Back to Journals » Nature and Science of Sleep » Volume 16
Self-Reported Symptoms of Obstructive Sleep Apnea are Associated with Increased Risk of Kidney Stones: A Cross-Sectional Study from NHANES 2015-2020
Authors Du D, Luo J, Cai W , Qin J, Yang Y, Hu X, Li X, Luo F, Shen Y
Received 15 August 2024
Accepted for publication 14 November 2024
Published 18 December 2024 Volume 2024:16 Pages 2099—2110
DOI https://doi.org/10.2147/NSS.S491657
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Ahmed BaHammam
Dongru Du,1– 3,* Jianjun Luo,1,4,* Weiling Cai,1,5 Jiangyue Qin,6 Yao Yang,1,2 Xueru Hu,1,2 Xiaohua Li,1,7 Fengming Luo,1– 3 Yongchun Shen1,2
1Department of Pulmonary and Critical Care Medicine, West China Hospital, Sichuan University, Chengdu, 610064, People’s Republic of China; 2State Key Laboratory of Respiratory Health and Multimorbidity, West China Hospital, Sichuan University, Chengdu, 610064, People’s Republic of China; 3Laboratory of Pulmonary Immunology and Inflammation, Frontiers Science Center for Disease-Related Molecular Network, Sichuan University, Chengdu, 610064, People’s Republic of China; 4Department of Intensive Care Unit, The People’s Hospital of Leshan, Leshan, 614000, People’s Republic of China; 5Department of Pulmonary and Critical Care Medicine, The People’s Hospital of Luojiang, Deyang, 618599, People’s Republic of China; 6General Practice Ward/International Medical Center Ward, General Practice Medical Center, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China; 7Department of Pulmonary and Critical Care Medicine, Sixth People’s Hospital of Chengdu, Chengdu, Sichuan, 610051, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Fengming Luo; Yongchun Shen, Email [email protected]; [email protected]
Objective: To investigate whether self-reported symptoms of obstructive sleep apnea (OSA), including snoring, snorting/stopping breathing, and sleepiness, are associated with increased risk of kidney stones.
Methods: This cross-sectional study was conducted based on the 2015– 2020 National Health and Nutrition Examination Survey (NHANES). Self-reported symptoms of OSA and history of kidney stones were diagnosed via questionnaires. Multivariable logistic regression was used to determine the associations between self-reported symptoms of OSA and kidney stones. Subgroup analyses and interaction tests were performed to address this issue further.
Results: A total of 9,973 participants were enrolled, and the prevalence of kidney stones was 10.76%. Although no significant association was observed between frequent snoring and kidney stones after covariate adjustments (OR 1.033, 95% CI 0.726, 1.469 p = 0.850), frequent snorting/stopping breathing was associated with a greater risk of kidney stones after covariate adjustments (OR 1.655, 95% CI 1.262, 2.172, p = 0.002). Participants who often or almost always felt sleepy also had a greater risk of kidney stones after covariate adjustment (OR 1.651, 95% CI 1.222, 2.229; p = 0.004). The interaction tests suggested that marital status (p = 0.015) and smoking status (p < 0.001) significantly interacted with the association between snorting/stopping breathing and kidney stones.
Conclusion: Self-reported frequent snorting/stopping breathing and sleepiness may be associated with increased risk of kidney stones. Although these findings may emphasize prevention of kidney stones in these people, further research was still needed to verify our results.
Keywords: obstructive sleep apnea, snoring, snorting/stopping breathing, sleepiness, kidney stones, NHANES
Introduction
Kidney stones, caused by the abnormal accumulation of crystalline substances in the kidney, are widely prevalent and pose a growing global public health challenge globally.1 A recent study revealed that the prevalence of kidney stones was 11.0%, and there was a substantially greater 12-month incidence of kidney stones in the United States.2 In China, a meta-analysis of 18 articles with 115,087 individuals revealed that the pooled overall prevalence of kidney stones reached 7.54%, which increased with age.3 Moreover, the economic burden of kidney stones is comparable to the combined cost of prostate and bladder cancers.4 However, the pathogenesis and risk factors of kidney stones are complicated and not fully understood.
