Back to Journals » Risk Management and Healthcare Policy » Volume 18
Risk Assessment and Prevention of Venous Thromboembolism in Critically Ill Patients in Tibet: A Prospective Cohort Study with Historical Controls
Authors Qiong J, Gu Y, Dekyi J, Dawa, Tsring P, Zhao M, Wang X, Li G, Liu H
Received 5 August 2024
Accepted for publication 28 March 2025
Published 3 April 2025 Volume 2025:18 Pages 1171—1179
DOI https://doi.org/10.2147/RMHP.S490160
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jongwha Chang
Ji Qiong,1,* Yanmei Gu,2,* Jampa Dekyi,1 Dawa,1 Phurbu Tsring,1 Min Zhao,1 Xin Wang,2 Guangming Li,2 Haixia Liu2
1Department of Critical Care Medicine, Lhasa People’s Hospital, Lhasa, 850000, People’s Republic of China; 2Department of Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, Beijing, 100069, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Haixia Liu, Department of Critical Care Medicine, Beijing Youan Hospital, Capital Medical University, 8 Xi Tou Tiao, You An Men Wai Street, Fengtai District, Beijing, 100069, People’s Republic of China, Tel +86 13521206692, Email [email protected]
Objective: Venous thromboembolism (VTE) is a significant concern in critically ill patients. However, the incidence and risk factors of VTE in high-altitude regions like Tibet remain unclear. This study aimed to assess the effectiveness and safety of standardized anticoagulation therapy in preventing VTE among intensive care unit (ICU) patients in Tibet.
Methods: This prospective controlled study included 78 patients in the treatment group receiving low molecular weight heparin (LMWH) and 56 patients in the control group without standardized VTE prophylaxis. VTE incidence, risk factors, and safety outcomes were compared between the two groups. Patients were followed up for a minimum of one week after ICU discharge to assess VTE outcomes.
Results: The incidence of VTE was significantly lower in the treatment group (35.9%) compared to the control group (42.9%, p< 0.05). Risk factors for VTE included mechanical ventilation. The most common VTE type observed was deep vein thrombosis (DVT), with pulmonary embolism (PE) occurring less frequently. No significant bleeding events were observed in the treatment group.
Conclusion: Standardized LMWH prophylaxis effectively reduces VTE incidence in critically ill patients in Tibet without increasing bleeding risk. Regular risk assessment and appropriate prophylaxis should be implemented in high-altitude ICU settings.
Keywords: venous thromboembolism, critical care, thromboprophylaxis, high altitude, Tibet
Introduction
Venous thromboembolism (VTE), encompassing deep vein thrombosis (DVT) and pulmonary embolism (PE), is a significant concern in critically ill patients admitted to intensive care units (ICUs). VTE is associated with increased morbidity, mortality, and healthcare costs.1 The risk of VTE in ICU patients is particularly high due to various factors, including prolonged immobilization, invasive procedures, underlying medical conditions, and the prothrombotic state induced by critical illness.2,3
The incidence of VTE in ICU patients varies widely in the literature, ranging from 5% to 31%, depending on the patient population, diagnostic methods, and prophylaxis strategies employed.4,5 This variability underscores the importance of understanding local epidemiology and risk factors to guide appropriate preventive measures.
While VTE prevention strategies have been well-established in many healthcare settings, their effectiveness and safety in high-altitude regions like Tibet remain understudied. The unique physiological adaptations to high altitude, including increased blood viscosity, enhanced erythropoiesis, and altered coagulation profiles, may influence the risk of VTE and the efficacy of standard prophylactic measures.6,7
High altitude exposure triggers several hematological changes that could potentially elevate the risk of thrombosis. The hypoxic environment stimulates erythropoiesis, resulting in increased hematocrit and blood viscosity due to a higher red blood cell mass.8 Hypoxia-induced endothelial dysfunction results in reduced nitric oxide (NO) production and increased expression of von Willebrand factor (vWF), promoting platelet adhesion and thrombosis. Studies have also reported that hypoxia upregulates procoagulant factors such as tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1), reducing fibrinolysis and promoting thrombosis.9,10 Systemic inflammation is exacerbated in hypoxic environments. Proinflammatory cytokines such as IL-6 and TNF-α are upregulated, increasing platelet activation and the production of procoagulant microparticles. Additionally, high-altitude exposure has been linked to increased levels of fibrinogen and factor VIII, further enhancing thrombogenicity.11 These physiological adaptations collectively increase the likelihood of thrombosis in high-altitude environments.
