Back to Journals » Drug Design, Development and Therapy » Volume 19
Semaglutide Alleviates Ovarian Oxidative Stress and Autophagy via the PI3K/AKT/mTOR Pathway in Mice with Polycystic Ovary Syndrome
Authors Guo S, Li X, Liu M, Feng M, Wang X, Xue H , Zhang L
Received 14 February 2025
Accepted for publication 5 May 2025
Published 23 May 2025 Volume 2025:19 Pages 4297—4310
DOI https://doi.org/10.2147/DDDT.S522730
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Muzammal Hussain
Sili Guo,* Xiaohan Li,* Mei Liu, Meiqi Feng, Xi Wang, Haibo Xue, Lei Zhang
Department of Endocrinology and Metabolism, The First School of Clinical Medicine, Binzhou Medical University Hospital, Binzhou Medicial University, Binzhou, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Lei Zhang, Department of Endocrinology and Metabolism, The First School of Clinical Medicine, Binzhou Medical University Hospital, Binzhou Medical University, Binzhou, Shandong, People’s Republic of China, Email [email protected] Haibo Xue, Department of Endocrinology and Metabolism, The First School of Clinical Medicine, Binzhou Medical University Hospital, Binzhou Medical University, Binzhou, Shandong, People’s Republic of China, Email [email protected]
Background: Polycystic ovary syndrome (PCOS) is a typical reproductive endocrine system disease with high incidence rate among childbearing age women. Several clinical data show that glucagon-like peptide-1 receptor agonists (GLP-1RAs) might have therapeutic effects on PCOS, but the mechanisms are still unclear. Here, we aim to assess the effects of semaglutide (a weekly preparation of GLP-1RAs) on PCOS in vivo.
Methods: C57BL/6J female mice aged 3 weeks were subcutaneously injected with dehydroepiandrosterone and fed high-fat diet for 3 weeks to establish PCOS model. Then, we randomly divided the modeled mice into PCOS group (n=6), S-Low group (n=6), and S-High group (n=6). Additionally, six normal mice served as controls. Mice in S-Low and S-High group were intraperitoneally injected with corresponding dose of semaglutide every week for 4 weeks. The estrus cycle was observed daily. At the end of the experiment, body weight, blood glucose, and serum hormone levels were measured. Ovarian morphology was also observed. Then, the oxidative stress markers, autophagy-related proteins and CYP19A1, StAR, and CYP17A1 expression in ovarian tissue were measured. Finally, we used Western blot to detect the expression of PI3K/AKT/mTOR and downstream proteins.
Results: After treatment with semaglutide, the estrous rhythm of PCOS mice was restored, the number of ovarian vesicles decreased, serum hormone imbalance corrected, and glucose tolerance improved. The relative expression of CYP17A1, StAR, Beclin-1, and LC3B, as well as MDA, were significantly reduced, while CYP19A1, p62, GSH, and SOD were significantly increased. Finally, semaglutide alleviates ovarian oxidative stress and autophagy via the PI3K/AKT/mTOR pathway.
Conclusion: Semaglutide alleviates autophagy and ovarian oxidative stress via the PI3K/AKT/mTOR pathway in mice with PCOS.
Keywords: polycystic ovary syndrome, semaglutide, oxidative stress, autophagy
Introduction
Polycystic ovary syndrome (PCOS), a typical reproductive endocrine system disease with high incidence rate among childbearing age women, has developed into one of the important pathological factors leading to female infertility. Approximately 15% of women of childbearing age globally are affected by this syndrome, which can potentially exert long-term and profound negative impacts on patients’ health.1 PCOS is primarily characterized by ovulatory dysfunction, hyperandrogenemia, and polycystic ovarian morphology. This condition often presents with symptoms such as metabolic disorders, irregular menstruation, and clinical manifestations associated with high androgen levels.2 However, the mechanisms underlying these impairments are not yet fully understood. Inflammation, insulin resistance, ovarian oxidative stress and autophagy are the common pathogenesis mechanisms associated with PCOS.3,4 Currently, no specific treatment for PCOS exists. Therefore, it is particularly necessary to seek new treatment strategy for PCOS.
The phenomenon of oxidative stress originates from the imbalance between intracellular oxidants and antioxidant systems, and this biochemical process is essential to the pathological mechanism of PCOS. Excessive reactive oxygen species (ROS) cannot be promptly eliminated, leading to a series of pathological reactions.5 ROS can trigger ion channel opening, lipid peroxidation, protein modification, DNA oxidation, resulting in damage to cell structure and function, and even leading to cell apoptosis.6 Literature has found that oxidative stress is closely associated with a series of negative impacts, including abnormal endocrine function, endometrial damage, poor follicular development, and decreased oocyte quality. These factors collectively contribute to the occurrence and progression of PCOS.7 Therefore, inhibiting oxidative stress is considered a potential means for the prevention and treatment of PCOS.