Accounting for 1/3 of human life, sleep is essential for maintaining multisystem homeostasis and the health status of humans.5 Sleep disorders, such as insomnia, circadian rhythm disruption and sleep-disordered breathing, are now recognized as essential health problems worldwide.6 Sleep disorders have been reported to be associated with multiple diseases such as chronic obstructive pulmonary disease and cancers.7,8 Recent studies have suggested a potential link between sleep disorders and kidney stones.9,10 A cross-sectional study revealed a greater risk of kidney stones in participants who slept less than seven hours per day.9 Another study indicated that circadian rhythm disturbances may affect the pathogenesis of kidney stones through multiple pathways, including microbiota dysbiosis and metabolic disorders.10 However, current studies in this field are still limited, and it is still unclear which kinds of sleep disorders significantly increase the risk of kidney stones.
Obstructive sleep apnea (OSA) was a highly prevalent sleep disorder characterized as persistent upper airway obstruction and intermittent hypoxia.11 Although OSA was reported to have shared risk factors of kidney stones such as male sex and obesity, whether OSA was associated with increased risk of kidney stones was still unclear.11,12 Therefore, this study tried to determine the association between OSA and kidney stones based on the National Health and Nutrition Examination Survey (NHANES). However, the accurate diagnosis of OSA was not available in NHANES due to lack of polysomnography results. To address this issue, the frequency of self-reported typical symptoms of OSA, including snoring, snorting/stopping breathing and excessive daytime sleepiness, was applied as an alternative to OSA diagnosis.
Methods
Study Population
The NHANES is designed to evaluate the health status of the American population and includes approximately 5000 participants to form a nationally representative sample each year.13 The NHANES program was approved by the Ethics Review Board of the National Center for Health Statistics (Protocol #2018-01 and Protocol #2011-17). In this study, data were obtained from NHANES 2015 to 2020, as only these cycles included complete data on self-reported symptoms of OSA. The exclusion criteria were as follows: (1) Unavailable questionnaire data on the occurrence of OSA-related symptoms. (2) Unavailable questionnaire data on the diagnosis of kidney stones. (3) Patients younger than 20 years, pregnant, or without data of other covariates.
Exposure and Outcome
Data on self-reported symptoms of OSA were obtained from the questionnaires. Participants enrolled in this study were asked, “How often do you snore? (Never/Rarely (1–2 times/week)/Occasionally (3–4 times/week)/Frequently (more than 5 times/week))”, “How often do you snort or stop breathing? (Never/Rarely (1–2 times/week)/Occasionally (3–4 times/week)/Frequently (more than 5 times/week))” and “How often do you feel overly sleepy during the day? (Never/Rarely (1/month)/Sometimes (2–4/month)/Often or almost always (more than 5/month))?”. The diagnosis of kidney stones was obtained from another questionnaire, “Ever had kidney stones (yes/no)”.
Covariates
Covariates, which included demographic characteristics (sex, age, race, education status, marital status, poverty-to-income ratio (PIR), body mass index (BMI), and smoking status), comorbidities (hypertension and diabetes), and biochemical profiles (serum calcium, creatine, and uric acid), were selected based on previous studies and data availability from the NHANES database. Never smoking was defined as smoking <100 cigarettes throughout one’s life. The diagnoses of comorbidities were obtained from the questionnaire data. Measurements of standard biochemical profiles can be found in P_BIOPRO (cdc.gov).
Statistical Analysis
Considering the complex probability sampling design of the NHANES database, all statistical analyses were conducted with regard to survey design parameters, including primary sample units and strata. As the NHANES 2017-March 2020 pre-pandemic cycle represented a period of 3.2 years, when combining the NHANES 2015–2016 cycle with this cycle, the weight of NHANES 2015–2016 cycle was multiplied by 2/5.2, while the weight of NHANES 2017-March 2020 pre-pandemic cycle was multiplied by 3.2/5.2, according to the NHANES guidelines (NHANES Analytic Guidance and Brief Overview for the 2017-March 2020 Pre-pandemic Data Files (cdc.gov)). Continuous variables were evaluated using t-tests and presented as means with standard errors (SEs). Categorical variables were evaluated using the chi-square test and presented as percentages with SEs. Multivariate logistic regression analysis was performed to evaluate the association between self-reported symptoms of OSA and kidney stones. The results were analyzed based on the three models. Model 1: Crude model without covariate adjustments. Model 2: Minimally adjusted for sex, age and race. Model 3: Fully adjusted for sex, age, race, education status, marital status, PIR, BMI, smoking status, hypertension status, diabetes status and serum calcium, creatine and uric acid levels. Subgroup analyses and interaction tests were performed to evaluate this association further. The sample size was estimated to ensure the statistical power and reduce the risk of type II errors. The specific effect sizes were evaluated referring to previous related publications, and the probability to detect type II errors was 0.2. All analyses were conducted using R and Empowerstats software (EmpowerStats | Data Analysis for Biostatistics & Epidemiology), and a p value <0.05 was regarded as statistically significant.