These physiological adaptations, combined with the known risk factors for VTE in critically ill patients, create a unique challenge in preventing thrombotic complications in high-altitude ICU settings.
Moreover, the ethnic composition of Tibet, with a predominant Tibetan population, introduces another layer of complexity. Tibetans have undergone genetic adaptations to high altitude over thousands of years, which have resulted in distinct physiological characteristics compared to lowland populations.12 These adaptations primarily involve the hypoxia-inducible factor (HIF) pathway and have been associated with lower hemoglobin concentrations compared to other high-altitude populations.13 However, the impact of these genetic adaptations on coagulation profiles and thrombotic risk remains largely unexplored.
The use of pharmacological thromboprophylaxis, particularly low molecular weight heparin (LMWH), has been shown to be effective in reducing VTE risk in critically ill patients at lower altitudes.14 Compared to unfractionated heparin (UFH), LMWH offers several advantages, including predictable pharmacokinetics, a lower incidence of heparin-induced thrombocytopenia (HIT), and reduced bleeding risks. Additionally, LMWH does not require routine monitoring of coagulation parameters, making it more feasible in resource-limited high-altitude ICU settings. However, the altered coagulation profiles observed at high altitudes have raised concerns regarding the appropriate use and safety of anticoagulants in this setting. The balance between reducing thrombotic risk and minimizing potential bleeding complications remains a challenge, leading to ongoing debate about the optimal thromboprophylaxis strategy for critically ill ICU patients at high altitudes.15
Given these unique considerations, there is a pressing need for research specifically addressing VTE risk and prevention strategies in high-altitude critical care environments.
We aim to provide crucial data to inform clinical practice and guide the development of tailored VTE prevention strategies for critically ill patients in Tibet and potentially other high-altitude regions.
Methods
Study Design and Setting
This prospective controlled study was conducted at the People’s Hospital of Lhasa, Tibet Autonomous Region, China. Lhasa is situated at an altitude of approximately 3650 meters (11,975 feet) above sea level, making it one of the highest cities in the world. The hospital is a tertiary care center serving a population of over 3 million people in the Tibet Autonomous Region.
The study protocol was approved by the Ethics Committee of the People’s Hospital of Lhasa. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. Written informed consent was obtained from all prospective patients in the treatment group before study participation. For the retrospective control group, individual informed consent was waived by the institutional ethics committee, as the study utilized anonymized medical records without direct patient involvement.
Participants
The study included two groups of patients: Treatment Group: This group consisted of critically ill ICU patients admitted between 2022 and 2023 who received standardized thromboprophylaxis with low molecular weight heparin (LMWH).
Control Group: This group included patients admitted between 2019 and 2021 who did not receive standardized VTE prophylaxis. This retrospective control group was included to compare VTE incidence with and without standardized prophylaxis. During this period, the study hospital had not yet implemented a formal institutional guideline for VTE prevention in critically ill patients at high altitudes. While some patients may have received thromboprophylaxis based on individual clinical decisions, there was no systematic protocol in place. Since 2022, a standardized thromboprophylaxis regimen has been introduced in response to the increasing recognition of high VTE risk in this population. Patients who met the inclusion and exclusion criteria were consecutively enrolled in the study during the specified period.
Inclusion criteria were:
- Age ≥18 years
- Body weight ≥45 kg
- Expected ICU stay ≥2 days
Exclusion criteria were:
- Presence of VTE at ICU admission
- Liver failure (defined as Child-Pugh class C cirrhosis or acute liver failure)
- End-stage renal failure (defined as estimated glomerular filtration rate <15 mL/min/1.73m2 or requiring dialysis)
- High bleeding risk, defined as: a) Active major bleeding b) History of heparin-induced thrombocytopenia c) Platelet count <50,000×109/L d) International Normalized Ratio (INR) >1.5 e) Recent major surgery (<48 hours) with high bleeding risk
- Pregnancy
- Known hypersensitivity to LMWH
Intervention
Patients in the treatment group received dalteparin 5000 IU subcutaneously once daily as thromboprophylaxis. The choice of dalteparin was based on its availability and established efficacy in VTE prevention in critically ill patients.16
The timing of prophylaxis initiation varied depending on the patient’s condition:
Medical patients: Prophylaxis was initiated immediately upon admission to the ICU, provided there were no contraindications.
