Back to Journals » Cancer Management and Research » Volume 17
Safety and Efficacy of Stereotactic Radiosurgery in the Management of Primary Spinal Cord Glioblastoma: A Case Report
Authors Lazzari G , Montagna A, Benevento I, D'Andrea B, Metallo V , Tucciariello R , Colamaria A, Di Perna G, Modano P, Bianculli A
Received 1 December 2024
Accepted for publication 10 February 2025
Published 19 February 2025 Volume 2025:17 Pages 349—355
DOI https://doi.org/10.2147/CMAR.S509635
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Grazia Lazzari,1 Antonietta Montagna,1 Ilaria Benevento,1 Barbara D’Andrea,1 Vito Metallo,1 Raffaele Tucciariello,2 Antonio Colamaria,3 Giuseppe Di Perna,3 Pasqualina Modano,4 Antonella Bianculli2
1Radiation Oncology Unit - IRCCS-CROB, Rionero in Vulture, PZ, 85028, Italy; 2Physic Unit - IRCCS-CROB, Rionero in Vulture, PZ, 85028, Italy; 3Neurosurgery Unit- Ospedali Riuniti -Policlinico Universitario, Foggia, FG, 71121, Italy; 4Emergency and Palliative Care Unit- IRCCS-CROB, Rionero in Vulture, PZ, 85028, Italy
Correspondence: Grazia Lazzari, Radiation Oncology Unit. IRCCS-CROB; Rionero in Vulture, PZ, Italy, Tel +39 3495300810 ; + 39 0972 726741, Email [email protected]
Objective: Primary spinal cord Glioblastoma multiforme (IV grade WHO), also known as Primary Spinal Cord Astrocytoma (SCA), accounts for 6– 8% of primary spinal cord tumors and up to 1.5% of all spinal cord tumors. However, owing to their rarity, no large studies or management consensus are available. Gross total resection (GTR) is the best advisable approach; however, in primary spinal tumors, this procedure is not always safe or feasible. Higher radiation doses with conventional radiotherapy (RT) are limited by the spinal cord’s radiation tolerance. Stereotactic radiosurgery (SRS) is an effective and safe alternative when administered with adequate real-time simulation and planning to minimize setup errors with risks to the normal spinal cord.
Case Presentation: Herein we present the case of a 32-year-old woman with primary grade IV glioblastoma (GBM) of the spinal cord at cervical C3-C6 (C3–C6) vertebrae level who underwent subtotal resection. The patient presented with neurological impairment in the neck, shoulder, and limbs. Sphincteric dysfunction and hyperaesthesia on the chest were also recorded. As adjuvant therapy, SRS with a dose of 14 Gy to the PTV was administered, ensuring a Dmax of 12 Gy to the spinal cord. Patient was treated in the same day of simulation and planning; hence, no set-up discrepancies were recorded on Cone Beam CT (CBCT) images during the RT delivery. Two days after SRS, the patient’s neurological symptoms improved with recovery of neck and shoulder motor functions followed by weak upper limb activity. Afterwards, 6 cycles of temozolomide were administered.
Conclusion: We described a case of grade IV glioblastoma multiforme after partial resection, that was safely treated with adjuvant SRS in real-time with simulation and planning. This modality could improve the safety of SRS in the treatment of such tumors.
Keywords: adjuvant therapy, glioblastoma, stereotactic radiosurgery, primary spinal cord tumors
Introduction
Extracranial glioblastoma is a rare condition comprising a wide spectrum of pathological entities that could affect secondary sites, such as spinal leptomeningeal metastasis, systemic metastasis (lymphatic or hematogenous spreads), as well as primary spinal cord glioblastoma. Secondary extracranial glioblastoma (GBM) with spinal dissemination has already been described in patients with primary cranial GBM. Its recurrence in bones has been reported in 31% of patients, with a predominance in the vertebral body, as recently reported in a case report by Colamaria et al.1 Beyond this rare occurence, primary spinal GBM should be considered, as shown in this paper.
