Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 17
Risk Factors for Non-Alcoholic Fatty Liver Disease in Patients with Bipolar Disorder: A Cross-Sectional Retrospective Study
Authors Wang Y, Li X , Gao Y, Zhang X , Liu Y , Wu Q
Received 5 April 2024
Accepted for publication 7 August 2024
Published 17 August 2024 Volume 2024:17 Pages 3053—3061
DOI https://doi.org/10.2147/DMSO.S463335
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Muthuswamy Balasubramanyam
Ying Wang,1,* Xuelong Li,2,* Yakun Gao,2 Xun Zhang,3 Yiyi Liu,3 Qing Wu1,3
1Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, Anhui Mental Health Center, Hefei Fourth People’s Hospital, Hefei, Anhui, People’s Republic of China; 2Qingdao Mental Health Center, Qingdao, Shandong, People’s Republic of China; 3School of Mental Health and Psychological Sciences, Anhui Medical University, Hefei, Anhui, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qing Wu, Department of Psychiatry, Affiliated Psychological Hospital of Anhui Medical University, Hefei Fourth People’s Hospital, 316 Huangshan Road, Hefei, 230022, People’s Republic of China, Tel +86-13856919530, Email [email protected]
Purpose: The co-morbidity of non-alcoholic fatty liver disease (NAFLD) in patients with bipolar disorder (BD) has a negative impact on patient treatment and prognosis. This study aimed to identify the prevalence of NAFLD in patients with BD and investigate the risk factors of NAFLD.
Patients and Methods: A total of 678 patients with BD were included in the study. Clinical data were obtained from the hospital’s electronic health record system. Data included fasting blood glucose, alanine aminotransferase, triglycerides, aspartate aminotransferase, high-density lipoprotein cholesterol (HDL), alkaline phosphatase, total cholesterol, glutamine transpeptidase, uric acid, apolipoprotein A1, apolipoprotein B, and liver ultrasound findings.
Results: The prevalence of NAFLD was 43.66% in patients with BD. Significant differences in body mass index (BMI), mean age, diabetes prevalence, course of BD, fasting blood glucose, alanine aminotransferase, HDL, alkaline phosphatase, triglycerides, aspartate aminotransferase, uric acid, glutamine transpeptidase, apolipoprotein B, total cholesterol, and apolipoprotein A1 were seen between the groups (all P< 0.01). Male sex, age, BMI, course of BD, alanine aminotransferase, fasting blood glucose, aspartate aminotransferase, diabetes, glutamine transpeptidase, total cholesterol, alkaline phosphatase, triglycerides, uric acid, apolipoprotein B, HDL, and apolipoprotein A1 levels were correlated with NAFLD (all P< 0.05). In patients with BD, diabetes (OR=6.412, 95% CI=1.049− 39.21), BMI (OR=1.398, 95% CI=1.306− 1.497), triglycerides (OR=1.456, 95% CI=1.036− 2.045), and apolipoprotein A1 (OR=0.272, 95% CI=0.110− 0.672) were risk factors for NAFLD (all P< 0.05).
Conclusion: Risk factors for NAFLD in patients with BD include diabetes, BMI, course of BD, and a low level of apolipoprotein A1. A proactive approach to disease management, such as appropriate physical activity and adoption of a healthy diet, and regular monitoring of changes in patient markers should be adopted to reduce the prevalence of NAFLD.