Surgical patients: The timing of initiation varied based on the type of surgery: a) Obstetric patients: 12 hours postoperatively b) Orthopedic patients: 8–12 hours postoperatively c) Neurosurgical patients: 24 hours postoperatively, after confirming hemostasis d) Non-surgical patients with intracranial hemorrhage: Early initiation after confirming hemorrhage stability (typically 48–72 hours after admission).
Anticoagulation treatment was discontinued if any of several critical conditions occurred. These included major bleeding, which was defined as overt bleeding associated with a decrease in hemoglobin level of 2 g/dL or more, the need for transfusion of 2 or more units of blood, or bleeding in a critical site such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular, pericardial, or intramuscular areas with compartment syndrome. Additionally, anticoagulation was stopped if the patient’s platelet count decreased to less than 50,000/mm3 or fell below 50% of the baseline value. Suspected heparin-induced thrombocytopenia was also grounds for discontinuation of anticoagulation therapy.
In cases where anticoagulation was discontinued, physical methods such as intermittent pneumatic compression devices were employed for VTE prevention when possible.
VTE Assessment
DVT was assessed using the following methods:
- Ultrasound evaluation: Initial assessment within 2 days of admission, followed by twice-weekly evaluations. Additional scans were performed if DVT was suspected. Trained ultrasonographers evaluated the proximal leg vein system at 1 cm intervals, assessing compressibility at six sites: common femoral vein, proximal, mid, and distal superficial femoral vein, popliteal vein, and venous trifurcation. Any partially or fully non-compressible venous segment was classified as DVT. Wall thickening alone was not considered diagnostic of DVT. Distal DVT was defined as thrombosis occurring below the popliteal vein (such as in the tibial and peroneal veins), while proximal DVT was defined as thrombosis involving the popliteal vein and above (including the femoral and iliac veins).
- Computed Tomography Pulmonary Angiography (CTPA): Used for diagnosing pulmonary embolism (PE) in patients with confirmed DVT or suspected PE with respiratory dysfunction.
- Clinical assessment: Trained researchers evaluated patients for the following signs and symptoms:
- Local temperature changes
- Pitting edema
- Swelling in each lower limb
The presence of one or more of these signs or symptoms was considered consistent with DVT.
Data Collection
Demographic characteristics: Gender, age, ethnicity, body mass index (BMI), primary diagnosis, and comorbidities.
Laboratory tests: White blood cell count (WBC), hemoglobin (HGB), platelet count (PLT), prothrombin time (PT), activated partial thromboplastin time (APTT), thrombin time (TT), fibrinogen (FIB), D-dimer, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), direct bilirubin (DBIL), serum creatinine (sCr), blood urea nitrogen (BUN), and serum sodium (Na+).
Treatment information: Total fluid input, mechanical ventilation, continuous renal replacement therapy (CRRT), use of vasoactive drugs, blood product transfusions (platelets, plasma, red blood cells), ICU length of stay, sedation, and central venous catheterization (internal jugular, subclavian, or femoral vein).
Scoring systems: Acute Physiology and Chronic Health Evaluation (APACHE) II score and Caprini VTE risk assessment score.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or median (interquartile range) based on their distribution. Categorical variables were presented as frequencies and percentages. The Student’s t-test or Mann–Whitney U-test was used to compare continuous variables between groups, while the chi-square test or Fisher’s exact test was used for categorical variables.
Logistic regression analysis was performed to adjust for potential confounding factors in the association between thromboprophylaxis and VTE incidence. Variables with p<0.1 in univariate analysis were included in the multivariate model. Results were presented as odds ratios (ORs) with 95% confidence intervals (CIs).
All statistical analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, USA). A two-tailed p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 134 patients were included in the study, with 78 adult patients (≥18 years old) admitted to the ICU between January 2022 and May 2023 in the treatment group and 56 patients admitted to the ICU between August 2019 and July 2021 in the control group. The baseline characteristics of both groups are presented in Table 1.
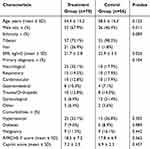 |
Table 1 Baseline Characteristics of the Study Population |
The treatment and control groups were generally well-matched, with no significant differences in age, primary diagnosis, comorbidities, APACHE II scores, or Caprini scores. However, there were significant differences in sex distribution (p=0.011) and BMI (p=0.026) between the two groups.
