Back to Journals » Therapeutics and Clinical Risk Management » Volume 21
Risk Factors for Postoperative Infections in Severe Traumatic Brain Injury Patients Undergoing Emergency Craniotomy
Received 17 December 2024
Accepted for publication 13 April 2025
Published 2 May 2025 Volume 2025:21 Pages 609—619
DOI https://doi.org/10.2147/TCRM.S512780
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Zhiyu Zhang,1,* Lin Xu,2,* Sheng Xu1
1Emergency Department, Brain Hospital of Hunan Province (The Second People’s Hospital of Hunan Province), Changsha, Hunan, People’s Republic of China; 2Department of Pediatrics, Brain Hospital of Hunan Province (The Second People’s Hospital of Hunan Province), Changsha, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Sheng Xu, Emergency Department, The Second People’s Hospital of Hunan Province (Brain Hospital of Hunan Province), No. 427, Section 3, Furong Middle Road, Yuhua District, Changsha, Hunan Province, 410007, People’s Republic of China, Email [email protected]
Background and Aim: Severe traumatic brain injury (TBI) patients undergoing emergency craniotomy are at high risk of postoperative infections. This study aims to identify the risk factors associated with these infections to improve patient outcomes.
Methods: A retrospective cohort study was conducted, including 312 severe TBI patients who underwent emergency craniotomy at Brain Hospital of Hunan Province between December 2019 and December 2021. Clinical data were collected, and both univariate and multivariate logistic regression analyses were performed to identify risk factors for postoperative infections.
Results: Among the 312 patients, 57 (18.3%) developed postoperative infections. Multivariate analysis identified several significant risk factors, including older age (OR=1.75, 95% CI: 1.23– 2.49), prolonged surgery duration (OR=2.01, 95% CI: 1.38– 2.92), presence of preoperative infection (OR=2.59, 95% CI: 1.64– 4.09), and lower Glasgow Coma Scale (GCS) score on admission (OR=1.82, 95% CI: 1.21– 2.74).
Conclusion: Identifying patients at high risk for postoperative infections can help guide preventive measures and improve outcomes in severe TBI patients undergoing emergency craniotomy.
Keywords: traumatic brain injury, craniotomy, postoperative infections, risk factors, Glasgow Coma Scale
Introduction
Severe traumatic brain injury (TBI) represents a major global health concern, significantly contributing to morbidity and mortality. According to the World Health Organization, TBI is projected to become the third leading cause of global disease burden. Falls, traffic accidents, and assaults are the primary causes of TBI, with the incidence particularly high among young adults and the elderly.1–3
Emergency craniotomy, a surgical procedure aimed at relieving intracranial pressure and preventing secondary brain injury, is frequently performed in severe TBI cases.4 Despite its life-saving potential, the procedure is associated with a range of complications, among which postoperative infections are notably prevalent.5 These infections can lead to prolonged hospital stays, increased healthcare costs, and higher mortality rates.6 Postoperative infections remain a major concern following emergency craniotomy in severe TBI patients, with surgical site infections (SSI), meningitis, ventriculitis, and bloodstream infections being among the most common complications. These infections significantly contribute to prolonged hospital stays, increased morbidity, and higher healthcare costs. Identifying risk factors for these infections is critical for implementing targeted infection prevention protocols, such as optimized perioperative antibiotic prophylaxis, strict aseptic techniques during surgery, and close postoperative monitoring. Furthermore, systemic infections before surgery, such as pneumonia or urinary tract infections, have been associated with increased postoperative complications, underscoring the need for rigorous preoperative infection control strategies.7–9 These factors include patient demographics (such as age and gender), preexisting medical conditions (such as diabetes and hypertension), injury severity, and perioperative management practices. For example, older age and prolonged surgical duration have been consistently associated with higher infection rates. Additionally, the presence of systemic infections before surgery has been recognized as a significant risk factor for postoperative complications.10,11
However, there is a scarcity of studies specifically focusing on the risk factors for postoperative infections in the context of emergency craniotomy for severe TBI. The unique physiological and pathological characteristics of severe TBI patients, combined with the urgent nature of the surgery, may influence infection risks differently compared to other surgical populations. Decompressive craniectomy (DC) treatment in the ICU is frequently required for severe TBI patients to manage intracranial hypertension. While DC can be lifesaving, it is also associated with an elevated risk of postoperative infections due to prolonged hospital stays, exposure to invasive medical devices, and impaired immune responses. Wettervik et al12 analyzed 61 TBI patients who received DC treatment in the ICU and concluded that there is a high risk of surgical complications and postoperative bleeding, necessitating special attention to hemostasis. Kourbeti et al13 through a retrospective study, found that respiratory tract infections are common in TBI patients undergoing surgery, with Acinetobacter species emerging as new pathogens. Postoperative and device-related cerebrospinal fluid and environmental transmission are significant risk factors for the development of SSI. However, previous studies have rarely explored the risk factors for postoperative infections in patients with severe traumatic brain injury undergoing emergency craniotomy. Understanding these specific risk factors is crucial for developing targeted preventive measures and improving patient outcomes. Identifying high-risk patients and understanding the modifiable risk factors for postoperative infections can lead to improved perioperative care strategies. For instance, optimizing surgical duration, managing preoperative infections effectively, and tailoring postoperative monitoring can significantly reduce infection rates.