Keywords: China, comorbidity, Psychiatry
Introduction
Bipolar disorder (BD) is an episodic psychiatric disorder characterized by recurrent episodes of depressive and manic symptoms that affects more than 1% of the global population.1 Due to several factors, including the use of antipsychotic drugs, unhealthy lifestyle habits, and unknown pathogenesis, the risk of comorbid somatic diseases such as metabolic syndrome and related cardiovascular diseases is markedly higher in patients with BD than in the general population.2,3 In addition, negative eating behaviours and drug dependence, often adopted by adolescents, especially those with BD, in pursuit of “pleasurable stimuli” in response to pressure, increase the prevalence of obesity and non-alcoholic fatty liver disease (NAFLD).4,5 BD is associated with a potential loss of approximately 10–20 years of life owing to physical disease such as cardiovascular disease, which severely worsens patient prognosis.6
NAFLD is currently the second most common liver disease in the world; NAFLD is characterized by excessive accumulation of fat in the liver and is closely associated with metabolic syndrome.7 NAFLD is an independent risk factor for cardiovascular diseases, such as subclinical coronary or carotid atherosclerosis, cardiomyopathy, and arrhythmias, and plays a negative role in patients’ life expectancy.8 The prevalence of NAFLD is increasing globally, with an average prevalence of over 30% as of 2019.9 The prevalence of NAFLD is expected to increase annually due to the rising global prevalence of diabetes, metabolic syndrome, and obesity.10 The incidence of advanced liver disease, such as cirrhosis and hepatocellular carcinoma, may be increased as well.11 Chinese patients with psychiatric disorders have widespread hepatocellular steatosis and liver fibrosis.12
Recent study has shown that NAFLD significantly increases the risk of developing BD compared to other psychiatric disorders, which is a gene-level effect, suggesting that it is important to explore the relationship between BD and NAFLD.13 Prevalence of NAFLD is higher in patients with BD (28.4%) and is related to sex, metabolic function, and antipsychotic medications.14,15 Our team has previously explored the risk factors for NAFLD in patients with BD of different genders, however, unlike the present study, the previous study has not explored the prevalence of and risk factors for NAFLD in BD patients as a whole, nor have they explored the in-depth mechanisms of risk factors and NAFLD.16 These shortcomings are also present in other study.2 NAFLD has a significant adverse effect on patients’ unnatural death and aggravates the disease burden. The aim of this study was to investigate the prevalence of and factors influencing NAFLD in patients with BD and provide a theoretical support for the development of future clinical treatment plans.
Materials and Methods
This retrospective cross-sectional study included first-episode and unmedicated inpatients with BD at the Hefei Fourth People’s Hospital (Hefei, China) between July 2020 and June 2022. Patients’ demographic and clinical data were collected in an anonymous format from an electronic health record system provided by Hefei Fourth People’s Hospital. On the basis of compliance with the Declaration of Helsinki, the Medical Ethics Committee of the Fourth People’s Hospital of Hefei City approved the study and waived the requirement for obtaining informed consent because the study was retrospective, informed consent could not be obtained from all subjects in its entirety, all data were collected anonymously, the study did not cause any harm to the patients, all data were encrypted, and all investigators signed confidentiality agreements.
The inclusion criteria were as follows: patients who 1) were diagnosed with BD by two or more attending physicians and had a BD diagnosis that met the 10th revision of the International Classification of Diseases (ICD-10) diagnostic criteria;17 2) were at least 18 years old; 3) had no diseases that cause fatty liver; 4) were not treated with liver-protective drugs; and 5) had no systematic diagnosis and treatment of BD and fatty liver within the past 3 months. The exclusion criteria were as follows: 1) a previous diagnosis of substance or drug abuse and 2) neurodegenerative diseases, including mental retardation and Alzheimer’s disease. In total, 678 participants were included.
Clinical Assessment
All data were collected from the electronic health record system. To assess the somatic status of newly admitted patients, biochemical tests and liver ultrasonography were routinely performed on all patients. Biochemical tests included fasting blood glucose, alanine transaminase (ALT), alkaline phosphatase (ALP), total cholesterol (TC), glutamyl transpeptidase (GGT), triglycerides (TG), aspartate aminotransferase (AST), high-density lipoprotein cholesterol (HDL), apolipoprotein A1 (Apo A1), uric acid (UA), and apolipoprotein B (Apo B). All patients had venous blood collected after 8 h of fasting between the night of admission and the early morning of the next day between 06:00 and 07:00. The blood samples were sent to the testing department of the hospital for biochemical tests within 1 h. An automatic biochemistry analyzer (AU480; Beckman Coulter, Brea, CA, USA) was used to measure the plasma biochemical parameters using commercial kits (Roche, Basel, Switzerland).
Definition of NAFLD
The diagnostic criteria for NAFLD include: 1) the presence of definitive hepatic steatosis as evidenced by histology or imaging and 2) the absence of other causes of hepatic fat accumulation, such as hepatitis, heavy alcohol consumption, hereditary diseases, or medications.18 After ensuring that the above criteria were met, all participants underwent liver ultrasonography, a fairly sensitive and non-invasive tool for diagnosing NAFLD, performed by a uniformly trained ultrasonographer.19 Based on the examination results, the participants were divided into two groups: NAFLD and non-NAFLD.
