Back to Journals » Cancer Management and Research » Volume 17
Secondary Analysis of PSA and BCR-Free Survival in Asian Prostate Cancer Patients
Authors Gao X , Fu Y , Mo Z , Ruan Y
Received 2 April 2025
Accepted for publication 2 June 2025
Published 24 June 2025 Volume 2025:17 Pages 1205—1214
DOI https://doi.org/10.2147/CMAR.S527092
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Xin Gao,1,* Yijun Fu,1,* Zimei Mo,1 Yongtong Ruan2
1Department of Urology, Yangjiang Hospital of Traditional Chinese Medicine, Affiliated to Guangzhou University of Chinese Medicine, Yangjiang, Guangdong, People’s Republic of China; 2Department of Urology, Yangjiang Hospital of Traditional Chinese Medicine, Yangjiang, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yongtong Ruan, Department of Urology, Yangjiang Hospital of Traditional Chinese Medicine, Shiwan North Road, Jiangcheng District, Yangjiang, 529500, People’s Republic of China, Tel +86-13432578081, Email [email protected]
Background: Prostate cancer remains a significant global health burden, with biochemical recurrence (BCR) affecting 20– 50% of patients post-radical prostatectomy. This study aimed to investigate the relationship between preoperative PSA levels and BCR-free survival in Asian populations.
Methods: We conducted a retrospective cohort study of 3,092 prostate cancer patients who underwent radical prostatectomy at Yonsei University College of Medicine, Korea (1992– 2014). The exposure variable was preoperative PSA level, and the primary outcome was BCR-free survival. Covariates included age, Gleason score, pathological stage, surgical margins, and other clinical factors. Patients were followed quarterly for two years post-surgery, semi-annually for three years, then annually thereafter (median follow-up: 66 months).
Results: Using piecewise linear regression, we identified a significant threshold effect at PSA 5.1 ng/mL. Above this threshold, each unit increase in LnPSA was associated with a 6.10-month reduction in BCR-free survival (95% CI: − 7.64 to − 4.56, P< 0.0001). Higher PSA levels correlated with increased adverse pathological features and shorter BCR-free survival across all risk groups.
Conclusion: This study establishes a critical PSA threshold of 5.1 ng/mL for BCR risk stratification in Asian populations. Above this threshold, each unit increase in LnPSA correlates with a 6.10-month reduction in BCR-free survival, providing valuable guidance for post-operative monitoring and personalized treatment strategies.
Keywords: prostate cancer, prostate-specific antigen, PSA, biochemical recurrence, nonlinearity
Introduction
Prostate cancer remains one of the most common malignancies among men worldwide.1 According to GLOBOCAN 2022 data, approximately 1.47 million new cases of prostate cancer are reported annually, ranking as the leading male cancer in 118 countries.2 The disease burden shows marked geographical variations, with significantly higher incidence rates in developed countries compared to developing regions.3 Recent epidemiological studies have revealed substantial racial and ethnic disparities in incidence and mortality rates.4 In China, the projected new cases for 2024 exceed 100,000, with an age-standardized incidence rate of 9.68 per 100,000.5 Recent preclinical studies have provided valuable molecular insights into PSA regulation in prostate cancer. Li et al (2024)6 demonstrated that microRNA-34a (miR-34a), frequently downregulated in prostate tumors with TP53 alterations (present in approximately 46% of primary prostate cancer patients), significantly influences PSA expression through posttranscriptional mechanisms. Their research revealed that miR-34a targets key pathways involved in cancer stem cell survival, potentially explaining inter-patient variations in PSA dynamics.7 This molecular heterogeneity exhibits population-specific characteristics influenced by ethnicity-specific genetic polymorphisms, providing a potential molecular basis for the different PSA thresholds observed between Asian and Western populations. These molecular mechanisms offer new frameworks for understanding the population-specific PSA behavior observed in our study.
Prostate-Specific Antigen (PSA), a glycoprotein secreted by prostatic epithelial cells, is a widely used biomarker for prostate cancer screening and diagnosis.8 While its primary clinical application is guiding biopsy decisions and confirming diagnosis, its potential value in predicting BCR-free survival remains an area of active research, despite recognized limitations in specificity and being influenced by multiple factors beyond cancer progression. According to the European Association of Urology guidelines, PSA plays a crucial role not only in early diagnosis but also in evaluating treatment efficacy and predicting recurrence risk.9 Studies have demonstrated that dynamic changes in PSA levels accurately reflect tumor burden, with elevations often indicating disease progression or increased recurrence risk.10,11 Multiple investigations have confirmed strong correlations between preoperative PSA levels and prognostic factors such as pathological stage and Gleason score.12–15
For patients undergoing radical prostatectomy (RP), biochemical recurrence (BCR) serves as a critical prognostic indicator.16,17 Recent 2024 studies indicate that even among patients maintaining consistently low post-operative PSA levels, approximately 15–20% may experience late BCR.18,19 Recent meta-analyses have demonstrated a significant positive correlation between preoperative PSA levels and BCR risk.20,21 However, some studies suggest this correlation may be influenced by factors such as tumor stage and surgical approach.22–24 These inconsistencies might be attributed to population heterogeneity, varying follow-up periods, and different BCR definitions, underscoring the need for further investigation.
