, Amissah R, et al. Acoustophoretic printing. Sci Adv. 2018;4(8):eaat1659. doi:10.1126/sciadv.aat1659
101. Chang CC, Boland ED, Williams SK, et al. Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res Part B. 2011;98(1):160–170. doi:10.1002/jbm.b.31831
102. Wang Z, Abdulla R, Parker B, et al. A simple and high-resolution stereolithography-based 3D bioprinting system using visible light crosslinkable bioinks. Biofabrication. 2015;7(4):045009.
103. Pepper ME, Seshadri V, Burg T, et al. Cell settling effects on a thermal inkjet bioprinter.
104. Gudapati H, Yan J, Huang Y, et al. Alginate gelation-induced cell death during laser-assisted cell printing. Biofabrication. 2014;6(3):035022. doi:10.1088/1758-5082/6/3/035022
105. de Armentia SL, Fernández-Villamarín S, Ballesteros Y, et al. 3D printing of a graphene-modified photopolymer using stereolithography for biomedical applications: a study of the polymerization reaction. Int J Bioprinting. 2022;8(1):503. doi:10.18063/ijb.v8i1.503
106. Viray CM, van Magill B, Zreiqat H, et al. Stereolithographic visible-light printing of Poly (-glutamic acid) hydrogel scaffolds. ACS Biomater Sci Eng 2022;8(3):1115–1131. doi:10.1021/acsbiomaterials.1c01519
107. Park JA, Lee HR, Park SY, et al. Self-organization of fibroblast-laden 3d collagen microstructures from inkjet-printed cell patterns. Adv Biosyst 2020;4(5):e1900280. doi:10.1002/adbi.201900280
108. Duffy GL, Liang H, Williams RL, et al. 3D reactive inkjet printing of poly-ɛ-lysine/gellan gum hydrogels for potential corneal constructs. Mater Sci Eng C Mater Biol Appl. 2021;131:112476. doi:10.1016/j.msec.2021.112476
109. Van Hoorick J, Tytgat L, Dobos A, et al. (Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. 2019;97:46–73. doi:10.1016/j.actbio.2019.07.035
110. Janarthanan G, Kim JH, Kim IG, et al. Manufacturing of self-standing multi-layered 3D-bioprinted alginate-hyaluronate constructs by controlling the cross-linking mechanisms for tissue engineering applications. Biofabrication. 2022;14(3):035013. doi:10.1088/1758-5090/ac6c4c
111. Wang Y, Chen Y, Zheng J, et al. Three-dimensional printing self-healing dynamic/photocrosslinking gelatin-hyaluronic acid double-network hydrogel for tissue engineering. ACS omega. 2022;7(14):12076–12088. doi:10.1021/acsomega.2c00335
112. Taebnia N, Zhang R, Kromann EB, et al. Dual-material 3d-printed intestinal model devices with integrated villi-like scaffolds. ACS Appl Mater Interfaces. 2021;13(49):58434–58446. doi:10.1021/acsami.1c22185
113. Hossain Rakin R, Kumar H, Rajeev A, et al. Tunable metacrylated hyaluronic acid-based hybrid bioinks for stereolithography 3D bioprinting. Biofabrication. 2021;13(4):044109. doi:10.1088/1758-5090/ac25cb
114. Mondschein RJ, Kanitkar A, Williams CB, et al. Polymer structure-property requirements for stereolithographic 3D printing of soft tissue engineering scaffolds. Biomaterials. 2017;140:170–188. doi:10.1016/j.biomaterials.2017.06.005
115. Dufour A, Gallostra XB, O’Keeffe C, et al. Integrating melt electrowriting and inkjet bioprinting for engineering structurally organized articular cartilage. Biomaterials. 2022;283:121405. doi:10.1016/j.biomaterials.2022.121405
116. Kamdem Tamo A, Doench I, Walter L, et al. Development of bioinspired functional chitosan/cellulose nanofiber 3d hydrogel constructs by 3d printing for application in the engineering of mechanically demanding tissues. Polymers. 2021;13(10):1663. doi:10.3390/polym13101663
117. Gruene M, Deiwick A, Koch L, et al. Laser printing of stem cells for biofabrication of scaffold-free autologous grafts. Tissue Eng Part C MethoD. 2011;17(1):79–87. doi:10.1089/ten.tec.2010.0359
118. Blanquer SBG, Gebraad AWH, Miettinen S, et al. Differentiation of adipose stem cells seeded towards annulus fibrosus cells on a designed poly (trimethylene carbonate) scaffold prepared by stereolithography. J Tissue Eng Regener Med. 2017;11(10):2752–2762. doi:10.1002/term.2170
119. Cui X, Breitenkamp K, Finn MG, et al. Direct human cartilage repair using three-dimensional bioprinting technology. Tissue Eng Part A. 2012;18(11–12):1304–1312. doi:10.1089/ten.tea.2011.0543
