Back to Journals » Journal of Pain Research » Volume 18
Risk Stratification for Postoperative Opioid Induced Respiratory Depression: A Retrospective Case-Control Analysis of Existing Validated Tools
Authors Hutcheson S , Pehrson A, Gassert RB, Guffey E, Shanahan PC, Sisk L, Patton S, Solla CA
Received 28 October 2024
Accepted for publication 1 April 2025
Published 28 April 2025 Volume 2025:18 Pages 2233—2240
DOI https://doi.org/10.2147/JPR.S495181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Timothy Atkinson
Sam Hutcheson, Aimee Pehrson, Robert B Gassert, Ethan Guffey, Paul C Shanahan, Laura Sisk, Samuel Patton, Che Antonio Solla
Department of Anesthesiology; University of Tennessee Graduate School of Medicine, Knoxville, TN, USA
Correspondence: Sam Hutcheson, Department of Anesthesiology; The University of Tennessee Graduate School of Medicine, 1924 Alcoa Hwy Box U-109, Knoxville, TN, 37920-9220, USA, Tel +1-205-240-2035, Email [email protected]
Purpose: Postoperative opioid-induced respiratory depression (POIRD) is a preventable perioperative cause of morbidity and mortality. A validated POIRD risk stratification tool could reduce these complications. 3 pre-existing validated opioid tools; and specific risk factors identified from these tools; were examined in this retrospective case-control study to determine if they could assess POIRD risk in patients discharged to hospital floors from the Post-Anesthesia Care Unit (PACU).
Patients and Methods: Our dataset includes 126 matched patients who underwent surgery at the University of Tennessee Medical Center from January 2019 to December 2021. All patients that were related to active traumas or burns were excluded from this study. Escalation of care secondary to respiratory failure (an increase in respiratory support with movement to an intensive care unit/stepdown unit or patient expiration secondary to respiratory failure) with and without naloxone administration was the primary endpoint; with the subgroup that received naloxone being the surrogate POIRD endpoint. Escalation of care secondary to respiratory failure; regardless of naloxone use; was a secondary endpoint.
Results: There was no association between the 3 opioid tools evaluated with the POIRD surrogate endpoint or escalation of care. Bipolar disorder (OR 3.68; 95% CI 1.11– 9.56) and a history of substance abuse (OR 26.33; 95% CI 5.18– 119.02) were significant risk factors that contributed to escalation of care secondary to respiratory failure. A history of substance abuse was found to have a significant association with escalation of care secondary to respiratory failure with naloxone administration (OR=6.886; 95% CI 2.02– 23.56).
Conclusion: While we were unable to identify a tool to stratify POIRD risk; patients with bipolar disorder and a history of substance abuse are at an increased risk of postoperative respiratory failure requiring escalation of care; with a history of substance abuse being associated with POIRD.
Keywords: preoperative risk stratification, ORT, PRODIGY, RIOSORD, retrospective case-control study
Introduction
Opioids are the foundation of perioperative analgesic therapy.1 While opioids are efficacious in perioperative pain management; they are known to suppress respiratory function in a dose-dependent manner.2 In the perioperative setting; the physiologic effects of surgery; anesthesia; and certain patient-specific co-morbidities can exacerbate this respiratory depressant effect; leading to escalation of care and subsequent increased costs resulting from length of stay and resource utilization.3 The temporal relationship between respiratory depression in the PACU and subsequent episodes of severe respiratory depression on the postoperative wards is supported by studies showing that severe ventilatory adverse events tend to occur within the first six to twelve hours of discharge from the PACU with 85% occurring within the first twenty-four hours.4–6 Additionally; a study of the American Society of Anesthesiologists Closed Claims database showed that 88% of respiratory depression events occurred within 24 hours of surgery; and 97% were likely preventable with better response and patient monitoring.7 The phenomenon of POIRD appears to be nearly ubiquitous in the PACU where patients are closely monitored for changes in vital signs during the immediate postoperative period. Chung et al; found that 95% of PACU patients monitored with capnography and pulse oximetry were found to have episodes of respiratory depression.8 Clinically significant opioid-induced respiratory depression has an incidence between 0.3% to 46%; depending on the clinical definition and detection method.9 While almost all of these episodes do not go on to develop into a critical respiratory adverse event; POIRD is still the fourth most common patient safety event in the postoperative period.10 Nonetheless; those cases of clinically significant POIRD had significant perioperative morbidity and mortality associated with increased length of stay (mean 5 additional days); readmission (15.8% vs 9.4% in patients without events); and cost (mean increase $10,000).11,12