This retrospective cohort study aims to conduct a secondary analysis of data from 3,092 prostate cancer patients who underwent radical prostatectomy at Yonsei University College of Medicine, Seoul, Korea, between 1992 and 2014. Our study features several distinctive strengths: first, it focuses on an Asian population, providing specifically relevant insights for Asian clinical practice; second, it employs a rigorous follow-up protocol (quarterly visits for the first two years post-surgery, semi-annual visits for the next three years, and annual visits thereafter) with a median follow-up duration of 66 months, ensuring data reliability and completeness; third, it offers innovative analysis of the relationship between PSA and BCR, accounting for various influencing factors. The findings from this study will contribute valuable evidence for developing individualized treatment strategies and follow-up protocols, particularly for Asian populations, while advancing our understanding of PSA’s role in predicting biochemical recurrence risk.
Methods
Study Population
This retrospective cohort study was conducted at Yonsei University College of Medicine, Seoul, Korea, enrolling patients who underwent radical prostatectomy between 1992 and 2014. Of the 4,404 patients initially identified, 3,092 were included in the final analysis after applying selection criteria. Inclusion criteria encompassed patients who underwent radical prostatectomy during the study period. Exclusion criteria comprised patients who received neoadjuvant therapy, those with incomplete pathological or follow-up data, and those with lymph node metastasis identified during surgery. All patient data were extracted from the institutional prostate cancer database using standardized data collection forms by trained research personnel. Patients were followed up until 2014, with a median follow-up duration of 66 months.
Variables
The primary exposure variable was preoperative PSA level, measured using immunoradiometric assay from venous blood samples collected within one week before surgery. The outcome variable was BCR-free survival, defined as the time from surgery to biochemical recurrence. Biochemical recurrence was defined as two consecutive PSA measurements ≥0.2 ng/mL after surgery. Follow-up assessments were conducted every three months for the first two years post-surgery, every six months for the subsequent three years, and annually thereafter. Covariates included age, RP Gleason score, pathological tumor stage, seminal vesicle invasion, positive surgical margins, extracapsular extension, preoperative risk grouping, year of surgery, and adverse pathological findings. These covariates were selected based on previous research evidence and clinical expert consensus to control for potential confounding factors. All pathological assessments were performed by dedicated genitourinary pathologists using standardized reporting templates. Missing data, which accounted for less than 5% of the total data, were handled using complete case analysis.
Ethic Statement
This study represents a secondary analysis of anonymized data published by Jang WS et al (2016)25 in PLOS ONE. The original data collection received institutional review board approval from Yonsei University College of Medicine (YUHS-2016-0724). In accordance with established research ethics guidelines, secondary analyses of publicly available anonymized datasets do not typically require additional ethical approval. We have properly cited the original source and adhered to PLOS ONE’s data usage policies throughout our analysis. The study was conducted in compliance with the Declaration of Helsinki and maintained patient privacy through secure data management protocols. All procedures followed applicable ethical standards for medical research involving human data.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (for normal distribution) or median (interquartile range), while categorical variables were presented as frequencies or percentages. PSA values were natural log-transformed (LnPSA) to improve normality. Between-group differences were assessed using chi-square test for categorical variables and Student’s t-test or Mann–Whitney U-test for continuous variables based on their distribution.
The analysis was conducted in three steps:
Step 1: Association Analysis
We employed univariate and multivariate linear regression models with progressive adjustment strategies:
- Model 1: Unadjusted model.
- Model 2: Adjusted for demographic characteristics.
- Model 3: Model 2 plus additional covariates presented in Table 1
 |
Table 1 Demographic and Clinical Characteristics of Study Population by LnPSA Tertiles |
The changes in effect estimates across different adjustment strategies were examined to assess the robustness of our findings. LnPSA was used as the exposure variable in all models.