Incidence of VTE
The overall incidence of VTE and the breakdown of DVT and PE in both groups are presented in Table 2.
 |
Table 2 Incidence of VTE in Treatment and Control Groups |
The incidence of VTE was significantly lower in the treatment group compared to the control group (35.9% vs 42.9%, p=0.041). While there was a trend towards lower DVT incidence in the treatment group, the difference did not reach statistical significance (33.3% vs 39.3%, p=0.077). Proximal DVT was observed in 12.8% of the treatment group and 16.1% of the control group, while distal DVT accounted for 20.5% and 23.2%, respectively. There was no significant difference in PE incidence between the two groups (5.1% vs 8.9%, p=0.387). Our subgroup analysis suggested that older age, male gender, and higher BMI were associated with a higher incidence of VTE.
Risk Factors for VTE
Univariate and multivariate logistic regression analyses were performed to identify independent risk factors for VTE. The results are presented in Table 3.
 |
Table 3 Risk Factors for VTE (Multivariate Logistic Regression Analysis) |
The multivariate analysis, after adjusting for potential confounders, identified mechanical ventilation (p=0.046) as a significant risk factor for VTE. Other risk factors, including advanced age (p=0.128), obesity (p=0.152), prolonged immobilization (p=0.837), malignancy (p=0.521) and sepsis (p=0.077), did not reach statistical significance after adjustment (p > 0.05), suggesting that they may not be independently associated with VTE in this study population.
Safety Outcomes
The safety outcomes, including bleeding events and other adverse effects, are summarized in Table 4.
 |
Table 4 Safety Outcomes in Treatment and Control Groups |
There were no significant differences in bleeding events or other adverse effects between the treatment and control groups. One major bleeding event occurred in the treatment group, but this was not statistically significant compared to the control group. Minor bleeding events and thrombocytopenia were slightly more common in the treatment group, but these differences were not statistically significant.
Discussion
This study provides valuable insights into the incidence, risk factors, and prevention of VTE in critically ill patients in Tibet, a high-altitude region where such data have been scarce. Our findings demonstrate that standardized thromboprophylaxis with LMWH significantly reduces the incidence of VTE in this population without increasing the risk of bleeding complications.
Our study revealed a high overall incidence of VTE, with 35.9% in the treatment group and 42.9% in the control group, significantly exceeding the typical range of 5% to 31% reported in ICU patients at lower altitudes.17,18 This elevated incidence can be attributed to several factors unique to our study population. Firstly, Tibet’s average elevation exceeds 4000 meters above sea level, which is associated with increased blood viscosity, enhanced coagulation, and impaired fibrinolysis, potentially contributing to a higher thrombosis risk.19 Secondly, the predominantly Tibetan ethnicity of our study population may play a role, as genetic adaptations to high altitude could influence coagulation profiles and thrombotic risk, though this area warrants further investigation.20 Additionally, many patients experienced prolonged immobilization due to their critical illness, a well-established risk factor for VTE.21 Lastly, a significant proportion of patients in both groups had central venous catheters, known to increase the risk of upper extremity DVT.22 These combined factors likely contributed to the notably high VTE incidence observed in our study.
The significant reduction in VTE incidence observed in the treatment group (35.9% vs 42.9%, p=0.041) underscores the importance of standardized thromboprophylaxis in this high-risk population. This finding is consistent with previous studies demonstrating the efficacy of LMWH in preventing VTE in critically ill patients.23,24 However, the persistence of a relatively high VTE rate despite prophylaxis suggests that additional preventive measures may be necessary for this unique population.
Our study investigated risk factors for VTE. After adjusting for confounding factors, mechanical ventilation was identified as an independent risk factor for VTE These findings suggest that critically ill patients requiring mechanical ventilation may be at higher risk of developing VTE and should receive careful monitoring and prophylactic interventions. Notably, several variables that were previously considered risk factors for VTE, such as age, BMI, malignancy and prolonged bed rest, were not significantly associated with VTE risk after adjustment. This suggests that their impact on VTE might be mediated by other clinical factors or that their effects were diluted in our study population. However, sepsis demonstrated a trend toward significance, indicating a potential association that requires further investigation.
In our study, no significant differences in major bleeding events or other adverse effects were observed between the LMWH prophylaxis and control groups. This is reassuring, given the concerns about potential bleeding risks associated with anticoagulation at high altitudes due to altered coagulation profiles.25 Our findings suggest that standard LMWH dosing regimens can be safely used in critically ill patients in Tibet, although close monitoring remains essential.