Autophagy is a crucial molecular mechanism aimed at maintaining the homeostasis of cells and the entire organism.8 This cellular process achieves the maintenance of homeostasis in cells, tissues, and organisms by forming autophagosomes and relying on the lysosomal pathway to degrade organelles and cytoplasmic proteins.9 Recent studies have shown that cells can recognize oxidative stress signals under ischemic and hypoxic environmental conditions, thereby promoting an increase in autophagy activity.10 Furthermore, there is a close association between autophagy and metabolic disorders caused by PCOS. As stated by Zhang et al,11 an increase in autophagy levels within granulosa cells exacerbates insulin resistance. Simultaneously, the accumulation of autophagosomes within the ovary may trigger the death of granulosa cells and inhibit the developmental potential of oocytes.12 In women with PCOS, the levels of autophagy marker proteins were observed to rise in a dose-dependent manner in response to varying concentrations of testosterone.13 PI3K/AKT/mTOR signaling pathway is considered to be the main pathway involved in the initiation and regulation of autophagy, and its activation can inhibit autophagy.14 Quinoa can ameliorate PCOS via regulating gut microbiota through PI3K/AKT/mTOR pathway and autophagy.15 PI3K/AKT/mTOR pathway is also an important signaling pathway to promote cell metabolism and regulate oxidative stress.16,17 Protocatechuic acid can alleviate the symptoms of PCOS in mice via PI3K signaling in granulosa cells (GCs) to relieve ROS pressure and apoptosis.18
GLP-1 receptor agonists (GLP-1RAs) are usually used to treat type 2 diabetes and its complications. Recently, research on GLP-1 RAs mainly focus on the field of obesity. GLP-1 RAs act on GLP-1R in the central and peripheral nervous system to control appetite and delay gastric emptying, thereby increasing satiety and achieving weight loss.19 Due to their significant positive effects on weight loss and metabolic abnormalities, GLP-1RAs serve as clinical treatment options for PCOS in recent years.20 However, the mechanism by which GLP-1RAs improve PCOS is not fully understood. Several studies attribute the improvement of PCOS by GLP-1 to its weight loss effect.20,21 Our previous study found that semaglutide, a novel GLP-1RA, could resist ovarian inflammation through AMPK/SIRT1/NF-κB pathway.22 Existing research have demonstrated that GLP-1RAs can effectively alleviate oxidative stress, decrease endoplasmic reticulum stress, regulate autophagy processes, and promote metabolic balance.23–26 This study aimed to explore whether semaglutide could improve PCOS-related symptoms by regulating ovarian oxidative stress and autophagy in vivo.
Materials and Methods
Animals
High-fat diet (67% maintenance diet for rats and mice +10% lard +20% sucrose +2.5% cholesterol +0.5% sodium cholate) feedstuff and 3-week-female C57BL/6J mice were purchased from Pengyue Experimental Animal Breeding Co Ltd. (License No. SCXK (Shandong) 2023–0002). The experimental environment is set at a temperature of 20°C and a relative humidity of 60% −80%. During the experiment, the mice can eat and drink freely.
PCOS Model Establishment and Intervention
The mice were randomly divided into 2 groups after adaptive feeding for 3 days, namely normal control group (NC, n=6) and model group (n=22). NC group: 0.2 mL sesame oil was subcutaneously injected into the back of neck every day and fed with ordinary feed; The model group was treated with dehydroepiandrosterone (DHEA) dissolved in 0.2 mL sesame oil at 60 mg/kg/d, subcutaneously injected into the back of neck every day, and fed with high-fat diet for 21 days.27,28 Four mice were randomly sacrificed to observe the ovarian morphology and evaluate the modeling. We randomly divided the successfully modeled PCOS mice into three groups: PCOS group (PCOS, n=6), PCOS+semaglutide 0.42 mg/kg/w group (S-Low, n=6), and PCOS+semaglutide 0.84 mg/kg/w group (S-High, n=6). Mice in S-Low and S-High groups were subcutaneously injected with semaglutide lasting for one month. The mice in the NC group and PCOS group were subcutaneously injected with the same volume of physiological saline. We measured the weight of mice once a week and collected vaginal smears every day until the experiment is completed. After the intervention, blood samples and ovarian tissue were obtained. The experimental flowchart is shown in Figure 1.
 |
Figure 1 The operational flowchart of the entire experiment. |
Estrous Cycle Observation
In the experimental operation, the vaginal area needs to be exposed first, and a cotton swab soaked in physiological saline should be used to wipe the external genitalia. Subsequently, inject 5 milliliters of physiological saline into the vagina and repeat this process 4 to 5 times. Next, the absorbed droplets were transferred to a slide and stained with basic methylene blue. The slides were placed under a light microscope to observe the estrous cycle. The estrus cycle is judged by the following: Proestrus, mainly composed of nuclear epithelial cells; Estrus, mainly composed of large, flat, irregularly anucleated keratinocytes; Diestrus, few white blood cells and nucleated epithelial cells appear; Metestrus, mainly composed of white blood cells and nucleated epithelial cells.
Intraperitoneal Glucose Tolerance Tests (IPGTT) and Body Weight Measurement
After fasting 12h, the weight of mice was weighed, and the blood glucose was measured before glucose injection. Glucose (0.2g/mL) was injected intraperitoneally according to body weight (100μL/10g). Tail blood glucose was measured at 15, 30, 60, 90, and 120 min after injection.
Ovarian Dehydration Embedding
Fresh ovarian tissue with 4% paraformaldehyde fixed for 24 hours, and then used tap water rinse 1–2 hours, reoccupy graded ethanol dehydration, embedding xylene to cool naturally. Finally, it was sliced at 4um and stored at 4 °C.
H&E Staining
The experiment was conducted according to the instructions of the HE staining kit (Solarbio, G1120).29 Ovarian tissue slices were first stored at 4 °C and then transferred to a 60 °C environment for baking for 3 to 4 hours. Next, xylene and ethanol would be used for dewaxing treatment. In the staining step, stained with hematoxylin for 2 to 20 minutes, then soaked the slices in distilled water for 2 minutes. Subsequently, the sections were differentiated with differentiation solution for 30 seconds and washed twice with tap water for 5 minutes each time. Then counterstained with eosin for 1 min. Finally, dehydrated with graded ethanol, made xylene transparent, sealed with neutral resin, and took photos under the microscope (Olympus, Japan).