Step 2: Non-Linear Relationship Assessment
Generalized additive models and smooth curve fitting (penalized spline method) were used to evaluate the non-linear relationship between LnPSA and outcomes. When non-linearity was detected, we calculated the inflection point using a recursive algorithm and constructed a two-piecewise linear model on both sides of the inflection point. The likelihood ratio test was used to compare the standard linear regression model with the two-piecewise model to determine which better explained the true association.
Step 3: Stratified Analysis
Stratified linear regression models or generalized additive models were used for subgroup analyses. For continuous stratification variables, we first converted them to categorical variables based on clinical cut-points or tertiles, followed by interaction tests. Effect modification in subgroup indicators was assessed using likelihood ratio tests.
Sensitivity Analysis
To verify the robustness of our findings, LnPSA was converted into a categorical variable for trend analysis, validating the continuous variable analysis results and exploring potential non-linear relationships.
All statistical analyses were performed using R statistical software (http://www.R-project.org, The R Foundation). Two-sided P-values < 0.05 were considered statistically significant.
Results
Baseline Characteristics of Participants
A total of 3092 patients were stratified into three tertiles based on LnPSA levels, with significant differences observed across multiple clinicopathological characteristics (all P<0.001). The high LnPSA tertile was characterized by older age (65.82±6.77 vs 64.55±7.30 years in low tertile) and significantly shorter biochemical recurrence-free survival (31.52±32.26 vs 45.51±30.90 months in low tertile). Notably, patients with higher LnPSA levels demonstrated more aggressive pathological features, evidenced by a higher proportion of Gleason score group 2 (35.3% vs 13.4% in low tertile), increased rates of seminal vesicle invasion (18.7% vs 2.9%), positive surgical margins (61.7% vs 35.7%), and extracapsular extension (70.3% vs 41.7%). The presence of adverse pathological features showed a significant ascending trend across LnPSA tertiles (54.2%, 69.7%, and 80.5%, respectively). Risk stratification analysis revealed that the proportion of high-risk patients increased markedly with higher LnPSA levels (71.2% in high tertile vs 31.4% in low tertile), with notably no patients in the high LnPSA tertile classified as low-risk, suggesting that elevated LnPSA levels are strongly associated with adverse pathological outcomes and higher risk classifications in prostate cancer patients (Table 1).
Multivariate Analysis
Multivariate regression analyses were performed to evaluate the association between LnPSA levels and biochemical recurrence-free survival (BCR-free survival). In the continuous variable analysis, LnPSA demonstrated a significant negative correlation with BCR-free survival across all models. After full adjustment for potential confounders including smoothed age and operation year (Adjust II), each unit increase in LnPSA was associated with a 2.58-month reduction in BCR-free survival (95% CI: −3.69 to −1.47, P<0.0001).
When analyzed by LnPSA tertiles, with the low tertile as reference, patients in the high tertile demonstrated significantly shorter BCR-free survival. This association remained robust after full adjustment for confounders, with the high LnPSA tertile showing a 7.91-month reduction in BCR-free survival (95% CI: −10.48 to −5.34, P<0.0001). Although patients in the middle tertile showed a trend toward decreased BCR-free survival (−1.92 months, 95% CI: −4.22 to 0.38), this association did not reach statistical significance (P=0.1026). These findings suggest that elevated PSA levels independently predict shorter biochemical recurrence-free survival, particularly in patients with PSA levels in the highest tertile, even after accounting for other established risk factors (Table 2).
 |
Table 2 Association Between PSA Levels and BCR-Free Survival |
Non-Linear Relationship Between LnPSA and BCR-Free Survival
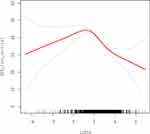
Smooth curve fitting analysis revealed a significant nonlinear relationship between LnPSA levels and biochemical recurrence-free survival. The curve demonstrated a consistent downward trend, with BCR-free survival decreasing as LnPSA levels increased. This negative association was particularly pronounced when LnPSA values exceeded 2, where the curve showed a steeper decline. The 95% confidence intervals (represented by the upper and lower bounds) remained relatively narrow throughout most of the LnPSA range, indicating the reliability of this association. Notably, the relationship appeared more stable in the middle range of LnPSA values (between 0 and 4), while showing wider confidence intervals at the extremes, particularly at higher LnPSA levels.(Figure 1)
Threshold Effect Analysis
In a cohort of 3,092 patients, we investigated the relationship between LnPSA levels and biochemical recurrence-free survival using both linear and piecewise linear regression models with adjustment for multiple clinicopathological factors. The initial linear model suggested a uniform negative association, with each unit increase in LnPSA corresponding to a 3.21-month decrease in BCR-free survival (95% CI: −4.34 to −2.08, P<0.0001).