Capnography is a standard monitor used during surgeries and could be used to monitor patients at higher risk of experiencing clinically significant POIRD; but without viable risk stratification; its use is limited due to cost. The average cost of continuous oxygen and capnography is estimated to be $52.73 per hospitalization; compared to $0.68 per stay with intermittent pulse oximetry.13 At the same time; intermittent pulse oximetry monitoring every 4–6 hours has been shown to miss >90% of episodes of prolonged hypoxia in patients in general floor rooms.14 Continuous pulse oximetry has its issues as well; as hypoxia is a late sign of respiratory depression; and can occur on average 60 seconds after evidence of respiratory depression was noted on capnography.15 A recent review from Al-Halawani et al showed that pulse oximetry consistently overestimated SpO2% (oxygen saturation) when compared to SaO2 obtained from blood gas samples in Black patients. These SpO2 readings were less precise as well in the same patient population.16
To avoid the potentially catastrophic consequences of clinically significant POIRD; preoperative risk stratification has been recommended.4 Although some risk factors for POIRD such as obstructive sleep apnea are well established in literature; limited tools exist in current practice to affect clinical outcomes.5 However; some pre-existing validated tools could be used to assess POIRD. The PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY (PRODIGY) tool is validated to predict opioid-induced respiratory depression based on a study where medical/surgical patients on the floor were monitored with continuous pulse oximetry and end-tidal capnography.14 A scoring system was developed based on risk factors associated with patients who had episodes of opioid-induced respiratory depression. However; this may be too broad a tool for risk stratification due to its high specificity and low sensitivity. For example; with the PRODIGY tool; any male over the age of 60 is considered high risk. Additionally; this tool did not assess the risk for escalation of care due to respiratory failure and did not exclusively focus on postoperative patients. Other tools include the Opioid Risk Tool (ORT) and the Risk Index for Overdose or Serious Opioid-induced Respiratory Depression (RIOSORD). The ORT uses a weighted scoring system based on specific risk factors to determine the risk for opioid abuse before initiation of opioids in the outpatient setting.17 The RIOSORD tool is validated for use in identifying patients at risk for opioid overdose or serious respiratory depression in the outpatient setting. With 15 independent variables; RIOSORD contains several established risk factors for POIRD.18 While there are validated tools to assess opioid-induced respiratory depression both inpatient and outpatient; there is a gap in the literature predicting escalation of care secondary to opioid-induced respiratory depression in the perioperative period.
This study assessed the ORT; PRODIGY; and RIOSORD to determine if these tools could be utilized to risk stratify patients for clinically significant POIRD. In addition to evaluating these three pre-existing tools; our study examined the individual risk factors used to develop the ORT; PRODIGY; and RIOSORD. To evaluate clinically significant POIRD in this study; we used postoperative escalation of care (defined as upgrading a patient to the ICU/stepdown unit from a medical/surgical floor with an increase in respiratory support or expiration secondary to respiratory failure) with and without naloxone as our primary endpoint; with the group receiving naloxone administration being our surrogate endpoint for clinically significant POIRD. We hypothesized that there would be significant associations between these three validated tools with clinically significant POIRD requiring escalation of care with and without naloxone administration. Postoperative respiratory failure secondary to respiratory distress; regardless of naloxone administration; was used as a secondary endpoint. We also had an exploratory aim to assess which individual risk factors identified between these three tools had significant relationships with the primary and secondary endpoints.
Methods
This retrospective case-control examines patients who underwent surgery with postoperative admission at the University of Tennessee Medical Center from January 2019 to December 2021. This study is in accordance with the Declaration of Helsinki and received approval from the University of Tennessee Graduate School of Medical Education Institutional Review Board (IRB number 4894). Data was collected from patient electronic health records after informed consent was waived by the University of Tennessee Graduate School of Medical Education IRB board as this retrospective study involved research that presented no more than a minimal risk to human subjects and materials that had already been collected for clinical care purposes. Inclusion criteria included adult patients (age 18 and older) who presented to the University of Tennessee Medical Center for surgery from January 2019-December 2021 and were admitted post-surgery for a minimum 23 hours. Exclusion criteria included patients receiving care for trauma and/or burns; cesarean sections; those under the age of 18; and those discharged home from recovery. We excluded all cases that were related to active traumas or burns as they were sent directly to an intensive care unit post-surgery and were more complex cases due to the nature of the injury or illness. Cesarean sections were excluded because these cases did not flow to the main PACU and recovery units. Escalation of care secondary to respiratory failure for this study is defined as the patient requiring admission to the intensive care/step-down unit with an increase in respiratory support; or patient expiration secondary to respiratory failure. History of substance abuse was self-reported and collected from the electronic health record.
