Back to Journals » Research and Reports in Tropical Medicine » Volume 16
Schistosomiasis Among Schoolchildren in Amd District of Hadhramout Governorate, East of Yemen: A Hotspot for Schistosoma haematobium Transmission
Authors Al-Bowri SS, Al-Mekhlafi AM, Abdul-Ghani R , Azazy AA
Received 5 April 2025
Accepted for publication 3 June 2025
Published 9 June 2025 Volume 2025:16 Pages 55—64
DOI https://doi.org/10.2147/RRTM.S532602
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Mario A. Rodríguez-Pérez
Saeed S Al-Bowri,1 Abdulsalam M Al-Mekhlafi,2 Rashad Abdul-Ghani,2,3 Ahmed A Azazy4
1Health Sciences Department, Faculty of Medicine and Health Sciences, Seiyun University, Seiyun, Yemen; 2Department of Medical Parasitology, Faculty of Medicine and Health Sciences, Sana’a University, Sana’a, Yemen; 3Tropical Disease Research Center, Faculty of Medicine and Health Sciences, University of Science and Technology, Sana’a, Yemen; 4Department of Laboratory Medicine, Faculty of Applied Medical Sciences, Al Baha University, Al Baha, Saudi Arabia
Correspondence: Rashad Abdul-Ghani, Department of Medical Parasitology, Faculty of Medicine and Health Sciences, Sana’a University, P.O. Box 13078, Sana’a, Yemen, Tel +967 775005239, Email [email protected]
Background: Schistosomiasis persists as a public health problem in Hadhramout, the largest governorate in the eastern part of Yemen. Despite its endemicity, epidemiological patterns in many districts remain unclear. Therefore, this study aimed to determine the prevalence and factors associated with schistosomiasis among schoolchildren in the Amd District of Hadhramout Valley.
Methods: A cross-sectional study was conducted with 380 schoolchildren aged 6– 16 years. Data on the children’s sociodemographic characteristics, infection-related behaviors, and environmental factors were collected using a structured questionnaire. Urine filtration and Kato-Katz techniques were used to detect and count the eggs of Schistosoma haematobium and S. mansoni, respectively. Data were analyzed using appropriate statistical tests, and multivariable binary logistic regression analysis was performed to identify predictors of schistosomiasis.
Results: In Amd District, 33.7% (95% CI: 28.9– 38.7) of schoolchildren had light-intensity infection with any Schistosoma species, indicating a moderate risk level, specifically S. haematobium among 31.6% (95% CI: 26.9– 36.3) and S. mansoni among 2.1% (95% CI: 0.7– 3.6) of children. Macrohematuria, microhematuria, and proteinuria were significantly associated with S. haematobium infection. However, neither hematochezia nor diarrhea was significantly associated with S. mansoni infection. Multivariable binary logistic regression analysis identified male gender (AOR = 4.2; 95% CI: 2.48– 7.12; P < 0.001), age ≥ 10 years (AOR = 3.1; 95% CI: 1.70– 5.56; P < 0.001), and contact with natural water sources (AOR = 2.0; 95% CI: 1.06– 3.58; P = 0.032) as independent predictors of schistosomiasis.
Conclusion: The risk of schistosomiasis in Amd District is moderate and predominated by S. haematobium, with light-intensity infections affecting approximately one-third of schoolchildren. Therefore, biannual preventive chemotherapy with praziquantel is recommended for all enrolled and non-enrolled school-age children. Macrohematuria, microhematuria, and proteinuria are important indicators of S. haematobium infection. Meanwhile, male gender, older age, and water contact can independently predict infection.