However, a more sophisticated piecewise linear regression analysis identified a significant threshold effect with a turning point at LnPSA of 1.63 (equivalent to approximately 5.1 ng/mL PSA). Below this threshold, a marginally positive association was observed, with each unit increase in LnPSA associated with a 2.31-month increase in BCR-free survival (95% CI: 0.01 to 4.61, P=0.0492). In contrast, beyond this threshold, a strong negative association emerged, with each unit increase in LnPSA corresponding to a 6.10-month decrease in BCR-free survival (95% CI: −7.64 to −4.56, P<0.0001). The difference in effects between these two segments was substantial at 8.41 months (95% CI: −11.46 to −5.36, P<0.0001). At the turning point, the predicted BCR-free survival was 46.78 months (95% CI: 45.22 to 48.34).
Notably, the likelihood ratio test (P<0.001) strongly supported the superiority of the piecewise linear model over the simple linear model, indicating that the relationship between PSA levels and biochemical recurrence-free survival is not uniformly linear but rather demonstrates a distinct threshold effect at approximately 5.1 ng/mL PSA, which may serve as a clinically relevant reference point for risk stratification (Table 3)
 |
Table 3 Association Between LnPSA and BCR-Free Survival: Linear and Piecewise Linear Regression Analysis |
Discussion
This study investigated the relationship between preoperative PSA levels (exposure variable) and biochemical recurrence-free survival (outcome variable) among 3,092 patients who underwent radical prostatectomy. Based on data from Yonsei University College of Medicine, Korea (1992–2014), the study features several notable strengths: a large Asian cohort, rigorous follow-up protocol (quarterly visits for first two years post-surgery, semi-annual visits for next three years, and annual thereafter), and a median follow-up duration of 66 months. The findings revealed a non-linear association between PSA levels and BCR-free survival, with a significant threshold effect at PSA 5.1 ng/mL, above which each unit increase in LnPSA was associated with a 6.10-month reduction in BCR-free survival (95% CI: −7.64 to −4.56, P < 0.0001). It is important to acknowledge that while our study demonstrates a significant relationship between PSA levels and BCR-free survival, PSA alone has recognized limitations as a predictor of recurrence. Traditionally, PSA has been primarily used for diagnosis rather than prognostication. However, our identification of a non-linear relationship and a specific threshold of 5.1 ng/mL offers a novel perspective that may enhance the utility of PSA in risk stratification when integrated with other established prognostic factors. This finding does not suggest replacing comprehensive risk assessment models with PSA alone, but rather indicates that PSA levels, particularly when above this threshold, may warrant more careful consideration within the broader clinical context. This nuanced understanding aligns with recent calls for more integrated approaches to prostate cancer management, where multiple biomarkers and clinical features are considered collectively for personalized risk assessment.
Our findings on the non-linear relationship between PSA levels and biochemical recurrence complement the latest research. According to a 2024 systematic review, approximately one-third of prostate cancer patients experience biochemical recurrence after definitive treatment, with PSA kinetics being a crucial prognostic indicator.26 Through piecewise linear regression analysis, our study is the first to establish a critical threshold of 5.1 ng/mL in Asian populations, providing a more precise early warning value for clinical risk stratification. This finding is particularly significant as recent meta-analyses demonstrate the importance of early identification of high-risk patients for subsequent treatment decisions.27 Notably, the 2024 European Association of Urology (EAU) guidelines emphasize the importance of considering multiple factors, including PSA doubling time and pathological features, when assessing biochemical recurrence risk.28 Our study’s unique strengths lie in its extended follow-up period (median 66 months), rigorous follow-up protocol, and the application of a piecewise linear regression model, which better captures the non-linear relationship between PSA levels and prognosis. These findings not only deepen our understanding of PSA’s importance as a prognostic marker but also provide more reliable reference points for individualized risk assessment in Asian populations.