Statistical Analyses
All analyses were performed using SPSS software (version 26.0; IBM Corp., Armonk, NY, USA). Categorical data are presented as numbers and percentages. Means and standard deviations, or medians and interquartile ranges, are used to represent continuous variables. Categorical variables were analyzed using the Chi-square test. Normality was tested using the Shapiro–Wilk test. The independent samples t-test and Mann–Whitney U-test were used to analyze continuous data. Cramer’s V correlation coefficient and Spearman’s rank correlation coefficient were used to determine the correlation between variables. Regression analysis was used to analyze the factors influencing NAFLD. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. All statistical tests were two-tailed with an alpha level of 0.05.
Results
Baseline Characteristics
Of the 678 participants, 343 were women (50.59%), and 335 were men (49.41%). The prevalence of NAFLD was 43.66%. The difference in the mean age between the NAFLD group (36.95±10.45) and non-NAFLD group (31.84±10.49) was significant (P<0.001). There were no differences in the marital status and years of education between the groups (all P>0.05) (Table 1).
 |
Table 1 Differences in Demographic Data Between the Groups |
Diabetes prevalence, body mass index (BMI), course of BD, blood glucose, ALT, apo B, TG, GGT, UA, ALP, TC, and AST were higher in the NAFLD group than in the non-NAFLD group (all P<0.05). Apolipoprotein A1 (apo A1) and HDL were lower in the NAFLD group than in the non-NAFLD group (all P<0.05). The psychotic symptoms and types of seizures did not differ significantly between the groups (P>0.05) (Table 2).
 |
Table 2 Differences in Clinical Data Between the Groups |
Correlation Analysis Between NAFLD and Variables
NAFLD status was positively and significantly correlated with male sex (r=0.129), diabetes (r=0.157), age (r=0.205), BMI (r=0.590), course of BD (r=0.186), blood glucose (r=0.245), ALT (r=0.334), ALP (r=0.187), AST (r=0.130), GGT (r=0.386), TC (r=0.198), TG (r=0.358), UA (r=0.209), and apo B (r=0.283) (all P<0.05). NAFLD status was negatively and significantly correlated with HDL (r=−0.229) and apo A1 levels (r=−0.120) (all P<0.05) (Table 3).
 |
Table 3 Correlations Between NAFLD and Other Variables |
Risk Factors for NAFLD
To further screen out factors for NAFLD, all variables were first included in unitary logistic regression one by one, and variables with statistically significant results were uniformly included in multiple logistic regression. The final results were as follows: diabetes (OR=6.412, 95% CI=1.049−39.215), BMI (OR=1.398, 95% CI=1.306−1.497), TG (OR=1.456, 95% CI=1.036−2.045), and low apo A1 levels (OR=0.272, 95% CI=0.110−0.672) were risk factors for NAFLD in patients with BD (all P<0.05) (Table 4).
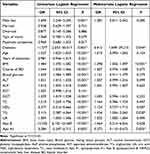 |
Table 4 Risk Factors for NAFLD in Patients with BD |
Discussion
The prevalence of NAFLD in the 678 participants was 43.66%, which is higher than the recent average reported among Chinese adults (29.2%).20 We found significant differences in diabetes prevalence, BMI, course of BD, blood glucose, ALT, ALP, AST, GGT, TC, TG, UA, and apo B levels between the groups. Moreover, male sex, diabetes, age, BMI, course of BD, blood glucose, ALT, ALP, AST, GGT, TC, UA, apo B, HDL, TG, and apo A1 levels were correlated with the prevalence of NAFLD. Furthermore, diabetes, BMI, TG, and low levels of apo A1 were risk factors for NAFLD in patients with BD.
BD adversely affects patients not only in terms of impairment of social functioning and cognitive abilities, but also in terms of many complex somatic disorders. Patients with BD are at high risk for NAFLD, hyperlipidemia, and ischemic heart disease.14,21,22 In up to 40% of individuals with nonpsychiatric disorders, portal vein and lobular inflammation and hepatocyte injury progress to nonalcoholic steatohepatitis; some of these patients develop progressive fibrosis and cirrhosis.23 Therefore, it is reasonable to speculate that NAFLD poses a serious health risk and is a significant adverse factor in the normal life expectancy of patients with BD.