Primary spinal cord GBM is a rare malignancy that includes “Primary Spinal Cord Astrocytomas (SCAs)” that account for approximately 1.5% of all spinal cord tumors,2 and are typically found in the thoracic or cervical column and less frequently in the conus medullaris.3 There is no consensus treatment guidelines for these tumors; however, the suggested approaches in retrospective studies include surgery and radiotherapy as the only available therapeutic options. The use of temozolomide therapy has also been reported but as a consolidation approach.4 There is a general consensus indicating that surgical decompression is the optimal treatment strategy for all spinal cord tumors, irrespective of whether they are secondary or primary lesions. In cases of primary spinal cord grade III and IV astrocytomas, surgery appears to achieve good local control and survival when gross total resection (GTR) is performed.5 GTR is always advisable and should be attempted; however, severe postoperative neurological sequelae could occur.6
Beside surgery, radiotherapy is another option. Numerous studies have recorded improved survival mainly in patients with WHO grade II–IV astrocytomas.7 Current advances in RT technology such as stereotactic radiosurgery and image-guided radiotherapy can aid in delivering therapeutic doses while sparing normal healthy tissue,8 such as the spinal cord.
However, the risk of direct radiation-induced injury to the spinal cord, such as radiation myelitis (RM), should be considered. Moreover, there is no consensus on the optimal timing for surgery, techniques, or radiation dose. Practically, doses above 45 Gy are administered with caution because of the poor tolerance of the spinal cord to doses of over 50 Gy according to the linear quadratic model.9 Stereotactic body radiotherapy (SBRT) and Stereotactic Radiosurgery (SRS) are currently standard techniques used to safely treat vertebral metastases and are capable of sparing the spinal cord. Translating this effect from spinal metastases palliation, the same efficacy and safety could be expected of its use in the management of primary spinal cord tumors due to its ability to deliver a high biologically equivalent dose (BED) in a very precise and conformal manner, thereby achieving a steep radiation dose gradient within the spinal cord.10 Moreover, previous retrospective studies have shown its efficacy in functional outcomes, response, and palliation with SRS at a single session with a median dose of 16 Gy.11 Based on available radiobiological models on the safety of SRS on the spinal cord, we decided to cautiously treat a patient with primary spinal cord glioblastoma with SRS, and carefully delivered the treatment on the same day of simulation and planning to avoid daily set-up errors on the spinal cord in a patient with quadriplegia. Using the CBCT images, the set-up errors were minimal, ensuring the exact planned fall off dose to the normal spinal cord. Importantly, no late spinal cord radiation-induced impairments were recorded several months after the procedure.
Case Presentation
Informed consent was obtained from the patient to publish this report. A 32-year-old obese woman was diagnosed with primary spinal cord glioblastoma (WHO grade IV) at the C3–C6 vertebral level. Two months prior, she had complained of paresthesia in the upper extremities and impaired neck movements. These symptoms worsened, leading to sphincteric incontinence and hyperesthesia of the chest. Cervical MRI showed an epidural mass on the anterior side of the spinal cord as a hypointense T1-weighted and hyperintense T2-weighted lesion with a heterogeneous pattern of contrast enhancement (Figure 1). The lesion was 35 mm long, 26 mm wide, and 18 mm anteroposteriorly. The patient initially underwent laminectomy with subtotal resection of the tumor. Histological examination of the biopsy specimen revealed a grade IV astrocytoma. Due to the patient’s weight and neurological conditions, repeated daily set-up were considered risky; therefore, SRS was chosen to be delivered on the same day of simulation and planning, thus minimizing the risk of repeated set-up errors that are unsafe for the spinal cord. The patient was transferred from the CT table to the Linac table with a transfer sled while waiting for SRS. Simulation CT scan images were acquired with a thickness of 2 mm. Pre- and postoperative MRI T1-T2 weight images were overlaid with simulation CT scans and applied to define the Gross Tumor Volume (GTV). T2-weighted MRI images were also used to delineate the entire cervical spinal cord and 20 mm below in the thoracic tract. GTV consisted of the residual mass on MRI T2-weighted images and measured 5.2 cc; afterwards, a 1-mm margin was added to GTV to obtain the Planning Target Volume (PTV) which was 10.8 cc. The prescription dose was 100% of the target mean and consisted of 15 Gy to the GTV with 14 Gy to the PTV, providing a dose that was less than 12 Gy Dmax to the surrounding normal spinal cord tissue (Figures 2 and 3).