The clinical value of our findings extends beyond statistical significance to practical applications in patient care. Our identified threshold of 5.1 ng/mL differs from the conventional 4 ng/mL cutoff used in Western populations.29,30 This finding aligns with studies showing Asian men demonstrate distinct PSA profiles compared to Western counterparts, with potentially different biochemical recurrence patterns.31 Recent research by Liu et al (2024)32 suggests integrating population-specific PSA thresholds could enhance current risk assessment frameworks like CAPRA and NCCN guidelines. Notably, studies from different Asian regions have reported variable thresholds—Japanese guidelines recommend 4 ng/mL,33 while Chinese studies suggest values between 4.0–6.3 ng/mL.34 These variations highlight heterogeneity even within Asian populations, underscoring the need for region-specific risk stratification approaches in prostate cancer management.35 We propose the following clinical applications of this threshold: (1) Post-operative monitoring frequency: Patients with preoperative PSA >5.1 ng/mL may benefit from more intensive follow-up protocols, such as monthly PSA measurements for the first six months post-surgery, followed by bimonthly testing for one year; (2) Integration with existing risk calculators: Our threshold can be incorporated into the Cancer of the Prostate Risk Assessment (CAPRA) score by adjusting the PSA stratification intervals specifically for Asian patients; (3) Adjuvant therapy considerations: For patients with preoperative PSA >5.1 ng/mL combined with other adverse features (positive margins, extracapsular extension), early adjuvant radiotherapy might be considered rather than salvage approaches; (4) Risk counseling: This threshold provides a valuable reference point for discussing recurrence risk with Asian patients, potentially improving shared decision-making about post-operative management options.
This study’s comprehensive analysis of PSA’s non-linear relationship with BCR-free survival, supported by robust long-term follow-up data, offers a more nuanced framework for clinical decision-making compared to traditional linear models. Our findings align with recent research indicating that Asian men generally present with different PSA profiles,29 suggesting the need for ethnicity-specific risk assessment approaches. Analysis of PSA thresholds across Asian populations reveals notable variations that reflect genetic and environmental heterogeneity. Down et al (2024)30 demonstrated significantly lower advanced prostate cancer incidence in Asian men (4.5%, 95% CI: 3.8–5.3%) compared to White men (7.5%, 95% CI: 7.2–7.7%) at equivalent PSA elevations. This finding corroborates observed differences in PSA dynamics between populations. The genetic basis for these variations has been documented, with Asian men exhibiting distinct polymorphisms in genes regulating androgen metabolism and PSA expression.36 Comparative studies consistently show that Asian men present with lower PSA levels for equivalent tumor volumes than Western cohorts, providing a biological rationale for why our 5.1 ng/mL threshold diverges from the conventional 4.0 ng/mL Western standard.37,38 These findings underscore the importance of developing population-specific risk stratification models rather than applying universal cutoffs across diverse ethnic groups.The study’s results advocate for more intensive monitoring and potentially earlier intervention strategies for patients with PSA levels above 5.1 ng/mL, particularly given the observation that Asian-American men often present with higher grade disease.39 Looking ahead, future research should focus on integrating this PSA threshold into existing risk calculators and validating its applicability across different Asian subpopulations, while also exploring the development of personalized follow-up protocols based on PSA kinetics.40
Our study demonstrates several significant methodological and operational strengths. First, we included a large cohort of 3,092 Asian prostate cancer patients, which is particularly valuable as recent research indicates that Asian patients exhibit distinct PSA patterns and disease characteristics compared to Western populations.41 Second, we implemented a rigorous follow-up protocol (quarterly visits for the first two years post-surgery, semi-annual visits for the next three years, and annual visits thereafter), with a median follow-up duration of 66 months. This systematic follow-up strategy aligns closely with the 2024 EAU guidelines for post-prostatectomy monitoring.42 Regarding data analysis, we innovatively employed a piecewise linear regression model, which has shown unique advantages in recent biomarker research by better capturing the non-linear relationship between PSA and prognosis.43 Furthermore, the study utilized a three-step analytical approach (association analysis, non-linear relationship assessment, and stratified analysis) to comprehensively evaluate the relationship between PSA and biochemical recurrence, providing valuable reference points for individualized risk assessment in Asian populations.
Several limitations of this study should be acknowledged. First, as a single-center study, the generalizability of our findings may be limited.44 Second, our exclusion of patients who received neoadjuvant therapy, those with lymph node metastasis, and those with incomplete follow-up data likely introduced selection bias toward lower-risk disease profiles. Recent studies by Liu et al (2024)45 demonstrate that PSA’s predictive value varies across risk groups, with paradoxically low PSA levels sometimes indicating worse outcomes in high-grade disease. For patients with advanced disease, Hung et al (2024)46 and Carrot et al (2024)47 suggest that PSA kinetics rather than absolute values may offer superior prognostic information. These findings indicate our exclusion criteria may have limited our evaluation of PSA dynamics in advanced disease states. Third, since the study population consisted exclusively of Korean patients, and recent research has shown significant differences in PSA screening patterns and prostate cancer characteristics between Asian and Western populations,48 caution should be exercised when extrapolating these findings to other populations. Fourth, as a retrospective observational study, we could only establish associations rather than causal relationships.49 Additionally, PSA screening itself serves as an important confounder that may influence the overall detection rate and proportion of low-risk cases.50 Finally, while we adjusted for known confounding factors, our study did not account for several potentially significant unmeasured variables. Recent evidence suggests that genetic markers, including family history and hereditary susceptibility, substantially influence prostate cancer risk.50 Lifestyle factors such as smoking status, dietary patterns, and medication use may modulate PSA levels independently of malignancy.51 Furthermore, metabolic comorbidities, particularly central adiposity and components of metabolic syndrome, have demonstrated associations with altered PSA concentrations.52 These unmeasured confounders potentially introduce bias in our interpretation. Future investigations should incorporate these genetic, environmental, and metabolic parameters to enhance risk assessment accuracy and PSA interpretation frameworks.