In this study, significant differences in BMI were noted between the NAFLD and non-NAFLD groups, and regression analysis revealed that BMI was a risk factor for NAFLD in patients with BD. As a recurrent mood disorder, BD is often accompanied by unhealthy lifestyle habits such as eating disorders and reduced physical activity during the long course of the disease, leading to metabolic dysfunction and cardiovascular disease.24 An increased BMI often implies the presence of obesity. An increase in BMI promotes the inward flow of fatty acids in hepatocytes, during which the overproduction of reactive oxygen species further leads to oxidative stress, which in turn leads to mitochondrial dysfunction when mitochondrial respiratory chain activity and ATP formation are impaired, further contributing to the accumulation of fat in the liver.25 This increases the risk of developing NAFLD. Patients with BD have a higher prevalence of obesity than the general population.26 Patients with BD show significantly more sedentary behavior and less physical activity than individuals without BD, and a higher BMI is associated with a decrease in physical activity above a moderate level.27 Physical inactivity has been confirmed to be an independent risk factor for NAFLD.28 A large study in the United States showed that people who were sedentary for more than 8 h per day had a 44% higher risk of developing NAFLD than healthy active people.29 Therefore, performing an appropriate amount of exercise could play a positive role in reducing the prevalence of NAFLD in patients with BD by lowering their BMI.
Studies have shown that people with BD are more likely to have abnormal weight than healthy individuals because alleles that are associated with BD are also associated with a higher BMI.30 Weight gain leads to adipose tissue dysfunction, resulting in local inflammation and the upregulation of cytokines that promote insulin resistance, which increases blood sugar concentration.31 Insulin resistance, in turn, impairs the ability of adipocytes to store fat, causing free fatty acids to be released into the bloodstream and absorbed by the liver, ultimately leading to fat accumulation in the liver.26 These results explain why TG is a risk factor for NAFLD in patients with BD. Simultaneously, the accumulation of fatty acids in hepatocytes promotes the synthesis of triglycerides, wherein diacylglycerol intermediates accumulate and weaken liver insulin signal transduction by activating the protein kinase c epsilon.32 This process further aggravates the body’s insulin resistance. In the context of obesity and hyperinsulinemia, the liver promotes NAFLD using carbohydrates to produce fat, which leads to steatosis.33
In the present study, diabetes was found to be a risk factor for NAFLD in patients with BD. A recent study showed that the risk of developing type 2 diabetes was 2.1 times higher in people with BD than in the healthy population, suggesting that people with BD face a serious risk of diabetes.34 These results can be corroborated by the results of Chen et al that identified a higher risk of diabetes for patients with BD up to three years before the onset of BD.35 After the first onset of BD, the risk of up to 15 chronic somatic diseases such as liver disease and diabetes continues to increase.36 A complex bidirectional relationship exists between diabetes mellitus and NAFLD. Diabetes exacerbates the impairment of the glucolipid metabolic pathways, significantly increasing the risk of NAFLD.37 In addition, NAFLD exacerbates insulin resistance, which increases the release of cytokines and hepatic factors, and promotes diabetes development.38 Insulin resistance leads to increased hepatic de novo lipogenesis as well as a reduction in insulin-inhibition of lipolysis in adipose tissue, with the net effect of increasing hepatic lipid content.19 Notably, insulin resistance-associated inflammation, oxidative stress, and mitochondrial dysfunction are widespread in the population of patient with BD, not only promoting the development of BD but also leading to NAFLD.19 The treatment and improvement of BD and diabetes are often synchronized, suggesting that equal attention should be paid to prevention and treatment.38
A previous study found that patients with BD had significantly lower levels of Apo A1 compared to healthy individuals.39 This phenomenon has been observed before the onset of the disease.40 Apo A1, the major apolipoprotein fraction of HDL, is a 243 amino acid polypeptide that is of fundamental importance in maintaining the structural integrity of HDL particles.41 Apo A1 has been shown to have a protective effect against NAFLD, effectively reducing steatosis, inflammation, liver coefficient, total liver cholesterol, triglyceride, and LDL levels to reduce liver fat accumulation through its anti-oxidative stress effects.42 Apo A1 may protect against hepatocyte lipid accumulation by inhibiting peroxisome proliferator-activated receptors, including peroxisome proliferator-activated receptors A, G, and D.43 Increased human apo A1 expression can lead to decreased levels of endoplasmic reticulum stress and lipid gene-related proteins, including acetyl-CoA carboxylase, sterol regulatory element-binding protein 1, and fatty acid synthase.44 In addition, some apoptotic gene products associated with endoplasmic reticulum stress are also affected by increased apo A1 expression, suggesting that increased apo A1 expression can reduce lipid levels and inhibit endoplasmic reticulum stress and adipogenesis in hepatocytes to reduce steatosis and the risk of NAFLD.44
Limitations
This study has some limitations. First, the effects of the antipsychotic drugs could not be completely excluded, which may have led to erroneous results. Second, the study did not include a healthy population as a control group. Therefore, we could not compare NAFLD prevalence between patients with BD and healthy individuals. Third, owing to its cross-sectional design, this study could not explore the causal relationships between the variables.