Keywords: schistosomiasis, schoolchildren, Yemen
Introduction
Schistosomiasis, a neglected tropical disease (NTD) caused by parasites of the genus Schistosoma, remains a major public health challenge in rural communities of resource-limited countries.1 In 2021, an estimated 251.4 million people in endemic countries with moderate-to-high transmission required preventive treatment to reduce and prevent disease-related morbidity.2
Yemen is one of the countries in the Middle East and North Africa region most highly endemic for NTDs, including schistosomiasis caused by Schistosoma haematobium and S. mansoni.3 Since 2010, the country has implemented a nationwide control strategy centered on preventive chemotherapy with praziquantel (PZQ) and delivered through mass drug administration (MDA) campaigns.4 Nevertheless, high-prevalence hotspots persist in endemic areas.5–10 In 2022, over 3.6 million people, including over 3 million school-age children (SAC), were estimated to require preventive chemotherapy with PZQ.11 However, the national coverage rates were 78.7% for all ages and 74.2% for SAC.11
Hadhramout, the largest governorate in Yemen, is located in the eastern part of the country and comprises 28 districts as its administrative units.12 The local workforce primarily engages in agriculture, fishing, and livestock rearing.12 These activities often involve frequent contact with water, exposing people to a high risk of waterborne infections such as schistosomiasis. Existing research on schistosomiasis prevalence remains limited to specific districts,10,13 leaving major gaps in the understanding of the epidemiological profile of the disease across the governorate. The national mapping of schistosomiasis in 2014 classified schistosomiasis risk among SAC in the governorate as low (prevalence <10%).9 Nonetheless, subsequent interruptions to MDA campaigns and deterioration of the healthcare structure as a result of the humanitarian crises may have altered the transmission dynamics across the districts of the governorate. For instance, a recent study in selected districts in Hadhramout identified moderate-risk levels of S. haematobium infection.10 Therefore, this study aimed to determine the prevalence and factors associated with schistosomiasis among schoolchildren in Amd District, Hadhramout Governorate, east of Yemen.
Methods
Study Design, Population and Area
A cross-sectional study was conducted among schoolchildren aged 6 to 16 years in Amd District in 2021. This rural agricultural district is located in the central-western part of Hadhramout Governorate at the coordinates 15°10′N, 47°55′E (Figure 1). It is one of the smallest districts in the Hadhramout Valley, spanning an area of 737 km2 and populated with over 31,000 inhabitants as of 2021.12
 |
Figure 1 Map of Yemen marking locations of Hadhramout Governorate and Amd District. |
Sample Size and Sampling Strategy
The required sample size was determined to be 334 schoolchildren using OpenEpi, version 3.01 (available at www.openepi.com), based on an anticipated prevalence of 31.8%, as previously reported for rural children across five of the country’s governorates,6 a confidence level of 95%, an absolute precision of 5%, and a design effect of 1.0 because of the assumed uniform risk across the district. However, 380 children were recruited for this study to enhance precision. A two-stage cluster sampling method was implemented. Initially, two schools were randomly selected from a list of primary schools in the district. The children were then selected using simple random sampling from the registers of eligible students. If a child was absent, declined to participate, or lacked informed consent from their parents or legal guardians, he or she was substituted with the next available child in the register.
Data Collection
A structured questionnaire was used to collect data on the children’s sociodemographic characteristics, history of antischistosomal therapy, behaviors, and environmental factors related to schistosomiasis. Additionally, the questionnaire included parasitological and laboratory data obtained after urine and stool analyses.
Sample Collection and Analysis
From January to March 2021, children were asked to collect urine and stool samples in pre-labeled containers between 10 am and 2 pm after explaining the proper method of sample collection to them. The samples were immediately transported to Al-Bowri Specialized Medical Laboratories in Seiyun City for analysis. Urine samples were macroscopically inspected for macrohematuria and tested for microhematuria using reagent strips (UroColorTM 9, Standard Diagnostics Inc., South Korea), according to the manufacturer’s instructions. Urine samples were processed using the filtration technique and then microscopically examined for S. haematobium eggs.14,15 Eggs were counted per 10 mL of urine (EP10mL),14,15 and the intensity of infection was then categorized as light (≤50 EP10mL) or heavy (>50 EP10mL).16 For the detection and quantification of S. mansoni eggs, two thick fecal smears were prepared and microscopically examined using the Kato-Katz technique.17 According to the number of eggs per gram of stool (EPG), the intensity of infection was classified as light (<100 EPG), moderate (100–399 EPG), and heavy (≥ 400 EPG).18
Data Analysis
Data were analyzed using IBM SPSS Statistics, version 22.0 (IBM Corp., Armonk, NY, USA) at P-values <0.05. The prevalence of schistosomiasis was calculated and reported with a 95% confidence interval (CI). In line with WHO guidelines,19 the level of infection risk in the district was classified as low (prevalence of 1% to <10%), moderate (prevalence of ≥10% to <50%), and high (prevalence of ≥50%).19 Univariate analysis, using Pearson’s chi-square or Fisher’s exact test, was used to test the association between schistosomiasis and independent variables, and the odds ratios (ORs) and corresponding 95% CIs of associations were reported. Variables with univariate P-values <0.2 were included in a multivariable binary logistic regression model to identify independent predictors of infection. The adjusted ORs (AORs) and their 95% CIs for the predictors were reported.