Results
Baseline Characteristics of Eligible Participants
A total of 9,973 eligible participants were selected from the NHANES 2015–2020, the process of which was presented in Figure 1. The baseline characteristics of eligible participants were presented in Table 1, with a weighted prevalence of kidney stones of 10.76%. Compared to those without kidney stones, the baseline characteristics of patients with kidney stones were significantly different, except for serum calcium (p = 0.252), PIR (p = 0.549), and snoring (p = 0.090).
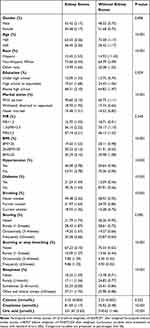 |
Table 1 Baseline Characteristics of Eligible Participants in This Cross-Sectional Study |
 |
Figure 1 Selection of participants from NHANES 2015–2020. |
Association Between Self-Reported Symptoms of OSA and Kidney Stones
Table 2 shows the associations between self-reported symptoms of OSA and the presence of kidney stones. Although frequent snoring (OR 1.425, 95% CI 1.042,1.948; p=0.033) was positively associated with kidney stones, this association disappeared after covariate adjustments (OR 1.033, 95% CI 0.726, 1.469; p = 0.850). Positive associations were observed for those who frequently snorted/stopped breathing (crude model: OR 2.248, 95% CI 1.711, 2.954, p < 0.001; minimally adjusted model: OR 2.153, 95% CI 1.620, 2.860, p < 0.001; fully adjusted model: OR 1.655, 95% CI 1.262, 2.172, p = 0.002) and those who often or almost always felt sleepy (crude model: OR 1.929, 95% CI 1.463, 2.544, p < 0.001; minimally adjusted model: OR 1.978, 95% CI 1.484, 2.638, p < 0.001; fully adjusted model: OR 1.651, 95% CI 1.222, 2.229, p = 0.004), which were still significant after covariate adjustments.
 |
Table 2 Relationship Between Symptoms of OSA and Kidney Stones |
Subgroup Analyses and Interaction Tests
The results of subgroup analyses and interaction tests are presented in Tables 3–4, with all covariates adjusted. Regardless of statistical significance, frequent snorting/stopping breathing was also associated with a greater risk of kidney stones in most subgroups, except for those who were never married (Table 3). Participants who often or almost always felt sleepy also had a greater risk of kidney stones than those who never felt sleepy in all subgroups (Table 4). The results of the interaction tests suggested that marital status (p = 0.015) and smoking status (p < 0.001) significantly interacted with the relationship between snorting/stopping breathing and kidney stones, while no variables significantly interacted with the relationship between sleepiness and kidney stones (all p > 0.05).
 |
Table 3 Subgroup Analysis of Relationship Between Snorting/Stopping Breathing and Kidney Stone |
 |
Table 4 Subgroup Analysis of Relationship Between Sleepiness and Kidney Stone |
Discussion
In this cross-sectional study, we observed that participants who frequently snorted/stopped breathing and often/almost always felt sleepy, rather than those who snored, were at greater risk of kidney stones in American adults. Results of this study suggest that prevention of kidney stones should be given more attention to those with obvious symptoms of snorting/stopping breathing and sleepiness.