Pharmacy provided the data of all patients who received naloxone post discharge from recovery during this time period (n=91). A random sample of 245 from the 71,591 surgeries that met inclusion criteria and did not require naloxone was used to ensure sufficient case-control matching and statistical power. A post-hoc power analysis was performed and revealed for 80% power based on an incidence rate of 24% of escalation of care in those with naloxone use for POIRD; minimum 13 matches are required in each group. Propensity score matching was then utilized to develop a matching cohort from the random sample of 245 patients using the nearest neighbor technique and a match caliper of 0.25. Our cohort included 63 patients receiving naloxone and 63 who did not; with 17 in each requiring escalation of care. We performed descriptive and frequency statistics on all continuous and categorical variables. Independent variables were the composite and individual risk factors included in the ORT; PRODIGY; and RIOSORD including age; sex; history of bipolar disorder; history of schizophrenia; self-reported substance abuse; self-reported alcohol abuse; congestive heart failure; kidney disease; sleep apnea; chronic hepatitis or cirrhosis; and chronic obstructive pulmonary disease (COPD). Dependent variables included escalation of care and the use of naloxone within 24-hours post-surgery. Differences between these risk factors between the escalation of care group and non-escalation of care group were assessed with a Chi Square test as found in Table 1. To assess the relationship between the composite and individual risk factors with escalation of care with and without naloxone use; we first performed a correlation analysis to determine the strength of association of the PRODIGY and RIOSORD score; individual risk factors; escalation of care; and the use of naloxone. A composite ORT score was not compiled as the electronic medical record did not include family history of alcohol or substance abuse. For this reason; we examined the patient only individual factors of the ORT (age; substance abuse; alcohol abuse; bipolar disorder; schizophrenia) that contributed to a score of moderate to high risk of opioid misuse. Spearman’s Rho was chosen for categorical and ordinal variables and a Pearson’s Moment correlation was utilized for continuous variables. After determining contributing risk factors with significant correlations; binary logistic regression analysis was completed; utilizing those variables. The Hosmer and Lemeshow test for goodness of fit was used to determine the acceptability of the model. Nagelkerke R-Square test was performed to determine the amount of variance explained by the model. Statistical significance was measured at a 95% confidence interval. ROC analysis was performed to evaluate the discriminatory ability of model variables in predicting naloxone use. Statistical power (0.8) was determined with an n=126 and moderate effect size (0.3). All statistics were computed using IBM SPSS Version 29 (2022).
 |
Table 1 Post-Operative Induced Respiratory Depression Risk Factors and Escalation of Care Post Surgery |
Results
This study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines for reporting observational studies. The STROBE checklist was used to ensure transparent and comprehensive reporting of study methods and findings. From January 2019- December 2021; 71,682 number of individuals presented to the University of Tennessee Medical Center for surgery; with 91 requiring naloxone for POIRD. 55 people were excluded for trauma surgery and burns prior to establishing a matched cohort (n=126). In the matched sample; there were 34 males with 10 requiring escalation of care and 92 females with 24 requiring escalation of care. Neither the PRODIGY or RIOSORD tools were associated with naloxone use or escalation of care as noted in Table 2.