Immunohistochemistry
The experiment was conducted according to the instructions of the immunohistochemistry kit (Boster Biological Technology, Wuhan, China. Catalog#SA1020).30 Ovarian sections were oven-dried at 60 °C for 3–4 hours, hyalinized with xylene twice for 10 minutes each, and then dehydrated through a graded ethanol series (75% ethanol for 2 minutes, 85% ethanol for 2 minutes, 90% ethanol for 2 minutes, 95% ethanol for 2 minutes, 100% absolute ethanol twice for 2 minutes). Subsequently, washed twice with phosphate buffered saline (PBS, Servicebio, CHINA), followed by a 10 minutes repair treatment with sodium citrate solution, and washed twice again with PBS. Subsequently, washed with peroxidase inhibitor for 10 minutes and blocked with bovine serum albumin (BSA, BOSTER, CHINA) for 30 minutes. Prepare the antibody at a dilution ratio of 1:100, then incubated overnight at 4 °C, and finally performed reheating treatment at room temperature. The antibodies used in the study were CYP19A1 antibodies (No. ba3704, BOSTER, China; dilution 1:50;), Beclin-1 antibodies (dilution 1:100; No.#3495, CST, USA), p62 antibodies (dilution 1:500; No. #23214, CST, USA), LC3B antibodies (dilution 1:200; No.#83506, CST, USA) was increased for half an hour and then observed.
Immunofluorescence
For immunofluorescence staining purposes, ovarian tissue sections were processed in the following manner. First performed was the deparaffinization and hydration steps. To block nonspecific binding, bovine serum albumin treatment was applied. Primary antibodies then were incubated overnight at 4°C - CYP17A1 (No. A00615-3, BOSTER, China; dilution 1:400) and StAR (No. 67130-1-ig, San Ying, China; dilution 1:400) Secondary antibodies subsequently were added, with incubation at room temperature lasting one hour. For nuclear counterstaining, 4′,6-diamidino-2-phenylindole(DAPI, #P0131, Beyotime) was used for five minutes. The slices treated with anti-fading sealant need to be sealed and observed under a fluorescence microscope (Olympus, Japan).31
Sex Hormone Measurement
A chemiluminescence immunoassay analyzer (Spring C2200, Zhonghong Detection, China) was used to determine serum estradiol (E2), progesterone hormone (P) and testosterone (T).
Oxidative Stress Index Analysis
Ovarian tissues were collected. Oxidative stress markers were measured with a lipid peroxidation (MDA) detection kit (A003-1-2, Nanjing Jiancheng Bioengineering Institute, China) Antioxidant markers, including superoxide dismutase (SOD) and glutathione (GSH), were evaluated using superoxide dismutase activity detection kit (A001-3-2, Nanjing Jiancheng Bioengineering Institute, China) and glutathione detection kit (A006-2-1, Nanjing Jiancheng Bioengineering Institute, China).
Western Blot
Mice ovarian tissues were immersed in Ripa buffer and phosphatase inhibitors and protease inhibitors were added. The tissue was lysed on ice for 30 min, then triturated with a tissue homogenizer and centrifuged to obtain the supernatant, which was called tissue protein sample. Protein concentration was measured with BCA protein assay reagent (No. AR0146, BOSTER, China). Then an appropriate amount of protein loading buffer was added and electrophoretic separation was performed on SDS-PAGE gels (7.5% or 15%). Proteins were electrophoresed at 80 V (stacking gel) and 120 V (separating gel) using SDS-PAGE. The target protein is then transferred onto a polyvinylidene fluoride (PVDF) membrane (Millipore, USA). Proteins were transferred to PVDF membranes at 400 mA constant current for 90 min in ice-cold transfer buffer. Subsequently, skim milk powder was used for sealing treatment at room temperature for a duration of 2 hours. After the membrane was cut according to the molecular size, the primary antibody was incubated. After sealing, the membrane was incubated with the following antigen-specific primary antibody at 4 °C overnight: PI3K (dilution 1:1000; No. 4292S, CST, USA), p-AKT (dilution 1:1000; No. 4060S, CST, USA), AKT (dilution 1:1000; No. 4691S, CST, USA), mTOR (diluted, 1:1000; No.# 2983, CST, USA), p-mTOR (diluted, 1:1000; No. #2971, CST, USA), p62 (diluted, 1:1000; No. #23214, CST, USA), LC3B (diluted, 1:1000; No.#83506, CST, USA), Beclin-1 (diluted, 1:1000; No.#3495, CST, USA), β-actin (dilution 1:5000; No. BM0627, BOSTER, China). After three membrane washing steps, a diluted secondary antibody solution was introduced and incubated at room temperature for 2 hours to detect the presence of specific reaction products.
Statistical Analysis
The SPSS software (version 26.0, IBM) was performed for all statistical analysis. P-value <0.05 was statistically significant (two side). Data compartment between two groups were used for the Student's t-test, and one-way ANOVA was used to compare data among multiple groups.