While postoperative infections in neurosurgery have been extensively studied, research specifically focusing on emergency craniotomy in severe TBI patients remains limited. Most existing studies have either analyzed postoperative infections in elective neurosurgical procedures or included emergency craniotomy cases without isolating severe TBI patients as a distinct subgroup. This lack of specificity makes it challenging to draw precise conclusions regarding the unique risk factors associated with emergency craniotomy in severe TBI patients. Severe TBI cases often require urgent surgical intervention under suboptimal conditions, such as emergency settings, increased intracranial pressure, polytrauma, and a compromised immune response. These factors may increase the risk of postoperative infections differently from elective surgeries, where preoperative preparation and infection control measures are more structured. Although a few studies have examined infection risks in neurosurgery, there is a lack of targeted research that specifically evaluates postoperative infection risk factors in patients undergoing emergency craniotomy for severe TBI. A more detailed understanding of these risk factors is critical for improving perioperative infection prevention protocols and optimizing postoperative patient outcomes.6
To rigorously evaluate the factors contributing to postoperative infections, univariate and multivariate logistic regression analyses were employed in this study. Univariate analysis allowed us to explore individual associations between risk factors and infection incidence, providing an initial screening of potential predictors. Multivariate analysis was then conducted to adjust for confounding variables, ensuring that only independent risk factors were identified. This statistical approach enhances the reliability of our findings and offers a more precise understanding of the key determinants of postoperative infections.14,15
Furthermore, this study aims to contribute to the broader field of neurosurgery by highlighting the importance of targeted infection prevention protocols in emergency surgical settings.
Materials and Methods
Patient Selection
This retrospective cohort study included TBI patients treated at the Department of Neurosurgery, Brain Hospital of Hunan Province, between December 2018 and December 2020. TBI was defined as brain injury caused by external forces, including brain contusion, traumatic epidural hemorrhage, traumatic subdural hematoma, and traumatic cerebral hemorrhage.16 Diagnosis was confirmed by two imaging specialists through brain CT and MRI findings.
Strict inclusion criteria were applied: (1) confirmed severe TBI diagnosis, and (2) no prior treatment before admission. Exclusion criteria included: (1) death within 24 hours of admission, (2) presence of malignancy, and (3) incomplete clinical information. Severe TBI is typically defined based on the Glasgow Coma Scale (GCS), which assesses a patient’s level of consciousness after a brain injury.17 The GCS scores patients on a scale of 3 to 15 based on their verbal, motor, and eye-opening responses to stimuli. Severe TBI is characterized by a GCS score of 8 or less. Diffuse Axonal Injury (DAI) was diagnosed based on characteristic MRI or CT findings, including multiple punctate hemorrhages in the white matter and corpus callosum, consistent with traumatic shearing injury. Patients with severe TBI often exhibit prolonged unconsciousness or coma, and they may experience significant neurological deficits and complications. The study received approval from the Ethics Committee of Brain Hospital of Hunan Province and adhered to the Declaration of Helsinki. All patients provided informed consent.
Upon emergency admission, laboratory tests (routine blood, urine, liver and kidney function, electrolytes, and blood and urine amylase) were performed within two hours. Imaging studies (head CT or MRI) were also conducted. The diagnosis of TBI was finalized through a multidisciplinary discussion involving neurologists, neurosurgeons, and imaging physicians.