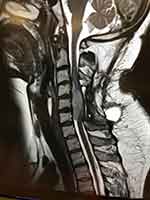 |
Figure 1 MRI sagittal image of the spinal cord mass after debulking surgery. |
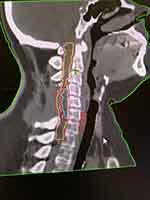 |
Figure 2 Sagittal image of the Gross Tumor Volume (GTV = azur); Planning Target Volume (PTV = red); spinal cord (yellow); and thecal sac (Orange). |
 |
Figure 3 SRS dose distribution in the sagittal plane. |
The treatment plan consisted of VMAT using 2 arcs and 6 MV photon beams. The reverence point was fixed to 100% of the target mean with a dose max of 104.8% providing a 95.2% normalization to the GTV. The mean dose to the GTV was 15 Gy; the mean dose to PTV was 14.52 Gy. Regarding the dose to the spinal cord, 0.1 cc received 12.5 Gy, while 0.1 cc of the thecal sac received 13 Gy. On CBCT images, the set-up error calculated at the vertebral contour and thecal sac was an average of 0.2 mm in every direction. High-dose steroids and mannitol were supplied in the following days. Neurological deficits of the limbs and necks improved within 2 days, with recovery of motor functions in the upper extremities and shoulders, and the patient’s incontinency was also ameliorated. Afterwards, she was started with 6 cycles of temozolomide. No features suggestive of neurological toxicities were observed. Six months later, cervical MRI showed a fibrotic lesion at the level of the C4–C6 vertebrae without contrast enhancement in the irradiated tumor bed (Figure 4). After 9 months of follow-up, the patient was alive but had impairment of in the lower extremities.
 |
Figure 4 MRI sagittal image after 6 months showing the absence of enhancement in the treated area that was treated with SRS. |
Discussion
Extracranial GBM is a rare disease that could be secondary in the bones or primary in the spinal cord. Extracranial GBM accounts for 0.4–2.0% of all intracranial GBM cases.12 Primary spinal GBM is a rare malignancy that includes “Primary Spinal Cord Astrocytomas (SCAs)”, which represent approximately 1.5% of all spinal cord tumors.2 Since 1938, at least 200 cases of primary spinal GBM have been reported. SCAs show a slight male preponderance and are more prevalent in young patients with a mean age of 26 years.3 The age at diagnosis and vertebral involved level appear to be the most important prognostic factors. However, although data regarding age are still controversial, available trends indicate that younger age at diagnosis is associated with a better median overall survival of approximately 14.3 months for patients less than 50 years old.4 The vertebral level of the lesion in the spine is also a prognostic factor. Tumors in the thoracic spine have been reported to have a better prognosis than cervical spine tumors, probably due to the availability of more effective surgery procedures in non-cervical spine regions.13 Our patient had a cervical tumor and was treated using a less demolitive surgery.