Results
Semaglutide Alleviates Sex Hormone Disorders
Serum sex hormones were detected (Figure 2A–C). Compared to NC mice, serum T content of PCOS mice increased significantly, serum E2 content decreased, and serum P content did not change significantly. After treatment with semaglutide, the T content was significantly reduced and a significant rise in E2 levels was dose dependent, but the content of P did not change significantly.
 |
Figure 2 (A–C) The change of sex hormones in peripheral blood. Data are presented as mean±SD. Vs NC, ##P <0.01; Vs PCOS, *P <0.05, **P <0.01; n=6 per group. |
Semaglutide Restores Ovarian Morphology and Estrous Cycle
As shown in Figure 3A, the ovaries of the control group mice exhibited normal follicle distribution at all developmental stages, and no cystic follicle formation was observed. In contrast, the PCOS model group mice exhibited significant structural abnormalities in their ovaries, characterized by an increase in the number of vesicles, a decrease in the number of corpora lutea, and a thinning of the granulosa cell layer. It is worth noting that after intervention with semaglutide, the number of corpus luteum in the experimental group of mice significantly increased, while the number of cystic follicles decreased significantly. This result confirms the reversal effect of semaglutide on the pathological development of ovarian tissue. Through the observation of vaginal smears in Figure 3B, it can be further found that the estrous cycle of the PCOS model group mice is significantly disrupted. As shown in Figure 3C, the estrous cycle of the normal control group mice was maintained within the physiological range of 4–5 days, while the PCOS model group mice showed sustained elongation of the estrous cycle. Experimental data show that semaglutide intervention can effectively improve the disorder of the estrous cycle, which is consistent with the expected results.
Semaglutide Improves Impaired Glucose Tolerance
PCOS mice body weight is significantly higher than in NC group (Figure 4A). The same trend appeared in the comparison of fasting glucose and AUC in IPGTT between the NC group and PCOS group (Figure 4B–C). After treatment with semaglutide, the body weight decreased in a dose-dependent manner. Simultaneously, the impaired glucose tolerance was also improved in a dose-dependent manner.
Semaglutide Regulates the Expression of Ovarian Steroidogenic Enzymes
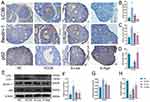
The immunofluorescence results of CYP17A1 and StAR are shown in (Figure 5A–C). As shown in the figure, the expression of CYP17A1 and StAR in the ovary of PCOS mice increased, while the expression of them decreased after semaglutide treatment. The results demonstrated a significant reduction of CYP19A1 in PCOS mice detected by immunohistochemistry technology. Further research found that after implementing intervention measures, the relative expression level of CYP19A1 showed a clear dose–response relationship, that is, with the increase of intervention dose, its expression level correspondingly increased (Figure 5D and E).
Semaglutide Inhibits Oxidative Stress
To evaluate the role of semaglutide on oxidative stress in ovarian tissues, we examined the levels of oxidative stress markers. Compared to NC mice, the mice in PCOS group had significantly higher MDA levels, while lower SOD and GSH levels. After semaglutide intervention, the content of the 3 markers showed dose-dependent improvement and tended towards normal (Figure 6A–C).
 |
Figure 6 (A) SOD activity; (B) GSH content; (C) MDA content. Data are presented as mean±SD. Vs NC, ##P <0.01; Vs PCOS, *P <0.05, **P <0.01; n=6 per group. |
Semaglutide May Regulate Autophagy in the Ovary of PCOS Mice
To evaluate the effect of semaglutide on ovarian autophagy, we detected the autophagy related proteins in the ovary by immunohistochemistry and Western blot. The content of Beclin-1 and LC3B in PCOS group was significantly higher than that in NC group, while the content of p62 was significantly lower than that in NC group. Compared with PCOS group, the expression of Beclin-1 and LC3B showed a significant downward trend after semaglutide treatment, while p62 was significantly higher than that in PCOS group (Figure 7A–H).
Effect of Semaglutide on PI3K/AKT/mTOR Pathway
To explore the mechanism by which semaglutide alleviates ovarian oxidative stress and inhibits autophagy, we detected PI3K/AKT and downstream signaling molecules. Compared to NC group, we found that PI3K/β-actin, p-AKT/AKT, and the ratio of p-mTOR/mTOR was down regulated, while semaglutide up-regulated the above proteins in a dose- response manner (Figure 8A–D).
 |
Figure 8 (A) Western blot of PI3K/AKT/mTOR signaling; (B–D) Protein quantification statistics. Data are presented as mean±SD. Vs NC, ##P <0.01; Vs PCOS, *P <0.05, **P <0.01; n=6 per group. |
Discussion
The abnormal synthesis and accumulation of androgens in PCOS may be the cause of hyperandrogenism in PCOS. The upregulation of CYP17A1 expression in follicular membrane cells of PCOS patients leads to excessive accumulation of androgens and dysregulation of endogenous steroidogenesis. Meanwhile, the downregulation of CYP19A1 expression in GCs inhibits the conversion of androgens to estrogens. Here, the application of semaglutide significantly reversed the effects. In the present study, we found that semaglutide can improve the characterization of PCOS mice by improving autophagy and oxidative stress of ovarian tissue. Mechanistically, semaglutide can upregulate the PI3K/AKT/mTOR signaling pathway to improve PCOS. Our data provide new theoretical basis for the promotion of semaglutide in the clinical application of PCOS.