Data Analysis
The statistical analysis was conducted in a systematic manner to identify significant risk factors for postoperative infections. The initial step involved univariate analysis, where each variable was analyzed independently to assess its association with postoperative infection status. In our statistical analysis, we classified variables into categorical and continuous types. Chi-square tests were used to assess the association between categorical variables (such as gender, preoperative infection status, and ICU admission) and the occurrence of postoperative infections. The t-test was used to compare continuous variables (such as age, surgery duration, and GCS scores) between the groups with and without postoperative infections. Categorical variables: Gender, preoperative infection, ICU admission, etc; Continuous variables: Age, surgery duration, Glasgow Coma Scale (GCS) scores, etc. This analysis utilized chi-square tests for categorical variables and t-tests for continuous variables, with a significance threshold set at p<0.05. Variables that showed a statistically significant association in the univariate analysis (p<0.05) were then included in the multivariate logistic regression model. This model was employed to determine the independent risk factors for postoperative infections while controlling for potential confounding variables. The logistic regression provided odds ratios (OR) with 95% confidence intervals (CI), quantifying the strength of association between each risk factor and the likelihood of developing an infection.
To ensure that our study was sufficiently powered to detect meaningful outcomes, a statistical power analysis was conducted. Based on the effect size, sample size, and significance level (α = 0.05), the study was powered at 80% to detect a moderate effect size of 0.5. This power analysis confirmed that the sample size was adequate for detecting significant associations between the identified risk factors and the development of postoperative infections.
Furthermore, receiver operating characteristic (ROC) curves were plotted for significant continuous variables to evaluate their predictive power. The area under the curve (AUC) was calculated to quantify the accuracy of these variables in predicting postoperative infections. All statistical analyses were performed using SPSS version 25.0 (IBM, Armonk, NY, USA). The significance level was maintained at p<0.05 for all tests, ensuring that the results were both statistically robust and clinically meaningful. By employing this rigorous analytical approach, the study aimed to identify key risk factors and provide actionable insights for improving patient outcomes in severe TBI cases requiring emergency craniotomy.
Results
Baseline Characteristics of Severe TBI Patients Undergoing Emergency Craniotomy
This study included a total of 348 patients based on strict inclusion and exclusion criteria, with 122 patients in the low frailty group and 226 patients in the high frailty group (Figure 1). In the low frailty group, there were 73 males (59.8%), whereas in the high frailty group, there were 165 males (73.0%), with a statistically significant difference between the two groups (p=0.015). Patients in the high frailty group were relatively older (p =0.041), had a higher proportion of hemostatic agent use upon admission (79.6%) (P=0.048), and had lower GCS scores at admission, with 28.3% of patients having a total score of 3–8, which was higher than in the low frailty group (p=0.047). Additionally, the high frailty group had a greater number of multiple injury sites, with 114 single injury sites (80.4%), compared to 69.7% in the low frailty group (p<0.001). At admission, highly frail patients had a higher median body temperature of 36.9°C (p =0.032), and 57 patients (25.2%) were admitted to the ICU, which was a higher proportion than in the low frailty group (p =0.009). There were no statistically significant differences between the two groups for the remaining variables (Table 1).
 |
Table 1 Baseline Characteristics of Severe TBI Patients Undergoing Emergency Craniotomy (n=312) |
 |
Figure 1 Inclusion and exclusion table for patients with severe TBI. |
Analysis of Risk Factors Affecting Postoperative Infections Using Univariate and Multivariate Logistic Regression
For all severe patients, univariate and multivariate logistic regression analyses were conducted. Variables with P < 0.05 in the univariate logistic regression results were included in the multivariate logistic regression model to identify risk factors for postoperative infection. Variables that were statistically significant in the univariate analysis included age (p = 0.002), BMI (p = 0.035), surgery duration (p < 0.001), preoperative infection (p < 0.001), GCS score on admission (p < 0.001), admission to ICU (P = 0.003), and hypoxia at admission (SpO2 < 90%) (p = 0.013). These variables were included in the multivariate logistic regression analysis, which showed that age > 80 (OR = 1.75, 95% CI: 1.23–2.49, p < 0.001 vs < 80), surgery duration (OR = 2.01, 95% CI: 1.38–2.92), Preoperative infection (OR=2.59, 95% CI: 1.64–4.09) and severe GCS score at admission (OR = 1.82, 95% CI: 1.21–2.74) were associated with postoperative infection (Table 2). For the specific variable of frailty, the multivariate OR was the largest among all variables. As shown in Figure 2, the odds ratios for age, surgery duration, preoperative infection, and GCS score are all above 1, indicating that these variables are positively associated with the likelihood of developing postoperative infections. This visualization provides a clear and effective way to interpret the strength of each risk factor’s association with postoperative infection risk and highlights the factors that should be prioritized in clinical settings for infection prevention strategies.