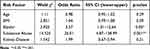 |
Table 2 Logistic Regression Model of Predictors of POIRD with Escalation of Care |
While none of the composite or individual risk factors had a statistically significant relationship with naloxone use; history of substance abuse did have a statistically significant association to escalation of care with the use of naloxone (r=0.304; p=<.001; χ²(1)=11.636; p=<.001; OR=6.886; 95% CI 2.02–23.56). Select risk factors were significantly associated with escalation of care: Age (r=−.221; p=0.01); BMI (r=0.215; p=0.02); Bipolar disorder (BP) (r=0.210; p=0.02); History of substance abuse (r=0.469; p<0.001); Kidney disease (r=0.215; p=0.02). These independent variables were included in the initial binary logistic regression model. The model fit was determined with Hosmer Lemeshow’s test for goodness of fit χ²(8) = 6.321 p = 0.611 and the Nagelkerke R-Square test (R2= 0.373) indicating 37% of the variance is explained in this model with model goodness of fit. Analysis of each independent variable revealed BP (Waldχ²(1)=3.925; OR 3.57; 95% CI 1.01–12.64; p=0.046) and history of substance abuse (Waldχ²(1)=14.525; OR 26.01 95% CI 4.87–138.99; p <0.001) were the only significant risk factors in the model holding all other variables constant. Follow-up ROC analysis on model predictors provided AUC values of age (0.346); BMI (0.650); BP (0.573); history of substance abuse (0.665); and kidney disease (0.606); indicating that individually BMI; BP; history of substance abuse and kidney disease have fair discrimination; but when combined in the multivariate model provide improved predictability. Of note; race did have a significant correlation with POIRD with escalation of care (r=0.368; p<0.001); however; we did not include this in the model because only two races were represented in the sample.
Discussion
Our study was unable to establish significant correlations between escalation of care requiring naloxone administration and current validated risk stratification tools. However; we were able to identify several independent risk factors associated with postoperative respiratory failure requiring escalation of care. While significant associations between age; BMI; BP; history of substance; and kidney disease exist; our regression model indicated that bipolar disorder and a history of substance abuse were the only significant predictor variables for escalation of care secondary to respiratory failure with or without naloxone use. A history of substance abuse was also associated with escalation of care secondary to respiratory failure with naloxone administration; our POIRD surrogate endpoint.
While we were unable to find a direct link between bipolar disorder and POIRD in this study; there is still utility in knowing these patients are at an increased risk of respiratory failure in the immediate postoperative period. At the same time; despite being unable to establish a link in this study; we suspect that POIRD does contribute to this elevated risk based on existing literature. Bipolar disorder was identified as both a risk factor for outpatient opioid abuse by the ORT and Revised ORT; as well as a risk factor for outpatient opioid overdose by RIOSORD.17–19 Armstrong et al found that seven risk factors were associated with increased postoperative pain at 20–24 hours and 24–48 hours postoperatively after elective orthopedic surgery; one of which was psychological conditions other than anxiety. This definition included bipolar disorder.20 Mulligan et al showed that mood disorders; such as bipolar disorder; were at an increased risk for narcotic use beyond 90 days postoperatively; as well as a higher visual analog pain score at follow-up one year postoperatively after major ankle/hindfoot reconstruction.21 With these patients experiencing higher pain scores in the immediate postoperative period; it is likely that these patients would also have increased morphine milligram equivalents (MME) requirements during this same period; leading to an increased risk of developing clinically significant POIRD.
A history of substance abuse is a wide-ranging term that includes abuse of alcohol; prescription as well as recreational drugs. In examining the validated risk tools; the PRODIGY tool specifically designed for opioid-induced respiratory depression does take into account current opioid prescriptions. Nonetheless; this tool does not take into account all substance abuse including illicit drugs or illegal use of opioids.14 Our results show that a history of substance abuse is a patient risk factor that contributes to critical respiratory depression following surgery; likely from the need for increased MME to control perioperative pain. Patients with a history of opioid abuse may be on maintenance therapies that make managing their perioperative pain regimen more challenging. For example; patients on naltrexone; an opioid antagonist; may require an increased amount of opioids due to this competitive antagonism.22 If naltrexone has been held before surgery; they are at an increased risk of overdose due to opioid receptor upregulation that is no longer occupied by naltrexone.22 With 86% of those with a history of substance abuse in this study requiring escalation of care; further research is needed to determine the underlying factors and how multimodal approaches would benefit patients who report a history of substance abuse.
Our results also showed Black patients were at higher risk of escalation of care secondary to respiratory failure when compared to other races. It is hypothesized that when pulse oximetry is the primary method for monitoring respiratory complications; Black patients are at a higher risk of developing critical respiratory events on medical/surgical floors secondary to unrecognized hypoxemia. While our sample size was small; this highlights that further research is needed to examine disparities in postoperatively respiratory complications when pulse oximetry is utilized to monitor these patients; including examining the potential role of capnography.