Although PCOS is a very common disorder among women of childbearing age, there are currently no pharmacological options available to address all clinical manifestations encountered in clinical practice. Clinical evidence has demonstrated the effectiveness of using GLP-1RAs to treat overweight/obese PCOS patients. Salamun et al found that liraglutide increases pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments.32 A randomized clinical trial from Denmark concluded that liraglutide therapy can improve the ovarian dysfunction in an overweight PCOS population and decrease free testosterone levels.33 Similar data was confirmed by a study from China. The research results indicate that for overweight PCOS patients, compared to the treatment plan of using metformin alone, the combination of metformin and liraglutide shows more significant therapeutic effects in regulating endocrine disorders and improving reproductive function.34 The above study attributed the improvement of PCOS symptoms by GLP-1RAs to their weight loss effect. In line with these findings, after treatment with semaglutide, our PCOS mice showed significant weight loss. Can GLP-1RAs treat PCOS through other mechanisms besides weight loss? Xiong et al35 concluded that both semaglutide and liraglutide can improve metabolic and reproductive disorders via modulating the whole structure of gut microbiota in PCOS. Findings from Sun et al suggest that the GLP-1/GLP-1R axis acts on ovarian granulosa cells by modifying the phosphorylation site of forkhead box protein O1 to promote their viability, thereby promoting oocyte maturation in PCOS.36 Besides, Zhang et al37 verified that GLP-1RAs induce browning of white adipose tissue and decrease hyperinsulinemia and hyperandrogenemia in PCOS mice. Our previous study confirmed semaglutide could alleviate ovarian inflammation through AMPK/SIRT1/NF-κB pathway.22 In addition to the mentioned effects, could GLP-1 RAs treat PCOS through other mechanisms?
PCOS is characterized by a state of persistent mild oxidative stress and inflammation, which is closely linked to a range of clinical and metabolic irregularities.38 MDA is an indicator of chronic oxidative stress caused by lipid peroxidation, commonly used to assess the effectiveness of antioxidant interventions.39,40 Antioxidants, including GSH, SOD, and CAT, are crucial for sustaining low levels of ROS. This regulation is essential for the proper functioning of cellular processes.41 Currently, PCOS is considered as a condition characterized by lower levels of antioxidants and oxidative stress.42 The research results showed that in the PCOS group, MDA levels significantly increased, while SOD and GSH activities decreased. The changes in this series of biochemical indicators confirm the crucial role of oxidative stress response in the formation of polycystic ovary syndrome in mice. Therefore, inhibiting oxidative stress may be a potentially effective strategy for the therapeutic of PCOS. Sun et al43 found that kisspeptin improves proliferation and alleviates apoptosis and oxidative stress in GCs. In detail, kisspeptin overexpression enhanced the levels of the antioxidants GSH, SOD and alleviated the accumulation of MDA and ROS in GCs, indicating that kisspeptin protects GCs from oxidative stress.43 PI3K/AKT/mTOR signaling pathway is the main regulatory pathway of oxidative stress.44 Protocatechuic acid can alleviate the symptoms of PCOS in mice via PI3K signaling in GCs to relieve ROS pressure and apoptosis.18 GLP-1 has been proven to improve endothelial cell function through antioxidant stress.45–47 Recently, Li et al48 declared that semaglutide attenuates the doxorubicin-induced mitochondrial damage through the PI3K/AKT pathway. Consistent with this, in our study, we also found that semaglutide can effectively reduce oxidative stress markers in ovarian tissue of PCOS mice.
During the process of follicular atresia, the accumulation of autophagosomes once reaching a certain level can induce apoptosis in GCs.49 In PCOS patients, the autophagic activity of GCs is abnormally enhanced, which is manifested in the significantly increased expression of autophagy-related protein Beclin1.50 In addition, the expression of Beclin1 was positively correlated with serum T and anti-Mullerian hormone. These findings suggest a significant association between abnormal regulation of autophagy in GCs and elevated androgen levels and follicular development disorders in PCOS patients.50 As is well known that the PI3K/AKT/mTOR signaling pathway plays a crucial role in regulating the autophagy process of GCs and oocytes.51 Autophagy is activated after inhibiting the PI3K/AKT/mTOR pathway, leading to degeneration and apoptosis of GCs cells, which in turn triggers follicular atresia.52 Some drugs regulate cell apoptosis and autophagy in PCOS through the PI3K pathway, thereby improving several pathological changes of PCOS. Xie et al53 found that melatonin regulates autophagy in PCOS through the PI3K/AKT pathway, thereby improving ovarian dysfunction. In addition to improving insulin resistance, metformin can also alleviate cellular autophagy via regulating the PI3K/AKT/mTOR pathway to improve ovarian function.54,55 GLP-1 RAs have been found to treat many diseases by alleviating autophagy.56,57 In line with the literatures, our data showed that the expression of autophagy-related proteins LC3II/I and Beclin1 decreased and the expression of autophagy substrate p62 increased after semaglutide treatment. Further, we ascertain that semaglutide inhibits ovarian autophagy by activating PI3K/AKT/mTOR pathway.
This study explored the effects of weekly formulations of GLP-1RAs in PCOS mice; however, there are also some limitations. First, the study only conducted in vivo experiments and lacked data from cellular experiments. Second, the intervention time is relatively short, and it has not been observed whether PCOS characterization in mice will reappear after stopping the intervention. Third, due to experimental conditions and funding constraints, our study did not use inhibitors of relevant signaling pathways to further validate the mechanism of action of semaglutide.
In conclusion, the current study probed the function and mechanism of semaglutide in improving PCOS in vivo experiments, and found that it can be achieved via regulating the PI3K/AKT/mTOR signaling pathway to reduce oxidative stress and autophagy (Figure 9). This study aims to establish a theoretical framework for the clinical application of GLP1-RAs in non-diabetes domains. Notably, these findings require further research to confirm.