120. Faramarzi N, Yazdi IK, Nabavinia M, et al. Patient-specific bioinks for 3d bioprinting of tissue engineering scaffolds. Adv Healthcare Mater. 2018;7(11):e1701347. doi:10.1002/adhm.201701347
121. Takagi D, Lin W, Matsumoto T, et al. High-precision three-dimensional inkjet technology for live cell bioprinting. Int J Bioprinting. 2019;5(2):208. doi:10.18063/ijb.v5i2.208
122. Teo MY, RaviChandran N, Kim N, et al. Direct patterning of highly conductive pedot: pss/ionic liquid hydrogel via microreactive inkjet printing. ACS Appl Mater Interfaces. 2019;11(40):37069–37076. doi:10.1021/acsami.9b12069
123. Iwanaga S, Saito N, Sanae H, et al. Facile fabrication of uniform size-controlled microparticles and potentiality for tandem drug delivery system of micro/nanoparticles. Colloids Surf, B. 2013;109:301–306. doi:10.1016/j.colsurfb.2013.04.007
124. Ferris CJ, Gilmore KJ, Beirne S, et al. Bio-ink for on-demand printing of living cells. Biomater Sci. 2013;1(2):224–230. doi:10.1039/C2BM00114D
125. Yoon S, Park JA, Lee HR, et al. Inkjet-spray hybrid printing for 3d freeform fabrication of multilayered hydrogel structures. Adv Healthcare Mater. 2018;7(14):e1800050. doi:10.1002/adhm.201800050
126. Gao XD, Zhang XB, Zhang RH, et al. Aggressive strategies for regenerating intervertebral discs: stimulus-responsive composite hydrogels from single to multiscale delivery systems. J Mat Chem B. 2022;10(30):5696–5722. doi:10.1039/D2TB01066F
127. Duan B, Hockaday LA, Kang KH, et al. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J Biomed Mater Res Part A. 2013;101(5):1255–1264. doi:10.1002/jbm.a.34420
128. Li M, Shi T, Yao D, et al. High-cytocompatible semi-IPN bio-ink with wide molecular weight distribution for extrusion 3D bioprinting. Sci Rep. 2022;12(1):6349. doi:10.1038/s41598-022-10338-1
129. Sahai N, Gogoi M, Tewari RP. 3D printed chitosan composite scaffold for chondrocytes differentiation. Curr Med Imaging Rev. 2021;17(7):832–842. doi:10.2174/1573405616666201217112939
130. Zhang C, Dong Q, Liang K, et al. Photopolymerizable thiol-acrylate maleiated hyaluronic acid/thiol-terminated poly (ethylene glycol) hydrogels as potential in-situ formable scaffolds. Int J Biol Macromol. 2018;119:270–277. doi:10.1016/j.ijbiomac.2018.07.153
131. Lin S, Li B, Yang L, et al. New method for reducing viscosity and shear stress in hydrogel 3D printing via multidimension vibration. Comput. Methods Biomech. Biomed. Eng. 2022;16:1–16.
132. Alizadehgiashi M, Nemr CR, Chekini M, et al. Multifunctional 3D-printed wound dressings. ACS nano. 2021;15(7):12375–12387. doi:10.1021/acsnano.1c04499
133. Li Q, Xu S, Feng Q, et al. 3D printed silk-gelatin hydrogel scaffold with different porous structure and cell seeding strategy for cartilage regeneration. Bioact Mater 2021;6(10):3396–3410. doi:10.1016/j.bioactmat.2021.03.013
134. Ghafari R, Jonoobi M, Amirabad LM, et al. Fabrication and characterization of novel bilayer scaffold from nanocellulose based aerogel for skin tissue engineering applications. Int J Biol Macromol. 2019;136:796–803. doi:10.1016/j.ijbiomac.2019.06.104
135. Gruene M, Unger C, Koch L, et al. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed Eng Online. 2011;10(1):19. doi:10.1186/1475-925X-10-19
136. Midha S, Dalela M, Sybil D, et al. Advances in three-dimensional bioprinting of bone: progress and challenges. J Tissue Eng Regener Med. 2019;13(6):925–945. doi:10.1002/term.2847
137. Gu Z, Fu J, Lin H, et al. Development of 3D bioprinting: from printing methods to biomedical applications. Asian J Pharm Sci. 2020;15(5):529–557. doi:10.1016/j.ajps.2019.11.003
138. Koch L, Kuhn S, Sorg H, et al. Laser printing of skin cells and human stem cells. Tissue Eng Part C MethoD. 2010;16(5):847–854. doi:10.1089/ten.tec.2009.0397
139. Burke G, Devine DM, Major I. Effect of stereolithography 3d printing on the properties of PEGDMA hydrogels. Polymers. 2020;12(9):2015. doi:10.3390/polym12092015
140. Annabi N, Nichol JW, Zhong X, et al. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng Part B, Rev. 2010;16(4):371–383. doi:10.1089/ten.teb.2009.0639