There are several types of kidney stones, the majority of which include calcium, uric acid, struvite, and cystine stones. Although previous studies have reported multiple factors that may contribute to kidney stone formation, the specific mechanisms involved warrant further investigation.14 Diet has been regarded as an important factor contributing to the pathogenesis of kidney stones. A recent umbrella review suggested that fructose intake and dietary sodium may serve as risk factors, while intake of coffee and alcohol may serve as protective factors for kidney stones.15 Another study revealed that dietary vinegar enhanced histone acetylation in tubular cells, which may enhance citrate excretion and inhibit calcium excretion in urine, thereby reducing formation of kidney stones.16 Apart from dietary effects, recent studies have also suggested a potential link between sleep disturbances and kidney stone development. Patients with obstructive sleep apnea were reported to have significantly greater levels of 24-h urinary oxalate, uric acid, sodium, potassium, phosphorous, chloride, and sulfate, which may promote kidney stone formation.17 A population-based cohort study suggested that sleep apnea is associated with an increased risk of nephrolithiasis, especially in male participants with metabolic-related comorbidities.18 Short sleep duration and circadian rhythm disruption may also serve as risk factors for kidney stones.9,10 Consistent with previous studies, this cross-sectional study indicated that self-reported snoring was not significantly associated with kidney stones, whereas frequent snorting/stopping breathing and often/always sleepiness were significantly associated with kidney stones, suggesting that severe symptoms of OSA may significantly increase the risk of nephrolithiasis.
The potential mechanisms by which sleep disorders affect the pathogenesis of kidney stones are summarized in Figure 2. Sleep disorders may increase the risk of kidney stones through multiple pathways including microbiota alteration, systematic inflammation, and circadian rhythm disruption. Alterations in the microbiota have been reported in patients with sleep disorders. Shimizu et al noted that shorter sleep duration led to alterations in the gut microbiota via reduced secretion of human defensin 5.19 Matenchuk et al suggested that hypothalamus-pituitary-adrenal axis activation and immune system regulation may serve as potential pathways between sleep disorders and microbiota alterations.20 Evidence also indicates the role of hypoxia in inducing gut microbiota dysbiosis.21 Moreover, alterations in microbiota may also be associated with the development of kidney stones. Al et al compared the gut, oral, and urinary microbiota of 30 healthy controls and 83 stone formers and suggested that multisite microbiota alterations may serve as effective indicators of kidney stone development.22 Mechanistically, oxalate-degrading bacteria, including Oxalobacter formigenes, have been shown to reduce the formation of kidney stones by promoting degradation and modulating the function of the oxalate transporter SLC26A6.9,23 Short-chain fatty acids, a common group of microbiota-derived metabolites, may also affect kidney stone formation via oxalate transporter modulation and GPR43-dependent immune regulation.24,25 Alteration of microbiota may reduce the abundance of oxalate-degrading bacteria and the levels of short-chain fatty acids, thereby leading to the development of kidney stones.26 Additionally, systematic inflammation, which also increases the risk of kidney stones, is widely observed in patients with sleep disorders.21,27 Patients with OSA were characterized as intermittent hypoxia, which then promote production of oxidative stress and sympathetic activation.28 Another study suggested that immune and inflammatory responses may contribute to the pathogenesis of insomnia.29 As for how systematic inflammation contributes to kidney stones, current evidence suggests that kidney crystal deposition may be associated with inflammasome activation, increased reactive oxygen species, and elevated expression of inflammation-related molecules.30 However, more in-depth investigations were still needed for specific mechanisms. Moreover, circadian rhythm disruption has also been reported in patients with sleep disorders.31 Elevated expressions of circadian clock genes were observed in patients with OSA in the morning, which may be associated with their comorbid affective disorders.32 Wang et al reported that the expression of brain and muscle Arnt-like protein 1 (BMAL1) may regulate the nuclear factor erythroid 2-related factor/heme oxygenase-1 (NRF2/HO-1) pathway, thereby reducing urinary stone formation, suggesting that circadian rhythm may also participate in the pathogenesis of kidney stones.33 Compared with the effects of sleep disorders over kidney stones, limited evidence has been reported on how kidney stones contribute to the pathogenesis of sleep disorders. Although it was well-acknowledged that the acute onset of kidney stones may directly disrupt the sleeping process via worsening pain, further research was still needed to understand the intricate pathological process.