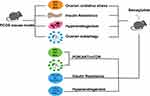 |
Figure 9 Mechanism diagram of semaglutide improving PCOS in mice. |
The mechanism by which semaglutide improves PCOS through the PI3K/AKT/mTOR pathway has potential, but its application needs to be optimized through more rigorous clinical research and precision medicine strategies. In the future, the focus should be on its reproductive safety, long-term efficacy, and integration with other therapies, ultimately achieving effective transformation from basic research to clinical practice.
Data Sharing Statement
Raw data are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
All protocols were approved by the Ethics Committee of Binzhou Medical University Hospital (Ethical Number: 20221014-22 and 20231208-50). All experimental techniques followed the guidelines for the Care and Use of Laboratory Animals and were conducted in compliance with the ARRIVE guidelines.58
Consent for Publication
All authors agree to this submission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by Scientific Research Fund of Binzhou Medical University (BY2021KYQD032); the Scientific Research Initiation Fund of Binzhou Medical University Hospital (2021-05); Shandong Province Medical and Health Technology Project (202403060432).
Disclosure
The authors declare no competing interests in this work.
References
1. Harada M. Pathophysiology of polycystic ovary syndrome revisited: current understanding and perspectives regarding future research. Reprod Med Biol. 2022;21:e12487. doi:10.1002/rmb2.12487
2. Rotterdam ESHRE/ASRM‐Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19:41–47. doi:10.1093/humrep/deh098
3. Manta A, Paschou SA, Isari G, Mavroeidi I, Kalantaridou S, Peppa M. Glycemic index and glycemic load estimates in the dietary approach of polycystic ovary syndrome. Nutrients. 2023;15(15):3483. doi:10.3390/nu15153483
4. Huang Q, Li Y, Chen Z, Ou H, Tan Y, Lin H. Bushenhuoluo decoction improves polycystic ovary syndrome by regulating exosomal miR-30a-5p/ SOCS3/mTOR/NLRP3 signaling-mediated autophagy and pyroptosis. J Ovarian Res. 2024;17:29. doi:10.1186/s13048-024-01355-x
5. Nakamura BN, Lawson G, Chan JY, et al. Knockout of the transcription factor NRF2 disrupts spermatogenesis in an age-dependent manner. Free Radic Biol Med. 2010;49:1368–1379. doi:10.1016/j.freeradbiomed.2010.07.019
6. Wohlgemuth SE, Calvani R, Marzetti E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J Mol Cell Cardiol. 2014;71:62–70. doi:10.1016/j.yjmcc.2014.03.007
7. Kaltsas A, Zikopoulos A, Moustakli E, et al. The silent threat to women’s fertility: uncovering the devastating effects of oxidative stress. Antioxidants. 2023;12(8):1490. doi:10.3390/antiox12081490
8. Klionsky DJ, Petroni G, Amaravadi RK, et al. Autophagy in major human diseases. EMBO j. 2021;40:e108863. doi:10.15252/embj.2021108863
9. Esmaeilian Y, Hela F, Bildik G, et al. Autophagy regulates sex steroid hormone synthesis through lysosomal degradation of lipid droplets in human ovary and testis. Cell Death Dis. 2023;14:342. doi:10.1038/s41419-023-05864-3
10. Guo QQ, Wang SS, Zhang SS, et al. ATM-CHK2-Beclin 1 axis promotes autophagy to maintain ROS homeostasis under oxidative stress. EMBO j. 2020;39:e103111. doi:10.15252/embj.2019103111
11. Zhang C, Hu J, Wang W, Sun Y, Sun K. HMGB1-induced aberrant autophagy contributes to insulin resistance in granulosa cells in PCOS. FASEB j. 2020;34:9563–9574. doi:10.1096/fj.202000605RR
12. Liu M, Zhu H, Zhu Y, Hu X. Guizhi Fuling Wan reduces autophagy of granulosa cell in rats with polycystic ovary syndrome via restoring the PI3K/AKT/mTOR signaling pathway. J Ethnopharmacol. 2021;270:113821. doi:10.1016/j.jep.2021.113821
13. Li X, Qi J, Zhu Q, et al. The role of androgen in autophagy of granulosa cells from PCOS. Gynecol Endocrinol. 2019;35:669–672. doi:10.1080/09513590.2018.1540567
14. Roy B, Pattanaik AK, Das J, et al. Role of PI3K/Akt/mTOR and MEK/ERK pathway in concanavalin A induced autophagy in HeLa cells. Chem Biol Interact. 2014;210:96–102. doi:10.1016/j.cbi.2014.01.003
15. Dou J, Wu Y, Hu R, et al. Quinoa ameliorates polycystic ovary syndrome via regulating gut microbiota through PI3K/AKT/mTOR pathway and autophagy. Nutr Metab. 2024;21:80. doi:10.1186/s12986-024-00855-3
16. Fojtík P, Beckerová D, Holomková K, Šenfluk M, Rotrekl V. Both hypoxia-inducible factor 1 and MAPK signaling pathway attenuate PI3K/AKT via suppression of reactive oxygen species in human pluripotent stem cells. Front Cell Dev Biol. 2020;8:607444. doi:10.3389/fcell.2020.607444
17. Lu Q, Wang WW, Zhang MZ, et al. ROS induces epithelial-mesenchymal transition via the TGF-β1/PI3K/Akt/mTOR pathway in diabetic nephropathy. Exp Ther Med. 2019;17:835–846. doi:10.3892/etm.2018.7014