Several limitations of our study should be acknowledged. The single-center design, conducted at one hospital in Lhasa, may limit the generalizability of our findings to other high-altitude regions or healthcare settings. Different high-altitude regions, such as the Andes and the Ethiopian Highlands, have unique environmental and genetic adaptations that may influence VTE risk. Additionally, variations in healthcare infrastructure and ICU management protocols across regions could impact VTE prevention strategies. While our study provides valuable data, the relatively small sample size restricts our ability to conduct robust subgroup analyses and potentially identify additional risk factors. The non-randomized design, utilizing a historical control group rather than randomization, introduces the potential for confounding factors that may have influenced the results. Our limited follow-up, focusing on VTE events during ICU stay and up to one week after discharge, may not capture the long-term protective effects of thromboprophylaxis. Additionally, we lacked comprehensive data on some potential VTE risk factors, such as genetic thrombophilias or detailed medication histories. Future research should incorporate multi-center studies across diverse high-altitude settings to determine whether our findings apply broadly and to develop tailored thromboprophylaxis guidelines for high-altitude ICU patients.
Despite these limitations, our study provides important evidence supporting the use of standardized thromboprophylaxis in critically ill patients in Tibet. Future research directions should encompass several key areas. Multicenter studies involving multiple hospitals in Tibet and other high-altitude regions would yield more generalizable results and allow for exploration of regional variations in VTE risk and prevention strategies. Optimization of prophylaxis regimens through studies comparing different dosing regimens or combinations of pharmacological and mechanical prophylaxis could help identify the most effective strategies for this high-risk population. Further investigation of altitude-specific risk factors, focusing on the physiological adaptations to high altitude and their impact on coagulation, could inform more tailored approaches to VTE prevention in these settings. Studies examining the long-term consequences of VTE in high-altitude populations, including the incidence of post-thrombotic syndrome and chronic thromboembolic pulmonary hypertension, would provide valuable insights for patient management. Lastly, cost-effectiveness analyses evaluating the economic impact of standardized thromboprophylaxis in this setting could inform policy decisions and resource allocation.
Conclusion
Venous thromboembolism represents a significant challenge in the care of critically ill patients in Tibet. Our study demonstrates that standardized thromboprophylaxis with low molecular weight heparin effectively reduces the incidence of VTE without increasing bleeding risk. Further research should also explore regional variations in VTE risk and the impact of different anticoagulation protocols across diverse high-altitude settings. Addressing these gaps will contribute to the development of more tailored and effective VTE prevention strategies for critically ill patients in high-altitude environments.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of Lhasa People’s Hospital. [SYLL2224019]. Written informed consent was obtained from all prospective patients in the treatment group before study participation. For the retrospective control group, individual informed consent was waived by the institutional ethics committee, as the study utilized anonymized medical records without direct patient involvement.
Funding
“Team Based” Medical Aid to Tibet Project of Natural Science Foundation of Xizang Autonomous Region (grant number XZ2022ZR-ZY21(Z)).
Disclosure
All of the authors had no any personal, financial, commercial, or academic conflicts of interest separately.
References
1. Wagner G, Steiner D, Ohrenberger G, et al. Prevalence and incidence of venous thromboembolism in geriatric patients admitted to long-term care hospitals. Sci Rep. 2024;14(1):17737. doi:10.1038/s41598-024-67480-1
2. Weatherald J, Wen C, Stickland MK, et al. Sex differences in venous thromboembolism after Covid-19 infection: a retrospective population-based matched cohort study. Ann Am Thorac Soc. 2024;21(11):1624–1628. doi:10.1513/AnnalsATS.202401-070RL
3. Phillips MR, Purcell LN, Charles AG. Pediatric Venous Thromboembolism-Understanding in Evolution. JAMA Surg. 2024;159(10):1156–1157. doi:10.1001/jamasurg.2024.2488
4. Jin J, Lu J, Su X, et al. Development and validation of an ICU-venous thromboembolism prediction model using machine learning approaches: a multicenter study. Int J Gen Med. 2024;17:3279–3292. doi:10.2147/IJGM.S467374
5. Narayan AS, Ramamoorthy JG, Parameswaran N, et al. Central venous catheter-associated venous thromboembolism in children: a prospective observational study. J Pediatr Hematol Oncol. 2024;46(7):e544–e549. doi:10.1097/MPH.0000000000002923
6. Anand AC, Jha SK, Saha A, et al. Thrombosis as a complication of extended stay at high altitude. Natl Med J India. 2001;14(4):197–201.
7. Bartsch P, Gibbs JS. Effect of altitude on the heart and the lungs. Circulation. 2007;116(19):2191–2202. doi:10.1161/CIRCULATIONAHA.106.650796
8. Mateo-Sidron JAR, Abalde FE, Hidalgo J. Introduction to high altitude medicine. In: High Altitude Medicine: A Case-Based Approach. 2023:1–15.