The results of interaction tests revealed that marital status and smoking significantly interacted with the relationship between snorting/stopping breathing and kidney stones (both p < 0.05). Previous studies have shown that smoke exposure is strongly associated with impaired health, including an increased risk of kidney stones. Huang et al analyzed data from 2007 to 2018 in the NHANES and reported that current smoking may be associated with an increased risk of kidney stones.34 Another study reported that secondhand smoke is an important risk factor for developing kidney stone disease and that the impact of secondhand smoke is not inferior to that of smoking.35 The mechanism by which smoke exposure causes kidney stones is complex, and harmful substances in tobacco smoke such as cadmium and lead may increase the risk of kidney stones. Additionally, smoking may increase vasopressin levels, which leads to a decrease in urine output and promotes stone formation. Smoking can also release reactive oxygen species, causing kidney damage and accelerating the development of chronic kidney disease, eventually leading to the development of kidney stones.34–37 Therefore, tobacco control is important for the management of kidney stone disease. However, few studies have explored the interactive role of marital status in this relationship, which warrants further investigation. Of course, there are many risk factors for kidney stone disease, and interpretation of the current findings should be combined with other clinical information of the patients.
This study had several limitations. (1) The study had a cross-sectional design, so we could not determine the causal relationship between OSA-related symptoms and kidney stones. Further longitudinal studies with large sample size are needed to verify these findings. (2) Although we adjusted for possible covariates, there were unadjusted confounding factors that may affect our results. For example, as diet plays a crucial role in the pathogenesis of kidney stones, inadequate adjustments of diet-related covariates may induce potential bias, thereby affecting the reliability of these results. Future researches may consider collecting dietary and hydration data from patients in their own centers via more detailed clinical consultation and laboratory tests to control these variables better. (3) The diagnoses of OSA-related symptoms and kidney stones were obtained via a questionnaire, and recall bias was inevitable, which may have affected our results. Moreover, participants with OSA-related symptoms or asymptomatic kidney stone formation may be unaware of their conditions, making it harder for questionnaire screening to reach accurate diagnoses.38 (4) Results of this study were achieved based on a single, cross-sectional dataset, which was associated with insufficient generalizability. (5) Data over subtypes of kidney stones were unavailable in this study, making it hard to determine the detailed relationship between self-reported symptoms of OSA and different types of kidney stones. Moreover, although circadian rhythm has been shown to affect the formation of calcium oxalate stones,33 whether different sleep disorders may interact differently with different types of kidney stones was still unclear. Further studies may record stone composition for patients with kidney stones and conduct clinical and mechanical investigations to address this intricate connection.
Conclusion
Taken together, based on data from NHANES, self-reported obvious snorting/stopping breathing and sleepiness may be associated with increased risk of kidney stones. However, considering the nature of cross-sectional design, potential risk of bias and insufficient generalizability of the results, future prospective, multi-center studies with large sample size are needed to verify our findings.
Data Sharing Statement
All data used in this cross-sectional study are available on the NHANES website (NHANES - National Health and Nutrition Examination Survey Homepage (cdc.gov)).
Ethnic Approval
Due to the approval of Ethics Review Board of National Center for Health Statistics (Protocol #2018-01 and Protocol #2011-17) and the well-obtained written informed consent from all participants in NHANES, the IRB of West China hospital of Sichuan University waived the ethical approval and written informed consent of this study.
Acknowledgments
We gratefully acknowledge the NHANES program and its participants. Figure 2 was created by figdraw.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the National Natural Science Foundation of China (82170046, 31871157, and 81830001) and Program from Science and Technology Department of Sichuan Province (2024NSFSC1522, 2024YFFK0279, and 2024YFHZ0362). These funding agencies were not involved in designing the study, collecting, or analyzing the data, writing the manuscript, or making decisions related to publication.
Disclosure
All authors declared no competing interest for this work.