Conclusion
The relationship between PSA levels and biochemical recurrence is non-linear. The relationship between PSA levels and biochemical recurrence-free survival is non-linear. When PSA levels exceeded 5.1 ng/mL, there was a significant negative association between PSA and BCR-free survival, with higher PSA levels corresponding to shorter BCR-free survival time.
Acknowledgments
First and foremost, I would like to express my sincere gratitude to my supervisor, Professor Ruan, for the invaluable guidance and academic inspiration throughout the entire research process. Special thanks are extended to my research team members for their close collaboration and constructive suggestions during the research.
Disclosure
The authors declare no competing financial interests.
References
1. Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820
2. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–263. doi:10.3322/caac.21834
3. Wu Y, He S, Cao M, et al. Comparative analysis of cancer statistics in China and the United States in 2024. Chinese Med J. 2024;137(24):3093–3100. doi:10.1097/CM9.0000000000003442
4. Stock SR, Burns MT, Waller J, et al. Racial and ethnic differences in prostate cancer epidemiology across disease states in the VA. JAMA Network Open. 2024;7(11):e2445505. doi:10.1001/jamanetworkopen.2024.45505
5. Chen J, He L, Ni Y, et al. Prevalence and associated risk factors of prostate cancer among a large Chinese population. Sci Rep. 2024;14(1):26338. doi:10.1038/s41598-024-77863-z
6. Li WJ, Wang Y, Liu X,et al. Developing folate-conjugated miR-34a therapeutic for prostate cancer: challenges and promises. Int J Mol Sci. 2024;25(4):2123.
7. Abdelaal AM, Sohal IS, Iyer SG, et al. Selective targeting of chemically modified Mir-34A to prostate cancer using a small molecule ligand and an endosomal escape agent. Mol Ther Nucleic Acids. 2024;35(2):102193. doi:10.1016/j.omtn.2024.102193
8. Chu Z, Xu Y, Yin Z, et al. Advances in prostate cancer biomarkers]. Sheng wu gong cheng xue bao. Chin J Biotechnol. 2024;40(11):3951–3973. doi:10.13345/j.cjb.240283
9. Hung S, Chang LW, Hsiao TH, et al. Predictive value of polygenic risk score for prostate cancer incidence and prognosis in the Han Chinese. Sci Rep. 2024;14(1):14. doi:10.1038/s41598-023-41906-8
10. Afriansyah A, Hamid AR, Mochtar CA, et al. Prostate Specific Antigen (PSA) kinetic as a prognostic factor in metastatic prostate cancer receiving androgen deprivation therapy. Sys Rev Meta-Analysis F1000Res. 2018;7.
11. Matsubara N, Chi KN, Özgüroğlu M, et al. Correlation of prostate-specific antigen kinetics with overall survival and radiological progression-free survival in metastatic castration-sensitive prostate cancer treated with abiraterone acetate plus prednisone or placebos added to androgen deprivation therapy: post hoc analysis of phase 3 LATITUDE study. Europ Urol. 2019;77(4):494–500. doi:10.1016/j.eururo.2019.11.021
12. Tilki D, Rouprêt M, Shariat SF, et al. Mortality risk for patients with biopsy gleason grade group 1 prostate cancer. Eur Urol Oncol. 2024;8(1):7. doi:10.1016/j.euo.2024.12.004
13. Hamdy FC, Donovan JL, Lane JA, et al. 10-year outcomes after monitoring, surgery, or radiotherapy for localized prostate cancer. N Engl J Med. 2016;375(15):1415–1424. doi:10.1056/NEJMoa1606220
14. Okwor CJ, Okwor VC, Meka IA, et al. Association between pre-operative total prostate-specific antigen and survivorship of prostate cancer following radical prostatectomy: a systematic review. Med Principl Prac. 2024;33.
15. Gillessen S, Turco F, Davis I, et al. Management of patients with advanced prostate cancer. Report from the 2024 Advanced Prostate Cancer Consensus Conference (APCCC). Europ Urol. 2025;87.