18. Wang F, Yin Y, Nie X, et al. Protocatechuic acid alleviates polycystic ovary syndrome symptoms in mice by PI3K signaling in granulosa cells to relieve ROS pressure and apoptosis. Gynecol Endocrinol. 2023;39:2228917. doi:10.1080/09513590.2023.2228917
19. Zhang ZY, Yan Q, Wu WH, Zhao Y, Zhang H, Li J. PPAR-alpha/gamma agonists, glucagon-like peptide-1 receptor agonists and metformin for non-alcoholic fatty liver disease: a network meta-analysis. J Int Med Res. 2023;51:3000605231177191. doi:10.1177/03000605231177191
20. Elkind-Hirsch KE, Chappell N, Shaler D, Storment J, Bellanger D. Liraglutide 3 mg on weight, body composition, and hormonal and metabolic parameters in women with obesity and polycystic ovary syndrome: a randomized placebo-controlled-phase 3 study. Fertil Steril. 2022;118:371–381. doi:10.1016/j.fertnstert.2022.04.027
21. Tang L, Yuan L, Yang G, et al. Changes in whole metabolites after exenatide treatment in overweight/obese polycystic ovary syndrome patients. Clin Endocrinol. 2019;91:508–516. doi:10.1111/cen.14056
22. Liu M, Guo S, Li X, et al. Semaglutide alleviates ovary inflammation via the AMPK/SIRT1/NF‑κB signaling pathway in polycystic ovary syndrome mice. Drug Des Devel Ther. 2024;18:3925–3938. doi:10.2147/dddt.S484531
23. Seufert J, Gallwitz B. The extra-pancreatic effects of GLP-1 receptor agonists: a focus on the cardiovascular, gastrointestinal and central nervous systems. Diabetes Obes Metab. 2014;16:673–688. doi:10.1111/dom.12251
24. Nuamnaichati N, Mangmool S, Chattipakorn N, Parichatikanond W. Stimulation of GLP-1 receptor inhibits methylglyoxal-induced mitochondrial dysfunctions in H9c2 cardiomyoblasts: potential role of Epac/PI3K/Akt pathway. Front Pharmacol. 2020;11:805. doi:10.3389/fphar.2020.00805
25. Mangmool S, Hemplueksa P, Parichatikanond W, Chattipakorn N. Epac is required for GLP-1R-mediated inhibition of oxidative stress and apoptosis in cardiomyocytes. Mol Endocrinol. 2015;29:583–596. doi:10.1210/me.2014-1346
26. Abdalla MA, Deshmukh H, Atkin S, Sathyapalan T. The potential role of incretin-based therapies for polycystic ovary syndrome: a narrative review of the current evidence. Ther Adv Endocrinol Metab. 2021;12:2042018821989238. doi:10.1177/2042018821989238
27. Ma Y, Ma Y, Li P, et al. Wnt5a alleviates the symptoms of PCOS by modulating PI3K/AKT/mTOR pathway-mediated autophagy in granulosa cells. Cell Signal. 2025;127:111575. doi:10.1016/j.cellsig.2024.111575
28. Lin B, Guo X, Lu W, et al. Dapagliflozin attenuates fat accumulation and insulin resistance in obese mice with polycystic ovary syndrome. Eur J Pharmacol. 2024;977:176742. doi:10.1016/j.ejphar.2024.176742
29. Sun Y, Chao S, Ouyang H, et al. Hybrid nanogenerator based closed-loop self-powered low-level vagus nerve stimulation system for atrial fibrillation treatment. Sci Bull. 2022;67:1284–1294. doi:10.1016/j.scib.2022.04.002
30. Li F, Deng L, Xu T, et al. Getah virus triggers ROS-mediated autophagy in mouse Leydig cells. Front Microbiol. 2024;15:1519694. doi:10.3389/fmicb.2024.1519694
31. Zeng Y, Yang Q, Ouyang Y, et al. Nickel induces blood-testis barrier damage through ROS-mediated p38 MAPK pathways in mice. Redox Biol. 2023;67:102886. doi:10.1016/j.redox.2023.102886
32. Salamun V, Jensterle M, Janez A, Bokal EV. Liraglutide increases IVF pregnancy rates in obese PCOS women with poor response to first-line reproductive treatments: a pilot randomized study. Eur J Endocrinol. 2018;179:1–11. doi:10.1530/Eje-18-0175
33. Nylander M, Frossing S, Clausen HV, Kistorp C, Faber J, Skouby SO. Effects of liraglutide on ovarian dysfunction in polycystic ovary syndrome: a randomized clinical trial. Reprod Biomed Online. 2017;35:121–127. doi:10.1016/j.rbmo.2017.03.023
34. Xing C, Zhao H, Zhang J, He B. Effect of metformin versus metformin plus liraglutide on gonadal and metabolic profiles in overweight patients with polycystic ovary syndrome. Front Endocrinol. 2022;13:945609. doi:10.3389/fendo.2022.945609
35. Xiong C, Wu J, Ma Y, et al. Effects of glucagon-like peptide-1 receptor agonists on gut microbiota in dehydroepiandrosterone-induced polycystic ovary syndrome mice: compared evaluation of liraglutide and semaglutide intervention. Diabetes Metab Syndr Obes. 2024;17:865–880. doi:10.2147/DMSO.S451129
36. Sun Z, Li P, Wang X, et al. GLP-1/GLP-1R signaling regulates ovarian PCOS-associated granulosa cells proliferation and antiapoptosis by modification of forkhead box protein O1 phosphorylation sites. Int J Endocrinol. 2020;2020:1484321. doi:10.1155/2020/1484321