Ethical Considerations
This study complied with the ethical guidelines of the Declaration of Helsinki for human participants. Ethical approval for this study was granted by the Postgraduate Research Committee of the Faculty of Medicine and Health Sciences at Sana’a University. Before enrollment, the aims and procedures of the study were thoroughly explained to the parents or legal guardians who provided written informed consent. The children also provided verbal assent to affirm their voluntary participation. All collected data were anonymized, and participant confidentiality and privacy were ensured at all stages of the study. Children diagnosed with schistosomiasis were administered a single weight-adjusted dose of PZQ in accordance with therapeutic protocols.
Results
Schoolchildren Characteristics
Most schoolchildren were males (60.3%), aged 10 years or younger (71.4%), with a mean age of 11.5 ± 2.8 years. More than half of the children came from households with more than five members, with a mean household size of 8.6 ± 2.8 members. Regarding parental literacy, the fathers of most children were literate (68.9%), while the mothers of more than half of the children were literate (53.7%). Most of the children’s fathers were employed (58.9%) compared to only 5.8% of their mothers (Table 1).
 |
Table 1 Characteristics of Schoolchildren Included in the Study* |
Prevalence and Intensity of Schistosomiasis
The overall prevalence of infection with any Schistosoma species among schoolchildren in Amd District was 33.7% (128/380; 95% CI: 28.9–38.7), indicating a moderate risk level. Specifically, S. haematobium was prevalent among 31.6% of the children (120/380; 95% CI: 26.9–36.3), while S. mansoni was prevalent among 2.1% of the children (8/380; 95% CI: 0.7–3.6). No co-infection with both types of infection was observed, and all children had light-intensity infections.
Clinical Specimen Indicators of Schistosomiasis
Macrohematuria (P = 0.029), microhematuria (P <0.001), and proteinuria (P <0.001) were significantly associated with S. haematobium infection among schoolchildren. Conversely, neither hematochezia (P = 1.000) nor diarrhea (P = 0.132) was significantly associated with S. mansoni infection (Table 2).
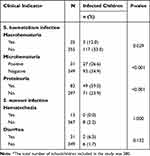 |
Table 2 Clinical Specimen Indicators of Schistosomiasis Among Schoolchildren in Amd District, Hadhramout Governorate, Yemen (2021)* |
Sociodemographic Factors Associated with Schistosomiasis
Both gender and age were significantly associated with schistosomiasis among schoolchildren. Specifically, males had significantly higher odds of infection than females (OR = 3.9, 95% CI: 2.35–6.34; P <0.001), with infection rates of 44.5% vs 17.2%. Similarly, children aged ≥10 years showed significantly higher odds of infection than those aged <10 years (OR = 3.1, 95% CI: 1.78–5.37; P <0.001), with infection rates of 39.8% vs 17.6%, respectively. In contrast, no significant association was observed between schistosomiasis and household size, parental literacy, or employment status (Table 3).