Conclusions
Patients with unmedicated BD have a higher risk of developing NAFLD than the general population. Risk factors include BMI, diabetes, course of BD, and a low level of apo A1. Special attention should be paid to the risk of NAFLD in patients with BD during the course of treatment, especially after the use of psychotropic medications. Blood lipids and BMI should be constantly monitored, especially in patients with diabetes and a long course of the disease, and risk factors should be actively eliminated. Simultaneously, attention should be paid to supervising patients with BD to maintain a healthy lifestyle and healthy dietary habits and engage in physical exercise in moderation to reduce the risk of NAFLD.
Abbreviations
ALP, Alkaline phosphatase; ALT, Alanine transaminase; Apo A1, Apolipoprotein A1; Apo B, Apolipoprotein B; AST, Aspartate aminotransferase; BD, Bipolar disorder; BMI, Body mass index; GGT, Glutamyl transpeptidase; HDL, High-density lipoprotein; NAFLD, Non-alcoholic fatty liver disease; TC, Total cholesterol; TG, Triglyceride; UA, Uric acid.
Data Sharing Statement
The datasets used and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee of the Hefei Fourth People’s Hospital. The Ethics Committee waived the requirement of obtaining informed consent because the study was retrospective, did not cause any injury to patients, and all data (including basic personal information and detailed medical records) were encrypted and all researchers signed a confidentiality agreement. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Acknowledgments
We thank the Anhui Mental Health Center for supporting this study. This study would not have been possible without the support of our hospital.
Funding
This research was funded by the Hefei Health Applied Medicine Research Project (grant number: Hwk2021yb015) and the Hospital Project of Hefei Fourth People’s Hospital (grant numbers: 2019001, HFSY2022YB08, HFSY2022ZD11, HFSY2023YB05 and HFSY2023ZD01). The funders had no role in the study design; collection, analysis, or interpretation of the data; writing of the manuscript; or in the decision to submit the article for publication.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Vieta E, Berk M, Schulze TG, et al. Bipolar Disorders. Nat Rev Dis Primers. 2018;4:18008. doi:10.1038/nrdp.2018.8
2. Vancampfort D, Vansteelandt K, Correll CU, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: a meta-analysis of prevalence rates and moderators. Am J Psychiatry. 2013;170(3):265–274. doi:10.1176/appi.ajp.2012.12050620
3. Wang Z, Li T, Li S, et al. The prevalence and clinical correlates of medical disorders comorbidities in patients with bipolar disorder. BMC Psychiatry. 2022;22(1):176. doi:10.1186/s12888-022-03819-0
4. Tarantino G, Cataldi M, Citro V. Could alcohol abuse and dependence on junk foods inducing obesity and/or illicit drug use represent danger to liver in young people with altered psychological/relational spheres or emotional problems? Int J Mol Sci. 2022;23(18). doi:10.3390/ijms231810406
5. Moon E, Chang JS, Choi S, et al. Characteristics of stress-coping behaviors in patients with bipolar disorders. Psychiatry Res. 2014;218(1–2):69–74. doi:10.1016/j.psychres.2014.03.047
6. McIntyre RS, Berk M, Brietzke E, et al. Bipolar Disorders. Lancet. 2020;396(10265):1841–1856. doi:10.1016/S0140-6736(20)31544-0
7. Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi:10.1016/j.jhep.2020.03.039
8. Sinn DH, Kang D, Chang Y, et al. Non-alcoholic fatty liver disease and progression of coronary artery calcium score: a retrospective cohort study. Gut. 2017;66(2):323–329. doi:10.1136/gutjnl-2016-311854