37. Zhang Y, Lin Y, Li G, et al. Glucagon-like peptide-1 receptor agonists decrease hyperinsulinemia and hyperandrogenemia in dehydroepiandrosterone-induced polycystic ovary syndrome mice and are associated with mitigating inflammation and inducing browning of white adipose tissuedagger. Biol Reprod. 2023;108:945–959. doi:10.1093/biolre/ioad032
38. Mousavi R, Alizadeh M, Asghari Jafarabadi M, et al. Effects of melatonin and/or magnesium supplementation on biomarkers of inflammation and oxidative stress in women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled trial. Biol Trace Elem Res. 2022;200:1010–1019. doi:10.1007/s12011-021-02725-y
39. Góth L, Rass P, Páy A. Catalase enzyme mutations and their association with diseases. Mol Diagn. 2004;8:141–149. doi:10.1007/bf03260057
40. Guentsch A, Preshaw PM, Bremer-Streck S, Klinger G, Glockmann E, Sigusch BW. Lipid peroxidation and antioxidant activity in saliva of periodontitis patients: effect of smoking and periodontal treatment. Clin Oral Investig. 2008;12:345–352. doi:10.1007/s00784-008-0202-z
41. Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–288. doi:10.1093/humupd/dms059
42. Macut D, Bjekić-Macut J, Savić-Radojević A. Dyslipidemia and oxidative stress in PCOS. Front Horm Res. 2013;40:51–63. doi:10.1159/000341683
43. Sun P, Zhang Y, Sun L, Sun N, Wang J, Ma H. Kisspeptin regulates the proliferation and apoptosis of ovary granulosa cells in polycystic ovary syndrome by modulating the PI3K/AKT/ERK signalling pathway. BMC Womens Health. 2023;23:15. doi:10.1186/s12905-022-02154-6
44. Liao J, Liu B, Chen K, et al. Galangin attenuates oxidative stress-mediated apoptosis in high glucose-induced renal tubular epithelial cells through modulating renin-angiotensin system and PI3K/AKT/mTOR pathway. Toxicol Res. 2021;10:551–560. doi:10.1093/toxres/tfab009
45. Anagnostis P, Athyros VG, Adamidou F, et al. Glucagon-like peptide-1-based therapies and cardiovascular disease: looking beyond glycaemic control. Diabetes Obes Metab. 2011;13:302–312. doi:10.1111/j.1463-1326.2010.01345.x
46. Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol. 2010;30:1407–1414. doi:10.1161/atvbaha.110.206425
47. Ceriello A, Novials A, Canivell S, et al. Simultaneous GLP-1 and insulin administration acutely enhances their vasodilatory, antiinflammatory, and antioxidant action in type 2 diabetes. Diabetes Care. 2014;37:1938–1943. doi:10.2337/dc13-2618
48. Li X, Luo W, Tang Y, et al. Semaglutide attenuates doxorubicin-induced cardiotoxicity by ameliorating BNIP3-Mediated mitochondrial dysfunction. Redox Biol. 2024;72:103129. doi:10.1016/j.redox.2024.103129
49. Li M, Xiao YB, Wei L, Liu Q, Liu PY, Yao JF. Beneficial effects of traditional Chinese medicine in the treatment of premature ovarian failure. Evid Based Complement Alternat Med. 2022;2022:5413504. doi:10.1155/2022/5413504
50. Li D, You Y, Bi FF, et al. Autophagy is activated in the ovarian tissue of polycystic ovary syndrome. Reproduction. 2018;155:85–92. doi:10.1530/rep-17-0499
51. Tong C, Wu Y, Zhang L, Yu Y. Insulin resistance, autophagy and apoptosis in patients with polycystic ovary syndrome: association with PI3K signaling pathway. Front Endocrinol. 2022;13:1091147. doi:10.3389/fendo.2022.1091147
52. Choi J, Jo M, Lee E, Choi D. AKT is involved in granulosa cell autophagy regulation via mTOR signaling during rat follicular development and atresia. Reproduction. 2014;147:73–80. doi:10.1530/REP-13-0386
53. Xie F, Zhang J, Zhai M, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162:73–82. doi:10.1530/REP-20-0643
54. Lu G, Wu Z, Shang J, Xie Z, Chen C, Zhang C. The effects of metformin on autophagy. Biomed Pharmacother. 2021;137:111286. doi:10.1016/j.biopha.2021.111286
55. Xu B, Dai W, Liu L, et al. Metformin ameliorates polycystic ovary syndrome in a rat model by decreasing excessive autophagy in ovarian granulosa cells via the PI3K/AKT/mTOR pathway. Endocr J. 2022;69:863–875. doi:10.1507/endocrj.EJ21-0480
56. Cai X, Li J, Wang M, et al. GLP-1 treatment improves diabetic retinopathy by alleviating autophagy through GLP-1R-ERK1/2-HDAC6 signaling pathway. Int J Med Sci. 2017;14:1203–1212. doi:10.7150/ijms.20962
57. Cai X, She M, Xu M, et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int J Biol Sci. 2018;14:1696–1708. doi:10.7150/ijbs.27774
58. Percie du Sert N, Hurst V, Ahluwalia A, et al. The ARRIVE guidelines 2.0: updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617–3624. doi:10.1111/bph.15193
 © 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2025 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, 4.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.