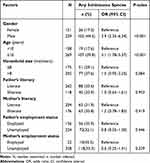 |
Table 3 Sociodemographic Factors Associated with Schistosomiasis Among Schoolchildren in Amd District, Hadhramout Governorate, Yemen (2021) |
Behavioral and Environmental Factors Associated with Schistosomiasis
Contact with natural water sources (valleys, streams, or ponds) was a statistically significant risk factor for schistosomiasis (OR = 2.6, 95% CI: 1.50–4.65; P = 0.001), with 38.5% of exposed children infected compared to 19.1% of unexposed children. However, no significant association was observed between schistosomiasis and the presence of natural water sources near households or schools, non-piped sources of household water, absence of latrines in households or schools, or non-intake of antischistosomal treatment in the six months preceding the survey (Table 4).
 |
Table 4 Behavioral and Environmental Risk Factors Associated with Schistosomiasis Among Schoolchildren in Amd District, Hadhramout Governorate, Yemen (2021) |
Independent Predictors of Schistosomiasis
Multivariable binary logistic regression analysis identified male gender (AOR = 4.2; 95% CI: 2.48–7.12; P <0.001), age ≥10 years (AOR = 3.1; 95% CI: 1.70–5.56; P <0.001), and contact with natural water sources (AOR = 2; 95% CI: 1.06–3.58; P = 0.032) as independent predictors of schistosomiasis among schoolchildren (Table 5).
 |
Table 5 Independent Predictors of Schistosomiasis Among Schoolchildren in Amd District, Hadhramout Governorate, Yemen (2021) |
Discussion
The present study revealed an overall prevalence of 33.7% for infection with any Schistosoma species among schoolchildren in Amd District, signifying a moderate level of infection risk based on WHO endemicity thresholds.19 This finding aligns with the district’s ecological and socioeconomic characteristics—natural water bodies, agricultural practices, and inadequate sanitation—which likely sustain transmission. Additionally, the protracted humanitarian crisis may have undermined previous control efforts. The moderate endemicity level revealed in the present study underscores the need for biannual preventive chemotherapy with PZQ for SAC, as per WHO guidelines.19 Although MDA has been implemented in Yemen since 2010, its effect on interrupting transmission remains limited without integrated strategies. Therefore, a sustainable approach should integrate water, sanitation, and hygiene (WASH) with preventive chemotherapy,20 besides health education and snail control. In line with the present study, the overall prevalence of infection with any Schistosoma species among rural children was 31.8% in five Yemeni governorates (Taiz, Ibb, Dhamar, Sana’a, and Hodeidah).6 However, a survey conducted in 2008 in Shara’b Al-Raona district of Taiz reported an overall prevalence of 24.6% for Schistosoma species among schoolchildren.5 The light infection intensities observed may reflect past MDA efforts,4 yet chronic low-level infections are still linked to morbidity.21–23
The predominance of S. haematobium (31.6%) over S. mansoni (2.1%) confirms that Amd District is a hotspot for the transmission of S. haematobium, underscoring the need for preventive chemotherapy and health education to pave the way for eliminating this disease as a public health problem. The prevalence of S. haematobium in the present study is higher than that (14.7%) recently reported from other selected rural communities of Hadhramout Governorate,10 but it is consistent with that recently reported in Kharif district, north of Yemen, where 34.8% of schoolchildren were found to be infected with S. haematobium.24 Lower prevalence rates were reported in a survey of five governorates in the country (23.8%),6 the southern governorate of Abyan (18%),25 the southwestern governorate of Taiz (3.5–18.6%),5,26,27 as well as the northern governorates of Hajjah (1.7%),28 and Sa’adah (3.3%).29 The low prevalence of S. mansoni in the present study is consistent with the lower rates reported among restaurant workers in Mukalla city of Hadhramout (0.8%),30 schoolchildren in Bani Mater district in rural Sana’a (1.4%).7 In contrast, higher rates were reported among rural children in Al Haymah Ad Dakhiliya district in Sana’a (33.9),7 in Taiz (6.9–31%),5,26,31 in five governorates of the country (9.3%),6 and in Ibb (6.5%).8
Microhematuria, macrohematuria, and proteinuria were significantly associated with S. haematobium infection, reinforcing their utility as field indicators. Likewise, a study in Abyan governorate showed the diagnostic utility of microhematuria, alone or with macrohematuria, in diagnosing S. haematobium infection in schoolchildren.25 However, a contrasting pattern was observed in Kharif district of Amran, where only microhematuria—not macrohematuria—showed a significant association with S. haematobium infection among schoolchildren.24 These findings align with the recommendations of the WHO Expert Committee, which advocates for microhematuria as a practical tool to estimate disease prevalence, detect infections, and assess intervention effectiveness.32 However, the performance of reagent strips in communities with mostly light infections warrants further evaluation.33 On the other hand, hematochezia and diarrhea were not significantly associated with S. mansoni infection, reflecting limited statistical power owing to the small number of cases detected and uniformly low infection intensities observed. In line with the present findings, the intensity of infection with S. mansoni was not found to be associated with morbidity measures among SAC in African countries.34 Notably, occult fecal blood, a potential diagnostic cue for S. mansoni infection,35 was not assessed in this study.