 |
Table 2 Univariate and Multivariate Logistic Regression Analysis of Risk Factors for Postoperative Infections in Severe TBI Patients |
 |
Figure 2 Odds ratio plot showing the strength of associations between risk factors (age, surgery duration, preoperative infection, GCS score) and postoperative infection risk. |
Comparison of Complications in TBI Patients With No Infection and Without Postoperative Infections
In the non-infection group, 15 patients (5.9%) experienced neurological complications; however, in the infection group, 18 patients (15.8%) experienced neurological complications, with a statistically significant difference between the two groups (p < 0.001). For non-neurological complications, there was a statistically significant difference in the proportion of respiratory-related complications between the two groups (p = 0.008), with the infection group having a higher incidence. There were no significant differences in the incidence rates of complications in other systems (Table 3).
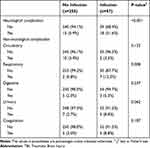 |
Table 3 Comparison of TBI Complications in Patients With and Without Postoperative Infections (n=312) |
Plotting Receiver Operating Curves (ROC) for Frailty Score as Well as Significant Variables
Using ROC curves to illustrate the predictive ability of various variables for postoperative infection in severe TBI patients, the horizontal axis represents 1-specificity and the vertical axis represents sensitivity. The ROC curves for age, surgery duration, preoperative infection, and GCS score are shown in Figure 2. The area under the curve (AUC) quantifies the ability of each variable to discriminate between patients with and without postoperative infections. The AUC values represent the accuracy of the continuous variables in predicting the likelihood of developing an infection, where a value of 0.5 indicates no discrimination (random prediction) and a value of 1.0 indicates perfect discrimination. For age, the AUC is 0.751 (95% CI: 0.582–0.893), indicating that age is a moderately good predictor of postoperative infection risk. For surgery duration, the AUC is 0.780 (95% CI: 0.515–0.912), suggesting that longer surgery duration is a stronger predictor for postoperative infections. For preoperative infection, the AUC is 0.772 (95% CI: 0.612–0.883), demonstrating that the presence of an infection before surgery is a significant predictor of postoperative infections. For GCS score, the AUC is 0.734 (95% CI: 0.599–0.886), indicating a fair ability to predict postoperative infection risk based on the initial GCS score. (Figure 3). Based on the maximum value of the Youden index (sensitivity + specificity - 1), the optimal cutoff values for each indicator were determined. At the optimal cutoff values, the sensitivity, specificity, accuracy, positive predictive value, and negative predictive value are as follows: Age: Sensitivity 0.705, Specificity 0.806, Accuracy 0.751, Positive Predictive Value 0.603, Negative Predictive Value 0.853; Surgery duration: Sensitivity 0.752, Specificity 0.802, Accuracy 0.780, Positive Predictive Value 0.652, Negative Predictive Value 0.859; Preoperative infection: Sensitivity 0.726, Specificity 0.803, Accuracy 0.770, Positive Predictive Value 0.634, Negative Predictive Value 0.836; and GCS score: Sensitivity 0.689, Specificity 0.789, Accuracy 0.586, Positive Predictive Value 0.812, Negative Predictive Value 0.734 (Table 4).
 |
Table 4 Predictive Value of Risk Factors for in-Hospital Mortality |
 |
Figure 3 Age, Surgery duration, preoperative infection and GCS of the receiver operating curve. |
Discussion
This study focuses on several important risk factors for postoperative infection in patients with severe traumatic brain injury (TBI) who undergo emergency craniotomy. The analysis shows that older age, longer surgery duration, the presence of preoperative systemic infection, and lower Glasgow Coma Scale (GCS) scores upon admission are independently associated with an increased risk of postoperative infection.