16. Hoeh B, Preisser F, Zattoni F, et al. Risk of biochemical recurrence and metastasis in prostate cancer patients treated with radical prostatectomy after a 10-year disease-free interval. Eur Urol Oncol. 2024.
17. Childs DS, Orme JJ, Ravi P. Delaying prostate-specific antigen progression in biochemically recurrent prostate cancer: is it clinically meaningful?. J Clin Oncol J Am Soc Clin Oncol. 2024;42(10):1095–1097. doi:10.1200/JCO.23.02410
18. Preisser F, Abrams-Pompe RS, Stelwagen PJ, et al. European association of urology biochemical recurrence risk classification as a decision tool for salvage radiotherapy-a multicenter study. Eur Urol. 2024;85(2):164–170. doi:10.1016/j.eururo.2023.05.038
19. Merriman KM, Harmon SA, Belue MJ, et al. Comparison of MRI-Based staging and pathologic staging for predicting biochemical recurrence of prostate cancer after radical prostatectomy. Am J Roentgenol. 2023;221.
20. Van den Broeck T, Van Den Bergh RC, Arfi N, et al. Prognostic value of biochemical recurrence following treatment with curative Intent for prostate cancer: a systematic review. Europ Urol. 2019;75.
21. Weiner AB, Kakani P, Armstrong AJ, et al. Risk stratification of patients with recurrence after primary treatment for prostate cancer: a systematic review. Eur Urol. 2024;86(3):200–210. doi:10.1016/j.eururo.2024.04.034
22. Luz FACD, Leung AA, Walker RL, et al. Analysis of the surgical approach in prostate cancer staging: results from the surveillance, epidemiology and end results program. Sci Rep. 2023;13(1):13. doi:10.1038/s41598-022-27264-x
23. Dilme RV, Rivas JG, Fernández Hernández L, et al. Oncological outcomes in robot-assisted radical prostatectomy: the value of PSA density as a preoperative predictive factor. Ther Adv Urol. 2024;16.
24. McLaughlin PW, Cousins MM, Tsodikov A, et al. Mortality reduction and Cumulative Excess Incidence (CEI) in the Prostate-Specific Antigen (PSA) screening era. Sci Rep. 2024;14(1):14.
25. Jang WS, Kim LHC, Yoon CY, et al. Effect of preoperative risk group stratification on oncologic outcomes of patients with adverse Pathologic findings at radical prostatectomy. PLoS One. 2016;11(10):e0164497. doi:10.1371/journal.pone.0164497
26. Sciarra A, Santarelli V, Salciccia S, et al. How the management of biochemical recurrence in prostate cancer will be modified by the concept of anticipation and incrementation of therapy. Cancers. 2024;16(4):764. doi:10.3390/cancers16040764
27. Shore ND, Moul JW, Pienta KJ, et al. Biochemical recurrence in patients with prostate cancer after primary definitive therapy: treatment based on risk stratification. Prostate Cancer Prostatic Dis. 2024;27(2):192–201. doi:10.1038/s41391-023-00712-z
28. Kranz J, Bartoletti R, Bruyère F, et al. European association of urology guidelines on urological infections: summary of the 2024 guidelines. Eur Urol. 2024;86(1):27–41. doi:10.1016/j.eururo.2024.03.035
29. Mochtar CA, Andika RS. The value of prostate-specific antigen in Asia. Ther Adv Urol. 2010;2(2):77–83. doi:10.1177/1756287210370329
30. Down L, Barlow M, Bailey SER, et al. Association between patient ethnicity and prostate cancer diagnosis following a prostate-specific antigen test: a cohort study of 730,000 men in primary care in the UK. BMC Med. 2024;22(1). doi:10.1186/s12916-024-03283-5
31. Lim J, Bhoo-Pathy N, Sothilingam S, et al. Ethnicity is an independent determinant of age-specific PSA level: findings from a multiethnic Asian setting. PLoS One. 2014;9(8):e104917–e104917. doi:10.1371/journal.pone.0104917
32. Liu J, Graff SL, Wang Y, et al. Predicting biochemical recurrence of prostate cancer post-prostatectomy using artificial intelligence: a systematic review. Cancers. 2024;17(1):16. doi:10.3390/cancers17010016
33. Kohjimoto Y, Uemura H, Yoshida M, et al. Japanese clinical practice guidelines for prostate cancer 2023. Int J Urol. 2024;31.