The male gender, age ≥10 years, and contact with natural water sources were independent predictors of schistosomiasis among schoolchildren, This finding corroborates previous studies conducted in Yemen and other endemic regions that identified the male gender as an independent predictor of infection.36–40 The gender disparity in risk may stem from sociocultural norms in conservative communities, where boys often engage in water-related recreational activities, such as swimming and bathing, more freely than girls, who usually face restrictions on outdoor water contact. Likewise, the significant association between older age and increased risk of infection in the present study aligns with the patterns observed in the districts of other Yemeni governorates,6 although there are some exceptions.7,24 This pattern might result from accumulated exposure as children age. On the other hand, the significant association between contact with natural water sources and the risk of infection with schistosomes in the present study agrees with the findings reported among children in Yemen and elsewhere.8,10,36–38,41,42 In contrast, swimming in ponds or dams was not significantly associated with intestinal schistosomiasis among rural schoolchildren in Sana’a.7
This study found no significant association between a larger household size and the risk of schistosomiasis among children. Similarly, household size demonstrated no statistically significant association with schistosomiasis prevalence among rural Yemeni children.6,7 Parental illiteracy also lacked a significant association with infection rates in this study, mirroring findings elsewhere in Yemen.7,36 In contrast, fathers’ illiteracy showed a significant association with infection risk among rural children across five Yemeni governorates.6 Schistosomiasis was also found to be significantly higher among schoolchildren with uneducated mothers in Sana’a and Abyan governorates.7,37 The role of confounders, such as economic status and water access, in masking the true association between parental illiteracy and an increased risk of infection in the present study could not be ruled out. On the other hand, the lack of a significant association between parental employment status and schistosomiasis in the present study aligns with that reported among rural children in five Yemeni governorates.6
In the present study, household water sources and sanitation facilities in households and schools were not significantly associated with schistosomiasis, which agrees with a finding among schoolchildren in Ibb.36 However, a divergent finding was found among rural schoolchildren from other Yemeni districts,6,7 where reliance on unsafe or non-piped water sources independently predicted infection. Geographical disparities in water source contamination and sanitation across districts could account for differences in their association with the risk of infection. These contrasting findings highlight the need for local environmental assessments in schistosomiasis control programs as transmission dynamics can vary substantially between communities with shared socioeconomic and demographic characteristics. Contrary to findings from districts in other governorates of Yemen,6,8,37 where proximity to water sources independently predicted schistosomiasis risk in children, the present study found no significant association between the risk of infection and the proximity of households or schools to natural water sources. It is worth noting that the ecology of these water sources is unknown, and the proximity to the water itself is not sufficient for transmission unless there are snail intermediate hosts. On the other hand, the risk of infection depends on the frequency and type of exposure to water sources, and not on the mere proximity of such sources.
The lack of a significant association between prior administration of antischistosomal medications and reduced risk of infection in this study reinforces the need for integrated control measures, including health education, snail control, safe water access and sanitation infrastructure, rather than relying solely on chemotherapy to sustain transmission interruption. In contrast to the present study, a history of treatment with PZQ was significantly associated with S. haematobium infection among schoolchildren in selected rural communities in Hadhramout.10 While PZQ is effective in reducing infection prevalence and intensity, reinfection rates remain a persistent challenge in endemic areas.43–45 Further investigations should adopt longitudinal methods to unravel the associations between therapeutic interventions, reinfection rates, and environmental factors.