141. Johnson AR, Caudill CL, Tumbleston JR, et al. Single-step fabrication of computationally designed microneedles by continuous liquid interface production. PLoS One. 2016;11(9):e0162518. doi:10.1371/journal.pone.0162518
142. Han D, Lu Z, Chester SA, et al. Micro 3D printing of a temperature-responsive hydrogel using projection micro-stereolithography. Sci Rep. 2018;8(1):1963. doi:10.1038/s41598-018-20385-2
143. Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science. 2019;364(6439):458–464. doi:10.1126/science.aav9750
144. Chen JY, Hwang JV, Ao-Ieong WS, et al. Study of physical and degradation properties of 3D-printed biodegradable, photocurable copolymers, PGSA-co-PEGDA and PGSA-co-PCLDA. Polymers. 2018;10(11):1263. doi:10.3390/polym10111263
145. Warner J, Soman P, Zhu W, et al. Design and 3D printing of hydrogel scaffolds with fractal geometries. ACS Biomater Sci Eng. 2016;2(10):1763–1770. doi:10.1021/acsbiomaterials.6b00140
146. Karakurt I, Aydoğdu A, Çıkrıkcı S, et al. Stereolithography (SLA) 3D printing of ascorbic acid loaded hydrogels: a controlled release study. Int J Pharm. 2020;584:119428. doi:10.1016/j.ijpharm.2020.119428
147. Anandakrishnan N, Ye H, Guo Z, et al. Fast stereolithography printing of large-scale biocompatible hydrogel models. Adv Healthcare Mater. 2021;10(10):e2002103. doi:10.1002/adhm.202002103
148. Tang A, Ji J, Li J, et al. Nanocellulose/PEGDA aerogels with tunable poisson’s ratio fabricated by stereolithography for mouse bone marrow mesenchymal stem cell culture. Nanomaterials. 2021;11(3):603. doi:10.3390/nano11030603
149. Tang A, Li J, Li J, et al. Nanocellulose/PEGDA aerogel scaffolds with tunable modulus prepared by stereolithography for three-dimensional cell culture. J biomater sci Poly ed. 2019;30(10):797–814. doi:10.1080/09205063.2019.1602904
150. Luo Y, Pan H, Jiang J, et al. Desktop-stereolithography 3d printing of a polyporous extracellular matrix bioink for bone defect regeneration. Front Bioeng Biotechnol. 2020;8:589094. doi:10.3389/fbioe.2020.589094
151. Serra T, Capelli C, Toumpaniari R, et al. Design and fabrication of 3D-printed anatomically shaped lumbar cage for intervertebral disc (IVD) degeneration treatment. Biofabrication. 2016;8(3):035001. doi:10.1088/1758-5090/8/3/035001
152. Marshall SL, Jacobsen TD, Emsbo E, et al. Three-dimensional-printed flexible scaffolds have tunable biomimetic mechanical properties for intervertebral disc tissue engineering. ACS Biomater Sci Eng. 2021;7(12):5836–5849. doi:10.1021/acsbiomaterials.1c01326
153. Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connective Tissue Res. 1989;23(1):75–88. doi:10.3109/03008208909103905
154. Zhang K, Xue C, Lu N, et al. Mechanical loading mediates human nucleus pulposus cell viability and extracellular matrix metabolism by activating of NF-κB. Exp Ther Med. 2019;18(3):1587–1594. doi:10.3892/etm.2019.7744
155. Wang B, Ke W, Wang K, et al. Mechanosensitive ion channel piezo1 activated by matrix stiffness regulates oxidative stress-induced senescence and apoptosis in human intervertebral disc degeneration. Oxid Med Cell Longev. 2021;2021(1):8884922. doi:10.1155/2021/8884922
156. Zhu M, Tan J, Liu L, et al. Construction of biomimetic artificial intervertebral disc scaffold via 3D printing and electrospinning. Mater Sci Eng C Mater Biol Appl. 2021;128:112310. doi:10.1016/j.msec.2021.112310
157. Christiani TR, Baroncini E, Stanzione J, et al. In vitro evaluation of 3D printed polycaprolactone scaffolds with angle-ply architecture for annulus fibrosus tissue engineering. Regen Biomater. 2019;6(3):175–184. doi:10.1093/rb/rbz011
158. Shamsah AH, Cartmell SH, Richardson SM, et al. Tissue engineering the annulus fibrosus using 3D rings of electrospun PCL: PLLA angle-ply nanofiber sheets. Front Bioeng Biotechnol. 2019;7:437. doi:10.3389/fbioe.2019.00437
159. Sun B, Lian M, Han Y, et al. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact Mater 2021;6(1):179–190. doi:10.1016/j.bioactmat.2020.06.022
160. Rosenzweig DH, Carelli E, Steffen T, et al. 3D-printed ABS and PLA scaffolds for cartilage and nucleus pulposus Tissue regeneration. Int J Mol Sci. 2015;16(7):15118–15135. doi:10.3390/ijms160715118
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.