Due to their origin from the cord parenchyma, these tumors often have a typical eccentric growth pattern, thus creating a focal expansion of the spinal cord’s diameter or displacement of the normal spinal parenchyma with a compressive effect. Consequently, severe neurological peripheral impairment due to a spinal cord compression occurs. There is a general consensus on surgical decompressive resection as the optimal treatment strategy for all spinal cord astrocytomas owing to its good local control and favorable impact on survival mainly in patients with grade III and IV spinal cord gliomas.4 Studies have shown a survival of 18.7 months post-surgery after accounting for approximately 10% of patients that received a gross total resection.6 Gross total resection should always be attempted, especially when there are good planes of dissection intraoperatively and when stable neuromonitoring is feasible, irrespective of the tumor grade.14 Nevertheless, in grade III and IV astrocytomas this approach is not always feasible. Reports show that gross total resection has been obtained in 0% of grade IV to only 12% in grade III tumors.7 These results are coherent with data from several studies, including a retrospective review of 22 patients with high-grade intramedullary astrocytomas in which gross total resection was obtained only in two patients.15 Moreover, severe postoperative neurological morbidities should be expected, as reported in a study where 37% out of 46 patients treated with GTR developed worse neurologic impairment as compared to their preoperative state.6,16 Our patient underwent partial tumor debulking resection and required adjuvant treatment.
Radiotherapy and temozolomide therapy were used in the management of the patient, similar to the treatment of cranial GBM. Adjuvant radiotherapy in spinal astrocytomas could be considered with caution because radiation-induced spinal cord damage could occur and is referred to as radiation myelitis (RM).9 A study by Li et al demonstrated that this damage occurs quickly after spinal cord radiation in rat models. The study also showed that endothelial apoptosis in the spinal cord occurred within 24 h of the radiation to the cord.17 However, there is no consensus on the optimal timing and radiation dose. In terms of conventional fractionation, a retrospective study by Corradini et al reported that a minimum median dose of 45 Gy was effective;18 however, its relationship with survival outcomes remains still unclear. In a study conducted on 29 patients treated with GTR or subtotal resection and adjuvant RT with 3D-IMRT modalities and a median dose of 50.4 Gy in standard fractionation, no clear association was found between the dose and OS and PFS.19 However, higher doses should be avoided to minimize the risk of RM, which varies across different vertebral levels. Studies have also established that the α/β spinal cord tolerance according to the linear quadratic model ranges from 2 to 4 Gy across the cervical, thoracic, and lumbar vertebrae.20 Using conventional fractionation, as reported by the QUANTEC model, the risk of RM is 0.03% at a Dmax of 45 Gy, 0.2% at 50 Gy, 1% at 54 Gy, and 10% at 61 Gy.21 Thus, effective directed radiation doses on the spinal cord above 50 Gy in conventional fractionation could be unsafe. A growing body of evidence on the safety of SBRT and SRS in spinal metastases encourages their use in the management of spinal cord tumors; this is backed by the reports of a few retrospective series conducted on radioresistant tumors such as cordomas or condrosarcomas. In a study by Chang et al,22 29 patients with different primary spinal cord tumors were treated with adjuvant or salvage SBRT. The doses used ranged from 12 to 50 Gy in two to six sessions. The mean radiation dose converted into a biological effective dose (BED) was 60 Gy (range, 43–105 Gy). The mean local progression-free survival was 56 months for patients with chordomas and 73 months for patients with sarcoma. Regarding SRS, in a report by Ryu et al, 26 patients with primary spinal cord tumors were treated with radiosurgery, which varied from a single session of 12–18 Gy to fractionated schedules in six lesions. The radiation dose was prescribed to the 90% isodose line, which encompassed the periphery of the target tumor, with at least 90% of the target volume receiving the prescribed dose. The overall local tumor control rate was 94%, of which complete response was seen in 26%, partial response in 26%, and stable findings in 42% of cases. Improvements in neurological status were observed in 56% of cases, and 28% were stable.23 Given the patient’s critical neurological condition, we decided to use SRS as adjuvant RT. A single dose was selected for its effectiveness in treating the tumor, while ensuring the safety of the normal spinal cord. In a