The absence of prior epidemiological data underscores the importance of this study in informing targeted interventions, at least to the best of our knowledge. In addition to contributing to the sparse literature on schistosomiasis in Hadhramout Governorate, this study can inform targeted control strategies in this district. While its findings fill an important knowledge gap, a few limitations should be considered when interpreting them. This study was school-based and recruited enrolled SAC only because of logistic challenges, possibly introducing a selection bias by excluding non-enrolled SAC in the community. This exclusion may inadvertently overlook vulnerable subgroups of children, including those from marginalized socioeconomic backgrounds or unstable living conditions, who may exhibit distinct behavioral or environmental exposure to infection. Consequently, extrapolating these findings to the broader pediatric population of the district requires caution, as unmeasured confounders in non-enrolled SAC could alter risk profiles. Therefore, future studies on SAC in the community are recommended to comprehensively map the transmission patterns and socioeconomic determinants of infection. On the other hand, reliance on self-reported data regarding behaviors related to infection might introduce recall bias, possibly leading to inaccuracies in recall or reporting by schoolchildren.
Conclusion
In Amd District, the risk level of schistosomiasis is moderate and predominated by S. haematobium, with light-intensity infections affecting approximately one-third of schoolchildren. Therefore, biannual preventive chemotherapy with PZQ is recommended for all enrolled and non-enrolled SAC. Macrohematuria, microhematuria, and proteinuria are important indicators of S. haematobium infection. Meanwhile, male gender, older age, and water contact can independently predict infection.
Acknowledgments
The authors thank the school administrators who facilitated this study by granting access to conduct it and assisting in the recruitment of children. The authors appreciate the children’s participation and acknowledge the consent provided by their parents or guardians to include them in the study.
Disclosure
The authors report no conflicts of interest in this work.
References
1. King CH. Toward the elimination of schistosomiasis. N Engl J Med. 2009;360(2):106–109. doi:10.1056/NEJMp0808041
2. World Health Organization. Fact sheets: Schistosomiasis; 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis.
3. Hotez PJ, Savioli L, Fenwick A. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PLoS Negl Trop Dis. 2012;6(2):e1475. doi:10.1371/journal.pntd.0001475
4. The World Bank. Yemen Schistosomiasis Control Project. Washington, DC: WB; 2018.
5. Abdulrab A, Salem A, Algobati F, Saleh S, Shibani K, Albuthigi R. Effect of school based treatment on the prevalence of schistosomiasis in endemic area in Yemen. Iran J Parasitol. 2013;8(2):219–226.
6. Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, Surin J. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis. 2013;7(8):e2377. doi:10.1371/journal.pntd.0002377
7. Al-Haidari SA, Mahdy MAK, Al-Mekhlafi AM, et al. Intestinal schistosomiasis among schoolchildren in Sana’a Governorate, Yemen: prevalence, associated factors and its effect on nutritional status and anemia. PLoS Negl Trop Dis. 2021;15(9):e0009757. doi:10.1371/journal.pntd.0009757
8. Al-Murisi WMS, Al-Mekhlafi AM, Mahdy MAK, Al-Haidari SA, Annuzaili DA, Thabit AAQ. Schistosoma mansoni and soil-transmitted helminths among schoolchildren in An-Nadirah District, Ibb Governorate, Yemen after a decade of preventive chemotherapy. PLoS One. 2022;17(8):e0273503. doi:10.1371/journal.pone.0273503
9. Johari NA, Annuzaili DA, El-Talabawy HF, et al. National mapping of schistosomiasis, soil-transmitted helminthiasis and anaemia in Yemen: towards better national control and elimination. PLoS Negl Trop Dis. 2022;16(3):e0010092. doi:10.1371/journal.pntd.0010092
10. Bayousuf FF, Bin-Hameed EA, Assakaf GM, Al-Bowri SS. Urinary schistosomiasis among school-age children in selected rural communities in Hadhramout governorate, Yemen. Univ J Pharm Res. 2024;9(1):44–51. doi:10.22270/ujpr.v9i1.1067
11. World Health Organization/The Global Health Observatory. Neglected tropical diseases: schistosomiasis. Available from: https://www.who.int/data/gho/data/themes/topics/schistosomiasis.