Age has consistently been considered an important risk factor for postoperative infection. In this study, elderly patients exhibited a higher likelihood of infection following emergency craniotomy. This finding aligns with existing literature, suggesting that aging is associated with immunosenescence and a diminished ability to respond to infections.18,19 Researchers such as Young et al20 have found that clinicians caring for the elderly need to consider not only the likelihood of microbial cure but also the need to balance maintaining functional status and overall quality of life to reduce postoperative complications. Therefore, elderly patients may require more intensive perioperative monitoring and tailored strategies to enhance their immune defenses and mitigate the risk of infection.
The duration of surgery is another key factor influencing infection rates. Prolonged surgeries increase the time of exposure to potential contaminants and lead to tissue trauma and immunosuppression. Cheng et al found that extended surgery time increases the risk of surgical site infections (SSI).21 Given the significance of SSI on patient outcomes and healthcare economics, hospitals should focus on reducing surgery time. Similarly, Qin et al discovered that longer surgery duration may independently increase the risk of postoperative urinary tract infections.22 Teo et al noted that the risk of infection in total knee arthroplasty is closely related to longer surgery duration.23 We hope that our findings will help guide decisions regarding the safety of combined surgeries and improve preoperative risk stratification. Our results support the view that minimizing surgery time, when feasible, can reduce the risk of infection. Implementing effective surgical techniques and ensuring timely decision-making during surgery may help mitigate infection risk.
The presence of preoperative systemic infection significantly increases the risk of postoperative infection.24 This underscores the importance of thorough preoperative evaluation and the necessity of addressing existing infections before performing emergency craniotomy. Strategies such as the prophylactic use of antibiotics and ensuring optimal preoperative health are crucial for preventing postoperative complications.25 The research team led by Donzé found that preoperative sepsis is a significant independent risk factor for arterial and venous thrombosis.26 The risk of thrombosis increases with the severity of the inflammatory response and is higher in both emergency and elective surgeries.27 High suspicion for thrombosis should be maintained for sepsis patients undergoing surgery. Similarly, Madsen’s study of 4.8 million individuals found a strong correlation between high preoperative risk and certain postoperative complications.28 Yang et al found that preoperative inflammatory markers are important factors associated with the occurrence of sepsis after bowel obstruction surgery.29 Therefore, strict control of preoperative inflammation is essential. This aligns with our study, which similarly emphasizes the need to reduce inflammation levels before surgery in severe TBI patients to improve their prognosis.
In this study, we have identified several key risk factors for postoperative infections in severe TBI patients undergoing emergency craniotomy, including age, surgery duration, preoperative infection, and GCS score. Each of these variables has significant implications for infection risk, with preoperative infection and surgery duration showing particularly strong associations. These findings highlight the critical role of preoperative infection control and timely surgical intervention in reducing the incidence of infections. The multivariate analysis confirmed that these factors are independently associated with postoperative infection, underlining their importance in clinical risk stratification and preventive measures.
Additionally, prophylactic antibiotic use is a cornerstone of infection prevention in neurosurgery. Administering antibiotics within an optimal window before surgery, typically within an hour of incision, can significantly reduce the risk of surgical site infections (SSIs). In this study, patients who received antibiotics prior to surgery demonstrated a lower incidence of infections. Furthermore, ensuring optimal preoperative health—such as managing diabetes, improving nutritional status, and addressing any ongoing infections—is essential for reducing infection risk. The control of preoperative inflammation also plays a significant role, as elevated inflammatory markers are associated with higher rates of postoperative infections. Anti-inflammatory strategies, including the use of appropriate medications to reduce systemic inflammation, can contribute to better outcomes and help mitigate the risk of infections following emergency craniotomy.
While this study provides significant insights into the risk factors for postoperative infections in severe traumatic brain injury (TBI) patients undergoing emergency craniotomy, several limitations should be acknowledged. Firstly, the retrospective nature of the study inherently carries limitations related to data accuracy and completeness, as it relies on existing medical records that may be incomplete or inconsistently documented, potentially leading to biases in data collection and analysis. Additionally, the study was conducted at a single center, which may limit the generalizability of the findings to other settings or populations, as different hospitals may have varying protocols, resources, and patient demographics that could influence the incidence and management of postoperative infections.