Limitations
POIRD is relatively difficult to diagnose and study due to the variations in individual risk factors and how they interact with perioperative medications. While 95% of patients in the PACU will develop POIRD due to the amount of intraoperative and postoperative opioids administered; most of these patients do not require naloxone.8 When this is considered; using naloxone administration alone as an endpoint for POIRD did not provide any statistically significant results other than in those with a history of substance abuse. To address this; we also examined escalation of care as a whole due to the impact on cost and quality of care.
This study was conducted at a single medical center just before and during the COVID-19 pandemic. This led to significant staffing shortages as well as patient visitation restrictions; which possibly impacted the monitoring of the patients included in this study. Another limitation is the retrospective nature of this study. Future research utilizing capnography that assesses the role substance use and the role comorbid mental health diagnosis play in the escalation of care postoperatively is needed. Additionally; our study did not examine all the individual risk factors in the Revised Opioid Risk Tool; an updated and validated version of the ORT; because the required data was not available in the electronic health record.
Conclusion
This study provides insight into independent risk factors that contributed to escalation of care secondary to respiratory failure; including bipolar disorder; a history of substance abuse; as well as race. There was a significant association with our POIRD surrogate endpoint and a history of substance abuse. While more POIRD predictors need to be identified; a history of substance abuse should be considered in any tool created to predict clinically significant POIRD moving forward.
While we believe that POIRD most likely contributed to the significant difference in escalation of care secondary to respiratory failure in bipolar disorder; future research should include the utilization of end-tidal capnography on medical/surgical floors after discharge from the PACU to determine how independent risk factors identified predict clinically significant POIRD. Research has shown that higher intra-operative MMEs are associated with higher postoperative pain scores as well as increased postoperative MME requirements at 2 hours postoperatively.23 Future research that examines perioperative opioid MME utilization in conjunction with predictive risk factors; will help guide the development of a more comprehensive risk tool for POIRD. By understanding how independent risks impact POIRD and MME utilization; we will be better able to enhance patient safety and incentivize more individualized multimodal analgesic strategies tailored to maximal benefit and minimal risk.
Abbreviations
POIRD, Postoperative opioid-induced respiratory depression; ORT, Opioid Risk Tool; PRODIGY, PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY; RIOSORD, Risk Index for Overdose or Serious Opioid-induced Respiratory Depression; PACU, Post-anesthesia care unit; ICU, Intensive care unit; MME, Morphine milligram equivalents.
Data Sharing Statement
De-identified patient data are available upon request to the corresponding author.
Acknowledgment
We would like to acknowledge Megan Hintz; PharmD; who collected data on patients who received naloxone postoperatively.
Author Contributions
All listed authors made a significant contribution to the work reported; whether in the conception; study design; execution; acquisition of data; analysis and interpretation; took part in drafting; revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The University of Tennessee Graduate School of Medical and Dental Education and the University of Tennessee Medical Center Department of Anesthesiology provided financial support for the publication of this article.
Disclosure
The authors have no relevant conflicts of interest to disclose for this study.
References
1. Shanthanna H, Ladha KS, Kehlet H, Joshi GP. Perioperative opioid administration. Anesthesiology. 2021;134(4):645–659. doi:10.1097/ALN.0000000000003572
2. Palkovic B, Marchenko V, Zuperku EJ, Stuth EAE, Stucke AG. Multi-level regulation of opioid-induced respiratory depression. Physiol. 2020;35(6):391–404. doi:10.1152/physiol.00015.2020
3. Zedler B, Xie L, Wang L, et al. Risk factors for serious prescription opioid-related toxicity or overdose among veterans health administration patients. Pain Med Malden Mass. 2014;15(11):1911–1929. doi:10.1111/pme.12480