12. Berghof Foundation, Political Development Forum Yemen. Local governance in Yemen: resource hub - Governorates. Available from: https://yemenlg.org/governorates/hadhramout/.
13. Al-Haddad AM, Baswaid SH. Frequency of intestinal parasitic infection among children in Hadhramout governorate (Yemen). J Egypt Soc Parasitol. 2010;40(2):479–488.
14. Peters PA, Warren KS, Mahmoud AA. Rapid, accurate quantification of schistosome eggs via nuclepore filters. J Parasitol. 1976;62(1):154–155. doi:10.2307/3279081
15. World Health Organization. Urine Filtration Technique of Schistosoma haematobium Infection. Geneva: WHO; 1983.
16. WHO Expert Committee. Prevention and control of schistosomiasis and soil-transmitted helminthiasis. World Health Organ Tech Rep Ser. 2002;912:1–57.
17. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400.
18. Montresor A, Crompton DWT, Hall A, Bundy DA, Savioli L. Guidelines for the Evaluation of Soil-Transmitted Helminthiasis and Schistosomiasis at Community Level: A Guide for Managers of Control Programmes. Geneva: World Health Organization; 1998.
19. World Health Organization. Helminth Control in School-Age Children: A Guide for Managers of Control Programmes. Geneva: WHO; 2011.
20. Campbell SJ, Biritwum NK, Woods G, Velleman Y, Fleming F, Stothard JR. Tailoring water, sanitation, and hygiene (WASH) targets for soil-transmitted helminthiasis and schistosomiasis control. Trends Parasitol. 2018;34(1):53–63. doi:10.1016/j.pt.2017.09.004
21. King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–1569. doi:10.1016/s0140-6736(05)66457-4
22. King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4(1):65–79. doi:10.1177/1742395307084407
23. King CH. It’s time to dispel the myth of “asymptomatic” schistosomiasis. PLoS Negl Trop Dis. 2015;9(2):e0003504. doi:10.1371/journal.pntd.0003504
24. Alansi DHZ, Mahdy MAK, Abdul-Ghani R, Azazy AA. School-based epidemiology of Schistosoma haematobium infection in Kharif District of Amran Governorate, north of Yemen: need for chemopreventive strategy revisiting. Infect Drug Resist. 2025;18:161–170. doi:10.2147/idr.s496484
25. Bassiouny HK, Hasab AA, El-Nimr NA, Al-Shibani LA, Al-Waleedi AA. Rapid diagnosis of schistosomiasis in Yemen using a simple questionnaire and urine reagent strips. East Mediterr Health J. 2014;20(4):242–249. doi:10.26719/2014.20.4.242
26. Al-Shamiri AH, Al-Taj MA, Ahmed AS. Prevalence and co-infections of schistosomiasis/hepatitis B and C viruses among school children in an endemic area in Taiz, Yemen. Asian Pac J Trop Med. 2011;4(5):404–408. doi:10.1016/s1995-7645(11)60113-2
27. Al-Kabab AA, El-Sheikh EI, Al-Mikhlafy AA. A community-based study of the prevalence and risk factor for Schistosoma haematobium in two endemic districts of Taiz Governorate, Yemen. J Med Pharm Sci. 2023;7(3):29–45. doi:10.26389/AJSRP.K020723
28. Alharbi RA, Alwajeeh TS, Assabri AM, Almalki SSR, Alruwetei A, Azazy AA. Intestinal parasitoses and schistosome infections among students with special reference to praziquantel efficacy in patients with schistosomosis in Hajjah governorate, Yemen. Ann Parasitol. 2019;65(3):217–223. doi:10.17420/ap6503.203
29. Raja’a YA, Mubarak JS. Intestinal parasitosis and nutritional status in schoolchildren of Sahar district, Yemen. East Mediterr Health J. 2006;12(Suppl 2):S189–194.
30. Baswaid SH, Al-Haddad A. Parasitic infections among restaurant workers in Mukalla (Hadhramout/Yemen). Iran J Parasitol. 2008;3(3):37–41.