retrospective analysis on 30 patients with primary spine tumors who were treated with SRS11 several single dose levels were tested with the Dmax to the spinal cord as lower as possible. Single doses ranged from 10 Gy in one fraction to 24 Gy in one fraction (median, 16 Gy in one fraction). All doses were prescribed to the 90% isodose line. The primary dose constraint for plan selection was to achieve the objective of 10 Gy to 10% of the partial volume of the spinal cord and a maximum point dose of 14 Gy. All doses were prescribed to the 90% isodose line. Post-treatment pain, neurological, and radiographic responses were the endpoints. As a result, pain control consisted of a response rate of 88% while neurological improvement was also observed in 65% of cases. Furthermore, local control was achieved in 77% of patients after long-term follow-ups. In regard to spinal cord SRS tolerance, the risk of RM in spinal SRS has been modeled by Shagal et al through the Hypofractionation Treatment Effects in the Clinic (HyTEC) report. According to the report, for de novo SBRT delivered in 1–5 fractions, the spinal cord Dmax associated with a 1–5% risk of RM was 12.4–14.0 Gy in one fraction, 17.0 Gy in two fractions, 20.3 Gy in three fractions, 23.0 Gy in four fractions, and 25.3 Gy in five fractions.24 In our case, the prescription was limited to 0.1 cc of the spinal cord to 12.5 Gy, while 0.1 cc of the PRV received 13 Gy to stay within the 1–5% RM calculated risk. Based on literature reports and considering the conditions of our patient, we opted for SRS with a dose prescription that was effective for tumor treatment and safe for the spinal cord in terms of a low risk of RM. The novelty of this report was the treatment of a patient on the same day of CT scan simulation and treatment planning to minimize the risk of set-up errors and to deliver the planned dose with minimal risk to the spinal cord.
Conclusion
Our report indicates that SRS is a safe and effective treatment option for primary SCAs. Although the HyTEC report is helpful in the decision-making process, a correct set-up should be provided. Same-day simulation and treatment can reduce setup errors, ensuring the calculated dose gradient accurate dose delivery to the tumor while minimizing risks to the spinal cord. Our report shows that this approach is effective and safe.
Consent for Publication
The authors have obtained informed consent from the patients for publication of the case details and any accompanying images. Institutional policy for approval by ethical committee was not required.
Acknowledgments
The authors have obtained informed consent from the patients for publication of the case details and any accompanying images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Colamaria A, Blagia M, Sacco M, Carbone F. Diffuse vertebral metastases from glioblastoma with vertebroepidural diffusion: a case report and review of the literature. Surg Neurol Intern. 2021;12:437. doi:10.25259/SNI_538_2021
2. Milano MT, Johnson MD, Sul J, et al. Primary spinal cord glioma: a surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83–92. doi:10.1007/s11060-009-0054-7
3. Shen CX, Wu JF, Zhao W, et al. Primary spinal glioblastoma multiforme: a case report and review of the literature. Medicine. 2017;96(16):6634–6640. doi:10.1097/MD.0000000000006634
4. Wong AP, Dahdaleh NS, Fessler RG, et al. Risk factors and long-term survival in adult patients with primary malignant spinal cord astrocytomas. J Neurooncol. 2013;115(3):493–503. doi:10.1007/s11060-013-1251-y
5. Hamilton KR, Lee SS, Urquhart JC, Jonker BP. A systematic review of outcome in intramedullary ependymoma and astrocytoma. J Clin Neurosci. 2019;63:168–175. [[PubMed: 30833131]]. doi:10.1016/j.jocn.2019.02.001
6. McGirt MJ, Goldstein IM, Chaichana KL, et al. Extent of surgical resection of malignant astrocytomas of the spinal cord. Neurosurgery. 2008;63(1):55–61. doi:10.1227/01.NEU.0000335070.37943.09
7. Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2009;73(3):727–733. doi:10.1016/j.ijrobp.2008.04.060
8. Boström A, Kanther NC, Grote A, Boström J. Management and outcome in adult intramedullary spinal cord tumours: a 20-year single institution experience. BMC Res Notes. 2014;7(1):908. doi:10.1186/1756-0500-7-908
9. Samartzis D, Gillis CC, Shih P, et al. Intramedullary spinal cord tumors: part I-epidem iology, pathophysiology, and diagnosis. Glob Spine J. 2015;5(5):425–435. doi:10.1055/s-0035-1549029
10. Guckenberger M, Mantel F, Gerszten PC, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: a multi-institutional analysis. Radiat Oncol. 2014;9(1):226–233.10.1186/s13014–014–0226–2. doi:10.1186/s13014-014-0226-2
11. Elibe E, Boyce-Fappiano D, Ryu S, et al. Stereotactic radiosurgery for primary tumors of the spine and spinal cord. J Radiosurg SBRT. 2018;5:107–113. PMID: 29657891.