4. Weingarten TN, Sprung J. An update on postoperative respiratory depression. Int Anesthesiol Clin. 2022;60(2):8–19. doi:10.1097/AIA.0000000000000362
5. Gupta K, Nagappa M, Prasad A, et al. Risk factors for opioid-induced respiratory depression in surgical patients: a systematic review and meta-analyses. BMJ Open. 2018;8(12):e024086. doi:10.1136/bmjopen-2018-024086
6. Driver CN, Laporta ML, Bergese SD, et al. Frequency and temporal distribution of postoperative respiratory depressive events. Anesth Analg. 2021;132(5):1206–1214. doi:10.1213/ANE.0000000000005478
7. Lee LA, Caplan RA, Stephens LS, et al. Postoperative opioid-induced respiratory depression: a closed claims analysis. Anesthesiology. 2015;122(3):659–665. doi:10.1097/ALN.0000000000000564
8. Chung F, Wong J, Mestek ML, Niebel KH, Lichtenthal P. Characterization of respiratory compromise and the potential clinical utility of capnography in the post-anesthesia care unit: a blinded observational trial. J Clin Monit Comput. 2020;34(3):541–551. doi:10.1007/s10877-019-00333-9
9. Urman RD, Khanna AK, Bergese SD, et al. Postoperative opioid administration characteristics associated with opioid-induced respiratory depression: results from the PRODIGY trial. J Clin Anesth. 2021;70:110167. doi:10.1016/j.jclinane.2021.110167
10. Rao VK, Khanna AK. Postoperative respiratory impairment is a real risk for our patients: the intensivist’s perspective. Anesthesiol Res Pract. 2018;2018:1–7. doi:10.1155/2018/3215923
11. Oderda GM, Gan TJ, Johnson BH, Robinson SB. Effect of opioid-related adverse events on outcomes in selected surgical patients. J Pain Palliat Care Pharmacother. 2013;27(1):62–70. doi:10.3109/15360288.2012.751956
12. Kessler ER, Shah M, Gruschkus SK, Raju A. Cost and quality implications of opioid-based postsurgical pain control using administrative claims data from a large health system: opioid-related adverse events and their impact on clinical and economic outcomes. Pharmacotherapy. 2013;33(4):383–391. doi:10.1002/phar.1223
13. Khanna AK, Jungquist CR, Buhre W, et al. Modeling the cost savings of continuous pulse oximetry and capnography monitoring of United States general care floor patients receiving opioids based on the PRODIGY trial. Adv Ther. 2021;38(7):3745–3759. doi:10.1007/s12325-021-01779-7
14. Khanna AK, Bergese SD, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective; observational trial. Anesth Analg. 2020;131(4):1012–1024. doi:10.1213/ANE.0000000000004788
15. Deitch K, Miner J, Chudnofsky CR, Dominici P, Latta D. Does end tidal CO2 monitoring during emergency department procedural sedation and analgesia with propofol decrease the incidence of hypoxic events? A randomized; controlled trial. Ann Emerg Med. 2010;55(3):258–264. doi:10.1016/j.annemergmed.2009.07.030
16. Al-Halawani R, Charlton PH, Qassem M, Kyriacou PA. A review of the effect of skin pigmentation on pulse oximeter accuracy. Physiol Meas. 2023;44(5):05TR01. doi:10.1088/1361-6579/acd51a
17. Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–442. doi:10.1111/j.1526-4637.2005.00072.x
18. Zedler B, Xie L, Wang L, et al. Development of a risk index for serious prescription opioid-induced respiratory depression or overdose in veterans’ health administration patients. Pain Med Malden Mass. 2015;16(8):1566–1579. doi:10.1111/pme.12777
19. Cheatle MD, Compton PA, Dhingra L, Wasser TE, O’Brien CP. Development of the revised opioid risk tool to predict opioid use disorder in patients with chronic nonmalignant pain. J Pain. 2019;20(7):842–851. doi:10.1016/j.jpain.2019.01.011
20. Armstrong AD, Hassenbein SE, Black S, Hollenbeak CS. Interdisciplinary pain team. risk factors for increased postoperative pain and recommended orderset for postoperative analgesic usage. Clin J Pain. 2020;36(11):845–851. doi:10.1097/AJP.0000000000000876
21. Mulligan RP, McCarthy KJ, Grear BJ, Richardson DR, Ishikawa SN, Murphy GA. Psychosocial risk factors for postoperative pain in ankle and hindfoot reconstruction. Foot Ankle Int. 2016;37(10):1065–1070. doi:10.1177/1071100716655142
22. Ward EN, Quaye ANA, Wilens TE. Opioid use disorders: perioperative management of a special population. Anesth Analg. 2018;127(2):539–547. doi:10.1213/ANE.0000000000003477
23. Albrecht E, Grape S, Frauenknecht J, Kilchoer L, Kirkham KR. Low- versus high-dose intraoperative opioids: a systematic review with meta-analyses and trial sequential analyses. Acta Anaesthesiol Scand. 2020;64(1):6–22. doi:10.1111/aas.13470
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