Conclusion
In conclusion, this study underscores the importance of identifying and addressing key risk factors for postoperative infections in severe TBI patients undergoing emergency craniotomy. By focusing on age, surgery duration, preoperative infections, and GCS scores, healthcare providers can implement targeted interventions to reduce infection rates and improve patient outcomes. Continued research and clinical innovations are essential to further enhance the care and recovery of this vulnerable patient population.
Future directions for this research could involve exploring the long-term effects of postoperative infections in TBI patients, as well as evaluating the impact of specific infection prevention protocols across different healthcare settings. Further studies could also investigate the potential role of novel biomarkers in predicting postoperative infection risk and assessing the effectiveness of personalized infection prevention strategies. Additionally, expanding the research to include multicenter cohorts could validate and generalize these findings to broader patient populations, providing a more comprehensive understanding of infection risk factors in TBI surgery.
Data Sharing Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Human Ethics and Consent to Participate Declarations
This retrospective study was approved by the Ethics Committee of Brain Hospital of Hunan Province and adhered to the ethical principles outlined in the Declaration of Helsinki. Informed consent forms were obtained from all patients.
Acknowledgments
Thanks to the nurses in the department for their help with the project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare.
References
1. Capizzi A, Woo J, Verduzco-Gutierrez M. Traumatic brain injury: an overview of epidemiology, pathophysiology, and medical management. Med Clin North Am. 2020;104(2):213–238. doi:10.1016/j.mcna.2019.11.001
2. Khellaf A, Khan DZ, Helmy A. Recent advances in traumatic brain injury. J Neurol. 2019;266(11):2878–2889. doi:10.1007/s00415-019-09541-4
3. Stocchetti N, Carbonara M, Citerio G, et al. Severe traumatic brain injury: targeted management in the intensive care unit. Lancet Neurol. 2017;16(6):452–464. doi:10.1016/S1474-4422(17)30118-7
4. Risdall JE, Menon DK. Traumatic brain injury. Philos Trans R Soc London, Ser B. 2011;366(1562):241–250. doi:10.1098/rstb.2010.0230
5. Chughtai KA, Nemer OP, Kessler AT, Bhatt AA. Post-operative complications of craniotomy and craniectomy. Emerg Radiol. 2019;26(1):99–107. doi:10.1007/s10140-018-1647-2
6. Feng W, Sun C, Hao S, et al. Risk assessment and pathogen profile of surgical site infections in traumatic brain injury patients undergoing emergency craniotomy: a retrospective study. Int Wound J. 2024;21(3):e14743. doi:10.1111/iwj.14743
7. Fernández-Ugidos P, Barge-Caballero E, Gómez-López R, et al. In-hospital postoperative infection after heart transplantation: risk factors and development of a novel predictive score. Transplant Infect Dis. 2019;21(4):e13104. doi:10.1111/tid.13104
8. Kew ME, Cancienne JM, Christensen JE, Werner BC. The timing of corticosteroid injections after arthroscopic shoulder procedures affects postoperative infection risk. Ame J Sports Med. 2019;47(4):915–921. doi:10.1177/0363546518825348
9. Tan T, Lee H, Huang MS, et al. Prophylactic postoperative measures to minimize surgical site infections in spine surgery: systematic review and evidence summary. Spine J. 2020;20(3):435–447. doi:10.1016/j.spinee.2019.09.013
10. Jeschke MG. The importance of sepsis in surgical patients. Surgery. 2023;174(2):398–399. doi:10.1016/j.surg.2023.04.036
11. Napoli F, Aleman R, Zadneulitca N, Navia J, Brozzi NA. Sepsis in cardiothoracic surgery. Surgery. 2024;175(2):556–558. doi:10.1016/j.surg.2023.10.015
12. Svedung Wettervik T, Lenell S, Enblad P, Lewén A. Decompressive craniectomy in traumatic brain injury-craniectomy-related and cranioplasty-related complications in a single center. World Neurosurg. 2021;148:e508–e517. doi:10.1016/j.wneu.2021.01.013