31. Al-Kabab AA, Elsheikh E, Al-Mikhlafy AA. Community-based prevalence of intestinal schistosomiasis and associated risk factors in two endemic districts of Taiz Governorate, Yemen. Med Updates. 2023;13(13):85–107. doi:10.21608/muj.2023.190463.1130
32. World Health Organization. The control of schistosomiasis. Second report of the WHO Expert Committee. World Health Organ Tech Rep Ser. 1993;830:1–86.
33. Degarege A, Animut A, Negash Y, Erko B. Performance of urine reagent strips in detecting the presence and estimating the prevalence and intensity of Schistosoma haematobium infection. Microorganisms. 2022;10(10):2062. doi:10.3390/microorganisms10102062
34. Wiegand RE, Secor WE, Fleming FM, et al. Associations between infection intensity categories and morbidity prevalence in school-age children are much stronger for Schistosoma haematobium than for S. mansoni. PLoS Negl Trop Dis. 2021;15(5):e0009444. doi:10.1371/journal.pntd.0009444
35. Nakamura I, Yagi K, Kumagai T, Ohta N. Positive fecal occult blood test as a diagnostic cue for Schistosoma mansoni infection in a developed country. IDCases. 2017;10:108–109. doi:10.1016/j.idcr.2017.10.005
36. Raja’a YA, Assiragi HM, Abu-Luhom AA, et al. Schistosomes infection rate in relation to environmental factors in school children. Saudi Med J. 2000;21(7):635–638.
37. Al-Waleedi AA, El-Nimr NA, Hasab AA, Bassiouny HK, Al-Shibani LA. Urinary schistosomiasis among schoolchildren in Yemen: prevalence, risk factors, and the effect of a chemotherapeutic intervention. J Egypt Public Health Assoc. 2013;88(3):130–136. doi:10.1097/01.epx.0000441277.96615.96
38. Amuta EU, Houmsou RS. Prevalence, intensity of infection and risk factors of urinary schistosomiasis in pre-school and school aged children in Guma Local Government Area, Nigeria. Asian Pac J Trop Med. 2014;7(1):34–39. doi:10.1016/s1995-7645(13)60188-1
39. Atalabi TE, Lawal U, Ipinlaye SJ. Prevalence and intensity of genito-urinary schistosomiasis and associated risk factors among junior high school students in two local government areas around Zobe Dam in Katsina State, Nigeria. Parasit Vectors. 2016;9(1):388. doi:10.1186/s13071-016-1672-5
40. Ayabina DV, Clark J, Bayley H, Lamberton PHL, Toor J, Hollingsworth TD. Gender-related differences in prevalence, intensity and associated risk factors of Schistosoma infections in Africa: a systematic review and meta-analysis. PLoS Negl Trop Dis. 2021;15(11):e0009083. doi:10.1371/journal.pntd.0009083
41. Abdel-Motaleb GS, El-Ghareeb AS, Aly NS, Salama NA. Present situation of Schistosoma haematobium infection among primary school aged children in some areas of Qualyobia governorate-Egypt. J Egypt Soc Parasitol. 2013;43(3):577–589. doi:10.12816/0006415
42. Senghor B, Diallo A, Sylla SN, et al. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit Vectors. 2014;7:5. doi:10.1186/1756-3305-7-5
43. N’Goran EK, Utzinger J, N’Guessan AN, et al. Reinfection with Schistosoma haematobium following school-based chemotherapy with praziquantel in four highly endemic villages in Côte d’Ivoire. Trop Med Int Health. 2001;6(10):817–825. doi:10.1046/j.1365-3156.2001.00785.x
44. Webster BL, Diaw OT, Seye MM, et al. Praziquantel treatment of school children from single and mixed infection foci of intestinal and urogenital schistosomiasis along the Senegal River Basin: monitoring treatment success and re-infection patterns. Acta Trop. 2013;128(2):292–302. doi:10.1016/j.actatropica.2012.09.010
45. Woldegerima E, Bayih AG, Tegegne Y, Aemero M, Jejaw Zeleke A. Prevalence and reinfection rates of Schistosoma mansoni and praziquantel efficacy against the parasite among primary school children in Sanja Town, northwest Ethiopia. J Parasitol Res. 2019;2019:3697216. doi:10.1155/2019/3697216
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