9. Richalet JP, Jeny F, Callard P, Bernaudin JF. High-altitude pulmonary edema: the intercellular network hypothesis. Am J Physiol Lung Cell Mol Physiol. 2023;325(2):L155–L173. doi:10.1152/ajplung.00292.2022
10. Mannucci PM, Gringeri A, Peyvandi F, et al. Short-term exposure to high altitude causes coagulation activation and inhibits fibrinolysis. Thromb Haemost. 2002;87(2):342–343.
11. Chohan IS, Singh I, Balakrishnan K. Fibrinolytic activity at high altitude and sodium acetate buffer. Thromb Diath Haemorrh. 1974;32(1):65–70.
12. Getu A. Ethiopian native highlander’s adaptation to chronic high‐altitude hypoxia. Biomed Res Int. 2022;2022:5749382. doi:10.1155/2022/5749382
13. O’Brien KA, Simonson TS, Murray AJ. Metabolic adaptation to high altitude. Curr Opin Endocr Metab Res. 2020;11:33–41.
14. Cook D, Meade M; PROTECT Investigators for the Canadian Critical Care Trials Group and the Australian and New Zealand Intensive Care Society Clinical Trials Group. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305–1314.
15. Xiong S, Hou J, Yang H, et al. The profiles of venous thromboembolism at different high altitudes. High Alt Med Biol. 2024;25(3):223–225. doi:10.1089/ham.2023.0081
16. Rodger MA, Kahn SR, Cranney A, et al. Long-term dalteparin in pregnancy not associated with a decrease in bone mineral density: substudy of a randomized controlled trial. J Thromb Haemost. 2007;5(8):1600–1606. doi:10.1111/j.1538-7836.2007.02634.x
17. Gao X, Zeng L, Wang H, et al. Prevalence of venous thromboembolism in intensive care units: a meta-analysis. J Clin Med. 2022;11(22):6691. doi:10.3390/jcm11226691
18. Tran A, Fernando SM, Rochwerg B, et al. Prognostic factors associated with development of venous thromboembolism in critically ill patients—a systematic review and meta-analysis. Crit Care Med. 2022;50(4):e370–e381. doi:10.1097/CCM.0000000000005382
19. Treml B, Wallner B, Blank C, Fries D, Schobersberger W. The influence of environmental hypoxia on hemostasis—A systematic review. Front Cardiovasc Med. 2022;9:813550. doi:10.3389/fcvm.2022.813550
20. Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol. 2006;46(1):18–24. doi:10.1093/icb/icj004
21. Rahmani J, Roudsari AH, Bawadi H, et al. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: a systematic review and dose-response meta-analysis of cohort studies among four million participants. Thromb Res. 2020;192:64–72. doi:10.1016/j.thromres.2020.05.014
22. Sakuraya M, Okano H, Yoshihiro S, Niida S, Kimura K. Insertion site of central venous catheter among hospitalized adult patients: a systematic review and network meta-analysis. Front Med. 2022;9:960135.
23. Smythe MA, Koerber JM. Dalteparin in critically ill patients. N Engl J Med. 2011;365(2):179–180. doi:10.1056/NEJMc1105423
24. Khorana AA, Spyropoulos AC, Zwicker J, Lyman GH, Francis CW. Preventing VTE in outpatients with cancer. Chest. 2012;142(1):265–266. doi:10.1378/chest.12-0423
25. Ninivaggi M, de Laat M, Lancé MM, et al. Hypoxia induces a prothrombotic state independently of the physical activity. PLoS One. 2015;10(10):e0141797. doi:10.1371/journal.pone.0141797
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Venous Thromboembolism Among Hospitalized Patients: Incidence and Adequacy of Thromboprophylaxis – A Retrospective Study
Ambra N, Mohammad OH, Naushad VA, Purayil NK, Mohamedali MG, Elzouki AN, Khalid MK, Illahi MN, Palol A, Barman M, Sharif M, Chalihadan S, Punnorath A, Mostafa A, Al Hariri B, Khidir TGM, Varikkodan I
Vascular Health and Risk Management 2022, 18:575-587
Published Date: 24 July 2022
Factors Associated with Elderly Health-Related Quality of Life in Tibet: A Cross-Sectional Study from a Health Ecological Perspective
Pan Q, Hu J, Yangzong, Zhang X, Zhaxidawa
Journal of Multidisciplinary Healthcare 2024, 17:177-190
Published Date: 25 January 2024