12. Goodwin CR, Liang L, Abu-Bonsrah N, et al. Extraneural glioblastoma multiforme vertebral metastasis. World Neurosurg. 2016;89:578–82.e3. doi:10.1016/j.wneu.2015.11.061
13. Ropper AE, Cahill KS, Hanna JW, et al. Primary vertebral tumors: a review of epidemiologic, histological, and imaging findings, Part I: benign tumors. Neurosurg. 2011;69(6):1171–1180. doi:10.1227/NEU.0b013e31822b8107
14. Ogunlade J, Wiginton IVJG, Elia C, et al. Primary spinal astrocytomas: a literature review. Cureus. 2019;11(7):e5247. doi:10.7759/cureus.5247
15. Raco A, Piccirilli M, Landi A, et al. High-grade intramedullary astrocytomas: 30 years’ experience at the neurosurgery department of the university of Rome “Sapienza”. J Neurosurg Spine. 2010;12(2):144–153. doi:10.3171/2009.6.SPINE.08910
16. Babu R, Karikari IO, Owens TR, Bagley CA. Spinal cord astrocytomas: a modern 20-year experience at a single institution. Spine. 2014;39(7):533–540. doi:10.1097/BRS.0000000000000190
17. Li YQ, Chen P, Jain V, et al. Early radiation-induced endothelial cell loss and blood-spinal cord barrier breakdown in the rat spinal cord. Radiat Res. 2004;161(2):143–152. doi:10.1667/RR3117
18. Corradini S, Hadi I, Hankel V, et al. Radiotherapy of spinal cord gliomas: a retrospective mono-institutional analysis. Strahlenther Onkol. 2016;192(139):45. doi:10.1007/s00066-015-0917-0
19. Upadhyay R, Khose S, Pokhylevych H, et al. Patterns of failure after radiation therapy in primary spinal high-grade gliomas: a single institutional analysis. Neurooncol Adv. 2022;20(4(1)):vdac129. doi:10.1093/noajnl/vdac129
20. Nieder CG, Grosu A, Andratschke NH, Molls M. Update of human spinal cord reirradiation tolerance based on additional data from 38 patients. Int J Radiation Oncology Biol Phys. 2006;66(5):1446–1449. doi:10.1016/j.ijrobp.2006.07.1383
21. Kirkpatrick JP, van der Kogel AJ, Schultheiss TE. Radiation dose volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42–S49.
22. Chang U-K, Lee DH, Kim MS. Stereotactic radiosurgery for primary malignant spinal tumors. Neurological Res. 2014;36(6):597–606. doi:10.1179/1743132814Y.0000000381
23. Ryu S, Biondo A, Rock J, et al. Stereotactic radiosurgery of primary spine and spinal cord tumors. J Radiosurg SBRT. 2013;2(2):127–133. PMID: 29296351.
24. Sahgal A, Chang JH, Ma L, et al. Spinal cord dose tolerance to stereotactic body radiation therapy. Int J Radiation Oncol Biol Phys. 2021;110(1):124–136. doi:10.1016/j.ijrobp.2019.09.038
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