References
1. Thongprayoon C, Krambeck AE, Rule AD. Determining the true burden of kidney stone disease. Nat Rev Nephrol. 2020;16(12):736–746. doi:10.1038/s41581-020-0320-7
2. Hill AJ, Basourakos SP, Lewicki P, et al. Incidence of kidney stones in the United States: the continuous national health and nutrition examination survey. J Urol. 2022;207(4):851–856. doi:10.1097/JU.0000000000002331
3. Wang W, Fan J, Huang G, et al. Prevalence of kidney stones in mainland China: a systematic review. Sci Rep. 2017;7:41630. doi:10.1038/srep41630
4. Geraghty RM, Cook P, Walker V, Somani BK. Evaluation of the economic burden of kidney stone disease in the UK: a retrospective cohort study with a mean follow-up of 19 years. BJU Int. 2020;125(4):586–594. doi:10.1111/bju.14991
5. Baranwal N, Yu PK, Siegel NS. Sleep physiology, pathophysiology, and sleep hygiene. Prog Cardiovasc Dis. 2023;77:59–69. doi:10.1016/j.pcad.2023.02.005
6. Latreille V. Sleep disorders. Am J Med. 2019;132(3):292–299. doi:10.1016/j.amjmed.2018.09.021
7. Du D, Zhang G, Xu D, et al. Prevalence and clinical characteristics of sleep disorders in chronic obstructive pulmonary disease: a systematic review and meta-analysis. Sleep Med. 2023;112:282–290. doi:10.1016/j.sleep.2023.10.034
8. Mogavero MP, DelRosso LM, Fanfulla F, Bruni O, Ferri R. Sleep disorders and cancer: state of the art and future perspectives. Sleep Med Rev. 2021;56:101409. doi:10.1016/j.smrv.2020.101409
9. Yin S, Wang J, Bai Y, Yang Z, Cui J, Wang J. Association between sleep duration and kidney stones in 34 190 American adults: a cross-sectional analysis of NHANES 2007-2018. Sleep Health. 2022;8(6):671–677. doi:10.1016/j.sleh.2022.08.003
10. He SK, Wang JH, Li T, et al. Sleep and circadian rhythm disturbance in kidney stone disease: a narrative review. Front Endocrinol. 2023;14:1293685. doi:10.3389/fendo.2023.1293685
11. Yeghiazarians Y, Jneid H, Tietjens JR, et al. Obstructive sleep apnea and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;144(3):e56–e67. doi:10.1161/CIR.0000000000000988
12. Khan SR, Pearle MS, Robertson WG, et al. Kidney stones. Nat Rev Dis Primers. 2016;2:16008. doi:10.1038/nrdp.2016.8
13. Luo Z, Wang T, Wu W, Yan S, Chen L. Association between weekend catch-up sleep and depressive symptoms in American adults: finding from NHANES 2017-2020. J Aff Disorders. 2024;354:36–43. doi:10.1016/j.jad.2024.03.008
14. Peerapen P, Thongboonkerd V. Kidney Stone Prevention. Adv Nutr. 2023;14(3):555–569. doi:10.1016/j.advnut.2023.03.002
15. Ma Y, Cheng C, Jian Z, et al. Risk factors for nephrolithiasis formation: an umbrella review. Int J Surg. 2024;110(9):5733–5744. doi:10.1097/JS9.0000000000001719
16. Zhu W, Liu Y, Lan Y, et al. Dietary vinegar prevents kidney stone recurrence via epigenetic regulations. EBioMedicine. 2019;45:231–250. doi:10.1016/j.ebiom.2019.06.004
17. Tallman JE, Stone BV, Sui W, Miller NL, Hsi RS. Association between obstructive sleep apnea and 24-h urine chemistry risk factors for urinary stone disease. Urolithiasis. 2023;51(1):46. doi:10.1007/s00240-023-01421-x
18. Tsai SH, Stoller ML, Sherer BA, Chao ZH, Tung TH. Risk of nephrolithiasis in patients with sleep apnea: a population-based cohort study. J Clin Sleep Med. 2018;14(5):767–773. doi:10.5664/jcsm.7102
19. Shimizu Y, Yamamura R, Yokoi Y, et al. Shorter sleep time relates to lower human defensin 5 secretion and compositional disturbance of the intestinal microbiota accompanied by decreased short-chain fatty acid production. Gut Microbes. 2023;15(1):2190306. doi:10.1080/19490976.2023.2190306
20. Matenchuk BA, Mandhane PJ, Kozyrskyj AL. Sleep, circadian rhythm, and gut microbiota. Sleep Med Rev. 2020;53:101340. doi:10.1016/j.smrv.2020.101340