9. Henry L, Paik J, Younossi ZM. Review article: the epidemiologic burden of non-alcoholic fatty liver disease across the world. Aliment Pharmacol Ther. 2022;56(6):942–956. doi:10.1111/apt.17158
10. Buzzetti E, Pinzani M, Tsochatzis EA. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism. 2016;65(8):1038–1048. doi:10.1016/j.metabol.2015.12.012
11. Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD Disease Burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi:10.1016/j.jhep.2018.05.036
12. Li H, Chen C, Chen Y, et al. High prevalence of metabolic diseases, liver steatosis and fibrosis among Chinese psychiatric patients. BMC Psychiatry. 2023;23(1):206. doi:10.1186/s12888-023-04684-1
13. Xu WM, Zhang HF, Feng YH, et al. Genetically predicted fatty liver disease and risk of psychiatric disorders: a Mendelian randomization study. World J Clin Cases. 2024;12(14):2359–2369. doi:10.12998/wjcc.v12.i14.2359
14. Ma Q, Yang F, Ma B, et al. Prevalence of nonalcoholic fatty liver disease in mental disorder inpatients in china: an observational study. Hepatol Int. 2021;15(1):127–136. doi:10.1007/s12072-020-10132-z
15. Godin O, Leboyer M, Belzeaux R, et al. Non-alcoholic fatty liver disease in a sample of individuals with bipolar disorders: results from the face-bd cohort. Acta Psychiatr Scand. 2021;143(1):82–91. doi:10.1111/acps.13239
16. Wang Y, Liu Y, Zhang X, et al. Sex-based differences and risk factors for comorbid nonalcoholic fatty liver disease in patients with bipolar disorder: a cross-sectional retrospective study. Diabet Metab Syndr Obes. 2023;16:3533–3545. doi:10.2147/DMSO.S428523
17. Kaltenboeck A, Winkler D, Kasper S. Bipolar and Related Disorders in Dsm-5 and Icd-10. CNS Spectr. 2016;21(4):318–323. doi:10.1017/S1092852916000079
18. Wong VW, Chan WK, Chitturi S, et al. Asia-Pacific working party on non-alcoholic fatty liver disease guidelines 2017-part 1: definition, risk factors and assessment. J Gastroenterol Hepatol. 2018;33(1):70–85. doi:10.1111/jgh.13857
19. Gangopadhyay A, Ibrahim R, Theberge K, et al. Non-Alcoholic fatty liver disease (nafld) and mental illness: mechanisms linking mood, metabolism and medicines. Front Neurosci. 2022;16:1042442. doi:10.3389/fnins.2022.1042442
20. Zhou F, Zhou J, Wang W, et al. Unexpected rapid increase in the burden of nafld in china from 2008 to 2018: a systematic review and meta-analysis. Hepatol. 2019;70(4):1119–1133. doi:10.1002/hep.30702
21. Hsu JH, Chien IC, Lin CH. Increased risk of hyperlipidemia in patients with bipolar disorder: a population-based study. Gen Hosp Psychiatry. 2015;37(4):294–298. doi:10.1016/j.genhosppsych.2015.04.003
22. Hsu JH, Chien IC, Lin CH. Increased risk of ischemic heart disease in patients with bipolar disorder: a population-based study. J Affect Disord. 2021;281:721–726. doi:10.1016/j.jad.2020.11.083
23. Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic Fatty Liver Disease. Nat Rev Dis Primers. 2015;1:15080. doi:10.1038/nrdp.2015.80
24. Aguglia A, Salvi V, Amerio A, et al. Number of episodes and duration of illness associated with hypertension and 10-year cardiovascular risk in patients with bipolar disorder Type I. Psychiatry Res. 2022;308:114344. doi:10.1016/j.psychres.2021.114344
25. Legaki AI, Moustakas II, Sikorska M, et al. Hepatocyte Mitochondrial Dynamics and Bioenergetics in Obesity-Related Non-Alcoholic Fatty Liver Disease. Curr Obes Rep. 2022;11(3):126–143. doi:10.1007/s13679-022-00473-1
26. Li X, Shi X, Tan Y, et al. Metabolic indexes of obesity in patients with common mental disorders in stable stage. BMC Psychiatry. 2022;22(1):91. doi:10.1186/s12888-022-03752-2