34. Liu Z, Sun Y-H, Xu C-L, et al. Age-specific PSA reference ranges in Chinese men without prostate cancer. Asian J Androl. 2009;11(1):100–103. doi:10.1038/aja.2008.17
35. Nath CK, Barman B, Phukan P, et al. Prostate-specific antigen density: a measurement to differentiate benign hypertrophy of prostate from prostate carcinoma. J Lab Phys. 2020;12.
36. Zhang K, Bangma CH, Roobol MJ. Prostate cancer screening in Europe and Asia. Asian J Urol. 2017;4(2):86–95.
37. Hinata N, Fujisawa M. Racial differences in prostate cancer characteristics and cancer-specific mortality: an overview. World J Mens Health. 2022;40(2):217.
38. Yii RSL, Lim J, Sothilingam S, et al. Predictive factors of prostate cancer diagnosis with PSA 4.0–10.0 Ng/mL in a multi-ethnic Asian population, Malaysia. Asian J Surg. 2020;43(1):87–94. doi:10.1016/j.asjsur.2019.02.014
39. Lichtensztajn DY, Gomez SL, Sieh W, et al. Prostate cancer risk profiles of Asian-American men: disentangling the effects of immigration status and race/ethnicity. J Urol. 2014;191(4):952–956. doi:10.1016/j.juro.2013.10.075
40. Ye D, Uemura H, Chung BH, et al. Prostate-specific antigen kinetics in Asian patients with metastatic castration-sensitive prostate cancer treated with apalutamide in the TITAN trial: a post hoc analysis. Int J Urol. 2025;32(2):164–172. doi:10.1111/iju.15615
41. Rubilotta E, Balzarro M, Gubbiotti M, et al. Role of bladder emptying on outcomes of transurethral resection of the prostate. Urology. 2023;175:25–28. doi:10.1016/j.urology.2023.02.012
42. Tilki D, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG guidelines on prostate cancer. part II-2024 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2024;86(2):164–182. doi:10.1016/j.eururo.2024.04.010
43. Beckabir W, Wobker SE, Damrauer JS, et al. Spatial relationships in the tumor microenvironment demonstrate association with pathologic response to neoadjuvant chemoimmunotherapy in muscle-invasive bladder cancer. Eur Urol. 2024;85(3):242–253. doi:10.1016/j.eururo.2023.11.008
44. De Nunzio C. Best of 2024 in prostate cancer and prostatic diseases. Prostate Cancer Prostatic Dis. 2025;28(1):1–5. doi:10.1038/s41391-025-00941-4
45. Liu Z, Hong P, He J, et al. Favorable prostate-specific antigen levels correlate with a worse prognosis in high-grade prostate cancer: a population-based analysis. Int J Surg. 2025;111.
46. Hung S, Lin Y-S, Chang Y-H, et al. Management of patients with advanced prostate cancer: report of the Taiwan advanced prostate cancer consensus conference 2022. Urological Sci. 2024;35(4):169–182. doi:10.1097/us9.0000000000000033
47. Carrot A, Oudard S, Colomban O, et al. Prognostic value of the modeled prostate-specific antigen KELIM confirmation in metastatic castration-resistant prostate cancer treated with taxanes in FIRSTANA. JCO Clin Cancer Inform. 2024; 8. doi:10.1200/CCI.23.00208
48. Jeong CW. Prostate-specific antigen-based prostate cancer screening: one for all or individualized for each race? – a narrative review. J Urologic Oncol. 2024;22(1):4–10. doi:10.22465/juo.244600220011
49. Goktas O. Prostate cancer screening with Prostate-Specific Antigen (PSA) testing: a retrospective study. Pak J Med Sci. 2024;40(10):2324–2330. doi:10.12669/pjms.40.10.8558
50. Bergengren O, Pekala KR, Matsoukas K, et al. 2022 update on prostate cancer epidemiology and risk factors-a systematic review. Eur Urol. 2023;84(2):191–206. doi:10.1016/j.eururo.2023.04.021
51. Gandaglia G, Leni R, Bray F, et al. Epidemiology and prevention of prostate cancer. Eur Urol Oncol. 2021;4.
52. Cariolou M, Markozannes G, Becerra-Tomás N, et al. Association between adiposity after diagnosis of prostate cancer and mortality: systematic review and meta-analysis. BMJ Med. 2023;2.
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
Recommended articles
The Prognostic Value of Perioperative Factors on Biochemical Recurrence in Patients Undergoing Radical Prostatectomy
Pyrgidis N, Weinhold P, Schulz GB, Chaloupka M, Berg E, Westhofen T, Rodler S, Keller P, Jokisch F, Stief CG, Marcon J, Bischoff R
Research and Reports in Urology 2025, 17:185-194
Published Date: 27 May 2025