13. Kourbeti IS, Papadakis JA, Neophytou C, et al. Infections in patients with traumatic brain injury who undergo neurosurgery. British J Neuro Surg. 2011;25(1):9–15. doi:10.3109/02688697.2010.500411
14. Wang L, Ji C, Zhang X, et al. Post-traumatic osteoarthritis after ankle fracture fixation: a minimum three-year follow-up. Foot Ankle Surg. 2025. doi:10.1016/j.fas.2025.02.014
15. Xia F, Zhang Q, Ndhlovu E, Zhang M, Zou Y. A novel nomogram to predict resectable gastric cancer based on preoperative circulating tumor cell. Clin Transl Gastroenterol. 2024;15(2):e00561. doi:10.14309/ctg.0000000000000561
16. Pavlovic D, Pekic S, Stojanovic M, Popovic V. Traumatic brain injury: neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary. 2019;22(3):270–282. doi:10.1007/s11102-019-00957-9
17. Rakhit S, Nordness MF, Lombardo SR, Cook M, Smith L, Patel MB. Management and challenges of severe traumatic brain injury. Semin Resp Crit Care Med. 2021;42(1):127–144. doi:10.1055/s-0040-1716493
18. Simon GI, Craswell A, Thom O, Chew MS, Anstey CM, Fung YL. Impacts of aging on anemia tolerance, transfusion thresholds, and patient blood management. Transfusion Med Rev. 2019;33(3):154–161. doi:10.1016/j.tmrv.2019.03.001
19. Stanojcic M, Chen P, Xiu F, Jeschke MG. Impaired immune response in elderly burn patients: new insights into the immune-senescence phenotype. Ann Surg. 2016;264(1):195–202. doi:10.1097/SLA.0000000000001408
20. Young MH, Washer L, Malani PN. Surgical site infections in older adults: epidemiology and management strategies. Drugs Aging. 2008;25(5):399–414. doi:10.2165/00002512-200825050-00004
21. Cheng H, Chen BP, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect. 2017;18(6):722–735. doi:10.1089/sur.2017.089
22. Qin C, de Oliveira G, Hackett N, Kim JY. Surgical duration and risk of urinary tract infection: an analysis of 1,452,369 patients using the national surgical quality improvement program (NSQIP). Int J Surg. 2015;20:107–112. doi:10.1016/j.ijsu.2015.05.051
23. Teo BJX, Yeo W, Chong HC, Tan AHC. Surgical site infection after primary total knee arthroplasty is associated with a longer duration of surgery. J Orthop Surg. 2018;26(2):2309499018785647. doi:10.1177/2309499018785647
24. Breaza GM, Hut FE, Cretu O, et al. Impact of preoperative biliary stenting on intestinal dysfunction and perioperative complications after pylorus-preserving pancreaticoduodenectomy. Medicina. 2025;61. doi:10.3390/medicina61030391
25. Islam N, Thalib L, Mahmood S, et al. Regional variations in incidence of surgical site infection and associated risk factors in women undergoing cesarean section: a systematic review and meta-analysis. Intens Crit Care Nurs. 2025;89:103951. doi:10.1016/j.iccn.2025.103951
26. Donzé JD, Ridker PM, Finlayson SR, Bates DW. Impact of sepsis on risk of postoperative arterial and venous thromboses: large prospective cohort study. BMJ. 2014;349:g5334. doi:10.1136/bmj.g5334
27. Ross SW, Kuhlenschmidt KM, Kubasiak JC, et al. Association of the risk of a venous thromboembolic event in emergency vs elective general surgery. JAMA Surg. 2020;155(6):503–511. doi:10.1001/jamasurg.2020.0433
28. Madsen HJ, Meguid RA, Bronsert MR, et al. Associations between preoperative risks of postoperative complications: results of an analysis of 4.8 Million ACS-NSQIP patients. Am J Surg. 2022;223(6):1172–1178. doi:10.1016/j.amjsurg.2021.11.024
29. Yang J, Ran T, Lin X, et al. Association between preoperative systemic immune inflammation index and postoperative sepsis in patients with intestinal obstruction: a retrospective observational cohort study. Immun Inflamm Dis. 2024;12(2):e1187. doi:10.1002/iid3.1187
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
Traumatic Brain Injury Outcomes After Recreational Cannabis Use
Szaflarski JP, Szaflarski M
Neuropsychiatric Disease and Treatment 2024, 20:809-821
Published Date: 3 April 2024