21. Zhang Y, Luo H, Niu Y, et al. Chronic intermittent hypoxia induces gut microbial dysbiosis and infers metabolic dysfunction in mice. Sleep Med. 2022;91:84–92. doi:10.1016/j.sleep.2022.02.003
22. Al KF, Joris BR, Daisley BA, et al. Multi-site microbiota alteration is a hallmark of kidney stone formation. Microbiome. 2023;11(1):263. doi:10.1186/s40168-023-01703-x
23. Knauf F, Brewer JR, Flavell RA. Immunity, microbiota and kidney disease. Nat Rev Nephrol. 2019;15(5):263–274. doi:10.1038/s41581-019-0118-7
24. Liu Y, Jin X, Ma Y, et al. Short-chain fatty acids reduced renal calcium oxalate stones by regulating the expression of intestinal oxalate transporter SLC26A6. mSystems. 2021;6(6):e0104521. doi:10.1128/mSystems.01045-21
25. Jin X, Jian Z, Chen X, et al. Short chain fatty acids prevent glyoxylate-induced calcium oxalate stones by GPR43-dependent immunomodulatory mechanism. Front Immunol. 2021;12:729382. doi:10.3389/fimmu.2021.729382
26. Miller AW, Penniston KL, Fitzpatrick K, Agudelo J, Tasian G, Lange D. Mechanisms of the intestinal and urinary microbiome in kidney stone disease. Nat Rev Urol. 2022;19(12):695–707. doi:10.1038/s41585-022-00647-5
27. Piber D, Cho JH, Lee O, Lamkin DM, Olmstead R, Irwin MR. Sleep disturbance and activation of cellular and transcriptional mechanisms of inflammation in older adults. Brain Behav Immun. 2022;106:67–75. doi:10.1016/j.bbi.2022.08.004
28. Lv R, Liu X, Zhang Y, et al. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduction Targeted Ther. 2023;8(1):218. doi:10.1038/s41392-023-01496-3
29. Mithani S, Yun S, Leete JJ, et al. Whole blood transcriptome analysis using RNA sequencing in individuals with insomnia disorder and good sleepers: a pilot study. Sleep Med. 2021;80:1–8. doi:10.1016/j.sleep.2021.01.013
30. Khan SR, Canales BK, Dominguez-Gutierrez PR. Randall’s plaque and calcium oxalate stone formation: role for immunity and inflammation. Nat Rev Nephrol. 2021;17(6):417–433. doi:10.1038/s41581-020-00392-1
31. Šmon J, Kočar E, Pintar T, Dolenc-Grošelj L, Rozman D. Is obstructive sleep apnea a circadian rhythm disorder? J Sleep Res. 2023;32(4):e13875. doi:10.1111/jsr.13875
32. Gabryelska A, Turkiewicz S, Kaczmarski P, et al. Circadian clock dysregulation: a potential mechanism of depression in obstructive sleep apnea patients. Transl Psychiatry. 2024;14(1):423. doi:10.1038/s41398-024-03134-0
33. Wang J, Bai Y, Yin S, et al. Circadian clock gene BMAL1 reduces urinary calcium oxalate stones formation by regulating NRF2/HO-1 pathway. Life Sci. 2021;265:118853. doi:10.1016/j.lfs.2020.118853
34. Huang Y, Wang H, Xu C, Zhou F, Su H, Zhang Y. Associations between smoke exposure and kidney stones: results from the NHANES (2007-2018) and Mendelian randomization analysis. Front Med. 2023;10:1218051. doi:10.3389/fmed.2023.1218051
35. Chen YH, Lee JI, Shen JT, et al. The impact of secondhand smoke on the development of kidney stone disease is not inferior to that of smoking: a longitudinal cohort study. BMC Public Health. 2023;23(1):1189. doi:10.1186/s12889-023-16116-6
36. Jones P, Karim Sulaiman S, Gamage KN, Tokas T, Jamnadass E, Somani BK. Do lifestyle factors including smoking, alcohol, and exercise impact your risk of developing kidney stone disease? Outcomes of a systematic review. J Endourol. 2021;35(1):1–7. doi:10.1089/end.2020.0378
37. Day PL, Wermers M, Pazdernik V, Jannetto PJ, Bornhorst JA. Detection of cadmium and lead in kidney stones. Associations with patient demographics, stone composition, and smoking. J App Lab Med. 2023;8(2):330–340. doi:10.1093/jalm/jfac089
38. Billings ME, Cohen RT, Baldwin CM, et al. Disparities in sleep health and potential intervention models: a focused review. Chest. 2021;159(3):1232–1240. doi:10.1016/j.chest.2020.09.249
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