27. Tew GA, Bailey L, Beeken RJ, et al. Physical activity in adults with schizophrenia and bipolar disorder: a large cross-sectional survey exploring patterns, preferences, barriers, and motivating factors. Int J Environ Res Public Health. 2023;20(3):2548. doi:10.3390/ijerph20032548
28. Kim D, Vazquez-Montesino LM, Li AA, et al. Inadequate physical activity and sedentary behavior are independent predictors of nonalcoholic fatty liver disease. Hepatol. 2020;72(5):1556–1568. doi:10.1002/hep.31158
29. Kim D, Konyn P, Cholankeril G, et al. Physical Activity is associated with nonalcoholic fatty liver disease and significant fibrosis measured by fibroscan. Clin Gastroenterol Hepatol. 2022;20(6):e1438–e1455. doi:10.1016/j.cgh.2021.06.029
30. Bahrami S, Steen NE, Shadrin A, et al. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry. 2020;77(5):503–512. doi:10.1001/jamapsychiatry.2019.4188
31. Maher JJ, Leon P, Ryan JC. Beyond Insulin Resistance: innate Immunity in Nonalcoholic Steatohepatitis. Hepatol. 2008;48(2):670–678. doi:10.1002/hep.22399
32. Samuel VT, Liu ZX, Wang A, et al. Inhibition of protein kinase cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J Clin Invest. 2007;117(3):739–745. doi:10.1172/JCI30400
33. Lambert JE, Ramos-Roman MA, Browning JD, et al. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterol. 2014;146(3):726–735. doi:10.1053/j.gastro.2013.11.049
34. Liu YK, Ling S, Lui LMW, et al. Prevalence of Type 2 diabetes mellitus, impaired fasting glucose, general obesity, and abdominal obesity in patients with bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2022;300:449–461. doi:10.1016/j.jad.2021.12.110
35. Chen PH, Tsai SY, Pan CH, et al. High risk and trajectories of physical illnesses before the diagnosis of bipolar disorder. J Affect Disord. 2021;281:99–108. doi:10.1016/j.jad.2020.11.127
36. Launders N, Kirsh L, Osborn DPJ, et al. The Temporal relationship between severe mental illness diagnosis and chronic physical comorbidity: a UK primary care cohort study of disease burden over 10 years. Lancet Psychiatry. 2022;9(9):725–735. doi:10.1016/S2215-0366(22)00225-5
37. Stefan N, Cusi K. A Global View of the Interplay between Non-Alcoholic Fatty Liver Disease and Diabetes. Lancet Diabetes Endocrinol. 2022;10(4):284–296. doi:10.1016/S2213-8587(22)00003-1
38. Targher G, Corey KE, Byrne CD, et al. The Complex Link between NAFLD and Type 2 Diabetes Mellitus - Mechanisms and Treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599–612. doi:10.1038/s41575-021-00448-y
39. Song YR, Wu B, Yang YT, et al. Specific alterations in plasma proteins during depressed, manic, and euthymic states of bipolar disorder. Braz J Med Biol Res. 2015;48(11):973–982. doi:10.1590/1414-431X20154550
40. Haenisch F, Alsaif M, Guest PC, et al. Multiplex immunoassay analysis of plasma shows prominent upregulation of growth factor activity pathways linked to gsk3beta signaling in bipolar patients. J Affect Disord. 2014;156:139–143. doi:10.1016/j.jad.2013.12.008
41. Deng S, Xu Y, Zheng L. HDL Structure. Adv Exp Med Biol. 2022;1377:1–11. doi:10.1007/978-981-19-1592-5_1
42. Wang W, Zhou W, Wang B, et al. Antioxidant effect of apolipoprotein a-i on high-fat diet-induced non-alcoholic fatty liver disease in rabbits. Acta Biochim Biophys Sin (Shanghai). 2013;45(2):95–103. doi:10.1093/abbs/gms100
43. Chen C, Li H, Song J, et al. Role of Apolipoprotein A1 in Ppar Signaling Pathway for Nonalcoholic Fatty Liver Disease. PPAR Res. 2022;2022:4709300. doi:10.1155/2022/4709300
44. Guo Q, Zhang C, Wang Y. Overexpression of apolipoprotein a-i alleviates endoplasmic reticulum stress in hepatocytes. Lipids Health Dis. 2017;16(1):105. doi:10.1186/s12944-017-0497-3
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

